Abstract
Highly pathogenic influenza A viruses cause acute severe pneumonia to which the occurrence of “cytokine storm” has been proposed to contribute. Here we show that interleukin-15 (IL-15) knockout (KO) mice exhibited reduced mortality after infection with influenza virus A/FM/1/47 (H1N1, a mouse-adapted strain) albeit the viral titers of these mice showed no difference from those of control mice. There were significantly fewer antigen-specific CD44+ CD8+ T cells in the lungs of infected IL-15 KO mice, and adoptive transfer of the CD8+ T cells caused reduced survival of IL-15 KO mice following influenza virus infection. Mice deficient in β2-microglobulin by gene targeting and those depleted of CD8+ T cells by in vivo administration of anti-CD8 monoclonal antibody displayed a reduced mortality rate after infection. These results indicate that IL-15-dependent CD8+ T cells are at least partly responsible for the pathogenesis of acute pneumonia caused by influenza A virus.
Highly pathogenic influenza A viruses cause acute severe pneumonia that results in high morbidity and significant mortality (11, 12, 24, 26). Elevated levels of serum cytokines and chemokines accompany these clinical manifestations, and the possibility that this “cytokine storm” contributes to increased severity of the disease caused by avian H5N1 virus and by other strains of influenza A virus has been proposed (10, 21, 33). In fact, CCR2-deficient mice [CCR2 is chemokine (C-C motif) receptor 2] were protected from early pathological manifestations despite higher pulmonary titers of the influenza virus A/PR/8/34 (H1N1) strain (7). Tumor necrosis factor receptor 1 (TNFR-1)-deficient mice exhibited significantly reduced morbidity following challenge with H5N1 virus (31). Other cytokines or chemokines have also been investigated (8, 28, 34, 35, 38). Thus, at least some of the elevated proinflammatory cytokines may contribute to the pathogenesis of influenza A virus.
Interleukin-15 (IL-15) is a pleiotropic cytokine involved in both innate and adaptive immune responses (20, 36). IL-15 utilizes the β-chain of the IL-2 receptor (IL-2R) (CD122) and the common cytokine receptor γ-chain (CD132) for signal transduction in lymphocytes and therefore shares many biological properties with IL-2 (3). Memory CD8+ T cells, natural killer (NK) cells, NKT cells, and intraepithelial lymphocyte (IEL) T cells (15, 23, 42) decrease in mice with defective IL-15 signaling, indicating the importance of IL-15 in their development and/or maintenance. IL-15 regulates not only the number of memory CD8+ T cells but also activation of their functions, including gamma interferon (IFN-γ) production and cytotoxic activity (40), which are important to target the virus (9). Therefore, it is possible that we may be able to use IL-15 as an immune-enhancing molecular adjuvant in vaccines for protection against various pathogens, including influenza A virus (37).
In the present study, we demonstrate that IL-15 knockout (KO) mice exhibited high resistance against infection with mouse-adapted influenza virus A/FM/1/47 (H1N1) strain. We show for the first time that IL-15-dependent CD8+ T cells are at least partly responsible for the pathogenesis of acute pneumonia caused by influenza A virus. In addition, our observations are important in the light of recent research into the use of IL-15 as an adjuvant for vaccination.
MATERIALS AND METHODS
Mice.
C57BL/6 mice in which IL-15 had been knocked out (IL-15 KO mice) (15) and β2-microglobulin (β2-m) KO mice (16) were purchased from Taconic (Germantown, NY). The mice were maintained in specific-pathogen-free conditions and used at 7 to 12 weeks of age. The study design was approved by the Committee of Ethics on Animal Experiments of the Faculty of Medicine, Kyushu University. Experiments were carried out under the Guidelines for Animal Experiments. Laboratory animals were cared for and used in accordance with the experimental animal standards of Japan (30a).
Reagents.
Fluorescein isothiocyanate (FITC)-conjugated anti-CD3ɛ (145-2C11), anti-CD11b, and anti-IFN-γ (XMG1.2) monoclonal antibodies (MAbs), phycoerythrin (PE)-conjugated anti-NK1.1 (PK136), anti-T-cell receptor γδ (anti-TCR γδ) (UC7), anti-major histocompatibility complex (anti-MHC) class II (M5/114.14.2), and anti-CD8α (53-6.7) MAbs, and allophycocyanin (APC)-conjugated anti-CD44 (IM7) MAb were purchased from eBioscience (San Diego, CA). Peridinin chlorophyll protein (PerCP)-Cy5.5-labeled anti-CD4 (L3T4 RM4-5) MAb was purchased from BD Biosciences (San Jose, CA). H-2Db tetramers were purchased from MBL (Nagoya, Japan). 2.4G2 (anti-Fcγ receptor II or III [anti-FcγRII/III]-specific MAb, rat IgG1, producing hybridoma) was obtained from the American Type Culture Collection.
Virus.
Madin-Darby canine kidney (MDCK) cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Grand Island, NY). Influenza virus A/FM1/47 (H1N1, a mouse-adapted strain) was provided by the Osaka Prefectural Institute of Public Health in Japan (6, 25) and intranasally infected on day 0 by dropping 20 μl of fluid containing 500 PFU influenza virus into each nostril.
Virus titer in the lungs.
The lungs were placed in homogenizers with 2 ml of phosphate-buffered saline (PBS) containing 0.05% Tween 80. The lungs were completely homogenized, and the homogenates were serially diluted with cold PBS. For a plaque assay, MDCK cells were plated at 1 × 106 cells in a flat-bottomed 6-well plate 24 h before infection. Supernatants from lung homogenates serially diluted were used to infect the MDCK cells at 37°C for 1 to 2 h. The cells were subsequently overlaid with DMEM (MP Biomedicals) mixed with 0.75% agarose (Lonza) in the presence of 1 μg/ml N-acetyltrypsin (Sigma). The plaques were visualized by staining the cells with naphthol blue black (Sigma), and the cells were counted 4 to 6 days after infection.
Histology.
