Abstract
A CD8+ T-cell response is critical for protection against Encephalitozoon cuniculi infection. However, the factors responsible for the generation of CD8+ T-cell immunity during E. cuniculi infection and the cytokines involved in this process have not been identified. In the present study, we demonstrated that p40-deficient animals, which are unable to produce interleukin-12 (IL-12), have a serious defect in expansion of the CD8+ T-cell response which compromises the survival of an infected host. Adoptive transfer of CD8+ T cells from immunocompetent donors protected SCID mice infected with E. cuniculi, whereas administration of CD8+ T cells from p40−/− mice failed to protect infected SCID mice. In vitro dendritic cell (DC) cultures from knockout mice pulsed with E. cuniculi spores were unable to develop a robust CD8+ T-cell immune response. Addition of exogenous IL-12 or transfer of CD8+ T cells that were initially primed with DC from p40−/− animals to DC cultures from immunocompetent mice (directly or via transwells) led to optimal expansion of these cells. This IL-12-mediated reinstatement of CD8+ T-effector immunity was independent of gamma interferon (IFN-γ) as addition of antibody to the cultures failed to have an effect. These studies demonstrated that IL-12 plays a predominant role in the expansion of effector CD8+ T-cell immunity against E. cuniculi, which is critical for host survival. These findings are very important for understanding the protective immune mechanisms needed to protect an immunocompromised host against an opportunistic infection and can be extended to other microsporidial pathogens.
Encephalitozoon cuniculi is an opportunistic pathogen recently associated with HIV infection, organ transplants, travelers, and the elderly (8, 37). Cell-mediated immunity has been shown to be critical for host resistance against E. cuniculi infection (32), and previous studies in our laboratory have suggested that CD8+ T cells play a predominant role during this type of infection (16). Mice lacking CD8+ T cells are unable to survive E. cuniculi challenge, and a CD8+ T-cell cytotoxic response is essential for protective immunity against this parasite.
Priming of the CD8+ T-cell response against several intracellular pathogens has been shown to be largely dependent on interleukin-12 (IL-12) (25, 40), a heterodimeric proinflammatory cytokine that has been postulated to serve as a bridge between the innate and adaptive immune responses (35). Formed by a light-chain p35 and a heavy-chain p40, this cytokine has multiple functions. It stimulates the development of Th1 lymphocytes (18), induces the production of gamma interferon (IFN-γ) by mouse CD4+ Th1 clones (12), and induces the production of cytotoxic CD8+ T lymphocytes (34). In the present study, we demonstrate that the absence of IL-12 causes a severe defect in the expansion of the effector CD8+ T-cell response against E. cuniculi infection. In vitro studies demonstrate that addition of exogenous IL-12 even 48 to 72 h postculture can reverse the defect in a CD8+ T-cell population primed with p40−/− dendritic cells (DC).
MATERIALS AND METHODS
Mice.
Six- to 7-week-old female C57BL/6 mice were obtained from the National Cancer Institute (Frederick, MD), and sex- and age-matched p40−/− animals were purchased from Jackson Laboratory (Bar Harbor, ME). The animals were housed under approved conditions at the Animal Research Facility at George Washington University (Washington, DC).
Parasites and infection.
A rabbit isolate of E. cuniculi (genotype II), kindly provided by L. M. Weiss (Albert Einstein College of Medicine, Bronx, NY), was used throughout this study. The parasites were maintained by continuous passage in rabbit kidney (RK-13) cells obtained from the American Type Culture Collection (Manassas, VA). The RK-13 cells were maintained in RPMI 1640 containing 10% fetal calf serum (FCS) (HyClone Laboratories, Logan, UT). Organisms were collected from the culture medium, centrifuged at 325 × g for 10 min, and washed twice with phosphate-buffered saline (PBS). The mice were infected via the intraperitoneal (i.p.) route using 107 spores/mouse.
Quantification of the parasite burden.
Tissues (livers and spleens) were harvested from C57BL/6 and p40−/− mice at 21 days postinfection (p.i.). DNA was extracted from tissues by using a DNeasy blood and tissue kit (Qiagen, Valencia CA), and 400 ng of each sample was assayed by real-time PCR using the following primers specific for E. cuniculi: ECUNF (5′-ATGAGAAGTGATGTGTGCG-3′) and ECUNR (5′-TGCCATGCACTCACAGGCATC-3′) (20). Amplification was performed with a Bio-Rad iCycler (Bio-Rad, Hercules, CA) using the following conditions: 40 cycles of 94°C for 45 s, 53°C for 1 min, and 72°C for 30s. The number of parasites was determined by using a standard curve obtained by amplification of DNA from known numbers of parasites.
Activation of CD8+ T cells in p40−/− animals.
Intracellular staining for IFN-γ and the proliferation marker KI-67 was performed to assess the CD8+ T-cell response during E. cuniculi infection. Briefly, splenocytes from wild-type (WT) or p40−/− mice were isolated at 14 days p.i. and stimulated in vitro with phorbol myristate acetate (PMA), ionomycin, and monensin for 4 h using a previously described protocol (21). Four-color flow cytometry analysis of the cells was performed as follows. The cell suspension was washed and labeled for CD8 and CD25 surface markers. Membrane permeabilization was performed with a Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) used according to the manufacturer's instructions. Staining for intracellular proliferation marker KI-67 (fluorescein isothiocyanate [FITC]) and IFN-γ (phycoerythrin [PE]) accumulation was then performed. Samples were acquired with a FACSCalibur (BD Biosciences) (30,000 events were collected), and data were analyzed using Flowjo (Tree Star Inc., Ashland, OR).
