Abstract
The oral bacterium Prevotella intermedia attaches to and invades gingival epithelial cells, fibroblasts, and endothelial cells. Several genes encoding proteins that mediate both the adhesion and invasion processes are carried on the genome of this bacterium. Here, we characterized one such protein, AdpC, belonging to the leucine-rich repeat (LRR) protein family. Bioinformatics analysis revealed that this protein shares similarity with the Treponema pallidum LRR (LRRTP) family of proteins and contains six LRRs. Despite the absence of a signal peptide, this protein is localized on the bacterial outer membrane, indicating that it is transported through an atypical secretion mechanism. The recombinant form of this protein (rAdpC) was shown to bind fibrinogen. In addition, the heterologous host strain Escherichia coli BL21 expressing rAdpC (V2846) invaded fibroblast NIH 3T3 cells at a 40-fold-higher frequency than control E. coli BL21 cells expressing a sham P. intermedia 17 protein. Although similar results were obtained by using human umbilical vein endothelial cells (HUVECs), only a 3-fold-increased invasion of V2846 into oral epithelial HN4 cells was observed. Thus, AdpC-mediated invasion is cell specific. This work demonstrated that AdpC is an important invasin protein of P. intermedia 17.
Prevotella intermedia, a black-pigmented, Gram-negative, anaerobic bacterium, is associated with periodontal disease. Lopez previously found a high prevalence of Porphyromonas gingivalis and P. intermedia in adult periodontitis lesions (31). Although P. intermedia is also found at healthy sites (30, 44), the profile of degradative enzymes produced by the organism varies depending on the site at which it is present (33). This suggests that under certain conditions, this organism alters its enzyme profile to become more virulent, which promotes the progression of periodontitis. In addition to periodontitis, P. intermedia has also been shown to be associated with endodontic infections such as root canal infection, apical periodontitis, and periapical lesions. In fact, Baumgartner et al. showed previously that 36% of samples from endodontic infections contain P. intermedia (1). Also, P. intermedia has been found in extraoral sites in bacterial tracheitis in children (7) and cancrum oris (also known as noma, an infection that destroys orofacial tissues) lesions (17, 18). Finally, previous studies suggested that chronic infections, including those associated with periodontitis, increase the risk of systemic diseases such as coronary heart disease and preterm delivery of low-birth-weight infants. Haraszthy et al. previously found periodontopathogens, including P. intermedia, in atherosclerotic plaques (22). In addition, Madianos et al. (32) previously demonstrated a significantly higher prevalence of positive fetal IgM to P. intermedia for preterm infants than for full-term infants.
P. intermedia invades a variety of nonphagocytic eukaryotic cells (16). The ability to invade these cells affords the bacteria access to a nutritionally rich environment as well as an escape from host immune defenses. The type C fimbriae have been shown to play a major role in the invasion process of epithelial cells, as the anti-type C fimbria antibody inhibited invasion (16). However, we postulated that other proteins mediating the attachment and invasion processes of P. intermedia remain unknown. The availability of the genomic sequence of P. intermedia 17 allowed us to apply genomic approaches to identify and characterize such candidates. Using proteomic approaches, we have identified and characterized the surface protein AdpB, which binds a variety of host extracellular matrix (ECM) components (47). Furthermore, analysis of the P. intermedia 17 genome sequence has revealed the presence of multiple genes encoding proteins with similarity to the leucine-rich repeat (LRR) family of proteins (27). These proteins have been demonstrated to be present in a variety of organisms, including viruses, bacteria, archaea, and eukaryotes, and have been shown to play a role in the immune response, apoptosis, adhesion, invasion, signal transduction, and DNA/RNA processing (5, 27). Thus, we reasoned that the P. intermedia 17 LRR-like proteins play a role in the attachment and invasion of the bacterium into host cells as well. Here, we report one such P. intermedia 17 protein, AdpC, encoded by PI0136 (www.oralgen.lanl.gov), that confers an invasive phenotype to Escherichia coli cells expressing the protein.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. intermedia strain 17, provided by Kai Leung (U.S. Army Dental Research Detachment, Great Lakes, IL), was used. Bacteria were maintained on blood agar plates (tryptic soy agar II [TSA II], 5% sheep blood; BBL, Cockeysville, MD) under anaerobic conditions in an atmosphere consisting of 80% N2, 10% H2, and 10% CO2 at 37°C. Broth cultures were prepared in brain heart infusion (BHI) broth (Difco Laboratories, Detroit, MI) supplemented with 5 μg/ml hemin, 5 mg/ml yeast extract, 1 mg/ml cysteine, and 1 μg/ml menadione. E. coli strains TOP10 (Invitrogen, Carlsbad, CA) and BL21(DE3) (Invitrogen, Carlsbad, CA), used for cloning and protein expression, respectively, were grown aerobically at 37°C in Luria-Bertani (LB) broth or on 1.5% agar plates with 50 μg/ml kanamycin. All bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain | Plasmid | Description | Source or reference |
|---|---|---|---|
| P. intermedia 17 | Parent strain | Kai Leung, U.S. Army Dental Research Detachment, Great Lakes, IL | |
| E. coli | |||
| One Shot | Chemically competent cell | Invitrogen | |
| TOP10 | pCR 2.1 | Cloning vector | Invitrogen |
| V2920 | pVA2920 | Knr; pCR2.1 containing the 0.9-kb adpC gene in TOP10 cells | This study |
| V2846 | pVA2846 | Knr; pET30a containing the 0.9-kb adpC gene in BL21(DE3) cells; expression construct | This study |
| V2720 | pVA2720 | Knr; pET30a | Novagen |
| V2848 | pVA2848 | Knr; pET30a containing the 0.9-kb PI1571 gene in BL21(DE3) cells; expression construct | This study |
| V2847 | pVA2847 | Knr; pET30a containing 0.9-kb PI2109 in BL21(DE3) cells; expression construct | This study |
| V2921 | pVA2921 | Knr; pET30a containing the 2-kb C-terminal region of pPI0035 in BL21(DE3) cells; expression construct | This study |
| V2922 | pVA2922 | Knr; pET30a containing 2-kb pPI0626 in BL21(DE3) cells; expression construct | This study |
Eukaryotic cells and growth conditions.
Human umbilical vein endothelial cells (HUVECs) were obtained from Cambrex Corporation (Walkersville, MD) and maintained in endothelial growth medium (EGM; Cambrex Corporation, Walkersville, MD) at 37°C in 95% air-5% CO2 under standard conditions as described previously (23). NIH 3T3 cells, a kind gift from Silvio Gutkind (OPBC, NIDCR, NIH), were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml penicillin-streptomycin at 37°C in 90% air-10% CO2. HN4 cells were derived from a primary tongue squamous cell carcinoma and were obtained from W. Andrew Yeudall of Virginia Commonwealth University (VCU) (Richmond, VA) (12). Cells were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin-streptomycin, 100 mM sodium pyruvate, and 100 mM HEPES at 37°C under an atmosphere of 90% air-10% CO2.
5′ RACE.
