Abstract
Yersinia adhesin A (YadA) is a trimeric autotransporter adhesin with multiple functions in host-pathogen interactions. The aim of this study was to dissect the virulence functions promoted by YadA in vitro and in vivo. To accomplish this, we generated Yersinia enterocolitica O:8 mutants expressing point mutations in YadA G389, a highly conserved residue in the membrane anchor of YadA, and analyzed their impact on YadA expression and virulence functions. We found that point mutations of YadA G389 led to impaired transport, stability, and surface display of YadA. YadA G389A and G389S mutants showed comparable YadA surface expression, autoagglutination, and adhesion to those of wild-type YadA but displayed reduced trimer stability and complement resistance in vitro and were 10- to 1,000-fold attenuated in experimental Y. enterocolitica infection in mice. The G389T, G389N, and G389H mutants lost trimer stability, exhibited strongly reduced surface display, autoagglutination, adhesion properties, and complement resistance, and were avirulent (>10,000-fold attenuation) in mice. Our data demonstrate that G389 is a critical residue of YadA, required for optimal trimer stability, transport, surface display, and serum resistance. We also show that stable trimeric YadA protein is essential for virulence of Y. enterocolitica.
Enteropathogenic Yersinia species Yersinia enterocolitica and Yersinia pseudotuberculosis are food-borne pathogens causing diarrhea, mesenteric lymphadenitis, and reactive arthritis (10). Upon ingestion of contaminated food by the host, the bacteria colonize the intestine and may invade M cells which overlie the Peyer's patches (PPs) (17). After translocation by M cells, Y. enterocolitica multiplies extracellularly in the adjacent tissue.
A major virulence determinant of Y. enterocolitica is the Yersinia adhesin A (YadA) (13). YadA belongs to the family of trimeric autotransporter adhesins (TAAs) (29). TAAs consist of an N-terminal head domain connected via an extended stalk to the C-terminal membrane anchor domain. The head domain of YadA is involved in binding to collagen or host cells, whereas the stalk domain is involved in serum resistance (34). Three membrane anchor domains of one YadA trimer build up a beta-barrel pore, which facilitates the transition of the passenger domains (stalk and head) onto the outer membrane (OM) (21). The exact mechanism of trimeric autotransport remains unclear. There do exist several models, among which the hairpin and threading models propose that the membrane anchor domains integrate into the OM and build up a beta-barrel pore. After that, the passenger domains traverse the pore, starting with either the N or C terminus (5).
Numerous efforts have attempted to elucidate the function of YadA in establishing infection with Y. enterocolitica. The domain-function relationships of YadA have been analyzed in detail (35, 36). There is striking evidence that the head domain of YadA is involved in binding to extracellular matrix proteins, such as collagen, and in binding to neutrophils (14, 20, 27, 34). Also, the autoagglutination capacity seems to involve at least parts of the head domain (44). In addition to adherence and autoagglutination, serum resistance seems to be an important function mediated by YadA for virulence of Y. enterocolitica in vivo. Via the binding of serum complement factor H and C4 binding protein (C4BP), YadA may prevent deposition of C3b on the bacterial surface and therefore block formation of membrane attack complexes and killing of the bacteria (6, 23). However, this function has so far been mapped only for factor H, which binds to the stalk domain of YadA (7). The binding region for C4BP is still unknown.
Recent studies by Ackermann et al. (1) have shown that only the factual membrane anchor domain of YadA is able to confer serum resistance and mouse virulence to Y. enterocolitica expressing chimeric fusion proteins of the N-terminal YadA passenger domain and the C-terminal membrane anchor domains of the TAAs UspA1 (Moraxella catarrhalis), EibA (Escherichia coli), and Hia (Haemophilus influenzae).
We previously showed that YadA and most other known TAAs have a G residue in the second beta-strand of the membrane anchor domain which forms the beta-barrel translocator pore; in the exceptional TAAs, it is either A, S, T, or N (19). This residue (G389 in YadA of Y. enterocolitica O:8) faces the lumen of the beta-barrel. According to a YadA beta-barrel model that was generated based on the Haemophilus influenzae Hia three-dimensional (3D) structure, the free space around G389 should be large enough to accommodate either A, S, C, D, P, N, T, V, E, or Q (in ascending order of side chain volume) without constraining the conformation of adjacent residues. An H residue would reach the limit of the pocket size but might still be accommodated with only minor local structural adjustments. We previously replaced G389 with the four naturally occurring residues (A, S, T, and N) and also with H in order to explore the upper size limit of the pocket. The side chain size of the residues increases in the order G < A < S < T < N < H, and polarity increases in the order A < G < S < T < N < H. When expressed in E. coli, these G389 substitutions were shown to influence YadA surface display and stability, to different degrees (19).
To elucidate which of the many functions of YadA in host-pathogen interaction in vitro are of relevance for the pathogenicity of Y. enterocolitica in vivo, we introduced the G389 substitution mutations into the Y. enterocolitica background and thoroughly analyzed trimer stability, accessibility by trypsin, outer membrane localization, adhesion to collagen and HeLa cells, Yop-mediated suppression of cytokine responses, autoagglutination, serum resistance, binding of complement regulatory factors (CRFs), and virulence in mice. From our results, we conclude that the stability of YadA trimers is decisive for the YadA-mediated serum resistance and virulence of Y. enterocolitica in mice.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C57BL/6 or BALB/c mice were purchased from Harlan Winkelmann (Borchen, Germany). The mice were kept under specific-pathogen-free conditions in positive-pressure cabinets (Techniplast) and provided with sterile food and water ad libitum.
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. The strains of Y. enterocolitica were grown overnight at 27°C in Luria-Bertani broth supplemented with nalidixic acid (10 μg/ml). The YadA-deficient mutant YadA0 was additionally supplemented with kanamycin (50 μg/ml). Y. enterocolitica strains expressing mutant versions of YadA were grown in the presence of nalidixic acid, kanamycin, and spectinomycin (50 μg/ml). A 1:20 dilution of the overnight Y. enterocolitica culture was incubated for an additional 2 to 3 h at 37°C. The bacteria were washed once with phosphate-buffered saline (PBS; Invitrogen), and the optical density at 600 nm (OD600) was determined.
TABLE 1.
Bacteria used in this work
| Bacterial strain | Description | Reference |
|---|---|---|
| WAP | Yersinia enterocolitica WA-314 serotype O:8 clinical isolate, virulent wild-type strain; pYV+ (YadA+) | 19a |
| WAC | Plasmidless derivative of WAP; pYV− (YadA−) | 38a |
| WAP Inv− | Invasin-deficient mutant of WAP; pYV+ (Inv−) | 36 |
| YadA0 | YadA-deficient mutant of WAP; pYV+ (YadA−) | 35 |
| YadAwt | As an internal control, the coding sequence of YadA was reinserted into the YadA0 strain | This study |
| G389A mutant | A point-mutated version of YadA was reintroduced into the YadA0 strain; glycine at position 389 was replaced by alanine | This study |
| G389S mutant | A point-mutated version of YadA was reintroduced into the YadA0 strain; glycine at position 389 was replaced by serine | This study |
| G389T mutant | A point-mutated version of YadA was reintroduced into the YadA0 strain; glycine at position 389 was replaced by threonine | This study |
| G389N mutant | A point-mutated version of YadA was reintroduced into the YadA0 strain; glycine at position 389 was replaced by asparagine | This study |
| G389H mutant | A point-mutated version of YadA was reintroduced into the YadA0 strain; glycine at position 389 was replaced by histidine | This study |
DNA manipulations and PCR.
Exchanges of single amino acids in the Y. enterocolitica yadA gene were introduced as described previously (19), using the plasmid pUC-A-1 (35), and were verified by DNA sequencing. The primer pairs used are available upon request. Using the EcoRI and SphI restriction sites, the point-mutated yadA genes were subcloned into the mobilizable suicide vector pGP704, resulting in the constructs pGP-YadAwt, pGP-YadA G389A, pGP-YadA G389S, pGP-YadA G389T, pGP-YadA G389N, and pGP-YadA G389H. Subsequently, a spectinomycin resistance cassette was inserted into the EcoRI restriction site, resulting in the plasmids pGPS-YadAwt, pGPS-YadA G389A, pGPS-YadA G389S, pGPS-YadA G389T, pGPS-YadA G389N, and pGPS-YadA G389H. After transformation into E. coli S17-1 λpir, the plasmids were mobilized into the YadA-deficient strain Y. enterocolitica YadA0 (35). Transconjugants harboring cointegrants (e.g., pYVO8-A-0::pGPS-YadAwt) were selected for kanamycin and spectinomycin resistance and verified by PCR and Western blotting.
Sample preparation for Western blot analysis.
Bacterial pellets were lysed in SDS sample buffer (5 × 107 Y. enterocolitica cells were loaded per lane) and incubated for 10 min at 95°C prior to loading if not indicated otherwise. For the preparation of unheated samples, alkaline lysis of approximately 1.5 × 109 Y. enterocolitica bacteria was performed using 180 μl of buffer 1 from a peqGOLD plasmid miniprep kit (Peqlab-Biotechnologie GmbH, Erlangen, Germany) and 20 μl of 1 M NaOH. After adjustment to pH 7.0, 20 μl of DNase I incubation buffer (10×) and 2 μl of DNase (10 U/ml; Roche Diagnostics GmbH, Mannheim, Germany) were added and left for 20 min at room temperature before SDS sample buffer was added. For denaturation of trypsin-digested proteins, the samples were heated in 5× Laemmli buffer supplemented with 6 M urea for 10 min at 95°C.
