Abstract
Stenotrophomonas maltophilia is a pathogen that causes infections mainly in immunocompromised patients. Despite increased S. maltophilia isolation from respiratory specimens of patients with cystic fibrosis (CF), the real contribution of the microorganism to CF pathogenesis still needs to be clarified. The aim of the present study was to evaluate the pathogenic role of S. maltophilia in CF patients by using a model of acute respiratory infection in DBA/2 mice following a single exposure to aerosolized bacteria. The pulmonary bacterial load was stable until day 3 and then decreased significantly from day 3 through day 14, when the bacterial load became undetectable in all infected mice. Infection disseminated in most mice, although at a very low level. Severe effects (swollen lungs, large atelectasis, pleural adhesion, and hemorrhages) of lung pathology were observed on days 3, 7, and 14. The clearance of S. maltophilia observed in DBA/2 mouse lungs was clearly associated with an early and intense bronchial and alveolar inflammatory response, which is mediated primarily by neutrophils. Significantly higher levels of interleukin-1β (IL-1β), IL-6, IL-12, gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), GROα/KC, MCP-1/JE, MCP-5, macrophage inflammatory protein 1α (MIP-1α), MIP-2, and TARC were observed in infected mice on day 1 with respect to controls. Excessive pulmonary infection and inflammation caused systemic effects, manifested by weight loss, and finally caused a high mortality rate. Taken together, our results show that S. maltophilia is not just a bystander in CF patients but has the potential to contribute to the inflammatory process that compromises respiratory function.
Cystic fibrosis (CF) has a peculiar set of bacterial pathogens, including Staphylococcus aureus, usually acquired in the early stages, and Pseudomonas aeruginosa, which will eventually infect up to 80% of adults with CF (21). Aggressive, early treatment with anti-pseudomonad antibiotics results in an improvement in lung function and prognosis; however, the selective pressure of antibiotics on bacterial species and the longer survival of patients have been associated with the emergence of new pathogens, such as Stenotrophomonas maltophilia (2, 14, 15, 50).
An increasing incidence of S. maltophilia isolates has been reported in some CF centers during the last decade (2, 9, 15, 22, 30). In the United States, the frequency of S. maltophilia isolation from CF patients increased rapidly, from around 3% in 1993 to 12.1% in 2006 (21). A prevalence of up to 25% has been reported in Europe (47), with rates ranging from 7 to 11% in different Italian centers (34, 46). Spicuzza et al. (46), in a retrospective evaluation of a cohort of Italian CF patients from 1996 to 2006, found that S. maltophilia was the only truly emerging pathogen, as it had never been isolated until 2004, when an incidence of 7% among all patients was recorded and remained constant through 2006. In non-CF patients (e.g., immunocompromised or intensive care unit patients), exposure to wide-spectrum antimicrobial drugs, long-term antimicrobial therapy, previous pulmonary infections, and chronic respiratory disease contribute to S. maltophilia acquisition and increase the risk for respiratory infection with this microorganism (49, 51, 52). All of these risk factors are present in the CF population (49).
Despite increased S. maltophilia isolation from CF patients, its potential for pathogenicity remains undetermined because of conflicting clinical results from studies investigating the correlation between the presence of this microorganism and lung damage. Although Karpati et al. (30) reported that prolonged infection with S. maltophilia was generally associated with worse lung function in CF patients, most studies reported only a mild effect on lung function (2, 14, 15, 29, 46, 49).
For these reasons, studies using in vivo models which more closely mimic CF pulmonary tissues are certainly needed to better clarify the pathogenic role of S. maltophilia in CF patients.
Therefore, in the present work, we infected DBA/2 mouse lungs with an S. maltophilia CF strain by using an aerosol delivery technology in order to simulate more closely the airborne transmission thought to be important in naturally occurring CF pulmonary infections (53). Using this approach, for the first time we developed a model of acute respiratory infection by S. maltophilia to investigate bacterial clearance, histological damage, and inflammatory response in the lungs of infected mice relative to those in normal controls.
MATERIALS AND METHODS
Mice.
Seven-week-old female (n = 46) and male (n = 46) specific-pathogen-free DBA/2 inbred mice (Charles River Laboratories Italia, Calco, Italy) were used in all experiments. Animals were housed, bred, and maintained in a barrier facility unit under specific-pathogen-free conditions with a 12-h light-dark cycle. Six to 12 animals were kept in polycarbonate sterile microisolator cages and maintained in a ventilated HEPA-filtered rack. All mice had access ad libitum to sterile acidified water and irradiated diet. All procedures involving mice were reviewed and approved by the Animal Care and Use Committee of G. d'Annunzio University of Chieti-Pescara.
Bacterial strain and growth conditions.
S. maltophilia strain OBGTC9, originally isolated from the sputum of a CF patient admitted to the Cystic Fibrosis Unit of the Pediatric Hospital Bambino Gesù of Rome, was used in all experiments. This strain was selected for its strong ability to adhere to CF-derived bronchial epithelial IB3-1 cells, a feature highly conserved in S. maltophilia CF strains (17). Bacterial stocks were maintained at −80°C until use.
To prepare the infectious dose for nebulization, S. maltophilia was grown with agitation (130 rpm) in 200 ml of Trypticase soy broth (Oxoid SpA, Garbagnate Milanese, Milan, Italy) for 16 h at 37°C and harvested by centrifugation at 3,220 × g for 10 min at 4°C. Pellets were then washed twice and resuspended in cold 1% phosphate-buffered saline (PBS). Cell density was adjusted to about 1.0 × 1010 to 3.0 × 1010 CFU/ml, and 8 ml of this standardized suspension was used for nebulization. The inoculum concentration was confirmed by serial plating after each experiment.
Experimental model of infection.
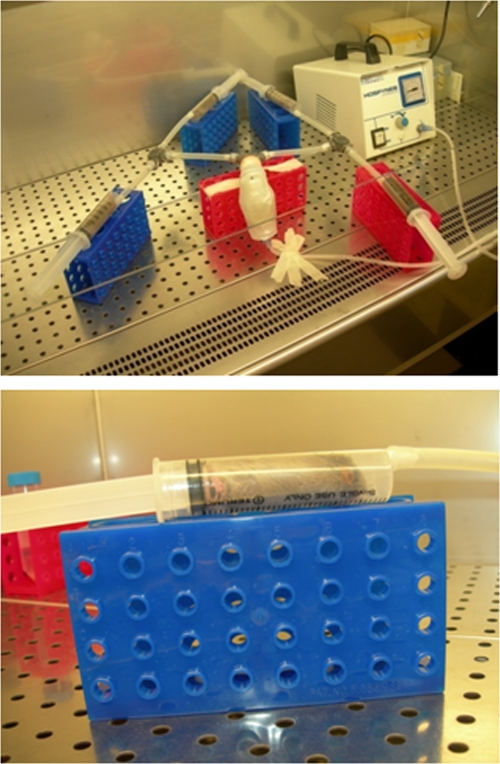
For airborne lung infection, a home-made aerosol dispersal system housed in a laminar-flow biological safety cabinet was used (Fig. 1). This device allows for infection of up to four mice simultaneously.
FIG. 1.
Aerosol delivery system. The system, housed in a biosafety cabinet, allowed us to simultaneously expose up to 4 mice to aerosol preparations. A small compressor transferred a bacterial suspension as an aerosol to each of the four inhalation chambers, each consisting of a 30-ml syringe. The standard aerosol exposure cycle was as follows: 60 min for nebulization (infection), 5 min for cloud decay, and UV irradiation for 5 min (decontamination).