5′ rapid amplification of cDNA ends (RACE) was performed as described previously (29). Briefly, total P. intermedia 17 RNA was prepared by using an RNeasy minikit (Qiagen). Five micrograms of total RNA and 200 pmol 5′-phosphorylated primer (Div-P) with the sequence 5′-GTTTCCTTGCCTTCAGCATC-3′ were used to generate cDNA. The cDNA was then purified with the MinElute PCR purification kit (Qiagen) and self-ligated with T4 RNA ligase (New England Biolabs) at an ambient temperature for 18 h. The ligated cDNA was amplified by a first PCR using a gradient annealing temperature ranging from 45°C to 60°C and primers Div-R1 (5′-GGTCTTAAGTTCTTCACCAAG-3′) and Div-F1 (5′-GCGCAAGCAAACGCGCTCG-3′). A 1:1,000 dilution of the primary PCR product was then used as a template to perform a secondary gradient PCR with an annealing temperature ranging from 45°C to 53°C with primers Div-R2 (5′-CTGGTTGGGCAACATCTATCG-3′) and Div-F2 (5′-CTTAAACCTTTTAGATGCTGC-3′). The presence and size of PCR products were determined by using 3% agarose gel electrophoresis. The sequences of the PCR products were determined by sequencing using DNA fragments extracted from gels as templates.
Cloning of adpC.
Primers for the full-length gene were designed to amplify the 900-bp gene (termed here adpC [ad for adhesin, p for Prevotella, and C because it is the third adhesin identified in P. intermedia 17]). The sequence of the forward primer was 5′-GTCACTACCATGGCTGTGTTCTTCAGTTGCACGC-3′, and the sequence of the reverse primer was 5′-GCTATCCTCGAGTATTGCGCCCGCCTTCACCTC-3′ (where the underlined sequences indicate NcoI and XhoI sites, respectively). P. intermedia 17 genomic DNA was used as a template for the amplification of the adpC gene. The PCR-amplified DNA was then cloned into a pCR 2.1 vector (Invitrogen, Carlsbad, CA) and transformed into TOP10 cells (Invitrogen, Carlsbad, CA). White colonies selected on LB agar plates containing kanamycin (50 μg/ml) and X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml) were examined for plasmid content. The NcoI/XhoI DNA fragment carrying the adpC gene was isolated from the recombinant pCR-adpC plasmid (pVA2920) and inserted into the pET30a expression vector (Novagen, Madison, WI) so that the expressed protein would fuse with the N-terminal hexahistidine and S tags as well as the C-terminal hexahistidine tag to facilitate detection and purification. DNA sequencing using the T7 promoter and the T7 terminator was used to verify the proper orientation of the insert and confirm that no changes were introduced into the adpC sequence.
Expression and purification of rAdpC.
The pET30a-adpC (pVA2846) construct was transformed into E. coli BL21(DE3) expression cells, and positive transformants were selected on LB agar plates containing kanamycin (30 μg/ml). A single colony was inoculated into 50 ml of LB medium containing 30 μg/ml of kanamycin and grown overnight at 37°C. This culture was then used to inoculate 1 liter of LB broth medium containing 30 μg/ml of kanamycin. The culture was incubated at 37°C until the optical density at 660 nm (OD660) reached about 0.6. AdpC expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to the culture, which was then allowed to incubate for an additional 4 h at 37°C. Cells were harvested by centrifugation at 7,500 rpm for 25 min. Recombinant AdpC (rAdpC) was purified by using nickel agarose (Ni-nitrilotriacetic acid [NTA]) affinity chromatography under native conditions according to the manufacturer's instructions (Qiagen, Valencia, CA). The purified protein was dialyzed against phosphate-buffered saline (PBS) (1× PBS, pH 7.4). The purity of the protein was assessed by using one-dimensional SDS-PAGE.
ELISA.
Ninety-six-well plates were coated with 100 μl of 1 μg/ml, 3 μg/ml, 6 μg/ml, 12.5 μg/ml, 25 μg/ml, 50 μg/ml, and 75 μg/ml of rAdpC overnight. The plates were then washed three times in PBS containing Tween 20 (PBS-T) (0.05%, vol/vol) and blocked with 1% nonfat milk in PBS for 2 h at 37°C. Fibrinogen (fraction I type I from human plasma), fibronectin (from human placenta), laminin (from human placenta), and collagen (type I from human skin) were purchased from Sigma-Aldrich (St. Louis, MO) and prepared in PBS (pH 7.4). Solutions (100 μl) containing 20 μg/ml of protein were then added to the wells and incubated at 37°C for 2 h. The plates were washed with 0.05% Tween 20 in PBS (pH 7.4). Following washing, the plates were incubated with antibody specific for fibronectin, fibrinogen, laminin, or collagen at 37°C for 2 h. After incubation, the plates were washed and incubated with a 1:2,000 dilution of secondary antibody (anti-rabbit IgG alkaline phosphatase [AP] conjugate) for 1 h at 37°C. Plates were then washed, and the color was developed by using a substrate for alkaline phosphatase (p-nitrophenylphosphate [PNPP] substrate solution; Bio-Rad, Hercules, CA). Fluorescent signals were detected at 405 nm with a Fluostar Galaxy spectrophotometer (BMG Labtechnologies Inc., Durham, NC). To determine the specificity of binding of AdpC to fibrinogen, a dose-dependent binding assay was done as described above by using bovine serum albumin (BSA) as a negative control. For competitive enzyme-linked immunosorbent assay (ELISA), a 96-well plate was coated with rAdpC (20 μg/ml) and incubated at 37°C for 2 h. Following washing with 1× PBS containing 0.05% Tween, the plate was blocked with 1% skim milk in 1× PBS overnight at 4°C, washed again as described above, and then bound with fibrinogen (5 μg/ml) preincubated with various amounts of rAdpC (0.25 mg/ml used to make dilutions ranging from 1 to 1:128). The plate was incubated overnight at 4°C, and the signal was developed as described above.
Bacterial cell fractionation.
P. intermedia 17 outer membrane proteins were isolated as described previously (36, 47). E. coli outer membranes were isolated as described previously by Carlone et al. (9). Cells were harvested and broken down by sonication. Membrane proteins were harvested by centrifugation in a microcentrifuge (three times at 15,000 × g at 4°C), and cytoplasmic proteins were solubilized with 2% Sarkosyl (in 10 mM HEPES, pH 7.4). Outer membranes were recovered by centrifugation as described above (15,000 × g at 4°C) and suspended in 10 mM HEPES buffer.
Anti-rAdpC antibody preparation.
The generation of rabbit polyclonal anti-rAdpC antibody was done commercially by Proteintech Group Inc. Briefly, 200 μg of rAdpC mixed with complete Freund's adjuvant was used to immunize two New Zealand White rabbits. The rabbits were then boosted twice with 100 μg of rAdpC in incomplete Freund's adjuvant at 2-week intervals. Blood was collected 2 weeks following the final boost. Sera were prepared, and the antibody was affinity purified using nitrocellulose membrane-immobilized rAdpC, as described previously (29, 41).
Western blot analysis.
Proteins from various E. coli V2846 and P. intermedia 17 cell fractions (whole-cell lysate or outer membrane) were separated on SDS-PAGE gels and transferred onto nitrocellulose membranes as described previously by Towbin et al. (45). Following blocking in Tris-buffered saline (TBS) with 1% (wt/vol) BSA, the membranes were incubated in TBS containing affinity-purified anti-rAdpC serum at a 1:500 dilution. The blot was then washed with TBS plus 0.05% Tween 20 and incubated with a 1:2,000 dilution of secondary antibody (horseradish peroxidase-conjugated anti-rabbit antibody; Promega, Madison, WI). Following three washes, protein bands were visualized with 4-chloro-naphthol in the presence of hydrogen peroxide.
