Abstract
Citrobacter rodentium, a murine model pathogen for enteropathogenic Escherichia coli, colonizes the surface of intestinal epithelial cells and causes mucosal inflammation. This bacterium is an ideal model for investigating pathogen-host immune interactions in the gut. It is well known that gene transcripts for Th1 cytokines are highly induced in colonic tissue from mice infected with C. rodentium. However, it remains to be seen whether the Th1 or Th2 cytokines produced by antigen-specific CD4+ T cells provide effective regulation of the host immune defense against C. rodentium infection. To investigate the antigen-specific immune responses, C. rodentium expressing ovalbumin (OVA-C. rodentium), a model antigen, was generated and used to define antigen-specific responses under gamma interferon (IFN-γ)-deficient or interleukin-4 (IL-4)-deficient conditions in vivo. The activation of antigen-specific CD4+ T cells and macrophage phagocytosis were evaluated in the presence of IFN-γ or IL-4 in vitro. IFN-γ-deficient mice exhibited a loss of body weight and a higher bacterial concentration in feces during OVA-C. rodentium infection than C57BL/6 (wild type) or IL-4-deficient mice. This occurred through the decreased efficiency of macrophage phagocytosis and the activation of antigen-specific CD4+ T cells. Furthermore, a deficiency in antigen-specific CD4+ T-cell-expressed IFN-γ led to a higher susceptibility to mucosal and gut-derived systemic OVA-C. rodentium infection. These results show that the IFN-γ produced by antigen-specific CD4+ T cells plays an important role in the defense against C. rodentium.
Enteropathogenic Escherichia coli (EPEC) is associated with significant morbidity and mortality in human populations worldwide. EPEC is a leading cause of bacterially induced diarrhea in developing countries and is responsible for approximately 1 million infant deaths per year (4, 8). Enteric bacteria, such as EPEC, evade many systemic host defense mechanisms by restricting their colonization to the luminal surface of the gastrointestinal epithelium (20). The strategies used by these pathogens include intimate adherence to the cecal and colonic epithelium and disruption of cellular structures through formation of attaching and effacing (A/E) lesions (20).
Citrobacter rodentium, a murine model pathogen for EPEC infection, similarly colonizes the surface of intestinal epithelial cells by utilization of A/E lesions in association with mucosal inflammation. Colonization of C. rodentium peaks 7 days after infection and is usually eradicated by day 28 after oral administration in immunocompetent mice. Bacterial colonization is limited to the intestinal mucosa, with a few bacteria reaching systemic sites. The infected mice exhibit a loss of body weight and diarrhea in association with crypt hyperplasia, a loss of goblet cells, and mucosal infiltration of the epithelium with lymphocytes, macrophages, neutrophils, and mast cells (10, 11).
The lymphocytic host response to C. rodentium is characterized by a large infiltration of CD4+ T cells into the colonic lamina propria, a modest increase in epithelial CD8+ T cells, and a highly polarized Th1 response (5, 6). Additionally, transcripts for Th1 cytokines such as gamma interferon (IFN-γ) and interleukin-12 (IL-12) are highly induced in colonic tissue of the infected mice, but those for the Th2 cytokine interleukin-4 (IL-4) are not (6). Recent reports have shown that Th17 cells are also involved in the host defense against C. rodentium infection because antigen-presenting cells (APCs) from C. rodentium-infected mice, when stimulated with microbial products such as lipopolysaccharide, peptidoglycans, and zymosan, produce significant amounts of interleukin-23 (IL-23) (9, 23, 24). Furthermore, IL-17 receptor A-deficient (IL-17RA−/−) mice and IL-23A−/− mice are more susceptible to C. rodentium infection (7, 12, 29). These studies indicate that Th17 cytokines and possibly Th1 cytokines play an important role in eradicating C. rodentium infection.
CD4+ T-cell- and B-cell-deficient mice also fail to eradicate C. rodentium infection. Among the factors produced by these cells, antibacterial IgG is particularly important. In contrast, mice lacking CD8+ T cells, IgA, secreted IgM, and proteins required for transport of IgA and IgM into the lumen (polymeric Ig receptor and J chain) clear C. rodentium normally (2, 11, 19, 27). Thus, it would appear that CD4+ T cells, B cells, and IgG, but not secretory IgA or IgM, play important roles in eradicating this pathogen. Consistent with this, neonatal Fc receptor (FcRn)-deficient mice were highly susceptible to C. rodentium infection in a pathway that depends upon antibacterial IgG (27).
The specific role of Th1 or Th2 cytokines produced by antigen-specific CD4+ T cells has not yet been examined in the regulation of C. rodentium infection. We therefore used C. rodentium expressing a model antigen, ovalbumin (OVA-C. rodentium), to investigate antigen-specific responses to C. rodentium in vivo in IFN-γ- or IL-4-deficient mice. We observed that IFN-γ effectively promoted the activation of antigen-specific CD4+ T cells and macrophage phagocytosis. Furthermore, the T-cell transfer models supported the functional role for IFN-γ but not IL-4 from the antigen-specific CD4+ T cells, the regulation of protective antibodies, the control of the pathogenic burden, and the resolution of infection. Our studies thus show that the IFN-γ produced by antigen-specific CD4+ T cells regulates the mucosal immune response to C. rodentium infection and, consequently, eradication of the mucosal pathogen.
MATERIALS AND METHODS
Animals.
Female or male 3- to 4-week-old C57BL/6 mice were purchased from CLEA Japan (Osaka, Japan). OT-II (1), IL-4−/− (25), and Rag1-deficient (Rag1−/−) mice (14) (C57BL/6 background) were obtained from The Jackson Laboratory (Bar Harbor, ME), and IFN-γ−/− mice (21) (C57BL/6 background) were kindly provided by S. Iwakura (Tokyo University, Japan). OT-II mice were crossed with IL-4−/− and IFN-γ−/− mice, and those with homozygous mutations in their IL-4 and IFN-γ genes (IL-4−/−/OT-II and IFN-γ−/−/OT-II, respectively) were used in this study. C57BL/6/OT-II, IL-4−/−/OT-II, and IFN-γ−/−/OT-II mice were used as donors in T-cell transfer studies. All mice were housed and bred in the Animal Unit of the Kobe University School of Medicine in a specific-pathogen-free facility under an approved experimental protocol.
Antibodies.
CD4-PE-Cy5.5 (clone RM4-5; PE is phycoerythrin) antibody was purchased from eBioscience (San Diego, CA). T-cell receptor (TCR) Vβ5.1-PE (clone MR9-4), TCR Vβ5.1-biotin (clone MR9-4), CD69-PE (clone H1.2F3), TCR Vα2-FITC (clone B20.1; FITC is fluorescein isothiocyanate), and streptavidin-PerCP-Cy5.5 CD11b-PE (clone M1/70; PerCP is peridinin chlorophyll protein) antibodies were obtained from BD Bioscience (San Jose, CA). Rabbit anti-OVA polyclonal antibody was purchased from Rockland (Gilbertsville, PA).
Establishment of C. rodentium expressing OVA and GFP.
C. rodentium strain DBS100 (catalog number 51459; ATCC) was kindly provided by Gad Frankel (Division of Cell and Molecular Biology, Imperial College London, United Kingdom). Constitutively OVA-expressing C. rodentium (OVA-C. rodentium) was created by electroporation of a plasmid vector encoding the chicken OVA construct (28) under the control of the two gal operon promoters and a kanamycin resistance gene into C. rodentium strain DBS100 (13). Green fluorescent protein (GFP)-expressing C. rodentium (GFP-C. rodentium) was kindly provided by C. Sasakawa (Tokyo University, Japan).
T-cell proliferation assay.