CTL assay.
A cytotoxic T-lymphocyte (CTL) assay was carried out according to a standard procedure published previously by our laboratory (16). Briefly, mouse peritoneal macrophages were harvested 2 days after i.p. inoculation with 1 ml of thioglycolate. The macrophages were washed three times in PBS and dispensed at a concentration of 5 × 104 cells/well in U-bottom 96-well plates. After overnight incubation, the cells were infected with 2 × 105 spores of E. cuniculi per well for 48 h and then washed extensively with PBS to remove the extracellular parasites. Macrophages were labeled with 51Cr (0.5 μCi/well) for 2 h at 37°C, washed five times with PBS, and incubated with splenocytes at various effector/target ratios in 200 μl (final volume) of culture medium. Each microtiter plate was centrifuged at 200 × g for 3 min and incubated at 37°C for 4 h. Samples (100 μl) were removed and assayed for released cpm by scintillation counting. The percentage of lysis was calculated as follows: [(mean cpm of test sample − mean cpm of spontaneous release sample)/(mean cpm of maximal release sample − mean cpm of spontaneous release sample)]/100.
CD8+ T-cell adoptive transfer.
C57BL/6 and p40−/− donor mice were sacrificed at day 14 p.i., splenocytes were collected, and CD8+ T cells were isolated with anti-CD8 magnetic beads used according to the manufacturer's instructions (Miltenyi, Auburn, CA). A total of 5 × 106 cells were adoptively transferred via the intravenous (i.v.) route to SCID mice, and recipient animals were challenged with 107 spores of E. cuniculi at 48 h posttransfer. Mice were monitored for morbidity and mortality daily until termination of the experiment.
Intracellular IL-12 detection.
Splenic dendritic cells were isolated from infected animals at days 2, 4, and 6 p.i. as previously described (22). Briefly, mice were sacrificed, spleens were harvested, and DC were isolated by enzymatic digestion (DNase I and collagenase D), followed by mechanical disruption. Cells were then labeled with anti-CD11c biotin-conjugated antibody (eBioscience, San Diego CA), followed by positive magnetic selection as described above. Further purification of DC was performed with a cell sorter after incubation of the cells with streptavidin-PE conjugate. FITC-conjugated anti-NK1.1 and CD19 antibodies were also used to exclude contaminating NK and B cells (FACSaria; BD Biosciences). Positively selected dendritic cells were plated at 5 × 105 DC/well and incubated with monensin (BD) overnight. The following day, cells were labeled with anti-CD11c and then fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions before incubation with anti-IL-12p40 antibodies (eBioscience). Cells were acquired using a FACSCalibur (BD Biosciences) (75,000 events were collected).
In vitro priming of T cells by splenic DC.
Purified splenic DC were incubated overnight with E. cuniculi spores as described above. The following day, the cultures were extensively washed, and purified CD8β+ T cells were added. CD8β+ T cells were isolated from spleens of naïve C57BL/6 mice (Thy1.1 or Thy1.2) by positive magnetic selection as previously described (23). In some experiments, naïve CD8+ T cells (CD44lo CD62Lhi) were purified further by cell sorting with a FACSaria (BD Biosciences). Cells were added to DC cultures at a physiologically relevant ratio (4 naive CD8+ T cells/DC or 10 total CD8+ T cells/DC). Thy1.1 CD8β+ T cells were added to a p40−/− DC culture, and Thy1.2 CD8β+ T cells were added to a WT DC culture. After 72 h of incubation, monensin (BD Biosciences) was added to each culture according to the manufacturer's instructions, and T-cell priming was assessed by intracellular staining for IFN-γ and KI-67 expression as previously described, as well as for CD25 and CD69 expression.
Different variations of this experiment were performed. In one set of experiments, Thy1.1 CD8+ T cells incubated with p40−/− DC were transferred at different time points after culturing (6, 12, 24, 48, or 72 h) to WT DC cultures containing Thy1.2 CD8β+ T cells. In another set of experiments, IFN-γ blockade (anti-IFN-γ clone XMG1.2; eBioscience) was performed at different time points after transfer of Thy1.1 CD8β+ T cells (6, 12, 24, 48, and 72 h). Also, murine recombinant IL-12p70 (rIL-12p70) (20 ng/ml; ProSpec-Tany TechnoGene Ltd., Israel) was added to Thy1.1 CD8β+ T cells primed by p40−/− DC at different time points (6, 12, 24, 48, and 72 h) postculture. For the transwell experiment, E. cuniculi-pulsed DC from WT animals were incubated in 12-well plates with CD8β+ T cells isolated from Thy1.2 mice. In another set of experiments, E. cuniculi-pulsed p40−/− DC were incubated with Thy1.1 CD8β+ cells. After 6 h of incubation, the Thy1.1 CD8β+ T cells were transferred to the top chamber of a transwell (0.4-μm-pore-size transwells; Corning Inc., Corning, NY). Cells were acquired as previously described (30,000 events were collected).