Dot blot analysis.
Bacterial cells from cultures grown to the mid-logarithmic phase were washed three times with PBS and adjusted to a final concentration of 0.45 as determined by measurements of the optical density at 660 nm. Four microliters of each serial dilution (1, 1:2, 1:4, and 1:8) was spotted in triplicate onto a nitrocellulose membrane. The membrane was air dried, blocked with 5% skim milk (2 h at room temperature [RT]), and then incubated with anti-rAdpC (1:500 dilution in PBS for 2 h at RT). Following incubation, the membrane was washed three times with PBS containing 0.05% Tween 20. Anti-rabbit IgG alkaline phosphatase-conjugated antibody at a 1:5,000 dilution was then added, and the blot was incubated for 1 h at RT. Following washing three times with PBS-Tween 20, the signals were visualized by incubation of the membrane with alkaline phosphatase substrate (nitroblue tetrazolium [NBT]/BCIP [5-bromo-4-chloro-3-indolylphosphate]).
S-tag analysis.
The presence of the S-tag fusion protein was assessed by using the FRETWorks S-tag fluorescent detection kit (Novagen, Madison, WI) according to the manufacturer's instructions. Studies were done by using whole bacterial cells.
E. coli interaction with host cells. (i) Plate assay.
(a) Total interaction. Mouse NIH 3T3 fibroblast cells, oral epithelial HN4 cells, and HUVECs were used for the study. The cells were prepared in 12-well tissue culture plates with 2.5 × 105 cells/well in cell-specific growth medium (e.g., EGM for HUVECs) and grown at 37°C in 95% air-5% CO2 under standard conditions. Host cells were washed twice with 1× PBS (pH 7.4) and suspended in serum-free medium. E. coli expressing the native AdpC protein (V2846) was used for the infection study. E. coli with only the pET30a vector (V2720) and E. coli expressing a protein encoded by PI1571 (V2848) were used as control strains. The bacterial cell types were grown in LB broth with 30 μg/ml of kanamycin at 37°C. Cultures were induced with 1 mM IPTG when the culture density reached the mid-logarithmic growth phase (OD660 of 0.5) and were grown for an additional 3 h. Bacterial cultures were harvested, washed twice with PBS (pH 7.4), and suspended in EGM (or NIH 3T3-specific medium). Host cells were then infected with the bacterial cells at a multiplicity of infection (MOI) of 1:100 and incubated at 37°C in 95% air-5% CO2 for 30 min. The cells were then washed with PBS and disrupted by two freeze-thaw cycles to break the eukaryotic cells. The mixture was harvested from plates, and 200 μl of serial dilutions in LB broth supplemented with 10 μg/ml of kanamycin was plated onto LB agar plates. Following overnight incubation at 37°C, the number of bacterial CFU was determined.
(b) Invasion. Eukaryotic cells were infected as described above. The extracellular bacteria were killed by the incubation of the cells with host cell medium supplemented with 100 μg/ml of carbenicillin. Carbenicillin is a very effective antibiotic against E. coli and at the same time is not internalized by nonphagocytic cells and, thus, does not kill intracellular bacteria. The cells were then washed with PBS, and intracellular bacteria were released and enumerated as described above.
(ii) Confocal imaging.
Infection was performed as described above, with some modifications. The E. coli cells were labeled by using 2,7-bis(2-carboxyethyl)-5 (and 6)-carboxyfluorescein (BCECF) prior to infection. HUVECs were prepared on glass coverslips, washed, and infected with the labeled bacterial cells at an MOI of 1:10. After 10 min, the HUVECs were fixed in PBS supplemented with 10% formaldehyde at room temperature for 15 min. Cells were then washed and incubated with 50 mM NH4Cl and 0.3% Tween 20 in PBS for 10 min at room temperature. Coverslips with HUVECs were washed, mounted with Antifade (Molecular Probes, Eugene, OR), and examined by using a Carl Zeiss (Bannockburn, IL) LSM 510 Meta confocal imaging microscope with a Zeiss PlanApo 63×/1.4-numerical-aperture oil objective.
Bioinformatics approaches.
Sequence similarity searches were done by using the BLAST program (NCBI [http://ncbi.nlm.nih.gov/]).
Statistical analysis.
Student's t test two-group comparisons were done to assess the statistical significance of our data.
RESULTS
Characterization of AdpC using bioinformatics approaches.
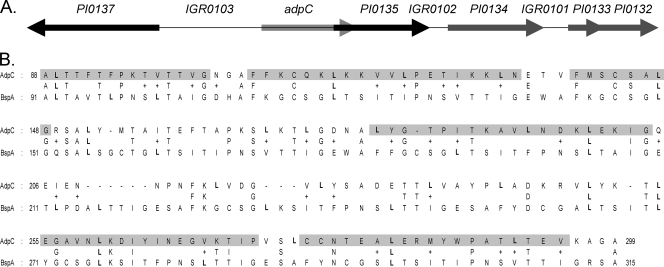
The PI0136 gene, which encodes the AdpC protein, is the first gene of a two-gene genomic locus located upstream of the PI0135 gene, which codes for a putative internalin-like protein (Fig. 1A). Upstream of adpC and separated by an 859-bp intergenic region (IGR0103) is a large open reading frame with the opposite orientation, PI0137, which codes for a lectin-like adhesin precursor. Located downstream of PI0135 by 230 bp (IGR0102) is PI0134, which codes for an ISPg-3-related transposase, followed by two open reading frames, PI0133 and PI0132, which encode hypothetical proteins. PI0134 is separated from PI0133 by 167 bp (IGR0101). The genetic arrangement of the open reading frames suggests that adpC is possibly cotranscribed with PI0135. AdpC shares the highest sequence similarity with the surface antigen BspA of Tannerella forsythia (formerly known as Bacteroides forsythus) (GenBank accession number AAC82625.1). Both proteins share 30% identity and 46% similarity over the 225-amino-acid region (Fig. 1B). This similarity is confined to the first LRR region of T. forsythia BspA containing 16 LRR motifs. The sequence of the motifs is XXLXLXXNXLXXLXXLXXLXXL, where L is leucine, valine, or isoleucine; N is asparagine, cysteine, or threonine; and X is any amino acid (40). Most of the lysine and isoleucine residues are conserved between the two proteins (Fig. 1B). Sequence analysis revealed that P. intermedia AdpC has six LRR repeats. The positions of the repeats in AdpC are shown in Fig. 1B. AdpC also showed significant similarity to a Bacteroides fragilis hypothetical protein (EMBL accession number BAD48495.1), a Bacteroides thetaiotaomicron hypothetical protein (GenBank accession number AA076347.1), the Streptococcus pneumoniae choline-binding protein PcpA (39), a Trichomonas vaginalis BspA-like surface antigen, and an Entamoeba histolytica BspA-like leucine-rich repeat protein (10, 15, 24).
FIG. 1.
Characteristics of P. intermedia 17 AdpC. (A) adpC genomic locus. Gene designations are according to the Oralgen database (www.oralgen.lanl.gov). (B) Alignment of P. intermedia 17 AdpC and Tannerella forsythia BspA. Leucine amino acid residues are shown in boldface type. The positions of the six LRR regions in AdpC are highlighted in gray.
Transcriptional start site.