Lung tissues were removed and fixed with 10% neutral buffered formalin and then embedded in paraffin. After the tissue was cut in round slices, the tissue sections were stained with hematoxylin and eosin (H&E) and examined microscopically.
Cell preparation.
Lung tissue samples were minced and incubated with stirring at 37°C for 30 min in HBSS with 1.3 mM EDTA, followed by treatment with 150 units/ml collagenase (Invitrogen) at 37°C for 1.5 h in RPMI 1640 with 10% fetal bovine serum (FBS). The resulting suspension was pelleted by centrifugation, resuspended in 45% Percoll layered on 66.6% Percoll, and centrifuged at 600 × g. Cells at the gradient interface were harvested and washed extensively before use. Lung cells at the gradient interface were harvested. Splenocytes were prepared by centrifugation and resuspended in RPMI 1640 supplemented with 10% FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and 10 mM HEPES.
Flow cytometric analysis.
Splenocytes or lung cells were preincubated with 2.4G2 culture supernatant to prevent nonspecific staining. After the cells were washed, they were stained with various combinations of MAbs. The stained cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). Data were analyzed with CellQuest software (BD Biosciences). For the intracellular cytokine staining (cytokine fluorescence-activated cell sorting [FACS]), lung cells were incubated with 10 μg/ml nuclear protein (NP)-specific peptide (ASNENMDTM) (30) and 10 μg/ml brefeldin A (Sigma-Aldrich) for 4 h at 37°C in 48-well flat-bottom plates at a concentration of 5 × 106/well in a volume of 500 μl of RPMI 1640 containing 10% fetal calf serum (FCS). After the cells were cultured, they were surface stained with various combinations of MAbs and then subjected to intracellular cytokine staining using the manufacturer's instructions (BD Biosciences). In brief, 100 μl of BD Cytofix/Cytoperm solution (BD Biosciences) was added to the cell suspension with mild mixing and placed for 20 min at 4°C. Fixed cells were washed with 250 μl of BD Perm/Wash solution (BD Biosciences) twice and were stained intracellularly with anti-IFN-γ MAb for 30 min at 4°C. Samples were acquired in a FACSCalibur flow cytometer and analyzed by CellQuest software.
In vivo cytotoxicity assay.
Analysis of in vivo cytolytic activity was carried out using a protocol similar to that previously reported (22). B6-Ly5.1+ splenocytes were divided into two populations and labeled with a high concentration of carboxyfluoroscein succinimidyl ester (CFSE) (5 μM) (CFSEhigh) and a low concentration of CFSE (0.5 μM) (CFSElow). Next, CFSEhigh cells were pulsed with 5 μg/ml NP peptide for 1 h at 37°C, while CFSElow cells were not pulsed. After the two cell groups were washed, they were mixed in equal proportions and then injected intravenously (i.v.) into mice infected with influenza virus 6 or 8 days previously. Spleens or lungs were obtained from recipients 24 h later for flow cytometric analysis to measure in vivo killing activities. Percent specific lysis was calculated according to the formula [1 − (ratio of primed cells/ratio of unprimed cells) × 100], where the ratio of unprimed cells = % CFSElow/% CFSEhigh cells remaining in noninfected recipients, and the ratio of primed cells = % CFSElow/% CFSEhigh cells remaining in infected recipients.
In vivo treatments.
Anti-CD8 MAb (clone 2.43) was intraperitoneally injected into mice to deplete CD8+ T cells. Isotype-matched control IgGs were obtained by BioLegend. For adoptive transfer, splenocytes were washed and passed through nylon wool columns. CD8+ T cells were negatively purified to >90% using autoMACS by depletion of the cells expressing CD4, B220, CD11c, NK1.1, TCR γδ, or MHC class II; the cells were resuspended in PBS and then transferred intravenously into IL-15 KO mice infected with influenza virus.
Statistical analysis.
The difference in survival rates was evaluated by the log rank test (Mantel-Cox). Differences in parametric data were evaluated by the Student t test. A P value of <0.05 was considered significant.
RESULTS
Survival rate and viral clearance in IL-15 KO mice inoculated intranasally with influenza virus A/FM/1/47.
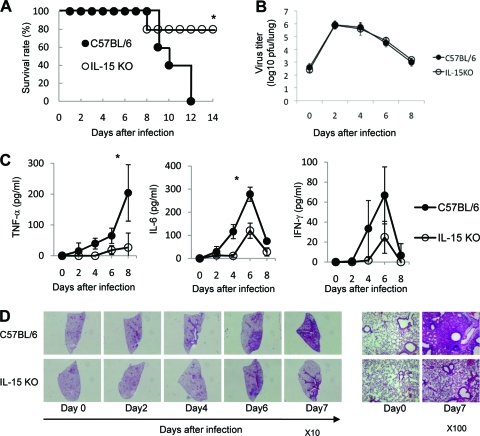
We first monitored the survival of IL-15 KO mice daily after influenza virus infection. All mice in the control group died within 14 days after intranasal infection with 100, 500, and 5,000 PFU of influenza virus A/FM/1/47 (H1N1, a mouse-adapted strain), suggesting that a mouse-adapted A/FM/1/47 strain was pathogenic to mice (data not shown). To determine the role of IL-15 in influenza A virus pathogenesis, we monitored the survival of IL-15 KO mice daily for 14 days after intranasal infection with influenza virus A/FM/1/47. All mice in the control group died within 14 days after intranasal inoculation with 500 PFU of influenza virus A/FM/1/47, while 80% of IL-15 KO mice survived beyond 8 days after infection (P < 0.05; Fig. 1A). The results clearly show that IL-15 deficiency protected mice from lethal viral effects. Next, we examined the kinetics of virus titers in lungs following intranasal challenge at 500 PFU of influenza virus A/FM/1/47. There was no significant difference in the viral load in the lungs during the course of infection with 500 PFU of influenza virus A/FM/1/47 between IL-15 KO and control mice (Fig. 1B). These results suggest that influenza virus-induced mortality was not directly related to an impaired host defense mechanism for elimination of the virus.
