Abstract
We set up a polarized cell culture model to study the pathogenicity of a common respiratory tract pathogen, Chlamydia pneumoniae. Immunofluorescence staining of ZO-1 (a tight junction protein) and Na+K+ ATPase (a protein pump localized at the basolateral membrane in the polarized epithelial cells), as well as TER measurements, suggested that the filter-grown Calu-3 cells, but not the A549 cells, were polarized when grown on collagen-coated membranes. Both the flat and the filter-grown cultures were infected with C. pneumoniae. Infection in the polarized Calu-3 cultures produced more C. pneumoniae genome equivalents than infection in the flat cultures. However, this progeny was not as infective as that in the flat cultures. The maximum amount of C. pneumoniae was detected at 6 days postinfection in the filter-grown A549 cells, indicating a slower developmental cycle than that observed in the flat A549 cultures. The effect of cycloheximide on the growth of C. pneumoniae in the polarized cells was negligible. Furthermore, the infection in the polarized Calu-3 cells was resistant to doxycycline, and several cytokines were released mainly on the apical side of the polarized cells in response to C. pneumoniae infection. These findings indicate that the growth of chlamydiae was altered in the filter-grown epithelial culture system. The diminished production of infective progeny of C. pneumoniae, together with the resistance to doxycycline and polarized secretion of cytokines from the infected Calu-3 cells, suggests that this model is useful for examining epithelial cell responses to C. pneumoniae infection, and it might better resemble in vivo infection in respiratory epithelial cells.
Chlamydia pneumoniae is a common respiratory tract bacterium that infects most people at some stage of their life. The clinical symptoms of C. pneumoniae infection can be negligible, or they may include respiratory tract diseases ranging from mild flu to severe pneumonia, the latter especially in elderly or immunocompromised patients (12). Although C. pneumoniae was identified as its own bacterial species within the genus Chlamydia 20 years ago (13), much of its pathogenicity still remains to be revealed.
The epithelium of the respiratory tract constitutes a physical barrier and the first line of host defense that C. pneumoniae encounters. It consists of ciliated pseudostratified/simple columnar epithelial cells and mucus-producing goblet cells. The alveoli are lined by squamous type 1 pneumocytes. Surfactant-producing type II pneumocytes are typically found at the alveolar-septal junctions, and macrophages are present in the alveoli. During murine experimental intranasal infection, C. pneumoniae can be cultured from lung homogenates (19, 30), and immunohistochemistry has revealed chlamydial inclusions in bronchial epithelial cells and macrophages (26, 43). Also in humans, the respiratory epithelial cells, as well as the alveolar macrophages, represent the primary target cells of C. pneumoniae, and yet the bacterium is able to invade and infect several different cell types (8, 11, 21).
Epithelial cells lining the respiratory tract are architecturally structured in a polarized orientation with distinct apical and basal faces. Lateral contacts between the epithelial cells, together with adhesion to the extracellular matrix, lead to a reorganization of the cell cytoskeleton and a distinct distribution of both apical and basolateral proteins on different cell membranes (44). Formation of a proper functional barrier between the apical and basal compartments of the epithelial cell layer is also crucial. In polarized epithelial cells, a tight junction serves as a specialized intercellular junctional complex that mediates adhesion between the cells and regulates diffusion through the intercellular space (33). Various cell culture models have been extensively used to study the characteristics and pathogenesis of chlamydial infections, but most published papers with epithelial cell lines describe infection in cell cultures grown on impermeable plastic or glass supports, in which case the membrane asymmetry of a polarized cell and proper cell-cell contacts that form the tight barrier across the cell layer are lacking.
One model that simulates naturally occurring cell polarization is achieved in vitro when the epithelial cells are cultured on permeable membranes that permit cells to feed basolaterally and allow formation of distinct apical and basolateral surfaces. Such polarized cell cultures more closely resemble the in vivo situation and have proven useful in studies on several aspects of C. trachomatis infection (38). For instance, C. trachomatis entry into epithelial cells (39), the growth and production of infective progeny (35, 39), properties of persistent infection (17, 18), and even antimicrobial drug effects (40) are altered in polarized cell cultures. Although the polarized epithelial cell culture model mimicking the natural epithelial cell barrier appears to be a rational choice for studying the pathogenesis of respiratory tract infections, there are no reports of its use with C. pneumoniae infection. Therefore, in the present study, we set up a polarized cell culture model for C. pneumoniae using two different transformed lung epithelial cell lines. Human lung carcinoma cell line A549 exhibits metabolic and transport properties of type II pulmonary epithelial cells (7). This cell line is widely used in polarized cell studies, although some reports show that these cells do not necessarily form functional tight junctions when grown on permeable supports (15, 37). Calu-3 cells, a lung adenocarcinoma cell line, possess characteristics of serous gland epithelial cells (5, 22). The Calu-3 cell line is reported to form a strong functional barrier in a polarized culture (6, 37). Both cell lines have previously been shown to be susceptible to C. pneumoniae infection when grown on impermeable supports (9, 42). The purpose of the present study was to characterize C. pneumoniae infection in filter-grown/polarized cultures of respiratory epithelial cells. We demonstrate that the epithelial cells cultured on a semipermeable membrane are a suitable model for studying C. pneumoniae infection. Our results show that the characteristics of infection (i.e., kinetics, production of infectious progeny, and sensitivity to antimicrobials) might drastically differ between models that use flat and filter-grown cells.
MATERIALS AND METHODS
Cell lines and culture.
A549 (ATCC CCL-185) and Calu-3 (ATCC HTB-55), obtained from the American Type Culture Collection (LGC Promochem AB), were used in these studies. The culture medium for A549 cells was F-12K nutrient mixture Kaighn's modification with l-glutamine (Gibco) and, for Calu-3 cells, minimal essential medium alpha medium with Earle's salts and l-glutamine was used. Both media were supplemented with 10% fetal bovine serum (FBS) and 20 μg of gentamicin/ml. HL cells (a human epithelial cell line susceptible to C. pneumoniae) were grown in Dulbecco modified Eagle medium (Sigma) with 10% FBS and 20 μg of gentamicin/ml. The cell cultures were maintained in a humidified air chamber at 37°C with 5% CO2. For the polarized cell experiments, the cell lines were transferred onto 6.5-mm Transwell permeable support inserts with a 0.4-μm pore size, coated with Collagen from rat tail (Sigma), and placed in 24-well plates. The epithelial cells were plated at a density of 2 × 105 cells per insert, and the Calu-3 cells were allowed to grow for 10 to 14 days to polarize. To compare the polarized cultures (referred to here as “filter grown”) to the conventional monolayer cultures (referred to here as “flat”), epithelial cells were also transferred to and grown on the bottom of the 24-well plates at a density of 5 × 105 cells per well. Alternatively, for microscopic visualization of the monolayer cultures, the cells were plated on glass coverslips (13 mm in diameter) in 24-well plates. Experiments with the monolayer cultures were started on the next day after plating. Fresh culture medium for the cell cultures in the 24 wells was changed two to three times per week. Millicell-ERS (electrical resistance system; Millipore) was used according to the manufacturer's instructions to measure the transepithelial resistance (TER) of the cells grown on the Transwell inserts to detect formation of tight junctions that suggests development of a polarized cell layer.