To determine the transcriptional start site of the adpC transcript, 5′ RACE was performed. Two major PCR amplification fragments were detected following secondary PCR using primers Div-R2 and Div-F2 (results not shown; primer positions are shown in Fig. 2). Sequence analysis of the larger fragment, approximately 330 bp, revealed that it was a concatemer composed of several adpC fragments. The smaller fragment, 110 bp in length, was a single circularized cDNA fragment composed of adpC sequences located upstream of the sequence designated Div-R2 and downstream of the sequence designated Div-F2 until the end of the Div-P sequence as well as 16 bp of sequence derived from an intergenic region located upstream of adpC, IGR0103 (Fig. 2). Precisely, the region containing adpC sequences started at the beginning of phosphorylated primer Div-P, which was used to amplify the transcript, and ended on the C residue located in the intergenic region 16 bp upstream of the translational start site of adpC. Such data indicated that self-ligation of the cDNA occurred during our study. Ultimately, these results demonstrated that the adpC transcript initiates 16 bp upstream of the translational start site at base pair C (Fig. 2).
FIG. 2.
Genetic organization of the adpC transcriptional start site. Sequences upstream of adpC are denoted in italic type. The transcriptional (+1) and translational (Met) start sites are indicated. Sequences used to design primers are underlined, and the designations of the primers are included underneath the sequences.
Purification of rAdpC.
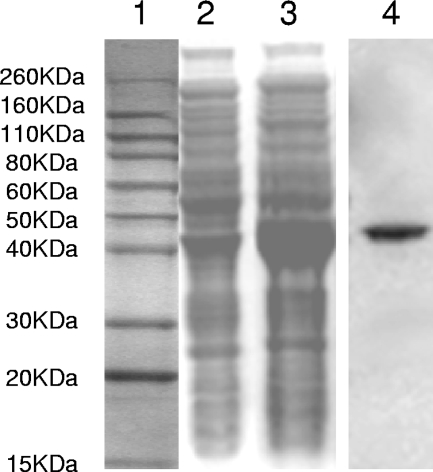
The gene encoding the entire protein, as annotated on the Oralgen website (www.oralgen.lanl.gov), was cloned into the pET30 vector, and the protein was expressed by using E. coli BL21(DE3) cells. The addition of IPTG to E. coli V2846 cells resulted in the expression of a distinct band at 42 kb corresponding to the expected size of rAdpC (Fig. 3, lane 3). This band was absent in the E. coli cell lysate prior to induction with IPTG (Fig. 3, lane 2). The cell lysates from IPTG-induced E. coli V2846 were applied onto a nickel affinity column, and AdpC was purified under native conditions. SDS-PAGE analysis of the elution fractions confirmed the presence of only one band, thus verifying that the protein was purified to homogeneity (Fig. 3, lane 4).
FIG. 3.
Purification of rAdpC. Shown is an SDS-PAGE analysis of the E. coli V2846 cell lysate before (lane 2) and after (lane 3) IPTG induction. Purified AdpC after nickel agarose (Ni-NTA) affinity chromatography is shown in lane 4. A molecular mass marker is shown in lane 1.
AdpC binds fibrinogen.
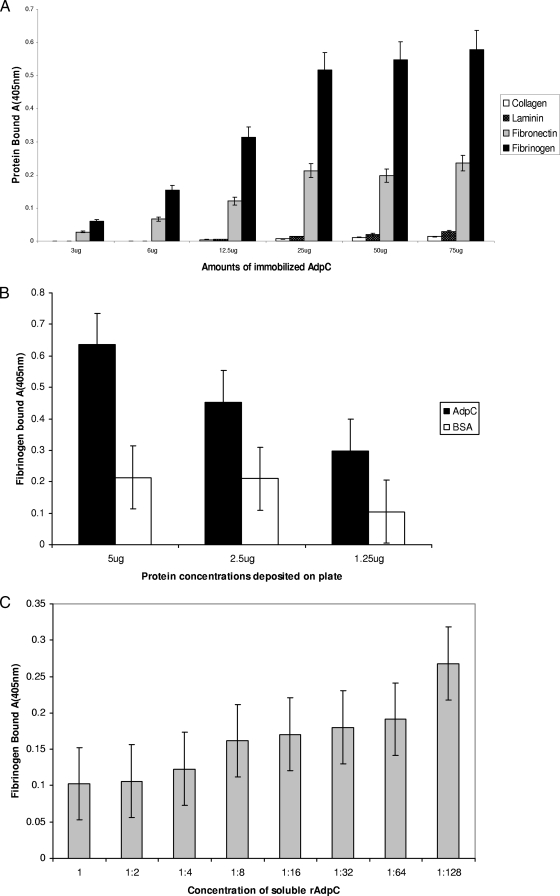
An enzyme-linked immunosorbent assay (ELISA) was performed to test the adherence of rAdpC to extracellular matrix (ECM) proteins. As shown in Fig. 4A, several proteins were examined. rAdpC bound fibrinogen with the highest affinity. Binding was also observed with fibronectin. However, no binding was detected with collagen and laminin. These results showed that AdpC binds fibrinogen and fibronectin. Furthermore, increasing amounts of rAdpC bound both fibrinogen and fibronectin in a saturable, dose-dependent manner, indicating that the binding was specific. To further confirm the specificity of AdpC binding to fibrinogen and fibronectin, the binding experiment was performed in the presence of a negative control, BSA. No significant differences in AdpC binding to BSA and to fibronectin were detected, thus demonstrating that the binding was not specific (results not shown). However, significant 2- to 3-fold differences were observed between AdpC binding to fibrinogen and to BSA. Therefore, AdpC is a fibrinogen-binding protein (Fig. 4B). Finally, a competitive ELISA performed in the presence of increasing amounts of soluble rAdpC confirmed the specificity of the binding of AdpC to fibrinogen (Fig. 4C).
FIG. 4.
rAdpC binding to ECM proteins. Shown is data from ELISA of rAdpC binding to various ECM ligands. (A) Binding of various ligands to rAdpC. Different amounts of rAdpC ranging from 3 to 75 μg were deposited into wells of a 96-well plate. Solutions (100 μl) containing 20 μg/ml of various ECM proteins were then added to the wells, and the binding was detected by using antibody specific for the ECM proteins followed by a secondary AP-conjugated antibody. The amounts of bound proteins were then determined based on the fluorescent signal intensity derived from a substrate for AP. (B) Specificity of AdpC binding to fibrinogen. Various amounts of rAdpC and BSA were deposited into the wells of a 96-well plate, and the plate was then incubated with solutions (100 μl) containing 20 μg/ml of fibrinogen. Bound fibrinogen was detected as described above for A. (C) Competitive inhibition of rAdpC binding to fibrinogen using various concentrations of soluble rAdpC. A 96-well plate coated with rAdpC (2 μg/well) was incubated with fibrinogen (5 μg/ml) mixed with various amounts of rAdpC (0.25 mg/ml used to make dilutions ranging from 1 to 1:128). Bound fibrinogen was detected as described above for A.
Cellular localization of AdpC in P. intermedia 17 and in E. coli.
