Abstract
Multiple pattern recognition systems have been shown to initiate innate immune responses to microbial pathogens. The degree to which these detection systems cooperate with each other to provide host protection is unknown. Here, we investigated the importance of several immune surveillance pathways in protecting mice against lethal infection by the intracellular pathogen Legionella pneumophila, the causative agent of a severe pneumonia called Legionnaires' disease. Rip2 and Naip5/NLRC4 signaling was found to contribute to the innate immune response generated against L. pneumophila in the lung. Elimination of Rip2 or Naip5/NLRC4 signaling in MyD88-deficient mice resulted in increased replication and dissemination of L. pneumophila and higher rates of mortality. Irradiated wild-type mice receiving bone marrow cells from pattern recognition receptor-deficient mice displayed L. pneumophila infection phenotypes similar to those of donor mice. Rip2 and Naip5/NLRC4 signaling provided additive effects in protecting MyD88-deficient mice from lethal infection by L. pneumophila, with the contribution of Naip5/NLRC4 being slightly greater than that of Rip2. Thus, activation of the Rip2, MyD88, and Naip5/NLRC4 signaling pathways triggers a coordinated and synergistic response that protects the host against lethal infection by L. pneumophila. These data provide new insight into how different pattern recognition systems interact functionally to generate innate immune responses that protect the host from lethal infection by activating cellular pathways that restrict intracellular replication of L. pneumophila and by recruiting to the site of infection additional phagocytes that eliminate extracellular bacteria.
To respond to diverse populations of microbes, the mammalian innate immune system utilizes germ line-encoded pattern recognition receptors (PRRs) that detect conserved molecular patterns associated with pathogens (38). The ectodomains of transmembrane Toll-like receptor (TLR) are involved in detecting microbes outside cells and within vacuoles, and the adapter protein MyD88 is used by many TLRs to transduce extracellular signals into functional responses (38). In contrast, the nucleotide-binding domain, leucine-rich repeat (NLR) proteins constitute a surveillance mechanism capable of responding to microbial products delivered into the host cytosol (27). The Nod1 and Nod2 proteins are PRRs that detect microbial products present in the cytosol and in response activate NF-κB and mitogen-activated protein kinase (MAPK) signaling pathways through an adapter serine-threonine kinase called Rip2 (11, 18, 25, 26, 28, 29, 33, 44, 46, 50).
The Gram-negative bacterium Legionella pneumophila is a useful model for investigating the initiation of the innate immune response. L. pneumophila persists in the environment as a parasite of freshwater protozoans (15); however, upon gaining access to the mammalian respiratory system through contaminated aerosols, the bacteria can infect and replicate within alveolar macrophages (17, 24, 37). Failure to treat infected individuals, especially those who are immunocompromised, with antibiotics can lead to the development of a severe pneumonia known as Legionnaires' disease (17, 37). Following phagocytosis by a macrophage, L. pneumophila generates a unique vacuole that evades fusion with lysosomes and accumulates endoplasmic reticulum (ER) protein markers, features that allow the compartment to support intracellular replication (12, 22, 23, 30, 56). L. pneumophila is able to perform this task by utilizing a type IV secretion system encoded by the dot and icm genes (36, 48, 57). The Dot/Icm secretion apparatus delivers bacterial proteins into the host cell cytosol that modulate normal endosomal trafficking and prevent lysosome-mediated killing of the bacteria (31, 41).
The proteins TLR2, TLR5, and TLR9 have been shown to recognize L. pneumophila during engulfment at the cell surface or in an early endosomal compartment (2, 6, 7, 19-21, 43). Mice deficient in TLR2 have a subtle defect in clearance of L. pneumophila from the lung after infection (6, 20). Surprisingly, defects in TLR5 and TLR9 signaling do not exacerbate this TLR2 defect significantly (5), suggesting that TLR signaling alone is not essential for host protection against L. pneumophila infection. Mice deficient for MyD88 have a profound defect in interleukin-12 (IL-12) and gamma interferon (IFN-γ) production (5, 6, 20, 54) and display high numbers of L. pneumophila CFU in the lungs compared to control mice (6, 20). MyD88 is required for signaling pathways stimulated by TLRs and for pathways activated by the IL-1 family of receptors (1), which is the likely reason why a deficiency in MyD88 results in a more severe L. pneumophila susceptibility phenotype than a deficiency in the three primary TLRs stimulated by L. pneumophila. Macrophages and NK cells have been implicated as cell types that utilize MyD88 for an in vivo response to L. pneumophila (5, 6, 20, 54); however, it remains to be determined which cell types play a protective role in the MyD88-dependent response.
In addition to activating MyD88-dependent pathways, virulent L. pneumophila activates cytosolic pattern recognition systems. The flagellin protein produced by L. pneumophila signals through the NLR proteins Naip5 and NLRC4 (also known as IPAF and CARD12), resulting in the activation of caspase-1 and other pathways that restrict intracellular replication of L. pneumophila in mouse macrophages (4, 34, 40, 45, 58). Increased replication of L. pneumophila in the lungs is observed after infection of mice deficient in Naip5 or NLRC4 signaling (4, 10, 34, 58); however, these mice are still able to clear the infection over a period of several days. The finding that L. pneumophila activates a Rip2-dependent signaling pathway in macrophages that mediates IκB degradation and NF-κB nuclear translocation suggests that the NLR proteins Nod1 and Nod2 are also involved in detection (35, 52). Whether Rip2 signaling is important for host protection against L. pneumophila, however, has not been addressed.
The ability of multiple pathogen recognition systems to respond to L. pneumophila makes this an attractive model to investigate whether these different signaling pathways play functionally independent or synergistic roles in stimulating the host defense to this intracellular pathogen. In this study, we used a mouse model of Legionnaires' disease to investigate the role of multiple microbial recognition systems in providing host protection against this intracellular pathogen.
MATERIALS AND METHODS
Bacterial strains.
L. pneumophila serogroup 1 strain JR32 (47), an flaA mutant (JR32 ΔflaA) (45), and serogroup 1 clinical isolate F2111 (13) were used in this study. L. pneumophila strains were cultured on charcoal-yeast extract (CYE) agar (14) for 2 days and then cultured overnight in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract (AYE) broth (10 g/liter yeast extract, 10 g/liter ACES, 0.4 g/liter l-cysteine HCl-H2O, 0.135 g/liter ferric nitrate) prior to use in experiments. For enzyme-linked immunosorbent assay (ELISA) studies and in vivo growth assays, bacteria were grown to an optical density at 600 nm of 1 in AYE broth. For ex vivo growth assays, bacteria were grown to an optical density of 3.4 in AYE broth.
Mice.
C57BL/6 (stock number 000664) mice were purchased from Jackson Laboratories. MyD88−/− (1) and Rip2−/− (33) mice in a C57BL/6 background have been described previously. MyD88−/− mice were provided by R. Medzhitov, and Rip2−/− mice were provided by R. Flavell. For experiments using mice with the nonfunctional Naip5 gene, MyD88−/− and Rip2−/− mice in a mixed 129/SvJ × C57BL/6 background were mated with A/J mice to generate mice that were homozygous for the A/J Naip5 allele.