Trypsin digestion.
Overnight cultures of Y. enterocolitica grown at 27°C were diluted to an OD600 of 0.1 in fresh medium and grown to exponential phase for 2 h at 27°C. Next, bacteria were grown for 2 h at 37°C to induce YadA expression. A total of 1.5 × 108 bacteria were pelleted and washed once with PBS. Bacteria were then resuspended in PBS containing 0.1 mg/ml trypsin (Applichem) and incubated on ice for 1 h. To stop tryptic digestion, a trypsin inhibitor (0.2-mg/ml final concentration; Sigma) was added. Bacteria and supernatant were separated by centrifugation. The bacterial pellet was resuspended in an equal volume of 5× concentrated Laemmli buffer, heated, and subjected to SDS-PAGE.
Western blot analysis.
After SDS-PAGE, proteins were transferred to nitrocellulose membranes. The membranes were blocked overnight with PBS-5% milk powder at 4°C. For the detection of YadA, a purified IgG fraction of polyclonal rabbit YadA antiserum (diluted 1:1,000) and a peroxidase-conjugated secondary anti-rabbit antibody (diluted 1:10,000; Dianova) were added and left for 1 h and for 45 min, respectively, at room temperature. Detection of bound antibodies was carried out using an ECL detection kit (Amersham Biosciences).
Surface localization of YadA by immunofluorescence.
Coverslips were coated overnight at 4°C with 10 μg/ml human collagen type I (Calbiochem/Merck, Darmstadt, Germany) in PBS. After being washed with PBS, 2 × 107 bacteria were centrifuged onto coverslips at 300 rpm for 5 min. After 1 h of incubation at 37°C, the coverslips were washed three times with PBS and then fixed with 4% paraformaldehyde (PFA). We tested if a periplasmic protease was stained in fixed but nonpermeabilized cells. This was not the case, and therefore only extracellular proteins were stained in this assay. Bacteria were incubated with a rabbit polyclonal antibody (PAb) (IgG fraction) directed against O:8 YadA (diluted 1:200) and a Cy2-conjugated secondary anti-rabbit antibody (diluted 1:200; Dianova) in PBS at room temperature for 1 h and for 45 min, respectively. Fluorescence images were obtained with a DMRE fluorescence microscope (Leica, Wetzlar, Germany).
Quantification of YadA surface localization by flow cytometry.
Overnight cultures were grown at 27°C. The next day, bacteria were diluted 1:20 and grown for 2 h at 37°C. After 2 h, 1 × 109 bacteria were harvested by centrifugation. Cells were washed once with PBS, fixed with 4% PFA, washed again, and resuspended in PBS. Samples were stained with a purified IgG fraction of a rabbit anti-YadA antiserum (1:200) and a Cy2-conjugated secondary antibody (1:100; Dianova) for 1 h at room temperature and then washed twice with PBS. Surface localization of YadA was measured by flow cytometry on a FACSCalibur flow cytometer (Becton Dickinson), and data were analyzed with WinMDI (J. Trotter) software. Data are means for 3 independent experiments.
Autoagglutination.
The autoagglutination of Y. enterocolitica was assayed essentially as described previously (25). The overnight cultures of Y. enterocolitica strains were diluted 1:20 in RPMI 1640 supplemented with 10% fetal calf serum (FCS) and 25 mM HEPES and were grown for 24 h at 37°C. The bacteria were then allowed to settle at room temperature. Autoagglutination was recorded as a reduction of the OD600 in the culture supernatant, measured in 15-min intervals. The clearance of the medium and aggregated bacteria at the bottom of the tubes were additionally recorded with a digital camera. For microscopic investigation, 5 μl of aggregate was pipetted onto a slide, sealed with a coverslip, and examined by phase-contrast microscopy.
Adhesion to collagen-coated slides.
Coverslips were coated overnight at 4°C with 10 μg/ml human collagen type I (Calbiochem/Merck, Darmstadt, Germany) in PBS. Bacteria were grown and harvested as described before. After being washed with PBS, 2 × 107 bacteria were centrifuged onto coverslips at 300 rpm for 5 min. After 1 h of incubation at 37°C, the coverslips were washed three times with PBS and then fixed with 4% PFA. Bacteria were stained with DAPI (4′,6-diamidino-2-phenylindole) and counted in three randomly selected fields of view obtained at a magnification of ×100 under a fluorescence microscope. Wild-type adhesion was set to 100%.
Adhesion to HeLa cells.
A total of 1 × 105 human HeLa cervical epithelial cells (ATCC CCL-2.1) per well were seeded on coverslips in a 24-well microplate and grown overnight in RPMI 1640 supplemented with 10% FCS and antibiotics. The next day, the cells were washed once with prewarmed PBS and grown for another 1 h in RPMI 1640 with 10% FCS and without antibiotics. Two wells were trypsinized, and the number of cells per well was determined. Bacteria grown overnight at 27°C were diluted 1:20 in fresh medium with antibiotics and grown for 2 to 3 h at 37°C to induce the expression of YadA. Afterwards, bacteria were harvested by centrifugation (5 min at 3,000 rpm in a tabletop centrifuge), washed once with prewarmed PBS, and resuspended in RPMI 1640 with 10% FCS. The OD600 was determined, and the HeLa cells were infected with bacteria at a multiplicity of infection (MOI) of 50. Bacteria were spun down on the cells by a short centrifugation step (1 min, 300 rpm) and were incubated for 30 min at 37°C. Nonadhering bacteria were removed by 3 washing steps with prewarmed PBS. HeLa cells with the attached bacteria were fixed with 4% PFA in PBS for 10 min at room temperature. The fixed samples were washed once with PBS and stained with an aqueous solution of fuchsine for 2 min. The stained samples were washed once in PBS and air dried. Finally, the coverslips were mounted in Entellan and examined with a light microscope. Pictures of randomly chosen areas were taken at a magnification of ×100. Approximately six images comprising about 100 cells, on average, were used to count cells and adherent bacteria. From these data, the number of bacteria per cell was calculated for each strain. The numbers given in diagrams are averages for three experiments. Adherence of Y. enterocolitica expressing wild-type YadA (YadAwt) was set to 100%, and all other values refer to this value.
Cell culture and IL-8 ELISA.
HeLa cells were cultivated in RPMI 1640 medium supplemented with 2 mM glutamine and 10% fetal calf serum (experiments were also performed with serum-starved cells and gave comparable results). Infection experiments and interleukin-8 (IL-8) enzyme-linked immunosorbent assay (ELISA) were carried out as described previously (39), using an MOI of 50. IL-8 concentrations were calculated using recombinant human IL-8 (BD Biosciences Pharmingen) as a standard.
Gentamicin killing assay.
Bacterial invasion was assessed 3 h after infection (MOI = 50). One hour after the infection, the cells were washed three times with phosphate-buffered saline, fresh medium with 100 μg of gentamicin/ml was added, and the cells were incubated for another 2 h at 37°C, lysed with 1% Triton X-100, and plated on selective LB agar plates at appropriate dilutions.
Serum resistance test.
Pooled normal human serum (NHS) from healthy donors was purchased from the transfusion medicine department of the university hospital in Tübingen, Germany, aliquoted, and stored at −80°C. Y. enterocolitica was grown to exponential phase at 37°C and washed once with PBS, and the OD600 was determined. A total of 3 × 106 bacteria were incubated in 25% NHS for 1 h at 37°C. As a control, bacteria were incubated in heat-inactivated serum (HIS). Complement activity was stopped by placing the samples on ice and by the addition of 1 volume of brain heart infusion medium. Serial dilutions (10−1 to 10−6) of the bacteria were plated on selective agar and incubated at 27°C for 48 h. The serum bactericidal effect was calculated as the survival percentage, taking the bacterial counts obtained with bacteria incubated in HIS as 100%. The killing experiment was repeated for each strain at least three times, starting from independent cultures.
Quantification of factor H/FHL-1 and C4BP binding by flow cytometry.
The capacity of Y. enterocolitica WAP, Y. enterocolitica YadA0, the reconstituted YadAwt-expressing strain (Y. enterocolitica YadAwt), and the different YadA G389 point mutants to bind factor H/FHL-1 or C4BP from HIS was analyzed by flow cytometry. The different strains in overnight cultures (grown at 27°C) were grown until mid-log phase and washed once in PBS containing 2% bovine serum albumin (BSA). Bacteria (108) were incubated with HIS for 1 h at 37°C. After being washed, bacteria were incubated with rabbit anti-factor H antiserum directed against short consensus repeats (SCRs) 1 to 4 (24) or with rabbit anti-C4BP PAb (Complement Technology, Tyler, TX) for 30 min on ice, followed by incubation with Alexa Fluor 488-conjugated swine anti-rabbit PAb. After two additional washes, bacteria were analyzed in a flow cytometer (FACScan LRII; Becton Dickinson, Mountain View, CA). All incubations were done in a final volume of 100 μl PBS-2% BSA, and the washings were done with the same buffer. The primary and secondary PAbs were added separately as a negative control for each strain analyzed.
Cofactor assay.
Y. enterocolitica WAP, YadA0, and YadAwt and the G389A mutant (5 × 108) were incubated with factor H (100 mg/ml) in Dulbecco's PBS (DPBS) for 1 h at 37°C. After being washed thoroughly in the same buffer, the bacteria were incubated with C3b (2.4 μg/ml) and factor I (4.8 μg/ml) for 15 min at 37°C. The reactions were terminated by the addition of SDS-PAGE sample buffer (RotiLoad1; Carl Roth GmbH, Karlsruhe, Germany). The samples were analyzed by SDS-PAGE and transferred to a nitrocellulose membrane. C3b degradation was analyzed using goat anti-human C3 PAb (Complement Technology), followed by horseradish peroxidase (HRP)-conjugated rabbit anti-goat PAb (Dakopatts). After additional washings, development was performed with ECL Western blotting detection reagent (Applichem).