Chlamydia strain and infection.
C. pneumoniae isolate K6 (3) (obtained from Pekka Saikku, University of Oulu, Oulu, Finland) was propagated in the HL cells and roughly purified by ultrasonication, followed by one cycle of low- and high-speed centrifugation to remove cell debris and concentrate chlamydiae. The obtained stock was diluted in sucrose phosphate glutamate (SPG) buffer, divided into aliquots, and stored at −70°C. C. pneumoniae and the cell lines were free of mycoplasma, as detected with a VenorGeM mycoplasma PCR detection kit (Minerva Biolabs).
If not stated otherwise, the epithelial cell cultures on the Transwell inserts and on the glass coverslips were inoculated with 2 × 105 and 5 × 105 inclusion-forming units (IFU), respectively, reaching a multiplicity of infection of ∼1. The plates were centrifuged at 515 × g for 1 h and incubated at 37°C for 1 h before a fresh culture medium with or without cycloheximide (0.5 μg/ml) was added. For cultures longer than 3 days, fresh culture medium was changed twice a week.
Analysis of chlamydial growth and production of infective progeny.
Filter-grown and flat cultures of both cell lines were infected with C. pneumoniae and incubated with or without cycloheximide for 2, 24, 48, or 72 h or for 6 days. At these times, infected cells were harvested by scraping mechanically with a pipette tip in 400 μl of SPG buffer and frozen at −70°C until further processed. In order to study infectivity, the collected infected cell samples were sonicated for 15 min in a water bath, and 50 μl of the sample was passaged onto a fresh layer of HL cells. Infection was performed as described above with centrifugation and incubation. The HL cells were incubated at 37°C in 5% CO2 for 72 h in the presence of cycloheximide to ensure optimal growth of the infectious progeny and collected using 400 μl of MagNA pure bacteria lysis buffer (Roche). To analyze the productivity of the infection, the amount of C. pneumoniae genome equivalents (GEs) obtained after the passage in the HL cells was adjusted to the whole sample size and divided by the number of GEs present in the primary culture (i.e., the fold change in GE numbers). According to our previous studies, the change in GE numbers can be used to estimate and compare amounts of infectious particles present in the primary cultures (24). All of the analyses were done in duplicate wells and were repeated once or twice with similar results.
Real-time quantitative PCR.
Total DNA from the samples collected after primary infection and repassage was isolated with a MagNA pure compact instrument using a MagNA pure compact nucleic acid isolation kit I and DNA Bacterial protocol (Roche Diagnostics). The concentration of total DNA in the extracted samples was measured by NanoDrop 1000 spectrophotometer (Thermo Scientific). C. pneumoniae genomes were quantified by using a real-time quantitative PCR targeting C. pneumoniae outer membrane protein gene ompA, as described in detail previously (24), with some modifications. The oligonucleotide primers and probe were from TAG Copenhagen, and the concentration of the probe in the analysis was 200 nM. Amplification and detection were performed by using an Applied Biosystems 7500 real-time PCR system, and absolute quantification of the results was analyzed with sequence detection software version 1.3.1.21 (Applied Biosystems). The results are presented as the number of C. pneumoniae GE/total DNA (ng) in the sample.
Immunofluorescence staining.
For confocal and immunofluorescence microscopy, the filter-grown or flat cell cultures were fixed with 3% paraformaldehyde for 15 min and stored in phosphate-buffered saline until stained. For optimal fluorescence staining, the fixed cells were first incubated in 0.01% citrate buffer in a boiling water bath for 1 h, permeabilized with 0.2% Triton-X, and blocked with bovine serum albumin and Tween 20. The primary antibodies used were mouse anti-C. pneumoniae, 41654-100 (Abcam); mouse anti-Na+K+ ATPase a-1, clone C464.6 (Millipore); and rabbit anti-ZO-1, 61-7300 (Zymed). The secondary antibodies were goat anti-mouse Alexa Fluor 488 and goat anti-rabbit Alexa Fluor 488 (Molecular Probes). Both the primary and the secondary antibodies were incubated for 1 h at room temperature, after which the nuclei were stained with either DAPI (4′,6′-diamidino-2-phenylindole) or Topro-3 (Molecular Probes).
Secreted inflammatory mediators.
Interleukin-8 (IL-8) in a cell culture medium was measured by using a commercial Human IL-8 enzyme-linked immunosorbent assay kit (Sanquin) according to the manufacturer's instructions. Samples for IL-8 assays were diluted 1:300, and a commercial TMB Xtra substrate (Kem-En-Tec) was used to detect the signal. A panel of cytokines, chemokines, and growth factors in the cell culture medium of flat and filter-grown cells was analyzed with a human cytokine Panel A Array kit (R&D Systems) according to the manufacturers' instructions using 1 ml of the cell culture supernatant as a sample. The cytokine spots were visualized with an Immun-Star chemiluminescence kit (Bio-Rad) and detected using a FluorChem HD2 imaging system (Alpha Innotech Corp.). Spot densitometry analysis was done with AlphaEase FC Software 6.0.0 (Alpha Innotech Corp.). The spot density data are presented by the software as integrated density values (IDVs). The IDV is the sum of all of the pixel values within a determined spot area after background correction. The obtained densitometry values were adjusted to the values of the positive control spots of each membrane, and the results are presented as a percentage of the positive control IDV.
In vitro activity of doxycycline against C. pneumoniae.
To analyze the MIC of doxycycline for C. pneumoniae in filter-grown and flat cell cultures, A549 cells were inoculated with 2 × 103 IFU per well, and Calu-3 cells were inoculated with 1 × 105 IFU per well, to obtain an optimal number of inclusions to be counted under fluorescence microscopy. The cultures were centrifuged and incubated as described above, after which fresh culture medium without gentamicin, but with 2-fold dilutions of doxycycline added. Concentrations of doxycycline (doxycycline hyclate; Sigma) between 0.031 and 2 μg/ml were analyzed. At 72 h postinfection (p.i.), the infected cells were fixed with 3% paraformaldehyde, permeabilized, and stained with Chlamydia species-specific fluorescein isothiocyanate-conjugated antibody (Pathfinder Chlamydia Culture Confirmation System; Bio-Rad Laboratories), and the inclusions were counted under fluorescence microscopy. The doxycycline concentration that reduced the inclusion count by at least 95% compared to the placebo-exposed control cells was set as the MIC.