FIG. 1.
Mortality and viral clearance in IL-15 KO mice after infection with influenza virus A/FM/1/47. (A) Survival rate of C57BL/6 mice (closed circles) and IL-15 KO mice (open circles) following intranasal infection with influenza virus A/FM/1/47 (H1N1) strain at 500 PFU. Each group consists of 10 mice. Values that are statistically significantly different (P < 0.05) are indicated by an asterisk. Data are representative of four independent experiments. (B) Virus titers in the lung after inoculation with 500 PFU of influenza virus A/FM/1/47. The mouse lungs were taken and homogenized on days 2, 4, 6, and 8 for quantifying the virus titers of C57BL/6 and IL-15 KO mice by a plaque assay in MDCK cells. Each point shows the mean ± standard deviation (SD) (error bar). Each group consists of 3 to 5 mice. Data are representative of at least three independent experiments. (C) Proinflammatory cytokines of IL-15 KO mice after infection with 500 PFU of influenza virus A/FM/1/47. Mouse sera were taken on days 2, 4, 6, and 8, and the levels of TNF-α, IL-6, and IFN-γ from C57BL/6 and IL-15 KO mice were quantified by enzyme-linked immunosorbent assay (ELISA). Each group consists of 5 mice. Statistically significant differences (P < 0.05) between C57BL/6 and IL-15 KO mice are indicated with an asterisk. Each point shows the mean ± standard deviation (SD). Data are representative of at least three independent experiments. (D) Histology of lung tissues stained with H&E on days 2, 4, 6, and 7 after infection with 500 PFU of influenza virus A/FM/1/47. Representative figures are shown. Original magnifications, ×10 (left) and ×100 (right).
Proinflammatory cytokines of IL-15 KO mice inoculated intranasally with influenza virus A/FM/1/47.
The occurrence of a “cytokine storm” has been proposed to contribute to the increased severity of disease caused by highly pathogenic influenza viruses (14, 33). To determine the proinflammatory cytokine levels following influenza virus A/FM/1/47 infection, we measured cytokine levels in the sera of IL-15 KO mice during the course of infection. The levels of tumor necrosis factor alpha (TNF-α) and IL-6 were significantly lower in IL-15 KO mice than control mice on day 6 after infection (P < 0.05; Fig. 1C). IL-1β was not detected in the infection (data not shown).
Lung injury of IL-15 KO mice inoculated intranasally with influenza virus A/FM/1/47.
We examined the morphological and histological changes in the lungs of IL-15 KO mice by day 7 after influenza A virus infection. As shown in Fig. 1D, which exhibits a typical gross morphological view of the lungs, the lungs of control mice appeared severely injured as assessed by massive and diffuse leukocyte accumulation, whereas those of IL-15 KO mice showed only restricted leukocyte accumulation during the course of influenza A virus infection. These results suggested that the lung injury induced by influenza A virus infection was reduced in the absence of IL-15.
Cell population infiltrating the lungs of IL-15 KO mice inoculated intranasally with influenza virus A/FM/1/47.
To determine which cell type can possibly participate in IL-15-regulated lung injury, lung cells were harvested from the lungs of IL-15 KO mice on days 2, 4, 6, and 8 after infection with influenza virus A/FM/1/47, and the number and phenotype of the cells were characterized by flow cytometric analysis. The total number of lung cells was significantly lower in IL-15 KO mice than in control mice on day 6 after infection (P < 0.05; Fig. 2A). The number of NK1.1+ cells was significantly lower in the lungs of IL-15 KO mice on days 4 and 6 after infection (P < 0.05; Fig. 2B), whereas the number of CD44+ CD4+ T cells was almost the same in IL-15 KO mice as in control mice (Fig. 2C). The number of CD44+ CD8+ T cells was considerably lower in the lungs of IL-15 KO mice on days 4, 6, and 8 after infection (P < 0.05; Fig. 2D).
FIG. 2.
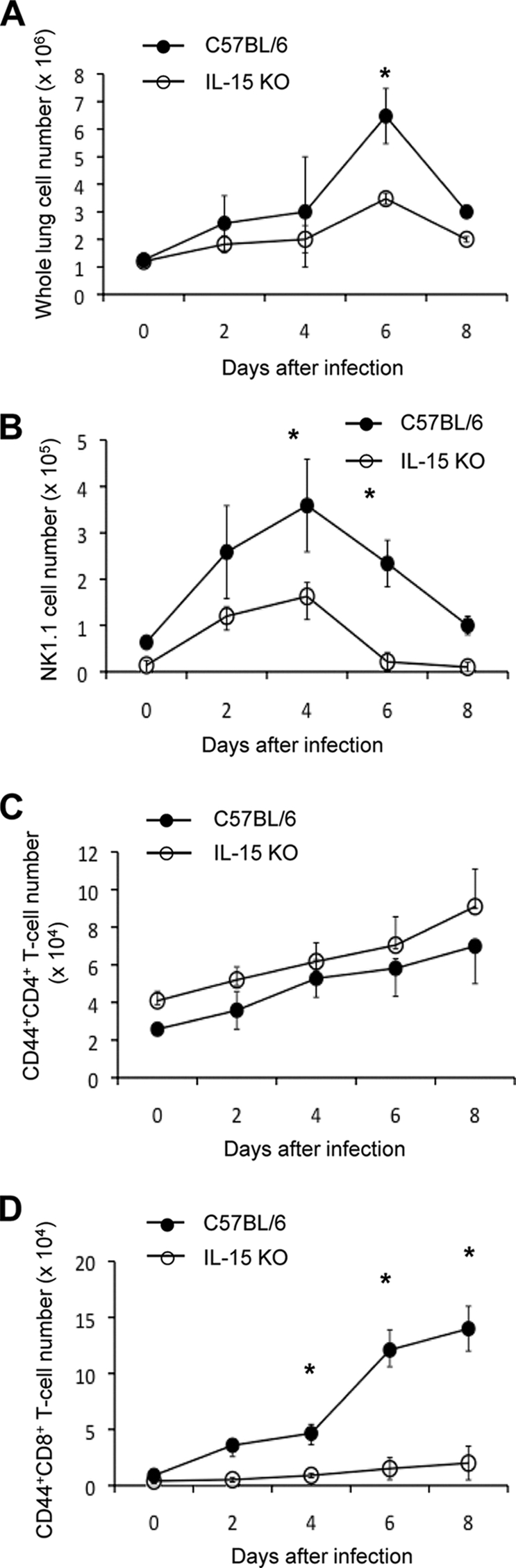
Cell population infiltrating the lungs of IL-15 KO mice after infection with influenza virus A/FM/1/47. The lung cells were isolated on days 2, 4, 6, and 8 after infection with 500 PFU of influenza virus A/FM/1/47, and the absolute number of lung cells was counted (A). The cells were stained with anti-NK1.1, CD4, CD8, or CD44 MAb and subjected to flow cytometric analysis. The absolute numbers of NK1.1+ cells (B), CD44+ CD4+ T cells (C), and CD44+ CD8+ T cells (D) were counted by multiplying the percentage of the cells by the absolute number of lung cells. Each group consisted of 5 mice. Statistically significant differences (P < 0.05) between C57BL/6 and IL-15 KO mice are indicated with n asterisk. Each point shows the mean ± standard deviation (SD) (error bar). Data are representative of at least three independent experiments.