Supernatants were collected at various time points following the addition of T cells (6, 12, 24, 48, and 72 h) and assayed for IL-12p40 production with enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, San Diego, CA) according to the manufacturer's protocols.
Statistical analysis.
Statistical analysis of the data was performed using a two-sample Student t test (27).
RESULTS
IL-12-deficient mice are unable to control E. cuniculi infection.
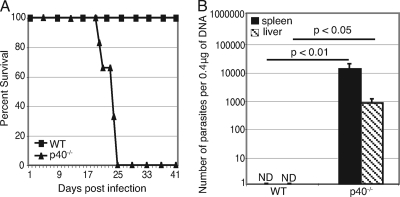
As previously described (30), our results demonstrated that all p40−/− mice succumbed to intraperitoneal E. cuniculi challenge by week 4 p.i., while WT C57BL/6 controls survived until the end of the experiment (Fig. 1A) (30). Parasite loads from tissues (spleens and livers) of infected animals were assayed by real-time PCR at day 21 p.i. As expected, WT mice were able to control the infection, and the parasites were cleared from both organs by day 21 p.i. (Fig. 1B). However, high numbers of E. cuniculi spores were detected in the spleens and livers from p40−/− animals. Unlike the control animals, knockout mice were unable to control replication of the parasites and ultimately succumbed to the challenge (Fig. 1B).
FIG. 1.
p40−/− mice are unable to control E. cuniculi infection. p40−/− and WT mice were infected i.p. with 107 E. cuniculi spores/mouse. (A) Animals (6 mice/group) were monitored daily for morbidity and mortality until termination of the experiment. (B) Animals (4 mice/group) were sacrificed at day 21 p.i., organs (livers and spleens) were harvested, and DNA was prepared. Parasite loads were quantified by real-time PCR. The number of parasites was determined from a standard curve after amplification from a known number of parasites. The results are representative of two experiments. ND, not determined.
p40−/− mice exhibit a downregulated CD8+ T-cell response to E. cuniculi infection.
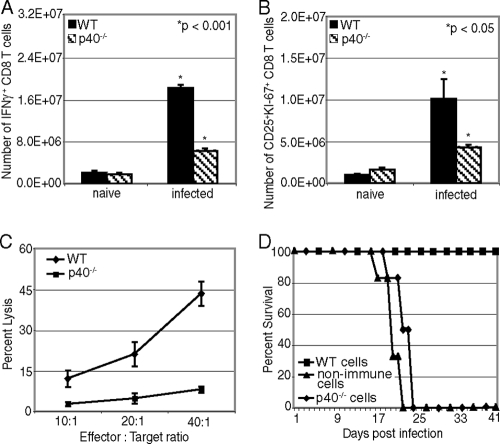
A CD8+ T-cell response against E. cuniculi is critical for protection against infection (1, 16, 28). Consequently, we analyzed the CD8+ T-cell response in p40−/− mice after i.p. infection. Fourteen days p.i, lower levels of IFN-γ-producing CD8+ T cells were detected in p40−/− mice than in WT animals (Fig. 2A). IL-12-depleted animals also contained a lower number of actively cycling CD8+ T cells, as demonstrated by the CD25+ KI-67+ CD8+ T-cell frequency (Fig. 2B). Similarly, the CTL activity of CD8+ T cells from p40−/− infected mice was decreased more than 4-fold compared to that in infected WT animals (Fig. 2C). Finally, immune CD8+ T cells isolated from p40−/− animals were unable to protect SCID recipients against a lethal E. cuniculi challenge after adoptive transfer, and all the animals succumbed to infection by day 25 p.i. (Fig. 2D). In comparison, immune CD8+ T cells isolated from WT mice conferred protection to SCID mice against E. cuniculi challenge. These results demonstrate that IL-12-deficient animals are unable to generate an optimal CD8+ T-cell immune response against E. cuniculi.
FIG. 2.
Defective CD8+ T-cell response from p40−/− mice after E. cuniculi infection. p40−/− and WT mice (4 mice/group) were sacrificed at day 14 p.i., and splenocytes were prepared. (A and B) The CD8+ T-cell response was assessed by intracellular staining for IFN-γ (A) and by CD25+ KI-67+ staining (B). (C) Cytotoxicity was evaluated by the chromium release assay. CD8+ T cells were isolated by magnetic sorting at day 14 p.i., purified cells (≥92% pure) were incubated with E. cuniculi-infected radiolabeled macrophages, and cytotoxicity was measured by chromium release in the supernatants. (D) At day 14 p.i., purified CD8 T cells from spleens of WT or p40−/− mice (6 mice/group) were adoptively transferred to SCID animals (n = 6) via the i.v. route (5 × 106 cells/mouse). The recipients were challenged 48 h later with 107 E. cuniculi spores. Morbidity and mortality were monitored daily until termination of the experiment. The data are representative of two experiments with similar results.
IL-12 is important for expansion of CD8+ T-cell effectors against E. cuniculi infection.
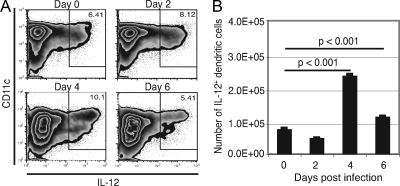
DC have been shown to be the main antigen-presenting cells during infection (24) and can produce IL-12 in response to various pathogens (35). The splenic DC response of WT mice was evaluated at different time points after E. cuniculi infection (days 2, 4, and 6), and the frequency of IL-12-producing cells was assessed by intracellular staining. Increases in both the number and the percentage of DC releasing IL-12 were detected as early as day 2 p.i., peaking at day 4 before starting to decline at day 6 p.i. (Fig. 3A and B). These results suggest that E. cuniculi infection induces strong IL-12 production by DC, implying that this cytokine has a role in host protection against the pathogen.