The standardized bacterial suspension (1.0 × 1010 to 3.0 × 1010 CFU/ml) was nebulized (minimum flow rate, 5 liters/min; operating pressure, 60 kPa) and transferred by a piston compressor (Hospyneb Professional; 3A Health Care) to each of the four inhalation chambers, each consisting of a 30-ml syringe (Terumo). Before exposure to infected aerosol, mice were weighed and then inserted into the syringe, introducing the head initially and then the rest of the body by pushing them gently and successively repositioning the plunger. The chamber was then connected to the device for nebulization.
A standard aerosol exposure cycle consisted of 60 min for nebulization and 5 min for cloud decay, and finally, mice were externally decontaminated by exposure for 5 min to UV irradiation.
This inhalation exposure system allowed us to deliver nebulized bacteria in distal airways uniformly throughout the lungs, as confirmed by histological analysis (data not shown). Nebulization of 8 ml of a suspension containing 1.0 × 1010 to 3.0 × 1010 CFU/ml delivered a reproducible number of bacteria in both lungs and among all animals simultaneously exposed, as confirmed by culture analysis, yielding low mouse-to-mouse variability in the initial doses delivered into the lung (mean ± standard deviation [SD], 4.8 × 106 ± 8.6 × 105 CFU/lung; coefficient of variation, 17.8% [results are from two independent experiments with eight animals each]).
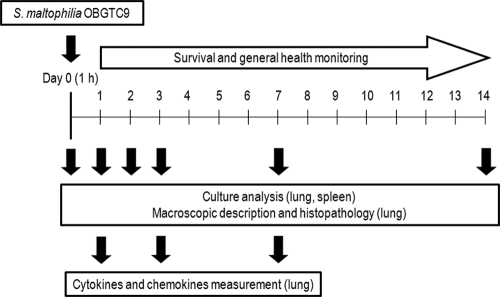
Experimental design.
The experimental layout is shown in Fig. 2. Age- and gender-matched DBA/2 mouse groups were randomized at the time of exposure to receive an aerosol with PBS containing S. maltophilia OBGTC9 (12 mice/group) or one with PBS only (6 mice/group). The general health of the mice was monitored daily, with mice considered unwell when a >10% weight loss or general ill appearance (ruffled coat, huddled position, or lack of retreat in handler's presence) was observed with respect to controls. Mice were sacrificed by CO2 inhalation at 1, 2, 3, 7, and 14 days postexposure. To determine the initial bacterial deposition in the lungs, mice (n = 8) were sacrificed immediately (1 h) after the standard exposure cycle. At each time point for each group, the lungs were observed in situ for macroscopic analysis and then removed en bloc from the chest via sterile excision. In each group, 8 (infected group) or 4 (control group) lungs were randomly assigned to one of the three following outcome measures: (i) histological analysis, (ii) quantitative bacteriology, and (iii) cytokine/chemokine measurements. To determine whether there had been dissemination of bacteria from the lung, resulting in bacteremia, at each time point the spleen of each mouse was homogenized and cultured for quantitative analysis.
FIG. 2.
Experimental design. Groups of mice were exposed to aerosol with sterile PBS alone (control group; 6 mice/group) or containing S. maltophilia OBGTC9 (infected group; 12 mice/group). On day 0 (1 h after exposure), the pulmonary bacterial load of DBA/2 mice (n = 8) was assessed. Mice were then sacrificed on days 1, 2, 3, 7, and 14 after exposure for microbiological analysis of the lung and spleen, macroscopic description and histopathology of lungs, and measurement of pulmonary cytokines/chemokines. All mice were further monitored daily for survival and general health.
Lung macroscopic and histopathological analyses.
The lungs were scored both in situ and after removal from the thoracic cavity according to the method proposed by Johansen et al. (27), as follows: +1, normal; +2, swollen lungs, hyperemia, and small atelectasis; +3, pleural adhesion, atelectasis, and multiple small abscesses; and +4, large abscesses, large atelectasis, and hemorrhages.
For histopathological examination, the isolated lungs were immediately fixed in 10% neutral buffered formalin, and each was then sectioned once along the long axis of the lobe. Tissue sections (thickness, 3 μm) were obtained from dorsal lung surfaces, throughout the tissue block and at regular intervals of 50 μm. Sections were then stained with Giemsa stain, and the degree of inflammation was scored by using a five-point system proposed by Dubin and Kolls (19). Ten fields were evaluated per lung, at low (×10), medium (×20), and high (×63 and ×100) magnifications. Histopathological evaluation was done by investigators who were blinded to the sample origin.
Quantitative bacteriology.
Pulmonary clearance and occurrence of disseminated infection were monitored by quantitative bacteriology of lung and spleen homogenates, respectively. Briefly, lungs were homogenized (24,000 × g/min) on ice in 2 ml of sterile PBS by use of an Ultra-Turrax T25-Basic homogenizer (IKA-Werke GmbH & Co. KG, Germany). The homogenizer was disinfected between each sample, using absolute ethanol (to avoid cross-contamination), and was rinsed twice with sterile PBS (to avoid inhibition of bacterial growth in the subsequent sample). Tenfold serial dilutions of lung homogenates were plated in triplicate on Mueller-Hinton agar (Oxoid SpA), and the number of colonies was counted 24 h after incubation at 37°C. Bacterial colony counts from each lung were normalized according to the wet weight of lung and then calculated as CFU/g, averaged, and compared between groups.
To assess bacterial dissemination, the spleen was also removed from each mouse in each group at each time point and homogenized as described for the lungs. Serial dilutions were also plated and analyzed for colony counts. The number of animals with a spleen positive for S. maltophilia was counted, and values were compared as percentages for each animal group and time point.
Cytokine and chemokine measurements.
A protease inhibitor cocktail (Pierce, Rockford, IL) was added to the lung samples immediately after collection. Lung homogenates were centrifuged at 1,500 × g at 4°C for 10 min, and then the supernatants were removed, aliquoted, and stored at −80°C until their use. The levels of 9 cytokines (interleukin-1β [IL-1β], IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p70, gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) and 9 chemokines (keratinocyte-derived cytokine [GROα/KC], monocyte chemotactic protein 1 [MCP-1/JE], macrophage chemoattractant protein 5 [MCP-5], macrophage inflammatory protein 1α [MIP-1α], MIP-2, regulated on activation, normal T-cell expressed and secreted [RANTES], thymus- and activation-regulated chemokine [TARC], eotaxin, and stromal cell-derived factor 1β [SDF-1β]) were simultaneously measured in supernatants from lung homogenates by a multiplex sandwich enzyme-linked immunosorbent assay (ELISA) system based on chemiluminescence detection (SearchLight chemiluminescent array kits; Endogen) according to the manufacturer's recommendations. The cytokine and chemokine levels were normalized according to the wet weight of lung tissue and are reported as pg/mg. The detection limits were 31.3 pg/ml (IFN-γ), 12.5 pg/ml (IL-1β and TNF-α), 3.1 pg/ml (IL-2, IL-4, IL-10, and IL-12 p70), 6.3 pg/ml (IL-5), 21.9 pg/ml (IL-6), 0.8 pg/ml (eotaxin), 1.6 pg/ml (MCP-5), 3.0 pg/ml (MIP-1α, RANTES, and MCP-1/JE), 6.0 pg/ml (MIP-2, TARC, and GROα/KC), and 37.5 pg/ml (SDF-1β).
Statistical analysis.
All analyses were conducted with GraphPad Prism 4.0 software (GraphPad Software Inc., San Diego, CA). Differences between studied groups were evaluated using unpaired Student's t test or analysis of variance (ANOVA) followed by Bonferroni's multiple comparison posttest for parametric data, the Kruskal-Wallis test followed by Dunn's multiple comparison posttest for nonparametric data, and the chi-square test for percentages. Survival of S. maltophilia-infected and control mice was compared by Kaplan-Meier survival analysis and the log rank test. With regard to cytokine and chemokine expression, cluster analysis was performed by exporting logarithmic ratios of exposed to control values at each time point for each cytokine/chemokine into PermutMatrix software, version 1.9.3 (http://www.lirmm.fr/∼caraux/PermutMatrix/). Hierarchical clustering was performed, using the Euclidean distance between pairs of observations and average linkages, to determine the degree of association (distance) between sets of observations. The results are presented as dendrograms.
