Abstract
Enterohemorrhagic Escherichia coli O157:H7, a world-wide human food-borne pathogen, causes mild to severe diarrhea, hemorrhagic colitis, and hemolytic uremic syndrome. The ability of this pathogen to persist in the environment contributes to its dissemination to a wide range of foods and food processing surfaces. Biofilms are thought to be involved in persistence, but the process of biofilm formation is complex and poorly understood in E. coli O157:H7. To better understand the genetics of this process, a mini-Tn5 transposon insertion library was constructed in strain EDL933 and screened for biofilm-negative mutants using a microtiter plate assay. Ninety-five of 11,000 independent insertions (0.86%) were biofilm negative, and transposon insertions were located in 51 distinct genes/intergenic regions that must be involved either directly or indirectly in biofilm formation. All of the 51 biofilm-negative mutants showed reduced biofilm formation on both hydrophilic and hydrophobic surfaces. Thirty-six genes were unique to this study, including genes on the virulence plasmid pO157. The type V secreted autotransporter serine protease EspP and the enterohemolysin translocator EhxD were found to be directly involved in biofilm formation. In addition, EhxD and EspP were also important for adherence to T84 intestinal epithelial cells, suggesting a role for these genes in tissue interactions in vivo.
Enterohemorrhagic Escherichia coli O157:H7 was first recognized as the probable cause of hemorrhagic colitis in humans in 1982 (50). Since then, this organism has emerged as a major cause of food-borne illness in countries around the world, including the United States (49), Europe (17, 18, 24, 36), Japan (39), and Australia (28). Outbreaks have been associated with a variety of food sources, including ground beef (50), green leafy vegetables (1, 41), and nonpasteurized milk (26), and environments such as municipal water and lakes (27, 55). Symptoms in infected humans range from mild diarrhea to severe, hemorrhagic colitis, with 5 to 10% of patients developing hemolytic uremic syndrome (HUS), making E. coli O157:H7 one of the leading causes of acute renal failure in children and the elderly (38).
Early studies have shown that some strains of E. coli O157:H7 form biofilms on both abiotic and biotic surfaces outside the host (15, 51, 58). Biofilms are exopolymeric matrix-enclosed bacterial populations that are firmly adherent to each other and/or to surfaces (9). Biofilms have been shown to be responsible for protection from a variety of environmental stresses, such as acidification, high temperatures, and desiccation (52). Moreover, microbes in biofilms are highly resistant to other adverse conditions, such as sanitizers and household cleaners (44, 45) as well as antibiotics (23, 34). The ability of this pathogen to form biofilms on a wide range of food surfaces as well as food processing surfaces makes E. coli O157:H7 problematic in both the health and food industries (29).
Biofilm formation is a dynamic and complex process and includes initial attachment of cells to the substratum, physiological changes within the organism, multiplication of the cells to form microcolonies, and eventually maturation of the biofilm (42). Because of this complexity, the process of biofilm formation and its regulation is poorly understood. Previous studies in E. coli O157:H7 have focused on individual genes and the specific genetic pathways that are responsible for biofilm formation (10, 51, 58, 60). In contrast, few studies have focused on studying genetic factors that control E. coli O157:H7 biofilm formation on a global scale, although studies of this type have been performed with other bacterial pathogens (43, 47, 59).
The goal of this study was to gain additional insights into biofilm formation in E. coli O157:H7. A global mutational approach with a mini-Tn5 transposon was used to study the process of biofilm formation in strain EDL933, a strong biofilm-forming strain. This strain was first isolated during a multistate outbreak involving contaminated hamburgers (50). A library of >11,000 mutants was generated and screened for a biofilm-negative phenotype. Our results reinforced the fact that biofilm formation is a complex process involving a large number of genes and genetic pathways. This study discovered several pO157 genes that were not previously known to be linked to biofilm formation.
MATERIALS AND METHODS
Bacteria.
The E. coli strains used in this study are shown in Table 1. A spontaneous nalidixic acid-resistant mutant of Escherichia coli O157:H7 strain EDL933 was selected to serve as a counterselection for these studies. The mutation in this mutant had no effect on biofilm formation or on the ability of EDL933 to colonize and persist in a sheep model of colonization (unpublished data). For all biofilm assays, the cultures were grown in Luria-Bertani (LB) broth for 24 h at 30°C under stationary conditions. For all other experiments, the cultures were grown overnight in LB broth at 37°C with shaking at 200 to 220 rpm. The temperature-sensitive E. coli strain harboring the pRedET plasmid (Gene Bridges GmbH, Dresden, Germany) was grown at 30°C. Antibiotic concentrations were 100 μg/ml for ampicillin, 50 μg/ml for kanamycin, and 20 μg/ml for nalidixic acid except where noted. All antibiotics were obtained from Sigma Chemical Co. (St. Louis, MO). The growth of individual mutants was assessed by measuring the growth endpoint optical density at 600 nm (OD600) after 24 h of growth in 96-well plates and compared to that of wild-type EDL933.
TABLE 1.
E. coli strains and plasmids
| Strain or plasmid | Genotype or descriptiona | Reference or source |
|---|---|---|
| Strains | ||
| BW19795 | RP4-2-tet::Mu-1kan::Tn7 integrant/srlC300 creC510 hsdR17 endA1 zbf-5 uidA(ΔMluI)::pir+thi | G. Phillips |
| DH5α | F− φ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | Invitrogen |
| ISM1536 | E. coli O157:H7 EDL933 | N. Cornick |
| ISM1191 | E. coli O157:H7 EDL933, Nalr | This study |
| ISM1205 | BW19795 pUTminiTn5Km2; Ampr Kanr | 40 |
| ISM1230 | E. coli O157:H7 EDL933 lacking plasmid pO157 | N. Cornick |
| ISM1893 | ISM1191/miniTn5Km2 in hlyB, Nalr Kanr | This study |
| ISM2014 | ISM1536 ΔhlyA Kanr | This study |
| ISM1211 | ISM1536 ΔhlyB Kanr | This study |
| ISM1216 | ISM1536 ΔhlyD Kanr | This study |
| ISM2013 | ISM1216/pISM30 Ampr Kanr | This study |
| ISM1978 | ISM1536/pISM31 Spcr | This study |
| ISM2015 | ISM1230/pISM31 Spcr | This study |
| ISM1944 | ISM1893/pISM31 Spcr Kanr | This study |
| ISM2016 | ISM 2014/pISM31 Spcr Kanr | This study |
| ISM2017 | ISM1211/pISM31 Spcr Kanr | This study |
| ISM2018 | ISM1216/pISM31 Spcr Kanr | This study |
| ISM2019 | ISM2013/pISM31 Spcr Kanr | This study |
| ISM1881 | ISM1191/miniTn5Km2 in L7020, Nalr Kanr | This study |
| ISM1919 | ISM1191/miniTn5Km2 in hlyC hlyA, Nalr Kanr | This study |
| ISM1227 | ISM1536 ΔL7020 Kanr | This study |
| Plasmids | ||
| pKD4 | Derivative of pANTSγ that contains an FLP recombination target-flanked Kanr gene from pCP15 | 13 |
| pRedET | Derivative of pSC101, Ampr, temp sensitive, carries lambda red recombinase | Gene Bridges |
| pBAD18 | Expression vector, Ampr | Beckwith lab |
| pISM30 | pBAD18 expressing ehxCABD operon | This study |
| pISM31 | pMHE6 expressing GFPuv | G. Phillips |
Nalr, nalidixic acid resistant; Ampr, ampicillin resistant; Kanr, kanamycin resistant; Spcr, spectinomycin resistant.