CD4+ T cells were positively selected by magnetic bead sorting with magnetic cell sorting (MACS) immunobeads (Miltenyi Biotec Inc., Bergisch Gladbach, Germany). The purity of the CD4+ T cells was usually over 95%, according to examination with a FACSCalibur flow cytometer (BectonDickinson, Franklin Lakes, NJ). For the T-cell proliferation assays, splenic CD4+ T cells (1 × 106 cells/ml) purified from OT-II mice were labeled with carboxyfluorescein succinimidyl ester (CFSE) (CellTrace CESE Cell Proliferation Kit; Molecular Probes, Eugene, OR). After APCs were incubated with bacterial sonicates from OVA-C. rodentium or wild-type (WT) C. rodentium, the APCs were incubated with CFSE-labeled CD4+ T cells for 24 h. The CFSE-labeled CD4+ T cells were stained with anti-CD4 and anti-TCR Vβ5.1 antibodies, and the CFSE intensity of the CD4+ T cells gated on TCR Vβ5.1-positive cells was measured by flow cytometry. Splenocytes (1 × 107 cells/ml) from C57BL/6 mice were used as APCs after irradiation with 70 Gy.
Protocol for the induction of colitis by OVA-C. rodentium infection.
OVA-C. rodentium was cultured in LB broth medium containing 20 μg/ml kanamycin (Wako, Osaka, Japan) for 6 h at 37°C with shaking. After 6 h, the bacterial density was assessed using absorbance at an optical density of 600 nm and confirmed by plating of serial dilutions. Four-week-old mice were orally inoculated with 1 × 109 CFU of OVA-C. rodentium using a gavage needle. To ensure maintenance of the plasmid during in vivo infection, 0.1 mg/ml kanamycin was added to the drinking water. Body weight, bacterial concentration in feces, and histological findings of the colon were assessed for 4 weeks after inoculation. The anti-OVA IgG levels in sera and feces were examined by an enzyme-linked immunosorbent assay (ELISA), as described below. Colonic tissues were stained with hematoxylin and eosin and evaluated as described in “Histological analysis of colon tissue” below. For the WT C. rodentium infection experiment, the mice were not given kanamycin in the drinking water.
Bacterial counts.
Fresh fecal pellets were collected from mice 7, 14, 21, and 28 days after infection, weighed, and dissolved in phosphate-buffered saline (PBS) at 100 mg/ml. The mixture was vortexed, and serial dilutions were plated on MacConkey agar plates containing 20 μg/ml kanamycin. Bacterial colonies were counted 24 h after culture start. OVA-C. rodentium cells harboring the kanamycin resistance gene were easily distinguished from commensal flora. The presence of C. rodentium in the feces of mice infected with OVA-C. rodentium was confirmed by PCR using Tir-specific primers (forward primer, GCGCGAATTCATGCCTATTGGTAATCTTGGTAATAATAAT; reverse primer, GCGCCCCGGGTTAGACGAAACGTTCAACTCCCGGTGTTGT) (26) after DNA was extracted from bacterial colonies which were selected at random.
Evaluation for the frequency of plasmid loss during infection.
C57BL/6 mice exposed to kanamycin in drinking water were orally infected with 1 × 109 CFU of OVA-C. rodentium. Fecal pellets treated as described above were plated on MacConkey agar plates with or without kanamycin, and bacterial colonies were counted. Approximately 150 colonies were selected at random, and the presence of C. rodentium in colonies was confirmed by PCR using Tir-specific primers. In PCR-positive colonies, the expression of OVA was confirmed by immunoblot analysis, and the positive rate of plasmid in feces during OVA-C. rodentium infection was evaluated.
Histological analysis of colon tissue.
For histological analysis, the terminal 0.5 cm of colon tissue was removed and soaked in 10% formalin in PBS. Next, paraffin-embedded sections were prepared and stained with hematoxylin and eosin. Histological analysis was performed according to a previous report (16). For histopathological grading of colitis, five criteria, i.e., hypervascularization, presence of mononuclear cells, epithelial hyperplasia, epithelial injury, and presence of granulocytes, were scored from 0 to 3, yielding an additive score between 0 (no colitis) and 15 (maximal colitis activity).
Determination of anti-OVA antibody in serum or fecal samples.
Whole blood and fecal pellets were collected from mice infected with OVA-C. rodentium. The serum was separated by centrifugation at 16,000 × g for 10 min and stored at −20°C until used in the following experiments. Fecal pellets were weighed and then added to sterile PBS containing 0.1% sodium azide at 100 mg/ml. The mixture was homogenized by continuous shaking for 10 min on a vortex mixer and centrifuged at 15,000 × g for 5 min. The supernatants obtained were collected and stored at −80°C. For analysis of the antigen-specific antibody response, 96-well plates were coated overnight at 4°C with 100 μl of a bicarbonate solution (pH 9.6) containing 100 μg/ml OVA. After being washed with PBS containing 0.05% Tween 20 (PBST), the plates were blocked by the addition of 1.5% (wt/vol) bovine serum albumin (BSA) in PBS for 1 h at 37°C. The plates were then washed twice with PBST before the sera or fecal lysates from individual mice were added and serially diluted in PBST containing 0.2% (wt/vol) BSA, and plates were then incubated for 2 h at 37°C. For the determination of specific IgG titers, the plates were washed with PBST, followed by addition of 100 μl of an anti-mouse IgG-specific horseradish peroxidase (HRP) conjugate (DakoCytomation; Denmark A/S) diluted 1:1,000 in PBST containing 0.2% (wt/vol) BSA. After incubation for 2 h at 37°C, the plates were washed with PBST, and then the bound antibody was detected by addition of o-phenylenediamine substrate and measurement of absorbance at 490 nm.
Antigenic challenge with OVA-C. rodentium and evaluation of early activation and cytokine production in OVA-specific OT-II CD4+ T cells.
OT-II mice (4 weeks old) were orally inoculated with 1 × 109 CFU of OVA-C. rodentium. Mesenteric lymph node (MLN) cells were collected from OT-II mice at days 0, 7, and 14 after OVA-C. rodentium infection. MLN cells were stained with anti-CD69, anti-TCR Vα2, and anti-TCR Vβ5.1 antibodies, and the activation of CD4+ T cells was determined after gating on TCR Vα2-positive and TCR Vβ5.1-positive cells as defined by flow cytometry. To evaluate cytokine production, OVA-specific CD4+ T cells were purified from MLN of the infected OT-II mice at days 0, 7, and 14 using MACS immunobeads. These cells were then restimulated with irradiated splenic APCs treated with 1 μM OVA peptide for 24 h in vitro. The production of IFN-γ and IL-4 from the OVA-specific CD4+ T cells was evaluated by intracellular cytokine staining by standard methods as defined by the manufacturer (Intracellular Cytokine Staining Starter Kit-Mouse; BD Biosciences, San Jose, CA). C57BL/6 mice (4 weeks old) that received 5 × 106 of CD4+ T cells from OT-II mice were orally inoculated with 1 × 109 CFU of OVA-C. rodentium, and then the expression of CD69 and the production of IFN-γ and IL-4 on OVA-specific CD4+ T cells were determined as described above.
Adoptive transfer and induction of OVA-C. rodentium infection.
CD4+ T cells were purified from the spleens of C57BL/6/OT-II, IFN-γ−/−/OT-II, and IL-4−/−/OT-II mice using MACS immunobeads as described above. Similarly, B cells were also purified from the spleens of C57BL/6 mice. Rag1−/− mice (8 to 10 weeks old) received 1 × 107 wild-type (C57BL/6) B cells and 5 × 106 CD4+ T cells from C57BL/6/OT-II, IFN-γ−/−/OT-II, or IL-4−/−/OT-II mice by intravenous injection. All recipients were orally inoculated with 1 ×109 CFU of OVA-C. rodentium at 5 days after the adoptive transfer. Body weight, bacterial concentration in feces, anti-OVA antibody level, and histological findings of the colon were examined for 4 weeks after the inoculation with OVA-C. rodentium as described above. Similarly, IFN-γ−/− mice that received C57BL/6/OT-II or IFN-γ−/−/OT-II cells were infected with 1 × 109 CFU of OVA-C. rodentium, and body weight, bacterial concentration in feces, serum anti-OVA IgG levels, and histological findings of colon tissue were evaluated. All the mice were given kanamycin in drinking water during infection.
Measurement of antigen-induced antigen-specific T-cell proliferation in the presence of IFN-γ or IL-4.
