Abstract
Toll-like receptors (TLRs) play a key role in the innate immune response by sensing bacterial ligands. The mechanisms involved in the TLR-mediated cytokine response are well established; however, the possible contribution of TLR-dependent recognition of bacteria to macrophage phagocytosis remains unclear. Listeria monocytogenes is an intracellular, parasitic, Gram-positive bacterium recognized mainly by TLR2. In this study, we investigated whether TLR2-dependent signaling is involved in the phagocytosis of L. monocytogenes by macrophages. We found no difference in the number of L. monocytogenes cells associating with wild-type (WT) and TLR2−/− macrophages 1 h after infection. However, the number of L. monocytogenes cells phagocytosed in TLR2−/− and MyD88−/− macrophages was significantly lower than that of WT macrophages. In addition, lipopolysaccharide (LPS) treatment restored impaired phagocytic activity of TLR2−/− macrophages but did not enhance the activity of MyD88−/− macrophages. The efficiency of phagocytosis was suppressed by inhibitors of phosphatidylinositol 3-kinase (PI3K) and the small Rho GTPases but not by cycloheximide. Moreover, functional activation of PI3K and Rac1 was impaired in TLR2−/− and MyD88−/− macrophages. In an in vivo infection model, we found significantly lower numbers of L. monocytogenes cells phagocytosed in peritoneal macrophages of TLR2−/− and MyD88−/− mice after intraperitoneal infection. Moreover, a lower number of bacteria were detected in the spleens of TLR2−/− mice 1 day after intravenous infection than in WT mice. These results clearly indicated that TLR2-MyD88-dependent signaling enhances the basal level of phagocytosis of L. monocytogenes by macrophages through activation of PI3K and Rac1, not by synthesis of proinflammatory cytokines or expression of phagocytic receptors.
Listeria monocytogenes is a Gram-positive, facultative, intracellular bacterium that causes severe disease (listeriosis) in humans and various animal species, with a mortality rate of approximately 30% (14, 38). L. monocytogenes can invade various types of cells, including epithelial cells, hepatocytes, endothelial cells, fibroblasts, and macrophages. After entry into host cells, L. monocytogenes is trapped temporarily in an endosome. However, the bacterium quickly escapes from the endosome into the cell cytoplasm, where it undergoes rapid replication by means of expressing various virulence genes, including prfA, plcA, hlyA, actA, mpl, and plcB, all of which are located in a locus called Listeria pathogenicity island 1 (14).
Accumulating evidence suggests that L. monocytogenes gains entry into nonprofessional phagocytes by utilizing bacterial invasion factors called internalins (11, 20, 24, 34, 42, 45). Indeed, internalin A mediates the entry of L. monocytogenes into human intestinal cells through binding to E-cadherin and the interaction of internalin B with c-Met (hepatocyte growth factor receptor) induces endocytosis of the bacterium into various types of cells (8, 25, 37). In comparison, professional phagocytes such as macrophages are able to phagocytose and subsequently kill various pathogens through phagolysosome fusion. Macrophages are known to express various receptors that recognize bacterial components or bind to opsonins attached to bacteria (54); a series of intracellular signaling pathways are then activated that lead to the dynamic and rapid reorganization of the actin cytoskeleton for phagocytic engulfment. Of these receptors, Fcγ receptor and complement receptor serve as opsonin receptors for IgG and C3b, respectively, that bind to the surface of bacteria (19, 33). Mannose receptor and CD14 can contribute to phagocytosis of bacteria through direct binding to mannosylated components (15, 17, 44). Scavenger receptor is also implicated in phagocytosis (43). These different cell surface receptors likely operate alone or in combination in the recognition and efficient internalization of bacteria into macrophages.
Toll-like receptors (TLRs) are type I integral membrane glycoproteins that are expressed in all lymphoid tissues, with the highest level of expression in peripheral blood leukocytes. TLRs are pattern recognition receptors (PRR) characterized by the presence of a Toll/IL-1 receptor (TIR) domain homologous to interleukin-1 receptor (IL-1R) in their cytoplasmic portion and a variable number of leucine-rich repeats (LRR) in their extracellular portion (31). There are more than 10 TLRs identified to date in mammals, and each TLR exhibits a distinct function in innate immune recognition (1, 5, 52). For example, TLR2 recognizes lipoteichoic acid, peptidoglycan, and lipoproteins of Gram-positive bacteria. TLR4 recognizes lipopolysaccharide (LPS) from Gram-negative bacteria, and TLR9 recognizes bacterial hypomethylated CpG DNA motifs. TLR-dependent recognition of bacteria induces a signal through myeloid differentiation factor 88 (MyD88) and is accompanied by inflammatory responses in macrophages (51). TLR2-MyD88 signaling is known to be critical for the proinflammatory cytokine response during L. monocytogenes infection. Several studies in vivo have revealed that TLR2 is required for optimum control of L. monocytogenes infection (29, 53). The increased susceptibility of MyD88−/− mice to L. monocytogenes infection may support the idea that TLR2 plays a role in resistance to L. monocytogenes (46). On the other hand, Edelson and Unanue showed that MyD88 was necessary for resistance to L. monocytogenes infection but TLR2 deficiency did not influence the propagation of L. monocytogenes in vivo (16). These conflicting findings appeared to indicate that though TLR2 participates in host resistance to L. monocytogenes through the induction of cytokine production in vivo, there are some signaling pathways that compensate for the lack of TLR2 to control bacterial infection.
In addition to this established function of TLRs in the host cytokine response, recent studies suggested that TLR signaling modulates the phagocytosis of pathogens (27, 41). Indeed, Blander and Medzhitov (6) reported that TLR-mediated signaling regulates phagolysosomal maturation in bone-marrow-derived macrophages after infection with Escherichia coli, heat-killed Salmonella enterica serovar Typhimurium, and Staphylococcus aureus. Letiembre et al. (35) reported that TLR2 promotes the phagocytosis of Streptococcus pneumoniae and killing of bacteria by polymorphonuclear leukocytes. Moreover, Luther et al. (39) have shown that TLR2, MyD88, and dectin-1 are required for efficient phagocytosis of Aspergillus fumigatus conidia. Doyle et al. (13) also reported that TLR-mediated enhancement of phagocytosis is due to the upregulation of scavenger receptors, and another recent report on MyD88-mediated phagocytosis of Borrelia burgdorferi has emphasized the importance of downstream signaling through phosphatidylinositol 3-kinase (PI3K) (48). From these findings it is likely that TLR signaling contributes to the phagocytosis and phagolysosome formation in professional phagocytes upon infection with extracellular parasitic bacteria or killed bacteria. On the other hand, little is known about whether the phagocytosis of L. monocytogenes, a representative Gram-positive intracellular parasitic bacterium, is dependent on the TLR signaling pathway. In this study, we analyze the contribution of TLR2-MyD88 signaling to phagocytosis of L. monocytogenes both in vitro and in vivo.
MATERIALS AND METHODS
Reagents.
Lipopolysaccharide (LPS) and Clostridium difficile toxin B, a small Rho GTPase inhibitor, were purchased from Sigma-Aldrich (Tokyo, Japan). Pam3CSK4 was purchased from Invivogen (San Diego, CA). LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor, was obtained from Calbiochem (Tokyo, Japan). Gentamicin was purchased from Wako Pure Chemical Industries (Osaka, Japan). Tryptic soy agar and thioglycolate medium were purchased from EIKEN Chemical (Tokyo, Japan). 5- or 6-(N-succinimidyloxycarbonyl)-fluorescein 3′,6′-diacetate (CFSE) and the Bacstain CTC (5-cyano-2,3-ditolyl-2H-tetrazolium chloride) rapid staining kit were purchased from DOJINDO (Kumamoto, Japan). Cycloheximide (CHX) and a protease inhibitor cocktail were purchased from Nacalai Tesque (Kyoto, Japan). Polystyrene microspheres (1 μm in diameter) were obtained from Polysciences (Warrington, PA). EZ-Detect Rac1 activation kit and EZ-Detect Cdc42 activation kit were purchased from Pierce Chemical (Rockford, IL). Recombinant mouse macrophage colony-stimulating factor (M-CSF) was purchased from R&D Systems (Minneapolis, MN).
Antibodies.