RESULTS
Mouse health and survival monitoring.
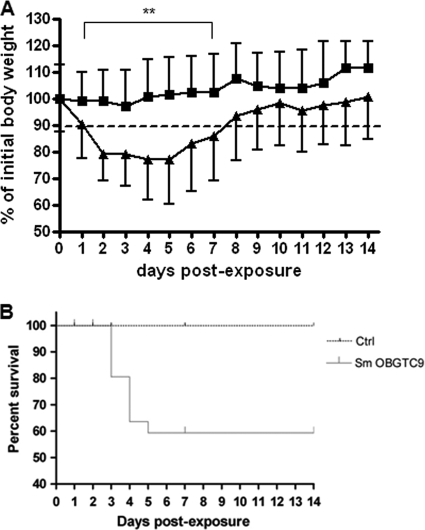
The mean body weight before infection was 19.1 ± 1.4 g for female mice and 23.3 ± 1.7 g for male mice. The changes in body weight of infected and control mice over time, expressed as percentages of initial body weights, are shown in Fig. 3A. Mice infected with S. maltophilia lost more than 10% of their body weight from day 1 through day 7, and during this period the mean weight of infected mice was significantly (P < 0.01) lower than that of control mice. By day 5, infected mice slowly started regaining weight, although they did not regain it completely during the period monitored. Control mice lost up to 5% of their body weight during the first 3 days and then started gaining weight, with their weight regained completely on day 4. The infected mice usually showed symptoms of slow responsiveness and piloerection from day 1 through day 6 and appeared healthy the next day.
FIG. 3.
(A) Weight monitoring during S. maltophilia lung infection. DBA/2 mice (n = 92) were exposed on day 0 to aerosolized S. maltophilia OBGTC9 (▴) or PBS only (▪) and were examined daily for weight loss during the course of infection. The dotted line shows a 10% weight loss with regard to mean body weight before infection. **, P < 0.01 for control versus infected mice (unpaired Student's t test). Error bars represent SD. (B) Survival of DBA/2 mice after pulmonary infection with S. maltophilia OBGTC9. Results are the combination of two independent experiments (n = 28 for uninfected mice; n = 56 for infected mice) monitored over 14 days. The difference in survival between infected and control mice was statistically significant (P < 0.01; log rank test).
The survival of DBA/2 mice was monitored over a period of 14 days (Fig. 3B). Among 56 DBA/2 mice infected with S. maltophilia OBGTC9, a total of 12 mice died (Kaplan-Meier survival proportion of 59.4%): 7 mice died on day 3 (80.5% survival proportion), 4 mice died on day 4 (63.6% survival proportion), and 1 mouse died on day 5 (59.4% survival proportion). Mortality was not observed in control mice. The difference in survival between infected and control mice was statistically significant (P < 0.01).
Kinetics of bacterial burden in the lungs and dissemination.
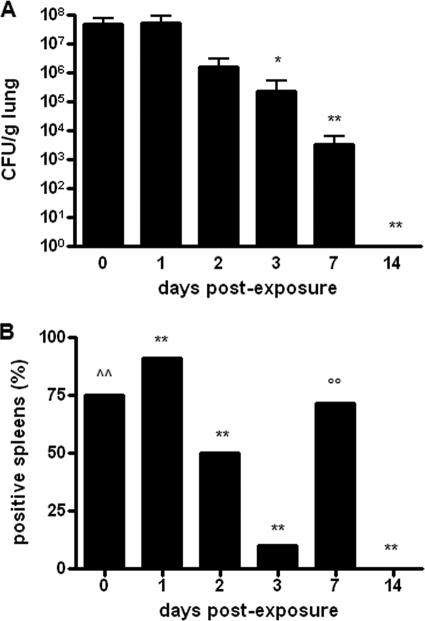
To explore the kinetics of S. maltophilia OBGTC9 infection in DBA/2 mouse lungs, bacterial loads were evaluated in lung tissue homogenates on day 0 (1 h), 1, 2, 3, 7, and 14 after a single exposure to S. maltophilia aerosol (Fig. 4A). The initial deposition of S. maltophilia observed after 1 h (day 0) was 4.9 × 107 ± 3.0 × 107 CFU/g. Bacterial loads decreased from day 1 to day 2, although not significantly (day 1, 5.2 × 107 ± 3.9 × 107 CFU/g; day 2, 1.5 × 106 ± 1.5 × 106 CFU/g; P > 0.05). A statistically significant decrease was observed from day 3 (2.3 × 105 ± 2.9 × 105 CFU/g; P < 0.05 versus day 0 and day 1) through day 7 (3.5 × 103 ± 3.0 × 103 CFU/g; P < 0.01 versus day 0 and day 1). On day 14, bacterial loads became undetectable in all infected mice. No S. maltophilia growth was observed at any time point in the lung homogenates from control mice.
FIG. 4.
Lung clearance and dissemination of S. maltophilia infection. (A) DBA/2 mouse (n = 92) lung clearance kinetics after respiratory exposure to S. maltophilia strain OBGTC9. Lungs were collected, homogenized, and cultured for bacterial counts at 0 (1 h), 1, 2, 3, 7, and 14 days postexposure. Results were normalized to the lung wet weight (CFU/g) and are shown as means + SD. *, P < 0.05; **, P < 0.01 versus day 0 (1 h) and day 1 (ANOVA [P < 0.0001] followed by Bonferroni's multiple comparison posttest). (B) Percentages of DBA/2 mice (n = 92) with spleens positive for S. maltophilia following lung infection. Spleens were collected, homogenized, and cultured for bacterial counts after 0 (1 h), 1, 2, 3, 7, and 14 days. Results are expressed as percentages of spleens positive for S. maltophilia at culture analysis. ∧∧, P < 0.001 versus day 1, day 2, day 3, and day 14; **, P < 0.001 versus each time point; ○○, P < 0.001 versus day 1, day 2, day 3, and day 14 (chi-square test).
To evaluate the invasiveness of S. maltophilia, we carried out microbiological analysis of spleen homogenates (Fig. 4B). Overall, S. maltophilia was found in 59.1% (26 of 44 spleens) of spleen samples tested, although the bacterial counts were generally very low at each time point, i.e., 1 h (positive spleens, 6 of 8 [75%]; bacterial load, 80 ± 83 CFU), day 1 (10 of 11 spleens [90.9%]; 7 ± 6 CFU), day 2 (4 of 8 spleens [50%]; 4 ± 2 CFU), day 3 (1 of 10 spleens [10%]; 1 CFU), and day 7 (5 of 7 spleens [71.4%]; 9 ± 6 CFU) after exposure. The highest percentage of S. maltophilia-positive spleens was observed on day 1 (P < 0.001), while no bacteria were isolated from spleens on day 14. Spleens from control mice were negative for S. maltophilia.
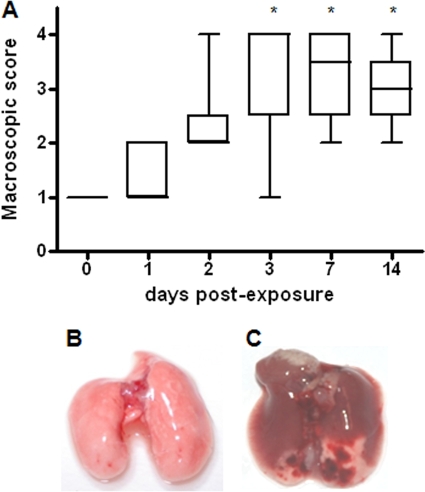
Macroscopic lung pathology.
