Abstract
A hallmark of alphaherpesviruses is their capacity to be neuroinvasive and establish latent infections in neurons. After primary replication in epithelial cells at the periphery, entry into nerve endings occurs, followed by retrograde transport of nucleocapsids to the nucleus where viral transcription, genome replication, and nucleocapsid formation take place. Translocation of nucleocapsids to the cytoplasm is followed by axonal transport to infect synaptically linked neurons. Two modes of intraaxonal anterograde herpesvirus transport have been proposed: transport of complete, enveloped virions within vesicles (“married model”), and separate transport of capsids and envelopes (“subassembly model”). To assess this in detail for the alphaherpesvirus pseudorabies virus (PrV), we used high-resolution transmission electron microscopy of primary neuronal cultures from embryonic rat superior cervical ganglia after infection with wild-type and gB-deficient PrV. Our data show that intranuclear capsid maturation, nuclear egress and cytoplasmic secondary envelopment occur as in cultured nonpolarized cells (H. Granzow, F. Weiland, A. Jöns, B. G. Klupp, A. Karger, and T. C. Mettenleiter, J. Virol. 71:2072-2082, 1997). PrV virions were present in axons as enveloped particles within vesicles associated with microtubules and apparently leave the neuron by exocytosis primarily at the growth cone. Only a few nonenveloped nucleocapsids were found in the axon. The same picture was observed after infection by phenotypically complemented gB-deficient PrV, which is able to complete only a single round of replication. Our data thus support intraaxonal anterograde transport of enveloped PrV virions within vesicles following the “married model.”
Most members of the subfamily Alphaherpesvirinae within the family Herpesviridae in the order Herpesvirales (8) are characterized by a pronounced neurotropism. They include the type member of the genus Simplexvirus, the human herpes simplex virus type 1 (HSV-1), as well as varicella-zoster virus, as type member of the genus Varicellovirus. After initial replication in mucosal membranes, these viruses infect sensory nerve endings and enter the peripheral nervous system by retrograde axonal transport. In sensory ganglia they replicate productively or establish latency (reviewed in reference 13). During occasional reactivation newly replicated virions are transported via fast anterograde axonal transport to the original site of infection causing recurrent herpetic lesions (reviewed in reference 11). In some cases, HSV-1 is transported to the central nervous system, causing viral encephalitis (13, 20). Suid herpesvirus 1, also designated as pseudorabies virus (PrV), is a member of the genus Varicellovirus within the Alphaherpesvirinae. In swine, its natural host, it causes Aujeszky's disease, which, depending on the age of the pig and the virulence of the virus, can manifest itself as a severe meningoencephalitis, rapidly leading to death of the animal. Besides pigs, PrV is able to productively infect a broad spectrum of mammalian species, not including equids and higher primates, resulting in invariably fatal infections (26).
HSV-1 and PrV have become models to analyze basic features of herpesvirus replication, including entry, virion formation, and egress (28). Infection occurs primarily by pH-independent fusion of the viral envelope with the plasma membrane. In the alphaherpesviruses four glycoproteins, gB, gD, and the gH/gL-complex have been shown to be required for entry (27). gB is essential for penetration and cell-to-cell spread and is the most highly conserved glycoprotein found in herpesviruses (27). Virus mutants lacking gB are no longer able to enter and spread between cultured epithelial cells (33, 36) or neurons (6) and cannot propagate in the nervous system in vivo (2). During penetration, part of the tegument dissociates from the incoming nucleocapsid which is then transported to the nuclear pore by dynein-mediated transport along microtubules (12). After docking at the nuclear pore, the viral genome is released into the nucleus, where transcription of viral genes, as well as viral genome replication, takes place. Nucleocapsids are assembled in the nucleus and subsequently translocated into the cytosol by a mechanism that involves budding at the inner nuclear membrane, which entails acquisition of a primary envelope, and subsequent deenvelopment by fusion of the primary envelope with the outer nuclear membrane. In the cytosol final capsid maturation includes apposition of tegument and acquisition of the final envelope by budding into vesicles derived from the trans-Golgi network. These vesicles are then transported to the cell surface, the vesicle membrane fuses with the plasma membrane, and the enveloped particle is released by exocytosis (reviewed in reference 28).