Mutant Library Construction.
Transposon mutagenesis was performed as described previously (22) with a few modifications. To obtain random mini-Tn5Km2 insertion mutants, the conjugal donor strain E. coli BW19795 containing the plasmid pUTmini-Tn5Km2 (supplied by David Holden, Imperial College, London, United Kingdom) was conjugated with E. coli O157:H7 EDL933. One milliliter of overnight culture of each strain was pelleted, washed three times with phosphate-buffered saline (PBS), resuspended in 5 ml of antibiotic-free LB broth, and incubated at 37°C with shaking until an OD600 of 0.7 to 1.0 was reached. The BW19795 donor strain was then transferred to stationary conditions for 30 min to allow regeneration of pili. Two hundred microliters of each strain was combined, and the mating mixture was plated onto a sterile membrane placed on a stack of sterile filter paper. Once the liquid medium was removed by capillary action, the membrane was transferred to an LB agar plate cell side up. Following overnight incubation at 37°C, membranes were vortexed with LB medium, and the suspension was incubated with shaking for 1 h at 37°C and then plated in 100-μl aliquots on LB plates containing kanamycin plus nalidixic acid as a counterselection against the donor strain. Each plate yielded 300 to 400 colonies of kanamycin-resistant mutants. Colonies were picked into 96-well plates, and each was scored for ampicillin resistance. The ampicillin-sensitive mutants were rearrayed into 96-well plates and stored at −70°C for further analysis. A total of 11,000 independent mutants were obtained.
Assays for biofilm formation.
The screen for the biofilm-negative phenotype was performed using a microtiter plate assay as described previously (16) with minor modifications. This assay is based on the ability of biofilm-forming bacteria to adhere to the wells of a 96-well microtiter plate, which are subsequently visualized by staining with crystal violet. Each plate of mini-Tn5 insertion mutants from an overnight LB culture was replica plated using a 96-prong replicator into fresh Costar 96-well, flat-bottom, nontreated polystyrene plates (Corning, Inc., New York, NY) containing 150 μl of LB broth per well. After 24 h of incubation at 30°C under stationary conditions, the plate was rinsed twice with water, and the adherent bacteria were stained with 0.01% crystal violet (175 μl/well) for 20 min. After staining, the plates were washed again twice with water. At this point, biofilms were visible as a violet ring on the side of each well and as a generalized staining of the well (Fig. 1). Mutants that lacked ring formation were scored as having a biofilm-negative phenotype. For growth assessment, a duplicate plate incubated overnight was briefly vigorously shaken on a microtiter plate shaker, and the OD600 was measured and compared with that of the wild-type control strain. Strains with growth that differed from wild-type growth (P < 0.01) were not considered further, as were mutants that showed inconsistent biofilm formation. In each plate, E. coli strain BW19795 was used as a negative control, and a well containing medium only was used as a blank.
FIG. 1.
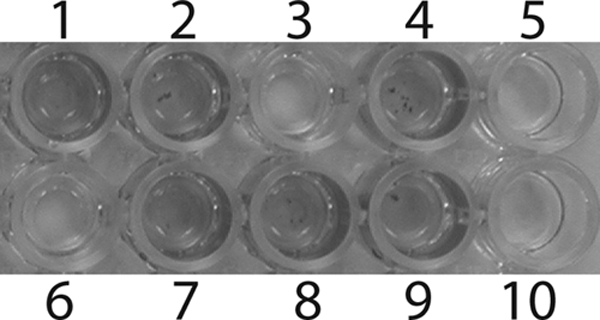
Microtiter plate assay showing screening of mini-Tn5Km2 mutants for the biofilm-negative phenotype. Wells 4 and 9 contained the wild-type biofilm-positive cells, wells 5 and 10 contained the biofilm-negative control (BW19795), wells 3 and 6 contained biofilm-negative transposon mutants, and wells 1, 2, 7, and 8 contained biofilm-positive transposon mutants.
For the quantitative biofilm assay (57), 12- by 75-mm polystyrene tubes (Fisher) were used. An overnight culture of each mutant, plus controls, was diluted 1:100 into 2 ml of LB broth and incubated at 30°C under stationary conditions for 24 h. The tubes were then rinsed twice with water and stained with 2.5 ml of 0.01% crystal violet for 20 min. After being washed three times with water, tubes were air dried and destained with 2.5 ml of 80% ethyl alcohol for 15 min. The tubes were vortexed, 100 μl was transferred to a new 96-well plate, and the optical density was measured at 595 nm using a Spectra MAX 190 spectrophotometer (Molecular Devices, Union City, CA). The optical density measurements were used as a measure of relative amounts of biofilms formed. All experiments were performed in triplicate.
Identification of mini-Tn5 insertion sites.
The mini-Tn5 insertion sites in biofilm-negative mutants were mapped by sequencing the amplified genomic region at the site of mini-Tn5 insertion. The amplification of the region of the DNA at the site of transposon insertion was done using either single-primer PCR(25) or Y-Linker PCR(30). The PCR products were then sequenced and analyzed using BLAST against the E. coli O157: H7 EDL933 genome sequence.
Construction of deletion mutants.