Virulence test.
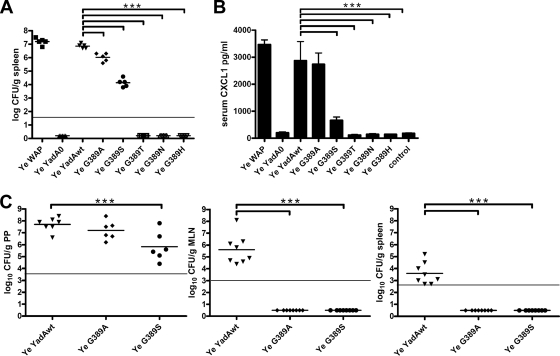
For systemic infection, bacteria were grown in Luria-Bertani broth at 27°C, harvested during the log phase, and frozen in 1-ml aliquots at −80°C. Prior to each experiment, an aliquot was thawed, washed, and resuspended in sterile PBS, pH 7.4. Mice were then injected with 1 × 105 bacteria in a volume of 100 μl into the tail vein. Twenty-four hours after infection, mice were sacrificed. Serum was collected and the spleen was removed. Half of the spleen was used to determine the bacterial load by plating the homogenate in serial dilutions on selective agar plates. The other half was used for immunohistology. For orogastric infection, bacteria were grown for 18 h at 27°C, diluted to an OD600 of 0.1, grown for another 2 to 3 h at 27°C, washed with PBS, and diluted to the appropriate infectious dose in PBS. Mice were infected intragastrically with 1 × 109 bacteria and were sacrificed on day 3 after infection (Animal Licensing Committee permission no. H5/08, Regierungspräsidium Tübingen, Tübingen, Germany). Organs were homogenized in 5 ml (spleen) or 3 ml (mesenteric lymph nodes [MLNs] and PPs) PBS containing 0.1% Tergitol TMN10 (Fluka) and 0.1% BSA (Biochrom). The number of bacteria was determined by plating 0.1-ml serial dilutions of the homogenates on Yersinia selective agar. The lower limit of detectable CFU per organ by this method was 50 (log10 50 = 1.7) for the spleen and 30 (log10 30 = 1.5) for PPs and MLNs (PPs and MLNs were pooled separately and are referred to as one organ). Higher values for the detection limits (Fig. 7) resulted from the fact that values are shown as CFU per gram of organ.
FIG. 7.
Virulence test and CXCL1 cytokine levels in sera of infected mice. (A) Numbers of bacteria (expressed as log10 CFU per gram of tissue) in the spleens of C57BL/6 mice 1 day after intravenous infection with 105 bacteria of different Yersinia strains. (B) Cytokine levels in sera of infected mice were determined by ELISA. Sera were obtained from mice that were killed 1 day after intravenous infection with 105 Y. enterocolitica cells. Values are means for at least five animal sera ± standard deviations. (C) Bacterial loads (expressed as log10 CFU per gram of tissue) in Peyer's patches (PP), mesenteric lymph nodes (MLN), and spleens after orogastric infection of BALB/c (dose of 109 CFU at 3 days postinfection) mice. ***, P < 0.001. The horizontal lines indicate the limits of detectable CFU per gram of tissue.
To ensure the presence of the recombinant virulence plasmid, bacteria were plated in duplicate on CIN agar and CIN agar supplemented with the appropriate antibiotics. In all experiments, bacterial counts were comparable on agar with and without selective antibiotics, indicating that the plasmid was still present. The CFU was determined after 2 days of incubation, as described previously (3).
Determination of CXCL1 production in serum.
The blood of sacrificed mice was collected immediately and left on wet ice for at least 1 h. Serum and clotted blood components were separated by centrifugation at 4°C and 2,500 × g for 10 min. The blood clot was removed, and the resulting serum was aliquoted and stored at −80°C. Serum CXCL1 levels in infected mice were determined by using a capture ELISA (KC-ELISA Duo set; R&D Systems, Wiesbaden, Germany).
Immunohistology.
For immunohistological analysis, the tissues were embedded in Tissue-Tek OCT compound (Nunc, Roskilde, Denmark), snap frozen in liquid nitrogen, and stored at −80°C. Frozen sections were prepared and stained by an immunoperoxidase method, using 3,3-diaminobenzidine-tetrahydrochloric acid (DAB; Sigma, Deisenhofen, Germany) as a chromogenic substrate. Nonspecific binding sites were blocked by incubation of the sections with PBS containing 10% FCS and 5% normal goat serum (NGS). Anti-Yersinia antibody WA-vital (antibody raised against whole bacteria) was diluted 1:200 in PBS containing 5% FCS and 5% NGS for 1 h at room temperature. The secondary antibody was peroxidase-conjugated affinity-purified F(ab′)2 fragment of goat anti-rabbit IgG (diluted 1:400; Jackson ImmunoResearch). Isotype-matched irrelevant rabbit IgG was used in controls and revealed no staining signal. The sections were counterstained with Mayer's hemalaun, mounted, and assessed microscopically by two independent investigators. Immunostaining of controls was negative for all groups tested.
Statistics.
The data shown in the figures are from representative experiments. Comparable results were obtained in at least two additional experiments. Differences between mean values were analyzed using one-way analysis of variance (ANOVA) and a Bonferroni posttest with a confidence interval of 95%. A P value of <0.05 was considered statistically significant (*), a P value of 0.001 to 0.01 was considered very significant (**), and a P value of <0.001 was considered extremely significant (***). Error bars represent mean values ± standard errors of the means (SEM).
RESULTS
Generation of Y. enterocolitica mutants expressing point-mutated versions of YadA.
In our previous work, we demonstrated that the replacement of a highly conserved glycine residue within the YadA membrane anchor domain by amino acids with larger side chains affects both the trimer stability and surface display of YadA expressed by E. coli BL21(DE3)Omp8 (19). In order to gain knowledge about the actual virulence functions of YadA, the point-mutated versions of YadA in which G389 was exchanged with A, S, T, N, and H were expressed in Y. enterocolitica and analyzed for virulence functions in vitro and in vivo.
Site-directed mutagenesis and generation of the Yersinia strains were performed essentially as described previously (19, 35). Fragments including the whole yadA gene and adjacent regions were subcloned into the suicide plasmid pGP-704. For later selection of recombined clones, a spectinomycin resistance cassette was introduced. The plasmids were transformed into E. coli SM17-1 λpir. As a recipient strain, we used Y. enterocolitica YadA0, in which the yadA gene is disrupted by the insertion of a kanamycin cassette. The wild-type yadA gene and the mutated yadA genes were introduced into the recipient strain via homologous recombination and cointegrant formation, respectively. By selection for resistance against spectinomycin and kanamycin, the transconjugants harboring cointegrants were identified. Single colonies were screened for the presence of the intact full-length yadA gene, and PCR products were sequenced to verify the point mutations.
Expression, trimer stability, and surface display of YadA are reduced in G389 mutants.
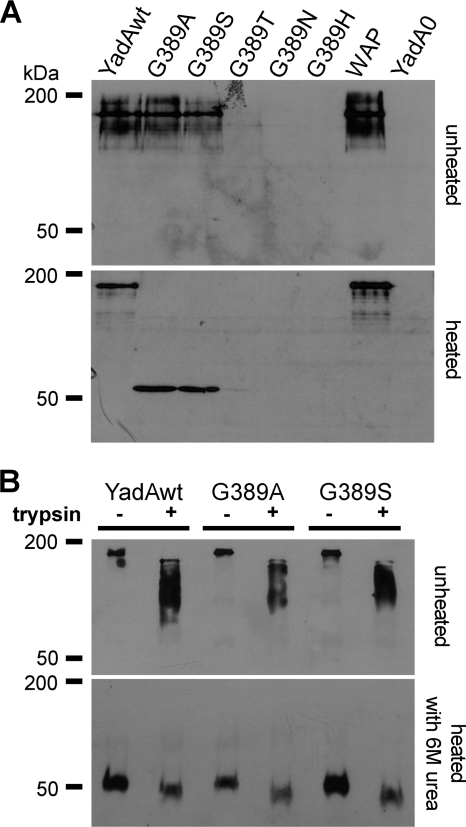
To assess expression, trimer formation, and trimer stability of YadA G389 mutants expressed in Y. enterocolitica, heated or unheated whole-cell lysates of Y. enterocolitica expressing wild-type YadA or G389 mutants were subjected to Western blot analysis. Figure 1A shows that a band of approximately 200 kDa could be observed for YadAwt, representing the trimeric form of YadA, despite the heating of samples. The G389A and G389S mutants also formed trimers, but their stability was reduced because in the heated samples only the monomeric form of YadA could be detected. We were not able to detect the G389T, G389N, and G389H mutants by Western blot analysis (Fig. 1A). Recent work suggests that this might be due to degradation of accumulated YadA mutant protein in the periplasmic space by proteases such as DegP (19).
FIG. 1.