To determine the minimal bactericidal concentration (MBC), the cell cultures were inoculated with C. pneumoniae and exposed to doxycycline as described above. After 72 h, the infected cells were scraped off mechanically in SPG and frozen at −70°C. The thawed samples were sonicated and inoculated onto fresh monolayers of HL cells grown on glass coverslips as described above, and fresh culture medium without antibiotics was added. After 72 h, the centrifugation and incubation were repeated, fresh culture medium was changed, and the epithelial cells were incubated for another 72 h to promote growth of possible latent chlamydial particles present in the passage. The infected cultures were then fixed and stained as described above. The MBC was set at the doxycycline concentration at which no inclusions were detected in the HL cells.
TEM.
Filter-grown A549 and Calu-3 cells were inoculated with 5 × 105 and 1 × 106 C. pneumoniae IFU, respectively, to obtain sufficient numbers of chlamydial inclusions for electron microscopy. At 72 h or at 6 days p.i., the infected cell cultures were washed and the samples for thin-sectioning were prepared as described previously by Lounatmaa (23). Briefly, the samples were prefixed in phosphate-buffered (pH 7.2) 2.5% glutaraldehyde with or without tannic acid for 2 h at room temperature. The fixed cells were washed three times with phosphate buffer. All of the samples were postfixed with phosphate-buffered 1% osmium tetroxide, dehydrated in an acetone series, and embedded in Taab resin. Cross-sections of the cell layer ca. 50 to 60 nm thick were cut, and the thin-sectioned cells were post-stained with uranyl acetate and lead citrate. The transmission electron microscopy (TEM) micrographs were taken with JEM-1200EX at 60 kV.
RESULTS
Development of cell polarization.
To analyze whether the studied epithelial cell cultures were truly polarized in our hands, the TER was measured across the filter-grown cell layer at different times after the epithelial cells were seeded on the membranes. The Calu-3 cells reached TER values between 180 and 305 Ω/cm2 at 14 days, whereas no increase in the TER was seen in the A549 cells during this observation period; the measured values ranged from 17 to 30 Ω/cm2.
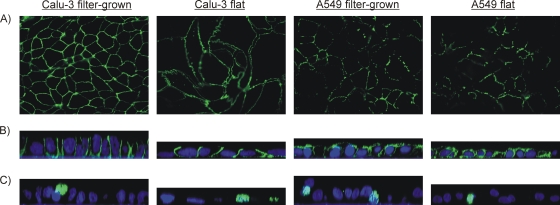
In addition, epithelial polarization was assessed by detecting the localization of certain cellular proteins. We stained for a tight junction protein zona occludens 1 (ZO-1), which is commonly used to visualize formation of proper tight junctions in cell cultures (36), and for the Na+K+ ATPase protein pump, known to localize at the basolateral membranes in polarized epithelial cells (29, 44). Both of these proteins were visualized with green Alexa 488-conjugated secondary antibodies. Fluorescence staining patterns of ZO-1 in flat and filter-grown Calu-3 cells showed a continuous net of ZO-1 staining (Fig. 1A), also suggesting the presence of functional tight junctions in the Calu-3 cells. In the A549 cultures, however, only fragmented nets of ZO-1 were seen. In addition, ZO-1 staining in the A549 cell layer fluctuated vertically, which made it difficult to focus on the net with fluorescence microscopy. In accordance with these findings, the localization of the Na+K+ ATPase pump on the basolateral membranes in the Calu-3 cells, as shown in Fig. 1B, indicates polarization of these cells but not of the A549 cells, where the staining for the Na+K+ ATPase pump was found in the apical membranes as well. Generally, the growth of Calu-3 cells on impermeable culture supports resembled that of polarized cells. However, these cells were not columnar and narrow in diameter like the polarized cells, but flat with a rather wide apical surface area.
FIG. 1.
Expression and localization of polarization marker proteins and C. pneumoniae by immunofluorescence staining of filter-grown and flat Calu-3 and A549 cell cultures. (A) Tight-junction protein ZO-1 with Alexa 488 examined by fluorescence microscopy; (B) Na+K+ ATPase pump with Alexa 488 (green) and nuclei with Topro-3 (blue) examined by confocal microscopy; (C) C. pneumoniae with Alexa 488 (green) and nuclei with Topro-3 (blue) examined by confocal microscopy.
Chlamydial growth.
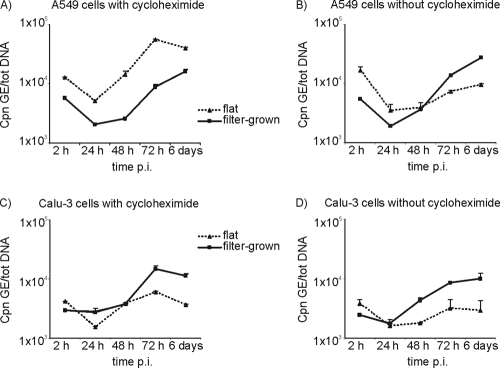
The growth of C. pneumoniae was studied in both the filter-grown and flat monolayer cultures of the A549 and Calu-3 cells. Cycloheximide significantly increased the growth of C. pneumoniae, measured as an increase in the C. pneumoniae GE numbers, in the flat cultures of both cell lines (Fig. 2). In the filter-grown A549 cultures, however, the lack of cycloheximide did not much affect the replication of C. pneumoniae, and nearly identical growth curves were obtained with or without the exposure (Fig. 2A versus 2B). The C. pneumoniae GE numbers at 6 days p.i. were 14.1 or 14.4 times higher than at 24 h p.i. in the filter-grown A549 cell cultures with or without cycloheximide, respectively. In the polarized Calu-3 cells, cycloheximide-exposed cultures produced higher genome numbers: at 6 days p.i. an 8.7-fold and a 4.2-fold increase in the genome numbers was seen with or without cycloheximide, respectively (P = 0.33 [Mann-Whitney U test]). Thus, cycloheximide had no effect on the replication of C. pneumoniae in the filter-grown A549 cultures and a slight effect on the polarized Calu-3 cells (Fig. 2C versus 2D).
FIG. 2.
C. pneumoniae (Cpn) growth curves presented as genome equivalents (GE) divided by the amount of total nucleic acids (tot DNA) per sample at 2, 24, 48, and 72 h and at 6 days p.i. Averages of duplicate samples and standard error of means are presented. Growth in flat (▴) and filter-grown (▪) A549 cells with (A) and without (B) cycloheximide and growth in flat (▴) and filter-grown (▪) Calu-3 cells with (C) or without (D) cycloheximide.