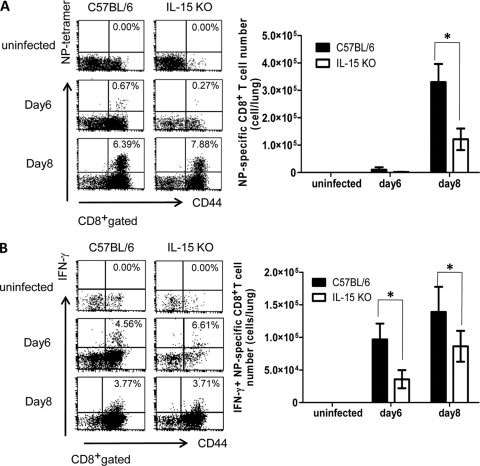
Antigen (Ag)-specific CD8+ T cells in IL-15 KO mice inoculated intranasally with influenza virus A/FM/1/47.
We examined the number of Ag-specific CD8+ T cells in the lungs of IL-15 KO mice after infection assessed by staining with an H-2Db tetramer coupled with a nuclear protein (NP)-derived ASNENMDTM peptide. Although the percentage of NP-specific CD8+ T cells seemed to be higher in IL-15 KO mice, the absolute number of NP-specific CD8+ T cells was significantly lower in the lungs of IL-15 KO mice than in control mice on day 8 after infection (P < 0.05; Fig. 3A). Next, we compared the functional activities of NP-specific CD8+ T cells between IL-15 KO mice and control mice infected with influenza virus A/FM/1/47, as assessed by intracellular cytokine flow cytometric analysis and in vivo cytotoxic assay. The percentage of IFN-γ+ CD8+ T cells did not differ in IL-15 KO mice from that in control mice (Fig. 3B), but the absolute number of NP-specific CD8+ T cells producing IFN-γ was significantly lower in the lungs of IL-15 KO mice on days 6 and 8 after infection (P < 0.05; Fig. 3B).
FIG. 3.
Ag-specific CD8+ T cells in the lungs of IL-15 KO mice after infection with influenza virus A/FM/1/47. (A) NP-specific CD8+ T cells on days 6 and 8 after infection with 500 PFU of influenza virus A/FM/1/47. The lung cells were stained with anti-CD8 MAb, anti-CD44 MAb, and NP-MHC class I tetramer. Samples were analyzed by flow cytometry. Dot plots are shown after gating CD8+ cells. The numbers indicate the percentage of cells in the corresponding quadrants. Absolute numbers were counted by multiplying the percentage of tetramer-positive cells by the absolute number of lung cells. Each group consisted of 5 mice. Statistically significant differences (P < 0.05) between C57BL/6 and IL-15 KO mice are indicated with an asterisk. Each point shows the mean plus standard deviation (SD) (error bar). Data are representative of at least three independent experiments. (B) IFN-γ-producing CD8+ T cells on days 6 and 8 after infection with 500 PFU of influenza virus A/FM/1/47. The lung cells were incubated with 10 μg/ml NP-derived ASNENMDTM peptide and 10 μg/ml brefeldin A for 4 h at 37°C, and expression of IFN-γ was detected by intracellular staining. Dot plots were shown after gating CD8+ cells. The numbers indicate the percentage of cells in the corresponding quadrants. The absolute number of IFN-γ-producing CD44+ CD8+ T cells was counted by multiplying the percentage of tetramer-positive cells by the absolute number of lung cells. Each group consisted of 5 mice. Statistically significant differences (P < 0.05) between C57BL/6 and IL-15 KO mice are indicated with an asterisk. Each point shows the mean plus standard deviation (SD). Data are representative of at least three independent experiments.
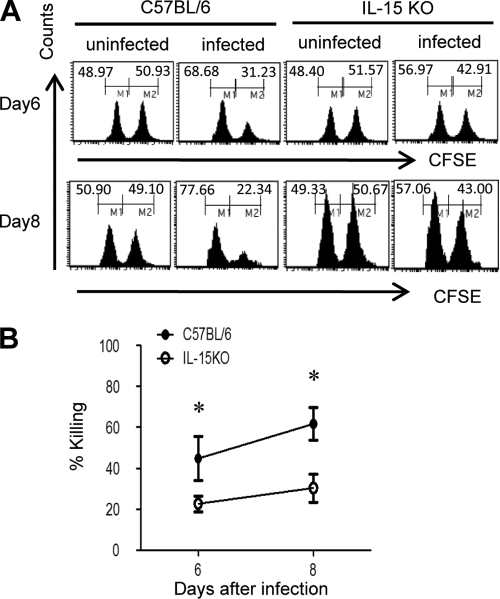
To directly detect the cytotoxic activity of CD8+ T cells in vivo, we measured the ability of CD8+ T cells to eliminate fluorescence-labeled spleen cells pulsed with NP peptides after infection. As shown in Fig. 4A, the elimination of NP-pulsed target cells was severely impaired in the lungs of IL-15 KO mice on days 6 and 8 after infection (P < 0.05; Fig. 4B), indicating that in vivo cytotoxic activity of NP-specific CD8+ T cells was severely reduced in IL-15 KO mice after infection. Taken together, the accumulation of Ag-specific CD44+ CD8+ T cells and their functions in lungs were significantly reduced in IL-15 KO mice after infection with influenza virus A/FM/1/47.