Whereas most studies on herpesvirus morphogenesis and transport use nonpolarized cultured cells, herpesviruses infect and replicate in highly polarized cells in vivo. This applies in particular to the neurotropic alphaherpesviruses which, during their infection of neurons, have to travel retrogradely for long distances to the cell body for replication, and anterogradely to synapses for viral spread. Whereas viral entry, transport to the cell body, nucleocapsid formation in the nucleus, nuclear egress, and virion formation in the cell body appear to be similar to those in nonpolarized cells (22, 31), axonal transport of virions during egress in neurons may be different (11). It had previously been hypothesized (reviewed in reference 13) that during anterograde axonal transport (tegumented) nucleocapsids and envelope proteins are transported separately (3, 17, 30, 35, 43) and that virion formation and exit occur along the axon in varicosities (10, 37) and at synapses or terminal growth cones (34, 43). This has been confirmed in several studies for HSV-1 (17, 21, 30, 38, 40), whereas some of the early data have recently been reinterpreted for PrV supported also by ultrastructural analysis (1, 5, 9, 14). Thus, for neuronal egress of PrV, enveloped viral particles are now suggested to be transported intraaxonally targeted from the assembly site in the cell body to axons by the viral envelope protein pUs9 (23), which binds to lipid rafts (25). The difference in cargo, i.e., nucleocapsids with only inner tegument proteins during entry, and enveloped particles in vesicles during egress could then determine the overall direction, retrograde transport during entry, and anterograde transport during egress (1). However, in HSV-1 pUS9 appears to promote separate transport of nucleocapsids and virion glycoproteins in axons (39). To address these issues, we set up preparative high-resolution transmission electron microscopy to visualize PrV transport in cultured primary neurons.
MATERIALS AND METHODS
Cells and viruses.
PrV strain Kaplan (PrV-Ka) (18) was used in these experiments, as was an isogenic PrV mutant, PrV-gB−, deficient in expression of the essential gB (32). PrV-Ka was propagated on porcine kidney cells (PSEK), whereas PrV-gB− was grown on gB-complementing rabbit kidney cell line RK13-gB (32). Cells were grown at 37°C in minimum essential medium (MEM) supplemented with 5 or 10% fetal calf serum (Invitrogen), respectively.
Neuronal cultures.
Dissection and culture of primary neuronal cells were essentially performed as described previously (4). Pregnant (E15.5) Sprague-Dawley rats (Charles River) were euthanized with CO2, and embryos were removed from the uterus. After transfer to a tissue culture dish and rinsing with Hanks balanced salt solution (Fisher Scientific) embryos were decapitated, and the mandibles were dissected by an incision ventral to the ear. Superior cervical ganglia (SCG) were located adjacent to the carotid arteries and the nodose ganglia, removed, and dissected. Dissected ganglia were plated on microscope slides coated with 500 μg of poly-dl-ornithine (Sigma)/ml and 10 μg of natural mouse laminin (Invitrogen)/ml. The neuronal culture medium consisted of Dulbecco modified Eagle medium with Ham F-12 (1:1) and Glutamax (Invitrogen) supplemented with 10 mg of bovine serum albumin (Sigma)/ml, 100 μg of human holotransferrin (Sigma)/ml, 16 μg of putrescine (Sigma)/ml, 4.6 mg of glucose (Gibco)/ml, 2 mM l-glutamine (Invitrogen), 10 μg of insulin (Sigma)/ml, 50 U of penicillin and streptomycin (Gibco)/ml, 25 U of kanamycin (Gibco)/ml, 30 nM selenium (Sigma), 20 nM progesterone (Sigma), and 50 ng of nerve growth factor 2.5S (Invitrogen)/ml. The neuronal cultures were incubated at 37°C, and neuronal culture medium was replaced every 3 days.
Infection with PrV.
Neurons were cultured for 5 to 7 days to allow axons to grow. For infection the viral inoculum was diluted in neuronal culture medium to infect cells with 3 × 104 PFU. After 1 h at 37°C the inoculum was removed and replaced with neuronal medium. The dishes were subsequently further incubated at 37°C.
Electron microscopy.
For electron microscopy, infected neuronal cultures on coverslips were fixed 18 h postinfection for 60 min with 2.5% glutaraldehyde (Fluka) buffered in 0.1 M sodium cacodylate (300 mOsmol, pH 7.2; Plano). After several washings in buffer the cultures were postfixed with 1% buffered OsO4 (Sigma) and stained with uranyl acetate (Plano). After stepwise dehydration in ethanol, cultures were cleared in propylene oxide (Serva), embedded in glycid ether 100 (Serva), and polymerized at 59°C for 4 days. After polymerization the coverslips were separated from the resin by plunging into liquid nitrogen, leaving the explant and axons exposed to the surface of the block. Ultrathin sections, cut in parallel to the surface, were counterstained with uranyl acetate and lead salts and examined by using a transmission electron microscope (Tecnai 12; Philips, the Netherlands). For PrV-Ka, ca. 200 thin sections from four independent experiments were analyzed, for PrV-gB− ca. 50 sections from two independent experiments. For antibody labeling a monospecific antiserum against the major capsid protein (19) and a monoclonal antibody against envelope glycoprotein gB (32) were used.