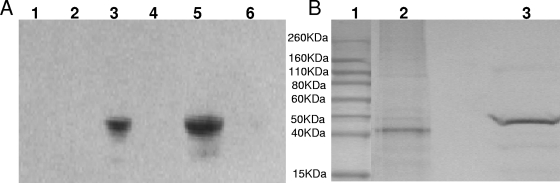
Although an analysis of cellular localization using the PSORT server (http://psort.ims.u-tokyo.ac.jp) failed to identify the typical N-terminal signal sequence that would target the protein to the outer membrane in this Gram-negative bacterium, we reasoned that AdpC was a surface protein, as are most of the proteins belonging to the LRR family. Thus, we examined various fractions derived from E. coli AdpC (V2846) for the presence of rAdpC using anti-rAdpC antibody (Fig. 5A). The presence of a signal corresponding to a 42-kDa protein in the cell lysate (Fig. 5A, lane 3) and the outer membrane (Fig. 5A, lane 5) demonstrated that AdpC is transported to the outer membrane. No AdpC-specific signal was detected in E. coli cell lysates expressing the C-terminal portion of a protein encoded by P. intermedia 17 pPI0035 (V2921) (Fig. 5A, lanes 1 and 2), thus confirming that the signal was specific to AdpC. Furthermore, outer membrane fractions of P. intermedia 17 were examined for the presence of AdpC. Again, a signal corresponding to 40 kDa was detected in this fraction (Fig. 5B, lane 2). The 2-kDa-smaller size of the detected protein than the rAdpC expressed in E. coli (Fig. 5B, lane 3) is in agreement with the absence of the His tag and S tag present in rAdpC expressed by E. coli V2846.
FIG. 5.
Cellular localization of AdpC. Proteins were separated by SDS-PAGE and analyzed by Western blotting. AdpC was detected with affinity-purified rabbit anti-rAdpC. (A) E. coli V2921 whole-cell lysate proteins (lanes 1, 2, 3, and 4) and outer membrane proteins (lanes 5 and 6), which were derived from cultures grown in the presence (lanes 1, 3, and 5) or absence (lanes 2, 4, and 6) of IPTG. Proteins were derived from E. coli V2921 (lanes 1 and 2) and E. coli V2846 (lanes 3, 4, 5, and 6). (B) P. intermedia 17 outer membrane proteins (lane 2) and E. coli V2846 cell lysate proteins (lane 3). A molecular mass marker is shown in lane 1.
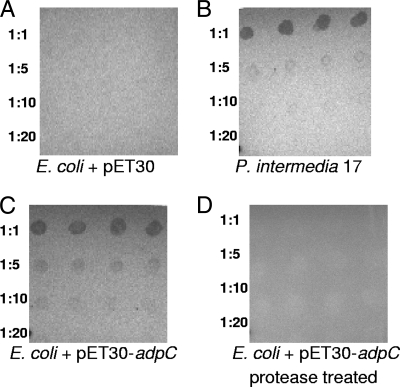
To determine the surface location of AdpC, a dot blot experiment was performed. As shown in Fig. 6, signal was detected when P. intermedia 17 and V2846 cells were used (Fig. 6B and C). No signal was observed when a control E. coli strain that does not express AdpC (V2720) was used (Fig. 6A), thus confirming that the observed signal was AdpC specific. Furthermore, protease-treated V2846 cells did not interact with anti-rAdpC, thus indicating that AdpC was degraded by the protease and no longer available for interactions with the antibody (Fig. 6D). Since in our experiment, intact bacterial cells were treated with protease, only surface proteins were substrates for degradation by the protease. Taken together, these data demonstrate that AdpC is a cell surface protein.
FIG. 6.
Surface location of AdpC determined by dot blot analysis. Serial dilutions of bacterial cells were deposited onto a nitrocellulose membrane, and the presence of AdpC was detected by using an anti-AdpC antibody. (A) E. coli containing plasmid pET30 (V2720). (B) Prevotella intermedia 17. (C) IPTG-induced E. coli containing recombinant plasmid pET30-adpC (V2846). (D) IPTG-induced E. coli containing recombinant plasmid pET30-adpC (V2846). The cells were protease treated prior to deposition onto the membrane.
We also performed an alternative assay to further confirm the cell surface location of AdpC. Using an S-tag fluorescence detection kit and whole bacterial cells, we detected signals at 520 nm in samples containing V2846 (Table 2). No signal above the background was detected in a sample containing control E. coli V2921, expressing the intracellular S-tag fusion protein (C-terminal portion of the pPI0035-endoded protein) (Table 2). These data again show that rAdpC is associated with the cell surface of E. coli V2846.
TABLE 2.
Cellular localization of rAdpC in E. coli V2846
| E. coli strain | Fluorescence | Presence of IPTG |
|---|---|---|
| V2921 | 14,680 | + |
| V2921 | 16,827 | − |
| V2846 | 33,563 | − |
| V2846 | 50,122 | + |
| + controla | 52,824 | |
| − controlb | 13,443 |
Manufacturer's positive control.
Manufacturer's negative control.
AdpC promotes invasion of E. coli expressing rAdpC.
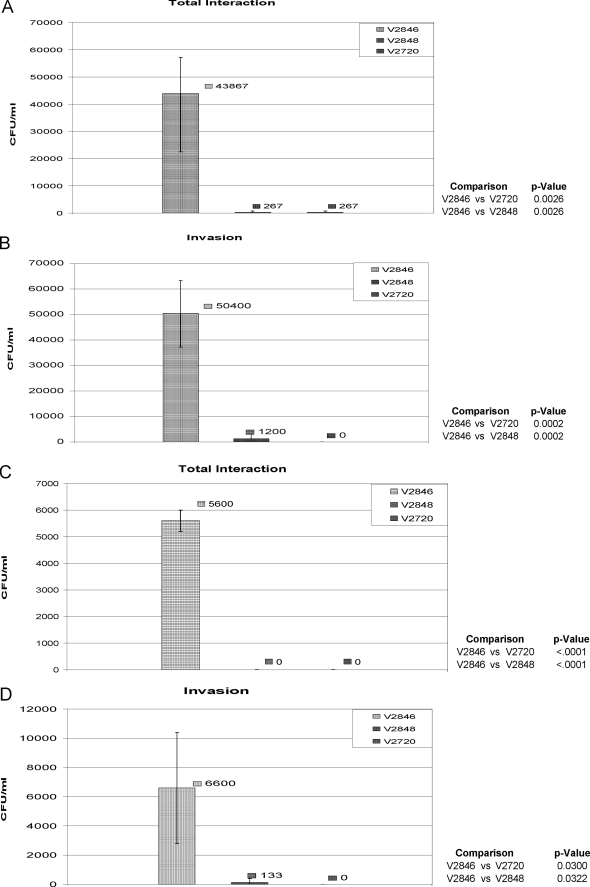
NIH 3T3 fibroblast cells, HUVECs, and oral epithelial HN4 cells were used for the study. First, we used an antibiotic protection assay where extracellular bacteria are killed by carbenicillin. At the same time, carbenicillin does not affect the viability of intracellular bacteria. As shown in Fig. 7A, E. coli cells expressing rAdpC (V2846) have a 70-fold-elevated ability to associate with fibroblasts compared to control bacteria containing vector alone or, as observed for V2848, which expresses another P. intermedia 17 LRR-like protein, PI1571. It is noteworthy that drastic invasion rates were also observed for V2846, while V2848 and V2720 did not invade fibroblast cells (Fig. 7B). Large differences in invasion rates were also obtained by infection of HUVECs; while control strains V2720 and V2848 were not able to associate with and invade host cells, rApdC-expressing strain V2846 readily invaded HUVECs (Fig. 7C and D).
FIG. 7.