FIG. 4.
In vivo cytotoxic activities of NP-specific CD8+ T cells in IL-15 KO mice after infection with influenza virus A/FM/1/47. (A) Histograms are gated on Ly5.1+ cells in the lung 24 h after coinjection with equal numbers of CFSEhigh-labeled and NP peptide-pulsed splenocytes and CFSElow-labeled and nonpulsed Ly5.1+ splenocytes into mice infected with 500 PFU of influenza virus A/FM/1/47 6 or 8 days previously. Values in the right corner of each panel represent the percentage of specific killing compared with nonpulsed cells. (B) The values of each panel represent the percentage of specific killing compared with nonpulsed cells. Each group consisted of 3 mice. Statistically significant differences (P < 0.05) between C57BL/6 and IL-15 KO mice are indicated with an asterisk. Each point shows the mean ± standard deviation (SD). Data are representative of at least three independent experiments.
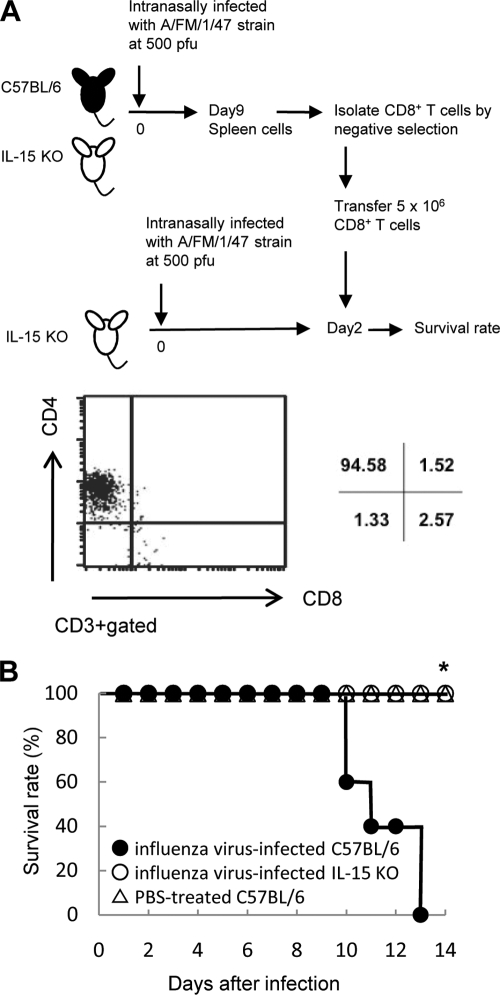
Adaptive transfer of CD8+ T cells into IL-15 KO mice inoculated intranasally with influenza virus A/FM/1/47.
To determine whether CD8+ T cells contributed to lung injury and mortality following infection, CD8+ T cells from the spleens of mice infected with influenza virus A/FM/1/47 9 days previously were transferred into IL-15 KO mice 2 days after infection, and the survival rate was monitored. CD8+ T cells were negatively purified to >90% using autoMACS (Fig. 5A). The survival rate of IL-15 KO mice was significantly decreased by adoptive transfer of CD8+ T cells from infected wild-type (WT) mice, but not by adaptive transfer of CD8+ T cells from infected IL-15 KO mice or from PBS-treated WT mice (P < 0.05; Fig. 5B). These results suggested that CD8+ T cells differentiating in the presence of IL-15 or CD8+ T cells activated in the presence of IL-15 are important in lethal viral effects following intranasal infection with 500 PFU of A/FM/1/47 strain.
FIG. 5.
Adoptive transfer of CD8+ T cells into IL-15 KO mice infected with influenza virus A/FM/1/47. (A) CD8+ T cells of splenocytes derived from C57BL/6 mice or IL-15 KO mice that had been infected with 500 PFU of influenza virus A/FM/1/47 9 days previously were adaptively transferred into IL-15 KO mice 2 days previously infected with 500 PFU of influenza virus A/FM/1/47. The purity of CD8+ T cells was confirmed by flow cytometry. (B) Survival rate of IL-15 KO mice transferred with CD8+ T cells from infected C57BL/6 mice (closed circles), infected IL-15 KO mice (open circles), or PBS-treated C57BL/6 mice (triangles). Each group consisted of 10 mice. Statistically significant differences (P < 0.05) are indicated with an asterisk. Data are representative of two independent experiments.
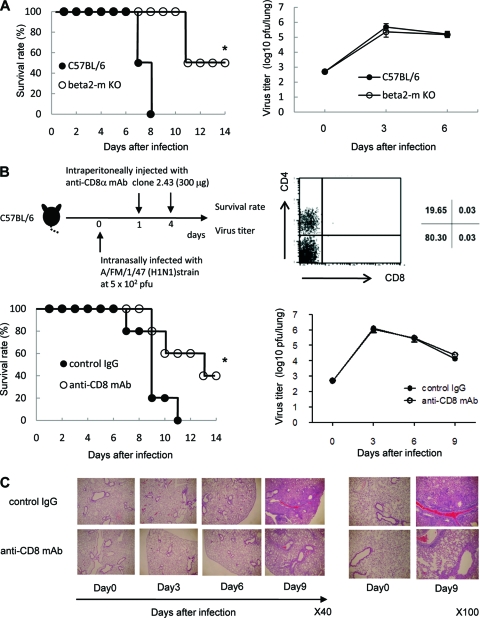
Survival of β2m KO mice or CD8+ T-cell-depleted mice inoculated intranasally with influenza virus A/FM/1/47.