Alexa Fluor 594-conjugated anti-rabbit IgG antibody (Ab) and Alexa Fluor 350-conjugated anti-rabbit IgG Ab were purchased from Invivogen (San Diego, CA). Anti-TLR2 monoclonal Ab (MAb, T2.5, mouse IgG1), fluorescein isothiocyanate (FITC)-conjugated anti-mouse TLR2 MAb (6C2, rat IgG2b), phycoerythrin (PE)-conjugated anti-F4/80 MAb (BM8, rat IgG2a), and isotype-matched control MAbs (rat IgG2b and rat IgG2a) were obtained from eBioscience (San Diego, CA). Affinity-purified mouse IgG was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA) and used as a control IgG for anti-TLR2 MAb (T2.5). Rabbit anti-Listeria polyclonal Ab (IgG) was purchased from VivoStat (Alleroed, Denmark). Rabbit anti-Akt polyclonal Ab (IgG) and rabbit anti-phosphorylated Akt MAb (IgG2b) were purchased from Cell Signaling Technology (Tokyo, Japan). Anti-Rac1 MAb (mouse IgG2b) and anti-Cdc42 MAb (mouse IgG1) were obtained from Pierce (Rockford, IL). Rat anti-mouse CD16/CD32 MAb (2.4G2, rat IgG2b), which specifically recognizes mouse FcγIII (CD16) and FcγII (CD32) receptors, was obtained from BD Biosciences (Tokyo, Japan).
Animals used in this study.
Wild-type female C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). TLR2−/−, TLR4−/−, and MyD88−/− mice on a C57BL/6 background were purchased from Oriental Bioservice (Kyoto, Japan). All mice were maintained in specific-pathogen-free conditions and used at 7 to 10 weeks of age. The Animal Ethics and Research Committee of Kyoto University Graduate School of Medicine (Kyoto, Japan) approved all of the experimental procedures performed.
Bacterial strains used in this study.
Listeria monocytogenes strain EGD was grown overnight in brain heart infusion (BHI) broth (EIKEN Chemical, Tokyo, Japan) at 37°C with shaking. One volume of the bacterial suspension was added to one hundred volumes of fresh BHI broth, and L. monocytogenes was cultured for 5 h. After being washed, bacteria were suspended in phosphate-buffered saline (PBS) supplemented with 10% glycerol and stored at −80°C in aliquots. The concentration of bacteria was determined by plating 10-fold serially diluted suspensions on tryptic soy agar plates and counting the number of colonies 24 h after incubation.
Enumeration of associating L. monocytogenes and L. monocytogenes phagocytosed by macrophages.
C57BL/6 WT, TLR2−/−, TLR4−/−, and MyD88−/− mice were injected intraperitoneally with 3 ml of 3% thioglycolate medium. Peritoneal exudate cells (PECs) were collected four days later. Bone marrow cells were collected from tibiae of mice and cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 5 μg ml−1 gentamicin, and recombinant mouse M-CSF (100 ng ml−1) for 7 days. After being washed with RPMI 1640 medium, adherent bone marrow-derived macrophages were collected. Cells were seeded in a 48-well plate at 2.5 × 105 cells well−1 and incubated at 37°C for 2 h in RPMI 1640 medium supplemented with 10% FBS. After being washed to remove nonadherent cells, adherent macrophages were infected with L. monocytogenes at a multiplicity of infection (MOI) of 20 at 37°C for 1 h in FBS-free RPMI 1640 medium. To enumerate L. monocytogenes associating with macrophages, cells were washed thoroughly to remove nonassociating bacteria and lysed in PBS containing 0.1% Triton X-100. The cell lysate was diluted with PBS and inoculated on tryptic soy agar plates. The CFU number was counted 24 h after incubation. To enumerate the internalized bacteria, macrophages were infected with L. monocytogenes as described above, washed, and cultured for 30 min in the presence of 20 μg ml−1 gentamicin. A preliminary experiment showed that extracellular bacteria are completely killed by this antibiotic treatment. Cells were washed and lysed in PBS containing 0.1% Triton X-100. The CFU number in the cell lysate was determined. In some experiments, macrophages were treated with 40 μg ml−1 anti-TLR2 MAb, 20 μM LY294002, 100 μg ml−1 CHX, or 40 ng ml−1 C. difficile toxin B for 1 h and infected with L. monocytogenes. Alternatively, WT, TLR2−/− and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h in the presence of 100 ng ml−1 LPS (TLR4 ligand) or 100 ng ml−1 Pam3CSK4 (TLR2 ligand) and the number of internalized L. monocytogenes was determined. Results were expressed as the relative phagocytic index, which was calculated using the following formula: phagocytic index = (number of phagocytosed L. monocytogenes cells/number of associating L. monocytogenes cells) × 100.
Discrimination between adherent and phagocytosed L. monocytogenes by immunofluorescence microscopy.
L. monocytogenes was labeled with 1 μg ml−1 CFSE for 15 min at room temperature with gentle shaking in the dark. Bacteria were washed twice with PBS and suspended in RPMI 1640 medium. PECs obtained from WT, TLR2−/−, and MyD88−/− mice were seeded at 2 × 105 cells well−1 in a 24-well plate in which a 12-mm glass coverslip was placed in each well and washed 2 h after incubation to remove nonadherent cells. Adherent macrophages were infected with CFSE-labeled L. monocytogenes for 1 h, washed, and fixed with PBS containing 1% paraformaldehyde for 5 min at room temperature. After confirming that the phagocytic efficiency was almost constant when cells were infected at an MOI of 20 to 50, we infected macrophages with L. monocytogenes at an MOI of 50, as this MOI enabled us to easily distinguish the associating bacteria from phagocytosed L. monocytogenes. To distinguish phagocytosed L. monocytogenes from adherent L. monocytogenes, infected cells were treated with rabbit anti-Listeria Ab and Alexa Fluor 594-conjugated anti-rabbit IgG Ab to stain only the extracellular L. monocytogenes as described previously (22). The numbers of associating L. monocytogenes cells and of L. monocytogenes cells adherent to macrophages were counted under a fluorescent microscope. Phagocytosed L. monocytogenes was enumerated using the following equation: number of phagocytosed L. monocytogenes cells = number of associating L. monocytogenes cells − number of adherent L. monocytogenes cells. In addition, macrophages were incubated with polystyrene microspheres for 2 h at a bead-to-cell ratio of 50 to 1, and the phagocytic activity was evaluated.
Viability of phagocytosed L. monocytogenes in macrophages.
Based on our preliminary finding that only the viable L. monocytogenes is able to convert CTC into fluorescent insoluble formazan, we employed the CTC staining kit to distinguish viable cells of L. monocytogenes from dead L. monocytogenes inside macrophages. To estimate the viability of phagocytosed L. monocytogenes in macrophages, therefore, PECs from WT, TLR2−/−, and MyD88−/− mice were infected with CFSE-labeled L. monocytogenes at an MOI of 50 for 1 h. After being washed, cells were incubated with CTC for 15 min to stain L. monocytogenes associating with macrophages. Cells were washed and fixed in PBS containing 1% paraformaldehyde. The extracellular L. monocytogenes was then stained with rabbit anti-Listeria Ab and Alexa Fluor 350-conjugated anti-rabbit IgG Ab. The numbers of viable and dead bacteria phagocytosed in macrophages were counted under a fluorescence microscope. The percent viability of L. monocytogenes was calculated as follows: (number of viable L. monocytogenes cells/total number of phagocytosed L. monocytogenes cells) × 100.
Flow cytometric analysis.
Peritoneal exudate macrophages (1 × 106 cells) were prepared and treated with anti-mouse CD16/CD32 MAb to block nonspecific binding to the Fc receptor. In some experiments, peritoneal exudate macrophages were incubated for 1 h with 20 μM LY294002 or 40 ng ml−1 toxin B before treatment with anti-mouse CD16/CD32 MAb. Cells were stained with anti-TLR2 MAb (6C2) at a concentration of 0.5 μg ml−1 for 30 min on ice and analyzed on a FACScalibur flow cytometer using the CELL Quest software (Becton Dickinson, Tokyo, Japan) (18). To determine the percentage of macrophages in PECs of WT, TLR2−/−, and MyD88−/− mice, PECs were collected, stained with PE-conjugated anti-F4/80 MAb, and analyzed by flow cytometry.
Determination of phosphatidylinositol 3-kinase (PI3K) activity by detection of phosphorylated Akt.
Akt is a downstream substrate of PI3K, and phosphorylation of Akt can be used as a marker of PI3K activation (2). Thus, we analyzed the amount of phosphorylated Akt semiquantitatively by Western blotting. Peritoneal exudate macrophages were prepared at 1 × 106 cells well−1 in a 24-well culture plate and infected with L. monocytogenes (MOI = 20) for 15 and 30 min. The cells were lysed in 100 μl of 2× SDS sample buffer containing a protease inhibitor cocktail. The cell lysate was sonicated for 5 min, boiled for 2 min, and then subjected to 10% SDS-polyacrylamide gel electrophoresis. The proteins were electroblotted to polyvinylidene difluoride (PVDF) membrane. The membrane was incubated with rabbit anti-Akt Ab or rabbit anti-phosphorylated Akt MAb for 1 h and with anti-rabbit IgG Ab conjugated with horseradish peroxidase. After treatment with Enhanced Chemiluminescence (ECL) detection reagents (Millipore, Billerica, MA), the bands representative of Akt and phosphorylated Akt were detected using LAS-4000 mini Fuji film (Tokyo, Japan).
Determination of Rac1 and Cdc42 activation in L. monocytogenes-infected macrophages.