Expression, stability, and trypsin accessibility of YadA and mutant versions expressed by Y. enterocolitica. Whole-cell lysates were prepared from Y. enterocolitica cultures grown for 2 to 3 h at 37°C. (A) Samples were either heated or not heated before separation by SDS-PAGE, and YadA was detected by Western blotting. Wild-type YadA appears as a trimer at ∼180 kDa, with monomers at 50 kDa. (B) Bacteria were grown as described above and incubated with or without 0.1 mg/ml trypsin for 1 h on ice. Afterwards, the bacterial pellet was resuspended in sample buffer. To disrupt protein trimers, samples were heated with 6 M urea to 95°C prior to loading, where indicated.
To investigate whether the YadA mutants were actually transported and exposed to the bacterial surface, we performed trypsin digestion experiments (Fig. 1B). Yersinia strains expressing YadAwt, YadA G389A, and YadA G389S were treated with trypsin and analyzed by Western blotting. Trypsin digestion resulted in one major truncation, leading to a shift from ∼200 kDa to ∼150 kDa for the trimers of YadAwt, YadA G389A, and YadA G389S (upper panel) and to a shift from ∼50 kDa to ∼45 kDa for the monomers (lower panel). These findings demonstrate that YadAwt, YadA G389A, and YadA G389S are accessible for trypsin and therefore are transported to and exposed on the bacterial outer membrane. Additionally, in order to find out if the mutants revealed comparable truncations to those of the YadA wild-type protein, digested samples were treated with 6 M urea prior to loading to disrupt YadA trimers. As shown in Fig. 1B (lower panel), the molecular masses of the resulting digestion products suggest that these proteins experienced comparable truncations.
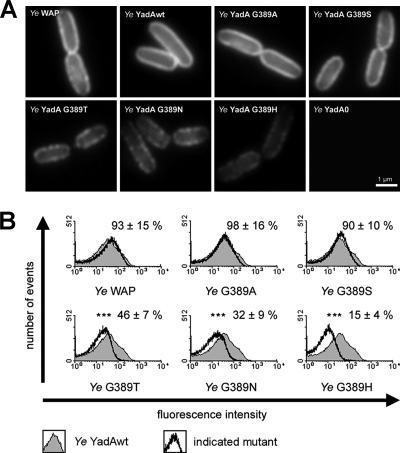
Immunofluorescence staining for YadA without permeabilization of the bacteria and subsequent microscopic analysis revealed that Y. enterocolitica WAP (parental YadA wild-type strain) and the mutant strains expressing YadAwt, YadA G389A, and YadA G389S displayed a strong, homogenous ring-shaped fluorescence pattern (Fig. 2A). In contrast, Y. enterocolitica strains expressing YadA G389T, YadA G389N, and YadA G389H revealed a weak and irregular staining pattern. This may reflect an irregular distribution of YadA at the bacterial surface for as yet unknown reasons. To quantify fluorescence intensity, we also performed flow cytometry analysis (Fig. 2B). The data revealed comparable mean fluorescence intensities for Y. enterocolitica WAP (93% ± 15%) and the G389A (98% ± 16%) and G389S (90% ± 10%) mutants to that for Y. enterocolitica YadAwt. In contrast, the G389T (46% ± 7%), G389N (32% ± 9%), and G389H (15% ± 4%) mutants showed significant reductions in mean fluorescence intensity compared to Y. enterocolitica YadAwt.
FIG. 2.
Immunofluorescence microscopy and flow cytometry analysis of Y. enterocolitica (Ye) expressing wild-type and mutant YadA. (A) Bacteria were grown for 2 h at 37°C to induce expression of YadA. YadA on the surfaces of bacteria was detected with anti-YadA antibodies and Cy2-coupled secondary antibodies. Y. enterocolitica YadA0 served as a negative control. (B) Histogram overlays of fluorescence intensity distributions in samples of bacteria expressing YadAwt or mutants. Histograms for bacteria expressing YadAwt (gray-filled area) were overlaid with the histograms for the individually indicated strains. Fluorescence of Y. enterocolitica YadA0 was also determined (not shown). The minute fluorescence of these samples was considered blank/background and subtracted from all other values. The mean fluorescence intensity of YadAwt was set to 100%. The histograms depict data for one representative experiment. Values are means with the YadA0 background subtracted ± standard deviations for three independent experiments.
Together, these data demonstrate that single point mutations of YadA G389 affect trimer stability and/or surface display of YadA and that Western blotting as well as immunofluorescence and flow cytometry analyses are required to address the various features of YadA.
YadA G389 point mutations affect autoagglutination and adhesion of Y. enterocolitica to host cells.
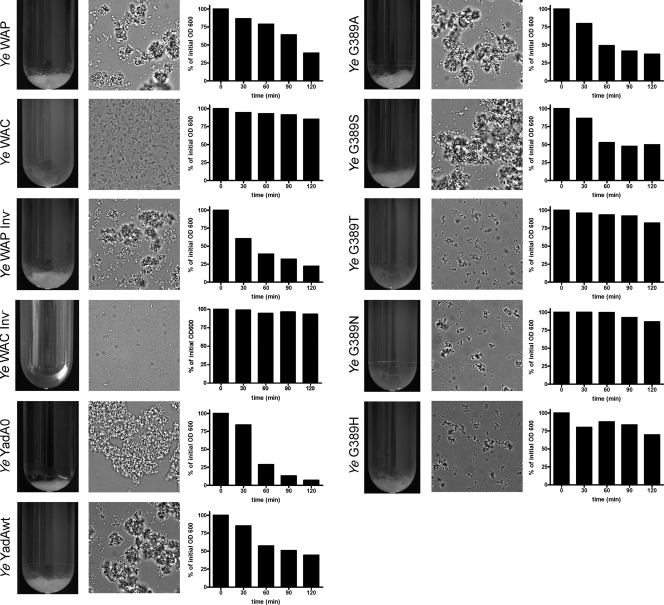
YadA has been demonstrated to promote autoagglutination of Y. enterocolitica (43). The ability of YadA-expressing Y. enterocolitica to form cell aggregates and macrocolonies may affect colonization and growth, and thus virulence (15). To address whether G389 point mutations affect autoagglutination, bacteria were grown in RPMI 1640 medium supplemented with 10% heat-inactivated FCS or minimal medium without FCS or LB medium at 37°C for 24 h according to the protocol published by Laird and Cavanaugh (25). Thereafter, sedimentation of bacteria, clearance of the culture medium, and the formation of bacterial aggregates were monitored macroscopically, densitometrically, and by microscopy. The same results were obtained with minimal medium or LB broth (data not shown). We found that Y. enterocolitica WAP rapidly settled from the suspension and formed a pellet in the culture tube (Fig. 3). The microscopic examination of the pellet revealed large clumps of aggregated bacteria. Autoagglutination could also be observed with Y. enterocolitica WAP Inv− (carrying a disrupted invasin gene), demonstrating that invasin is dispensable for autoagglutination. The control strain Y. enterocolitica WAC (which lacks the virulence plasmid, including yadA) did not form a pellet, and single, highly mobile bacteria could be observed by microscopy. Accordingly, Y. enterocolitica WAC Inv− did not show autoagglutination. Surprisingly, Y. enterocolitica YadA0, lacking YadA expression, rapidly agglutinated, and a pellet consisting of large clumps of bacteria was observed. Likewise, Y. enterocolitica YadAwt and the G389A and G389S mutants displayed autoagglutination comparable to that of Y. enterocolitica WAP. In contrast, the G389T, G389N, and G389H mutants did not display autoagglutination.
FIG. 3.
Autoagglutination of Y. enterocolitica. Cultures were grown overnight at 37°C in medium containing FCS (the same results were obtained with medium without FCS [data not shown]). The tubes were then incubated at room temperature without agitation, and bacteria were allowed to settle. Photographs of culture tubes were taken after 90 min of settling. WAP, Y. enterocolitica wild-type strain; WAC, Y. enterocolitica without the virulence plasmid; WAP Inv−, Y. enterocolitica wild-type strain with disrupted inv gene; WAC Inv−, virulence plasmid-cured strain with disrupted inv gene. The middle columns show microscopic pictures of bacterial aggregates which were taken from the sediments in the culture tubes. The right columns show time courses of bacterial sedimentation. Optical densities of bacterial culture supernatants were determined after bacteria were allowed to settle for the indicated times.
YadA specifically binds with high affinity to the alpha 1 chain of collagen type I in a triple-helical conformation and also to triple-helical collagen-like peptides (27, 41). This binding activity is conformation dependent and resides within the YadA head domain (35). Adhesion to extracellular matrix proteins has been implicated to be an important prerequisite for Yersinia virulence in vivo. Therefore, we wanted to test if Y. enterocolitica mutant strains which display reduced YadA surface levels are still able to bind to collagen type I and to HeLa cells. For this purpose, coverslips coated with collagen type I or confluent with HeLa cells were incubated with Y. enterocolitica wild-type and mutant strains. Adherent Y. enterocolitica cells were stained with DAPI or fuchsine and counted on microscopic images (Fig. 4). We found that the adhesion to both collagen type I and HeLa cells was unaltered for Y. enterocolitica YadAwt and the G389A and G389S mutants compared with Y. enterocolitica WAP, whereas adhesion was largely abolished in Y. enterocolitica YadA0. Adhesion was significantly reduced in the G389T, G389N (P < 0.01), and G389H (P < 0.001) mutants.
FIG. 4.
Adhesion of Y. enterocolitica to collagen type I and HeLa cells. Y. enterocolitica strains expressing wild-type and mutant YadA were allowed to adhere to collagen-coated coverslips (A) or to HeLa cells (B). Weakly bound bacteria were washed off, and adhering bacteria were counted. Y. enterocolitica YadAwt adherence was set to 100%. The values are means ± standard deviations and are representative of three independent experiments.