To confirm that CD8+ T cells contributed to the lung injury and mortality following infection with influenza virus A/FM/1/47, we examined the survival rates in β2m KO mice, in which major histocompatibility complex (MHC) class I-restricted CD8+ T cells cannot develop without MHC class I molecules (16). All mice in the control group died within 8 days after intranasal infection with 500 PFU of A/FM/1/47 strain, whereas β2m KO mice survived beyond 12 days after A/FM/1/47 infection (P < 0.05; Fig. 6A). There was no significant difference in the viral load in the lungs during the course of infection in β2m KO and WT mice.
FIG. 6.
CD8+ T cells contribute to influenza virus A/FM/1/47-induced lethality. (A) Survival rate and viral clearance in β2m KO mice inoculated intranasally with influenza virus. C57BL/6 mice and β2m KO mice were intranasally infected with 500 PFU of influenza virus. Each group consisted of 10 mice. Statistically significant differences (P < 0.05) are indicated with an asterisk. Data are representative of two independent experiments. After inoculation with A/FM/1/47 virus, the mouse lungs were taken and homogenized on days 3 and 6. The homogenates were used for quantifying the virus titer of C57BL/6 and β2m KO mice by a plaque assay. Each group consisted of 5 mice. Each point shows the mean ± standard deviation (SD) (error bar). Data are representative of two independent experiments. (B) Survival rate and virus titer of mice treated with anti-CD8 MAb (open circles) and control mice treated with isotype-matched IgG (closed circles) after infection with influenza virus A/FM/1/47. C57BL/6 mice were intranasally infected with 500 PFU of influenza virus A/FM/1/47 (H1N1) strain. Anti-CD8 MAb (clone 2.43) or isotype-matched control IgGs were intraperitoneally injected into the mice on day 1 and 4 after infection, respectively. Depletion of CD8+ Τ cells in the spleen was confirmed by flow cytometry 3 days after a single injection of 300 μg anti-CD8 MAb. Each group consisted of 10 mice. Statistically significant differences (P < 0.05) are indicated with an asterisk. Data are representative of two independent experiments. After inoculation with A/FM/1/47 influenza virus, the mouse lungs were taken and homogenized on days 3, 6, and 9. The homogenates were used for quantifying the virus titer by a plaque assay. Each group consisted of 5 mice. Each point shows the mean ± standard deviation (SD). Data are representative of two independent experiments. (C) Histology of lung tissues stained with H&E on days 3, 6, and 9 after infection with 500 PFU of influenza virus A/FM/1/47. Representative figures are shown. Original magnifications, ×40 (left) and ×100 (right).
Last, we examined the survival rates in CD8+ T-cell-depleted control mice by in vivo administration of anti-CD8 MAb after the mice were infected with influenza virus A/FM/1/47. CD8+ T cells were mostly abolished until 3 days after in vivo administration of 300 μg anti-CD8 MAb (Fig. 6B). All mice in the control group died within 8 days after they were infected with influenza virus A/FM/1/47, whereas in vivo administration of 300 μg anti-CD8 MAb on days 1 and 4 after infection protected mice from mortality following infection with A/FM/1/47 (P < 0.05; Fig. 6B). There was no significant difference in the viral load in the lungs during the course of infection between mice treated with anti-CD8 MAb and control mice (Fig. 6B). We examined the morphological and histological changes in the lungs of the mice treated with anti-CD8 MAb by day 7 after influenza A virus infection. As shown in Fig. 6C, which shows a typical gross morphological view of the lungs, the lungs of control mice appeared severely injured as assessed by the massive and diffuse accumulation of leukocytes, whereas the lungs of infected mice showed only restricted leukocyte accumulation during the course of influenza A virus infection. Thus, the lung injury induced by influenza A virus infection was reduced in the absence of CD8+ T cells. We confirmed that CD8+ T cells were mostly abolished until days after infection (unpublished data). There were no differences in the numbers of NK1.1+ cells and CD44+ CD4+ T cells in mice treated with anti-CD8 MAb and in control mice during the course of infection (unpublished data). These results clearly show that CD8+ T cells accumulating and/or expanding in the lungs after infection are responsible for lethal viral effects following influenza A virus infection.
DISCUSSION
Influenza A viruses are finally eliminated by adaptive immune mechanisms in which cytotoxic CD8+ T lymphocytes as Ag-specific effectors target the virus (10, 17, 30). On the other hand, it has been reported that excessive activation of Ag-specific CD8+ T cells is harmful and pathogenic by destroying infected alveolar epithelial cells in an Ag-specific manner (29, 30, 39, 43). We found in the present study that the absolute number of NP-specific CD8+ T cells and their functions were significantly lower in IL-15 KO mice following influenza A virus infection. Our results suggest that excessive activation of Ag-specific CD8+ T cells may be harmful and pathogenic by destroying infected alveolar epithelial cells.
Recent studies have demonstrated that primary responses to lymphocytic choriomeningitis virus (LCMV) were readily generated in IL-15 KO mice or IL-15Rα KO mice to a level equal to that in control mice (2). Thus, IL-15 is not required for generation of effector CD8+ T cells during the expansion phase after infection. However, we have recently reported that IL-15 plays a critical role in protecting effector CD8+ T cells from apoptosis during the contraction phase following microbial infection via inducing anti-apoptotic molecules (41). The contraction of effector T cells is an important process for termination of immune responses to avoid excessive inflammation after the battle against a pathogen has been won (13). We speculate that effector CD8+ T cells surviving in the presence of IL-15 may be pathogenic in lung injury following highly pathogenic influenza A virus infection. IL-15 deficiency may promote apoptosis of the pathogenic CD8+ T cells, inhibiting acute lung injury caused by influenza virus infection.