The growth of C. pneumoniae in the flat and filter-grown A549 cell cultures was similar when analyzed as C. pneumoniae GEs at the indicated times. The highest number of C. pneumoniae GEs on the filter-grown cultures was detected at 6 days p.i., indicating a slower growth rate and developmental cycle in this culture model (Fig. 2A). Times between 72 h and 6 days, i.e., at 84 and 96 h, were analyzed as well to determine whether the increase between 3 and 6 days p.i. was due to a second developmental cycle. However, this was not the case, since no decrease in the intracellular chlamydia GEs was seen during this period of infection (results not shown).
The Calu-3 cells and especially the polarized Calu-3 cells were more resistant to chlamydial infection than the A549 cells, and there were markedly fewer C. pneumoniae GEs detected at 2 h p.i. (after inoculation and washing) than in the corresponding A549 cultures, suggesting lower numbers of EBs attached to the Calu-3 cells than to the A549 cells (Fig. 2A versus 2C and Fig. 2B versus 2D). However, the polarized Calu-3 cell cultures produced similar levels of chlamydia GEs at 72 h p.i. as the filter-grown A549 at this time and, overall, more chlamydiae than the flat Calu-3 cultures (Fig. 2C).
Production of infective progeny.
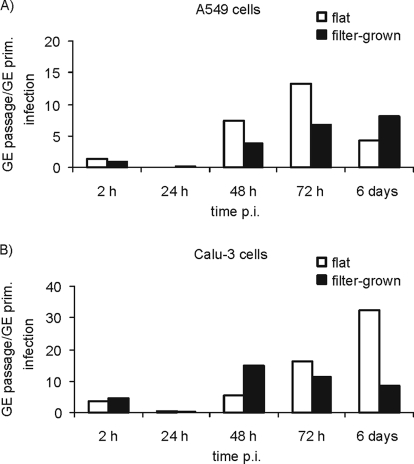
In accordance with the above results from the primary infection, the flat A549 cultures produced higher amounts of infectious C. pneumoniae EB than the filter-grown A549 cultures. This is shown as a fold change in the number of GEs (the number of GEs after passage versus the number of GEs in the primary culture) in Fig. 3A. In the filter-grown A549 cultures, the highest amount of infectious particles, detected as a fold change in GEs, was present at 6 days p.i., further suggesting a slower growth rate of C. pneumoniae in this model. In the Calu-3 cells, the chlamydiae detected in the polarized cultures at 72 h and at 6 days p.i. were not as infectious as those detected in the flat cultures at 72 h and at 6 days p.i. (Fig. 3B).
FIG. 3.
Fold change in C. pneumoniae GE numbers after passage of flat (□) and filter-grown (▪) primary cell cultures at different times postinfection. The numbers of C. pneumoniae genome equivalents (GEs) obtained after passage on HL cells were adjusted to the whole sample size and divided by the number of GEs present in the primary culture. Results obtained with A549 cells (A) and Calu-3 cells (B) grown with cycloheximide are shown.
Five-week follow-up.
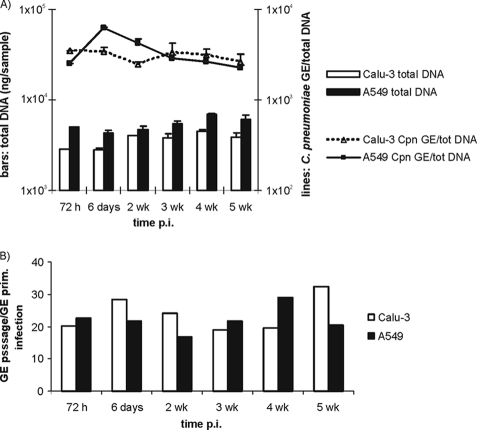
In a separate experiment, C. pneumoniae infection without cycloheximide exposure was monitored up for 5 weeks after the primary infection in both of the filter-grown cell lines to analyze chlamydial growth over a longer term. As shown in Fig. 4A, the number of cells in both cell lines remained steady, as measured by the amount of DNA, throughout the 5-week follow-up. The number of C. pneumoniae GEs in the Calu-3 cells were sustained at a rather constant level, whereas in the A549 cells, the numbers of chlamydiae slowly decreased after the peak reached at 6 days. In the A549 cultures, the cells grew on multiple layers, and dead cell debris was released into the supernatant throughout the follow-up period, as detected by light microscopy. Analysis of the samples inoculated onto fresh HL cells revealed a 20- to 30-fold increase in the amount of chlamydial GEs upon repassage, suggesting that rather stable amounts of infectious chlamydial particles were present in both cell cultures during the extended follow-up period (Fig. 4B).
FIG. 4.
Five-week follow-up of C. pneumoniae (Cpn) infection in filter-grown Calu-3 and A549 cell cultures. (A) Bars represent the amount of total DNA per sample in Calu-3 (□) and A549 (▪) cultures collected at different times postinfection. Lines represent the number of C. pneumoniae genome equivalents (GE) divided by the amount of the total DNA. Averages of duplicate samples and standard errors of the mean are presented. (B) Fold change in C. pneumoniae GE numbers after passage from a 5-week follow-up calculated as described in Fig. 3.
Secretion of inflammatory mediators.
IL-8 is a common inflammatory cytokine produced by epithelial cells in response to chlamydial infections. We analyzed the levels of IL-8 in the cell culture supernatants of both the flat and the filter-grown cultures. A cumulative increase in IL-8 levels between 2 h and 72 h p.i. was seen in both epithelial cell lines and culture models (results not shown). Interestingly, the polarized Calu-3 cell cultures secreted higher amounts of IL-8 to the apical than to the basal medium of the culture, as shown in Fig. 5. This was not seen in the infected A549 cultures, where most of the IL-8 was found in the basal medium (results not shown). Of note, also the uninfected filter-grown Calu-3 cells produced measurable IL-8 levels at 72 h after mock inoculation and again, preferably into the apical medium (Fig. 5).
FIG. 5.