To generate deletion mutations, a one-step gene inactivation method adapted from that described by Datsenko and Wanner (13) was used. The temperature-sensitive plasmid pRedET (Gene Bridges, Dresden, Germany), encoding lambda red recombinase, was transformed into E. coli O157:H7 EDL933. The kanamycin resistance gene was amplified from pKD4 (13) using primers shown in Table 2. Each primer sequence contained target-homologous sequences as well as sequences for amplification of the kanamycin gene. The products of this reaction were electroporated (2,000 V, 129 Ω) using a BTX electrocell manipulator (model 600; Harvard Apparatus, Holliston, MA) into E. coli O157:H7 EDL933/pRedET that was previously induced with 0.4% l-arabinose for 1 h. The cells were incubated in SOC medium (20 g tryptone, 5 g yeast extract, 2 g MgCl2·6H2O, 2.5 g MgSO4·7H2O, and 3.6 g glucose per liter; pH 7.5) for 1 h and then plated on selective medium (LB supplemented with 25 μg/ml of kanamycin) at 37°C.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′ → 3′) |
|---|---|
| P6 U | CGAGCTCGAATTCGGCCTAG |
| P7 U | CTGCAGGCATGCAAGCTTCG |
| P6 M | GCCAGATCTGATCAAGAGAC |
| P7 M | GCCGAACTTGTGTATAAGAGTC |
| Tn5 | GGCCAGATCTGATCAAGAGA |
| Y-Linker | CTGCTCGAATTCAAGCTTCT |
| Test F | TGTGTAGGCTGGAGCTGCTTC |
| ehxA F | AGTAAAAAACAGACAAGATTTTAATTTTAATATTGAGAAAGAAAACTAATTGTGTAGGCTGGAGCTGCTTC |
| ehxA R | TATAACGATGACCATTCCTCCTGGAATGGCCATCACCTCCTCTTTTAGTCCATATGAATATCCTCCTTA |
| ehxA test R | AGAACTACATTTACTCATCA |
| ehxB F | AGACTAAAAGAGGAGGTGATGGCCATTCCAGGAGGAATGGTCATCGTTATTGTGTAGGCTGGAGCTGCTTC |
| ehxB R | TTATAACGGCAAACCAAATCCCATAAACCTTTCATGTAAAAGCGCATACGCATATGAATATCCTCCTTA |
| ehxB test R | AGACATCAGAAAAAACCGTC |
| ehxD F | CCAAAAAAGACAGTTTATATGCATATTTATATCAGTTGCAGGCATAACGTTGTGTAGGCTGGAGCTGCTTC |
| ehxD R | ATTATTTCAGAAATCTATATCATATAAAAAGCCAATATGTTATTTATATAATATGAATATCCTCCTTA |
| ehxD test R | ACATAGATAA TCTTTACAAG |
| L7020F | ATGAATAAAA TATACTCTCT TAAATACAGC CATATTACAG GAGGGTTAATCGCTGTTTCTTGTGTAGGCTGGAGCTGCTTC |
| L7020 R | GCAATTTTAT TGAAAGACTG ATTATATCCG TTCAGGTCAA GAGTTCCGCCATTTTTCGCACATATGAATATCCTCCTTA |
| L7020 test R | TACAGCACCTGAATCAGTTG CA |
| ehx kpn1 F | CTCAGGTACCTAGATGCTTCTTGCTTAAAA |
| ehx xba1 R | GATCTCTAGACTAACGTTCACGTAAACTTT |
Confirmation of mutant constructions and determination of the locations of the kanamycin gene insertions were done by PCR. Primer F (which has homology within the kanamycin cassette) and primer R (which has homology immediately downstream of the gene sequences that were being replaced) were used to generate PCR products. To ensure curation of the temperature-sensitive pRedET plasmid, confirmed mutants were first grown at 42°C for 2 h and then plated on LB plates and incubated overnight at 37°C. The isolated colonies were picked and screened for kanamycin resistance and ampicillin sensitivity. All of the primers used are shown in Table 2.
Construction of plasmid pISM30 for genetic complementation.
The ehxCABD operon was amplified using primers that also incorporated upstream KpnI and downstream XbaI restriction sites (Table 2). The PCR was done using LongAmpTaq DNA polymerase (New England Biolabs) with the following parameters: 2 min at 95°C; 30 cycles of 95°C for 30 s, 58°C for 7 min, and 72°C for 1 min; and 5 min of extension at 72°C. The fragments were digested with KpnI and XbaI, cloned into vector pBAD18, and then transformed into E. coli DH5α. One plasmid, designated pISM30, was confirmed by restriction digestion and was electroporated into E. coli O157:H7 EDL933 ΔehxD.
Cell culture.
The T84 human colonic adenocarcinoma cells were maintained in 25-cm2 (Falcon) tissue culture flasks as monolayers at 37°C with 5% CO2. The cell cultures were grown in Dulbecco modified Eagle medium (DMEM)-F-12 medium (Invitrogen) supplemented with 2.5 mM l-glutamine, 5% fetal bovine serum, and gentamicin (50 μg/ml). The cells were passed every 7 days by treatment with 0.5% trypsin, and the medium was replaced every other day.
Bacterial adhesion assay.
Quantitative adhesion assays were performed using monolayers of T84 cells grown on glass coverslips. The glass coverslips were treated with 1 N HCl for 10 min, washed three times with sterile water, and placed in six-well polystyrene tissue culture plates (Costar, Corning, Corning, NY). T84 cells (4 × 105) were seeded onto the glass coverslips in each well and allowed to attach overnight. The monolayers were then washed with Hanks balanced salt solution and replenished with 1 ml of culture medium without antibiotics. An overnight culture of bacteria was diluted 1:20 in fresh LB and grown for another 2 h. One hundred microliters of this culture (approximately 4 × 106 bacteria) was added to each well containing T84 monolayer cultures. Bacterial cultures were serially diluted and plated to enumerate bacteria added. The tissue culture plates were then incubated at 37°C with 5% CO2 for 1.5 h. The coverslips were washed three times with PBS to remove nonadhered bacteria, and then the glass coverslip was transferred to a fresh six-well tissue culture plate. The T84 cells were then detached and lysed using 1 ml of 0.1% Triton X-100 for 15 min. In preliminary studies, this concentration of Triton X-100 had no effect on viability of E. coli O157:H7 EDL933. This solution was serially diluted in PBS and spread onto LB agar to enumerate the bacteria adhered to T84 cells. The percentage of adherence was calculated as number of bacteria adhered/number of bacteria added to the well × 100, and the relative percentage of adherence was calculated as percentage of adherence of mutant/percentage of adherence of wild type × 100. All experiments were done in triplicate. The paired Student t test was performed to identify statistical differences.
For microscopic analysis, bacteria were transformed with pISM31, a derivative of pMHE6 (20) expressing GFPuv (12). The T84 cells were seeded as described above and grown for 48 h until they were semiconfluent. Bacterial cultures were prepared, and the adherence assays were performed as described above. The plates were then incubated at 37°C with 5% CO2 for 1.5 h. Following this, the coverslips were washed three times with PBS, and the cells were fixed using 4% paraformaldehyde in PBS for 10 min. The coverslips were washed twice with PBS, and treated with BSP buffer (250 mg bovine serum albumin and 100 mg saponin per 100 ml PBS) for 5 min, and then washed twice with BSP. The cells were stained for F-actin (54) with Alexa Fluor 546-labeled phalloidin (Invitrogen) (1:200 dilution in BSP) for 1 h, washed twice with BSP, and then mounted on a glass slide using mounting solution with DAPI (4′,6′-diamidino-2-phenylindole). Once the slides were dry, the coverslips were sealed using clear nail polish, and images were captured using green and red filters on an Olympus IX70 inverted fluorescence microscope equipped with a DP70 digital camera. The images were merged using ImageJ software (National Institutes of Health).