OVA-specific CD4+ T cells were purified from the spleens of OT-II mice using MACS immunobeads and were then labeled with CFSE. After APCs were incubated with the bacterial sonicates from OVA-C. rodentium for 16 h with or without 100 U/ml recombinant murine IFN-γ (rIFN-γ) (Peprotech, Rocky Hill, NJ) or 30 ng/ml recombinant murine IL-4 (rIL-4) (Peprotech), OVA-specific CD4+ T cells were incubated with the APCs for 24 h and stained with anti-CD4 and anti-TCR Vβ5.1 antibodies. T-cell proliferation was determined by measuring the CFSE intensity of CD4+ T cells after they were gated on TCR Vβ5.1-positive cells by flow cytometry.
Evaluation of proliferation in IFN-γ- or IL-4-deficient antigen-specific CD4+ T cells.
OVA-specific CD4+ T cells were purified from the spleens of IFN-γ−/−/OT-II, C57BL/6/OT-II, and IL-4−/−/OT-II mice using MACS immunobeads and were then labeled with CFSE. APCs cultured with the bacterial sonicates from OVA-C. rodentium were incubated with the respective CFSE-labeled OVA-specific CD4+ T cells for 24 h and then stained with anti-CD4 and anti-TCR Vβ5.1 antibodies. Proliferation of T cells was determined by measuring the CFSE intensity of CD4+ T cells after they were gated on TCR Vβ5.1-positive cells by flow cytometry.
Preparation of peritoneal macrophages and evaluation of phagocytic activity.
Macrophages were prepared as described previously (13). Briefly, C57BL/6 mice were injected with 1 ml of 3% thioglycolate into the peritoneal cavity. The peritoneal cells were harvested by peritoneal lavage with 10 ml of cold sterile PBS twice. Peritoneal macrophages were treated with 100 U/ml rIFN-γ or 30 ng/ml rIL-4 for 16 h and incubated with GFP-C. rodentium for 60 min. The cells were then stained with TOPRO-3 and Alexa Fluor 546-conjugated phalloidin and examined by confocal microscopy (LSM5Pascal; Carl Zeiss, Germany). The cells were also stained with anti-CD11b-PE antibody, and phagocytic activity was determined as green fluorescence intensity per CD11b-positive cell by flow cytometry.
Statistics.
Statistical significance was determined by a two-tailed Student's t test. P values of less than 0.05 were considered significant.
RESULTS
Immunogenicity of C. rodentium constitutively expressing OVA.
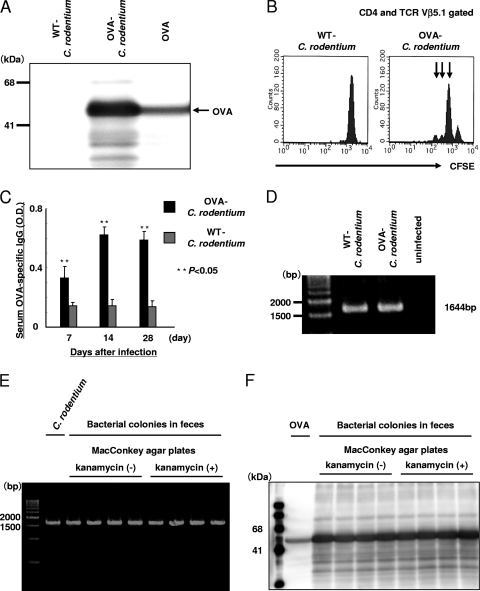
To assess the host antigen-specific T-cell responses during C. rodentium infection, C. rodentium constitutively expressing OVA was generated as previously described (13, 27). As shown in Fig. 1A, OVA protein expression could be detected in OVA-C. rodentium but not in WT C. rodentium. The bacterial sonicates from OVA-C. rodentium when presented by APCs were also able to stimulate OVA-specific CD4+ T cells from OT-II mice in vitro, as defined by division of the CFSE signal consistent with proliferation (Fig. 1B). These results indicate that the OVA expressed by C. rodentium is recognized by OVA-specific CD4+ T cells.
FIG. 1.
Immunogenicity of C. rodentium constitutively expressing OVA. (A) Establishment of a genetically engineered C. rodentium strain that constitutively produces OVA. The expression of OVA in C. rodentium was confirmed by immunoblot analysis. As a positive control, OVA proteins were used. The band was not detected by immunoblotting with secondary antibody only (data not shown). (B) APCs were cultured with bacterial sonicates from OVA-C. rodentium or WT C. rodentium, and then the CFSE-labeled OVA-specific CD4+ T cells purified from OT-II mice were incubated with APCs for 24 h. The number of OVA-specific CD4+ T cells was examined by flow cytometry. The arrow indicates increasing rounds of cell division. (C) The serum OVA-specific IgG level in C57BL/6 mice at 7, 14, and 28 days after infection with OVA-C. rodentium was evaluated by ELISA. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference from the values for the mice infected with WT C. rodentium (P < 0.05, Student's t test). OD, optical density. The OVA-specific IgG in serum from the uninfected mice was not detected by ELISA (data not shown). (D) DNA was extracted from colonies isolated from the feces of mice infected with OVA-C. rodentium or WT C. rodentium, and PCR was performed with Tir-specific primers. (E) Fecal pellets from mice infected with OVA-C. rodentium were plated on MacConkey agar plates with or without kanamycin, and then the presence of C. rodentium in bacterial colonies was confirmed by PCR using Tir-specific primers. (F) The expression of OVA in PCR-positive colonies in plates with kanamycin or without kanamycin was confirmed by immunoblot analysis.
Given these results, the antigenicity of OVA-C. rodentium in vivo was also investigated by measuring the serum anti-OVA IgG levels in C57BL/6 mice after infection with OVA-C. rodentium or WT C. rodentium. As shown in Fig. 1C, the serum anti-OVA IgG levels were significantly elevated in mice infected with OVA-C. rodentium at 7, 14, and 28 days after infection. The presence of C. rodentium in the feces of mice infected with OVA-C. rodentium at 7, 14, and 28 days after infection was confirmed by PCR of bacterial colonies using Tir-specific primers (26) (Fig. 1D and E). The protein expression of OVA in these bacterial colonies was also confirmed by immunoblot analysis (Fig. 1F). Thus, OVA-C. rodentium possesses OVA-specific immunogenicity, which makes it an appropriate model to assess C. rodentium-specific immune responses in vivo. Furthermore, to determine the frequency of plasmid loss during infection, C57BL/6 mice were orally infected with 1 × 109 CFU of OVA-C. rodentium in the presence of kanamycin supplied in the drinking water. Fecal pellets were plated on MacConkey agar plates with or without kanamycin, and then the number of bacterial colonies, Tir prevalence, and OVA prevalence were examined. Results showed a comparable rate of bacterial colonies (data not shown), and the prevalences of Tir and OVA were observed (Table 1 and Fig. 1E and F). When C57BL/6 mice exposed to kanamycin in the drinking water were orally infected with 1 × 109 CFU of WT C. rodentium, there were few bacterial colonies in fecal pellets of the mice for 4 weeks after inoculation, and the colonic tissue was in a healthy state (data not shown). These results suggest that the frequency of plasmid loss during infection is low and that the colonic damage is not caused by C. rodentium lacking plasmid even though there is some plasmid loss with OVA-C. rodentium infection.
TABLE 1.
The prevalence of Tir and OVA in bacterial colonies in feces
| Gene product | % Positive colonies on MacConkey agar plates at:a |
|||
|---|---|---|---|---|
| Day 7 |
Day 14 |
|||
| + Kanamycin | − Kanamycin | + Kanamycin | − Kanamycin | |
| Tirb | 100 | 98.0 | 100 | 97.3 |
| OVAc | 98.6 | 96.6 | 99.3 | 97.2 |
All mice were given kanamycin in drinking water during OVA-C. rodentium infection.
Percentages were calculated as [(the number of Tir-positive colonies)/(the total number of colonies)] × 100.
Percentages were calculated as [(the number of OVA-positive colonies)/(the number of Tir-positive colonies)] × 100.
OVA-C. rodentium can induce colitis similar to WT C. rodentium.