FIG. 3.
Early production of IL-12 by splenic DC after i.p. E. cuniculi infection. p40−/− and WT mice were infected i.p. with 107 E. cuniculi spores/mouse. At different time points (days 2, 4, and 6 p.i.), animals were sacrificed (3 mice/group), splenic cell suspensions were prepared, and intracellular IL-12 was measured. The results are presented as dot blots (A) and the absolute number of IL-12+ DC (B). The experiment was performed twice, and the data are representative of two experiments.
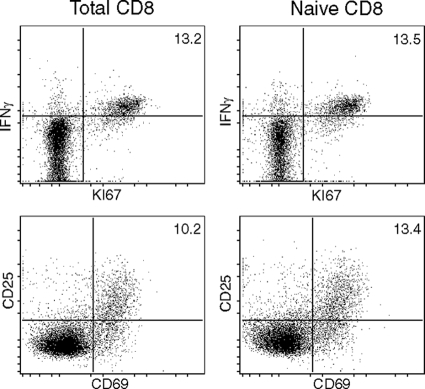
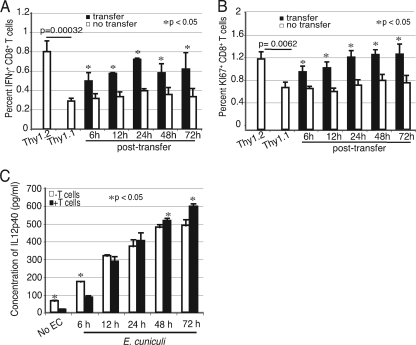
To further address the function of IL-12 in the CD8+ T-cell response during E. cuniculi infection, in vitro CD8+ T-cell priming experiments were performed. Previous studies from our laboratory have demonstrated that CD8+ T cells from naïve animals can be primed by E. cuniculi-pulsed DC. The effector response was measured by using antigen-specific proliferation and cytotoxicity (22, 23). As shown in Fig. 4, an experiment performed with the total splenic CD8+ T population or sorted naïve CD8+ T cells (CD44lo CD62Lhi) showed no significant difference in IFN-γ, KI67, CD25, and CD69 expression. Hence, all further experiments were carried out with total splenic CD8+ T cells. In the first set of experiments, CD8+ T cells isolated from naïve Thy1.1 mice were stimulated with p40−/− DC in the presence of E. cuniculi spores. In parallel, cells from congenic Thy1.2 mice were incubated with WT DC. At different time points (6 to 72 h) postculture, Thy1.1 CD8+ T cells were harvested and added to cultures containing Thy1.2 CD8+ T cells. The frequency of the IFN-γ-producing and actively cycling population (KI-67+ CD8+ T cells) was assessed 96 h post-initial culture. E. cuniculi- stimulated DC from p40−/− and WT DC and the appropriate CD8+ T cells (Thy1.1 and Thy1.2, respectively) served as controls. As shown in Fig. 5A, after stimulation with WT DC, over 3% of Thy1.2+ CD8+ T cells from control wells were positive for IFN-γ. Conversely, p40−/− DC were unable to induce comparable levels of IFN-γ+ Thy1.1 CD8+ T cells. However, a significant increase in the IFN-γ+ Thy1.1 CD8 T+ population was detected when these cells were transferred to Thy1.2 cultures (containing WT DC and Thy1.2 CD8+ T cells). Compared to the results for nontransferred controls, increases at all time points posttransfer were observed. Similarly, transfer of Thy1.1 cells to WT DC cultures led to an increase in the number of actively cycling cells, as determined by expression of the proliferation marker KI-67+ (Fig. 5B). The IL-12 levels in supernatants from WT DC cultures were monitored at different time points. Interestingly, increasing levels of IL-12 were detected up to 72 h (Fig. 5C), explaining the ability of these cells to rescue the CD8+ T cells transferred at later time points.
FIG. 4.
In vitro priming of total splenic CD8+ T cells or naïve CD8+ T cells by DC. Splenic DC from WT mice (4 mice/group) were isolated and incubated overnight with E. cuniculi spores. The next day, purified naïve CD8+ T cells or total splenic CD8+ T cells were added to the culture. After 72 h of incubation, cells were assessed for IFN-γ, KI67, CD25, and CD69 expression by flow cytometry. The experiment was performed twice, and the data are representative of one experiment.
FIG. 5.
p40−/− DC are unable to expand the initial CD8+ T-cell response against E. cuniculi infection. Splenic DC from WT and p40−/− mice (9 mice/group) were isolated and incubated overnight with E. cuniculi spores. The next day, CD8+ T cells from Thy1.1 and Thy1.2 animals (5 mice/group) were prepared and added to the p40−/− and WT DC cultures, respectively. At different time points following addition of T cells (6, 12, 24, 48, and 72 h), Thy1.1 CD8+ T cells were transferred from the p40−/− DC culture to a WT DC culture. At 96 h, cells were assayed for IFN-γ by intracellular staining (A) and for proliferation by using expression of the KI-67 marker (B). Supernatant from the WT DC culture was assayed for IL-12 by ELISA at different time points after addition of the T cells (6, 12, 24, 48, and 72 h) (C). Experiments were performed twice, and the results are representative of one experiment. EC, E. cuniculi.