Taken together, these results suggest the following. (i) YadA may promote autoagglutination in all tested strains, except for Y. enterocolitica YadA0, and the degree of autoagglutination is associated with the amount of YadA surface display. (ii) There may be other, as yet unknown factors encoded by the virulence plasmid which, in the absence of YadA, also can mediate autoagglutination. However, YadA, even if present at low quantities on the bacterial surface, may mask this effect. This assumption remains speculative at present and needs further exploration in future studies. (iii) Similar to autoagglutination, adhesion to collagen I or HeLa cells is abolished in those YadA mutants with significantly reduced YadA surface display.
Mutations of G389 of YadA do not affect the ability of Y. enterocolitica to suppress host cell cytokine responses and host cell invasion.
Upon the engagement of host cells, several components of Y. enterocolitica, including YadA, invasin protein, lipopolysaccharide (LPS), and YopB, have been demonstrated to activate NF-κB, which subsequently gives rise to a proinflammatory host cell response, including production of, e.g., IL-8 (17, 39, 45-47). NF-κB is a key transcription factor involved in innate immune responses to pathogens (9). However, virulent Y. enterocolitica may suppress NF-κB activation and cytokine production of host cells (2). The ability to suppress cytokine responses has been assigned to the presence of the Yersinia outer protein P (YopP) (37, 38).
Sufficient attachment to host cells is believed to be a prerequisite for cell invasion and the injection of Yops (11). To assess whether Y. enterocolitica G389 mutants with unaltered or reduced adherence to HeLa cells are affected in the ability to suppress host cell cytokine responses and invasion, HeLa cells were exposed to Y. enterocolitica wild-type and G389 mutant strains and IL-8 levels were determined in the cell culture supernatants after 6 h (Fig. 5A). The numbers of internalized bacteria were determined by a gentamicin killing assay. In agreement with previously published data (18), the avirulent strain Y. enterocolitica WAC (without the pYV plasmid encoding YadA) triggered production of IL-8 (∼1,500 pg/ml) and was internalized efficiently, while the virulent strain Y. enterocolitica WAP totally suppressed production of IL-8 and was significantly less internalized (∼2.4% [WAC] versus ∼0.3% [WAP] invasion [percentage of inoculum after 3 h]). Moreover, the absence of YadA did not affect the suppression of cytokine production by Yops. In fact, Y. enterocolitica YadA0 as well as all G389 mutant strains efficiently suppressed IL-8 production in HeLa cells. In addition, compared to Y. enterocolitica WAC, all other mutant strains were significantly less internalized. Taken together, these data suggest that despite reduced YadA surface display and strongly reduced host cell adhesion, all YadA mutant strains could inject enough Yops into host cells to suppress IL-8 release and to resist internalization by host cells.
FIG. 5.
Yersinia-induced IL-8 secretion and cell invasion in vitro. (A) HeLa cells were incubated with Y. enterocolitica for 1 h, and then bacteria were killed and cells were incubated for a further 6 h. IL-8 levels in the cell culture supernatants were determined by ELISA. Tumor necrosis factor alpha (TNF-α) was used as a positive control. As a negative control, cells were incubated without bacteria. Data are means and standard deviations for 2 independent experiments. (B) Uptake of bacteria into HeLa cells was analyzed by determining the number of intracellularly viable bacteria, using a gentamicin killing assay.
Mutations of YadA G389 interfere with serum resistance and binding to factor H and C4BP.
YadA, in addition to Ail, mediates protection of Y. enterocolitica against the host complement system (6-8, 12, 23, 33). Furthermore, LPS may also contribute to complement resistance of Y. enterocolitica. A protective effect has been shown for LPS of Y. enterocolitica serotype O:9 (42) but not for Y. enterocolitica strains of serotypes O:3 and O:8 (4, 8). To test the ability of YadA G389 mutant proteins to protect cells from complement killing, bacteria were incubated for 1 h in 25% NHS or HIS and thereafter plated on LB agar. Survival was calculated as a percentage of the bacterial count obtained with HIS (100% survival). The data depicted in Fig. 6A demonstrate that compared to Y. enterocolitica WAP, Y. enterocolitica YadAwt displayed an increased ability to survive in NHS, whereas the G389A and G389S mutants displayed a decreased ability to survive in NHS; the G389T, G389N, and G389H mutants were as sensitive to complement killing as the YadA-deficient strain Y. enterocolitica YadA0.
FIG. 6.
Serum resistance and binding of complement regulatory components factor H and C4BP are mediated by YadA. (A) Equal numbers of bacteria were incubated with 25% NHS or HIS for 1 h at 37°C and then plated on LB agar. The bacterial survival was calculated as a percentage, taking the bacterial counts obtained with HIS as 100%. (B) Binding of serum-derived factor H and C4BP to bacteria expressing or not expressing YadA was assessed by flow cytometry. Histograms for control samples (stained with primary and secondary antibodies only; light lines) were overlaid with histograms for samples specifically stained for factor H and C4BP (bold lines). The figure depicts the histograms from one representative experiment. (C) Binding of serum-derived factor H and C4BP was assessed for all strains. Bars show background-subtracted mean fluorescence intensities. Background-subtracted signal intensities for both factor H and C4BP staining for Y. enterocolitica YadA0 and the G389T, G389N, and G389H mutants sometimes reached values below zero due to variable signal noise. To make bars visible in the diagram, these values were set to 50. The data are means ± standard deviations for two independent experiments. (D) Cofactor activities of factor H bound to Y. enterocolitica WAP, YadA0, and YadAwt and the G389A mutant. The bacteria were incubated with (+) or without (−) factor H and then intensively washed. Afterwards, the bacteria were exposed to factor I and C3b. Control reactions (controls) were carried out without bacteria. C3b and the cleavage products resulting from membrane-bound factor H cofactor activity were detected with a polyclonal antibody directed against human C3b. Inactivation of C3b is indicated by the appearance of C3b α′-chain cleavage fragments of 68, 43, and 41 kDa and by reduction of the intensity of the C3b α′-chain band.
Recently, it was shown that the ability of YadA to protect Y. enterocolitica from killing by complement depends on its ability to bind the complement regulatory components factor H and C4BP. By this means, Y. enterocolitica prevents deposition of activated C3 on the bacterial membrane (6, 23). To investigate the binding of factor H and C4BP to Y. enterocolitica strains, we tested both whole serum and purified proteins. Because binding of purified protein was much lower than binding of serum-derived factor H and C4BP, we carried out all binding studies with HIS as a source of complement factors. To address if disturbed binding of factor H and/or C4BP in YadA G389 mutants is decisive for their survival in serum, we analyzed the binding of serum factor H and C4BP by flow cytometry. For analysis of factor H binding, we also performed binding assays by ELISA and obtained comparable results (not shown). The results revealed that Y. enterocolitica WAP, Y. enterocolitica YadAwt, and the YadA G389A and G389S mutants bound factor H in a similar manner (Fig. 6C). Compared to these strains, Y. enterocolitica YadA0 and all the other mutant strains (G389T, G389N, and G389H) displayed virtually no factor H binding. Similar to factor H, C4BP was bound by Y. enterocolitica WAP, Y. enterocolitica YadAwt, and the G389A mutant, in comparable and substantial amounts, and to lesser extents by the G389S mutant, whereas only residual binding of C4BP could be observed with the G389T, G389N, and G389H mutants. Factor H regulates the alternative pathway activity by serving as a cofactor for factor I in the degradation of C3b. The G389A mutant revealed impaired serum resistance, though factor H was bound at the wild-type level by this mutant. Therefore, we wanted to analyze if factor H bound to the G389A mutant actually has cofactor activity. We also tested Y. enterocolitica WAP and Y. enterocolitica YadA0, as positive and negative controls, respectively, because it was shown previously for Y. enterocolitica O:3 that YadA serves as the main factor H binder (6). Bacteria were incubated in the presence or absence of factor H and intensively washed, and then factor I and C3b were added. Following incubation for 15 min, all lysates were separated by SDS-PAGE. The cleavage of C3b was then analyzed by Western blotting. The cleavage products generated in the presence of surface-bound factor H and factor I showed comparable sizes to those of the fragments generated by factor I in combination with factor H in the fluid phase. When C3b was incubated in the presence of factor H bound to the bacterial surface, cleavage products of 43 and 41 kDa appeared (Fig. 6D). However, almost no cleavage products were seen with bacteria only. Factor H bound to the surface of Y. enterocolitica WAP, and Y. enterocolitica YadAwt was functionally active and exhibited substantial cofactor activity, whereas Y. enterocolitica YadA0 did not bind factor H, and therefore no factor H-mediated C3b cleavage could be detected. The G389A mutant-bound factor H retained cofactor activity to degrade C3b comparable to that of Y. enterocolitica WAP and Y. enterocolitica YadAwt. Taken together, the most interesting finding of all these assays is that the G389A mutant showed reduced serum resistance although it recruited similar amounts of factor H and C4BP to those obtained with Y. enterocolitica YadAwt.
Mutation of YadA G389 leads to significantly reduced virulence of Y. enterocolitica in mice.