IL-15 regulates not only the number of CD8+ T cells but also their activation (40). IL-15 plays an important role in early activation of Ag-specific memory CD8+ T cells following infection with microbes (27). IL-15 has been reported to induce IFN-γ production of CD44+ CD8+ T cells (5) and to directly upregulate expression of cytotoxic molecules such as granzyme B and perforin that are closely correlated with the cytotoxic effector function of CD8+ memory cells in vitro (19). Consistently, our present results revealed that total activities of NP-specific CD8T cells, including IFN-γ production and cytotoxic activity in vivo, were severely impaired in infected IL-15 KO mice. IL-15 may also play a crucial role of early activation of the pathogenic CD44+ CD8+ T cells following influenza A virus infection.
Innate immunity is the first line of defense against viral infection (4). Cells of innate immunity express pattern recognition receptors (PRRs) that induce proinflammatory cytokines (1). Among PRRs, influenza A virus, a single-stranded RNA virus, has been shown to trigger type I interferon (IFN) through recognition by Toll-like receptor 3 (TLR3) and retinoic acid-inducible gene I (RIG-I) in dendritic cells (DCs), fibroblasts, or alveolar epithelial cells and through TLR7 in plasmacytoid DCs (pDCs) (4, 32). The invasion of viruses is initially sensed by the host innate immunity system, triggering rapid antiviral responses that involve the release of proinflammatory cytokines and leading to the subsequent activation of adaptive immune responses. Recently, TLR3-deficient mice had an expected survival advantage accompanied by significantly reduced inflammatory mediators, including RANTES (CCR5L), IL-6, and IL-12, a lower number of CD8+ T cells in the bronchoalveolar air spaces and increased viral titers following influenza virus A/Scotland/20/74 (H3N2) infection (18). TLR3 has been demonstrated to interact with influenza A virus in specific cell populations, including pulmonary epithelial cells and alveolar macrophages (1). TLR3 recruits Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF) to mediate cytokine production via activation of IFN regulatory factor 3 (IRF3), which is ubiquitously expressed and responsible for IL-15 production (32). We speculate that impaired production of IL-15 may be associated with decreased CD8+ T-cell responses and an increased survival of TLR3-deficient mice following influenza A virus infection.
In conclusion, we observed that IL-15 KO mice were protected against highly pathogenic influenza A virus infection accompanied by a drastic decrease in infiltration of CD44+ CD8+ T cells in the lung despite no differences in the viral titer in the lung. Adaptive transfer of CD44+ CD8+ T cells into IL-15 KO mice deteriorated the survival rate following influenza virus infection. Hence, to our knowledge, our findings show for the first time that IL-15-dependent CD8+ T cells play a critical role in the pathogenesis of acute pneumonia caused by highly pathogenic influenza A virus. IL-15 may be a candidate target for immunomodulatory therapy for highly pathogenic influenza virus.
Acknowledgments
We thank Tang Ce, Momoe Itsumi, and Takahiro Ishikawa for technical assistance and valuable discussions.
This work was supported by a Grant-in-Aid for Young Scientists (B) (to N. Maeda), and for Scientific Research on Priority Areas (to Y. Yoshikai) by the Japan Society for the Promotion of Science (JSPS) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan and by the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases, which was launched as a project commissioned by the Ministry of Education, Culture, Sports, Science and Technology of Japan (to Y. Yoshikai).
All authors do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 24 March 2010.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 2.Becker, T. C., E. J. Wherry, D. Boone, K. Murali-Krishna, R. Antia, A. Ma, and R. Ahmed. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195:1541-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodnar, A., E. Nizsaloczki, G. Mocsar, N. Szaloki, T. A. Waldmann, S. Damjanovich, and G. Vamosi. 2008. A biophysical approach to IL-2 and IL-15 receptor function: localization, conformation and interactions. Immunol. Lett. 116:117-125. [DOI] [PubMed] [Google Scholar]
- 4.Bowie, A. G., and L. Unterholzner. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat. Rev. Immunol. 8:911-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyman, O., J. F. Purton, C. D. Surh, and J. Sprent. 2007. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 19:320-326. [DOI] [PubMed] [Google Scholar]
- 6.Brown, E. G. 1990. Increased virulence of a mouse-adapted variant of influenza A/FM/1/47 virus is controlled by mutations in genome segments 4, 5, 7, and 8. J. Virol. 64:4523-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson, T. C., M. A. Beck, W. A. Kuziel, F. Henderson, and N. Maeda. 2000. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 156:1951-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, R., M. Lu, C. Korteweg, Z. Gao, M. A. McNutt, J. Ye, T. Zhang, and J. Gu. 2008. Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J. Pathol. 216:328-336. [DOI] [PubMed] [Google Scholar]
- 9.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 10.Doherty, P. C., S. J. Turner, R. G. Webby, and P. G. Thomas. 2006. Influenza and the challenge for immunology. Nat. Immunol. 7:449-455. [DOI] [PubMed] [Google Scholar]
- 11.Gambotto, A., S. M. Barratt-Boyes, M. D. de Jong, G. Neumann, and Y. Kawaoka. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464-1475. [DOI] [PubMed] [Google Scholar]
- 12.Gillim-Ross, L., and K. Subbarao. 2006. Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19:614-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harty, J. T., and V. P. Badovinac. 2008. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8:107-119. [DOI] [PubMed] [Google Scholar]