RESULTS
Ultrastructure of noninfected explanted primary rat neurons.
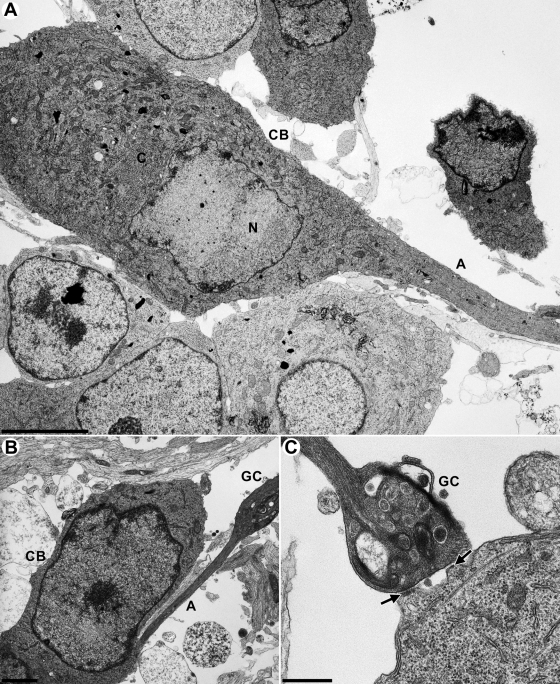
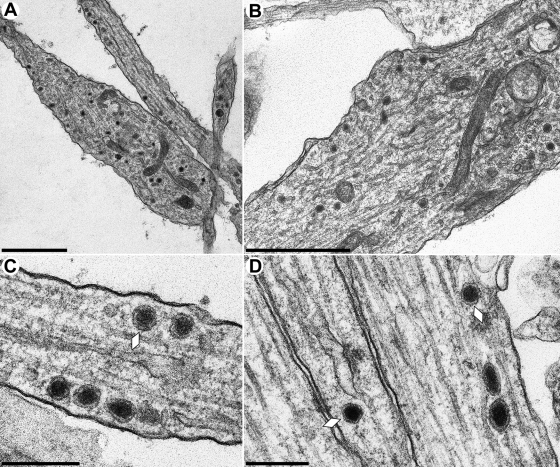
Explanted neurons could easily be distinguished from nonneuronal cells by their distinct morphology (Fig. 1A and B) differentiating into cell body, axons, and growth cones (Fig. 1). Axons could be identified by the presence of microtubules in parallel orientation and the lack of a rough endoplasmic reticulum (44). Not readily forming synapses in vitro, axons mostly terminate in bulbous growth cones (Fig. 1B) (11, 44). Very rarely connections between growth cones and other cells, which morphologically resemble synapses, could be visualized (Fig. 1C, black arrows). Cell bodies, axons and growth cones of noninfected cells contained electron-dense vesicular structures (Fig. 2), which are associated intraaxonally with microtubules (Fig. 2C and D, white lozenges), and may be confused with viral particles. However, due to their lack of an angular shape, as is characteristic for herpesvirus capsids, and their enclosure with only one lipid envelope they were identified as neurovesicles, and clearly differentiated from viral particles.
FIG. 1.
Ultrastructure of noninfected explanted primary rat neurons. (A and B) Cross sections of primary neurons at day 5 after removal from the SCG. A, axon; CB, cell body; N, nucleus; C, cytoplasm; GC, growth cone. (C) Growth cone (GC) attaching to a cell body with possible synapses (black arrows). Bars: 5 μm in panel A, 1.5 μm in panel B, and 500 nm in panel C.
FIG. 2.
Ultrastructure of noninfected in vitro-cultivated rat SCG neurons. Neurons were explanted and analyzed as described in Materials and Methods. Electron-dense membranous particles in growth cones (A and B) and along axons (C and D, white lozenges) represent neurovesicles. Bars: 1.2 μm in panels A and B and 300 nm in panels C and D.
Ultrastructure of primary rat neurons infected with PrV-Ka.