Activated Rac1 and Cdc42 were detected using EZ-Detect Rac1 and Cdc42 activation kits. Briefly, peritoneal exudate macrophages were prepared at 2 × 106 cells well−1 in a 24-well culture plate and infected with L. monocytogenes (MOI = 20) for 15 and 30 min. The cells were lysed in a lysis/binding/wash buffer (Pierce) containing a protease inhibitor cocktail, and the supernatant was collected after centrifugation (16,000 × g) at 4°C for 15 min. Activated Rac1 and Cdc42 were precipitated with glutathione S-transferase (GST-human Pak1-PBD) and detected with mouse anti-Rac1 MAb and anti-Cdc42 MAb by Western blotting, respectively.
Phagocytosis of L. monocytogenes by macrophages during in vivo infection.
WT, TLR2−/−, and MyD88−/− mice were injected intraperitoneally (i.p.) with 3 ml of 3% thioglycolate medium and infected i.p. with 1 × 108 CFU of L. monocytogenes 4 days later. PECs were harvested 5 min after infection, washed, and cultured in RPMI 1640 medium containing gentamicin for 30 min to kill extracellular L. monocytogenes. Cells were washed and lysed by 0.1% Triton X-100. The number of phagocytosed L. monocytogenes cells was determined as described above. Alternatively, WT and TLR2−/− mice were injected intravenously (i.v.) with 104 CFU of L. monocytogenes. Spleen was collected 1 day after infection and homogenized in PBS. The number of viable bacteria in the homogenates was determined.
Statistical analysis.
The statistical significance of the data was determined using a two-tailed Student t test. A P value of <0.05 was considered statistically significant. All data represent the results of two to three independent experiments.
RESULTS
TLR2, but not TLR4, is involved in the phagocytosis of L. monocytogenes by macrophages.
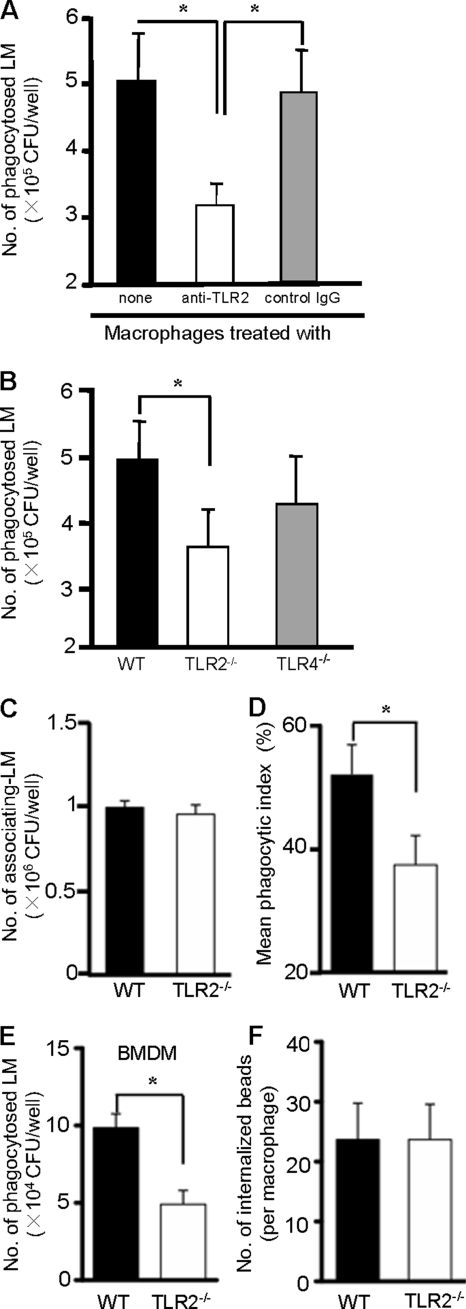
To analyze the role of TLR2 in the phagocytosis of L. monocytogenes, we infected macrophages with L. monocytogenes (5 × 106 CFU) in the presence or absence of anti-TLR2 MAb and determined the number of internalized bacteria using the gentamicin protection assay. As shown in Fig. 1A, WT macrophages were able to phagocytose about 5 × 105 CFU of L. monocytogenes, which was equivalent to 10% of the total bacteria added to the culture. Treatment with anti-TLR2 MAb significantly reduced the number of internalized bacteria, while mouse IgG, used as a control, did not affect the ability of macrophages to phagocytose bacteria (Fig. 1A). To confirm the involvement of TLR2 in the phagocytosis of L. monocytogenes, macrophages from WT, TLR2−/−, and TLR4-deficient (TLR4−/−) mice were compared. As expected, the phagocytic ability of TLR2−/− macrophages was significantly lower than that of WT macrophages (Fig. 1B). On the other hand, TLR4−/− macrophages did not show any defect in phagocytic ability compared with WT macrophages. As TLR2 has been shown to recognize components of L. monocytogenes (39), it is postulated that TLR2 serves as a phagocytic receptor that traps L. monocytogenes directly on macrophages. Alternatively, the intracellular signaling that is triggered by recognition of L. monocytogenes by TLR2 may be critical for the phagocytosis of L. monocytogenes. To investigate this, we infected WT and TLR2−/− macrophages with L. monocytogenes and determined the numbers of both L. monocytogenes associating with macrophages and phagocytosed bacteria. There was no difference in the numbers of associating L. monocytogenes cells in WT macrophages and in the TLR2−/− macrophages, indicating that the bacterium adhered to macrophages irrespective of the expression of TLR2 (Fig. 1C). On the other hand, WT macrophages phagocytosed about 55% of L. monocytogenes out of all associating bacteria, and TLR2−/− macrophages exhibited a significantly lower phagocytic index (Fig. 1D). When bone marrow-derived macrophages (BMDM) were used, a similar difference was observed between WT BMDM and TLR2−/− BMDM (Fig. 1E). To rule out the possibility of a general impairment in phagocytic ability, we analyzed the internalization of polystyrene beads, which is not dependent on any TLRs. As shown in Fig. 1F, the internalization of polystyrene beads was found to be identical in WT and TLR2−/− macrophages. These results showed that TLR2, but not TLR4, might play a role in the phagocytosis of L. monocytogenes by macrophages.
FIG. 1.
Difference in the phagocytosis of Listeria monocytogenes (LM) between WT and TLR2−/− macrophages. (A) WT macrophages were incubated with 40 μg ml−1 anti-TLR2 MAb (IgG1) or mouse control IgG for 1 h and infected with L. monocytogenes at an MOI of 20 for 1 h. Cells were then treated with 20 μg ml−1 gentamicin for 30 min, and the number of phagocytosed bacteria was determined. (B) WT, TLR2−/−, and TLR4−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h. The number of phagocytosed bacteria was determined after treatment with gentamicin for 30 min. (C and D) WT and TLR2−/− macrophages were infected with L. monocytogenes as mentioned above, and the number of associating L. monocytogenes cells (C) and the mean phagocytic index (D) were determined. The mean phagocyte index was calculated as follows: (number of phagocytosed L. monocytogenes cells/number of associating L. monocytogenes cells) × 100. (E) Bone marrow-derived macrophages from WT and TLR2−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h, and the number of phagocytosed bacteria was determined. (F) WT and TLR2−/− macrophages were cultured with polystyrene beads for 2 h at a bead-to-cell ratio of 50 to 1. After being washed, cells were fixed with 1% paraformaldehyde and the number of internalized beads was counted under fluorescence microscope. Data are expressed as the means ± standard deviations (SD) of results for triplicate cultures. Similar results were obtained in three independent experiments. *, P < 0.05.
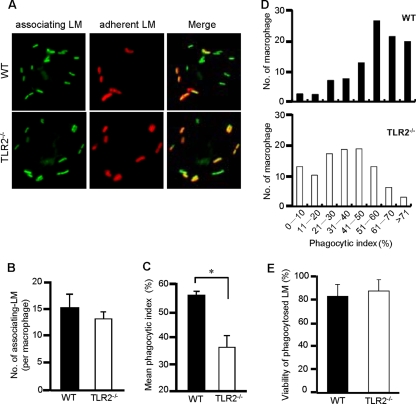
To further confirm the involvement of TLR2 in the phagocytosis of L. monocytogenes, the phagocytic efficiency was assessed by fluorescence microscopy. We infected WT and TLR2−/− macrophages with CFSE-labeled L. monocytogenes. Cells were then treated with rabbit anti-Listeria Ab and Alexa Fluor 594-conjugated anti-rabbit IgG Ab. This procedure enabled us to distinguish internalized bacteria from bacteria adhering to the surface of macrophages under fluorescence microscope (Fig. 2A). The efficiency of phagocytosis was determined in 500 infected macrophages. As with the result shown in Fig. 1C, there was no difference in the total numbers of associating L. monocytogenes cells between WT and TLR2−/− macrophages (Fig. 2B). On the other hand, WT macrophages exhibited a higher level of phagocytic activity than TLR2−/− macrophages. The phagocytic index was found to be significantly lower in TLR2−/− macrophages than in WT macrophages (Fig. 2C). We further compared the phagocytic efficiencies of individual macrophages between the two groups. The results depicted in Fig. 2D, illustrating the numbers of macrophages grouped according to their phagocytic index in WT and TLR2−/− macrophages, clearly show that most of the WT macrophages had a higher phagocytic index whereas most of the TLR2−/− macrophages had a lower phagocytic index. Furthermore, it is probable that the difference in the phagocytic efficiencies of WT and TLR2−/− macrophages might be attributable to the bactericidal activity of these macrophages. To test the hypothesis, the viability of L. monocytogenes inside macrophages was measured after 1 h of phagocytosis. We found that most of the bacteria inside WT and TLR2−/− macrophages were viable and there was no significant difference in viability between these two groups (Fig. 2E). These results indicated that TLR2 participates in the enhancement of the basic phagocytosis of L. monocytogenes by macrophages while TLR2 itself does not participate in the direct adhesion of L. monocytogenes.