To reveal the importance of YadA G389 mutations in the virulence of Y. enterocolitica in vivo, mice were intravenously infected with Y. enterocolitica wild-type and mutant strains and the bacterial burden in the spleen was determined (Fig. 7A). Y. enterocolitica WAP and Y. enterocolitica YadAwt resulted in bacterial burdens of 6.7 to 7.5 log10 CFU per gram of tissue, while Y. enterocolitica YadA0 did not colonize the spleen (P < 0.01). Compared to Y. enterocolitica YadAwt, the G389A mutant was significantly attenuated, with the bacterial burden reduced by ∼84% (5.6 to 6.3 log10 CFU). The G389S mutant was even more attenuated, with the bacterial burden reduced by ∼99.8% (∼4.1 log10 CFU). The G389T, G389N, and G389H mutants were totally avirulent. CXCL1 (KC) is a functional homologue of human IL-8 and is associated with neutrophil recruitment and inflammation. Influx of neutrophils from the blood and their interaction with vascular endothelium at the site of infection are early steps of acute inflammation (16). Since the Y. enterocolitica YadA G389 mutants seemed to be significantly reduced in virulence and abscess formation, we wanted to find out if they induced a systemic inflammatory reaction in infected mice. Therefore, we analyzed CXCL1 levels in the sera of systemically infected mice by ELISA (Fig. 7B). We observed comparably increased levels of CXCL1 in sera of mice infected with Y. enterocolitica WAP (∼3,500 pg/ml), Y. enterocolitica YadAwt (∼2,900 pg/ml), or the G389A mutant (∼2,800 pg/ml). In contrast, the G389S mutant triggered a significantly lower level of CXCL1 (∼600 pg/ml), although this level was higher than that in uninfected controls. Only background levels of CXCL1 could be detected in mice infected with the G389T, G389N, and G389H mutants or with Y. enterocolitica YadA0. To further analyze the consequences of YadA G389 mutations during the natural route of a Yersinia infection, we orogastrically administered 5 × 109 bacteria to mice (Fig. 7C). Three days after infection, mice were sacrificed. PPs, MLNs, and spleens were removed, and the bacterial load of each organ was determined. PPs revealed comparable bacterial loads in mice infected with the Y. enterocolitica YadA wild-type strain (log10 CFU, ∼8) and the G389A (log10 CFU, ∼7) and G389S (log10 CFU, ∼6) mutants. Nevertheless, only Y. enterocolitica YadAwt (log10 CFU, ∼5.5) could be detected in mesenteric lymph nodes, indicating that efficient dissemination was impaired in the mutant strains. Accordingly, only Y. enterocolitica YadAwt (log10 CFU, ∼3.5), not the G389A and G389S colonized the spleens of orally infected mice.
In conclusion, the data indicate that the virulence of Y. enterocolitica YadA wild-type and G389 mutant strains in mice is closely associated with their ability to cause systemic infection, including a significant bacterial burden in the spleen, abscess formation, and systemic CXCL1 serum levels.
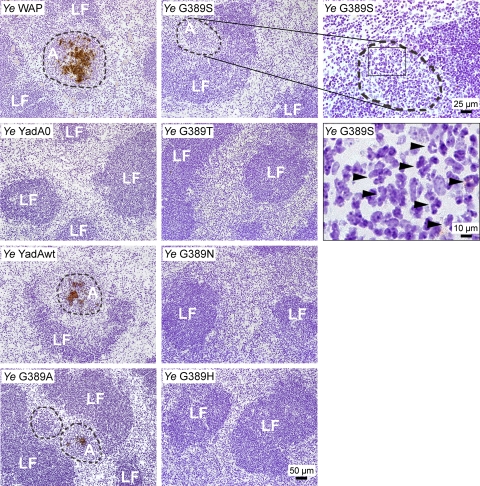
After systemic infection, Y. enterocolitica may induce the formation of abscesses in the spleen that consist of granulocytes, macrophages, and bacteria. To investigate whether and how YadA G389 mutations may affect abscess morphology, cryosections from the infected spleens of mice were prepared and subjected to immunohistochemistry analysis using anti-Yersinia antibodies (Fig. 8). Numerous abscesses with diameters of approximately 40 to 100 μm could be observed in mice infected with Y. enterocolitica WAP and YadAwt. Abscesses were made up of a central mass of bacteria infiltrated with inflammatory cells. The whole area was surrounded by a broad border of inflammatory cells and was located, in most cases, adjacent to lymphoid follicles. In mice infected with the G389A mutant, fewer and smaller abscesses could be detected. Upon infection with the G389S mutant, only a few very small abscesses were found; however, lymphoid follicles were infiltrated by numerous polymorphonuclear leukocytes. Neither abscesses nor signs of neutrophil inflammatory infiltrations were seen in mice infected with Y. enterocolitica YadA0 or the G389T, G389N, or G389H mutant.
FIG. 8.
Immunohistochemistry of spleens of intravenously infected mice. The spleens of Y. enterocolitica-infected mice were embedded in TissueTek and shock frozen. Seven-micrometer cryosections were stained with an antibody against Y. enterocolitica and a secondary antibody coupled to peroxidase. DAB was used as a chromogenic substrate and forms a brown precipitate. Bacteria and abscesses appear in brown. Cells were counterstained with hematoxylin. LF, lymph follicle; A, abscess. Abscesses are demarcated by dashed lines. Arrowheads point to polymorphonuclear leukocytes, found in large numbers in spleens of mice infected with the Y. enterocolitica G389S mutant, despite the fact that abscess formation was not detected.
DISCUSSION
In the previous work, we identified a highly conserved glycine residue (G389) within the second beta-sheet of the YadA membrane anchor domain and demonstrated that it is involved in YadA autotransport and trimer stability and in YadA-mediated serum resistance when expressed in E. coli (19). In the present study, we wanted to clarify two issues, as follows. (i) What is the impact of G389 on the YadA-associated in vitro virulence functions of Y. enterocolitica? To address this, we introduced the G389 substitution mutations G389A, G389S, G389T, G389N, and G389H into Y. enterocolitica and tested the bacteria for YadA expression, trimer stability, trypsin accessibility, outer membrane localization, autoagglutination, adherence, host cell cytokine induction, serum resistance, and binding of the serum complement regulatory components factor H and C4BP. (ii) Are the in vitro virulence assays performed with the YadA point mutants relevant to the virulence of Y. enterocolitica in the mouse model? To elucidate this, we infected mice with Y. enterocolitica wild-type bacteria and mutants expressing the YadA point mutants and compared bacterial burdens in several organs, serum cytokine levels, and morphological changes in the spleen.
The most salient finding of this study is that all YadA mutants displayed a reduction of trimer stability; however, their abilities to mediate autoagglutination, adherence, serum resistance, and virulence differed significantly in the various yadA mutant strains (a summary of the phenotypes of all strains is given in Table 2). Moreover, the data obtained by both the intravenous and orogastric mouse infection models suggest that only a few of the in vitro virulence traits actually are closely associated with the virulence of Y. enterocolitica in mice, thus challenging their meaningfulness. In fact, apparently only trimer stability and serum resistance, not the other in vitro virulence traits, are closely associated with the virulence functions in vivo. Finally, we can conclude that minor changes in the membrane anchor of YadA may directly or indirectly affect the complement resistance of Y. enterocolitica, the key virulence function mediated by YadA. However, at present, we can only speculate about the molecular basis for the reduced virulence and serum resistance. The current YadA membrane anchor model is based on the Hia structure (19, 30) and suggests that at position G389, larger side chain residues could be accommodated. The reasons why and how the larger side chains disturb the protein's stability and autotransport function need further investigation. Thus, the molecular structure of the YadA membrane anchor needs to be elucidated either by X-ray crystallography or by nuclear magnetic resonance (NMR) experiments. From the high level of conservation of the Gly residue (19), it can be assumed that the absence of a large side chain might be very important either in the folding process, in the autotransport process, or for final thermodynamic stability (or a combination of these factors). How mutations of this conserved residue finally may affect the recruitment of complement regulatory factors remains to be elucidated.
TABLE 2.
Summary of phenotypes of various mutantsa
| Strain | YadA expression (WB) | Trimer stability (WB) | Surface display (IF) | Autoagglutination | Adhesion (to collagen or HeLa cells) | Induction of IL-8 and invasion | Serum resistance | Binding of factor H/binding of C4BP | Intravenous virulence/ oral virulence |
|---|---|---|---|---|---|---|---|---|---|
| WAP | + | + | ++ | + | + | − | ++ | +/+ | ++/NA |
| YadA0 | 0 | NA | 0 | + | − | − | − | −/− | −/− |
| WAC | NA | NA | NA | − | NA | + | NA | NA | NA |
| YadAwt | + | + | ++ | + | + | − | ++ | +/+ | ++/++ |
| G389A mutant | + | − | ++ | + | + | − | ± | +/+ | +/− |
| G389S mutant | + | − | + | + | + | − | ± | +/± | ±/− |
| G389T mutant | − | NA | ± | − | ± | − | − | 0 | −/− |
| G389N mutant | − | NA | ± | − | − | − | − | 0 | −/− |
| G389H mutant | − | NA | − | − | − | − | − | 0 | −/− |
++, very high; +, high; ±, intermediate; −, low; 0, below detection limit; WB, Western blotting; IF, immunofluorescence; NA, not available.
In analyzing the expression of YadA, we were not able to detect the G389N, G389T, and G389H mutants by Western blotting of whole-cell lysates of Yersinia. When the SDS gels were overloaded, occasionally very faint bands of YadA trimer or monomer could be observed in nonheated, but not heated, samples of the G389N, G389T, and G389H mutants (data not shown), suggesting that the G389N, G389T, and G389H mutants have reduced trimer stability and are readily degraded in Y. enterocolitica. Nevertheless, we could demonstrate expression of the G389N, G389T, and G389H mutants by immunofluorescence microscopy and flow cytometry, indicating that these methods are more sensitive than Western blotting. In E. coli, we were able to rescue expression of yadA G389 mutants by knocking out the periplasmic protease DegP (19). DegP is part of the periplasmic stress response and is responsible for degradation of misfolded proteins, including autotransporter proteins accumulating in the periplasm (22, 32). Analogous to our experiments with E. coli, future experiments with a Y. enterocolitica ΔhtrA mutant strain will have to demonstrate whether the closely related periplasmic protease HtrA (28) is involved in degradation of YadA G389 mutants.