Bone marrow-derived macrophages (BMMs).
Bone marrow was collected from the femurs and tibiae of mice. Cells were plated on petri dishes and incubated at 37°C in RPMI 1640 medium containing 20% fetal bovine serum (FBS), 30% macrophage colony-stimulating factor (M-CSF)-conditioned medium, and 1% penicillin-streptomycin. At day 6, cells were harvested and resuspended in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 5% M-CSF. Cells were then plated in 24-well tissue culture-treated plates and incubated at 37°C. M-CSF was obtained from an L-929 fibroblast cell line (ATCC).
Ex vivo macrophage infections and growth assays.
BMMs were added to 24-well plates at a concentration of 2 × 105 cells/well. The cells were infected with either the L. pneumophila JR32 wild-type (WT) or JR32 Δfla strain at a multiplicity of infection (MOI) of 5 and incubated at 37°C. BMMs were washed at 1 h postinfection with warm Dulbecco's phosphate-buffered saline (PBS) to remove extracellular bacteria. Then either the BMMs were lysed immediately with sterile H2O (day 0) or fresh medium was added until the cells were harvested at 24, 48, and 72 h postinfection. Cell lysates were plated on CYE agar to determine the number of bacterial CFU. Each data point is the value for one mouse for which the average bacterial content of three independent wells was determined. The fold differences were determined by dividing the values obtained at 24, 48, and 72 h by the values obtained on day 0. Ex vivo growth assays were repeated at least once, and the results obtained were similar.
In vivo mouse infections.
Mice were anesthetized by intraperitoneal injection of a ketamine (100 mg/kg)-xylazine (10 mg/kg)-PBS solution and infected intranasally with wild-type L. pneumophila strain JR32, the isogenic ΔflaA derivative of JR32, or the clinical isolate F2111 in 40 μl of PBS. For in vivo bacterial growth assays, mice were euthanized by CO2 either at 4 h postinfection (day 0) or at the times indicated in the figures. Lungs, spleens, or livers were harvested and placed in 10 ml of sterile double-distilled H2O and homogenized using a PowerGen 125 handheld homogenizer (Fisher) for 30 s. Lysates were plated on CYE agar to determine the numbers of bacterial CFU. Each data point in the figures is the CFU count for a single mouse. All experiments were repeated at least one time independently, and similar results were obtained. The lower limit of detection in the assay was 100 CFU of L. pneumophila.
To determine cytokine levels in bronchoalveolar lavage fluid (BALF), mice were intranasally infected with either 1 × 106 or 4 × 104 CFU of L. pneumophila Δfla. Mice were euthanized by intraperitoneal injection of a ketamine (250 mg/kg)-xylazine (25 mg/kg)-PBS solution. The mouse lungs were lavaged once with 500 μl of PBS, and the BALF was stored at −80°C. IL-12 p40, IL-6, and IFN-γ levels in the BALF were determined by ELISA using BD Pharmingen IL-12 (p40/p70), IL-6, and IFN-γ reagents, respectively. Monocyte chemoattractant protein-1 ([MCP-1] BD OptEIA kit; BD Pharmingen) and keratinocyte-derived chemokine [KC] DuoSet Mouse KC; R&D Systems) levels were also determined by ELISA.
Lymphocyte isolation and intracellular cytokine staining.
Lymphocytes were isolated from lungs by digesting minced lungs for 1 h in a 37°C shaking incubator in collagenase buffer (RPMI 1640 medium, 100 U/ml collagenase I [Gibco], 5% fetal bovine serum, 0.1% CaCl2, 0.1% MgCl2). Lysates were put through a 70-μm-pore-size nylon cell strainer (BD Falcon), and cells were isolated using a Percoll (Sigma) gradient. Red blood cells were lysed with red blood cell lysing buffer (Sigma). Isolated lymphocytes were resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS, 1% FBS, 0.025% sodium azide) and blocked with purified rat anti-mouse CD16/CD32 (BD Pharmingen). Cells were stained with rat anti-mouse CD11b (Caltag Laboratories), rat anti-mouse Ly-6C/G (Gr-1) (Caltag Laboratories), and rat anti-mouse F4/80 (Caltag Laboratories). Neutrophils were gated as CD11b+, Gr-1+, and F4/80−. Inflammatory monocytes were gated as CD11b+, Gr-1+, and F4/80+. Data were collected using a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Generation of bone marrow-chimeric mice.
Recipient C57BL/6 (expressing the Ly5.1 antigen) mice were lethally irradiated with two doses of 500 rads over a 5-h period using a 137Cs γ-source and reconstituted intravenously with 1 × 107 bone marrow cells from donor C57BL/6 (expressing the Ly5.2 gene) wild-type, MyD88−/−, or Rip2−/− MyD88−/− mice. Mice were placed on Sulfatrim (40 mg/ml sulfamethoxazole-8 mg/ml trimethoprim) for 8 weeks following transplantation of bone marrow cells, and chimerism was confirmed by flow cytometry after differential staining of blood lymphocytes for Ly5.1 and Ly5.2. Mice were infected as described above.
Statistical analysis.
A two-tailed, Mann-Whitney U test was used to analyze the significance of differences in means between groups. Survival curves were generated using the Kaplan-Meier method, and the significance of differences was calculated by a log rank test. Differences were considered statistically significant if the P value was <0.05.
RESULTS
L. pneumophila activates Rip2-dependent pathways in vivo.
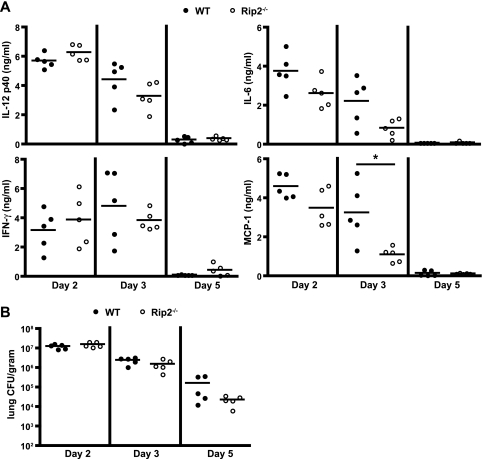
The activation of NF-κΒ by a Rip2-dependent pathway was shown following L. pneumophila infection of MyD88-deficient macrophages ex vivo (35, 52), suggesting that Nod1 and Nod2 are able to detect the presence of L. pneumophila products within the host cell cytosol (52). To determine whether Rip2 signaling is important for in vivo responses to L. pneumophila, wild-type (WT) and Rip2-deficient mice in the C57BL/6 background were infected intranasally with a flagellin-deficient (ΔflaA) strain of L. pneumophila. The L. pneumophila ΔflaA strain was used to evade activation of the Naip5 and NLRC4 signaling pathways. Mice were sacrificed at days 2, 3, and 5 postinfection, and inflammatory cytokine levels were measured in the BALF by ELISA. No significant difference was observed in the levels of IL-12 p40, IFN-γ, or IL-6 in the lung of Rip2-deficient mice compared to WT mice (Fig. 1A). A significant decrease was observed consistently in the levels of the chemokine MCP-1 (Fig. 1A), indicating a role for Rip2 in the detection of L. pneumophila in vivo. To determine whether the absence of Rip2 results in enhanced L. pneumophila replication in the lung, bacterial numbers were measured. No significant difference was detected in the number of L. pneumophila cells in the lung of Rip2-deficient mice compared to WT mice, and the rate of clearance from the lungs was also similar (Fig. 1B). To control for the possibility that the nonmotile phenotype exhibited by L. pneumophila deficient in flagellin might influence these results, the Rip2 deletion was introduced into mice that were deficient in Naip5 signaling as a result of being homozygous for the hypomorphic Naip5 allele from the A/J mouse (34). Similar results were obtained using Rip2-deficient mice having a defect in Naip5 signaling following infection with a clinical isolate of L. pneumophila that produces flagellin (see Fig. S1 in the supplemental material). Thus, bacterial motility and the production of flagellin did not affect the Rip2-dependent response.