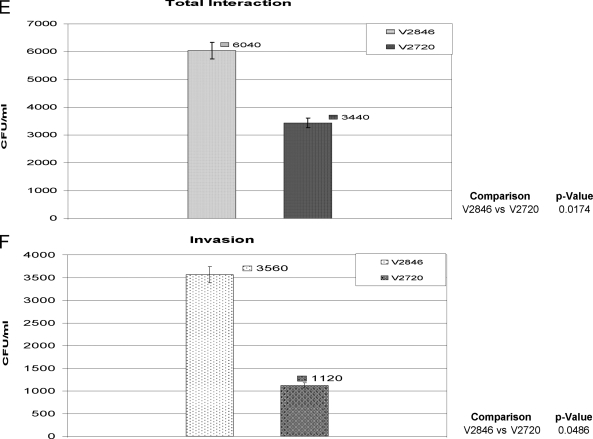
Interaction of E. coli strains with eukaryotic cells. Eukaryotic cells were infected with E. coli V2846 (AdpC), V2848 (AdpD), and V2720 (pET30 only) as described in Materials and Methods. Total association (A, C, and E) and invasion (B, D, and F) of E. coli strains into host cells were determined by the quantification of viable bacteria by counting of CFU. Results from an experiment performed in triplicate are shown. (A and B) NIH 3T3 fibroblasts. (C and D) HUVECs. (E and F) Oral epithelial HN4 cells.
We also examined the ability of AdpC to mediate the invasion of bacteria into oral epithelial cells. As shown in Fig. 7E, V2846 had an approximately 2-fold-elevated ability to interact with cells compared to control E. coli containing the pET30 vector only. Furthermore, V2846 invaded HN4 cells at a 3-fold-higher rate than V2720, demonstrating that AdpC slightly promotes the invasion of E. coli cells into oral epithelial cells (Fig. 7F). Considering the drastic differences in invasion rates observed between the control strain and V2846 when NIH 3T3 cells and HUVECs were used, we do not consider the slight change in the invasion of HN4 cells very biologically significant, and we conclude that the AdpC-mediated cell invasion is cell specific. Furthermore, since E. coli strains expressing other P. intermedia 17 LRR-like proteins, including PI1564 (Fig. 7A to D), PI0493, PI1571, and PI2109 (data not shown), did not invade eukaryotic cells, we conclude that the ability to confer an invasive phenotype on otherwise noninvasive bacteria is AdpC specific.
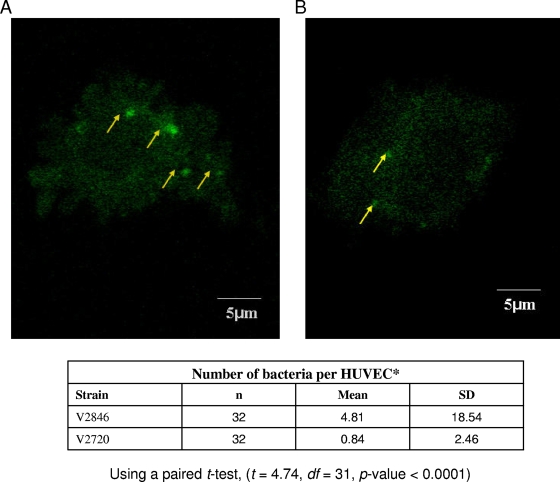
The results for AdpC-mediated bacterial invasion were further confirmed by using confocal microscopy, which demonstrated higher numbers of internalized bacteria for E. coli strain V2846 than for control strain V2720 (Fig. 8). We observed that while 48% of bacteria expressing AdpC were able to invade HUVECs, only 8% of control bacteria invaded the cells. These results indicated that the presence of AdpC increased bacterial invasion 6-fold. Collectively, the above-described results indicated that AdpC promotes the bacterial invasion of eukaryotic cells.
FIG. 8.
Interaction of E. coli strains with HUVECs. HUVECs were infected with fluorescently labeled E. coli V2846 (AdpC) and V2720 (pET30 only) as described in Materials and Methods. Bacterial invasion was determined by visualization of the cells using confocal microscopy and counting of fluorescently labeled bacteria at 0.5-μm intervals for each HUVEC. Bacterial invasion of at least 30 HUVECs was assessed.
DISCUSSION
This is the first report describing an LRR-like protein of P. intermedia 17. Proteins of the LRR family are involved mainly in protein-protein interactions (27). The LRR motif of these proteins forms a structural arrangement consisting of alpha-helices and beta-strands that are joined by loops formed by the LRR units (40). Such an arrangement results in a “horseshoe-shaped” molecule that provides a versatile scaffold for protein-protein interactions. Multiple genes encoding proteins with characteristics of the LRR family of proteins are present in the genome of P. intermedia 17. We began to investigate the role of the proteins from the protein encoded by PI0136, which was designated AdpC. Bioinformatics analysis showed that AdpC contains the characteristic features of the LRR family of proteins. There are several families of LRR proteins, and based on sequence similarities, AdpC belongs to the Treponema pallidum LRR protein (LRRTP) subfamily. This family, originally identified in Treponema pallidum, is comprised of proteins that are located on the cell surface (26, 27). Furthermore, LRRTP proteins were shown previously to play a role in surface adherence and aggregation (26, 37, 38). AdpC contains six typical LRR motifs in which most of the leucine-like residues are conserved compared to the BspA protein of T. forsythia (37, 42).
In contrast to other members of the LRR family of proteins that are usually surface proteins, AdpC is classified as being located in the cytoplasm. Most of the reported LRR proteins contain an N-terminal signal peptide that marks them for secretion across the bacterial membrane (cytoplasmic membrane) as well as a C-terminal membrane attachment region such as the LPxTG motif followed by a hydrophobic transmembrane region observed for LnlA of Listeria monocytogenes (2). AdpC lacks the classical signal sequence; therefore, it is classified as a cytoplasmic protein according to bioinformatics approaches. However, our data demonstrated that it is located in the outer membrane of P. intermedia, which suggests that mechanisms independent from the signal sequence play a role in the transport of the protein across bacterial membranes. Recently, a novel type II mechanism for the secretion of nonclassical bacterial proteins to the outer surface of the cell was reported (13, 19). This system involves the targeting of the protein to the outer membrane by an uncleaved N-terminal Tat anchor sequence. It is possible that a similar mechanism is also responsible for the secretion of AdpC, and future studies are planned to further explore this possibility. Such studies may also be of broader importance, as our data show that the secretion mechanism targeting proteins to the outer membrane is functional in other bacteria. Not only did we localize AdpC on the outer membrane of P. intermedia, but it was also located on the outer membrane and surface when it was expressed in a heterologous host, E. coli. Thus, this may be a general mechanism for protein secretion.
Our study showed that rAdpC binds fibrinogen. The characteristic sigmoidal shape of the binding curve further indicated that the binding was specific and, thus, receptor mediated. This was in agreement with the previously demonstrated role of other LRR family members that bind protein partners (27). For example, the BspA protein of T. forsythia was shown previously to bind fibronectin and fibrinogen (42).