Macroscopic DBA/2 mouse lung pathologies, assessed by using a four-point scoring system (19), are summarized in Fig. 5. More-severe changes in lung pathology were observed in infected mice on days 3, 7, and 14 than on day 1 (P < 0.05). In particular, a median score of +1 (normal lungs) was observed after 1 h and on day 1, a median score of +2 (swollen lung, hyperemia, and small atelectasis) was observed on day 2, the maximum score of +4 (large atelectasis and hemorrhages) was observed on day 3 (Fig. 5C), and finally, a median score of +3 (pleural adhesion and atelectasis) was observed on days 7 and 14. Necropsy of mice that succumbed to infection revealed a consolidated lung, marked edema, and hemorrhages. Control mice showed a score of +1 (normal lungs) during the 14 days monitored (Fig. 5B).
FIG. 5.
Macroscopic pathology of DBA/2 mouse lungs infected with S. maltophilia strain OBGTC9. (A) Macroscopic lung pathology in DBA/2 mice (n = 92) assessed on day 0 (1 h), 1, 2, 3, 7, and 14 postexposure by use of a four-point scoring system proposed by Dubin and Kolls (19). Results are shown as follows: the line within each box is the median; the upper and lower lines of the box are the 75th and 25th percentiles, respectively; and the whiskers are the highest and lowest values. *, P < 0.05 versus day 1 (Kruskal-Wallis test [P < 0.01] followed by Dunn's multiple comparison posttest). (B) Photograph of uninfected mouse lung on day 3. (C) Photograph of S. maltophilia-infected mouse lung on day 3.
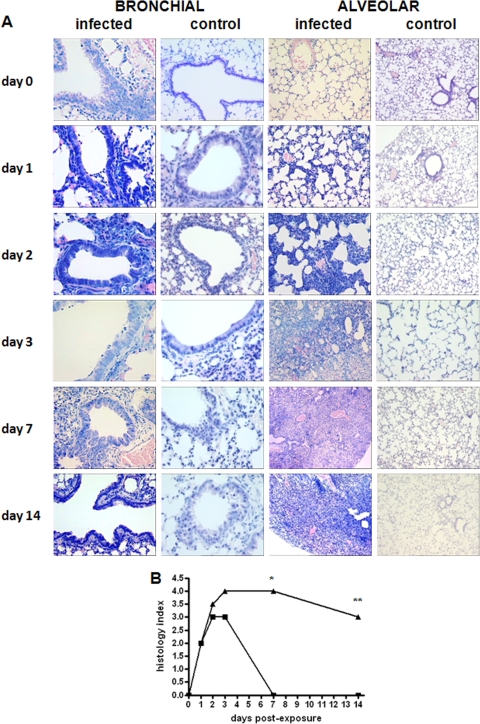
Lung histopathology.
The kinetics of lung histopathology and sections illustrating lung inflammation during the study period are shown in Fig. 6. Since there were no significant differences in the degree of inflammation at any of the time points by evaluating the left and right lungs separately, the data were pooled and considered representative of both lungs.
FIG. 6.
(A) Tissue histopathology of lungs of DBA/2 mice infected with S. maltophilia strain OBGTC9. Lung sections of DBA/2 mice (n = 92) were stained with Giemsa stain and are representative of eight (infected group) or four (control group) mice per group studied at each time point (1 h and 1, 2, 3, 7, and 14 days). Magnification, ×20 (bronchial sections) and ×10 (alveolar sections). (B) Microscopic DBA/2 mouse lung pathology following infection with S. maltophilia strain OBGTC9. Lung pathologies of DBA/2 mice (n = 92) were assessed by use of a five-point scoring system proposed by Johansen et al. (27). Results are shown as median values. *, P < 0.05; **, P < 0.01 for bronchial (▪) versus alveolar (▴) sections (Kruskal-Wallis test [P < 0.01] followed by Dunn's multiple comparison posttest).
In infected mice, we observed endobronchial inflammation, predominantly mononuclear, increasing from day 1 to day 3; thereafter, the infection moved toward the parenchyma, increased in inflammation score, involved alveolar walls and alveolar sacs, in succession, and induced increasing of septal thickening, edema, obliteration of the majority of alveolar spaces, and fibrosis. In particular, for bronchial lumens obtained from day 1 through day 3, we observed a predominantly polymorphonuclear infiltrate in 60% (day 1; score of 2), 30% (day 2; score of 3), and 45% (day 3; score of 3) of visualized lumens. On day 7, there was no sign of inflammation in the bronchial lumen (score of 0).
On day 1, in the alveolar parenchyma, we observed an increase of interstitial cellularity and increased thickness of the alveolus-capillary barrier (score of 2). Starting from day 2, edematous bleeding and obliteration of an increasing proportion of alveolar ducts were evident, in 15% (day 2; score of 2) to 80% (day 14; score of 3) of cases.
Generally, lungs of untreated control animals had little or no evidence of inflammation in the airways or lung parenchyma. Necropsy of mice that succumbed to infection revealed marked to severe alveolar hemorrhages and numerous neutrophils in the alveolar compartment.
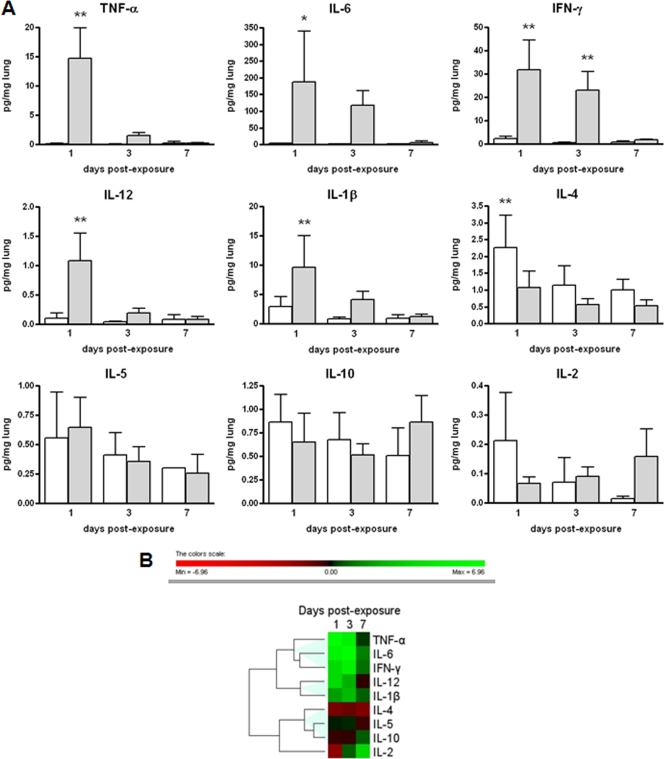
Cytokine and chemokine levels in mouse lungs.
To gain insight into the difference between the immunological reactions of the infected and uninfected DBA/2 mice, we measured the levels of 9 cytokines and 9 chemokines in the lung homogenates on days 1, 3, and 7. The expression levels of cytokines in the lungs of S. maltophilia-infected and control mice are reported in Fig. 7. Statistically higher levels of the following cytokines were observed in infected mice on day 1 than in controls: IL-1β (9.6 ± 4.1 pg/mg versus 2.9 ± 0.7 pg/mg, respectively; P < 0.01), IL-6 (187.8 ± 152.0 pg/mg versus 3.2 ± 1.2 pg/mg, respectively; P < 0.05), IL-12 (1.1 ± 0.2 pg/mg versus 0.1 ± 0.05 pg/mg, respectively; P < 0.01), IFN-γ (31.9 ± 12.6 pg/mg versus 2.3 ± 1.0 pg/mg, respectively; P < 0.01), and TNF-α (14.7 ± 5.1 pg/mg versus 0.1 ± 0.08 pg/mg, respectively; P < 0.01) (Fig. 7A). On day 3, cytokine levels in infected mice were also higher than those in controls, although the differences were not statistically significant, with the exception of IFN-γ (23.0 ± 7.9 pg/mg versus 0.5 ± 0.2 pg/mg, respectively; P < 0.01). IL-4 levels on day 1 were statistically higher in control than in infected mice (2.2 ± 0.9 pg/mg versus 1.0 ± 0.5 pg/mg, respectively; P < 0.01). All other cytokines measured were not significantly different between infected and control mice at the time points tested. Hierarchical cluster analysis of cytokine levels, based on the log ratios for infected versus control mice, revealed three different expression profiles: (i) TNF-α, IL-6, and IFN-γ; (ii) IL-12 and IL-1β; and (iii) IL-4, IL-5, and IL-10 (Fig. 7B).