FIG. 1.
L. pneumophila activates Rip2-dependent pathways in vivo. WT and Rip2-deficient (Rip2−/−) mice in a C57BL/6 background were given a high intranasal dose (1 × 106 CFU) of L. pneumophila ΔflaA. For each group, five mice were sacrificed at days 2, 3, and 5 postinfection as indicated below each graph. (A) BALF from infected mice was assayed for the indicated cytokines by ELISA. Each circle represents data obtained from a single mouse. The lines indicate the mean values calculated from the data for the two groups of mice. *, P < 0.05. (B) Bacterial numbers were determined from lung lysates at the indicated time points. There was no statistical significance between the two groups of mice (P > 0.05).
Rip2 is important for suppression of L. pneumophila replication in MyD88-deficient mice.
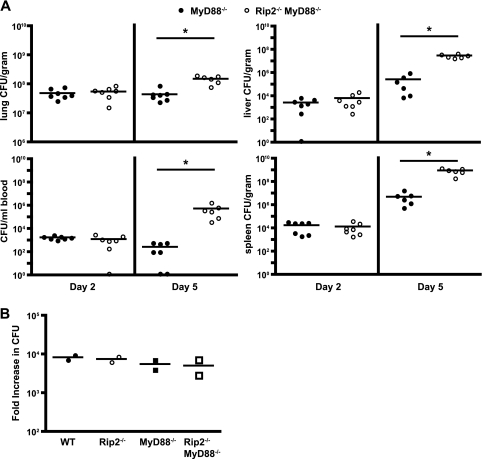
Because MyD88-dependent signaling contributes significantly to the innate immune response directed against L. pneumophila, host responses mediated by Rip2 may be masked by MyD88-dependent signaling pathways. To investigate this possibility, mice deficient in both Rip2 and MyD88 were generated in the C57BL/6 background and infected with L. pneumophila ΔflaA. At day 2 postinfection, there was no significant difference between the number of L. pneumophila ΔflaA CFU in the Rip2- and MyD88-deficient (Rip2/MyD88-deficient) mice and in the MyD88-deficient mice (Fig. 2A). At day 5, however, the Rip2/MyD88-deficient mice had slightly higher CFU counts in the lungs, liver, spleen, and blood than mice deficient in only MyD88 (Fig. 2A). This difference was statistically significant. Rip2/MyD88-deficient mice infected with motile wild-type L. pneumophila also had higher CFU counts than MyD88-deficient mice 5 days after infection, indicating that the contribution of Rip2 was independent of flagellin sensing by the Naip5/NLRC4 pathway (see Fig. S2 in the supplemental material). These data suggest that Rip2 signaling contributes to restricting L. pneumophila replication in vivo when MyD88-dependent responses are absent.
FIG. 2.
Rip2 is important for suppression of L. pneumophila replication in MyD88-deficient mice. (A) MyD88-deficient (MyD88−/−) and Rip2/MyD88-deficient (Rip2−/−MyD88−/−) mice in a C57BL/6 background were given a low intranasal dose (4 × 104 CFU) of L. pneumophila ΔflaA. At day 2 and day 5 postinfection, seven mice from each group were sacrificed, and bacterial numbers were determined from the lung, blood, liver, and spleen. Each point represents data from a single mouse. The lines indicate the mean values calculated from the data for the two groups of mice. The difference between the two groups of mice was statistically significant at day 5. *, P < 0.005. (B) BMMs from WT (filled circles), Rip2−/− (open circles), MyD88−/− (filled squares), and Rip2−/− MyD88−/− (open squares) mice were infected with L. pneumophila ΔflaA. The numbers of bacterial CFU were determined at 1 h and 48 h postinfection. Intracellular growth is expressed as the fold increase in the number of CFU detected over this period. Each point represents data for BMMs derived from a single mouse. All data points represent the average increase in the number of bacterial CFU determined from three wells infected independently. Each line indicates the mean calculated from the data for the two different mice.
To determine whether higher levels of replication in Rip2/MyD88-deficient mice result from an intrinsic defect in the ability of macrophages to restrict L. pneumophila multiplication, bone marrow-derived macrophages (BMMs) from WT, Rip2-deficient, MyD88-deficient, and Rip2/MyD88-deficient mice were infected with L. pneumophila ΔflaA, and intracellular growth was monitored for 3 days. L. pneumophila replication in macrophages lacking Rip2 was comparable to that in MyD88-deficient and WT cells (Fig. 2B). Similar results were observed using macrophages deficient in Naip5 signaling and infected with motile L. pneumophila producing flagellin (see Fig. S3 in the supplemental material). Thus, the enhanced replication of L. pneumophila observed in the Rip2/MyD88-deficient mice was not due to intrinsic differences in the ability of naive macrophages derived from these mice to support intracellular replication.
Cellular recruitment defects in Rip2/MyD88-deficient mice after L. pneumophila infection.