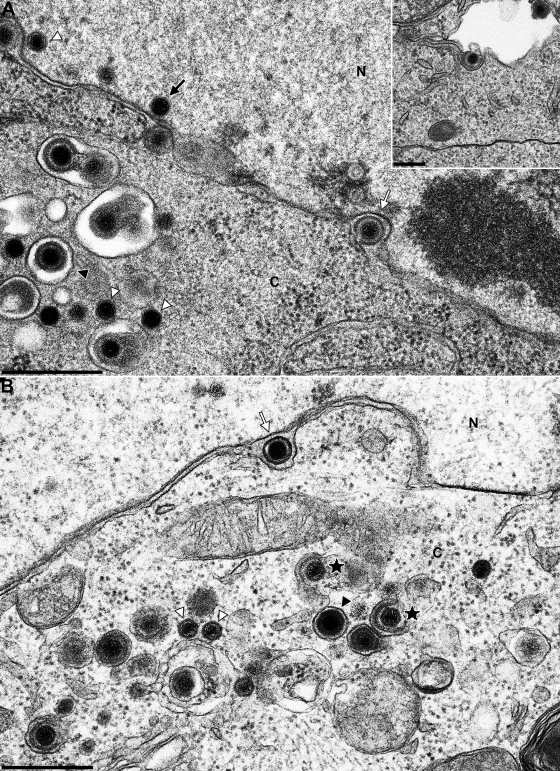
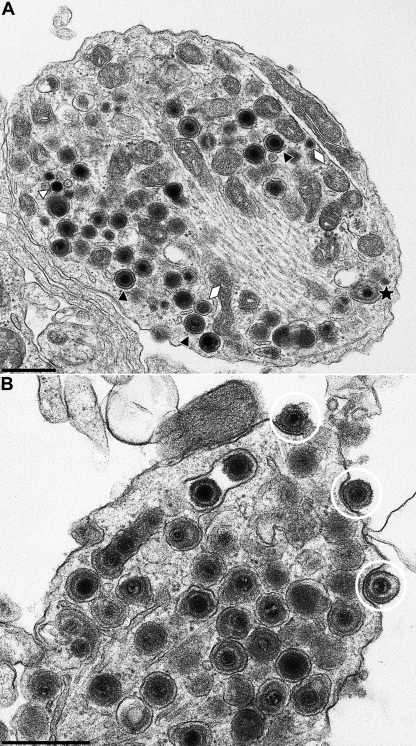
Primary neuronal cultures from rat superior cervical ganglia (SCG) were cultivated for 5 to 7 days and infected with 3 × 104 PFU of wild-type PrV-Ka (18). After 1 h adsorption at 37°C, the inoculum was removed and replaced by neuronal culture medium. After incubation for 18 h at 37°C cells were fixed, embedded in epoxy resin, and analyzed by electron microscopy. Virion morphogenesis in the cell body was similar to that observed in nonpolarized cells (15, 16). In the nucleus, different stages of nucleocapsid assembly were detected. Nucleocapsids were present in association with the inner nuclear membrane (Fig. 3A, black arrow), and primary enveloped virions were observed in the perinuclear cleft (Fig. 3A and B, white arrows). Deenvelopment at the outer nuclear membrane (Fig. 3A, white arrow), intracytoplasmic nucleocapsids (Fig. 3A and B, white triangles), as well as their secondary envelopment in the cytoplasm (Fig. 3B, stars), was also seen, as were mature enveloped virions located in vesicles (Fig. 3, black triangles). Consistent with the egress in nonneuronal cells mature virions in vesicles were released by exocytosis (Fig. 3A, inset).
FIG. 3.
Virion morphogenesis in the cell body of PrV-infected in vitro-cultivated rat SCG neurons. SCG were explanted, infected with PrV-Ka, and analyzed 18 h after infection. White arrows indicate primary enveloped virions in the perinuclear cleft, and black triangles point to enveloped virions in vesicles in the cytoplasm. White triangles highlight nonenveloped nucleocapsids in the nucleus and cytoplasm. Stars denote secondary envelopment stages. The black arrow in panel A highlights an intranuclear nucleocapsid closely apposed to the inner nuclear membrane. Inset in panel A shows exocytosis of an enveloped virion. N, nucleus; C, cytoplasm. Bars: 500 nm in panels A and B and 250 nm in the panel A inset.