FIG. 7.
Time course expression of cytokines in control and infected lungs. (A) Cytokine levels measured on days 1, 3, and 7 postexposure in lung homogenates from control (white bars) and infected (gray bars) mice (n = 54). Results were normalized to the lung wet weight (pg/mg) and are shown as means plus SDs. Statistically significant (*, P < 0.05; **, P < 0.01 [ANOVA followed by Bonferroni's multiple comparison posttest]) differences were observed in the levels of protein expression between infected and control mice for IL-1β, IL-4, IL-6, IL-12, IFN-γ, and TNF-α. (B) Hierarchical clustering expression plot. Different colors in the rectangles represent the average log ratios, defined as log2 (infected value/control value) for each cytokine. The dendrogram illustrates the degrees of similarity in expression between the cytokines tested. The color bar beneath the dendrogram represents the logarithmic expression values.
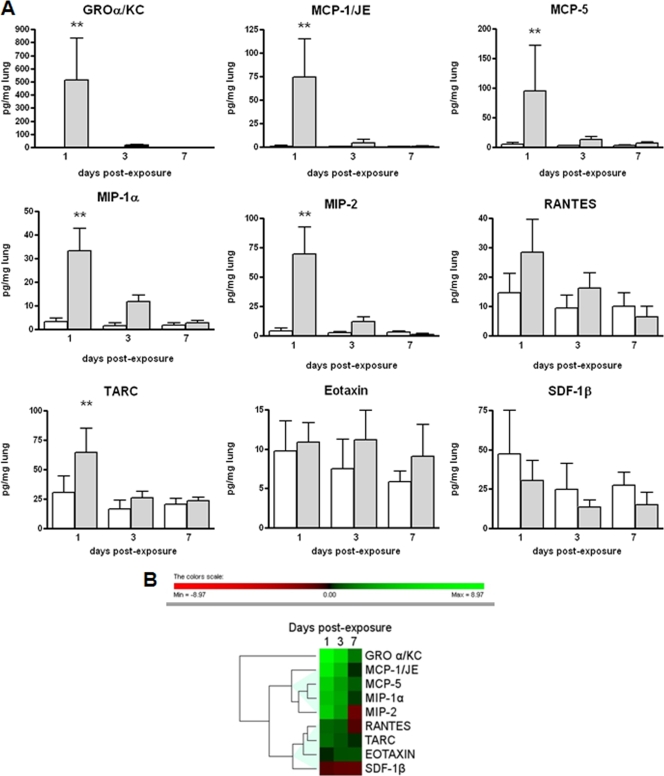
The expression levels of chemokines in the lungs of S. maltophilia-infected and control mice are represented in Fig. 8. Statistically (P < 0.01) higher levels of the following chemokines were observed in infected mice on day 1 than in controls: GROα/KC (514.8 ± 316.8 pg/mg versus 1.0 ± 0.4 pg/mg, respectively), MCP-1/JE (74.2 ± 40.9 pg/mg versus 1.1 ± 0.8 pg/mg, respectively), MCP-5 (95.2 ± 76.8 pg/mg versus 5.4 ± 2.3 pg/mg, respectively), MIP-1α (33.1 ± 9.6 pg/mg versus 3.1 ± 1.5 pg/mg, respectively), MIP-2 (69.4 ± 23.0 pg/mg versus 4.2 ± 2.4 pg/mg, respectively), and TARC (64.5 ± 20.5 pg/mg versus 30.8 ± 13.9 pg/mg, respectively) (Fig. 8A). After 3 and 7 days, chemokine levels in infected mice were also higher than those in controls, although the differences were not statistically significant. All other chemokines measured were not significantly different between infected and control mice at the time points tested. Hierarchical cluster analysis of chemokine levels, based on the log ratios for infected versus control mice, revealed two different expression profiles: (i) JE/MCP1, MIP-2, MIP-1α, and MCP-5; and (ii) eotaxin, TARC, RANTES, and SDF-1β (Fig. 8B).
FIG. 8.
Time course expression of chemokines in control and infected lungs. (A) Chemokine levels measured at 1, 3, and 7 days postexposure in the lung homogenates from control (white bars) and infected (gray bars) mice (n = 54). Results were normalized to the lung wet weight (pg/mg) and graphed as means plus SD. Statistically significant (**, P < 0.01 [ANOVA followed by Bonferroni's multiple comparison posttest]) differences were observed in the levels of protein expression between infected and control mice for GROα/KC, MCP-1/JE, MCP-5, MIP-1α, MIP-2, and TARC. (B) Hierarchical clustering expression plot. Different colors in the rectangles represent the average log ratios, defined as log2 (infected value/control value) for each chemokine. The dendrogram illustrates the degrees of similarity in expression between the chemokines tested. The color bar beneath the dendrogram represents the logarithmic expression values.
DISCUSSION
Robust experimental pulmonary infection models are a prerequisite for the understanding of the pathogenicity of microorganisms in the lung and the related host defense mechanisms. However, the route of administration is often associated with technical difficulties, especially in the case of small experimental animals such as mice.
Different techniques have been employed to introduce pathogens into the lung, such as intratracheal instillation (10), intranasal application (54), and application via aerosol (55, 56). Intratracheal administration allows the colonization of the lung by a large fraction of the inoculum, with a potentially low level of extrapulmonary contamination. However, the immobilization of bacteria on agar beads is time-consuming and could be associated with a low level of reproducibility in bacterial lung load, probably because of its difficult standardization. Furthermore, the trauma caused by the surgery involved in the tracheotomy often results in moribundity or death of animals.
The intranasal instillation and aerosol administration methods require little experimental effort and are the least invasive methods. However, low reproducibility and contamination of the lung by nasal and oropharyngeal flora are the major disadvantages of nasal instillation. In contrast, administration via aerosol by our inhalation system ensures high reproducibility (both between different experiments and between animals in a given experiment) and no lung contamination, and it is not time-consuming because it permits infection of up to four untrained mice simultaneously. Furthermore, we also assessed that bacteria are not lysed by shear forces when pressed through the nebulizer, thus not causing exposure to a high antigen load (data not shown). In contrast to tracheal surgery, the use of aerosolization as the method of exposure also avoids the use of anesthetic agents, which are known to affect pulmonary clearance (23), and permits the exclusion of surgery-related inflammation while trying to study the inflammatory response to bacterial infection. Lastly, the aerosol delivery technology simulates the natural route for acquiring deep-lung infections in humans.
Both acute and chronic models of lung infection have been established in several animal species, including rats, guinea pigs, hamsters, mice, mink, sheep, rabbits, and monkeys (48). The DBA/2 inbred mouse strain, a model already used for the study of pathogenesis in CF (4, 7, 36, 45), is characterized by an autosomal recessive mutation of the gene controlling the hemolytic activity of the serum, resulting in a deficiency in the production of the C5 component. This component participates in the host defense against infection and in the inflammatory response by means of multiple biological activities, such as chemoattraction, stimulation of phagocytes to release cytokines (TNF-α and IL-1), granule enzymes, and oxygen metabolites, and enhancement of antibody formation (28).