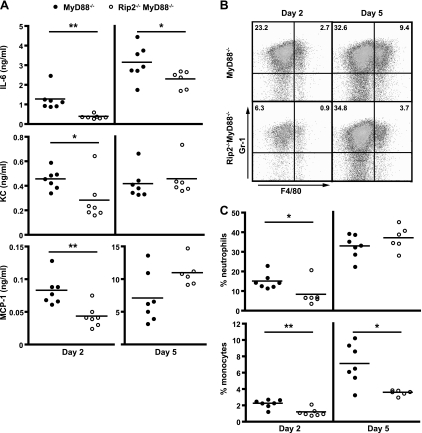
The cytokine IL-6 and chemokines KC and MCP-1 are detected at low levels after L. pneumophila infection of MyD88-deficient mice (Fig. 3A). To determine whether the higher bacterial numbers observed in the Rip2/MyD88-deficient mice correspond with a defect in production of proinflammatory chemokines or cytokines, MyD88-deficient and Rip2/MyD88-deficient mice were infected intranasally with L. pneumophila ΔflaA. Mice were sacrificed at days 2 and 5 postinfection, and ELISA measurements of BALF were used to assess the host response to infection. The levels of IL-6 at days 2 and 5 postinfection and of KC and MCP-1 at day 2 postinfection were reduced significantly in Rip2/MyD88-deficient mice compared to MyD88-deficient mice (Fig. 3A). Because the KC and MCP-1 attract neutrophils and inflammatory monocytes to infected tissues, respectively (9, 49), we examined whether the reduction of these chemoattractants in Rip2/MyD88-deficient mice early upon infection correlate with differences in cellular recruitment to the lung of infected mice. Rip2/MyD88-deficient mice had a significant reduction in the proportion of neutrophils (CD11b+, Gr-1+, and F4/80−) in the lung at day 2 postinfection and fewer inflammatory monocytes (CD11b+, Gr-1+, and F4/80+) at days 2 and 5 postinfection than MyD88-deficient mice (Fig. 3B and C). Thus, Rip2 signaling contributes to cytokine production and cellular recruitment to the lung of L. pneumophila-infected mice.
FIG. 3.
Cellular recruitment defects in Rip2/MyD88-deficient mice after L. pneumophila infection. MyD88−/− and Rip2−/− MyD88−/− mice in a C57BL/6 background were given a low intranasal dose (4 × 104 CFU) of L. pneumophila ΔflaA. Seven mice from each group were sacrificed at days 2 and 5 postinfection. (A) BALF from infected mice was assayed for the indicated cytokines by ELISA. Each point represents data from a single mouse. The horizontal lines indicate the mean values calculated from the data for the two groups of mice. (B and C) Cell suspensions from isolated lungs were stained for CD11b, Gr-1, and F4/80 and examined by flow cytometry. (B) Representative plots indicating the frequency of neutrophils (CD11b+, Gr-1+, and F4/80−; upper-left quadrant) and inflammatory monocytes (CD11b+, Gr-1+, and F4/80+; upper-right quadrant) from an individual MyD88−/− or Rip2−/− MyD88−/− mouse sacrificed at day 2 or day 5. (C) Average percentage of neutrophils or inflammatory monocytes from individual MyD88−/− or Rip2−/− MyD88−/− mice sacrificed at day 2 or day 5. Plots for mouse groups are arranged as in panel A. *, P < 0.05; **, P < 0.005.
Mice deficient for both Rip2 and MyD88 are highly susceptible to lethal infection by L. pneumophila.
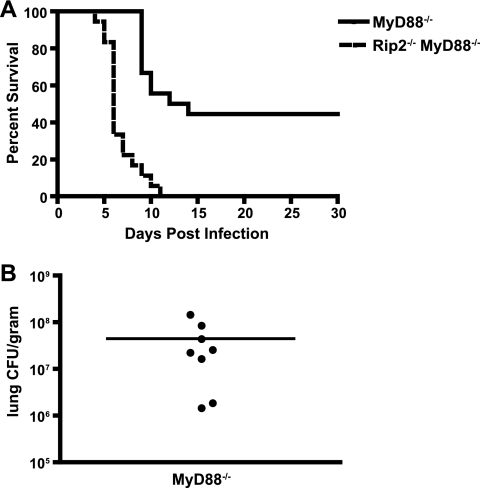
To investigate whether Rip2 signaling contributes to host protection upon infection by L. pneumophila, MyD88-deficient and Rip2/MyD88-deficient mice were infected with L. pneumophila ΔflaA and monitored for survival. Most of the infected Rip2/MyD88-deficient mice died within the first 7 days, and all of the mice died by day 11 (Fig. 4A). In contrast, all of the infected MyD88-deficient mice were alive at day 9, and 50% of the MyD88-deficient mice survived infection and appeared healthy at day 30. The surviving MyD88-deficient mice were sacrificed, and the numbers of bacteria in the lungs, liver, spleen, and blood were determined. The surviving MyD88-deficient mice had high levels of L. pneumophila in the lung, but bacteria were not detected in the liver, spleen, or blood (Fig. 4B). Similar findings were observed for Rip2/MyD88-deficient mice having a defect in Naip5 signaling and infected with motile L. pneumophila producing flagellin (see Fig. S4 in the supplemental material). These data indicate that Rip2 signaling in response to L. pneumophila activates pathways that provide host protection against infection and that these pathways play a critical role in host survival when MyD88 signaling is disrupted.
FIG. 4.
Mice deficient for both Rip2 and MyD88 are highly susceptible to lethal infection by L. pneumophila. MyD88−/− and Rip2−/− MyD88−/− mice in a C57BL/6 background were given a low intranasal dose (4 × 104 CFU) of L. pneumophila ΔflaA. (A) The graph represents the percentage of MyD88−/− (n = 19) and Rip2−/− MyD88−/− (n = 18) mice surviving at the time points indicated. The difference between the two groups of mice was significant (P < 0.0001). (B) Surviving MyD88−/− mice were sacrificed at day 30 postinfection, and the average number of CFU in the lung was measured. There were no detectible bacteria in either the liver or the spleen of these mice, and there were no surviving Rip2/MyD88-deficient mice to analyze. These data are in contrast to immune-sufficient mice, which did not have detectible bacteria in the lung at day 30 postinfection. Each point represents data from a single MyD88−/− mouse, and the line represents the average from all mice.
Host protection to L. pneumophila infection requires activation of innate signaling pathways in bone marrow-derived cells.
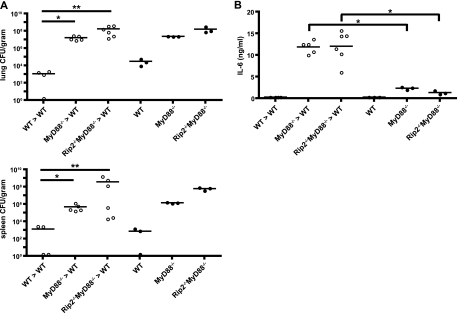
To elucidate cell types in the lung that require MyD88 and Rip2 signaling to direct a protective host response during infection by L. pneumophila, chimeric mice were generated by irradiating wild-type C57BL/6 mice and repopulating these mice with bone marrow cells obtained from either wild-type mice, MyD88-deficient mice, or Rip2/MyD88-deficient mice. Data examining L. pneumophila numbers at day 5 postinfection revealed that the chimeric mice had similar phenotypes as their respective bone marrow donor mice (Fig. 5A). Bacterial loads in the lung and spleen were significantly higher in the chimeric mice receiving MyD88-deficient or Rip2/MyD88-deficient bone marrow cells than in control mice receiving wild-type bone marrow cells. Importantly, bacterial colonization in the chimeric mice was not significantly different from that of their respective bone marrow-donor mice. High IL-6 levels were present in BALF from chimeric mice receiving MyD88-deficient or Rip2/MyD88-deficient bone marrow cells but not in BALF from respective donor MyD88-deficient or Rip2/MyD88-deficient mice (Fig. 5B), which indicates that a significant amount of Rip2/MyD88-dependent production of IL-6 is mediated by a radiation-resistant population of cells in the chimeric mice. Thus, even though radiation-resistant cells are contributing to the immune response, innate immune signaling pathways in the bone marrow-derived cells are critical for host protection against L. pneumophila.