- 14.Julkunen, I., T. Sareneva, J. Pirhonen, T. Ronni, K. Melen, and S. Matikainen. 2001. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 12:171-180. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy, M. K., M. Glaccum, S. N. Brown, E. A. Butz, J. L. Viney, M. Embers, N. Matsuki, K. Charrier, L. Sedger, C. R. Willis, K. Brasel, P. J. Morrissey, K. Stocking, J. C. Schuh, S. Joyce, and J. J. Peschon. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191:771-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koller, B. H., P. Marrack, J. W. Kappler, and O. Smithies. 1990. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248:1227-1230. [DOI] [PubMed] [Google Scholar]
- 17.La Gruta, N. L., K. Kedzierska, J. Stambas, and P. C. Doherty. 2007. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 85:85-92. [DOI] [PubMed] [Google Scholar]
- 18.Le Goffic, R., V. Balloy, M. Lagranderie, L. Alexopoulou, N. Escriou, R. Flavell, M. Chignard, and M. Si-Tahar. 2006. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, K., M. Catalfamo, Y. Li, P. A. Henkart, and N. P. Weng. 2002. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl. Acad. Sci. U. S. A. 99:6192-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma, A., R. Koka, and P. Burkett. 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 24:657-679. [DOI] [PubMed] [Google Scholar]
- 21.Maines, T. R., K. J. Szretter, L. Perrone, J. A. Belser, R. A. Bright, H. Zeng, T. M. Tumpey, and J. M. Katz. 2008. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol. Rev. 225:68-84. [DOI] [PubMed] [Google Scholar]
- 22.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 23.Nakazato, K., H. Yamada, T. Yajima, Y. Kagimoto, H. Kuwano, and Y. Yoshikai. 2007. Enforced expression of Bcl-2 partially restores cell numbers but not functions of TCRgammadelta intestinal intraepithelial T lymphocytes in IL-15-deficient mice. J. Immunol. 178:757-764. [DOI] [PubMed] [Google Scholar]
- 24.Ng, W. F., K. F. To, W. W. Lam, T. K. Ng, and K. C. Lee. 2006. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1—a review. Hum. Pathol. 37:381-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuno, Y., K. Matsumoto, Y. Isegawa, and S. Ueda. 1994. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J. Virol. 68:517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiris, J. S., M. D. de Jong, and Y. Guan. 2007. Avian influenza virus (H5N1): a threat to human health. Clin. Microbiol. Rev. 20:243-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schluns, K. S., K. Williams, A. Ma, X. X. Zheng, and L. Lefrancois. 2002. Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 168:4827-4831. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz, N., M. Kurrer, M. F. Bachmann, and M. Kopf. 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small, B. A., S. A. Dressel, C. W. Lawrence, D. R. Drake III, M. H. Stoler, R. I. Enelow, and T. J. Braciale. 2001. CD8(+) T cell-mediated injury in vivo progresses in the absence of effector T cells. J. Exp. Med. 194:1835-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stambas, J., C. Guillonneau, K. Kedzierska, J. D. Mintern, P. C. Doherty, and N. L. La Gruta. 2008. Killer T cells in influenza. Pharmacol. Ther. 120:186-196. [DOI] [PubMed] [Google Scholar]
- 30a.Standards Relating to the Care and Management of Experimental Animals. 1980. Prime Minister's Office. Notice 6. Prime Minister's office, Tokyo, Japan.
- 31.Szretter, K. J., S. Gangappa, X. Lu, C. Smith, W. J. Shieh, S. R. Zaki, S. Sambhara, T. M. Tumpey, and J. M. Katz. 2007. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81:2736-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi, O., and S. Akira. 2009. Innate immunity to virus infection. Immunol. Rev. 227:75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taubenberger, J. K., and D. M. Morens. 2008. The pathology of influenza virus infections. Annu. Rev. Pathol. 3:499-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Der Sluijs, K. F., L. J. Van Elden, R. Arens, M. Nijhuis, R. Schuurman, S. Florquin, J. Kwakkel, S. Akira, H. M. Jansen, R. Lutter, and T. Van Der Polls. 2005. Enhanced viral clearance in interleukin-18 gene-deficient mice after pulmonary infection with influenza A virus. Immunology 114:112-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Sluijs, K. F., L. J. van Elden, Y. Xiao, R. Arens, M. Nijhuis, R. Schuurman, S. Florquin, H. M. Jansen, R. Lutter, and T. van der Poll. 2006. IL-12 deficiency transiently improves viral clearance during the late phase of respiratory tract infection with influenza A virus in mice. Antiviral Res. 70:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waldmann, T. A. 2006. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6:595-601. [DOI] [PubMed] [Google Scholar]
- 37.Waldmann, T. A., and Y. Tagaya. 1999. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu. Rev. Immunol. 17:19-49. [DOI] [PubMed] [Google Scholar]
- 38.Wareing, M. D., A. B. Lyon, B. Lu, C. Gerard, and S. R. Sarawar. 2004. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J. Leukoc. Biol. 76:886-895. [DOI] [PubMed] [Google Scholar]
- 39.Xu, L., H. Yoon, M. Q. Zhao, J. Liu, C. V. Ramana, and R. I. Enelow. 2004. Pulmonary immunopathology mediated by antigen-specific expression of TNF-alpha by antiviral CD8+ T cells. J. Immunol. 173:721-725. [DOI] [PubMed] [Google Scholar]
- 40.Yajima, T., H. Nishimura, S. Sad, H. Shen, H. Kuwano, and Y. Yoshikai. 2005. A novel role of IL-15 in early activation of memory CD8+ CTL after reinfection. J. Immunol. 174:3590-3597. [DOI] [PubMed] [Google Scholar]
- 41.Yajima, T., K. Yoshihara, K. Nakazato, S. Kumabe, S. Koyasu, S. Sad, H. Shen, H. Kuwano, and Y. Yoshikai. 2006. IL-15 regulates CD8+ T cell contraction during primary infection. J. Immunol. 176:507-515. [DOI] [PubMed] [Google Scholar]
- 42.Zhao, H., H. Nguyen, and J. Kang. 2005. Interleukin 15 controls the generation of the restricted T cell receptor repertoire of gamma delta intestinal intraepithelial lymphocytes. Nat. Immunol. 6:1263-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou, J., M. Matsuoka, H. Cantor, R. Homer, and R. I. Enelow. 2008. Engagement of NKG2A on CD8+ effector T cells limits immunopathology in influenza pneumonia. J. Immunol. 180:25-29. [DOI] [PubMed] [Google Scholar]