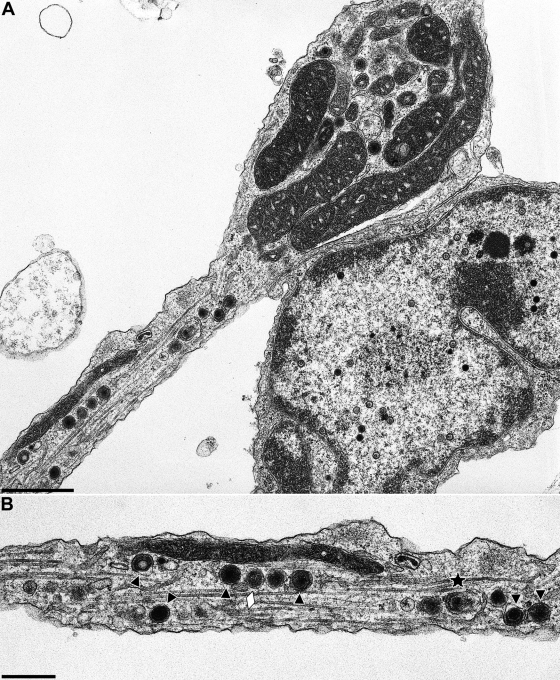
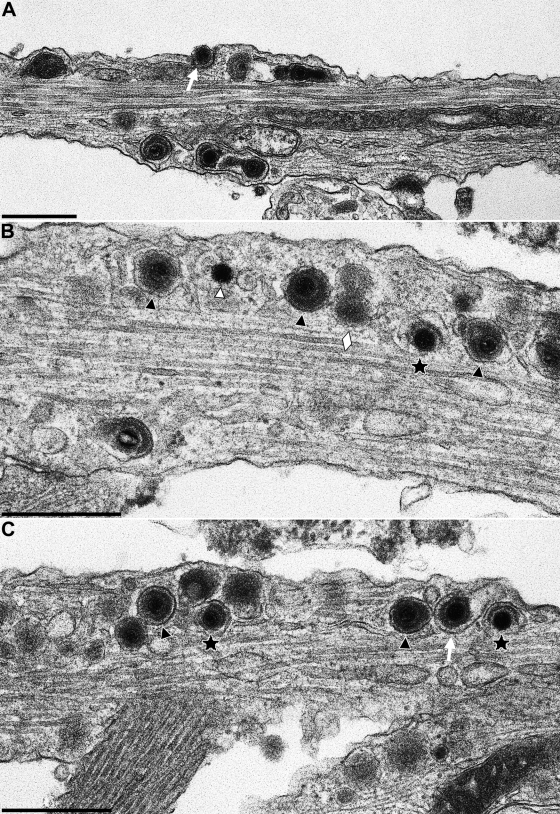
Viral particles were associated with microtubules along the axon and frequently lined-up within the plane of section (Fig. 4). They mostly consisted of enveloped particles (Fig. 4A and 5B and C, black triangles). Partially enveloped particles which were either in the process of deenvelopment or envelopment, were observed infrequently (Fig. 4B and 5B and C, stars). As is inherent in electron microscopy, directionality could not be established from the micrographs. Rarely, but consistently, nonenveloped nucleocapsids were also observed within the axon (Fig. 5B, white triangle). They amounted to less than 10% of total viral particles. Viral particles could be differentiated from neurovesicles (Fig. 4B and 5B, white lozenges) since the latter lack typical virion morphology.
FIG. 4.
Axon and growth cone of a PrV-infected in vitro-cultivated rat neuron. Neurons were explanted, infected with PrV-Ka, and analyzed 18 h after infection. (A) shows a section including terminal axon and growth cone of a neuron, the latter juxtaposed to the cell body of another neuron. The axonal part is enlarged in panel B. The black triangles in panel B point to enveloped virions, and the white lozenges indicate neurovesicles. The star denotes a partially enveloped particle. Bars: 1.0 μm in panel A and 500 nm in panel B.
FIG. 5.
Nucleocapsids and enveloped virions in axons of PrV-infected in vitro-cultivated rat neurons. Neurons were explanted, infected with PrV-Ka, and analyzed 18 h after infection. (A to C) Three representative sections are shown. Stars indicate partially enveloped particles, white triangle highlights a nucleocapsid, black triangles show enveloped virions, and lozenges denote neurovesicles. The white arrow in panel A marks a virion in egress. Bars: 500 nm.
Within the growth cones, again mostly enveloped virions within vesicles were observed (Fig. 6A, black triangles; and Fig. 6B), which were apparently released by exocytosis (Fig. 6B, encircled). As in the axon, nonenveloped nucleocapsids were also occasionally detected in the growth cone (Fig. 6A, white triangle), as were neurovesicles (Fig. 6A, white lozenges) and partially enveloped particles (Fig. 6A, star). Thus, these data indicate that the overwhelming majority of viral particles, which are transported along the axon to the growth cone consist of enveloped viral particles within vesicles, similar to the situation in egress from nonpolarized cells.
FIG. 6.