FIG. 2.
Fluorescence microscopic analysis for the phagocytosis of L. monocytogenes (LM). (A) WT and TLR2−/− macrophages were cultured with CFSE-labeled L. monocytogenes at an MOI of 50 for 1 h. After being washed, cells were fixed and incubated with rabbit anti-Listeria Ab and anti-rabbit IgG Ab conjugated with Alexa Fluor 594 to stain adherent L. monocytogenes to macrophages. According to the procedure, phagocytosed L. monocytogenes (green spots) was distinguished from adherent L. monocytogenes (yellow spots) in merged fluorescence images. (B and C) After infected L. monocytogenes cells were stained with rabbit anti-Listeria Ab and Alexa Fluor 594-conjugated anti-rabbit IgG Ab, the numbers of associating L. monocytogenes and adherent L. monocytogenes cells were counted in 500 WT and TLR2−/− macrophages, and the number of phagocytosed L. monocytogenes cells was determined. The phagocytic index (a percentage) was calculated as (number of phagocytosed L. monocytogenes cells/number of associating L. monocytogenes cells) × 100. The average number of associating L. monocytogenes cells (B) and the mean phagocyte index (C) are depicted. *, P < 0.05. (D) Columns depict the exact distribution of the phagocytic index in WT and TLR2−/− macrophages. The experiment was repeated three times, and data are expressed as the means ± standard errors for results of three independent experiments. (E) WT and TLR2−/− macrophages were infected with CFSE-labeled L. monocytogenes at an MOI of 50 for 1 h and incubated with CTC for 15 min. Cells were fixed and treated with rabbit anti-Listeria Ab and Alexa Fluor 350-conjugated anti-rabbit IgG Ab. The numbers of viable and dead bacteria inside macrophages were counted. The viability of intracellular bacteria (%) was calculated as follows: (number of viable L. monocytogenes cells/number of viable and dead L. monocytogenes cells) × 100.
MyD88 is required for efficient TLR2-dependent phagocytosis of L. monocytogenes.
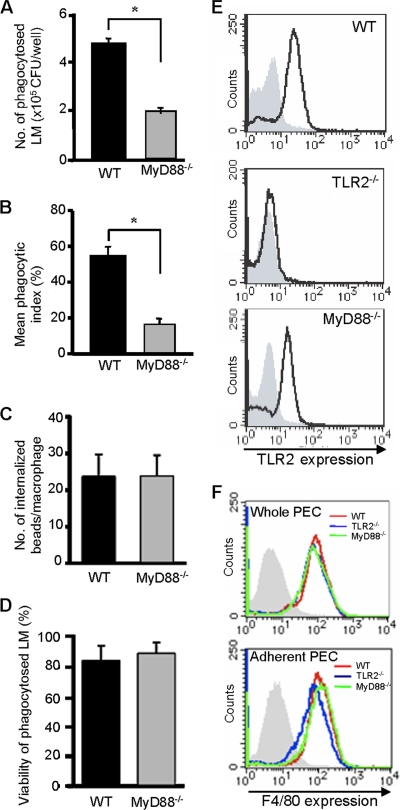
We next investigated whether TLR2-dependent signaling is required for phagocytosis of L. monocytogenes. As MyD88 is an essential adaptor protein for TLR2-dependent signaling, we examined the phagocytic ability of macrophages from MyD88−/− mice. As shown in Fig. 3A, the number of phagocytosed L. monocytogenes cells in MyD88−/− macrophages was significantly lower than that in WT macrophages. In addition, fluorescence microscopy revealed that MyD88−/− macrophages had a significantly lower phagocytic index than WT macrophages (Fig. 3B). The absence of any reduction in the phagocytosis of polystyrene beads supported the view that the effect seen in MyD88−/− macrophages was not due to some general impairment of macrophage function (Fig. 3C). In addition, we found that there was no difference in the viability of bacteria in WT and MyD88−/− macrophages. The majority of phagocytosed bacteria were viable in two groups of macrophages (Fig. 3D). These results indicated that TLR2- and MyD88-dependent signaling enhanced the phagocytosis of L. monocytogenes by macrophages. To rule out the possibility that the reduced phagocytosis of L. monocytogenes observed in MyD88−/− macrophages is due to the reduced expression of TLR2, we carried out fluorescence-activated cell sorter (FACS) analysis to analyze the expression level of TLR2 on these macrophages. As shown in Fig. 3E, TLR2 was expressed on WT but not TLR2−/− macrophages. In MyD88−/− macrophages, the expression level of TLR2 was slightly reduced but a substantial expression was detected. In addition, we determined the percentage of macrophages in whole and adherent PECs of WT, TLR2−/−, and MyD88−/− mice by measuring the number of F4/80-positive cells. There was no difference in the purity of macrophages in whole and adherent PECs of these mice (Fig. 3F). From these findings, it was clear that the reduced phagocytic efficiency in MyD88−/− macrophages was not due to the reduced expression of the extracellular domain of TLR2, a general impairment in phagocytosis, or a difference in bactericidal activity. It was also clear that the TLR-MyD88 signaling pathway plays a potentially important role in intracellular engulfment of bacteria adhering to the macrophage surface.
FIG. 3.
Difference in the phagocytosis of L. monocytogenes (LM) between WT and MyD88−/− macrophages. (A) WT and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h. After being washed, cells were treated with gentamicin for 30 min and the number of phagocytosed bacteria was determined. Data are expressed as the means ± standard deviations (SD) for results of triplicate cultures. Similar results were obtained in three independent experiments. *, P < 0.05. (B) WT and MyD88−/− macrophages were infected with CFSE-labeled L. monocytogenes at an MOI of 50 for 1 h. The numbers of phagocytosed L. monocytogenes and adherent L. monocytogenes cells were evaluated after immunostaining. Data are expressed as the means ± SD for results of triplicate cultures. Similar results were obtained in two independent experiments. *, P < 0.05. (C) WT and MyD88−/− macrophages were incubated with polystyrene beads for 2 h. After being washed, cells were fixed and the number of internalized beads was counted. (D) WT and MyD88−/− macrophages were infected with CFSE-labeled L. monocytogenes at an MOI of 50 for 1 h and treated similarly to those shown in Fig. 2E. The viability of intracellular bacteria was calculated as a percentage. (E) WT, TLR2−/−, and MyD88−/− macrophages were stained with FITC-conjugated anti-TLR2 MAb (IgG2b) or control IgG2b, and the level of TLR2 expression was analyzed by FACS. The histogram with the bold line shows the level of TLR2 expression on the three types of macrophages. The hatched area represents the basal fluorescent intensity in cells treated with control Ab. (F) Whole PEC and adherent PEC obtained from WT (red), TLR2−/− (blue), and MyD88−/− (green) mice were stained with PE-conjugated anti-F4/80 MAb (IgG2a). The expression of F4/80 was analyzed by FACS. The hatched area represents the basal fluorescent intensity in cells treated with control IgG2a.
Involvement of downstream signaling from TLR-MyD88 but not cytokine or other gene expression in phagocytosis of L. monocytogenes by macrophages.