FIG. 5.
Host protection from L. pneumophila infection requires activation of innate signaling pathways in bone marrow-derived cells. Bone marrow-chimeric mice were generated by irradiating wild-type (WT) mice and reconstituting them with donor bone marrow from either WT (WT>WT), MyD88-deficient (MyD88−/−>WT), or Rip2/MyD88-deficient (Rip2−/− MyD88−/−>WT) mice. Mice were given a low intranasal dose (4 × 104 CFU) of L. pneumophila ΔflaA. (A) Bacterial CFU in the lung and spleen was measured at 5 days postinfection for irradiated mice (open circles) receiving bone marrow from wild-type, MyD88−/−, or Rip2−/− MyD88−/− donor mice and for mice with the same genetic background as the donors (filled circles). (B) BALF from infected mice was assayed for IL-6. Each point represents data from a single mouse. The lines indicate the means calculated from data for each group of mice. *, P < 0.05; **, P < 0.01.
A functional hierarchy of pattern recognition receptors generates a protective immune response against L. pneumophila.
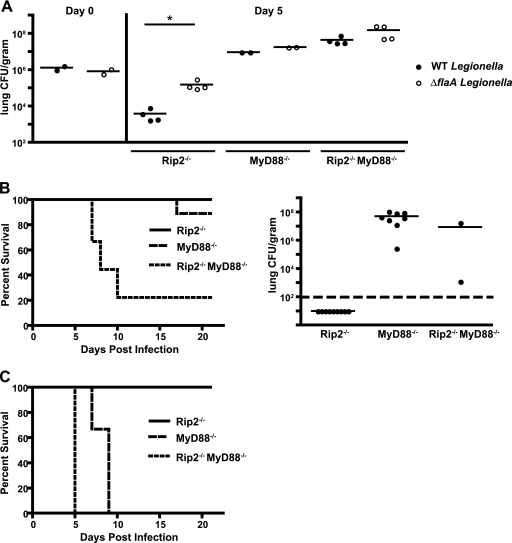
Rip2, MyD88, and Naip5/NLRC4 are activated during infection of macrophages by L. pneumophila, and each signaling pathway contributes to host protection. Although MyD88-dependent responses provide protection against flagellin-deficient L. pneumophila that fails to activate Naip5/NLRC4, flagellin-deficient L. pneumophila replicates to higher numbers and persists longer in the lungs of wild-type mice than isogenic L. pneumophila that produces flagellin (4, 5, 10, 34, 40), indicating that the contribution of Naip5/NLRC4 to bacterial clearance is not masked by a strong MyD88-dependent response. This is in contrast to results shown here for Rip2, where no significant difference was observed in L. pneumophila numbers when infected Rip2-deficient mice were compared to control wild-type mice. To determine whether the Naip5/NLRC4 pathway and the Rip2 pathway have functionally redundant or independent roles in providing host protection, immune-deficient mice were infected with a high dose of wild-type L. pneumophila or an isogenic ΔflaA strain, and bacterial colonization and host survival were compared.
Similar to what has been observed in wild-type mice, there were significantly more L. pneumophila ΔflaA bacteria in the lung of Rip2-deficient mice at day 5 postinfection than in wild-type L. pneumophila-infected mice (Fig. 6A). Rip2-deficient mice were able to control infection by either strain, as indicated by a decrease in the number of wild-type L. pneumophila and ΔflaA bacteria in the lung at day 5 compared to the count at 4 h postinfection (Fig. 6A). Additionally, all of the Rip2-deficient animals survived a high-dose infection by either wild-type L. pneumophila or the ΔflaA strain (Fig. 6B and C). In both MyD88-deficient and Rip2/MyD88-deficient mice, bacterial numbers were higher at day 5 than at 4 h postinfection, and the difference between the numbers of wild-type and ΔflaA bacteria was not as great as that which was observed in Rip2-deficient mice (Fig. 6A), indicating that MyD88-deficient mice are unable to suppress replication of these strains in vivo. Importantly, the majority of MyD88-deficient mice (9/10) survived a high-dose infection by wild-type L. pneumophila, whereas most Rip2/MyD88-deficient mice (8/10) died within the first 10 days of infection by this strain (Fig. 6B). Measurements of the number of wild-type L. pneumophila bacteria in the lungs of the surviving mice sacrificed 21 days after infection revealed that the Rip2-deficient mice were able to clear bacteria from the lungs, whereas persistent colonization of the lung by wild-type L. pneumophila was observed in the MyD88-deficient and Rip2/MyD88-deficient survivors (Fig. 6B). All of the MyD88-deficient and Rip2/MyD88-deficient mice receiving a high dose of the L. pneumophila ΔflaA strain died (Fig. 6C) although, consistent with what was observed for mice that succumbed following a low-dose infection, the Rip2/MyD88-deficient mice had a shorter mean time to death than the MyD88-deficient mice. Thus, Rip2 and Naip5/NLRC4 have additive and nonoverlapping roles in contributing to host protection following L. pneumophila infection.
FIG. 6.
Cooperative signaling between multiple pattern recognition systems is important for a protective immune response against L. pneumophila. Rip2−/−, MyD88−/−, and Rip2−/− MyD88−/− mice in a C57BL/6 background were given a high intranasal dose (1 × 106 CFU) of either wild-type L. pneumophila or L. pneumophila ΔflaA. (A) At day 5 postinfection bacterial CFU were measured in the lung. Data in the left panel (day 0) represent CFU measured in the lungs of control animals sacrificed 4 h after intranasal infection. Each point represents data from a single mouse. The lines indicate the means calculated from the data for the two groups of mice. The dashed line indicates the lower limit of detection. *, P < 0.05 (B) The graph on the left indicates the survival of Rip2−/−, MyD88−/−, and Rip2−/− MyD88−/− mice after a high intranasal dose (1 × 106 CFU) of wild-type L. pneumophila (n = 9 mice for each group). The difference between the MyD88−/− and Rip2−/− MyD88−/− mice was significant (P < 0.01). The graph on the right shows the number of L. pneumophila bacteria in the lungs of mice surviving until day 21. (C) Survival of Rip2−/−, MyD88−/−, and Rip2−/− MyD88−/− mice after a high intranasal dose (1 × 106 CFU) of L. pneumophila ΔflaA (n = 3 mice for each group). The difference between the MyD88−/− and Rip2−/− MyD88−/− mice was significant (P < 0.05).