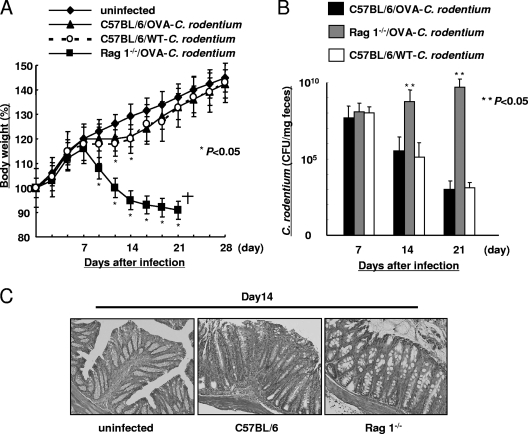
It was reported that T cells and B cells play important roles in eradicating C. rodentium (19, 22). To evaluate the roles of T cells and B cells in OVA-C. rodentium infection, C57BL/6 mice and Rag1−/− mice were orally infected with 1 × 109 CFU of OVA-C. rodentium. Rag1−/− mice exhibited a loss of body weight (Fig. 2A) and higher bacterial concentrations than C57BL/6 mice in their feces at 14 and 21 days after infection (Fig. 2B). Moreover, C. rodentium infection was lethal in Rag1−/− mice by 21 days after OVA-C. rodentium infection. This was associated with decreased histological injury in the Rag1−/− mice in comparison to the C57BL/6 mice as assessed at day 14 of infection (Fig. 2C). Together, these studies in Rag1−/− mice show the importance of adaptive immunity in clearing C. rodentium infection.
FIG. 2.
OVA-C. rodentium can induce colitis. Susceptibility to infection with 1 × 109 CFU of OVA-C. rodentium in Rag1−/− mice. Rag1−/− mice and C57BL/6 mice were infected with OVA-C. rodentium or WT C. rodentium at 1 × 109 CFU. In the case of WT C. rodentium infection, the mice were not given kanamycin in the drinking water. (A) Body weight changes in Rag−/− mice and C57BL/6 mice. Data are shown as means ± standard deviations (n = 6), and asterisks indicate a significant difference from the values for the uninfected mice (P < 0.05, Student's t test). (B) The bacterial loads (CFU/mg) of OVA-C. rodentium or WT C. rodentium in feces at 7, 14, and 21 days after infection. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference from the values for the C57BL/6 mice infected with WT C. rodentium (P < 0.05, Student's t test). No colonies were detected in feces of the respective uninfected mice (data not shown). (C) Histological findings of the colons uninfected mice and of Rag1−/− mice and C57BL/6 mice at 14 days after OVA-C. rodentium infection. Magnification, ×100.
When C57BL/6 mice were examined for responses to WT C. rodentium and OVA-C. rodentium, comparable degrees of body weight loss (Fig. 2A), burden of bacteria (Fig. 2B), and pathology (Fig. 2C and data not shown) were observed. These results were consistent with a previous report by Vallance et al. (22) and indicate that OVA-C. rodentium has the same pathogenicity as WT C. rodentium.
Susceptibility to C. rodentium is enhanced in the absence of IFN-γ.
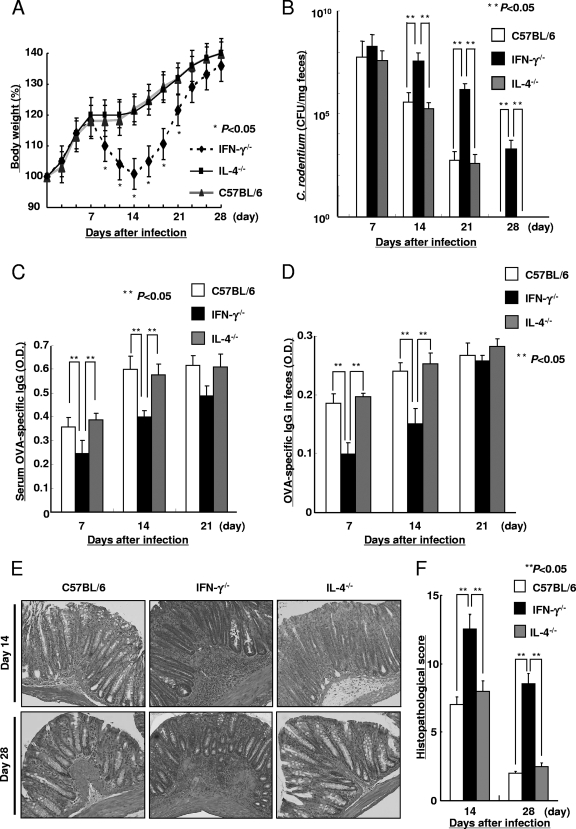
To determine whether Th1 and/or Th2 cytokines are important for protection against C. rodentium infection, the IFN-γ−/−, IL-4−/−, and C57BL/6 mice were orally infected with OVA-C. rodentium. IFN-γ−/− mice exhibited a loss of body weight (Fig. 3A) and a higher bacterial concentration in their feces at 14, 21, and 28 days after infection than IL-4−/− and C57BL/6 mice (Fig. 3B). Although the response was delayed, the IFN-γ−/− mice were able to successfully clear OVA-C. rodentium from their feces by 35 days after infection (data not shown). The IFN-γ−/− mice also exhibited a significant delay in the development of antigen-specific IgG levels at days 7 and 14 after infection in both serum and feces in comparison to the IL-4−/− and C57BL/6 mice (Fig. 3C and D). Macroscopic and microscopic injury was also more severe in the IFN-γ−/− mice than that observed in the IL-4−/− and C57BL/6 mice at days 14 and 28. The submucosa and lamina propria of the infected IFN-γ−/− mice exhibited a greater number of mononuclear cells and neutrophils in the tissue and increased crypt hyperplasia (Fig. 3E). This was supported by a quantitative evaluation of the histological findings, as shown in Fig. 3F. Consistent with this, the colons of the IFN-γ−/− mice were shorter and more edematous than those of the IL-4−/− and C57BL/6 mice (data not shown). In contrast, there was no inflammatory response observed in the IL-4−/− or C57BL/6 mice on day 28 after infection. These results indicate that IFN-γ−/− mice are more susceptible to C. rodentium infection than either IL-4−/− or C57BL/6 mice.
FIG. 3.
Susceptibility to OVA-C. rodentium in the absence of IFN-γ. Susceptibility to infection with 1 × 109 CFU of OVA-C. rodentium in IFN-γ−/− mice, IL-4−/− mice, and C57BL/6 mice. (A) Body weight changes in IFN-γ−/− mice, IL-4−/− mice, and C57BL/6 mice during OVA-C. rodentium infection. Data are shown as means ± standard deviations (n = 8), and asterisks indicate a significant difference from the values for the C57BL/6 mice (P < 0.05, Student's t test). (B) The bacterial loads (CFU/mg) of OVA-C. rodentium in feces at 7, 14, 21, and 28 days after infection. Data are shown as means ± standard deviations (n = 8), and two asterisks indicate a significant difference. No colonies were detected in feces of the respective uninfected mice (data not shown). (C and D) The OVA-specific IgG levels in serum and feces at 7, 14, and 21 days after infection were measured by ELISA. Data are shown as means ± standard deviations (n = 8), and two asterisks indicate a significant difference. The OVA-specific IgG in serum and feces from the respective uninfected mice were not detected by ELISA (data not shown). (E and F) Histological findings (E) and the histological score (F) of the colonic tissue from IFN-γ−/−, IL-4−/−, and C57BL/6 mice were evaluated at 14 and 28 days after infection. Magnification, ×100. Data are shown as means ± standard deviations (n = 8), and two asterisks indicate a significant difference. The histological score in the colonic tissue from the uninfected mice was 0, i.e., no colitis (data not shown).
Th1 or Th2 cytokine production from antigen-specific CD4+ T cells during C. rodentium infection.