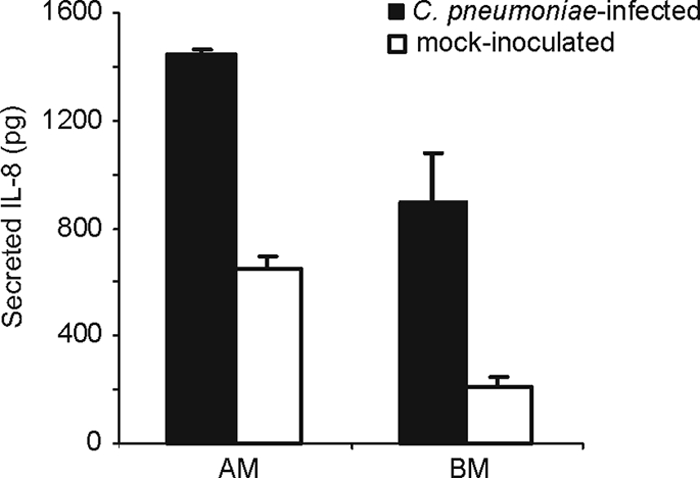
Cumulative secretion of IL-8 into apical media (AM) and basal media (BM) of filter-grown Calu-3 cells at 72 h after C. pneumoniae infection (▪) or inoculation with sucrose phosphate-buffered salt solution (SPG) used to dilute the chlamydia stock (□). Averages of four parallel samples and standard errors of the mean are presented.
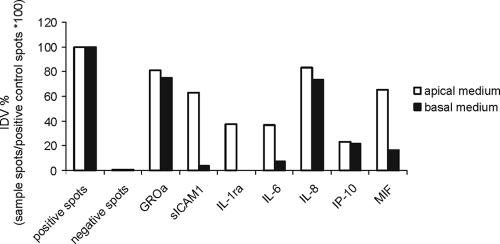
To study whether other inflammatory mediators were secreted in a polarized manner, we analyzed the apical and basal medium of the polarized Calu-3 cells at 24 and at 72 h p.i. with a cytokine array kit capable of detecting 36 cytokines from a single supernatant sample. The cytokines and chemokines that were repeatedly detected in marked amounts in the culture medium at 24 h p.i. were growth-related oncogene alpha (GROα), soluble intercellular adhesion molecule-1 (sICAM1), IL-8, and macrophage migration inhibitory factor (MIF). Cytokines detected at 72 h p.i. included IL-1 receptor antagonist (IL-1ra), IL-6, and gamma interferon-inducible protein-10 (IP-10), in addition to those already detected at 24 h. Figure 6 shows the IDVs for chemiluminescence spots for the above-mentioned cytokines and chemokines in the apical and basal medium of a polarized Calu-3 culture at 72 h p.i. Four of these—sICAM1, IL-1ra, IL-6, and MIF—were found to be more apically than basally secreted. All of the above-mentioned inflammatory mediators were also found in the medium of the flat Calu-3 cell cultures. Therefore, the secreted cytokine profiles of the filter-grown and flat Calu-3 cell cultures do not seem to differ.
FIG. 6.
Expression of cytokines and chemokines in apical (□) and basal (▪) media of a polarized Calu-3 culture at 72 h after C. pneumoniae infection. Expression was analyzed with a cytokine array kit producing spots that are visualized by chemiluminescence. IDVs from the spots are adjusted with the positive control spots of each membrane, and the results are presented as a percentage of the positive control spot IDV.
Doxycycline susceptibility.
The doxycycline MIC and MBC for C. pneumoniae as determined in the present study are presented in Table 1. The MIC values in both cell lines and culture models were from 0.125 to 0.25 μg/ml. However, C. pneumoniae growing in the polarized Calu-3 cultures appeared to be very resistant to doxycycline exposure (MBC > 2 μg/ml), whereas in the flat Calu-3 cultures, an MBC as low as 0.06 μg/ml was detected. The inhibitory concentrations are in accordance with those reported earlier for some C. pneumoniae isolates (10) but are somewhat higher than the more recently reported doxycycline MIC values for C. pneumoniae (2, 34), most probably due to the different evaluation methods used.
TABLE 1.
MICs and MBCs of doxycycline on C. pneumoniae in flat- and filter-grown A549 and Calu-3 cultures
| Cell line and culture model | Concn (μg/ml) |
|
|---|---|---|
| MIC | MBC | |
| A549 | ||
| Flat | 0.25 | 0.5 |
| Filter grown | 0.125 | 0.125 |
| Calu-3 | ||
| Flat | 0.125 | 0.06 |
| Filter grown | 0.125 | >2 |
Electron microscopy.
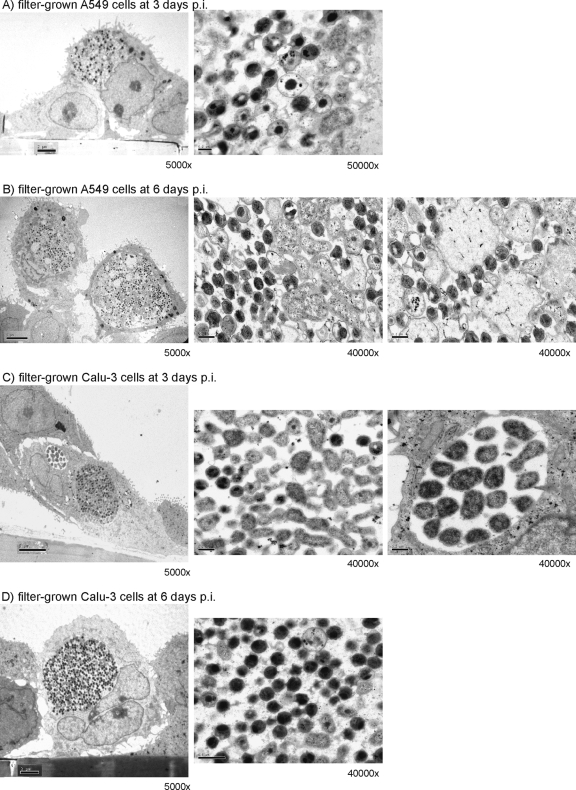
TEM of the filter-grown infected A549 cells showed immature chlamydial inclusions with significant amounts of dividing chlamydial particles, reticulate bodies (RBs), within the inclusions at 72 h p.i. (Fig. 7A). This is in accordance with the findings on chlamydial growth indicating a delayed or longer developmental cycle in the filter-grown A549 cells. After 6 days p.i., more mature elementary bodies (EBs) were seen, but also wide inclusion areas were filled with a granular matrix (Fig. 7B). In the filter-grown infected Calu-3 cells, large inclusions with high numbers of mature EB particles were found at 3 days p.i., but also small inclusions with low numbers of dividing RBs were present, indicating asynchronous growth (Fig. 7C). At 6 days p.i., large inclusions filled mostly with EB particles were detected in these cultures (Fig. 7D).
FIG. 7.
TEM images of C. pneumoniae inclusions in filter-grown A459 and Calu-3 cells at 72 h and at 6 days p.i. (A) A549 cells at 3 days p.i.; (B) A549 cells at 6 days p.i.; (C) Calu-3 cells at 3 days p.i.; (D) Calu-3 cells at 6 days p.i. The scale bar at the ×5,000 magnification is 2 μm, and at the ×40,000 and ×50,000 magnifications it is 0.2 μm.