DISCUSSION
Innate immune responses controlled by MyD88, Naip5/NLRC4, and Rip2 are activated by PRRs that detect bacterially derived molecules. How these systems interact to generate host immunity to bacterial pathogens has been an important question that has not been investigated systematically. Here, we addressed the importance of these pathways in providing protection against an intracellular pathogen using a mouse model of Legionnaires' disease. Our data suggest that these systems are responding independently to different microbial stimuli and that these individual responses are important elements of a complex functional hierarchy that regulates a multifaceted innate immune response, providing host protection against lethal infection by the intracellular pathogen L. pneumophila.
MyD88-dependent responses to L. pneumophila were analyzed previously both in macrophages cultured ex vivo and in mouse infection models (5, 6, 20, 54). These studies showed that MyD88 has a central role in generating a protective innate immune response to L. pneumophila. Extracellular L. pneumophila and bacteria that reside in endocytic vacuoles initiate MyD88-dependent responses through stimulation of TLR molecules, which mediate macrophage production of several key proinflammatory cytokines and chemokines through the activation of regulatory factors including NF-κΒ and MAPKs. The cytokines released by macrophages during L. pneumophila infection are important for the production of IFN-γ, a process that requires IL-18 activation of an MyD88-dependent pathway for IFN-γ expression by NK and NK T cells. Here, we show that Rip2-deficient mice infected with the L. pneumophila ΔflaA mutant were able to clear bacteria from the lung, indicating that the MyD88-dependent axis is able to protect the host against L. pneumophila infection in the absence of Rip2 and Naip5/NLRC4 stimulation. Thus, the MyD88 signaling module resides near the top of a hierarchical structure of signaling components critical for generating a protective innate immune response against L. pneumophila.
The Naip5/NLRC4 axis stimulated by bacterial flagellin was found to provide a level of protection against L. pneumophila that was measurable in all of the mouse genetic backgrounds examined in this study. Caspase-1 is activated upon stimulation of the Naip5/NLRC4 signaling pathway, and this response is important for the secretion of bioactive IL-1β and IL-18 from infected macrophages (4, 10, 34, 40, 45, 53, 58). This connects the Naip5/NLRC4 and MyD88 signaling pathways because increased production of pro-IL-1β is mediated by an MyD88-dependent pathway, and the receptors that respond to IL-1β and IL-18 utilize MyD88 for signaling (1, 39). Naip5/NLRC4 also controls MyD88-independent functions that result in a cell-autonomous response that limits intracellular replication of L. pneumophila. Caspase-1, caspase-7, interferon regulatory factor 1 (IRF1), and IRF8 have all been implicated in the cell-autonomous response regulated by Naip5/NLRC4 signaling (3, 4, 10, 16, 34, 40, 45, 53, 58); however, the exact mechanism by which Naip5/NLRC4 restricts replication of L. pneumophila is not completely clear. Stimulation of the Naip5/NLRC4 pathway in MyD88-deficient macrophages cultured ex vivo is sufficient to restrict replication of L. pneumophila (40). In contrast, we observed that in MyD88-deficient mice, wild-type bacteria grew to levels that were similar to those of the L. pneumophila ΔflaA strain, which indicates that replication of wild-type L. pneumophila in the lung was not restricted severely by the Naip5/NLRC4 pathway. Interestingly, the majority of MyD88-deficient mice survived a high-dose challenge by wild-type L. pneumophila, whereas most MyD88-deficient mice died after infection with the isogenic ΔflaA mutant. From these data we conclude that in the lung the Naip5/NLRC4 axis provides a level of host protection that is independent of MyD88 and that may extend beyond a cell-autonomous response that limits replication in macrophages.
Rip2 was found to be involved in the innate immune response to L. pneumophila. Previously, it was shown that Rip2 participates in the activation of NF-κΒ following infection of macrophages by L. pneumophila; however, this activation pathway was masked by the MyD88-dependent pathway of NF-κΒ activation (35, 52). Consistent with what was observed using mouse macrophages cultured ex vivo, Rip2-deficient mice had a very subtle phenotype compared to wild-type mice. IL-6 and MCP-1 levels were slightly diminished in the lungs of Rip2-deficient mice, but no significant differences in bacterial loads or host susceptibility were detected following infection by L. pneumophila. Our results using L. pneumophila differ slightly from data obtained recently for the intracellular pathogen Chlamydophila pneumoniae, where delayed pathogen clearance from the lung and an increased lethality were observed after infection of Rip2-deficient mice (51). This might relate to the fact that C. pneumoniae is an obligate intracellular pathogen that continues to evolve in association with mammalian hosts and thereby has acquired mechanisms to interfere with host immune signaling pathways. In contrast, L. pneumophila is an organism that normally lives in freshwater and soil and has coevolved with unicellular protozoan hosts, so it would be less likely to interfere with signaling pathways unique to mammalian hosts. Thus, the enhanced importance of Rip2 signaling in the host response to C. pneumoniae compared to the response to L. pneumophila may reflect differences in the ability of these two organisms to modulate innate immune pathways rather than fundamental differences in the role Rip2 plays in responding to pathogen infection.
Rip2 signaling was found to be very important in host protection in MyD88-deficient mice. MyD88 and Rip2 signaling both converge on the NF-κΒ activation pathway. Thus, it is not unexpected that Rip2 and MyD88 would have partially overlapping functions. An important difference between these two pathways lies in the mechanism by which they are activated in response to infection by L. pneumophila. MyD88-dependent NF-κΒ activation occurs after TLR engagement by either virulent or avirulent L. pneumophila. In contrast, Rip2-dependent NF-κΒ activation occurs after intracellular infection by L. pneumophila encoding a functional Dot/Icm type IV secretion system (52), which presumably translocates into the cytosol bacterial products that activate the PRRs Nod1 and Nod2. Thus, the MyD88 and Rip2 signaling systems cooperate with each other to mediate sustained activation of NF-κΒ during the course of macrophage infection by virulent L. pneumophila and enable the host to distinguish between nonpathogenic microbes and pathogens that have evolved mechanisms to establish direct communication with the host cytosol.
Although a defect in sustained NF-κΒ activation could account for the difference observed in the bulk levels of IL-6 and MCP-1 in the lungs of infected Rip2-deficient mice, it seems unlikely that the main role for Rip2 would be to increase the cellular output of cytokines. Rather, it seems more likely that Rip2 signaling provides a mechanism that allows infected cells to maintain a cytokine gradient that would enable immune effector cells to migrate efficiently to the location where infected macrophages reside. We hypothesize that for an intracellular pathogen like L. pneumophila, which completes a cycle of growth in macrophages rather quickly, the contribution of Rip2 in maintaining a cytokine gradient may not be as important because bacteria will be unable to evade MyD88-dependent detection for an extended period of time. Our hypothesis predicts a more critical role for Rip2 signaling in host protection against pathogens that persist intracellularly for extended periods of time and replicate slowly. This might also contribute to the more pronounced role for Rip2 in mediating protection against C. pneumoniae.