For these reasons, in the present study we used aerosol delivery technology for the deposition of S. maltophilia into DBA/2 mouse lungs. Using this model of respiratory infection, we investigated bacterial clearance, histopathology, and the inflammatory response in the mouse lungs following a single exposure to aerosolized S. maltophilia.
Unlike chronic models using agar beads that hold the bacteria in the airway by mechanical means, thus bypassing initial bacterial attachment and retention, our acute model requires S. maltophilia suspended in PBS, thus allowing us to test the ability of the animals to clear bacteria from the lung and to establish the relative importance of antibacterial factors in early development of pulmonary disease in CF. Our results showed that almost all (>99.99%) of the organisms initially deposed in the lung were killed within the first 7 days after inoculation, thus greatly reducing the inciting stimulus. On day 14, bacterial loads became undetectable in all infected DBA/2 mice, suggesting that they were able to resolve S. maltophilia lung colonization in a period ranging from 8 to 14 days.
The clearance of S. maltophilia from the lungs of DBA/2 mice was clearly associated with the profile of the inflammatory response observed in the DBA/2 lung tissue, which consisted of an early and intense, but detrimental, bronchial and alveolar inflammatory response, mediated primarily by neutrophils—the prominent inflammatory cell in the lungs of CF patients, even in individuals with minimal pulmonary involvement (32)—followed shortly by an influx of inflammatory macrophages.
Since DBA/2 mice are one of several C5-deficient inbred mouse strains (55), thus predicting a reduction in neutrophil recruitment to the lung tissues, the increased influx of inflammatory leukocytes we observed suggested that the loss of C5a may be compensated by the host by increasing the production of redundant/alternative neutrophil chemotactic factors.
In the present study, we evaluated the magnitude of the inflammatory response provoked by S. maltophilia exposure by measuring the DBA/2 mouse pulmonary levels of 9 cytokines and 9 chemokines, selected on the basis of the specific proinflammatory protein profiles found in infected CF patients (6, 11, 26, 31, 33).
In agreement with what has been observed in lung secretions from patients with CF with respect to healthy controls (6), we found statistically (P < 0.01) higher levels of TNF-α, IL-1β, and IL-6 in infected mice than in controls.
TNF-α has been implicated in the inflammation in and clearance of P. aeruginosa from the murine lung (39). TNF-α contributes to the restriction of microbial growth by upregulating adhesion molecules, such as ICAM-1, involved in polymorphonuclear leukocyte (PMN) recruitment and by amplifying the innate clearance mechanisms (40). Waters et al. (54) recently found that S. maltophilia induced substantially more TNF-α expression by macrophages than did P. aeruginosa, probably due to the high degree of lipid A heterogeneity.
IL-6 is constitutively upregulated in CF patients, leading to increased neutrophil recruitment and further enhancement of inflammation in the lung (16, 26).
We also found significantly higher IFN-γ and IL-12 levels in infected lungs than in controls. The inflammatory cytokine IFN-γ is produced by T lymphocytes and natural killer (NK) cells and is able to activate the microbicidal function of macrophages and NK cells (5). Furthermore, IFN-γ can enhance the chemotaxis and phagocytosis of PMNs to pathogens (37). We found that IFN-γ was the only cytokine with persistent hyperexpression, since levels in infected mice were significantly higher than those in controls until day 3.
IL-12 (IL-12 p70) is secreted mainly by peripheral lymphocytes after bacterial induction and stimulates production of IFN-γ by NK and T cells (5). Although IL-12 is essential for the host defense against various pathogens, this cytokine is not likely to be a major player in the host response to P. aeruginosa lung infection (42).
The increases in IFN-γ and IL-12 were in agreement with the decrease, though not statistically significant, of IL-10 in lung tissues, since IL-10 suppresses the synthesis of proinflammatory cytokines by inhibiting IL-12 and IFN-γ production (5). In addition, we observed that levels of the pleiotropic cytokine IL-4, a product of Th2 lymphocytes that inhibits the differentiation of Th1 cells (5, 24), were also reduced, even though this difference was not statistically significant.
Among chemokines, statistically (P < 0.01) higher levels were observed in infected mice on day 1 than in controls for GROα/KC, MCP-1/JE, MCP-5, MIP-1α, MIP-2, and TARC. GROα/KC and MIP-2—murine homologues of human IL-8 known to be expressed differently in the lungs of CF patients (16, 26, 33)—are overexpressed in murine keratinocytes, monocytes, and macrophages and are involved in chemotaxis and cell activation of neutrophils in the mouse (44). MIP-1α, produced by macrophages following their stimulation with bacterial endotoxins and involved in the cell activation and recruitment of neutrophilic granulocytes, is elevated in the lungs of young children with CF, even in the absence of pulmonary infection (8). MCP-5 and MCP-1/JE are potent chemoattractants for peripheral blood monocytes only, while increased levels of TARC suggest a T-cell chemoattraction and adhesion of monocytes to the endothelium.
Taken together, our results suggest that the immune response to acute infection with S. maltophilia is predominantly a Th1-type response characterized by the recruitment of PMNs to the lung tissue as a result of the excessive production of TNF-α, IL-1β, and the PMN chemoattractants MIP-1α, MIP-2, and GROα/KC. Similarly, higher levels of MCP-1/JE and MCP-5 are responsible for monocyte recruitment.
An indirect indicator of the failure of mice to control a Gram-negative infection is weight loss due to the induction of cachexia by LPS-induced inflammatory cytokines. Cachexia observed in CF patients might be the outcome of excessive concentrations of TNF-α (20). Interestingly, our results showed that the timing of the most severe mouse weight loss immediately followed the most dramatic difference in TNF-α concentration, thus confirming that it is a pivotal proinflammatory cytokine that plays an important role in inducing the excessive inflammatory response in the CF lung, as well as additional systemic effects.
The potential of S. maltophilia to cause invasive infections has been reported previously (35, 38). Recently, we showed that S. maltophilia is able to invade cultured A549 respiratory epithelial cells, although at very low levels (18). The results from the present study confirm the scant invasiveness of S. maltophilia, as suggested by a transient and minimal presence of the microorganism in the spleens of DBA/2 mice. In agreement with the work of Waters et al. (54), it is plausible that during pulmonary infection, the few bacteria crossing the epithelial barrier are readily cleared, if not by lytic effects of serum, then by phagocytosis, and they do not produce sufficient concentrations in the blood to provoke sepsis. The sequenced S. maltophilia genome has few regions with low levels of homology to any of the P. aeruginosa type III secretion genes (12). Type III secretion systems mediate bacterial interactions with host cytoskeletal components in many Gram-negative pathogens, and in P. aeruginosa, they highly correlate with invasive infection (25). Thus, the potential lack of type III secretion genes in S. maltophilia may contribute to its limited invasive capabilities.
S. maltophilia generally causes infections that result in increased morbidity, but not usually in mortality, in the immunocompromised host. Our results showed that excessive pulmonary infection and inflammation had systemic effects, manifested by weight loss, and ultimately caused a large number of deaths of infected mice compared to the control animals. The high overall mortality rate we observed in the present study indicates the severity of S. maltophilia infection, as confirmed by the macroscopic in situ lung analysis, revealing edema, hemorrhage, atelectasis, consolidated areas, and fibrinous adhesion to the thoracic wall. Contrarily to our findings, Waters et al. (54) found that S. maltophilia CF strains caused no mortality in a neonatal mouse model of respiratory tract infection.
While infection disseminated, as suggested by the presence of bacteria in the spleens of DBA/2 mice, the death of mice appeared to be due primarily to the complication in the lungs, indicating that the DBA/2 mice were unable to overcome the increased and prolonged inflammatory response provoked by S. maltophilia infection.