DISCUSSION
In the present study, C. pneumoniae infection was characterized in two human respiratory epithelial cell lines, A549 and Calu-3. These cells were grown on impermeable glass or plastic surfaces and on semipermeable filters that allow formation of apical and basolateral faces, as well as tight junctions between the cells to mimic the airway epithelial barrier. In accordance with earlier studies, the Calu-3 cells grown on the filters developed TER after 2 weeks of culture, whereas the A549 cells grown on filters failed to polarize (7, 37). Formation of continuous tight junctions between cells separating the apical and basolateral plasma membranes is highly important for maintaining polarization and vectorial transport in epithelial cells. Proper cell-cell contacts in the polarizing culture initiates distribution of plasma membrane proteins on either apical or basolateral domains. After cytoskeletal reassembly in a polarized epithelial cell, a fodrin-based membrane skeleton is localized to the sites of cell adhesion at cadherin/catenin complexes and the Na+K+ ATPase is bound to these sites with high affinity through ankyrin (44). Immunofluorescence staining of the Na+K+ ATPase, as well as the tight-junction protein ZO-1, clearly showed that filter-grown Calu-3 cells, but not A549 cells, were truly polarized. Other commonly used human epithelial cell lines, HL and HeLa 299, which are very susceptible to C. pneumoniae, failed to develop TER (results not shown).
The polarized Calu-3 cells produced more C. pneumoniae than the flat cultures, as has been reported earlier for C. trachomatis grown in polarized human endometrial epithelial cells (38). However, C. pneumoniae present in the polarized Calu-3 cells were not very infectious in our study, especially at the later times. This finding is in contrast to the results reported by Wyrick et al. (39) with C. trachomatis; these authors also detected an increase in the infectivity of chlamydiae propagated in polarized cultures. Another interesting finding in the present study was that C. pneumoniae growing in the polarized Calu-3 cells was highly resistant to doxycycline exposure. Taken together, the presence of low numbers of infectious particles and the observed high doxycycline MBC suggest the presence of metabolically inactive, persistent C. pneumoniae particles in the polarized Calu-3 cultures. This is also supported by the results from a 5-week follow-up experiment, where the Calu-3 cell cultures remained stable and constant chlamydia genome numbers, as well as infectious bacteria, could be recovered during the whole observation period. However, aberrant forms of chlamydial particles were not found by electron microscopy. Small and immature chlamydial inclusions containing low numbers of dividing RB particles were detected by TEM at 72 h p.i, together with larger inclusions with a high number of RB particles and few EBs. However, at 6 days p.i., the C. pneumoniae particles inside the inclusions were mostly EBs. The TEM findings indicate that the developmental cycle of C. pneumoniae in the polarized Calu-3 cells is longer than 72 h, although the highest number of chlamydial GEs was detected at 72 h. The presence of immature inclusions and high numbers of RB particles explains well the low infectivity detected at 72 h. Wyrick and Knight (41) have previously speculated that the presence of intact EB particles due to an asynchronous chlamydial developmental cycle might explain treatment failures. This might be one possible explanation for the high MBC detected in the polarized Calu-3 culture in our study, since inclusions containing C. pneumoniae at very different developmental stages were detected by TEM.
In the present study, the developmental cycle of C. pneumoniae in the filter-grown A549 cells appeared to be delayed and the highest chlamydiae genome numbers were detected at 6 days p.i. Inclusions detected by TEM at 72 h p.i. with high amounts of dividing RB particles support this finding. Previously, Guseva et al. (14) reported that the growth rate of C. trachomatis serovar E was faster in polarized human epithelial endometrium-derived HEC-1B cells, most probably due to the shorter lag phase between chlamydial entry into the cell and the beginning of chlamydial cell division. However, the growth rate of an invasive C. trachomatis serovar L2 was not affected by different culture conditions, as reported later by the same group (1). The delayed developmental cycle of C. pneumoniae detected in both cell lines in our study is divergent to these findings but may well be explained by the different chlamydial species and cell lines used. Interestingly, Guseva et al. (14) also reported “patchy” and clustered EB attachment of C. trachomatis serovar E in their three-dimensional culture system, but this was not detected in the case of serovar L2 (1). We found a similar, clustered appearance of C. pneumoniae inclusions in both the polarized and the flat Calu-3 cultures but not in either of the A549 cultures (results not shown). This phenomenon should be further studied in order to determine whether there are certain receptor molecules enriched on various areas of an apical membrane where some chlamydia serovars or species are able to attach, as suggested by Dessus-Babus et al. (1).
The use of cycloheximide did not bring a significant advantage to the growth of chlamydiae in the filter-grown cultures. In the flat monolayer cultures with cell lines such as HL and HeLa 299, chlamydial growth is enhanced by suppressing the host cell functions, for example, with cycloheximide (20, 21). In the case of the A549 cells, cycloheximide had no effect at all on C. pneumoniae growth in the filter-grown cultures, and only a slight increase in the chlamydial GE numbers was seen in the polarized Calu-3 cells when cycloheximide was used. Unlike the filter-grown A549 cultures, the polarized Calu-3 cells do not divide after reaching confluence, and a single cell layer is sustained naturally. Thus, there is no need for host cell suppressing agents in order to enhance chlamydial multiplication. This is an advantage, e.g., when host cell functions such as the secretion of proinflammatory mediators in response to infection are studied.
Analysis of the apical and the basal medium by enzyme immunoassay (for IL-8) and the human cytokine array panel kit suggested that the infected Calu-3 cells secreted newly synthesized cytokines and other mediators of inflammation in a polarized manner. The majority of IL-8, sICAM1, IL-1ra, IL-6, and MIF was secreted apically at 72 h p.i., whereas GROα and IP-10 were secreted into both the apical and the basal media. Such a polarized secretion of IL-8 was not seen in the filter-grown A549 culture. In the present study we describe for the first time that MIF is secreted by epithelial cells upon C. pneumoniae infection; secretion of the other mediators detected here upon chlamydial infection has been reported earlier (4, 16, 25, 27, 31, 32). The significance of the apically directed secretion of the proinflammatory mediators IL-1ra, IL-6, IL-8, and MIF, as well as that of sICAM-1, observed here is not clear and requires further studies. In an earlier report, apical IL-8 expression was suggested to increase transepithelial migration of leukocytes into alveolar spaces (28). On the other hand, insignificant basolateral secretion might aid in suppressing immunological defense, which could thus promote persistence of the bacteria inside the cells. Our hypothesis is that such processes could influence the outcome of C. pneumoniae infection in vivo.