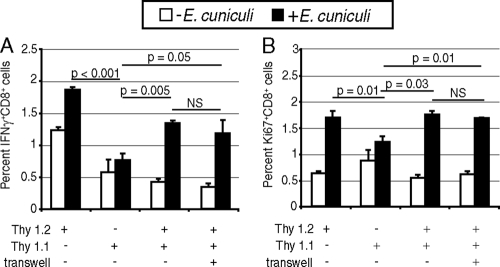
To determine that activation of transferred Thy1.1 T cells was not dependent on contact with WT DC (i.e., de novo priming), experiments involving transwell inserts, which prevent direct contact between cells, were performed. Cultures containing Thy1.1 CD8+ T cells in combination with p40−/− DC and Thy1.2 CD8+ T cells in combination with WT DC were established as described above. After 6 h of incubation, Thy1.1 cells were transferred to a Thy1.2 culture with the cells in direct contact or in the top chamber of a transwell insert. As shown in Fig. 6A and B, no difference in the incidence of IFN-γ-producing or actively cycling cells between Thy1.1 T cells placed in a transwell chamber and Thy1.1 T cells in direct contact with Thy1.2 T cells and WT DC was observed. These findings suggest that replenishment of the effector response of Thy1.1 CD8+ T cells initially cultured with p40−/− DC, after transfer to WT DC cultures, is contact independent and more likely mediated by IL-12.
FIG. 6.
Restoration of the CD8+ T-cell response by WT DC is contact independent. DC from WT and p40−/− animals (6 mice/group) were isolated and incubated with E. cuniculi spores as described in Materials and Methods. CD8+ T cells isolated from spleens of Thy1.1 and Thy1.2 mice (4 mice/group) were prepared and added to the p40−/− and WT DC cultures, respectively. After 6 h of incubation, Thy1.1 CD8+ T cells were transferred from the p40−/− DC culture to a WT DC culture with or without a transwell. At 96 h, the CD8+ T-cell response was assessed by detection of intracellular IFN-γ (A) and KI-67 (B). The experiment was performed twice, and the results are representative of one experiment. NS, not significant.
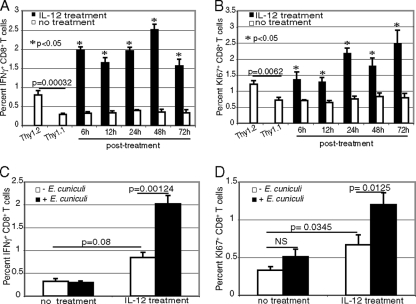
To further establish the role of IL-12 during priming of the CD8+ T-cell response against E. cuniculi, Thy1.1 CD8+ T cells cultured with p40−/− DC were treated exogenously with recombinant IL-12p70. As shown in Fig. 7A and B, addition of the cytokine to CD8+ T cells primed with DC from p40−/− mice led to a significant increase in the number of IFN-γ-producing and actively cycling cells. Although IL-12 treatment induced an increase in the response of CD8+ T cells, it was significantly less than that after IL-12 treatment in conjunction with E. cuniculi-pulsed DC (Fig. 7C and D). These findings corroborate well with the studies described above in which the defect in an IFN-γ-producing or actively cycling CD8+ T-cell population cultured initially with p40−/− DC was reversed upon transfer to cultures containing WT DC. These observations strongly suggest that the primary defect in expansion of the CD8+ T-cell response is due to the inability of p40−/− DC to produce IL-12, and availability of the cytokine can abrogate this defect by ensuring development of optimal CD8+ T-cell immunity.
FIG. 7.
IL-12 treatment can restore the immune response of CD8+ T cells primed by p40−/− DC. Splenic DC from WT and p40−/− animals (9 mice/group) were incubated with E. cuniculi spores as described in the legend to Fig. 6. Affinity-purified CD8+ T cells from Thy1.1 and Thy1.2 animals (5 mice/group) were added to p40−/− and WT cultures, respectively. p40−/− DC cultures were treated with recombinant murine IL-12p70 (20 ng/ml) at different time points after addition of Thy1.1 T cells (6, 12, 24, 48, and 72 h). Thy1.2 CD8+ T cells and a WT DC culture were used as positive controls in this experiment. At 96 h, the CD8+ T-cell response was assessed by intracellular IFN-γ (A) and KI-67 (B). The nonspecific increase in the CD8+ T-cell response by IL-12 treatment alone was measured by intracellular IFN-γ (C) and KI-67 (D) expression (NS, not significant). The experiment was performed twice, and the results are representative of one experiment.
Role of IL-12 in priming of the CD8+ T-cell response is IFN-γ independent.