Virions in growth cones of PrV-infected in vitro-cultivated rat neurons. Neurons were explanted, infected with PrV-Ka, and analyzed 18 h after infection. The black triangles in panel A indicate enveloped virions, the white triangle denotes a naked nucleocapsid, and the white lozenges show neurovesicles. The star denotes a partially enveloped particle. In panel B, three virions which apparently are released via exocytosis are marked by white circles. Enveloped virions within vesicles are abundant. Bars: 500 nm.
Intraaxonal transport after infection with PrV-gB−.
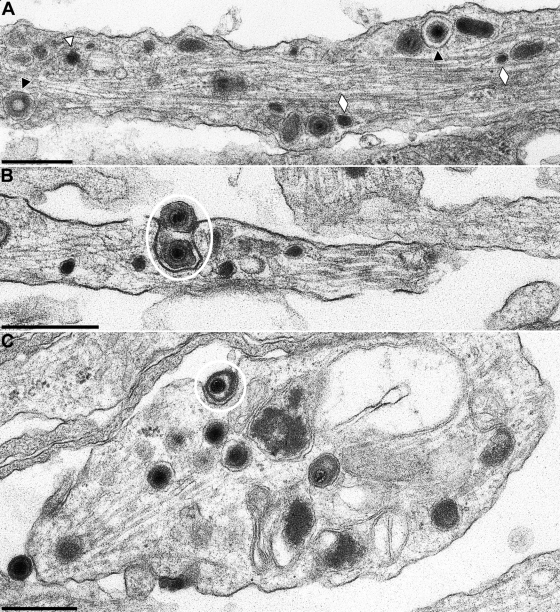
To distinguish exiting virions (anterograde transport) from incoming capsids (retrograde transport), neuronal cultures were infected with phenotypically complemented PrV-gB−. After replication on gB-expressing cells, transcomplemented mutant virions are able to enter neurons and undergo a complete replication cycle. However, neither direct spread from infected to noninfected cells nor infectious entry after release of gB-negative virions is possible (33, 36). Moreover, gB has been shown to be required also for transneuronal spread of PrV (2, 6). Thus, primary neuronal cultures from embryonic rats were infected with 3 × 104 PFU of transcomplemented PrV-gB− for 1 h and analyzed by transmission electron microscopy 18 h after infection. Virion formation in the cell body was similar as in wild-type PrV infection (data not shown). Intraaxonally, enveloped virions within vesicles associated with microtubules were observed (Fig. 7A, black triangles), as were neurovesicles (Fig. 7A, white lozenges), and, rarely, single nonenveloped capsids (Fig. 7A, white triangle). Egress of virions occurred predominantly at varicosities along the axons (Fig. 7B) and at growth cones (Fig. 7C) by exocytosis.
FIG. 7.
In vitro-cultivated PrV-gB− infected neuron. Neurons were explanted, infected with phenotypically complemented PrV with gB deleted that is unable to reenter cells after a first round of replication, and analyzed 18 h after infection. (A) Representative section through an axon. Black triangles indicate enveloped virions, the white triangle denotes a naked capsid, white lozenges highlight neurovesicles. (B and C) Viral egress by exocytosis along the axon (B) and at the growth cone (C). Egress stages are circled. Bars: 500 nm.
Immunoelectron microscopy of PrV-infected rat primary neurons.
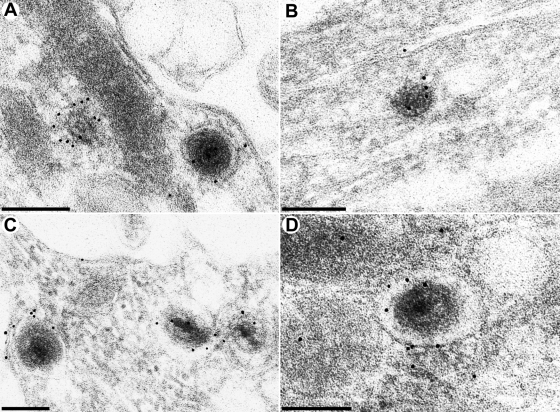
To unequivocally identify the observed structures, we analyzed ultrathin sections of Lowicryl embeddings by immunoelectron microscopy. As shown in Fig. 8, the observed particles labeled with a monospecific antiserum against the major capsid protein confirming that they contain virus capsids (Fig. 8A and B). Moreover, label with a monoclonal antibody against the envelope glycoprotein gB was also observed, thereby supporting our interpretation from ultrastructure that they represent enveloped viral particles (Fig. 8C and D).
FIG. 8.
Immuno-electron microscopy of PrV-infected neurons. Neurons were explanted, infected with PrV-Ka, and analyzed 18 h after infection by immunoelectron microscopy as detailed in Materials and Methods. Labeling was performed with a monospecific antiserum recognizing the major capsid protein pUL19 (A and B) or a monoclonal antibody directed against envelope glycoprotein B (C and D). Bars: 200 nm in panel A and 150 nm in panels B to D.