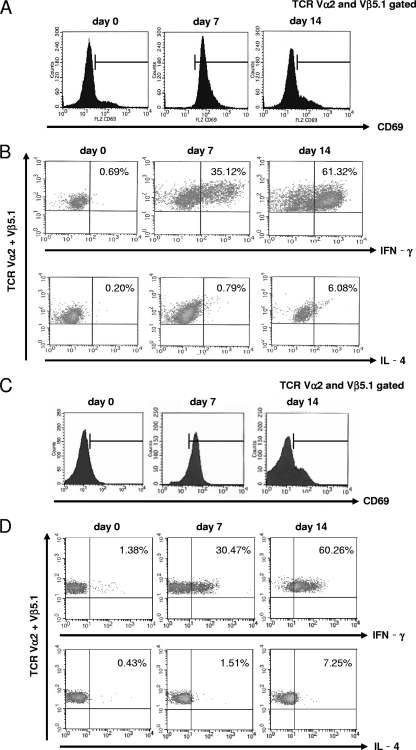
To assess the cytokine profile from antigen-specific CD4+ T cells during native C. rodentium infection, OT-II mice were orally infected with OVA-C. rodentium. First, to determine the early activation of CD4+ T cells, the expression of CD69 on OVA-specific OT-II CD4+ T cells purified from MLN during OVA-C. rodentium infection was investigated. At day 7 after infection, the expression levels of CD69 were elevated but were observed to decrease at day 14 after infection (Fig. 4A). Next, OVA-specific OT-II CD4+ T cells were purified from MLN at days 7 and 14 after OVA-C. rodentium infection. These cells were restimulated with APCs that were treated with OVA peptide for 24 h in vitro before the measurement of IFN-γ and IL-4 by an intracellular cytokine staining method. At days 7 and 14, the IFN-γ levels produced by OVA-specific OT-II CD4+ T cells were observed to be progressively elevated (Fig. 4B). In contrast, there was no evidence of IL-4 production of OVA-specific OT-II CD4+ T cells during OVA-C. rodentium infection (Fig. 4B). However, the exaggerated responses might be induced in OT-II mice infected with OVA-C. rodentium for the reason that the precursor frequency of OVA-specific CD4+ T cells in these T-cell receptor transgenic mice is too high. Therefore, OVA-specific OT-II CD4+ T cells were transferred into C57BL/6 mice, and then these C57BL/6 recipients were infected with OVA-C. rodentium, followed by measurement of the expression levels of CD69, IFN-γ, and IL-4 on OVA-specific OT-II CD4+ T cells. The expression levels of CD69, IFN-γ, and IL-4 were consistent with results of OT-II mice directly infected with OVA-C. rodentium (Fig. 4C and D). These results indicate that IFN-γ is a dominant cytokine produced by antigen-specific CD4+ T cells in response to C. rodentium infection.
FIG. 4.
Cytokine production in antigen-specific CD4+ T cells during C. rodentium infection. OT-II mice were orally inoculated with 1 × 109 CFU of OVA-C. rodentium. (A) Evaluation of CD69 expression on OVA-specific CD4+ T cells after in vivo stimulation with OVA-C. rodentium. (B) After in vivo stimulation with OVA-C. rodentium, the OVA-specific CD4+ T cells were treated with OVA peptide for 24 h in vitro. The production of IFN-γ and IL-4 was examined by an intracellular cytokine staining method using flow cytometry. (C and D) C57BL/6 mice that received 5 × 106 CD4+ T cells from OT-II mice were orally infected with 1 × 109 CFU of OVA-C. rodentium, and the expression of CD69 (C) and the production of IFN-γ and IL-4 (D) on OVA-specific CD4+ T cells were evaluated. Typical images are shown from at least triplicate determinations.
IFN-γ produced by antigen-specific CD4+ T cells plays an important role in the prevention of C. rodentium infection.
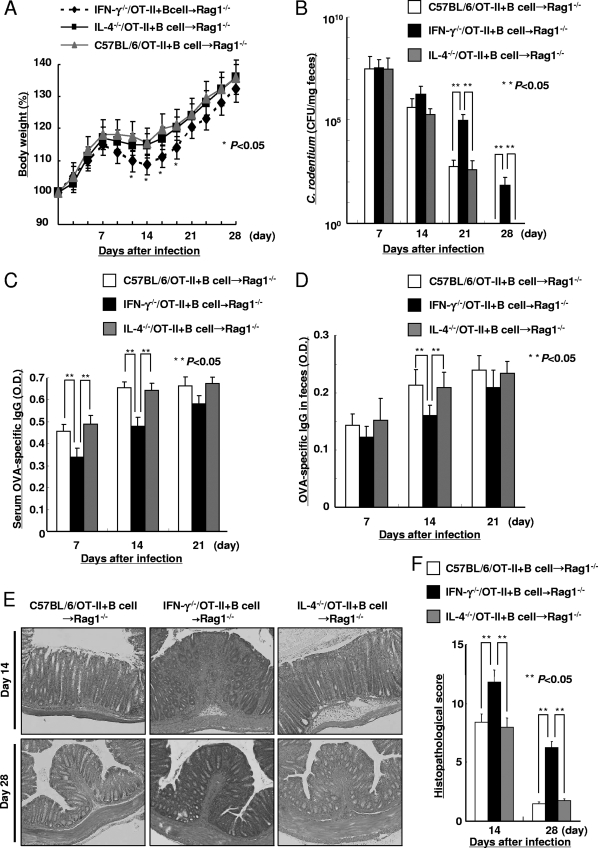
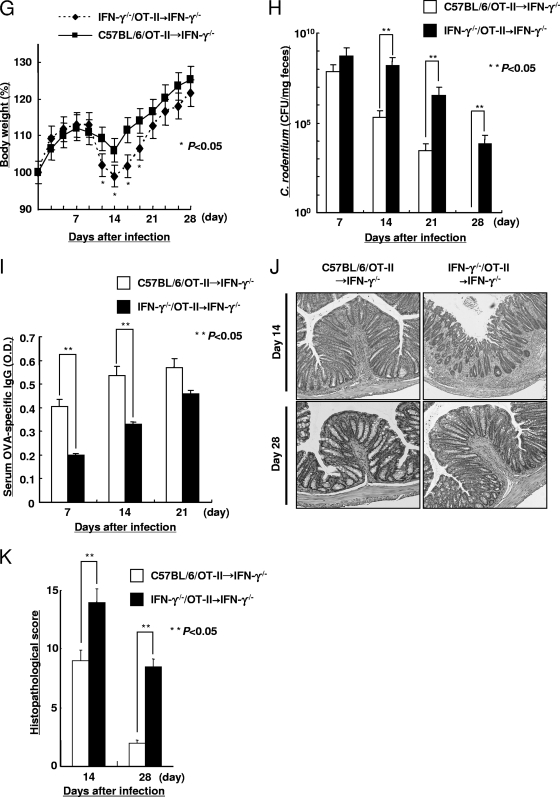
CD4+ T cells and B cells play important roles in clearance of C. rodentium because CD4+ T-cell- and B-cell-deficient mice failed to eradicate C. rodentium infection (2, 11, 19). To determine the contribution of antigen-specific CD4+ T cell-expressed IFN-γ and IL-4, IFN-γ−/−/OT-II, IL-4−/−/OT-II, and C57BL/6/OT-II cells were adoptively transferred into Rag1−/− recipients that received wild-type B cells. Subsequently, these Rag1−/− recipients were infected with OVA-C. rodentium. The mice that received the IFN-γ−/−/OT-II cells, despite the presence of B cells, exhibited a significant loss of body weight (Fig. 5A) and greater concentration of C. rodentium in their feces at 21 and 28 days after infection (Fig. 5B) than Rag1−/− mice that received either IL-4−/−/OT-II or C57BL/6/OT-II cells. Importantly, Rag1−/− mice that received IL-4−/−/OT-II or C57BL/6/OT-II cells exhibited similar body weight loss and bacterial burdens. Consistent with these observations, the Rag1−/− mice that received IFN-γ−/−/OT-II cells exhibited a decease in OVA-specific IgG levels on days 7 and 14 in serum and on day 14 in feces in comparison to the Rag1−/− mice that received C57BL/6/OT-II or IL-4−/−/OT-II cells (Fig. 5C and D). Consistent with the increased pathogen burden, the mice that received IFN-γ−/−/OT-II cells demonstrated more severe histological injury than the mice that received C57BL/6/OT-II or IL-4−/−/OT-II cells on days 14 and 28 after infection (Fig. 5E and F). By 35 days after infection, the Rag1−/− mice that received IFN-γ−/−/OT-II cells were able to clear infection. However, in these models, IFN-γ produced by cells other than CD4+ T cells, for example, NK cells, neutrophils, macrophages, and dendritic cells, might also promote key functions against C. rodentium infection. To remove the possible effects of IFN-γ produced from cells other than CD4+ T cells, IFN-γ−/− mice that received IFN-γ−/−/OT-II cells or C57BL/6/OT-II cells were infected with OVA-C. rodentium, and the body weight, bacterial concentration in feces, serum anti-OVA IgG levels, and histological findings of colon tissue were assessed for 4 weeks after inoculation. The IFN-γ−/− mice that received C57BL/6/OT-II cells exhibited a significant increase in the body weight (Fig. 5G) and a lower concentration of C. rodentium in feces at 14, 21, and 28 days after infection (Fig. 5H) than the IFN-γ−/− mice that received IFN-γ−/−/OT-II cells. Consistent with these observations, the IFN-γ−/− mice that received C57BL/6/OT-II cells revealed an increase in serum OVA-specific IgG levels on days 7 and 14 in comparison to the IFN-γ−/− mice that received IFN-γ−/−/OT-II cells (Fig. 5I). In addition, the IFN-γ−/− mice that received C57BL/6/OT-II cells exhibited milder histological injury than the IFN-γ−/− mice that received IFN-γ−/−/OT-II cells (Fig. 5J and K). These results indicate that the IFN-γ produced by antigen-specific CD4+ T cells plays an important role in the susceptibility to C. rodentium at a critical period after infection.