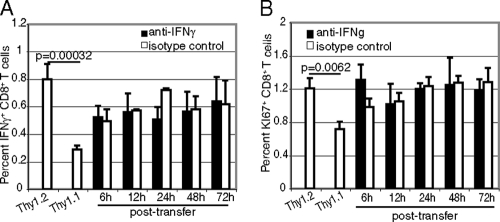
As IFN-γ has been reported to be involved in CD8+ T-cell expansion in viral and parasitic models (9, 38), next we determined if restoration of the effector response by IL-12 in CD8+ T cells primed with p40−/− DC is dependent on IFN-γ. Transfer of Thy1.1 CD8+ T cells primed by p40−/− DC to a culture containing Thy1.2 CD8+ T cells and WT DC was performed as described above. Anti-IFN-γ antibody was added to some of the wells at the time of transfer. As shown in Fig. 8A and B, treatment with anti-IFN-γ antibody had no effect on the frequency of IFN-γ-producing or actively cycling Thy1.1 CD8+ T cells. These results suggest that IL-12-mediated expansion of the CD8+ T-cell response during E. cuniculi infection is independent of IFN-γ.
FIG. 8.
Augmentation of the IL-12-mediated CD8+ T-cell response against E. cuniculi is independent of IFN-γ. DC from WT and p40−/− animals (9 mice/group) were isolated and incubated with E. cuniculi spores. CD8+ T cells from Thy1.1 or Thy1.2 mice (5 mice/group) were added to the p40−/− or WT cultures, respectively. WT DC cultures were treated with anti-murine IFN-γ (5 μg/ml) at different time points after addition of the Thy1.2 CD8+ T cells (6, 12, 24, 48, and 72 h). Thy1.1 CD8+ T cells and a p40−/− DC culture were used as positive controls for this experiment. At 96 h, the CD8+ T-cell response was assessed by intracellular IFN-γ (A) and KI-67 (B) expression. The experiment was performed twice, and the results are representative of one experiment.
DISCUSSION
IL-12 plays an important role in the response to infections, and mice lacking the gene for this cytokine have been shown to be susceptible to a wide array of intracellular pathogens, including Leishmania major, Toxoplasma gondii, Mycobacterium tuberculosis, Listeria monocytogenes, and E. cuniculi (10, 13, 14, 30). Similar to a previous study showing that p40−/− mice are unable to survive an E. cuniculi infection (30), our data confirm that these immunodeficient mice are unable to withstand a challenge with this pathogen. For the first time, we demonstrate that p40−/− mice display a defective CD8+ T-cell response against E. cuniculi, which is manifested by decreased cytotoxic activity and a reduced number of IFN-γ-producing cells within this population. Moreover, CD8+ T cells from p40−/− mice fail to protect SCID animals against a lethal challenge, further emphasizing the suboptimal effector functions. In vitro studies demonstrated that CD8+ T cells primed with DC from p40-deficient mice can be rescued by IL-12 treatment. Transfer of these cells to a culture containing WT DC restored their function, as demonstrated by IFN-γ and KI-67 expression.
The importance of CD8+ T cells in protection against E. cuniculi infection has been well established by studies conducted by us and in other laboratories (1, 2, 16, 20, 21). CD8+ T-cell-deficient animals are highly susceptible to this pathogen, and infected mice exhibit severe morbidity before death. Inability to clear E. cuniculi infection was also demonstrated for perforin knockout mice, suggesting the importance of the cytotoxic function of CD8+ T cells against the pathogen (16). In the present study, we observed that in the absence of IL-12, the host CD8+ T-cell effector response was severely compromised, which led to the death of infected animals. The role of IL-12 in induction of the CD8+ T-cell response has been described in both tumor and infectious disease models. Priming of naïve CD8+ T cells in the presence of IL-12 selectively results in superior antitumor activity in a tolerogenic model (7). Similarly, IL-12-dependent expansion of the primary CD8+ T-cell response during the initial stages of L. major and T. gondii infection has been described previously (26, 39).
In the present study, we demonstrated that DC isolated from p40−/− mice seemingly appear to be able to initiate an in vitro CD8+ T-cell response against E. cuniculi. This was demonstrated by the observation that transfer of Thy1.1 CD8+ T cells primed with p40−/− DC to a WT DC culture with or without a transwell insert led to an optimal CD8+ T-cell response. Thus, expansion of the CD8+ T-cell response appears to be strongly restricted by a lack of IL-12 since addition of recombinant cytokine to nontransferred cultures can also reverse the defect. This is in agreement with a report by Curtsinger et al., in which the requirement for IL-12 as a third signal to differentiate between tolerance with proliferation of CD8+ T cells and development of a cytotoxic function has been demonstrated (5). Moreover, it has been suggested that in addition to T-cell receptor (TCR) engagement and costimulation, IL-12 can act as a third signal necessary for expansion of CD8+ T cells (36). Also, IL-12 has previously been shown to act directly on CD8+ T cells in vivo and in vitro to promote proliferation, survival, and differentiation into effector cells capable of cytolysis (6, 31). However, most of the studies regarding the role of IL-12 during the CD8+ T-cell response were carried out with OT-1 CD8+ T cells stimulated with OVA peptides and might differ from an infectious disease model (4, 6, 36). Also, timing for IL-12 signaling is very important for the optimal CD8+ T-cell response. Recent in vitro experiments showed that addition of IL-12 had to occur no later than 18 h after antigen encounter and for a minimum of 40 h (4). In fact, our results differ from this report and show that addition of recombinant IL-12 can enhance the CD8+ T-cell response against E. cuniculi even as late as 48 or 72 h after antigen encounter. Addition of recombinant IL-12 caused a significant increase in the frequency of IFN-γ-producing effectors in a CD8+ T-cell population primed by p40−/− DC. Similarly, transfer of CD8+ T cells from a knockout to a WT culture restored the CD8+ T-cell effector response. Also, this cytokine has recently been shown to prevent activation-induced cell death (5), leading to enhanced survival and thus clonal expansion of the CD8+ T-cell population.