RESULTS
Identification of biofilm genes in E. coli O157:H7 EDL933.
In previous studies, E. coli O157:H7 EDL933 was shown to form biofilms on inert surfaces (15). Since there have been no reports of studies performed on a global scale to identify biofilm-linked genes in E. coli O157:H7, we conducted a global mutational study using mini-Tn5Km2 to identify genes involved either directly or indirectly in biofilm formation. During the initial screen, 114 mutants (1.04% of the total library) had a biofilm-negative phenotype (Fig. 1). Following the confirmation of the phenotype, the growth of each strain was assessed. After elimination of mutants with inconsistent biofilm formation or a growth deficiency, 95 mutants that were biofilm negative (0.86% of the library) were studied further. For convenience, these mutants were designated biofilm-negative phenotype (Bnp) mutants.
The precise mini-Tn5 insertion sites of these Bnp mutants were identified by DNA sequencing. Our results indicated that there was only a single transposon insertion within the genome in each mutant. This was evident from the Y-Linker PCR, which showed amplification of only one fragment, and from the DNA sequence trace chromatograms, which gave a single sequence. The mini-Tn5 insertion sites in 19 of the Bnp mutants could not be identified due to a failure to amplify the region at the transposon insertion sites despite repeated attempts.
The 76 insertions that could be identified were distributed randomly throughout the genome of E. coli O157:H7 EDL933. In some cases, there were multiple insertions in the same gene coding region but at different locations. Fifty-one distinct genes/intergenic regions were identified (Table 3). Thirty-two insertions were in coding sequences of known function, and 19 were in hypothetical genes whose functions are yet to be assigned. Twenty-five insertions occurred in sequences having homology to E. coli K-12 sequences, and 19 occurred in sequences not shared with K-12 (O islands); three are on the pO157 plasmid, and five are phage carried. The functions of these genes vary; they include genes that encode structural components (ecpD, csgG, csgB, csgA, tolQ, waaL, and waaP), enzymes (yahF, yaiH, galU, cls, manC, wbdQ, fcl, aroC, relA, rfaC, and dsbA), regulators (Z2086, yihF, and hns), receptors (Z1178, Z0700, and Z3635), and hypothetical proteins. Three independent insertions were identified in the curli pilus operon (csgG, csgB, and csgA) and four independent insertions in the lipopolysaccharide (LPS) biosynthesis operon (waaL, waaP, waaD, and waaJ).
TABLE 3.
Mini-Tn5 transposon biofilm-negative mutants of E. coli O157:H7 EDL933e
| Mutant | Locus tag | Gene name | Product or function |
|---|---|---|---|
| Bnp1a | Z3917b | Hypothetical protein | |
| Bnp2a | Z4881b | Putative aldolase | |
| Bnp3a | Z5856 | Putative aspartate carbamoyltransferase | |
| Bnp4a | Z2256b | Unknown protein associated with Rhs element | |
| Bnp5a | Z5890b | Partial putative integrase | |
| Bnp6a | L7020d | espP | Putative exoprotein precursor |
| Bnp7a | Z3635 | Putative receptor protein | |
| Bnp8a | Z3182b | hisD | l-histidinal:NAD+ oxidoreductase |
| Bnp9a | Z4625 | acrE | Protein affects cell membrane permeability |
| Bnp10 | Z0472 | yaiH | Putative enzyme |
| Bnp11a | Z1555b | Hypothetical protein | |
| Bnp12a | Z2436 | ynaJ | Hypothetical protein |
| Bnp13a | Z1921c | Unknown protein encoded by CP933X | |
| Bnp14a | Z0151 | ecpD | Putative fimbrial chaperone protein |
| Bnp15a | Z1456c/Z1457c | Hypothetical protein encoded by BP-933W/putative DNA binding protein of BP-933W | |
| Bnp16 | Z3592 | aroC | Chorismate synthase |
| Bnp17 | Z2026 | cls | Cardiolipin synthase |
| Bnp18a | L7049d | hlyB | Hemolysin transport protein |
| Bnp19a | Z3497 b | glpQ | Glycerophosphodiester phosphodiesterase |
| Bnp20 | Z4099 | relA | (p)ppGpp synthetase I |
| Bnp21 | Z5049 | waaL | Surface polymer ligase |
| Bnp22a | Z3199b/Z3200b | wbdP/per | Putative glycosyl transferase/perosamine synthetase |
| Bnp23 | Z5050b | waaD | LPS biosynthesis enzyme |
| Bnp24a | Z2163 | ydeH | Hypothetical protein |
| Bnp25 | Z1675/Z1676 | csgB/csgA | Minor curlin subunit precursor/curlin major subunit, coiled surface structures |
| Bnp26a | Z2086c | Putative regulator | |
| Bnp27a | Z3660 | yfeA | Hypothetical protein |
| Bnp28a | Z5214b | Hypothetical protein | |
| Bnp29 | Z5051b | waaJ | LPS α1,2-glucosyltransferase |
| Bnp30 | Z1670 | csgG | Curli production assembly/transport component |
| Bnp31a | Z1212b/Z1213b | Hypothetical protein | |
| Bnp32a | Z1213b | Hypothetical protein | |
| Bnp33 | Z2012/Z2013 | galU/hns | Glucose-1-phosphate uridylyltransferase/DNA binding protein; pleiotropic regulator |
| Bnp34 | Z3195/Z3196 | manC/wbdQ | Mannose-1-phosphate guanosyltransferase/GDP-mannose mannosylhydrolase |
| Bnp35a | Z0340c | Unknown protein encoded in prophage CP933I | |
| Bnp36 | Z1676 | csgA | Curlin major subunit, coiled surface structures |
| Bnp37 | Z0905 | tolQ | Inner membrane protein |
| Bnp38 | Z5054 | waaP | LPS biosynthesis enzyme |
| Bnp39a | Z4328b | Hypothetical protein | |
| Bnp40a | Z0700b | Putative receptor | |
| Bnp41a | Z3918b | Putative chaperone protein | |
| Bnp42a | Z1494c | Unknown protein encoded by BP-933W | |
| Bnp43a | Z0021b | Hypothetical protein | |
| Bnp44a | L7047/L7048d | hlyC/hlyA | Hemolysin protein |
| Bnp45a | Z1977 | ychM | Hypothetical protein |
| Bnp46a | Z0408/Z0409 | yahE/yahF | Hypothetical protein/putative oxidoreductase subunit |
| Bnp47a | Z3197 | fcl | Fucose synthetase |
| Bnp48a | Z0904 | ybgC | Hypothetical protein |
| Bnp49a | Z4327b | Hypothetical protein | |
| Bnp50 | Z5392 | dsbA | Protein disulfide isomerase I |
| Bnp51a | Z1177b/Z1178b | Partial putative phage inhibition protein/putative receptor |
Genes not previously shown to be involved with biofilm formation.