FIG. 5.
Cytokines produced from antigen-specific CD4+ T cells regulate C. rodentium infection. Susceptibility to infection with 1 × 109 CFU of OVA-C. rodentium in Rag1−/− mice receiving IFN-γ−/−/OT-II, IL-4−/−/OT-II, and C57BL/6/OT-II cells. IFN-γ−/−/OT-II, IL-4−/−/OT-II, and C57BL/6/OT-II cells were transferred into Rag1−/− mice, which had previously been injected with C57BL/6 B cells, and then these mice were infected with 1 × 109 CFU of OVA-C. rodentium. (A) The body weights of the mice were measured. Data are shown as means ± standard deviations (n = 6), and asterisks indicate a significant difference from the values for the Rag1−/− mice receiving C57BL/6/OT-II cells (P < 0.05, Student's t test). (B) The bacterial loads (CFU/mg) of OVA-C. rodentium in feces at 7, 14, 21, and 28 days after infection. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference. No colonies were detected in feces of the respective uninfected mice (data not shown). (C and D) The OVA-specific IgG levels in serum (C) and feces (D) at 7, 14, and 21 days after infection were evaluated by ELISA. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference. The OVA-specific IgG in serum and feces from the respective uninfected mice were not detected by ELISA (data not shown). (E and F) Histological findings (E) and the histological score (F) of colonic tissue from the respective Rag1−/− recipients were evaluated at 14 and 28 days after infection. Magnification, ×100. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference. The histological score in the colonic tissue from the uninfected mice was 0, i.e., no colitis (data not shown). Susceptibility to infection with 1 × 109 CFU of OVA-C. rodentium in IFN-γ−/− mice receiving IFN-γ−/−/OT-II or C57BL/6/OT-II cells. (G) The body weights of the mice were measured. Data are shown as means ± standard deviations (n = 6), and asterisks indicate a significant difference from the values for the IFN-γ−/− mice receiving C57BL/6/OT-II cells (P < 0.05, Student's t test). (H) The bacterial loads (CFU/mg) of OVA-C. rodentium in feces at 7, 14, 21, and 28 days after infection. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference. (I) The OVA-specific IgG levels in serum at 7, 14, and 21 days after infection were evaluated by ELISA. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference. (J and K) Histological findings (J) and the histological score (K) of colonic tissue from the respective IFN-γ−/− recipients were evaluated at 14 and 28 days after infection. Magnification, ×100. Data are shown as means ± standard deviations (n = 6), and two asterisks indicate a significant difference.
IFN-γ effectively induced antigen-specific T-cell activation.
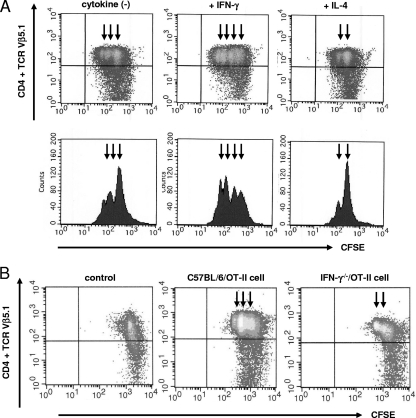
These studies show that susceptibility to C. rodentium is dependent upon IFN-γ. Therefore, antigen-specific T-cell responses during C. rodentium infection in the presence of IFN-γ were evaluated. APCs were cultured with bacterial sonicates from OVA-C. rodentium for 16 h with or without IFN-γ or IL-4. Subsequently, the antigen-loaded APCs were incubated with CFSE-labeled OVA-specific CD4+ T cells from OT-II mice for 24 h. T-cell responses were then examined by flow cytometry. The presence of IFN-γ led to proliferation of OVA-specific CD4+ T cells, as demonstrated by increased cell divisions compared to APCs that were loaded with OVA-C. rodentium without IFN-γ (Fig. 6A). In contrast, the presence of IL-4 decreased induction of OVA-specific CD4+ T-cell proliferation by OVA-C. rodentium-loaded APCs (Fig. 6A). APCs that were loaded with WT C. rodentium did not enhance the proliferation of OVA-specific CD4+ T cells (data not shown). Furthermore, to examine whether IFN-γ produced by antigen-specific CD4+ T cells is involved in infection-induced acquired immune responses, the proliferation of OVA-specific CD4+ T cells from C57BL/6/OT-II, IFN-γ−/−/OT-II, and IL-4−/−/OT-II mice was evaluated in vitro. APCs cultured with bacterial sonicates from OVA-C. rodentium were incubated with CFSE-labeled OVA-specific CD4+ T cells from IFN-γ−/−/OT-II, C57BL/6/OT-II, or IL-4−/−/OT-II mice for 24 h. The proliferation of OVA-specific CD4+ T cells was upregulated in C57BL/6/OT-II mice compared with that in IFN-γ−/−/OT-II mice, as shown by an enhancement of cell divisions (Fig. 6B). On the other hand, IL-4−/−/OT-II cells led to a degree of cell proliferation similar to that of C57BL/6/OT-II cells (data not shown). These results indicate that IFN-γ produced by antigen-specific CD4+ T cells can upregulate the ability of APCs to stimulate the proliferation of antigen-specific T cells and effectively induce acquired immune responses during C. rodentium infection.
FIG. 6.
IFN-γ effectively induces antigen-specific T-cell activation. (A) APCs were cultured with bacterial sonicates from OVA-C. rodentium for 16 h with or without IFN-γ or IL-4, and then CFSE-labeled OVA-specific OT-II CD4+ T cells from OT-II mice were incubated with the APCs for 24 h. The number of OVA-specific CD4+ T cells in the presence of IFN-γ or IL-4 was examined by flow cytometry. (B) APCs cultured with bacterial sonicates from OVA-C. rodentium were incubated with CFSE-labeled OVA-specific CD4+ T cells from IFN-γ−/−/OT-II, IL-4−/−/OT-II, or C57BL/6/OT-II mice for 24 h, and T-cell proliferation was evaluated by flow cytometry. IL-4−/−/OT-II cells and C57BL/6/OT-II cells had similar degrees of proliferation(data not shown). Arrows indicate increasing rounds of cell division. Typical images are shown from at least triplicate determinations.
IFN-γ enhances the macrophage phagocytic activity that is directed against C. rodentium infection.