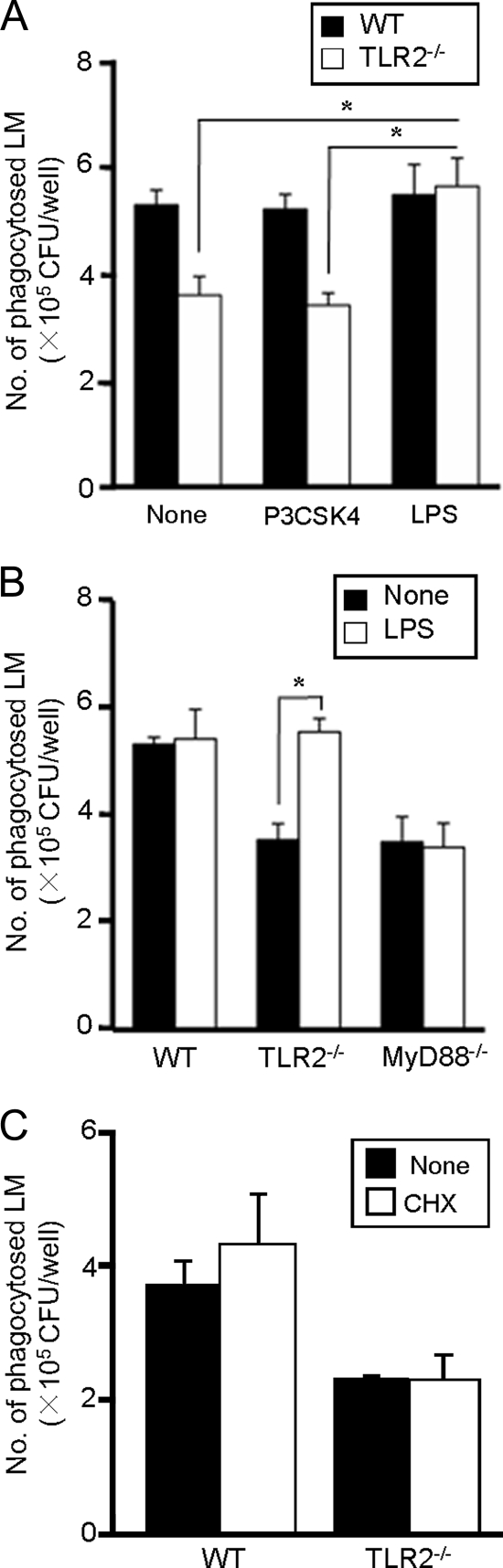
LPS is known to stimulate the cytokine response through the TLR4-MyD88-dependent signaling pathway. To confirm the importance of the MyD88-dependent signaling pathway, we tested whether stimulation with LPS restores the level of L. monocytogenes phagocytosis in TLR2−/− or MyD88−/− macrophages to WT levels. WT, TLR2−/−, and MyD88−/− macrophages were infected with L. monocytogenes in the presence or absence of LPS or Pam3CSK4 (an authentic TLR2 ligand), and the efficiency of phagocytosis was examined. The phagocytic ability of WT macrophages was not influenced by treatment with either Pam3CSK4 or LPS. Again, TLR2−/− macrophages exhibited a decrease in the phagocytic index compared with WT macrophages, as shown previously. However, the reduced phagocytic ability of TLR2−/− macrophages was significantly increased by treatment with LPS, while Pam3CSK4 did not show such an enhancing effect (Fig. 4A). On the other hand, treatment with LPS never enhanced the phagocytic efficiency of MyD88−/− macrophages (Fig. 4B). These results strongly suggested that activation of the MyD88-dependent signaling pathway is required for the enhancement of basic phagocytosis of L. monocytogenes by macrophages.
FIG. 4.
The effect of LPS and cycloheximide on the phagocytosis of L. monocytogenes (LM) by TLR2−/− and MyD88−/− macrophages. (A) WT and TLR2−/− macrophages were infected with L. monocytogenes at an MOI of 20 in the presence or absence of 100 ng ml−1 Pam3CSK4 or 100 ng ml−1 LPS for 1 h, and the number of phagocytosed L. monocytogenes cells was determined. Data are expressed as the means ± standard deviations (SD) for results of triplicate cultures. *, P < 0.05. (B) WT, TLR2−/−, and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 in the presence or absence of 100 ng ml−1 LPS for 1 h, and the number of phagocytosed L. monocytogenes cells was counted. Data are expressed as the means ± SD for results of triplicate cultures. *, P < 0.05. (C) WT and TLR2−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h in the presence or absence of CHX, and the number of phagocytosed bacteria was determined. Data are expressed as the means ± SD for results of triplicate cultures. Similar results were obtained in three independent experiments.
It has been shown that activation of the TLR2 signaling pathway induces the production of cytokines, including tumor necrosis factor-α (TNF-α), which causes macrophage activation. Doyle et al. (13) recently reported that stimulation of macrophages with TLR ligands induces the expression of several scavenger receptors that serve as phagocytic receptors. Therefore, it could be postulated that signals downstream of the TLR-MyD88 pathway, leading to expression of cytokines or some phagocytic receptors after L. monocytogenes infection, might contribute to phagocytosis. To test this, we determined the effect of CHX on phagocytosis. CHX treatment mostly inhibited the production of IL-6 and TNF-α from infected macrophages, whereas cell viability was not affected (data not shown). We found that CHX treatment did not alter the number of bacteria phagocytosed by WT and TLR2−/− macrophages (Fig. 4C), suggesting that the phagocytosis of L. monocytogenes is independent of protein synthesis and that TLR2-dependent enhancement of phagocytosis is not due to cytokine production or expression of phagocytic receptors.
Phosphatidylinositol 3-kinase (PI3K) is implicated in TLR2-dependent enhancement of phagocytosis of L. monocytogenes.
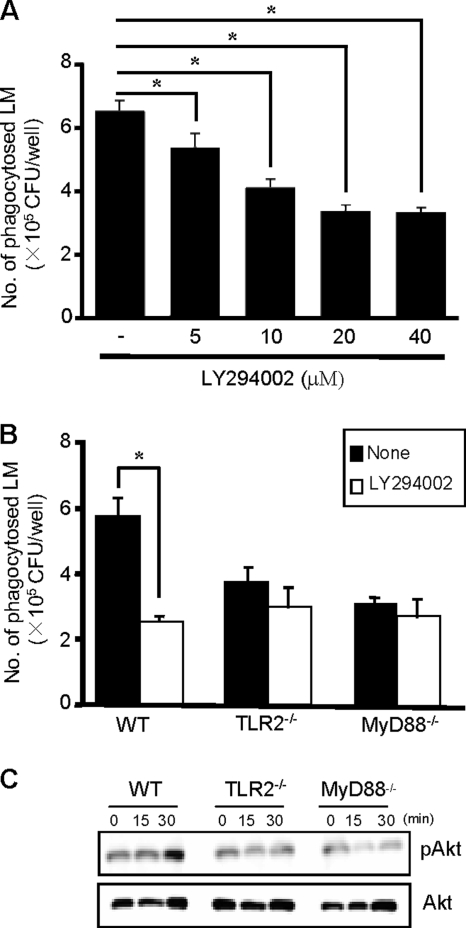
Phagocytosis of bacteria requires the activation of a number of signaling pathways that regulate the rearrangement of the actin cytoskeleton and extension of the plasma membrane (54). It has been shown that PI3K plays an important role in phagocytosis and TLR-dependent signaling induces activation of PI3K (32). These findings prompted us to determine whether PI3K contributes to the TLR2-dependent phagocytosis of L. monocytogenes. We infected WT, TLR2−/−, and MyD88−/− macrophages with L. monocytogenes in the presence or absence of LY294002, a PI3K inhibitor, and measured the number of phagocytosed bacteria. In WT macrophages, PI3K inhibitor reduced the efficiency of phagocytosis in a dose-dependent manner (Fig. 5A). We found that treatment of WT macrophages with LY294002 decreased the number of phagocytosed bacteria to almost the basal level observed in TLR2−/− and MyD88−/− macrophages. However, the inhibitor did not affect the phagocytosis by TLR2−/− and MyD88−/− macrophages (Fig. 5B). To confirm whether activation of PI3K is actually induced in infected macrophages, we analyzed the amount of phosphorylated Akt, one of the substrates of PI3K known to be an important signaling molecule for the induction of phagocytosis (2). During infection of WT macrophages with L. monocytogenes, the amount of phosphorylated Akt (pAKT) was increased 30 min after infection. However, no increase in pAKT was detected in TLR2−/− and MyD88−/− macrophages (Fig. 5C). These results indicated that L. monocytogenes-induced TLR2 signaling enhances phagocytosis through activation of PI3K and Akt phosphorylation.
FIG. 5.
The effect of LY294002 on the phagocytosis of L. monocytogenes (LM) and difference in the phosphorylation of Akt between WT, TLR2−/−, and MyD88−/− macrophages after L. monocytogenes infection. (A) WT macrophages were infected with L. monocytogenes at an MOI of 20 in the presence of graded concentrations of LY294002, a PI3K inhibitor, and the number of phagocytosed bacteria was determined. Data are expressed as the means ± standard deviations (SD) for results of triplicate cultures. (B) WT, TLR2−/−, and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h in the presence or absence of 20 μM LY294002, and the number of phagocytosed bacteria was determined. Data are expressed as the means ± SD for results of triplicate cultures. Similar results were obtained in two independent experiments. *, P < 0.05. (C) WT, TLR2−/−, and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 15 and 30 min. Cells were lysed, and phosphorylated Akt was evaluated by Western blotting. Total Akt was used as a loading control.
Rac1 but not Cdc42 activation is important for the phagocytosis of L. monocytogenes by macrophages.