On O islands.
Carried in prophage.
On pO157.
A slash indicates that the transposon site was between the genes indicated.
Plasmid pO157 genes involved in biofilm formation.
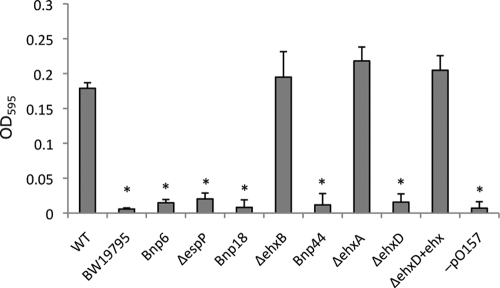
In our mutational analysis we identified three independent insertions of mini-Tn5km2 in pO157, one in the type V secreted serine protease espP (7) and two in the enterohemolysin operon ehxCABD (3, 4) (one in ehxB and one between ehxC and ehxA). We confirmed the role of pO157 in biofilm formation by testing strain ISM1230, a derivative of EDL933 lacking the plasmid. This strain failed to make biofilms (Fig. 2). To confirm the linkage between specific genes and the biofilm phenotype, deletion mutants were constructed for three of the genes that were biofilm negative during screening, i.e., espP, ehxB, and ehxA. The deletion mutants were then compared for their growth and biofilm phenotype using a quantitative tube assay with the corresponding mini-Tn5km2 Bnp mutants (Bnp6, Bnp18, and Bnp44, respectively) (Fig. 2). Two of the deletion mutants (the ΔehxB and ΔehxA mutants) showed no difference in biofilm formation compared to the wild type, but their corresponding mini-Tn5km2 Bnp mutants, Bnp18 and Bnp44, were deficient in biofilm formation. The other deletion-insertion mutant (the ΔespP mutant) behaved in manner similar to that of its corresponding mini-Tn5km2 Bnp mutant (Bnp6). One explanation for the inconsistency in ehxCABD mutations is that the insertion of the transposon caused polarity effects on downstream expression of ehxD (8) that would not occur with the deletion mutations. All deletions were constructed in frame, and the kanamycin marker used in the deletion reaction lacks a transcriptional stop signal, allowing for continued transcription and expression of downstream genes. Thus, these results indicate that ehxD, and not ehxA or ehxB, may be the critical element in biofilm formation. To test this hypothesis, we constructed a ΔehxD mutant and tested it for the biofilm phenotype. Our quantitative assay showed that the deletion of ehxD caused a negative biofilm phenotype (Fig. 2). To further confirm this linkage, we constructed a plasmid for genetic complementation by cloning the ehxCABD operon under control of its native promoter into plasmid pBAD18. When this plasmid was transformed in the ΔehxD mutant (ΔehxD+ pISM30), the biofilm phenotype was restored to levels comparable to those for the wild type (Fig. 2).
FIG. 2.
Comparison of biofilm formation on polystyrene by E. coli O157:H7 EDL933 transposon insertion and deletion mutants along with wild-type (WT) and negative-control (BW19795) strains. Data represent means plus standard errors for three replicates. Bnp6, espP::Tn5; Bnp18, ehxB::Tn5; Bnp44, Tn5 inserted at the ehxC-ehxA junction; ΔehxD+ehx, ΔehxD complemented with pISM30. *, significantly different from the wild-type control (P < 0.01).
Role of pO157 genes in adherence to T84 cells.
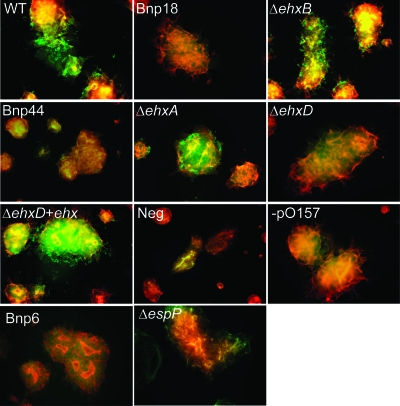
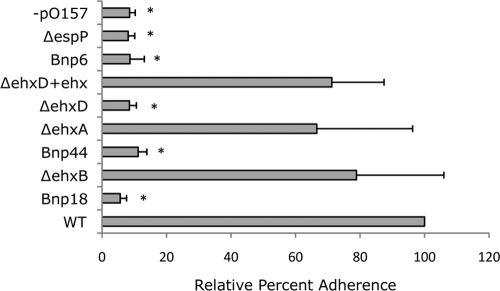
To analyze the role of ehxCABD in adherence to T84 cells, wild-type E. coli O157:H7 EDL933, the wild type without pO157, and the Bnp6, Bnp18, Bnp44, ΔespP, ΔehxA, ΔehxB, ΔehxD, ΔehxD+pISM30 mutants were tested for adherence to T84 cells. Both microscopic and quantitative analyses were done. For microscopic analysis, all of the bacterial strains were transformed with a green fluorescent protein-expressing plasmid, pISM31. As shown in Fig. 3, wild-type E. coli O157:H7 EDL933 and the ΔehxA, ΔehxB, and ΔehxD+pISM30 mutants adhered to T84 cells, while E. coli O157:H7 EDL933 without pO157 and the Bnp6, Bnp18, Bnp44, ΔespP, and ΔehxD mutants failed to adhere. The quantitative analysis also showed that there is no significant difference in relative adherence to T84 cells between wild-type E. coli O157:H7 EDL933 and the ΔehxA, ΔehxB, and ΔehxD+pISM30 mutants but that there was a significant reduction in the relative adherence of E. coli O157:H7 EDL933 without pO157, and the Bnp6, Bnp18, Bnp44, ΔespP, and ΔehxD mutants (Fig. 4). For this experiment, the percentage of wild-type bacteria adhering to the T84 monolayers was 32.6%. Strains with less than 4% adherence were considered negative.
FIG. 3.
Fluorescence microscopic pictures of GFPuv, expressing wild-type E. coli O157:H7 EDL933 and transposon insertion and deletion mutants, adhering to T84 cells. All pictures are merged images of DAPI (blue), F-actin (orange), and GFPuv-expressing bacterium (green) staining.
FIG. 4.