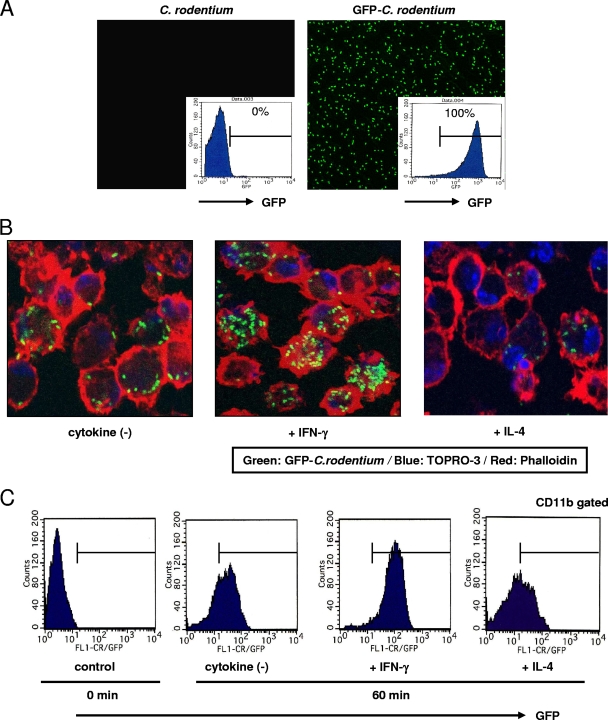
Finally, the phagocytic activity of macrophages in response to C. rodentium in the presence of IFN-γ or IL-4 was investigated. A genetically engineered C. rodentium strain that constitutively expresses GFP (GFP-C. rodentium) was used. Flow cytometry analysis and confocal microscopy confirmed the expression of GFP in GFP-C. rodentium but not in control C. rodentium (Fig. 7A). Peritoneal macrophages from C57BL/6 mice were collected after peritoneal injection of thioglycolate and cultured with GFP-C. rodentium for 60 min with or without IFN-γ or IL-4. The cells were stained with TOPRO-3 and phalloidin, and the number of GFP signals per macrophage was examined by confocal microscopy. IFN-γ increased the number of GFP-C. rodentium bacteria incorporated into macrophages compared to uptake by the vehicle control (Fig. 7B). On the other hand, a marked decrease in the GFP-C. rodentium uptake by macrophages after treatment with IL-4 was observed. In addition, similar results were obtained by measurement of the phagocytic activity as defined by flow cytometry, which detected the GFP signal in CD11b-positive cells (Fig. 7C). These results indicate that IFN-γ enhances the phagocytic activity of macrophages in response to C. rodentium.
FIG. 7.
Phagocytosis of C. rodentium by macrophages is regulated by IFN-γ and IL-4. (A) The expression of GFP in C. rodentium was confirmed by flow cytometry and confocal microscopy. (B) Peritoneal macrophages were incubated with GFP-C. rodentium for 60 min with IFN-γ or IL-4 and then were subjected to confocal microscopy with TOPRO-3 and phalloidin (green, GFP; blue, nuclei; red, actin). Magnification, ×630. (C) Peritoneal macrophages were incubated with GFP-C. rodentium for 60 min with IFN-γ or IL-4 and then were subjected to flow cytometry to evaluate the phagocytic activity of macrophages. Typical images are shown from at least triplicate determinations.
DISCUSSION
In this work, we demonstrated that IFN-γ produced by antigen-specific CD4+ T cells regulates the mucosal immune response to C. rodentium infection. From the experiments using immune cell-deficient mice, CD4+ T cells, B cells, and IgG, but not secretory IgA or IgM, were reported to play critical roles in the eradication of C. rodentium (15). This pathogen is therefore appropriate for evaluating the roles of CD4+ T cells and, moreover, the Th1 and Th2 cytokines produced by CD4+ T cells in defense of the mucosal tissue against bacterial infection. In our studies, C. rodentium expressing OVA, a model antigen, was used to investigate antigen-specific immune responses. The pathogenesis exerted by OVA-C. rodentium was similar to that of C. rodentium. This experimental model has at least three benefits. First, adaptive immunity to C. rodentium requires the development of systemic and CD4+ T-cell-dependent antibody responses (3). The OVA tag expressed by OVA-C. rodentium is non-cross-reactive with antigens in the colonic fluid, and so antigen-specific antibody responses can be assessed without affecting other factors. Second, our model can be used to evaluate various aspects of antigen-specific CD4+ T-cell functions relevant to antimicrobial immunity with transgenic mice such as OT-II mice. Finally, OVA-C. rodentium possesses a kanamycin resistance gene. Therefore, OVA-C. rodentium can be easily distinguished from commensal flora. Furthermore, the antibiotic treatment diminishes the load of bacteria in the colon, which also may have abolished secondary actions caused by other bacteria in the colon with a disrupted epithelial barrier. Thus, our experimental model is suitable for examining antigen-specific immune responses against C. rodentium infection.
IFN-γ is an immunoregulatory cytokine crucially involved in a wide range of infectious diseases. It has been reported that IFN-γ promotes immune responses at the initiation of several bacterial infections, i.e., macrophage activation, neutrophil recruitment, Th1 cell development, Th2 response inhibition, and epithelial defense, including the induction of antimicrobial defenses (17, 20). The colonic tissue from mice infected with C. rodentium exhibited increased expression of IFN-γ and more mucosal damage than that of uninfected mice (6). The importance of IFN-γ expression was confirmed by the marked increase in the susceptibility of IFN-γ−/− mice to OVA-C. rodentium infection. Moreover, IFN-γ promoted the efficacy of antigen-specific T-cell activation and macrophage phagocytosis during C. rodentium infection as well as the production of antibacterial IgG. The deficiency of IFN-γ may lead to a decrease in efficiency of various aspects of antigen-presenting cell functions relevant to antimicrobial immunity, including endocytosis, phagocytosis, and antigen-presenting cell activation, resulting in a less mature phenotype of these types of cells. Consistent with these phenomena, in the absence of IFN-γ produced by antigen-specific CD4+ T cells, there is less stimulation of antigen-specific T cells and, thus, of adaptive immunity. This stimulation of T cells is associated with increased B-cell production of bacterium-specific IgGs. The increased susceptibility to C. rodentium infection in the context of IFN-γ deficiency can thus be explained by a broad decline in the efficacy of the immune response against this mucosal pathogen.
The lymphocytic host responses to C. rodentium are characterized by a large infiltration of CD4+ T cells into the colonic lamina propria and highly polarized Th1 responses (5, 6). CD4+ T-cell-deficient mice failed to clear C. rodentium infection, and their titers of serum anti-C. rodentium antibody were significantly reduced compared to those of the control mice. CD4+ T cells thus play an important role in the mucosal and systemic immune response to this bacterium. In addition, our studies demonstrated that the level of IFN-γ was higher than that of other cytokines produced by antigen-specific CD4+ T cells during C. rodentium infection. Moreover, our adaptive transfer model demonstrated that IFN-γ deficiency from antigen-specific CD4+ T cells was more susceptible to this bacterial infection and revealed a significant delay in the development of antigen-specific IgG levels. In contrast to our results, a previous report showed that IFN-γ deficiency did not affect the bacterial burden or the development of protective antibodies in IFN-γ−/− mice and adoptively transferred CD4−/− recipients during C. rodentium infection (3). We have speculated about some possible explanations for these distinct results, but the reason for this discrepancy is unclear. First, the amount of C. rodentium bacteria administered in our experiments was more than double the amount used in experiments by Bry et al. (3), possibly resulting in the difference in bacterial burdens. Second, T cells other than CD4+ T cells, the involvement of which was excluded in our experimental conditions (Fig. 5A to F), might be involved in development of protective antibodies in the experiments of Bry et al. Finally, and most important, our unique experimental system using C. rodentium expressing OVA might have allowed for an enhanced activation of antigen-specific CD4+ T cells, leading to strong immune responses for the eradication of C. rodentium. Furthermore, adoptive transfer of OVA-specific CD4+ T cells from C57BL/6/OT-II mice into IFN-γ−/− recipients decreased the susceptibility to OVA-C. rodentium (Fig. 5G to K). Therefore, it is suggested that IFN-γ from antigen-specific CD4+ T cells promotes the key function against C. rodentium infection.