Overall, these data suggest that the innate immune response to L. pneumophila can be divided into three phases. Initially, macrophages are infected by L. pneumophila and initiate the production of cytokines. Previous studies and data using chimeric mice presented here suggest that MyD88, Rip2, and Naip5/NLRC4 all participate in this early process of pathogen recognition by macrophages. Cytokine production by macrophages is important for the recruitment of additional immune effector cells into the lung and for the activation of IFN-γ by NK and NK T cells recruited to the site of infection (5, 54). MyD88 and Rip2 signaling were shown here and in previous studies (5, 6, 20, 52, 54) to mediate responses important for upregulation of cytokine expression, and Naip5/NLRC4 signaling contributes to the processing and secretion of several cytokines. The second phase involves restricting the intracellular replication of L. pneumophila in macrophages, which both MyD88 and Naip5/NLRC4 appear to have a direct role in mediating. Activation of macrophages by IFN-γ is clearly one of the most potent and critical effector responses that limit intracellular replication of L. pneumophila in macrophages (8, 42), and the most abundant source of IFN-γ produced during infection comes from NK and NK T cells (5, 54). IFN-γ production by these cells involves MyD88-dependent signaling in response to IL-18 (5), which is secreted by macrophages and neutrophils after Naip5/NLRC4-mediated caspase-1 activation (10, 55). Naip5/NLRC4 also plays a role in activating a cell-autonomous response that limits replication in infected macrophages, but the molecular mechanism by which this occurs remains unknown. The third phase of the innate immune response to L. pneumophila is characterized by the recruitment of neutrophils to the site of infection. L. pneumophila is unable to replicate within neutrophils, and these highly chemotactic and phagocytic cells play an important role in eliminating extracellular L. pneumophila (32). MyD88-dependent production of chemokines contributes significantly to neutrophil recruitment. Rip2-deficient mice infected with L. pneumophila were also found to have a defect in chemokine production, and there was a delay in the recruitment of neutrophils to the lung, suggesting that Rip2 and MyD88 signaling plays a cooperative role in directing neutrophils to the site of infection.
In conclusion, these data provide a detailed picture of how different pattern recognition systems function coordinately to generate a robust and efficient innate immune response capable of limiting the replication of an intracellular pathogen. The susceptibility phenotypes observed for immune-deficient mice could be explained by modeling the overall contribution of these pattern recognition receptor systems to the generation of the three phases of a protective immune response. The increase in the total number of L. pneumophila bacteria observed in mice deficient in MyD88 or Naip5/NLRC4 signaling would result primarily from an inability of macrophages to restrict bacterial intracellular replication. IFN-γ production in mice deficient in Naip5/NLRC4 signaling eventually compensates for the defect that macrophages from these mice have in restricting intracellular replication of L. pneumophila, which explains why bacterial numbers eventually decline in the lung of mice infected with the ΔflaA strain when MyD88 signaling remains operational. In the absence of both MyD88 and Naip5/NLRC4 signaling, pattern recognition receptors that signal through Rip2 provide a measure of host protection by assisting in the recruitment of neutrophils. Rapid neutrophil recruitment mediated by Rip2 signaling served to help contain the infection in the lung and prevent progression to a systemic disease that results in host mortality, resulting in mice that showed persistent lung colonization but remained viable 3 weeks after infection.
Supplementary Material
Acknowledgments
We thank S. Akira (Osaka, Japan) for permission to use MyD88-deficient mice in this study, R. Medzhitov (Yale University) for providing mice, H. Shuman (Columbia University) for providing the JR32 strain, and P. Edelstein (University of Pennsylvania) for providing the F2111 isolate. We also thank S. Shin (Yale University) for helpful comments and discussions and T. Hand, W. Cui, and P. Bongiorni (Yale University) for technical assistance with chimeric studies.
This work was supported by NIH grant R01-AI048770 to C.R.R.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 29 March 2010.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akamine, M., F. Higa, N. Arakaki, K. Kawakami, K. Takeda, S. Akira, and A. Saito. 2005. Differential roles of Toll-like receptors 2 and 4 in in vitro responses of macrophages to Legionella pneumophila. Infect. Immun. 73:352-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akhter, A., M. A. Gavrilin, L. Frantz, S. Washington, C. Ditty, D. Limoli, C. Day, A. Sarkar, C. Newland, J. Butchar, C. B. Marsh, M. D. Wewers, S. Tridandapani, T. D. Kanneganti, and A. O. Amer. 2009. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog. 5:e1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amer, A., L. Franchi, T. D. Kanneganti, M. Body-Malapel, N. Ozoren, G. Brady, S. Meshinchi, R. Jagirdar, A. Gewirtz, S. Akira, and G. Nunez. 2006. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281:35217-35223. [DOI] [PubMed] [Google Scholar]
- 5.Archer, K. A., L. Alexopoulou, R. A. Flavell, and C. R. Roy. 2009. Multiple MyD88-dependent responses contribute to pulmonary clearance of Legionella pneumophila. Cell Microbiol. 11:21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer, K. A., and C. R. Roy. 2006. MyD88-dependent responses involving Toll-like receptor 2 are important for protection and clearance of Legionella pneumophila in a mouse model of Legionnaires' disease. Infect. Immun. 74:3325-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhan, U., G. Trujillo, K. Lyn-Kew, M. W. Newstead, X. Zeng, C. M. Hogaboam, A. M. Krieg, and T. J. Standiford. 2008. Toll-like receptor 9 regulates the lung macrophage phenotype and host immunity in murine pneumonia caused by Legionella pneumophila. Infect. Immun. 76:2895-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhardwaj, N., T. W. Nash, and M. A. Horwitz. 1986. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 137:2662-2669. [PubMed] [Google Scholar]
- 9.Bozic, C. R., L. F. Kolakowski, Jr., N. P. Gerard, C. Garcia-Rodriguez, C. von Uexkull-Guldenband, M. J. Conklyn, R. Breslow, H. J. Showell, and C. Gerard. 1995. Expression and biologic characterization of the murine chemokine KC. J. Immunol. 154:6048-6057. [PubMed] [Google Scholar]
- 10.Case, C. L., S. Shin, and C. R. Roy. 2009. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect. Immun. 77:1981-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamaillard, M., M. Hashimoto, Y. Horie, J. Masumoto, S. Qiu, L. Saab, Y. Ogura, A. Kawasaki, K. Fukase, S. Kusumoto, M. A. Valvano, S. J. Foster, T. W. Mak, G. Nunez, and N. Inohara. 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4:702-707. [DOI] [PubMed] [Google Scholar]
- 12.Derre, I., and R. R. Isberg. 2004. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect. Immun. 72:3048-3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelstein, P. H., and M. A. Edelstein. 1989. WIN 57273 is bactericidal for Legionella pneumophila grown in alveolar macrophages. Antimicrob. Agents Chemother. 33:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feeley, J. C., R. J. Gibson, G. W. Gorman, N. C. Langford, J. K. Rasheed, D. C. Mackel, and W. B. Baine. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286-290. [DOI] [PubMed] [Google Scholar]