Adherence of wild-type E. coli O157:H7 EDL933 and transposon insertion and deletion mutants to T84 cells shown quantitatively. Data represent means plus standard errors for three replicates. *, significantly different from the wild-type control (P < 0.01).
DISCUSSION
Earlier studies with Pseudomonas fluorescens and Staphylococcus aureus showed that the process of biofilm formation is complex and involves several convergent and divergent pathways (43, 59). To obtain an overall idea of the genes required for biofilm formation in the human pathogen E. coli O157:H7 EDL933, a global mutagenesis approach with mini-Tn5km2 was performed.
Previous studies with E. coli have shown that Tn5 and its minitransposon derivatives insert randomly in the genome (14). Based on the assumption that there are 1,000 essential genes out of 5,361 open reading frames in the genome of E. coli O157:H7 EDL933 (46), the generation of approximately 11,000 random mutants should give at least a 99% probability of inactivating 90% of nonessential genes. These studies uncovered several new genes that are involved in biofilm formation. Our approach was not complete, however, as we also missed some genes already shown to be involved in biofilm formation in E. coli O157:H7, such as csgD, ompA, and cadA (58). This is because our screen was not saturating. In fact, approximately 55,000 random mutants would need to be generated for inactivation of 100% of all nonessential genes with a 99% probability.
This study identified 51 Bnp genes in E. coli O157:H7, of which 19 were O-island pathogen-associated genes, five were phage carried, and three were located on pO157 (Table 3). This suggests that some of the regulatory pathways involved in biofilm formation in E. coli O157:H7 are unique to that serotype. Previous studies have shown that Shiga toxin-producing E. coli strains, including the O157:H7 serotype, are retained or persist in the ruminant gastrointestinal tract better than other E. coli pathotypes (11). The reason for this is unknown, but it is possible that genes unique to the O157:H7 serotype that are involved in biofilm development may enhance persistence in specific environmental situations such as those encountered in the bovine gastrointestinal tract or on food processing surfaces.
Of the 51 Bnp genes identified, 20 have defined functions and 31 either are hypothetical or have only putative functions assigned. Among those 20 genes with known functions are those already demonstrated to have a role in biofilm formation in other organisms. relA was shown to be required for efficient biofilm formation in Listeria monocytogenes, Streptococcus mutans, and E. coli (2, 32, 56). It has also been demonstrated that relA mutants show lower levels of (p)ppGpp and higher levels of LuxS under amino acid starvation conditions (32, 56). The changes in the levels of LuxS and (p)ppGpp affect pathways that are required for biofilm formation. Our study showed that in E. coli O157:H7, relA is also involved in biofilm formation. A more thorough transcriptional analysis of this gene may lead to the discovery of additional determinants of biofilm formation for E. coli O157:H7.
Four independent insertions in the lipopolysaccharide (LPS) biosynthesis operon (waaL, waaP, waaD, and waaJ) that resulted in the loss of biofilm formation were identified. In Gram-negative bacteria, LPS influences the physiochemical characteristics of the cell surface. In Pseudomonas aeruginosa it has been shown that the production of A band or B band LPS influences the surface characteristics and modifies the binding capabilities of the bacterium (35). The presence of four independent insertions in the LPS biosynthesis operon strongly suggests that LPS is directly involved in biofilm formation in E. coli O157:H7 as well. Thus, a more thorough study of this operon is needed to more accurately describe its role in biofilm development.
In Vibrio cholerae, galU, another Bnp gene, was shown to be essential for the formation of biofilms (5). In several organisms, including E. coli O157:H7, galU encodes glucose-1-phosphate uridylyl transferase and is responsible for synthesis of UDP glucose. The synthesis of UDP galactose via UDP-glucose is necessary for biosynthesis of exopolysaccharide, which is a binding substrate in biofilms. In Streptococcus mutans, aroC (chorismate synthase) has been shown to be involved in biofilm formation (53). cls (cardiolipin synthase) has also been shown to be involved in biofilm formation in Mycobacterium ulcerans (37). Lee et al. showed that disulfide bond isomerase A (dsbA) was involved in biofilm formation on abiotic surfaces in E. coli O157:H7 and that ΔdsbA strains were reduced in attachment to HT-29 epithelial cells and virulence in Caenorhabditis elegans (31).
Another set of interesting genes identified in this study are the csg genes, which are responsible for the production of the curli pilus, a coiled surface structure produced by various microbes. Two operons, csgBA and csgDEFG, are necessary for curli formation. csgA encodes the curlin subunit, CsgB is thought to nucleate CsgA curlin fibers, CsgD is a transcriptional activator of the csgBA operon, and CsgE, CsgF, and CsgG are three putative curli assembly factors (61). Curli pili are highly adhesive proteinaceous structures required for bacterial adherence to surfaces as well as bacterium-to-bacterium binding (62), and they are critical for both primary surface colonization and subsequent biofilm development in the formation of microcolonies (48). More-detailed genetic studies showed that CsgD is a control unit for biofilm formation and coordinates both positive (csgAB and yaiC) and negative (pepD and yagS) determinants of biofilm formation (6, 21). It is possible that the curli pilin subunit (CsgA and CsgB) is directly involved in the attachment of the organism to the surface and that the regulator CsgD is involved in the maturation of the biofilm. Although this gene is one of the best-studied biofilm-associated genes in E. coli O157:H7, questions remain about the actual mechanism of attachment of organisms to the substratum through curli pili.
Several Bnp genes identified in this study (Table 3) are uniquely associated with biofilm formation. Most of these either are hypothetical genes or have been assigned putative functions based on homology studies. Determination of how these genes might function in the development or maintenance of biofilms will require further study.
Three of the 51 Bnp genes obtained in the initial screening, Bnp6, Bnp18, and Bnp44, were chosen for further study because of their location on the plasmid pO157. A role of translocator EhxD in biofilm formation was confirmed by deleting the gene ehxD (strain ISM1216) and complementing the biofilm-negative phenotype with plasmid pISM30, expressing the ehxCABD operon (strain ISM2013). The role of ehxD in biofilm formation is unknown and needs further study. One possible hypothesis is the translocator EhxD functions independently of EhxB and transports factors that are critical to biofilm formation. Our study is the first to show the involvement of the plasmid pO157-carried enterohemolysin operon ehxCABD in biofilm formation in E. coli O157:H7, although a recent study reported the importance of pO157 in biofilm development (33). In addition to ehxD, espP was also important to biofilm development. We can only speculate on how this type V secreted serine protease (7, 19) might be involved in biofilm formation, but it must play an important role.