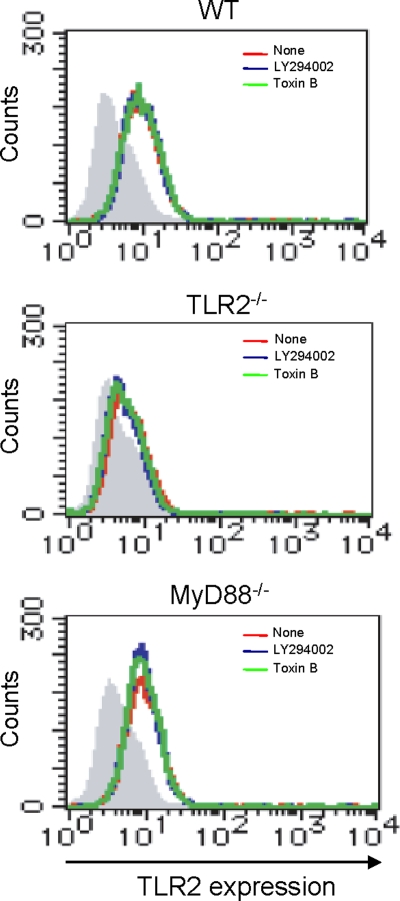
PI3K signaling has been reported to play an important role in actin polymerization through activation of the Rho family of GTPases that subsequently recruit the Arp2/3 complex to form the actin nucleation (7). In order to determine the possible involvement of these Rho family GTPases in the PI3K-directed actin polymerization during phagocytosis of L. monocytogenes, we tested the effect of C. difficile toxin B, an inhibitor specific for the Rho family GTPases (9, 30). We infected WT, TLR2−/−, and MyD88−/− macrophages with L. monocytogenes in the presence or absence of toxin B, and we measured the number of phagocytosed bacteria. Toxin B markedly reduced the efficiency of phagocytosis in WT macrophages but did not affect phagocytosis by TLR2−/− and MyD88−/− macrophages (Fig. 6A). We also examined whether Rac1 activation is induced in infected macrophages by analyzing the activated GTP-bound form of Rac1 or Cdc42. According to Western blot analysis (Fig. 6B), an increased level of GTP-bound Rac1 was detected 30 min after infection in WT macrophages whereas it was almost undetectable in TLR2−/− and MyD88−/− macrophages. Cdc42 did not appear to be involved in the process because there was no difference in the levels of GTP-bound Cdc42 between WT macrophages and TLR2−/− or MyD88−/− macrophages. In addition, the expression level of TLR2 on WT, TLR2−/−, and MyD88−/− macrophages was not affected by treatment with LY294002 or toxin B (Fig. 7). Taken together, these results indicated that L. monocytogenes-induced TLR2-MyD88 signaling enhances the phagocytosis through activation of PI3K and Rac1.
FIG. 6.
The effect of toxin B on the phagocytosis of L. monocytogenes (LM) and difference in the phosphorylation of Rho family GTPase proteins between WT, TLR2−/−, and MyD88−/− macrophages after L. monocytogenes infection. (A) WT, TLR2−/−, and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 1 h in the presence or absence of 40 ng ml−1 toxin B, and the number of phagocytosed bacteria was determined. Data are expressed as the means ± standard deviations (SD) for results of triplicate cultures. Similar results were obtained in two independent experiments. *, P < 0.05. (B) WT, TLR2−/−, and MyD88−/− macrophages were infected with L. monocytogenes at an MOI of 20 for 15 and 30 min. The GTP-bound forms of Rac1 and Cdc42 were precipitated using glutathione S-transferase (GST)-bound p21-activated kinase 1 (PAK1) and quantified by Western blotting using anti-Cdc42 (IgG1) and anti-Rac1 (IgG2b) MAbs. Total Rac1 and Cdc42 in the cell lysate were used as a loading control.
FIG. 7.
The effect of LY294002 and toxin B on the expression of TLR2 on macrophages. WT, TLR2−/−, and MyD88−/− macrophages were incubated with 20 μM LY294002 or 40 ng ml−1 toxin B for 1 h and treated with anti-CD16/32 MAb (IgG2b), followed by staining with FITC-conjugated anti-TLR2 MAb (IgG2b) or control IgG2b. The level of TLR2 expression was analyzed by FACS. The histogram with the red line shows the level of TLR2 expression on macrophages without treatment, the blue line represents the level of TLR2 expression on macrophages treated with LY294002, and the green line shows the level of TLR2 expression on macrophages treated with toxin B. The hatched area represents the basal fluorescent intensity in cells treated with control IgG2b.
TLR-MyD88 signaling is important for phagocytosis during L. monocytogenes infection in vivo.
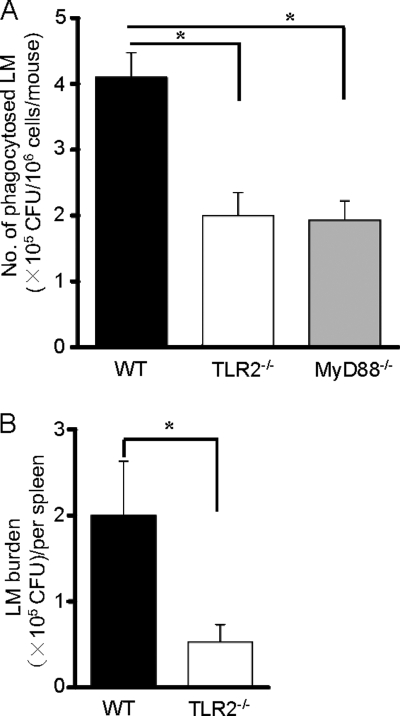
In order to confirm the biological significance of our in vitro data during L. monocytogenes infection in vivo, we compared the number of phagocytosed bacteria in WT, TLR2−/−, and MyD88−/− mice i.p. infected with L. monocytogenes. As shown in Fig. 8A, the number of L. monocytogenes cells entrapped by a given number of macrophages recovered after i.p. infection was significantly lower in TLR2−/− and MyD88−/− mice than in macrophages from WT mice. It has been shown that L. monocytogenes is captured mainly by various types of macrophages in the periarteriolar lymphoid sheath within 24 h after infection (4). Therefore, we intravenously infected WT and TLR2−/− mice with L. monocytogenes, and the CFU in spleen were enumerated 1 day later. The CFU number in spleens of TLR2−/− mice was approximately four times as low as that in spleens of WT mice (0.5 × 105 CFU versus 1.9 × 105 CFU, respectively) (Fig. 8B). Thereafter, L. monocytogenes replicated similarly in spleens of both WT and TLR2−/− mice and the CFU number reached 1 × 107 CFU in WT and 0.8 × 107 CFU in TLR2−/− mice at 3 days after infection. These results suggest that the TLR2-dependent signaling actually contributes to the phagocytosis of macrophages in vivo and the phagocytic activity of TLR2−/− macrophages might result in a lower bacterial burden within 1 day after L. monocytogenes infection whereas the difference in the phagocytic activity did not affect the final bacterial growth at the late phase of infection.
FIG. 8.
Difference in the phagocytosis of L. monocytogenes (LM) and bacterial burden between WT and TLR2−/− mice after L. monocytogenes infection in vivo. (A) WT, TLR2−/−, and MyD88−/− mice were injected i.p. with 3 ml of 3% thioglycolate medium and infected i.p. with 1 × 108 CFU of L. monocytogenes 4 days later. Five minutes after infection, PECs were collected, and the number of phagocytosed L. monocytogenes was determined. *, P < 0.05. (B) WT and TLR2−/− mice were infected intravenously with 104 CFU of L. monocytogenes. The spleens were removed 1 day after infection, and the number of viable bacteria in spleen was determined. *, P < 0.05.
DISCUSSION
Increasing evidence indicates that TLR-dependent signaling promotes not only cytokine production but also phagocytosis of bacteria by macrophages. The effect of TLR on phagocytosis of some extracellular bacteria has previously been reported (6, 13, 35). TLR2 expressed on macrophages plays a key role in the cellular response to components of Gram-positive bacteria, including L. monocytogenes (28, 36). However, there have been no reports to date showing that TLR2 signaling is involved in the phagocytosis of L. monocytogenes by macrophages. The results obtained in this study have clearly shown that although L. monocytogenes is able to invade macrophages independently of TLR2 and MyD88, TLR2, but not TLR4, enhances the basic level of phagocytosis of L. monocytogenes by macrophages.