In conclusion, our results taken together show that the lungs of DBA/2 mice undergoing a single aerosol exposure to S. maltophilia in suspension share many characteristics with the pulmonary disease in CF patients: (i) the lung contains a large number of neutrophils, the prominent inflammatory cells in CF lungs; (ii) the remarkably high levels of proinflammatory mediators, especially chemoattractant cytokines, in the lungs are similar to those observed in infants and young children with CF, even when controlled for bacterial burden (1, 3, 6, 41), which may perpetuate the robust inflammatory response; and (iii) additional CF-like systemic effects, such as weight loss and mortality, were also observed.
By using our model, this study provides a framework for understanding the role that S. maltophilia plays in pulmonary CF disease. The severe pathology and high mortality we observed in infected animals contrast with the view that S. maltophilia may be just a bystander in CF patients, supporting the idea that this opportunistic pathogen has the potential to contribute to the inflammatory process responsible for the deterioration of lung function in CF patients.
Since S. maltophilia can often be found in CF lungs together with other CF pathogens, in particular P. aeruginosa (43), further studies will be needed to assess the contribution of S. maltophilia to worsening the inflammation resulting from infection with other pathogens.
Acknowledgments
We thank Paola Ascione (Department of Oncology and Neurosciences, G. d'Annunzio University of Chieti-Pescara, Chieti, Italy) for her technical help in preparing lung sections for histological analysis. We also thank Andreina Santoro for contributing to the revision of the manuscript.
This work was supported in part by the Fondazione Italiana per la Ricerca sulla Fibrosi Cistica (grant FFC#7/2007) and in part by the Ministero Italiano dell'Università e della Ricerca Scientifica (MIUR) (grant PRIN2007; protocol 2007LXNYS7).
Editor: B. A. McCormick
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Armstrong, D. S., K. Grimwood, R. Carzino, J. B. Carlin, A. Olinsky, and P. D. Phelan. 1995. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. Br. Med. J. 310:1571-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballestero, S., I. Virseda, H. Escobar, L. Lucrecia, and F. Baquero. 1995. Stenotrophomonas maltophilia in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 14:728-779. [DOI] [PubMed] [Google Scholar]
- 3.Balough, K., M. McCubbin, M. Weinberger, W. Smits, R. Ahrens, and R. Fick. 1995. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Pediatr. Pulmonol. 20:63-70. [DOI] [PubMed] [Google Scholar]
- 4.Barclay, N. G., J. C. Spurrell, T. F. Bruno, D. G. Storey, D. E. Woods, and C. H. Mody. 1999. Pseudomonas aeruginosa exoenzyme S stimulates murine lymphocyte proliferation in vitro. Infect. Immun. 67:4613-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belardelli, F. 1995. Role of interferons and other cytokines in the regulation of the immune response. Acta Pathol. Microbiol. Immunol. Scand. 103:161-179. [DOI] [PubMed] [Google Scholar]
- 6.Bonfield, T. L., J. R. Panuska, M. W. Konstan, K. A. Hillard, J. B. Hillard, H. Ghnaim, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 7.Boucher, J. C., H. Yu, M. H. Mudd, and V. Deretic. 1997. Mucoid Pseudomonas aeruginosa in cystic fibrosis: characterization of muc mutations in clinical isolates and analysis of clearance in a mouse model of respiratory infection. Infect. Immun. 65:3838-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan, S., P. D. Sly, C. L. Gangell, N. Sturges, K. Winfield, M. Wikstrom, S. Gard, J. W. Upham, et al. 2009. Alveolar macrophages and CC chemokines are increased in children with cystic fibrosis. Eur. Respir. J. 34:655-661. [DOI] [PubMed] [Google Scholar]
- 9.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 10.Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. [DOI] [PubMed] [Google Scholar]
- 11.Chmiel, J. F., and P. B. Davis. 2003. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir. Res. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crossman, L. C., V. C. Gould, J. M. Dow, G. S. Vernikos, A. Okazaki, M. Sebaihia, D. Saunders, C. Arrowsmith, T. Carver, N. Peters, E. Adlem, A. Kerhornou, A. Lord, L. Murphy, K. Seeger, R. Squares, S. Rutter, M. A. Quail, M. A. Rajandream, D. Harris, C. Churcher, S. D. Bentley, J. Parkhill, N. R. Thomson, and M. B. Avison. 2008. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 9:R74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Demko, C. A., R. C. Stern, and C. F. Doershuk. 1998. Stenotrophomonas maltophilia in cystic fibrosis: incidence and prevalence. Pediatr. Pulmonol. 25:304-308. [DOI] [PubMed] [Google Scholar]
- 15.Denton, M. 1997. Stenotrophomonas maltophilia: an emerging problem in cystic fibrosis patients. Rev. Med. Microbiol. 8:15-19. [Google Scholar]
- 16.De Rose, V. 2002. Mechanisms and markers of airway inflammation in cystic fibrosis. Eur. Respir. J. 19:333-340. [DOI] [PubMed] [Google Scholar]
- 17.Di Bonaventura, G., A. Pompilio, C. Picciani, M. Nicoletti, R. Zappacosta, and R. Piccolomini. 2008. Adhesion to and biofilm formation on IB3-1 bronchial cells by Stenotrophomonas maltophilia: implications in cystic fibrosis. Clin. Microbiol. Infect. 14(Suppl. 7):S178. [Google Scholar]
- 18.Di Bonaventura, G., G. Prosseda, F. Del Chierico, S. Cannavacciuolo, P. Cipriani, A. Petrucca, F. Superti, M. G. Ammendolia, C. Concato, E. Fiscarelli, M. Casalino, R. Piccolomini, M. Nicoletti, and B. Colonna. 2007. Molecular characterization of virulence determinants of Stenotrophomonas maltophilia strains isolated from patients affected by cystic fibrosis. Int. J. Immunopathol. Pharmacol. 20:529-537. [DOI] [PubMed] [Google Scholar]
- 19.Dubin, P. J., and J. K. Kolls. 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 292:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elborn, J. S., S. M. Cordon, P. J. Western, I. A. MacDonald, and D. J. Shale. 1993. Tumor necrosis factor-alpha, resting energy expenditure and cachexia in cystic fibrosis. Clin. Sci. (London) 85:563-568. [DOI] [PubMed] [Google Scholar]
- 21.Gibson, R. L., J. L. Burns, and B. W. Ramsey. 2003. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 168:918-951. [DOI] [PubMed] [Google Scholar]
- 22.Gladman, G., P. J. Connor, R. F. Williams, and T. J. David. 1993. Controlled study of Pseudomonas maltophilia in cystic fibrosis. Arch. Dis. Child. 67:192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein, E., E. S. Munson, C. Eagle, R. Martucci, and P. D. Hoeprich. 1970. Influence of anesthetic agents on murine pulmonary bactericidal activity. Antimicrob. Agents Chemother. 10:231-235. [PubMed] [Google Scholar]
- 24.Hart, P. H., G. F. Vitti, D. R. Burgess, G. A. Whitty, D. S. Piccoli, and J. Hamilton. 1989. Potential anti-inflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. U. S. A. 86:3803-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser, A. R., E. Cobb, M. Bodi, D. Mariscal, J. Valles, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 26.Ionescu, A. A., T. D. Mickleborough, C. E. Bolton, M. R. Lindley, L. S. Nixon, G. Dunseath, S. Luzio, D. R. Owens, and D. J. Shale. 2006. The systemic inflammatory response to exercise in adults with cystic fibrosis. J. Cyst. Fibros. 5:105-112. [DOI] [PubMed] [Google Scholar]
- 27.Johansen, H. K., F. Espersen, S. S. Pedersen, H. P. Hougen, J. Rygaard, and N. Høiby. 1993. Chronic Pseudomonas aeruginosa lung infection in normal and athymic rats. APMIS 101:207-225. [PubMed] [Google Scholar]
- 28.Johnston, R. B., Jr. 1993. The complement system in host defense and inflammation: the cutting edges of a double edged sword. Pediatr. Infect. Dis. J. 12:933-941. [DOI] [PubMed] [Google Scholar]
- 29.Jones, A. M., M. E. Dodd, and J. R. Govan. 2004. Burkholderia cenocepacia and Burkholderia multivorans: influence on survival in cystic fibrosis. Thorax 59:948-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karpati, F., A. S. Malmborg, H. Alfredsson, L. Hjelte, and B. Strandvik. 1994. Bacterial colonization with Xanthomonas maltophilia: a retrospective study in a cystic fibrosis patient population. Infection 22:258-263. [DOI] [PubMed] [Google Scholar]
- 31.Khan, T. Z., J. S. Wagener, T. Bost, J. Martinez, F. J. Accurso, and D. W. H. Riches. 1995. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 151:1075-1082. [DOI] [PubMed] [Google Scholar]
- 32.Konstan, M. W., K. A. Hilliard, T. M. Norvell, and M. Berger. 1994. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am. J. Respir. Crit. Care Med. 150:448-454. [DOI] [PubMed] [Google Scholar]
- 33.Kube, D., U. Sontich, D. Fletcher, and P. B. Davis. 2001. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L493-L502. [DOI] [PubMed] [Google Scholar]
- 34.Lambiase, A., V. Raia, M. Del Pezzo, A. Sepe, V. Carnovale, and F. Rossano. 2006. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect. Dis. 6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landrum, M. L., N. G. Conger, and M. A. Forgione. 2005. Trimethoprim-sulfamethoxazole in the treatment of Stenotrophomonas maltophilia osteomyelitis. Clin. Infect. Dis. 40:1551-1552. [DOI] [PubMed] [Google Scholar]
- 36.Leverkoehne, I., H. Holle, F. Anton, and A. D. Gruber. 2006. Differential expression of calcium-activated chloride channels (CLCA) gene family members in the small intestine of cystic fibrosis mouse models. Histochem. Cell Biol. 126:239-250. [DOI] [PubMed] [Google Scholar]
- 37.Liu, J. H., and J. Y. Djeu. 1995. Role of cytokines in neutrophil functions, p. 71-86. In B. B. Aggarwal and R. K. Puri (ed.), Human cytokines: their role in disease and therapy. Blackwell Science Inc., Cambridge, United Kingdom.