YadA is the major adhesin of Y. enterocolitica and has been demonstrated to mediate autoagglutination (43). The exact mechanism of this phenomenon is not understood but is most likely due to the hydrophobic nature of the bacterial surface and due to homologous interaction of highly concentrated outer membrane proteins, as YadA-expressing bacteria readily settle out of a suspension. However, the role of autoagglutination per se for virulence of Y. enterocolitica is not known. Bacteria bearing the pYV virulence plasmid and expressing YadA in 3D collagen gels grow as densely packed microcolonies, while bacteria carrying the pYV plasmid but lacking YadA grow as loosely packed microcolonies (15). There appears to be a correlation between the autoagglutination phenotype and growth behavior in collagen gels (15). Formation of densely packed microcolonies, however, did not depend on the ability of YadA to bind collagen (15). It is conceivable that densely packed microcolonies might protect bacteria from complement attack and from phagocytosis. The results of the present study clearly demonstrate that autoagglutination may also occur in the absence of YadA and that the autoagglutination of Y. enterocolitica wild-type or mutant strains is not associated with the ability to exert a systemic infection in an experimental mouse infection model or to induce tissue abscesses including microcolonies in vivo. In fact, Y. enterocolitica WAP, WAP Inv−, and YadAwt and the G389A and G389S mutants displayed autoagglutination, suggesting that YadA, but not invasin, contributes to this effect. The G389T, G389N, and G389H mutants showed no significant sedimentation, most probably due to the small quantity of YadA available for hydrophobic interactions. Y. enterocolitica YadA0, however, displayed rapid autoagglutination in our hands. To rule out the possibility that this rather unexpected result emanated from our experimental setting, we repeated the experiment under serum-free conditions with minimal medium and LB but gained the same outcome. Therefore, we can exclude the possibility that autoagglutination of Y. enterocolitica YadA0 is mediated by serum-originating components. We rather think that there are other factors on the surface of Y. enterocolitica which are also capable of mediating autoagglutination and that these factors are masked by YadA. If there are only some residual molecules of YadA present, as in the G389T, G389N, and G389H mutants, then efficient interaction of these unknown autoagglutination factors is blocked. The identification of these factors is the subject of future experiments.
In vitro, YadA induces cytokine production (e.g., IL-8 production) in host cells (39, 40). This host cell response can be suppressed by injection of Yops via the type III secretion system into the host cells. To our surprise, a YadA-deficient strain also efficiently suppressed secretion of IL-8 by HeLa cells. Accordingly, none of our mutant strains, differing only in the amount of YadA presented on the surface, resulted in significant IL-8 secretion, suggesting that few adhering bacteria translocated enough effector proteins to exert inhibition of IL-8 secretion. Thus, in vitro YadA does not play an essential role in Yop translocation if sufficient adherence is ensured. This result is in accordance with the observation that small amounts of YadA can mediate adhesion to host cells. Assuming that YadA is a major determinant for virulence and that a YadA-deficient Y. enterocolitica strain might also be able to translocate Yop effectors efficiently in vivo but is nevertheless severely attenuated, other YadA-mediated effects must be decisive for YadA-dependent survival of Y. enterocolitica in the mouse model. Surprisingly, all of our mutant strains displayed comparable invasion behaviors. This might be explained by the fact that invasion reflects the net balance of adhesion (triggering uptake) and Yop injection (preventing uptake by disrupting the host cell actin cytoskeleton). Although we observed significantly reduced adhesion to HeLa cells with some of our mutants, these strains seemed to have injected enough Yops to efficiently suppress internalization.
The action of complement is one of the first host immune barriers to invading Y. enterocolitica. Complement resistance is thus a crucial feature of invasive, extracellularly located pathogens such as Y. enterocolitica. Consequently, numerous pathogenic microorganisms have developed mechanisms to evade the complement system (26, 48). An important function of YadA is the prevention of membrane attack complex (MAC) formation on the bacterial surface. Y. enterocolitica evades the complement system by the recruitment of two major complement regulatory proteins, namely, factor H and C4BP. Factor H prevents C3 deposition on the bacterial surface in vitro by inhibiting the binding of factor B to C3b, by supporting the dissociation of the C3bBb complex (decay accelerating activity), and by its function as a cofactor for the cleavage of C3b by factor I (1, 6, 8, 12, 23, 33). C4BP is a fluid-phase complement regulator that downregulates classical and lectin pathway complement activity by preventing the assembly and accelerating the decay of the C3 convertase.
It has been shown in vitro that both the stalk and the membrane anchor are involved in serum resistance (34). By means of the Y. enterocolitica YadA G389 mutants, we were able to test whether the quantity of YadA is decisive for complement resistance. Serum resistance levels of Y. enterocolitica WAP and Y. enterocolitica YadAwt were comparable. All of the other mutant strains had significantly reduced serum resistance, irrespective of the amount of YadA displayed on the outer membrane. Therefore, it is assumed that the exchange of G389 abrogates efficient interaction of YadA with complement regulators and thus leads to complement killing of Y. enterocolitica. Obviously, this complement regulatory factor (CRF) is neither factor H nor C4BP, because both are bound to almost wild-type levels by the G389A mutant and at least factor H exhibits full cofactor activity when bound to this strain. Nevertheless, interaction with other CRFs could be disturbed by modulation of binding to YadA. It has been shown for Ail that single point mutations within loop 2, which is exposed on the surface, can abrogate serum resistance (31). Studies by Ackermann et al. (1) support a scenario where specific interaction between the C terminus of YadA and complement inhibitory factors is inhibited. Analyses of serum resistance of Y. enterocolitica strains expressing no YadA, wild-type YadA, or chimeras revealed that the exchange of the YadA C terminus with that of UspA1 (Moraxella catarrhalis), EibA (Escherichia coli), or Hia (Haemophilus influenzae), respectively, resulted in a significant loss of survival in normal human serum. These results demonstrate that the C-terminal membrane anchor domain is involved in serum resistance either directly or indirectly, a finding also supported by this study.
Systemic infection of mice with Y. enterocolitica YadAwt and the G389 mutant strains revealed that only YadAwt was highly pathogenic in vivo and was associated with a high splenic bacterial burden, abscess formation, and high CXCL1 serum levels, while Y. enterocolitica YadA0 and the YadA G389T, G389N, and G389H mutants did not induce significant changes. Both the YadA G389A and G389S mutants were attenuated but retained the ability to induce abscesses and had increased CXCL1 serum levels. Thus, Y. enterocolitica YadA0 and the YadA G389T, G389N, and G389H mutants are rapidly killed by the first line of host defense (most likely complement), are not able to establish abscess formation, and do not lead to major inflammatory events, including cytokine production. The YadA G389A and G389S mutants cannot be killed as efficiently, probably due to their still-present, though reduced, serum resistance. In an orogastric mouse infection model, Y. enterocolitica YadAwt and the G389A and G389S mutants were able to colonize the Peyer's patches even though the YadA G389A and G389S mutants were found in slightly reduced numbers. Interestingly, only Y. enterocolitica YadAwt, not the G389A and G389S mutants, was found in mesenteric lymph nodes and could disseminate into the spleen at the time points investigated. Although this may be the result of (i) less efficient uptake by M cells, (ii) more efficient killing by the host, or (iii) delayed bacterial growth within the host tissue, scenario ii might be the most tempting one and will be pursued by exploring the virulence of YadA mutants in mice deficient in complement system components.
In summary, we demonstrated that exchange of the highly conserved single amino acid glycine within the YadA membrane anchor abrogates serum resistance. Moreover, virulence tests in the mouse model have shown that YadA-mediated serum resistance is decisive for Y. enterocolitica virulence, rather than other YadA-mediated functions, such as adhesion, which were demonstrated in vitro. These data suggest that in vivo assays provide the most sensitive assays to address minor changes in virulence factors.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 766 [bacterial cell envelope] and GKI 685 [infection biology: human- and plant-pathogenic bacteria and fungi]).