With this study we present the characterization of C. pneumoniae infection in a polarized cell culture model that more closely resembles the in vivo situation. The lung adenocarcinoma cell line Calu-3 was shown to polarize and form functional tight junctions when grown on a semipermeable membrane. The polarized cell layer was more resistant to C. pneumoniae infection, and the production of infective chlamydiae was suppressed in this model. The polarized Calu-3 cell culture was also highly resistant to the antibacterial effect of doxycycline, and polarized secretion of several inflammatory mediators was detected. Although the A549 cells did not produce similar tight functional barriers and polarized monolayers when grown on semipermeable filters, the characteristics of C. pneumoniae infection in the filter-grown cells were different from those in the flat cultures. Despite several drawbacks, including tedious manipulations of the filter-grown cells, difficulties in enumerating inclusions in the filter-grown cells, and economical aspects, infections in the filter-grown cell cultures might more closely mimic the interaction between epithelial cells and C. pneumoniae infection and can thus serve as a useful model. Further, the observed differences in the infection characteristics should be taken into consideration when planning further in vitro experiments on C. pneumoniae infection.
Acknowledgments
This research was supported by the Academy of Finland (grant 110340) and in the framework of the ERA-NET PathoGenoMics, grants 217554/ECIBUG and 130043/ChlamyTrans. This study was also supported by grants from the Jenny and Antti Wihuri Foundation (E.M.).
We thank Tuula Penttilä for the scientific conversations and Anu Haveri for excellent technical assistance.
Editor: B. A. McCormick
Footnotes
Published ahead of print on 29 March 2010.
REFERENCES
- 1.Dessus-Babus, S., C. G. Moore, J. D. Whittimore, and P. B. Wyrick. 2008. Comparison of Chlamydia trachomatis serovar L2 growth in polarized genital epithelial cells grown in three-dimensional culture with non-polarized cells. Microbes Infect. 10:563-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donati, M., G. M. Pollini, M. Sparacino, M. T. Fortugno, E. Laghi, and R. Cevenini. 2002. Comparative in vitro activity of garenoxacin against Chlamydia spp. J. Antimicrob. Chemother. 50:407-410. [DOI] [PubMed] [Google Scholar]
- 3.Ekman, M. R., J. T. Grayston, R. Visakorpi, M. Kleemola, C. C. Kuo, and P. Saikku. 1993. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin. Infect. Dis. 17:420-425. [DOI] [PubMed] [Google Scholar]
- 4.Entrican, G., S. Wattegedera, M. Rocchi, D. C. Fleming, R. W. Kelly, G. Wathne, V. Magdalenic, and S. E. Howie. 2004. Induction of inflammatory host immune responses by organisms belonging to the genera Chlamydia/Chlamydophila. Vet. Immunol. Immunopathol. 100:179-186. [DOI] [PubMed] [Google Scholar]
- 5.Finkbeiner, W. E., S. D. Carrier, and C. E. Teresi. 1993. Reverse transcription-polymerase chain reaction (RT-PCR) phenotypic analysis of cell cultures of human tracheal epithelium, tracheobronchial glands, and lung carcinomas. Am. J. Respir. Cell Mol. Biol. 9:547-556. [DOI] [PubMed] [Google Scholar]
- 6.Forbes, B., and C. Ehrhardt. 2005. Human respiratory epithelial cell culture for drug delivery applications. Eur. J. Pharm. Biopharm. 60:193-205. [DOI] [PubMed] [Google Scholar]
- 7.Foster, K. A., C. G. Oster, M. M. Mayer, M. L. Avery, and K. L. Audus. 1998. Characterization of the A549 cell line as a type II pulmonary epithelial cell model for drug metabolism. Exp. Cell Res. 243:359-366. [DOI] [PubMed] [Google Scholar]
- 8.Gaydos, C. A., J. T. Summersgill, N. N. Sahney, J. A. Ramirez, and T. C. Quinn. 1996. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect. Immun. 64:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gencay, M. M., M. Tamm, A. Glanville, A. P. Perruchoud, and M. Roth. 2003. Chlamydia pneumoniae activates epithelial cell proliferation via NF-κB and the glucocorticoid receptor. Infect. Immun. 71:5814-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnarpe, J., K. Eriksson, and H. Gnarpe. 1996. In vitro activities of azithromycin and doxycycline against 15 isolates of Chlamydia pneumoniae. Antimicrob. Agents Chemother. 40:1843-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godzik, K. L., E. R. O'Brien, S. K. Wang, and C. C. Kuo. 1995. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J. Clin. Microbiol. 33:2411-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayston, J. T., L. A. Campbell, C. C. Kuo, C. H. Mordhorst, P. Saikku, D. H. Thom, and S. P. Wang. 1990. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 161:618-625. [DOI] [PubMed] [Google Scholar]
- 13.Grayston, J. T., C. C. Kuo, L. A. Campbell, and S. P. Wang. 1989. Chlamydia pneumoniae sp. nov. for Chlamydia sp. strain TWAR. Int. J. Syst. Bacteriol. 39:88-90. [Google Scholar]
- 14.Guseva, N. V., S. Dessus-Babus, C. G. Moore, J. D. Whittimore, and P. B. Wyrick. 2007. Differences in Chlamydia trachomatis serovar E growth rate in polarized endometrial and endocervical epithelial cells grown in three-dimensional culture. Infect. Immun. 75:553-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermanns, M. I., R. E. Unger, K. Kehe, K. Peters, and C. J. Kirkpatrick. 2004. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab. Invest. 84:736-752. [DOI] [PubMed] [Google Scholar]
- 16.Högdahl, M., G. Söderlund, and E. Kihlström. 2008. Expression of chemokines and adhesion molecules in human coronary artery endothelial cells infected with Chlamydia (Chlamydophila) pneumoniae. APMIS 116:1082-1088. [DOI] [PubMed] [Google Scholar]
- 17.Kane, C. D., and G. I. Byrne. 1998. Differential effects of gamma interferon on Chlamydia trachomatis growth in polarized and nonpolarized human epithelial cells in culture. Infect. Immun. 66:2349-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kane, C. D., R. M. Vena, S. P. Ouellette, and G. I. Byrne. 1999. Intracellular tryptophan pool sizes may account for differences in gamma interferon-mediated inhibition and persistence of chlamydial growth in polarized and nonpolarized cells. Infect. Immun. 67:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaukoranta-Tolvanen, S. S., A. L. Laurila, P. Saikku, M. Leinonen, L. Liesirova, and K. Laitinen. 1993. Experimental infection of Chlamydia pneumoniae in mice. Microb. Pathog. 15:293-302. [DOI] [PubMed] [Google Scholar]
- 20.Kuo, C. C., and J. T. Grayston. 1988. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J. Clin. Microbiol. 26:812-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuo, C. C., and J. T. Grayston. 1990. A sensitive cell line, HL cells, for isolation and propagation of Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 162:755-758. [DOI] [PubMed] [Google Scholar]
- 22.Loman, S., J. Radl, H. M. Jansen, T. A. Out, and R. Lutter. 1997. Vectorial transcytosis of dimeric IgA by the Calu-3 human lung epithelial cell line: upregulation by IFN-γ. Am. J. Physiol. 272:L951-L958. [DOI] [PubMed] [Google Scholar]
- 23.Lounatmaa, K. 1985. Electron microscopic methods for the study of bacterial surface structures, p. 243-261. In T. K. Korhonen, E. A. Dawes, and P. H. Mäkelä (ed.), Enterobacterial surface antigens: methods for molecular characterization. Elsevier, Amsterdam, Netherlands.