A unique aspect of our study is the regulation of antibacterial IgG by IFN-γ from antigen-specific CD4+ T cells. The protective effects of IgG might contribute to the complement-fixing activity of high-affinity binding to activated FcγRs, such as FcγRI and FcγRII/III (13, 18). Bacterium-specific IgG plays a critical role in C. rodentium eradication. Therefore, the decreased production of IgG may be associated with susceptibility to C. rodentium infection. The results from these studies support an important role for the IFN-γ produced by antigen-specific CD4+ T cells in coordinating the innate and adaptive immune responses to this pathogen.
In conclusion, IFN-γ produced by antigen-specific CD4+ T cells regulates a variety of important processes in the protection against C. rodentium infection. This indicates the development of cellular and humoral (IgG) immunity and the enhancement of microbicidal activity by immune cells. Thus, IFN-γ produced by antigen-specific CD4+ T cells in response to C. rodentium regulates innate and adaptive immune pathways in response to primary infection at the mucosal surface.
Acknowledgments
We thank Chihiro Sasakawa (The University of Tokyo, Japan) for kindly providing GFP-C. rodentium cells.
This work was supported, in part, by grants for the Global COE Program “Global Center of Excellence for Education and Research on Signal Transduction Medicine in the Coming Generation” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by the Nagao Memorial Fund, Grant-in-Aids for Scientific Research and for Scientific Research in Priority Areas “Membrane Traffic” from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, and by the Foundation of Advancement of International Science (M.Y.). This work was also supported by a grant for the Education Program for Specialized Clinicians in the Support Program for Improving Graduate School Education from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H.S.).
We have no conflicts of interest.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 29 March 2010.
REFERENCES
- 1.Barnden, M. J., J. Allison, W. R. Heath, and F. R. Carbone. 1998. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76:34-40. [DOI] [PubMed] [Google Scholar]
- 2.Bry, L., and M. B. Brenner. 2004. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. J. Immunol. 172:433-441. [DOI] [PubMed] [Google Scholar]
- 3.Bry, L., M. Brigl, and M. B. Brenner. 2006. CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium. Infect. Immun. 74:673-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higgins, L. M., G. Frankel, I. Connerton, N. S. Goncalves, G. Dougan, and T. T. MacDonald. 1999. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285:588-591. [DOI] [PubMed] [Google Scholar]
- 6.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishigame, H., S. Kakuta, T. Nagai, M. Kadoki, A. Nambu, Y. Komiyama, N. Fujikado, Y. Tanahashi, A. Akitsu, H. Kotaki, K. Sudo, S. Nakae, C. Sasakawa, and Y. Iwakura. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30:108-119. [DOI] [PubMed] [Google Scholar]
- 8.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 9.LeibundGut-Landmann, S., O. Gross, M. J. Robinson, F. Osorio, E. C. Slack, S. V. Tsoni, E. Schweighoffer, V. Tybulewicz, G. D. Brown, J. Ruland, and C. Reis e Sousa. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat. Immunol. 8:630-638. [DOI] [PubMed] [Google Scholar]
- 10.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 11.Maaser, C., M. P. Housley, M. Iimura, J. R. Smith, B. A. Vallance, B. B. Finlay, J. R. Schreiber, N. M. Varki, M. F. Kagnoff, and L. Eckmann. 2004. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infect. Immun. 72:3315-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-β induces development of the TH17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 13.Masuda, A., M. Yoshida, H. Shiomi, S. Ikezawa, T. Takagawa, H. Tanaka, R. Chinzei, T. Ishida, Y. Morita, H. Kutsumi, H. Inokuchi, S. Wang, K. Kobayashi, S. Mizuno, A. Nakamura, T. Takai, R. S. Blumberg, and T. Azuma. 2008. Fcγ receptor regulation of Citrobacter rodentium infection. Infect. Immun. 76:1728-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mombaerts, P. 1995. Lymphocyte development and function in T-cell receptor and RAG-1 mutant mice. Int. Rev. Immunol. 13:43-63. [DOI] [PubMed] [Google Scholar]
- 15.Mundy, R., T. T. MacDonald, G. Dougan, G. Frankel, and S. Wiles. 2005. Citrobacter rodentium of mice and man. Cell Microbiol. 7:1697-1706. [DOI] [PubMed] [Google Scholar]
- 16.Neurath, M. F., B. Weigmann, S. Finotto, J. Glickman, E. Nieuwenhuis, H. Iijima, A. Mizoguchi, E. Mizoguchi, J. Mudter, P. R. Galle, A. Bhan, F. Autschbach, B. M. Sullivan, S. J. Szabo, L. H. Glimcher, and R. S. Blumberg. 2002. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn's disease. J. Exp. Med. 195:1129-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohteki, T., T. Fukao, K. Suzue, C. Maki, M. Ito, M. Nakamura, and S. Koyasu. 1999. Interleukin 12-dependent interferon gamma production by CD8α+ lymphoid dendritic cells. J. Exp. Med. 189:1981-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravetch, J. V., and J. P. Kinet. 1991. Fc receptors. Annu. Rev. Immunol. 9:457-492. [DOI] [PubMed] [Google Scholar]
- 19.Simmons, C. P., S. Clare, M. Ghaem-Maghami, T. K. Uren, J. Rankin, A. Huett, R. Goldin, D. J. Lewis, T. T. MacDonald, R. A. Strugnell, G. Frankel, and G. Dougan. 2003. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons, C. P., N. S. Goncalves, M. Ghaem-Maghami, M. Bajaj-Elliott, S. Clare, B. Neves, G. Frankel, G. Dougan, and T. T. MacDonald. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 168:1804-1812. [DOI] [PubMed] [Google Scholar]
- 21.Tagawa, Y., K. Sekikawa, and Y. Iwakura. 1997. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice: role for IFN-γ in activating apoptosis of hepatocytes. J. Immunol. 159:1418-1428. [PubMed] [Google Scholar]
- 22.Vallance, B. A., W. Deng, L. A. Knodler, and B. B. Finlay. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beelen, A. J., Z. Zelinkova, E. W. Taanman-Kueter, F. J. Muller, D. W. Hommes, S. A. Zaat, M. L. Kapsenberg, and E. C. de Jong. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity 27:660-669. [DOI] [PubMed] [Google Scholar]
- 24.Veldhoen, M., R. J. Hocking, R. A. Flavell, and B. Stockinger. 2006. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat. Immunol. 7:1151-1156. [DOI] [PubMed] [Google Scholar]
- 25.von der Weid, T., M. Kopf, G. Kohler, and J. Langhorne. 1994. The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur. J. Immunol. 24:2285-2293. [DOI] [PubMed] [Google Scholar]
- 26.Wei, O. L., A. Hilliard, D. Kalman, and M. Sherman. 2005. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect. Immun. 73:1978-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida, M., K. Kobayashi, T. T. Kuo, L. Bry, J. N. Glickman, S. M. Claypool, A. Kaser, T. Nagaishi, D. E. Higgins, E. Mizoguchi, Y. Wakatsuki, D. C. Roopenian, A. Mizoguchi, W. I. Lencer, and R. S. Blumberg. 2006. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J. Clin. Invest. 116:2142-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida, M., Y. Shirai, T. Watanabe, M. Yamori, Y. Iwakura, T. Chiba, T. Kita, and Y. Wakatsuki. 2002. Differential localization of colitogenic Th1 and Th2 cells monospecific to a microflora-associated antigen in mice. Gastroenterology 123:1949-1961. [DOI] [PubMed] [Google Scholar]
- 29.Zheng, Y., P. A. Valdez, D. M. Danilenko, Y. Hu, S. M. Sa, Q. Gong, A. R. Abbas, Z. Modrusan, N. Ghilardi, F. J. de Sauvage, and W. Ouyang. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14:282-289. [DOI] [PubMed] [Google Scholar]