It has been shown that intracellular parasitic bacteria are capable of modulating the bactericidal activity of macrophages in order to grow and reside in infected cells. L. monocytogenes is known to survive inside macrophages by escaping from phagosomes into the cytosol before phagolysosome formation takes place. However, Alvarez-Dominguez et al. (3) and Henry et al. (23) suggested that virulent L. monocytogenes has the ability to delay phagosome maturation. Although it has not yet been elucidated how L. monocytogenes directly manipulates the bactericidal mechanism of professional phagocytes, it is clear that L. monocytogenes has the potential to modulate the intracellular killing mechanism. Therefore, we determined the contribution of TLR2 signaling in the phagocytosis of L. monocytogenes by macrophages. Here we have shown that peritoneal macrophages from TLR2−/− and MyD88−/− have markedly reduced phagocytic efficiency compared with that of WT macrophages. A similar reduction was observed in bone marrow-derived macrophages from these knockout mice, although the number of phagocytosed L. monocytogenes was 5-fold lower than that of peritoneal macrophages (data not shown). Therefore, it is clear that the TLR2-MyD88-dependent signaling pathway is critical for the efficient phagocytosis of L. monocytogenes.
Doyle et al. (13) showed that pretreatment with TLR ligands enhances phagocytosis by macrophages due to upregulation of specific phagocytic receptors such as scavenger receptors, including scavenger receptor-A (SR-A), a macrophage receptor with a collagenous structure (MARCO). However, in our experiments, the difference in the efficiency of phagocytosis between WT and TLR2−/− macrophages could be observed as early as 1 h after infection. In addition, treatment with CHX did not affect the levels of phagocytosis in WT and TLR2−/− macrophages. Although any level of contribution of known phagocytic receptors that are constitutively expressed cannot be completely excluded, these results strongly imply that TLR2-dependent enhancement of phagocytosis of L. monocytogenes is not simply due to the production of cytokines or expression of phagocytic receptors such as scavenger receptors after L. monocytogenes infection.
In studying the possible mechanism involved in the TLR-mediated enhanced phagocytosis of L. monocytogenes by macrophages, fluorescence microscopy was useful in distinguishing L. monocytogenes cells outside the macrophages from those inside the macrophages. From our findings, TLR2 is unlikely to be acting as a phagocytic receptor that directly captures L. monocytogenes on macrophage surfaces. Shin et al. (47) have shown that signaling through TLR adaptor MyD88 plays an important role in the uptake of Borrelia. Our results showing the reduced efficiency of phagocytosis in MyD88−/− macrophages suggested a similar role of TLR-MyD88 signaling in phagocytosis of L. monocytogenes by macrophages. Moreover, the restoration of reduced phagocytosis in TLR2−/− but not MyD88−/− macrophages by addition of the TLR4 ligand LPS confirmed the importance of downstream signaling through MyD88 in the enhancement of phagocytosis.
MyD88 is an adaptor protein involved in a variety of downstream signaling pathways (1, 41), and some reports have suggested the link between PI3 kinases and phagocytosis (49, 54). PI3K catalyzes the phosphorylation of several species of phosphatidylinositol that are important in recruiting signaling molecules such as Akt/PKB to specific regions of the membrane. It is also involved in membrane extension, fusion behind bound particles, and insertion of new membrane at the site of particle internalization (54). Recent studies have shown that PI3K is involved in the induction of TLR-mediated cellular responses, including uptake of TLR ligands and cytokine production (32, 50). In our study we found that preincubation with LY294002, a PI3K inhibitor, resulted in impaired phagocytosis of L. monocytogenes by WT macrophages in a dose-dependent manner. However, treatment with p38 and ERK inhibitors did not result in a reduction in phagocytosis of L. monocytogenes by macrophages (data not shown). Furthermore, there was a significant difference in the magnitude of PI3K activation between WT and TLR2−/− or MyD88−/− macrophages after L. monocytogenes infection, indicating that PI3K is critically involved in the downstream signaling pathway during phagocytosis of L. monocytogenes by WT macrophages.
The Rho GTPases, Rac1 and Cdc42, are widely known as the critical regulators of actin cytoskeletal rearrangements during the phagocytic response to a variety of extracellular stimuli (10, 12). It has been reported that PI3K signaling is involved in the activation of the Rho family GTPases, including Rac1 and Cdc42, leading to the initiation of actin polymerization (21). In our study, we found that inhibition of the Rho family GTPases by toxin B reduced phagocytosis of L. monocytogenes and that activation of Rac1, but not Cdc42, was far less significant in TLR2−/− and MyD88−/− macrophages. In addition, the inhibitors for PI3K and Rac1 never affected the expression of TLR2 on macrophages. Therefore, it is likely that TLR2-MyD88 mediates an enhancement of phagocytosis through PI3K and Rac1 activation in WT macrophages after infection with L. monocytogenes. This is consistent with the recent report by Shin et al. showing that downstream signaling of MyD88-mediated phagocytosis is dependent on PI3K and Rac1 activation during Borrelia infection (47). However, one recent study showed that a MyD88-independent activation of the actin-Cdc42/Rac pathway is required for TLR-mediated phagocytosis (30). In fact, the authors determined the phagocytosis of GFP-E. coli after LPS treatment, which is different from our experiment that directly focuses on the role of TLR2 on early infection (after 1 h) without treatment. Furthermore, Braun et al. (8) reported that L. monocytogenes induces activation of PI3K to infect Caco-2 cells via InlB protein. Therefore, activation of PI3K appears to be important for the internalization of L. monocytogenes irrespective of the type of host cell. Small Rho GTPases are upstream activators of the Arp2/3 complex in many actin polymerization events such as cell-cell adhesion, cell movement, and phagocytosis (28). Moreover, Sousa et al. (48) reported that Rac1 promotes recruitment of cortactin and activation of Arp2/3 in E-cadherin-mediated Listeria entry. A possibility that Arp2/3 might be the downstream molecule for phagocytosis of L. monocytogenes by macrophages is to be explored in a further study.
Although our results clearly indicate that TLR2-MyD88-dependent signals leading to PI3K and Rac1 activation enhance the phagocytosis of L. monocytogenes by macrophages, the exact interaction between TLR2 and its ligand for the induction of the MyD88-PI3K-Rac1 pathway still remains to be determined. Recent studies highlighted the immunological function of lipoproteins derived from Gram-positive bacteria, and lipoproteins have been shown to induce the host immune response via activation of the TLR2 signaling pathway. Machata et al. (40) have shown that surface lipoproteins derived from L. monocytogenes activate the TLR2 signaling pathway during the early phase of infection. It is possible that the interaction of TLR2 with lipoprotein expressed on the surface of L. monocytogenes cells is involved in the TLR2-dependent enhancement of phagocytosis that was observed in our study. On the other hand, little is known about receptors that contribute to the phagocytosis of L. monocytogenes. It seems that one or more receptors, which are constitutively expressed on macrophages, including type I and type II scavenger receptors, may serve as phagocytic receptors for L. monocytogenes (26). In this study, we found that CHX never affected TLR2-dependent phagocytosis. This result suggests that enhancement of the phagocytosis is not attributable to the newly synthesized phagocytic receptors after L. monocytogenes infection. The exact manner of direct interaction between macrophages and L. monocytogenes remains to be elucidated.
There is increasing evidence suggesting the involvement of TLR signaling in phagocytosis of bacterial pathogens in vitro (41). However, the functional significance of these findings in vivo has yet to be determined in many cases. In the present study, we showed that macrophages from TLR2−/− and MyD88−/− mice harbor significantly lower numbers of phagocytosed L. monocytogenes cells after i.p. and i.v. infection with L. monocytogenes compared with macrophages from WT mice in the early period of L. monocytogenes infection. This finding clearly indicates that the TLR2-MyD88-mediated enhancement of phagocytosis is not a phenomenon observed only under specific experimental conditions in vitro but is also applicable to in vivo infection.
Acknowledgments
This study was supported by a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Culture and Sports of Japan; grants-in-aid for scientific research (B and C); a grant-in-aid for young scientists (B) from the Japanese Society for the Promotion of Science; and a grant-in-aid for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare of Japan and a grant-in-aid from the Waksman Foundation of Japan.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 5 April 2010.