Importantly, this study was also the first to identify and characterize a P. intermedia 17 protein that mediates the invasion of an otherwise noninvasive E. coli strain into nonphagocytic eukaryotic cells, including endothelial cells and fibroblast cells. The direct verification of the role of AdpC in the interaction of P. intermedia 17 with host cells should be accomplished by using an isogenic mutant deficient in the protein. However, efforts to generate a P. intermedia 17 isogenic mutant have been unsuccessful in several laboratories. Thus, we have used the heterologous host E. coli as a host for our study. The results of our invasion studies are very drastic: while no bacterial invasion was observed using control E. coli strains, high invasion rates were observed with the rAdpC-expressing strain. Such results indicated that this protein plays a significant role in mediating bacterial invasion. It is also noteworthy that four other LRR-like proteins encoded on the genome of P. intermedia 17 (PI1564, PI0493, PI1571, and PI2109) did not confer invasive potential to E. coli, thus demonstrating that this capability is specific for AdpC (J. Lewis et al., unpublished observations). Its capacity to mediate invasion into a variety of eukaryotic cells by different bacteria indicates that it may be involved in mediating a general aspect of microbial invasion. Further work aimed at identifying the AdpC ligands is expected to shed light on the molecular mechanisms of the AdpC-mediated invasion of eukaryotic cells by microorganisms.
AdpC did not significantly promote the internalization of E. coli into oral epithelial cells. Thus, AdpC-mediated invasion appeared to be cell specific. Such findings suggested that AdpC interacts with ligands that are present in only certain cell types and are absent in others. P. intermedia 17 is internalized by oral epithelial cells, and such an invasion is probably mediated by other invasins present in this organism. In addition to adpC, four other genes on the P. intermedia 17 genome encode internalin-related proteins. Several putative internalin-like proteins, such as the ones encoded by PI0023 and PI0212, share similarity with the P. gingivalis putative internalins InlJ (PG0350) and Irp (PG1374), which were shown previously to play a role in biofilm formation and the invasion of P. gingivalis into epithelial KB cells, respectively (8, 14). As demonstrated previously for P. gingivalis and L. monocytogenes, these proteins may also target P. intermedia to various niches (3, 21), including the epithelial cells examined in our study. The PI0135 gene, located downstream of adpC, codes for an internalin-like protein similar to the L. monocytogenes internalin LnlA, a major invasion protein required for the internalization of bacteria by intestinal epithelial cells (20, 28, 34, 35, 40). Thus, it is possible that PI0135-encoded internalin mediates the internalization of P. intermedia 17 by oral epithelial cells.
In conclusion, we have identified AdpC as a major protein candidate of P. intermedia 17 that is responsible for the internalization of bacteria by a variety of host cells. Future work will focus on an elucidation of the detailed mechanism of the AdpC-mediated invasion of E. coli, including the identification of the eukaryotic binding partners and protein structure characterization. In light of the multiple potential invasins present in P. intermedia, the use of E. coli as a surrogate host appears to be the best approach to start such investigations. The results from these investigations will then be applied to determine the role of AdpC in the pathogenesis of P. intermedia. As LRR proteins are versatile ligands, these studies will also contribute to a better understanding of the functions of the eukaryotic receptors involved in these interactions.
Acknowledgments
This research was supported by USPHS grants R01DE016124 and R01DE018039 from the National Institute of Dental and Craniofacial Research awarded to Janina Lewis. The genomic sequence of P. intermedia 17 was obtained from the J. Craig Venter Institute (formerly TIGR) (http://www.jcvi.org) and the Los Alamos Oral Pathogen Sequence Database (http://www.oralgen.lanl.gov). The confocal laser scanning fluorescent microscopy experiments were performed by the VCU Flow Cytometry and Imaging Shared Resource Facility. These experiments were supported in part by NIH grant P30CA16059.
We are grateful for the assistance and contributions from the members of the Todd Kitten laboratory (for use of the Molecular Imager and Fluor Star Galaxy Reader). We also thank Tiana Wyant for her help with tissue culture.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 22 March 2010.
REFERENCES
- 1.Baumgartner, J. C., B. J. Watkins, K. S. Bae, and T. Xia. 1999. Association of black-pigmented bacteria with endodontic infections. J. Endod. 25:413-415. [DOI] [PubMed] [Google Scholar]
- 2.Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, the European Listeria Gene Consortium, L. Jansch, F. Garcia-del Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. [DOI] [PubMed] [Google Scholar]
- 3.Bierne, H., C. Sabet, N. Personnic, and P. Cossart. 2007. Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect. 9:1156-1166. [DOI] [PubMed] [Google Scholar]
- 4.Reference deleted.
- 5.Braun, L., and P. Cossart. 2000. Interactions between Listeria monocytogenes and host mammalian cells. Microbes Infect. 2:803-811. [DOI] [PubMed] [Google Scholar]
- 6.Reference deleted.
- 7.Bumann, D., S. Aksu, M. Wendland, K. Janek, U. Zimny-Arndt, N. Sabarth, T. F. Meyer, and P. R. Jungblut. 2002. Proteome analysis of secreted proteins of the gastric pathogen Helicobacter pylori. Infect. Immun. 70:3396-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capestany, C. A., M. Kuboniwa, I. Y. Jung, Y. Park, G. D. Tribble, and R. J. Lamont. 2006. Role of the Porphyromonas gingivalis InlJ protein in homotypic and heterotypic biofilm development. Infect. Immun. 74:3002-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlone, G. M., M. L. Thomas, H. S. Rumschlag, and F. O. Sottnek. 1986. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J. Clin. Microbiol. 24:330-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlton, J. M., R. P. Hirt, J. C. Silva, A. L. Delcher, M. Schatz, Q. Zhao, J. R. Wortman, S. L. Bidwell, U. C. Alsmark, S. Besteiro, T. Sicheritz-Ponten, C. J. Noel, J. B. Dacks, P. G. Foster, C. Simillion, Y. Van de Peer, D. Miranda-Saavedra, G. J. Barton, G. D. Westrop, S. Muller, D. Dessi, P. L. Fiori, Q. Ren, I. Paulsen, H. Zhang, F. D. Bastida-Corcuera, A. Simoes-Barbosa, M. T. Brown, R. D. Hayes, M. Mukherjee, C. Y. Okumura, R. Schneider, A. J. Smith, S. Vanacova, M. Villalvazo, B. J. Haas, M. Pertea, T. V. Feldblyum, T. R. Utterback, C. L. Shu, K. Osoegawa, P. J. de Jong, I. Hrdy, L. Horvathova, Z. Zubacova, P. Dolezal, S. B. Malik, J. M. Logsdon, Jr., K. Henze, A. Gupta, C. C. Wang, R. L. Dunne, J. A. Upcroft, P. Upcroft, O. White, S. L. Salzberg, P. Tang, C. H. Chiu, Y. S. Lee, T. M. Embley, G. H. Coombs, J. C. Mottram, J. Tachezy, C. M. Fraser-Liggett, and P. J. Johnson. 2007. Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reference deleted.