- 38.Miyairi, I., J. A. Franklin, M. Andreansky, K. M. Knapp, and R. T. Hayden. 2005. Acute necrotizing ulcerative gingivitis and bacteremia caused by Stenotrophomonas maltophilia in an immunocompromised host. Pediatr. Infect. Dis. J. 24:181-183. [DOI] [PubMed] [Google Scholar]
- 39.Morissette, C., C. Francoeur, C. Darmond-Zwaig, and F. Gervais. 1996. Lung phagocyte bactericidal function in strains of mice resistant and susceptible to Pseudomonas aeruginosa. Infect. Immun. 64:4984-4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulligan, M. S., A. A. Vaporciyan, M. Miyasaka, T. Tamatani, and P. A. Ward. 1993. Tumor necrosis factor regulates in vivo intrapulmonary expression of ICAM-1. Am. J. Pathol. 142:1739-1744. [PMC free article] [PubMed] [Google Scholar]
- 41.Noah, T. L., H. R. Black, P. W. Cheng, R. E. Wood, and M. W. Leigh. 1997. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J. Infect. Dis. 175:638-647. [DOI] [PubMed] [Google Scholar]
- 42.O'Sullivan, R., S. O. Carrigan, J. S. Marshall, and T. J. Lin. 2008. Signal transducer and activator of transcription 4 (STAT4), but not IL-12 contributes to Pseudomonas aeruginosa-induced lung inflammation in mice. Immunobiology 213:469-479. [DOI] [PubMed] [Google Scholar]
- 43.Ryan, R. P., Y. Fouhy, B. F. Garcia, S. A. Watt, K. Niehaus, L. Yang, T. Tolker-Nielsen, and J. M. Dow. 2008. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 68:75-86. [DOI] [PubMed] [Google Scholar]
- 44.Schall, T. J. 1994. The chemokines, p. 419-460. In A. W. Thomson (ed.), The cytokine handbook, 2nd ed. Academic Press, San Diego, CA.
- 45.Speert, D. P., B. Steen, K. Halsey, and E. Kwan. 1999. A murine model for infection with Burkholderia cepacia with sustained persistence in the spleen. Infect. Immun. 67:4027-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spicuzza, L., C. Sciuto, G. Vitaliti, G. Di Dio, S. Leonardi, and M. La Rosa. 2008. Emerging pathogens in cystic fibrosis: ten years of follow-up in a cohort of patients. Eur. J. Clin. Microbiol. Infect. Dis. 28:191-195. [DOI] [PubMed] [Google Scholar]
- 47.Steinkamp, G., B. Wiedemann, E. Rietschel, A. Krahl, J. Gielen, H. Barmeier, and F. Ratjen. 2005. Prospective evaluation of emerging bacteria in cystic fibrosis. J. Cyst. Fibros. 4:41-48. [DOI] [PubMed] [Google Scholar]
- 48.Stotland, P. K., D. Radzioch, and M. M. Stevenson. 2000. Mouse models of chronic lung infection with Pseudomonas aeruginosa: models for the study of cystic fibrosis. Pediatr. Pulmonol. 30:413-420. [DOI] [PubMed] [Google Scholar]
- 49.Talmaciu, I., L. Varlotta, J. Mortensen, and D. V. Schidlow. 2000. Risk factors for emergence of Stenotrophomonas maltophilia in cystic fibrosis. Pediatr. Pulmonol. 30:10-15. [DOI] [PubMed] [Google Scholar]
- 50.Tan, K., S. P. Conway, K. G. Brownlee, C. Etherington, and D. G. Peckham. 2002. Alcaligenes infection in cystic fibrosis. Pediatr. Pulmonol. 34:101-104. [DOI] [PubMed] [Google Scholar]
- 51.Van Couwenberghe, C. J., T. B. Farver, and S. H. Cohen. 1997. Risk factors associated with isolation of Stenotrophomonas (Xanthomonas) maltophilia in clinical specimens. Infect. Control Hosp. Epidemiol. 18:316-321. [DOI] [PubMed] [Google Scholar]
- 52.Villarino, M. E., L. E. Stevens, B. Schable, G. Mayers, J. M. Miller, J. P. Burke, and W. R. Jarvis. 1992. Risk factors for epidemic Xanthomonas maltophilia infection/colonization in intensive care unit patients. Infect. Control Hosp. Epidemiol. 13:201-206. [DOI] [PubMed] [Google Scholar]
- 53.Wainwright, C. E., M. W. France, P. O'Rourke, S. Anuj, T. J. Kidd, M. D. Nissen, T. P. Sloots, C. Coulter, Z. Ristovski, M. Hargreaves, B. R. Rose, C. Harbour, S. C. Bell, and K. P. Fennelly. 2009. Cough-generated aerosols of Pseudomonas aeruginosa and other Gram-negative bacteria from patients with cystic fibrosis. Thorax 64:926-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waters, V. J., M. I. Gómez, G. Soong, S. Amin, R. K. Ernst, and A. Prince. 2007. Immunostimulatory properties of the emerging pathogen Stenotrophomonas maltophilia. Infect. Immun. 75:1698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson, K. R., J. M. Napper, J. Denvir, V. E. Sollars, and H. D. Yu. 2007. Defect in early lung defence against Pseudomonas aeruginosa in mice is associated with acute inflammatory lung injury and reduced bactericidal activity in naive macrophages. Microbiology 153:968-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu, H., S. Z. Nasr, and V. Deretic. 2000. Innate lung defenses and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratory infections in cystic fibrosis. Infect. Immun. 68:2142-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]