REFERENCES
- 1.Akira, S., and K. Takeda. 2004. Toll-like receptor signaling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., and P. Cohen. 1998. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8:55-62. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dominguez, C., R. Roberts, and P. D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731-743. [DOI] [PubMed] [Google Scholar]
- 4.Aoshi, T., J. A. Carrero, V. Konjufca, Y. Koide, E. R. Unanue, and M. J. Miller. 2009. The cellular niche of Listeria monocytogenes infection changes rapidly in the spleen. Eur. J. Immunol. 39:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutler, B. 2004. Innate immunity: an overview. Mol. Immunol. 40:845-859. [DOI] [PubMed] [Google Scholar]
- 6.Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304:1014-1018. [DOI] [PubMed] [Google Scholar]
- 7.Bosse, T., J. Ehinger, A. Cauchra, S. Benesch, A. Steffen, X. Wu, K. Schloen, H. H. Niemann, G. Scita, T. E. Stradal, C. Brakebusch, and K. Rottner. 2007. Cdc42 and phosphoinositide 3-kinase drive Rac-mediated actin polymerization downstream of c-Met in distinct and common pathways. Mol. Cell. Biol. 19:6615-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun, L., H. Ohayon, and P. Cossart. 1998. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol. Microbiol. 27:1077-1087. [DOI] [PubMed] [Google Scholar]
- 9.Carabeo, R. A., S. S. Grieshaber, A. Hasenkrug, C. Dooley, and T. Hackstadt. 2004. Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic 5:418-425. [DOI] [PubMed] [Google Scholar]
- 10.Caron, E., and A. Hall. 1998. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPase. Science 282:1717-1721. [DOI] [PubMed] [Google Scholar]
- 11.Cossart, P., J. Pizarro-Cerda, and M. Lecuit. 2003. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell. Biol. 13:23-31. [DOI] [PubMed] [Google Scholar]
- 12.Cox, D., P. Chang, Q. Zhang, P. G. Reddy, G. M. Bokoch, and S. Greenberg. 1997. Requirement for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186:1487-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doyle, S. E., R. M. O'Connell, G. A. Miranda, S. A. Vaidya, E. K. Chow, P. T. Liu, S. Suzuki, N. Suzuki, R. L. Modlin, W. C. Yeh, T. F. Lane, and G. Cheng. 2004. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dussurget, O., J. Pizarro-Cerda, and P. Cossart. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58:587-610. [DOI] [PubMed] [Google Scholar]
- 15.Dziarski, R., R. I. Tapping, and P. S. Tobias. 1998. Binding of bacterial peptidoglycan to CD14. J. Biol. Chem. 273:8680-8690. [DOI] [PubMed] [Google Scholar]
- 16.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophages listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 17.Ezekowitz, R. A., K. Sastry, P. Bailly, and A. Warner. 1990. Molecular characterization of the human macrophage mannose receptor: demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J. Exp. Med. 172:1785-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes, E. K., C. Sander, E. O. Ronan, H. McShane, A. V. Hill, P. C. Beverley, and E. Z. Tchilian. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Garcia, E., and C. Rosales. 2002. Signal transduction during Fc receptor-mediated phagocytosis. J. Leukoc. Biol. 72:1092-1108. [PubMed] [Google Scholar]
- 20.Hamon, M., H. Bierne, and P. Cossart. 2006. Listeria monocytogenes: a multifaceted model. Nat. Rev. Microbiol. 4:423-434. [DOI] [PubMed] [Google Scholar]
- 21.Han, J., K. Luby-Phelps, B. Das, X. Shu, Y. Xia, R. D. Mosteller, U. M. Krishna, J. R. Falck, M. A. White, and D. Broek. 1998. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279:558-560. [DOI] [PubMed] [Google Scholar]
- 22.Hara, H., K. Tsuchiya, T. Nomura, I. Kawamura, S. Shoma, and M. Mitsuyama. 2008. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J. Immunol. 180:7859-7868. [DOI] [PubMed] [Google Scholar]
- 23.Henry, R., L. Shaughnessy, M. J. Lossner, C. Alberti-Segui, D. E. Higgins, and J. A. Swanson. 2006. Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell. Microbiol. 8:107-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ireton, K. 2007. Entry of the bacterial pathogen Listeria monocytogenes into mammalian cells. Cell. Microbiol. 9:1365-1375. [DOI] [PubMed] [Google Scholar]
- 25.Ireton, K., B. Payrastre, H. Chap, W. Ogawa, H. Sakaue, M. Kasuga, and P. Cossart. 1996. A role for phosphoinositide 3-kinase in bacterial invasion. Science 274:780-782. [DOI] [PubMed] [Google Scholar]
- 26.Ishiguro, T., M. Naito, T. Yamamoto, G. Hasegawa, F. Gejyo, M. Mitusyama, H. Suzuki, and T. Kodama. 2001. Role of macrophage scavenger receptors in response to Listeria monocytogenes infection in mice. Am. J. Pathol. 158:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii, K. J., C. Coban, and S. Akira. 2005. Manifold mechanisms of Toll-like receptor-ligand recognition. J. Clin. Immunol. 25:511-521. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe, A. B., and A. Hall. 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21:247-269. [DOI] [PubMed] [Google Scholar]
- 29.Janot, L., T. Secher, D. Torres, I. Maillet, J. Pfeilschifter, V. F. Quesniaux, R. Landmann, B. Ryffel, and F. Erard. 2008. CD14 works with Toll-like receptor 2 to contribute to recognition and control of Listeria monocytogenes infection. J. Infect. Dis. 198:115-124. [DOI] [PubMed] [Google Scholar]
- 30.Kong, L., and B. X. Ge. 2008. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell. Res. 18:745-755. [DOI] [PubMed] [Google Scholar]
- 31.Kopp, E. B., and R. Medzhitov. 1999. The Toll-like receptor family and control of innate immunity. Curr. Opin. Immunol. 11:13-18. [DOI] [PubMed] [Google Scholar]
- 32.Kuo, C. C., W. T. Lin, C. M. Liang, and S. M. Liang. 2006. Class I and III phosphatidylinositol 3-kinase play distinct roles in TLR signaling pathway. J. Immunol. 176:5943-5949. [DOI] [PubMed] [Google Scholar]
- 33.Kwiatkowska, K., and A. Sobota. 1999. Signaling pathways in phagocytosis. Bioessays 21:422-431. [DOI] [PubMed] [Google Scholar]
- 34.Lecuit, M., S. Dramsi, C. Gottardi, M. Fedor-Chaiken, B. Gumbiner, and P. Cossart. 1999. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J. 18:3956-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letiembre, M., H. Echchannaoui, P. Bachmann, F. Ferracin, C. Nieto, M. Espinosa, and R. Landmann. 2005. Toll-like receptors 2 deficiency delays pneumococcal phagocytosis and impairs oxidative killing by granulocytes. Infect. Immun. 73:8397-8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 37.Lingnau, A., E. Domann, M. Hudel, M. Bock, T. Nichterlein, J. Wehland, and T. Chakraborty. 1995. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect. Immun. 63:3896-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low, J. C., and W. Donachie. 1997. A review of Listeria monocytogenes and listeriosis. Vet. J. 153:9-29. [DOI] [PubMed] [Google Scholar]
- 39.Luther, K., A. Torosantucci, A. A. Brakhage, J. Heesemann, and F. Ebel. 2007. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell. Microbiol. 9:368-381. [DOI] [PubMed] [Google Scholar]
- 40.Machata, S., S. Tchatalbachev, W. Mohamed, L. Jänsch, T. Hain, and T. Chakraborty. 2008. Lipoproteins of Listeria monocytogenes are critical for virulence and TLR2-mediated immune activation. J. Immunol. 181:2028-2035. [DOI] [PubMed] [Google Scholar]
- 41.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 42.Mengaud, J., H. Ohayon, P. Gounon, R.-M. Mege, and P. Cossart. 1996. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84:923-932. [DOI] [PubMed] [Google Scholar]
- 43.Peiser, L., S. Mukhopadhyay, and S. Gordon. 2002. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14:123-128. [DOI] [PubMed] [Google Scholar]
- 44.Schiff, D. E., L. Kline, K. Soldau, J. D. Lee, J. Pugin, P. S. Tobias, and R. J. Ulevitch. 1997. Phagocytosis of Gram-negative bacteria by a unique CD14-dependent mechanism. J. Leukoc. Biol. 62:786-794. [DOI] [PubMed] [Google Scholar]
- 45.Schubert, W. D., C. Urbanke, T. Ziehm, V. Beler, M. P. Machner, E. Domann, J. Wehland, T. Chakraborty, and D. W. Heinz. 2002. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell 111:825-836. [DOI] [PubMed] [Google Scholar]
- 46.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 47.Shin, O. S., L. S. Miller, R. L. Modlin, S. Akira, S. Uematsu, and L. T. Hu. 2009. Downstream signals for MyD88-mediated phagocytosis of Borrelia burgdorferi can be initiated by TRIF and are dependent on PI3K. J. Immunol. 183:491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sousa, S., D. Cabanes, L. Bougneres, M. Lecuit, P. Sansonetti, G. Tran-Van-Nhleu, and P. Cossart. 2007. Src, cortactin and Arp2/3 complex are required for E-cadherin-mediated internalization of Listeria into cells. Cell. Microbiol. 11:2629-2643. [DOI] [PubMed] [Google Scholar]
- 49.Stephens, L., C. Ellson, and P. Hawkins. 2002. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr. Opin. Cell Biol. 14:203-213. [DOI] [PubMed] [Google Scholar]
- 50.Strassheim, D., K. Asehnoune, J. S. Park, J. Y. Kim, Q. He, D. Richter, K. Kuhn, S. Mitra, and E. Abraham. 2004. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J. Immunol. 172:5727-5733. [DOI] [PubMed] [Google Scholar]
- 51.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 52.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 53.Torres, D., M. Barrier, F. Bihl, V. J. Quesniaux, I. Maillet, S. Akira, B. Ryffel, and F. Erard. 2004. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect. Immun. 72:2131-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]