- 12.Christofakis, E. P., H. Miyazaki, D. S. Rubink, and W. A. Yeudall. 2008. Roles of CXCL8 in squamous cell carcinoma proliferation and migration. Oral Oncol. 44:920-926. [DOI] [PubMed] [Google Scholar]
- 13.Coulthurst, S. J., and T. Palmer. 2008. A new way out: protein localization on the bacterial cell surface via Tat and a novel type II secretion system. Mol. Microbiol. 69:1331-1335. [DOI] [PubMed] [Google Scholar]
- 14.Dashper, S. G., C. S. Ang, P. D. Veith, H. L. Mitchell, A. W. Lo, C. A. Seers, K. A. Walsh, N. Slakeski, D. Chen, J. P. Lissel, C. A. Butler, N. M. O'Brien-Simpson, I. G. Barr, and E. C. Reynolds. 2009. Response of Porphyromonas gingivalis to heme limitation in continuous culture. J. Bacteriol. 191:1044-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis, P. H., Z. Zhang, M. Chen, X. Zhang, S. Chakraborty, and S. L. Stanley, Jr. 2006. Identification of a family of BspA like surface proteins of Entamoeba histolytica with novel leucine rich repeats. Mol. Biochem. Parasitol. 145:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorn, B. R., K. L. Leung, and A. Progulske-Fox. 1998. Invasion of human oral epithelial cells by Prevotella intermedia. Infect. Immun. 66:6054-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enwonwu, C. O., W. A. Falkler, and E. O. Idigbe. 2000. Oro-facial gangrene (noma/cancrum oris): pathogenetic mechanisms. Crit. Rev. Oral Biol. Med. 11:159-171. [DOI] [PubMed] [Google Scholar]
- 18.Falkler, W. A., Jr., C. O. Enwonwu, and E. O. Idigbe. 1999. Microbiological understandings and mysteries of noma (cancrum oris). Oral Dis. 5:150-155. [DOI] [PubMed] [Google Scholar]
- 19.Ferrandez, Y., and G. Condemine. 2008. Novel mechanism of outer membrane targeting of proteins in Gram-negative bacteria. Mol. Microbiol. 69:1349-1357. [DOI] [PubMed] [Google Scholar]
- 20.Gaillard, J. L., P. Berche, C. Frehel, E. Gouin, and P. Cossart. 1991. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65:1127-1141. [DOI] [PubMed] [Google Scholar]
- 21.Galan, J. E. 2000. Alternative strategies for becoming an insider: lessons from the bacterial world. Cell 103:363-366. [DOI] [PubMed] [Google Scholar]
- 22.Haraszthy, V. I., J. J. Zambon, M. Trevisan, M. Zeid, and R. J. Genco. 2000. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 71:1554-1560. [DOI] [PubMed] [Google Scholar]
- 23.He, J., H. Miyazaki, C. Anaya, F. Yu, W. A. Yeudall, and J. P. Lewis. 2006. Role of Porphyromonas gingivalis FeoB2 in metal uptake and oxidative stress protection. Infect. Immun. 74:4214-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirt, R. P., N. Harriman, A. V. Kajava, and T. M. Embley. 2002. A novel potential surface protein in Trichomonas vaginalis contains a leucine-rich repeat shared by micro-organisms from all three domains of life. Mol. Biochem. Parasitol. 125:195-199. [DOI] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Ikegami, A., K. Honma, A. Sharma, and H. K. Kuramitsu. 2004. Multiple functions of the leucine-rich repeat protein LrrA of Treponema denticola. Infect. Immun. 72:4619-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobe, B., and J. Deisenhofer. 1994. The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19:415-421. [DOI] [PubMed] [Google Scholar]
- 28.Lecuit, M., H. Ohayon, L. Braun, J. Mengaud, and P. Cossart. 1997. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect. Immun. 65:5309-5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis, J. P., K. Plata, F. Yu, A. Rosato, and C. Anaya. 2006. Transcriptional organization, regulation and role of the Porphyromonas gingivalis W83 hmu haemin-uptake locus. Microbiology 152:3367-3382. [DOI] [PubMed] [Google Scholar]
- 30.Lo Bue, A. M., G. Nicoletti, M. A. Toscano, B. Rossetti, G. Cali, and F. Condorelli. 1999. Porphyromonas gingivalis prevalence related to other micro-organisms in adult refractory periodontitis. New Microbiol. 22:209-218. [PubMed] [Google Scholar]
- 31.Lopez, N. J. 2000. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J. Periodontol. 71:948-954. [DOI] [PubMed] [Google Scholar]
- 32.Madianos, P. N., S. Lieff, A. P. Murtha, K. A. Boggess, R. L. Auten, Jr., J. D. Beck, and S. Offenbacher. 2001. Maternal periodontitis and prematurity. Part II. Maternal infection and fetal exposure. Ann. Periodontol. 6:175-182. [DOI] [PubMed] [Google Scholar]
- 33.Maeda, N., M. Okamoto, K. Kondo, H. Ishikawa, R. Osada, A. Tsurumoto, and H. Fujita. 1998. Incidence of Prevotella intermedia and Prevotella nigrescens in periodontal health and disease. Microbiol. Immunol. 42:583-589. [DOI] [PubMed] [Google Scholar]
- 34.Mengaud, J., M. Lecuit, M. Lebrun, F. Nato, J. C. Mazie, and P. Cossart. 1996. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect. Immun. 64:5430-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mengaud, J., H. Ohayon, P. Gounon, R.-M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 36.Molloy, M. P., B. R. Herbert, M. B. Slade, T. Rabilloud, A. S. Nouwens, K. L. Williams, and A. A. Gooley. 2000. Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 267:2871-2881. [DOI] [PubMed] [Google Scholar]
- 37.Onishi, S., K. Honma, S. Liang, P. Stathopoulou, D. Kinane, G. Hajishengallis, and A. Sharma. 2008. Toll-like receptor 2-mediated interleukin-8 expression in gingival epithelial cells by the Tannerella forsythia leucine-rich repeat protein BspA. Infect. Immun. 76:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid, S. D., A. G. Montgomery, J. M. Voyich, F. R. DeLeo, B. Lei, R. M. Ireland, N. M. Green, M. Liu, S. Lukomski, and J. M. Musser. 2003. Characterization of an extracellular virulence factor made by group A Streptococcus with homology to the Listeria monocytogenes internalin family of proteins. Infect. Immun. 71:7043-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Beato, A. R., R. Lopez, and J. L. Garcia. 1998. Molecular characterization of PcpA: a novel choline-binding protein of Streptococcus pneumoniae. FEMS Microbiol. Lett. 164:207-214. [DOI] [PubMed] [Google Scholar]
- 40.Schubert, W. D., C. Urbanke, T. Ziehm, V. Beier, M. P. Machner, E. Domann, J. Wehland, T. Chakraborty, and D. W. Heinz. 2002. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell 111:825-836. [DOI] [PubMed] [Google Scholar]
- 41.Sexton, J. A., J. S. Pinkner, R. Roth, J. E. Heuser, S. J. Hultgren, and J. P. Vogel. 2004. The Legionella pneumophila PilT homologue DotB exhibits ATPase activity that is critical for intracellular growth. J. Bacteriol. 186:1658-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma, A., H. T. Sojar, I. Glurich, K. Honma, H. K. Kuramitsu, and R. J. Genco. 1998. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 66:5703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reference deleted.
- 44.Teanpaisan, R., C. W. Douglas, and T. F. Walsh. 1995. Characterisation of black-pigmented anaerobes isolated from diseased and healthy periodontal sites. J. Periodontal Res. 30:245-251. [DOI] [PubMed] [Google Scholar]
- 45.Towbin, H., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. 1979. Biotechnology 24:145-149. [PubMed] [Google Scholar]
- 46.Reference deleted.
- 47.Yu, F., D. Iyer, C. Anaya, and J. P. Lewis. 2006. Identification and characterization of a cell surface protein of Prevotella intermedia 17 with broad-spectrum binding activity for extracellular matrix proteins. Proteomics 6:6023-6032. [DOI] [PubMed] [Google Scholar]