We were also interested in testing whether ehxD and espP might have a role in adherence to T84 colonic adenocarcinoma cells, testing the hypothesis that biofilm formation and cellular adherence to epithelial cells are linked in E. coli O157. A previous study by Dziva et al. showed that EspP was critical to adherence to a bovine primary rectal epithelial cell line (19), so it was reasonable to think that other products of genes on pO157 might also be involved in cell adherence. Clearly, cellular adherence occurs to T84 cells in vitro at 1 h after inoculation (Fig. 4). The loss of ehxD or espP, however, resulted in E. coli O157:H7 being incapable of T84 cell adherence. This was also true for a pO157-negative strain. Complementation of the ΔehxD strain resulted in adherence, suggesting that this protein, along with EspP (19), has an important role in cellular adherence and possibly tissue interactions in vivo.
In summary, through random mutagenesis we were able to identify genes not previously known to be involved in biofilm formation in E. coli O157. Two of these genes, espP and ehxD, are located on the virulence plasmid pO157. In addition to biofilm formation, these genes are important for adherence to T84 colonic epithelial cells. Further analysis of these gene products and the pathways involved will provide a better understanding of the process of biofilm formation and colonization by E. coli O157:H7, which in turn should help in the development of methods to decrease its prevalence in food animals and to control its dissemination in the environment.
Acknowledgments
This study was funded in part by the U.S. Department of Agriculture, Agricultural Research Service grant no. 58-3625-4-173, and by the Iowa State University Institute for Food Safety and Security.
We thank Gregory Phillips for assistance with deletion mutagenesis and the plasmid pISM31 and Bryan Bellaire for assistance with microscopy.
Editor: S. M. Payne
Footnotes
Published ahead of print on 29 March 2010.
REFERENCES
- 1.Ackers, M. L., B. E. Mahon, E. Leahy, B. Goode, T. Damrow, et al. 1998. An outbreak of Escherichia coli O157:H7 infections associated with leaf lettuce consumption. J. Infect. Dis. 177:1588-1593. [DOI] [PubMed] [Google Scholar]
- 2.Balzer, G. J., and R. J. McLean. 2002. The stringent response genes relA and spoT are important for Escherichia coil biofilms under slow-growth conditions. Can. J. Microbiol. 48:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, M. E., and R. A. Welch. 1996. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 64:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerlin, P., S. Chen, J. K. Colbourne, R. Johnson, S. De Grandis, et al. 1998. Evolution of enterohemorrhagic Escherichia coli hemolysin plasmids and the locus for enterocyte effacement in Shiga toxin-producing E. coli. Infect. Immun. 66:2553-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohringer, J., D. Fischer, G. Mosler, and R. Hengge-Aronis. 1995. UDP-glucose is a potential intracellular signal molecule in the control of expression of sigma S and sigma S-dependent genes in Escherichia coli. J. Bacteriol. 177:413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brombacher, E., C. Dorel, A. J. Zehnder, and P. Landini. 2003. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 149:2847-2857. [DOI] [PubMed] [Google Scholar]
- 7.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 8.Burland, V., Y. Shao, N. T. Perna, G. Plunkett, H. J. Sofia, et al. 1998. The complete DNA sequence and analysis of the large virulence plasmid in Escherichia coli O157:H7. Nucleic Acids. Res. 26:4196-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpentier, B., and O. Cerf. 1993. Biofilms and their consequences, with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 75:499-511. [DOI] [PubMed] [Google Scholar]
- 10.Cookson, A. L., W. A. Cooley, and M. J. Woodward. 2002. The role of type 1 and curli fimbriae of Shiga toxin-producing Escherichia coli in adherence to abiotic surfaces. Int. J. Med. Microbiol. 292:195-205. [DOI] [PubMed] [Google Scholar]
- 11.Cornick, N. A., S. L. Booher, T. A. Casey, and H. W. Moon. 2000. Persistent colonization of sheep by Escherichia coli O157:H7 and other E. coli pathotypes. Appl. Environ. Microbiol. 66:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crameri, A., E. A. Whitehorn, E. Tate, and W. P. C. Stemmer. 1996. Improved green fluorescent protein by molecular evolution using DNA shuffling. Nat. Biotechnol. 14:315-319. [DOI] [PubMed] [Google Scholar]
- 13.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehio, C., and M. Meyer. 1997. Maintenance of broad-host-range incompatibility group P and group Q plasmids and transposition of Tn5 in Bartonella henselae following conjugal plasmid transfer from Escherichia coli. J. Bacteriol. 179:538-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewanti, R., and A. C. Wong. 1995. Influence of culture conditions on biofilm formation by Escherichia coli O157:H7. Int. J. Food Microbiol. 26:147-164. [DOI] [PubMed] [Google Scholar]
- 16.Djordjevic, D., M. Wiedmann, and L. A. McLandsborough. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68:2950-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doorduyn, Y., C. M. de Jager, W. K. van der Zwaluw, I. H. Friesema, A. E. Heuvelink, et al. 2006. Shiga toxin-producing Escherichia coli (STEC) O157 outbreak, The Netherlands, September-October 2005. Euro Surveill. 11:182-185. [PubMed] [Google Scholar]
- 18.Dundas, S., W. T. Todd, A. I. Stewart, P. S. Murdoch, A. K. Chaudhuri, et al. 2001. The central Scotland Escherichia coli O157:H7 outbreak: risk factors for the hemolytic uremic syndrome and death among hospitalized patients. Clin. Infect. Dis. 33:923-931. [DOI] [PubMed] [Google Scholar]
- 19.Dziva, F., A. Mahajan, P. Cameron, C. Currie, I. J. McKendrick, et al. 2007. EspP, a type V-secreted serine protease of enterohaemorrhagic Escherichia coli O157:H7, influences intestinal colonization of calves and adherence to bovine primary intestinal epithelial cells. FEMS Microbiol. Lett. 271:258-264. [DOI] [PubMed] [Google Scholar]
- 20.Fodor, B. D., A. T. Kovacs, R. Csaki, E. Hunyadi-Gulyas, E. Klement, et al. 2004. Modular broad-host-range expression vectors for single-protein and protein complex purification. Appl. Environ. Microbiol. 70:712-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerstel, U., and U. Romling. 2003. The csgD promoter, a control unit for biofilm formation in Salmonella typhimurium. Res. Microbiol. 154:659-667. [DOI] [PubMed] [Google Scholar]
- 22.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, et al. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 23.Hoyle, B. D., and J. W. Costerton. 1991. Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37:91-105. [DOI] [PubMed] [Google Scholar]
- 24.Jensen, C., S. Ethelberg, A. Gervelmeyer, E. M. Nielsen, K. E. Olsen, et al. 2006. First general outbreak of Verocytotoxin-producing Escherichia coli O157 in Denmark. Euro Surveill. 11:55-58. [PubMed] [Google Scholar]