Another interesting observation made in the present study is that IL-12-dependent CD8+ T-cell expansion does not appear to be dependent on IFN-γ, as reported in previous studies (9, 11). Our laboratory has demonstrated that a compromised CD8+ T-cell response against T. gondii infection in p40-deficient mice is due to severe downregulation of IFN-γ levels in these animals, which can be reversed by exogenous IFN-γ treatment. Additionally, IFN-γ has been reported to stimulate CD8+ T-cell expansion after infection with lymphocytic choriomeningitis virus (38). Although the importance of IFN-γ for protection against E. cuniculi infection has been reported by us and other workers (15, 29, 30), this cytokine seems to play a very limited role in expansion of the CD8+ T-cell response against the pathogen, as anti-IFN-γ antibody treatment of cells transferred from p40−/− to WT DC cultures did not prevent restoration of the effector response. As the indispensable role played by IL-12 in expansion of the CD8+ T-cell response (17, 26, 39, 40) is becoming increasingly evident, the mechanism involved in this process is being investigated. It has been recently demonstrated that following the termination of TCR signaling, IL-12 alone is needed for the maintenance of the second phase of T-bet expression and stabilization of the TH1 response (33). As T-bet is also an important transcription factor for CD8+ T cells (19), the same phenomenon may occur with this population.
Overall, the importance of these findings is as follows. Our data, for the first time, link the regulation of CD8+ T-cell immunity during E. cuniculi infection to IL-12. They demonstrate that IL-12 treatment can restore the effector CD8+ T-cell response even after a prolonged period of culture in an IL-12-deficient environment (48 to 72 h), suggesting that this cytokine may be involved more in expansion of the CD8+ T-cell effector response than in the initiation of this response. Furthermore, as stated above, regulation of CD8+ T-cell immunity is not dependent on IFN-γ, as reported for other infections (9, 11). Recent studies have suggested that due to its ability to expand the CD8+ T-cell population, IL-12 treatment has a strong therapeutic potential in diseases where these cells are critical for the host defense (3, 27). As CD8+ T-cell immunity plays a central role in protection against E. cuniculi challenge (1, 2, 16), the observations described above have strong implications in developing strategies for designing immunotherapeutic agents for HIV-infected and elderly individuals who are prone to this type of infection.
Acknowledgments
We thank Teresa Hawley for her help with flow cytometry.
This work was supported by National Institutes of Health grant AI 043693 to I.A.K.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Braunfuchsova, P., J. Salat, and J. Kopecky. 2001. CD8+ T lymphocytes protect SCID mice against Encephalitozoon cuniculi infection. Int. J. Parasitol. 31:681-686. [DOI] [PubMed] [Google Scholar]
- 2.Braunfuchsova, P., J. Salat, and J. Kopecky. 2002. Comparison of the significance of CD4+ and CD8+ T lymphocytes in the protection of mice against Encephalitozoon cuniculi infection. J. Parasitol. 88:797-799. [DOI] [PubMed] [Google Scholar]
- 3.Cocco, C., V. Pistoia, and I. Airoldi. 2009. New perspectives for melanoma immunotherapy: role of IL-12. Curr. Mol. Med. 9:459-469. [DOI] [PubMed] [Google Scholar]
- 4.Curtsinger, J. M., C. M. Johnson, and M. F. Mescher. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165-5171. [DOI] [PubMed] [Google Scholar]
- 5.Curtsinger, J. M., D. C. Lins, and M. F. Mescher. 2003. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197:1141-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtsinger, J. M., C. S. Schmidt, A. Mondino, D. C. Lins, R. M. Kedl, M. K. Jenkins, and M. F. Mescher. 1999. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 162:3256-3262. [PubMed] [Google Scholar]
- 7.Diaz-Montero, C. M., S. El Naggar, A. Al Khami, R. El Naggar, A. J. Montero, D. J. Cole, and M. L. Salem. 2008. Priming of naive CD8+ T cells in the presence of IL-12 selectively enhances the survival of CD8+ CD62Lhi cells and results in superior anti-tumor activity in a tolerogenic murine model. Cancer Immunol. Immunother. 57:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Didier, E. S. 2005. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 94:61-76. [DOI] [PubMed] [Google Scholar]
- 9.Ely, K. H., L. H. Kasper, and I. A. Khan. 1999. Augmentation of the CD8+ T cell response by IFN-gamma in IL-12-deficient mice during Toxoplasma gondii infection. J. Immunol. 162:5449-5454. [PubMed] [Google Scholar]
- 10.Feng, C. G., D. Jankovic, M. Kullberg, A. Cheever, C. A. Scanga, S. Hieny, P. Caspar, G. S. Yap, and A. Sher. 2005. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J. Immunol. 174:4185-4192. [DOI] [PubMed] [Google Scholar]
- 11.Fruh, K., and Y. Yang. 1999. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr. Opin. Immunol. 11:76-81. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 13.Jankovic, D., A. Sher, and G. Yap. 2001. Th1/Th2 effector choice in parasitic infection: decision making by committee. Curr. Opin. Immunol. 13:403-409. [DOI] [PubMed] [Google Scholar]