- 24.Mannonen, L., E. Kamping, T. Penttilä, and M. Puolakkainen. 2004. IFN-gamma induced persistent Chlamydia pneumoniae infection in HL and Mono Mac 6 cells: characterization by real-time quantitative PCR and culture. Microb. Pathog. 36:41-50. [DOI] [PubMed] [Google Scholar]
- 25.Molestina, R. E., D. Dean, R. D. Miller, J. A. Ramirez, and J. T. Summersgill. 1998. Characterization of a strain of Chlamydia pneumoniae isolated from a coronary atheroma by analysis of the omp1 gene and biological activity in human endothelial cells. Infect. Immun. 66:1370-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mygind, T., S. Birkelund, E. Falk, and G. Christiansen. 2001. Evaluation of real-time quantitative PCR for identification and quantification of Chlamydia pneumoniae by comparison with immunohistochemistry. J. Microbiol. Methods 46:241-251. [DOI] [PubMed] [Google Scholar]
- 27.Nagarajan, U. M., D. M. Ojcius, L. Stahl, R. G. Rank, and T. Darville. 2005. Chlamydia trachomatis induces expression of IFN-gamma-inducible protein 10 and IFN-beta independent of TLR2 and TLR4, but largely dependent on MyD88. J. Immunol. 175:450-460. [DOI] [PubMed] [Google Scholar]
- 28.Nasreen, N., K. A. Mohammed, J. Hardwick, R. D. Van Horn, K. L. Sanders, C. M. Doerschuk, J. W. Hott, and V. B. Antony. 2001. Polar production of interleukin-8 by mesothelial cells promotes the transmesothelial migration of neutrophils: role of intercellular adhesion molecule-1. J. Infect. Dis. 183:1638-1645. [DOI] [PubMed] [Google Scholar]
- 29.Nelson, W. J., and P. J. Veshnock. 1987. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature 328:533-536. [DOI] [PubMed] [Google Scholar]
- 30.Penttilä, J. M., M. Anttila, M. Puolakkainen, A. Laurila, K. Varkila, M. Sarvas, P. H. Mäkelä, and N. Rautonen. 1998. Local immune responses to Chlamydia pneumoniae in the lungs of BALB/c mice during primary infection and reinfection. Infect. Immun. 66:5113-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Invest. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupp, J., H. Kothe, A. Mueller, M. Maass, and K. Dalhoff. 2003. Imbalanced secretion of IL-1β and IL-1RA in Chlamydia pneumoniae-infected mononuclear cells from COPD patients. Eur. Respir. J. 22:274-279. [DOI] [PubMed] [Google Scholar]
- 33.Shin, K., V. C. Fogg, and B. Margolis. 2006. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22:207-235. [DOI] [PubMed] [Google Scholar]
- 34.Siewert, K., J. Rupp, M. Klinger, W. Solbach, and J. Gieffers. 2005. Growth cycle-dependent pharmacodynamics of antichlamydial drugs. Antimicrob. Agents Chemother. 49:1852-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam, J. E., S. T. Knight, C. H. Davis, and P. B. Wyrick. 1992. Eukaryotic cells grown on microcarrier beads offer a cost-efficient way to propagate Chlamydia trachomatis. Biotechniques 13:374-378. [PubMed] [Google Scholar]
- 36.Wan, H., H. L. Winton, C. Soeller, G. A. Stewart, P. J. Thompson, D. C. Gruenert, M. B. Cannell, D. R. Garrod, and C. Robinson. 2000. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o. Eur. Respir. J. 15:1058-1068. [DOI] [PubMed] [Google Scholar]
- 37.Winton, H. L., H. Wan, M. B. Cannell, D. C. Gruenert, P. J. Thompson, D. R. Garrod, G. A. Stewart, and C. Robinson. 1998. Cell lines of pulmonary and non-pulmonary origin as tools to study the effects of house dust mite proteinases on the regulation of epithelial permeability. Clin. Exp. Allergy 28:1273-1285. [DOI] [PubMed] [Google Scholar]
- 38.Wyrick, P. 2006. Genomics and pathogenesis, p. 323-338. In P. Bavoil and P. Wyrick (ed.), Chlamydia. Horizon Bioscience, Norfolk, United Kingdom.
- 39.Wyrick, P. B., J. Choong, C. H. Davis, S. T. Knight, M. O. Royal, A. S. Maslow, and C. R. Bagnell. 1989. Entry of genital Chlamydia trachomatis into polarized human epithelial cells. Infect. Immun. 57:2378-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyrick, P. B., C. H. Davis, S. T. Knight, and J. Choong. 1993. In-vitro activity of azithromycin on Chlamydia trachomatis infected, polarized human endometrial epithelial cells. J. Antimicrob. Chemother. 31:139-150. [DOI] [PubMed] [Google Scholar]
- 41.Wyrick, P. B., and S. T. Knight. 2004. Pre-exposure of infected human endometrial epithelial cells to penicillin in vitro renders Chlamydia trachomatis refractory to azithromycin. J. Antimicrob. Chemother. 54:79-85. [DOI] [PubMed] [Google Scholar]
- 42.Yang, J., W. C. Hooper, D. J. Phillips, M. L. Tondella, and D. F. Talkington. 2003. Induction of proinflammatory cytokines in human lung epithelial cells during Chlamydia pneumoniae infection. Infect. Immun. 71:614-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, Z. P., P. K. Cummings, D. L. Patton, and C. C. Kuo. 1994. Ultrastructural lung pathology of experimental Chlamydia pneumoniae pneumonitis in mice. J. Infect. Dis. 170:464-467. [DOI] [PubMed] [Google Scholar]
- 44.Yeaman, C., K. K. Grindstaff, and W. J. Nelson. 1999. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 79:73-98. [DOI] [PubMed] [Google Scholar]