DISCUSSION
Elucidation of the mechanisms of anterograde neuronal transport of herpesviruses is important in order to understand recurrent infections of mucous membranes or spread to the central nervous system. However, these transport processes are still controversially discussed. Two models for intraaxonal anterograde transport have been developed (7): the “subassembly model,” which has initially been proposed for HSV-1 (34; reviewed in reference 11), and subsequently also for PrV (reviewed in references 7, 13, and 27) entails separate transport of viral nucleocapsids and envelopes with virion formation only occurring at varicosities along the axon (10, 30) and/or at the synapse or growth cone (30). In contrast, in recent years the so-called “married model” has been proposed for PrV after new evidence had been obtained leading to reinterpretation of earlier data (1, 5, 7, 14, 24). This model proposes intravesicular anterograde transport of enveloped virions similar to egress in nonpolarized cells. These divergent models were partly based on electron microscopy (5, 14, 29, 37), partly on imaging of fluorescently labeled virus particles (1, 9, 38, 40). To substantiate or disprove these earlier findings, we analyzed the intraaxonal transport of the alphaherpesvirus PrV in explanted embryonic rat SCG neurons by preparative high-resolution transmission and immunoelectron microscopy.
In the nucleus and cell body of infected neurons the same stages of virion formation were observed as previously described for nonpolarized, nonneuronal cells (15, 16). Nuclear egress occurred by primary envelopment of intranuclear nucleocapsids at the inner nuclear membrane, followed by deenvelopment through fusion of the primary envelope with the outer nuclear membrane. After translocation into the cytosol, secondary envelopment was observed in abundance, resulting in the presence of enveloped virus particles within vesicles. These vesicles apparently either travel to the plasma membrane surrounding the cell body for exocytosis of the enveloped virus particles or are transported to the axon. Within the axons of PrV-infected neurons the overwhelming majority of viral particles were enveloped virions within vesicles associated with the microtubular system as would be expected for fast axonal transport which had been suggested for PrV and HSV-1 (Fig. 4 and 5) (12, 31, 35, 41). Although electron micrographs do not give directionality but provide “snapshots” of an ongoing movement, our interpretation that the observed particles are indeed in the process of anterograde axonal transport is supported by studies of neurons infected with a phenotypically complemented gB− PrV mutant (33, 36). Within the growth cone enveloped virions within vesicles accumulate prior to being released by exocytosis. Exocytosis could be differentiated from endocytotic events by the lack of a clathrin coat around the vesicle membrane (Fig. 6B and 7C). Whether exocytosis from growth cones in vitro mimics the situation found in vivo at synapses is unclear. However, exocytosis and reentry would be congruent with the finding that fusion-promoting PrV glycoproteins gB, gH, and gL are required for transneuronal spread (2, 6).
In contrast, only very few nonenveloped nucleocapsids (<10% of total viral particles) were observed intraaxonally, although they were also present adjacent to microtubules. These naked nucleocapsids may also be transported anterogradely, followed by envelopment either along the axon (Fig. 5) or in the growth cone (Fig. 6), which can be observed, although less frequently. However, we cannot exclude that these nucleocapsids are actually in retrograde transport due to incomplete synchronization of the infectious process or after abortive secondary envelopment. Although infrequently, secondary envelopment (or accidental deenvelopment) stages have been observed both along axons and in growth cones (Fig. 5 and 6).
To differentiate between entry and egress, compartmentalized Campenot chambers had been used (reviewed in reference 7). In a different approach, we infected neurons with a phenotypically complemented gB-negative PrV mutant. This virus is able to infect neurons due to the presence of gB provided by the transcomplementing cell, but it produces noninfectious progeny (33, 36). Thus, only a single round of productive infection can occur. gB is also required for transneuronal spread of PrV (2, 6), so that after the first round of replication in neuronal cells no further infection should be possible. Analysis of neurons infected by this mutant also showed an abundance of enveloped virions within vesicles (Fig. 7), supporting our claim that they represent virus particles during anterograde transport for egress. As in wild-type virus infection, we occasionally also observed naked capsids (Fig. 7A). Thus, the frequency of detection of enveloped virions within vesicles (abundant) and naked nucleocapsids (rare) was similar in wild-type PrV-Ka and PrV-gB− infection, demonstrating that they are not due to reentry of released infectious virions. Release by exocytosis was observed along the axon (Fig. 7B) and from the growth cone (Fig. 7C).