- 16.Fortier, A., K. Doiron, M. Saleh, S. Grinstein, and P. Gros. 2009. Restriction of Legionella pneumophila replication in macrophages requires concerted action of the transcriptional regulators Irf1 and Irf8 and Nod-like receptors Naip5 and Nlrc4. Infect. Immun. 77:4794-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser, D. W., T. R. Tsai, W. Orenstin, W. E. Parken, H. J. Beechan, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 18.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 19.Hawn, T. R., W. R. Berrington, I. A. Smith, S. Uematsu, S. Akira, A. Aderem, K. D. Smith, and S. J. Skerrett. 2007. Altered inflammatory responses in TLR5-deficient mice infected with Legionella pneumophila. J. Immunol. 179:6981-6987. [DOI] [PubMed] [Google Scholar]
- 20.Hawn, T. R., K. D. Smith, A. Aderem, and S. J. Skerrett. 2006. Myeloid differentiation primary response gene (88)- and Toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J. Infect. Dis. 193:1693-1702. [DOI] [PubMed] [Google Scholar]
- 21.Hawn, T. R., A. Verbon, K. D. Lettinga, L. P. Zhao, S. S. Li, R. J. Laws, S. J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, A. Ozinsky, K. D. Smith, and A. Aderem. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to Legionnaires' disease. J. Exp. Med. 198:1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz, M. A. 1983. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158:1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Invest. 66:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inohara, N., T. Koseki, L. del Peso, Y. Hu, C. Yee, S. Chen, R. Carrio, J. Merino, D. Liu, J. Ni, and G. Nunez. 1999. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-κB. J. Biol. Chem. 274:14560-14567. [DOI] [PubMed] [Google Scholar]
- 26.Inohara, N., T. Koseki, J. Lin, L. del Peso, P. C. Lucas, F. F. Chen, Y. Ogura, and G. Nunez. 2000. An induced proximity model for NF-κB activation in the Nod1/RICK and RIP signaling pathways. J. Biol. Chem. 275:27823-27831. [DOI] [PubMed] [Google Scholar]
- 27.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 28.Inohara, N., Y. Ogura, F. F. Chen, A. Muto, and G. Nunez. 2001. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J. Biol. Chem. 276:2551-2554. [DOI] [PubMed] [Google Scholar]
- 29.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 30.Kagan, J. C., and C. R. Roy. 2002. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4:945-954. [DOI] [PubMed] [Google Scholar]
- 31.Kagan, J. C., M. P. Stein, M. Pypaert, and C. R. Roy. 2004. Legionella subvert the functions of Rab1 and Sec22b to create a replicative organelle. J. Exp. Med. 199:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz, S. M., and S. Hashemi. 1982. Electron microscopic examination of the inflammatory response to Legionella pneumophila in guinea pigs. Lab Invest. 46:24-32. [PubMed] [Google Scholar]
- 33.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194-199. [DOI] [PubMed] [Google Scholar]
- 34.Lightfield, K. L., J. Persson, S. W. Brubaker, C. E. Witte, J. von Moltke, E. A. Dunipace, T. Henry, Y. H. Sun, D. Cado, W. F. Dietrich, D. M. Monack, R. M. Tsolis, and R. E. Vance. 2008. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 9:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losick, V. P., and R. R. Isberg. 2006. NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J. Exp. Med. 203:2177-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marra, A., S. J. Blander, M. A. Horwitz, and H. A. Shuman. 1992. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc. Natl. Acad. Sci. U. S. A. 89:9607-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDade, J. E., C. C. Shepard, D. W. Fraser, T. R. Tsai, M. A. Redus, and W. R. Dowdle. 1977. Legionnaires' disease: isolation of a bacterium and demonstration of its role in other respiratory diseases. N. Engl. J. Med. 297:1197-1203. [DOI] [PubMed] [Google Scholar]
- 38.Medzhitov, R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819-826. [DOI] [PubMed] [Google Scholar]
- 39.Medzhitov, R., P. Preston-Hurlburt, E. Kopp, A. Stadlen, C. Chen, S. Ghosh, and C. A. Janeway, Jr. 1998. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2:253-258. [DOI] [PubMed] [Google Scholar]
- 40.Molofsky, A. B., B. G. Byrne, N. N. Whitfield, C. A. Madigan, E. T. Fuse, K. Tateda, and M. S. Swanson. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203:1093-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 42.Nash, T. W., D. M. Libby, and M. A. Horwitz. 1988. IFN-gamma-activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J. Immunol. 140:3978-3981. [PubMed] [Google Scholar]
- 43.Newton, C. A., I. Perkins, R. H. Widen, H. Friedman, and T. W. Klein. 2007. Role of Toll-like receptor 9 in Legionella pneumophila-induced interleukin-12 p40 production in bone marrow-derived dendritic cells and macrophages from permissive and nonpermissive mice. Infect. Immun. 75:146-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 45.Ren, T., D. S. Zamboni, C. R. Roy, W. F. Dietrich, and R. E. Vance. 2006. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabbah, A., T. H. Chang, R. Harnack, V. Frohlich, K. Tominaga, P. H. Dube, Y. Xiang, and S. Bose. 2009. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10:1073-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. U. S. A. 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serbina, N. V., and E. G. Pamer. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7:311-317. [DOI] [PubMed] [Google Scholar]
- 50.Shaw, M. H., T. Reimer, C. Sanchez-Valdepenas, N. Warner, Y. G. Kim, M. Fresno, and G. Nunez. 2009. T cell-intrinsic role of Nod2 in promoting type 1 immunity to Toxoplasma gondii. Nat. Immunol. 10:1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimada, K., S. Chen, P. W. Dempsey, R. Sorrentino, R. Alsabeh, A. V. Slepenkin, E. Peterson, T. M. Doherty, D. Underhill, T. R. Crother, and M. Arditi. 2009. The NOD/RIP2 pathway is essential for host defenses against Chlamydophila pneumoniae lung infection. PLoS Pathog. 5:e1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin, S., C. L. Case, K. A. Archer, C. V. Nogueira, K. S. Kobayashi, R. A. Flavell, C. R. Roy, and D. S. Zamboni. 2008. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 4:e1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silveira, T. N., and D. S. Zamboni. 2010. Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect. Immun. 78:1403-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sporri, R., N. Joller, U. Albers, H. Hilbi, and A. Oxenius. 2006. MyD88-dependent IFN-γ production by NK cells is key for control of Legionella pneumophila infection. J. Immunol. 176:6162-6171. [DOI] [PubMed] [Google Scholar]
- 55.Sporri, R., N. Joller, H. Hilbi, and A. Oxenius. 2008. A novel role for neutrophils as critical activators of NK cells. J. Immunol. 181:7121-7130. [DOI] [PubMed] [Google Scholar]
- 56.Swanson, M. S., and R. R. Isberg. 1995. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect. Immun. 63:3609-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 58.Zamboni, D. S., K. S. Kobayashi, T. Kohlsdorf, Y. Ogura, E. M. Long, R. E. Vance, K. Kuida, S. Mariathasan, V. M. Dixit, R. A. Flavell, W. F. Dietrich, and C. R. Roy. 2006. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7:318-325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.