- 14.Khan, I. A., T. Matsuura, and L. H. Kasper. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62:1639-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan, I. A., and M. Moretto. 1999. Role of gamma interferon in cellular immune response against murine Encephalitozoon cuniculi infection. Infect. Immun. 67:1887-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan, I. A., J. D. Schwartzman, L. H. Kasper, and M. Moretto. 1999. CD8+ CTLs are essential for protective immunity against Encephalitozoon cuniculi infection. J. Immunol. 162:6086-6091. [PubMed] [Google Scholar]
- 17.Li, Q., C. Eppolito, K. Odunsi, and P. A. Shrikant. 2006. IL-12-programmed long-term CD8+ T cell responses require STAT4. J. Immunol. 177:7618-7625. [DOI] [PubMed] [Google Scholar]
- 18.Manetti, R., P. Parronchi, M. G. Giudizi, M. P. Piccinni, E. Maggi, G. Trinchieri, and S. Romagnani. 1993. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 177:1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer, K. D., K. Mohrs, W. Reiley, S. Wittmer, J. E. Kohlmeier, J. E. Pearl, A. M. Cooper, L. L. Johnson, D. L. Woodland, and M. Mohrs. 2008. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J. Immunol. 180:693-697. [DOI] [PubMed] [Google Scholar]
- 20.Moretto, M., L. Casciotti, B. Durell, and I. A. Khan. 2000. Lack of CD4+ T cells does not affect induction of CD8+ T-cell immunity against Encephalitozoon cuniculi infection. Infect. Immun. 68:6223-6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moretto, M., B. Durell, J. D. Schwartzman, and I. A. Khan. 2001. Gamma delta T cell-deficient mice have a down-regulated CD8+ T cell immune response against Encephalitozoon cuniculi infection. J. Immunol. 166:7389-7397. [DOI] [PubMed] [Google Scholar]
- 22.Moretto, M. M., E. M. Lawlor, and I. A. Khan. 2008. Aging mice exhibit a functional defect in mucosal dendritic cell response against an intracellular pathogen. J. Immunol. 181:7977-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretto, M. M., L. M. Weiss, C. L. Combe, and I. A. Khan. 2007. IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection. J. Immunol. 179:2485-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser, M. 2001. Dendritic cells and the control of the immune response. Pathol. Biol. (Paris) 49:450-453. [DOI] [PubMed] [Google Scholar]
- 25.Park, A. Y., B. Hondowicz, M. Kopf, and P. Scott. 2002. The role of IL-12 in maintaining resistance to Leishmania major. J. Immunol. 168:5771-5777. [DOI] [PubMed] [Google Scholar]
- 26.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 27.Sabel, M. S., A. Arora, G. Su, K. A. Griffith, E. Mathiowitz, J. J. Reineke, and A. E. Chang. 2007. Generation of a tumor-specific systemic response after intratumoral injection of IL-12 and IL-18-loaded polylactic acid microspheres. J. Immunother. 30:808-816. [DOI] [PubMed] [Google Scholar]
- 28.Salat, J., P. Braunfuchsova, J. Kopecky, and O. Ditrich. 2002. Role of CD4+ and CD8+ T lymphocytes in the protection of mice against Encephalitozoon intestinalis infection. Parasitol. Res. 88:603-608. [DOI] [PubMed] [Google Scholar]
- 29.Salat, J., J. Jelinek, J. Chmelar, and J. Kopecky. 2008. Efficacy of gamma interferon and specific antibody for treatment of microsporidiosis caused by Encephalitozoon cuniculi in SCID mice. Antimicrob. Agents Chemother. 52:2169-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salat, J., B. Sak, T. Le, and J. Kopecky. 2004. Susceptibility of IFN-gamma or IL-12 knock-out and SCID mice to infection with two microsporidian species, Encephalitozoon cuniculi and E. intestinalis. Folia Parasitol. (Praha) 51:275-282. [PubMed] [Google Scholar]
- 31.Schmidt, C. S., and M. F. Mescher. 1999. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J. Immunol. 163:2561-2567. [PubMed] [Google Scholar]
- 32.Schmidt, E. C., and J. A. Shadduck. 1984. Mechanisms of resistance to the intracellular protozoan Encephalitozoon cuniculi in mice. J. Immunol. 133:2712-2719. [PubMed] [Google Scholar]
- 33.Schulz, E. G., L. Mariani, A. Radbruch, and T. Hofer. 2009. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity 30:673-683. [DOI] [PubMed] [Google Scholar]
- 34.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 36.Valenzuela, J., C. Schmidt, and M. Mescher. 2002. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 169:6842-6849. [DOI] [PubMed] [Google Scholar]
- 37.Weber, R., P. Deplazes, and D. Schwartz. 2000. Diagnosis and clinical aspects of human microsporidiosis. Contrib. Microbiol. 6:166-192. [DOI] [PubMed] [Google Scholar]
- 38.Whitmire, J. K., J. T. Tan, and J. L. Whitton. 2005. Interferon-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J. Exp. Med. 201:1053-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson, D. C., S. Matthews, and G. S. Yap. 2008. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii infection. J. Immunol. 180:5935-5945. [DOI] [PubMed] [Google Scholar]
- 40.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]