We thank J. Heesemann and N. Ackermann (LMU, Munich, Germany) for providing plasmids and J. Klenk, B. Hackl, and G. Haerer for excellent technical assistance.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Ackermann, N., M. Tiller, G. Anding, A. Roggenkamp, and J. Heesemann. 2008. Contribution of trimeric autotransporter C-terminal domains of oligomeric coiled-coil adhesin (Oca) family members YadA, UspA1, EibA, and Hia to translocation of the YadA passenger domain and virulence of Yersinia enterocolitica. J. Bacteriol. 190:5031-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol. Chem. 380:795-802. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., A. Tingle, A. Reske-Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengoechea, J. A., H. Najdenski, and M. Skurnik. 2004. Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors. Mol. Microbiol. 52:451-469. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein, H. D. 2007. Are bacterial ‘autotransporters’ really transporters? Trends Microbiol. 15:441-447. [DOI] [PubMed] [Google Scholar]
- 6.Biedzka-Sarek, M., H. Jarva, H. Hyytiainen, S. Meri, and M. Skurnik. 2008. Characterization of complement factor H binding to Yersinia enterocolitica serotype O:3. Infect. Immun. 76:4100-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biedzka-Sarek, M., S. Salmenlinna, M. Gruber, A. N. Lupas, S. Meri, and M. Skurnik. 2008. Functional mapping of YadA- and Ail-mediated binding of human factor H to Yersinia enterocolitica serotype O:3. Infect. Immun. 76:5016-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biedzka-Sarek, M., R. Venho, and M. Skurnik. 2005. Role of YadA, Ail, and lipopolysaccharide in serum resistance of Yersinia enterocolitica serotype O:3. Infect. Immun. 73:2232-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonizzi, G., and M. Karin. 2004. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280-288. [DOI] [PubMed] [Google Scholar]
- 10.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd, A. P., N. Grosdent, S. Totemeyer, C. Geuijen, S. Bleves, M. Iriarte, I. Lambermont, J. N. Octave, and G. R. Cornelis. 2000. Yersinia enterocolitica can deliver Yop proteins into a wide range of cell types: development of a delivery system for heterologous proteins. Eur. J. Cell Biol. 79:659-671. [DOI] [PubMed] [Google Scholar]
- 12.China, B., M. P. Sory, B. T. N′Guyen, B. M. De, and G. R. Cornelis. 1993. Role of the YadA protein in prevention of opsonization of Yersinia enterocolitica by C3b molecules. Infect. Immun. 61:3129-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.el Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 14.el Tahir, Y. E., P. Kuusela, and M. Skurnik. 2000. Functional mapping of the Yersinia enterocolitica adhesin YadA. Identification of eight NSV. Mol. Microbiol. 37:192-206. [DOI] [PubMed] [Google Scholar]
- 15.Freund, S., B. Czech, K. Trulzsch, N. Ackermann, and J. Heesemann. 2008. Unusual, virulence plasmid-dependent growth behavior of Yersinia enterocolitica in three-dimensional collagen gels. J. Bacteriol. 190:4111-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godiska, R., D. Chantry, G. N. Dietsch, and P. W. Gray. 1995. Chemokine expression in murine experimental allergic encephalomyelitis. J. Neuroimmunol. 58:167-176. [DOI] [PubMed] [Google Scholar]
- 17.Grassl, G. A., E. Bohn, Y. Muller, O. T. Buhler, and I. B. Autenrieth. 2003. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int. J. Med. Microbiol. 293:41-54. [DOI] [PubMed] [Google Scholar]
- 18.Grassl, G. A., M. Kracht, A. Wiedemann, E. Hoffmann, M. Aepfelbacher, C. von Eichel-Streiber, E. Bohn, and I. B. Autenrieth. 2003. Activation of NF-kappaB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell. Microbiol. 5:957-971. [DOI] [PubMed] [Google Scholar]
- 19.Grosskinsky, U., M. Schutz, M. Fritz, Y. Schmid, M. C. Lamparter, P. Szczesny, A. N. Lupas, I. B. Autenrieth, and D. Linke. 2007. A conserved glycine residue of trimeric autotransporter domains plays a key role in Yersinia adhesin A autotransport. J. Bacteriol. 189:9011-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Heesemann, J., and R. Laufs. 1983. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J. Bacteriol. 155:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heise, T., and P. Dersch. 2006. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc. Natl. Acad. Sci. U. S. A. 103:3375-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, I. R., F. Navarro-Garcia, M. Desvaux, R. C. Fernandez, and D. a'Aldeen. 2004. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 68:692-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jong, W. S., C. M. ten Hagen-Jongman, T. den Blauuwen, D. J. Slotboom, J. R. Tame, D. Wickstrom, J. W. de Gier, B. R. Otto, and J. Luirink. 2007. Limited tolerance towards folded elements during secretion of the autotransporter Hbp. Mol. Microbiol. 63:1524-1536. [DOI] [PubMed] [Google Scholar]
- 23.Kirjavainen, V., H. Jarva, M. Biedzka-Sarek, A. M. Blom, M. Skurnik, and S. Meri. 2008. Yersinia enterocolitica serum resistance proteins YadA and Ail bind the complement regulator C4b-binding protein. PLoS Pathog. 4:e1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn, S., and P. F. Zipfel. 1996. Mapping of the domains required for decay acceleration activity of the human factor H-like protein 1 and factor H. Eur. J. Immunol. 26:2383-2387. [DOI] [PubMed] [Google Scholar]
- 25.Laird, W. J., and D. C. Cavanaugh. 1980. Correlation of autoagglutination and virulence of yersiniae. J. Clin. Microbiol. 11:430-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambris, J. D., D. Ricklin, and B. V. Geisbrecht. 2008. Complement evasion by human pathogens. Nat. Rev. Microbiol. 6:132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leo, J. C., H. Elovaara, B. Brodsky, M. Skurnik, and A. Goldman. 2008. The Yersinia adhesin YadA binds to a collagenous triple-helical conformation but without sequence specificity. Protein Eng. Des. Sel. 21:475-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, S. R., N. Dorrell, P. H. Everest, G. Dougan, and B. W. Wren. 1996. Construction and characterization of a Yersinia enterocolitica O:8 high-temperature requirement (htrA) isogenic mutant. Infect. Immun. 64:2088-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linke, D., T. Riess, I. B. Autenrieth, A. Lupas, and V. A. Kempf. 2006. Trimeric autotransporter adhesins: variable structure, common function. Trends Microbiol. 14:264-270. [DOI] [PubMed] [Google Scholar]
- 30.Meng, G., N. K. Surana, J. W. St. Geme III, and G. Waksman. 2006. Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter. EMBO J. 25:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, V. L., K. B. Beer, G. Heusipp, B. M. Young, and M. R. Wachtel. 2001. Identification of regions of Ail required for the invasion and serum resistance phenotypes. Mol. Microbiol. 41:1053-1062. [DOI] [PubMed] [Google Scholar]
- 32.Pallen, M. J., and B. W. Wren. 1997. The HtrA family of serine proteases. Mol. Microbiol. 26:209-221. [DOI] [PubMed] [Google Scholar]
- 33.Pilz, D., T. Vocke, J. Heesemann, and V. Brade. 1992. Mechanism of YadA-mediated serum resistance of Yersinia enterocolitica serotype O3. Infect. Immun. 60:189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roggenkamp, A., N. Ackermann, C. A. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 185:3735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roggenkamp, A., H. R. Neuberger, A. Flugel, T. Schmoll, and J. Heesemann. 1995. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol. Microbiol. 16:1207-1219. [DOI] [PubMed] [Google Scholar]
- 36.Roggenkamp, A., K. Ruckdeschel, L. Leitritz, R. Schmitt, and J. Heesemann. 1996. Deletion of amino acids 29 to 81 in adhesion protein YadA of Yersinia enterocolitica serotype O:8 results in selective abrogation of adherence to neutrophils. Infect. Immun. 64:2506-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 38.Ruckdeschel, K., K. Richter, O. Mannel, and J. Heesemann. 2001. Arginine-143 of Yersinia enterocolitica YopP crucially determines isotype-related NF-kappaB suppression and apoptosis induction in macrophages. Infect. Immun. 69:7652-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Ruckdeschel, K., A. Roggenkamp, S. Schubert, and J. Heesemann. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 64:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid, Y., G. A. Grassl, O. T. Buhler, M. Skurnik, I. B. Autenrieth, and E. Bohn. 2004. Yersinia enterocolitica adhesin A induces production of interleukin-8 in epithelial cells. Infect. Immun. 72:6780-6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulte, R., P. Wattiau, E. L. Hartland, R. M. Robins-Browne, and G. R. Cornelis. 1996. Differential secretion of interleukin-8 by human epithelial cell lines upon entry of virulent or nonvirulent Yersinia enterocolitica. Infect. Immun. 64:2106-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze-Koops, H., H. Burkhardt, J. Heesemann, K. von der Mark, and F. Emmrich. 1995. Characterization of the binding region for the Yersinia enterocolitica adhesin YadA on types I and II collagen. Arthritis Rheum. 38:1283-1289. [DOI] [PubMed] [Google Scholar]
- 42.Skurnik, M., M. Biedzka-Sarek, P. S. Lubeck, T. Blom, J. A. Bengoechea, C. Perez-Gutierrez, P. Ahrens, and J. Hoorfar. 2007. Characterization and biological role of the O-polysaccharide gene cluster of Yersinia enterocolitica serotype O:9. J. Bacteriol. 189:7244-7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skurnik, M., I. Bolin, H. Heikkinen, S. Piha, and H. Wolf-Watz. 1984. Virulence plasmid-associated autoagglutination in Yersinia spp. J. Bacteriol. 158:1033-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamm, A., A. M. Tarkkanen, T. K. Korhonen, P. Kuusela, P. Toivanen, and M. Skurnik. 1993. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol. Microbiol. 10:995-1011. [DOI] [PubMed] [Google Scholar]
- 45.Viboud, G. I., and J. B. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 46.Viboud, G. I., E. Mejia, and J. B. Bliska. 2006. Comparison of YopE and YopT activities in counteracting host signalling responses to Yersinia pseudotuberculosis infection. Cell. Microbiol. 8:1504-1515. [DOI] [PubMed] [Google Scholar]
- 47.Viboud, G. I., S. S. So, M. B. Ryndak, and J. B. Bliska. 2003. Proinflammatory signalling stimulated by the type III translocation factor YopB is counteracted by multiple effectors in epithelial cells infected with Yersinia pseudotuberculosis. Mol. Microbiol. 47:1305-1315. [DOI] [PubMed] [Google Scholar]
- 48.Zipfel, P. F., R. Wurzner, and C. Skerka. 2007. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol. Immunol. 44:3850-3857. [DOI] [PubMed] [Google Scholar]