- 25.Karlyshev, A. V., M. J. Pallen, and B. W. Wren. 2000. Single-primer PCR procedure for rapid identification of transposon insertion sites. Biotechniques 28:1078-1082. [DOI] [PubMed] [Google Scholar]
- 26.Keene, W. E., K. Hedberg, D. E. Herriott, D. D. Hancock, R. W. McKay, et al. 1997. A prolonged outbreak of Escherichia coli O157:H7 infections caused by commercially distributed raw milk. J. Infect. Dis. 176:815-818. [DOI] [PubMed] [Google Scholar]
- 27.Keene, W. E., J. M. McAnulty, F. C. Hoesly, L. P. Williams, Jr., K. Hedberg, et al. 1994. A swimming-associated outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7 and Shigella sonnei. N. Engl. J. Med. 331:579-584. [DOI] [PubMed] [Google Scholar]
- 28.Kulkarni, H., P. N. Goldwater, A. Martin, and K. A. Bettelheim. 2002. Escherichia coli ‘O’ group serological responses and clinical correlations in epidemic HUS patients. Comp. Immunol. Microbiol. Infect. Dis. 25:249-268. [DOI] [PubMed] [Google Scholar]
- 29.Kumar, C. G., and S. K. Anand. 1998. Significance of microbial biofilms in food industry: a review. Int. J. Food. Microbiol. 42:9-27. [DOI] [PubMed] [Google Scholar]
- 30.Kwon, Y. M., and S. C. Ricke. 2000. Efficient amplification of multiple transposon-flanking sequences. J. Microbiol. Methods 41:195-199. [DOI] [PubMed] [Google Scholar]
- 31.Lee, Y. J., Y. Kim, S. Yeom, S. H. Kim, S. Park, et al. 2008. The role of disulfide bond isomerase A (DsbA) of Escherichia coli O157:H7 in biofilm formation and virulence. FEMS Microbiol. Lett. 278:213-222. [DOI] [PubMed] [Google Scholar]
- 32.Lemos, J. A., T. A. Brown, Jr., and R. A. Burne. 2004. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect. Immun. 72:1431-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim, J. Y., H. J. La, H. Sheng, L. J. Forney, and C. J. Hovde. 2010. Influence of plasmid pO157 on Escherichia coli O157:H7 Sakai biofilm formation. Appl. Environ. Microbiol. 76:963-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 35.Makin, S. A., and T. J. Beveridge. 1996. The influence of A-band and B-band lipopolysaccharide on the surface characteristics and adhesion of Pseudomonas aeruginosa to surfaces. Microbiology 142:299-307. [DOI] [PubMed] [Google Scholar]
- 36.Mannix, M., D. Whyte, E. McNamara, N. O'Connell, R. Fitzgerald, et al. 2007. Large outbreak of E. coli O157 in 2005, Ireland. Euro Surveill. 12. [DOI] [PubMed]
- 37.Marsollier, L., P. Brodin, M. Jackson, J. Kordulakova, P. Tafelmeyer, et al. 2007. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathog. 3:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mermin, J. H., and P. M. Griffin. 1999. Public health in crisis: outbreaks of Escherichia coli O157:H7 infections in Japan. Am. J. Epidemiol. 150:797-805. [DOI] [PubMed] [Google Scholar]
- 40.Metcalf, W. W., W. H. Jiang, and B. L. Wanner. 1994. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6Kλ origin plasmids at different copy numbers. Gene 138:1-7. [DOI] [PubMed] [Google Scholar]
- 41.Michino, H., K. Araki, S. Minami, S. Takaya, N. Sakai, et al. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150:787-796. [DOI] [PubMed] [Google Scholar]
- 42.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 43.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 44.Pan, Y., F. Breidt, Jr., and S. Kathariou. 2006. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microbiol. 72:7711-7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng, J. S., W. C. Tsai, and C. C. Chou. 2002. Inactivation and removal of Bacillus cereus by sanitizer and detergent. Int. J. Food Microbiol. 77:11-18. [DOI] [PubMed] [Google Scholar]
- 46.Perna, N. T., G. I. Plunkett, V. Burland, B. Mau, J. D. Glasner, et al. 2001. Genome sequence of enterohaemorrahgic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 47.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 48.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, et al. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 49.Rangel, J. M., P. H. Sparling, C. Crowe, P. M. Griffin, and D. L. Swerdlow. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, et al. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 51.Ryu, J. H., H. Kim, J. F. Frank, and L. R. Beuchat. 2004. Attachment and biofilm formation on stainless steel by Escherichia coli O157:H7 as affected by curli production. Lett. Appl. Microbiol. 39:359-362. [DOI] [PubMed] [Google Scholar]
- 52.Scher, K., U. Romling, and S. Yaron. 2005. Effect of heat, acidification, and chlorination on Salmonella enterica serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 71:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shemesh, M., A. Tam, and D. Steinberg. 2007. Differential gene expression profiling of Streptococcus mutans cultured under biofilm and planktonic conditions. Microbiology 153:1307-1317. [DOI] [PubMed] [Google Scholar]
- 54.Snider, J. L., C. Allison, B. H. Bellaire, R. L. Ferrero, and J. A. Cardelli. 2008. The beta1 integrin activates JNK independent of CagA, and JNK activation is required for Helicobacter pylori CagA+-induced motility of gastric cancer cells. J. Biol. Chem. 283:13952-13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swerdlow, D. L., B. A. Woodruff, R. C. Brady, P. M. Griffin, S. Tippen, et al. 1992. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann. Intern. Med. 117:812-819. [DOI] [PubMed] [Google Scholar]
- 56.Taylor, C. M., M. Beresford, H. A. Epton, D. C. Sigee, G. Shama, et al. 2002. Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. J. Bacteriol. 184:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tischler, A. D., and A. Camilli. 2004. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53:857-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres, A. G., C. Jeter, W. Langley, and A. G. Matthysse. 2005. Differential binding of Escherichia coli O157:H7 to alfalfa, human epithelial cells, and plastic is mediated by a variety of surface structures. Appl. Environ. Microbiol. 71:8008-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tu Quoc, P. H., P. Genevaux, M. Pajunen, H. Savilahti, C. Georgopoulos, et al. 2007. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect. Immun. 75:1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uhlich, G. A., P. H. Cooke, and E. B. Solomon. 2006. Analyses of the red-dry-rough phenotype of an Escherichia coli O157:H7 strain and its role in biofilm formation and resistance to antibacterial agents. Appl. Environ. Microbiol. 72:2564-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vianney, A., G. Jubelin, S. Renault, C. Dorel, P. Lejeune, et al. 2005. Escherichia coli tol and rcs genes participate in the complex network affecting curli synthesis. Microbiology 151:2487-2497. [DOI] [PubMed] [Google Scholar]
- 62.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, et al. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]