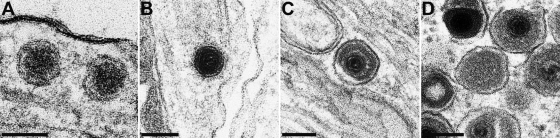
Intraaxonal virus structures may be confused with neurovesicles, which both contain an electron-dense core. However, from our micrographs it is clear that the angular, icosahedral herpesvirus nucleocapsid can clearly be differentiated from the spherical electron-dense core of neurovesicles (Fig. 9). Moreover, whereas nucleocapsids in anterograde transport are surrounded by two membranes, i.e., the virion envelope and the vesicle membrane, as are capsidless light (L) particles, neurovesicles only specify a single membrane (Fig. 9).
FIG. 9.
Direct comparison between neurovesicles and viral particles. Images of two neurovesicles without angular center and surrounded by a single membrane (A), an electron-dense angular viral capsid (B), an enveloped virion within a vesicle (C), and an L-particle (D, center) enclosed in two membranes and surrounded by enveloped virions within vesicles are shown. Bars: 100 nm in panels A and B and 150 nm in panels C and D.
Immunoelectron microscopic analyses demonstrated that the vesicles containing enveloped virions reacted with a monospecific antiserum against the major capsid protein of PrV, as well as with a monoclonal antibody against gB (Fig. 8), thereby differentiating them from neurovesicles and supporting our assumption that the membrane around the nucleocapsids is indeed a bona fide herpesvirus envelope.
Our results on PrV neuronal egress are different from those observed by others in ultrastructural analyses of HSV-1 infections (20, 30, 37). In axons of HSV-1-infected neurons, predominantly nonenveloped capsids were observed, and enveloped particles were primarily found at varicosities and in growth cones, which represent exit sites. These data correlate with the analysis of fluorescently labeled virion structural components of HSV-1, which also indicate separate intraaxonal transport of viral envelopes and capsids (29, 38, 40). Although somewhat difficult to accept based on the rather close genetic relationship between HSV-1 and PrV, it thus appears that intraaxonal transport of herpesviruses can be effected by different means, one entailing separate transport of the envelope and capsid components and formation of infectious virions along (varicosities) or at the end of the axon (synapses, growth cones), and the other relying on intraaxonal transport of complete virions within vesicles, as occurs in nonpolarized cells and in the cell body of neurons. It is conceivable that the mode of transport is actually determined by the efficiency of secondary envelopment in the cell body and the amount of intracytoplasmic viral assemblies or subassemblies available for translocation to the axon. A direct comparison between the different viruses which also includes viral mutants with defects in virion formation should resolve this issue.
In summary, our data support a model for anterograde axonal transport of PrV virions within vesicles which, in turn, attach to the microtubular system. Morphologically, this “cargo” cannot be differentiated from that of produced and transported virions/particles in infected nonpolarized cells and in the cell body of infected neurons. Thus, it remains to be analyzed whether there is indeed a specific sorting of these transport vesicles to the axon, presumably involving the pUS9 membrane protein (24, 25, 42), and which structure, viral or cellular, is recognized at the vesicle membrane to effect axonal transport.
In the future we will assay neuronal infection and intraaxonal transport of other herpesviruses, in particular HSV-1, as well as PrV mutants which have been described to be deficient in anterograde transport (3, 14, 24).
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG Me 854/9).
We thank Petra Meyer and Silvia Schuparis for expert technical assistance and Mandy Jörn for photographic help. The generous assistance of Lynn Enquist, Princeton University, in the establishment of the neuronal culture system is greatly appreciated.
Footnotes
Published ahead of print on 17 March 2010.
REFERENCES
- 1.Antinone, S. E., and G. A. Smith. 2006. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J. Virol. 80:11235-11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babic, N., T. C. Mettenleiter, A. Flamand, and G. Ugolini. 1993. Role of essential glycoproteins gII and gp50 in transneuronal transfer of pseudorabies virus from the hypoglossal nerves of mice. J. Virol. 67:4421-4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ch'ng, T. H., and L. W. Enquist. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J. Virol. 79:8835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ch'ng, T. H., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292:299-316. [DOI] [PubMed] [Google Scholar]
- 5.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curanovic, D., and L. W. Enquist. 2009. Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus. J. Virol. 83:7796-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curanovic, D., and L. W. Enquist. 2009. Directional transneuronal spread of α-herpesvirus infection. Fut. Virol. 4:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davison, A. J., R. Eberle, B. Ehlers, G. S. Hayward, D. J. McGeoch, A. C. Minson, P. E. Pellett, B. Roizman, M. J. Studdert, and E. Thiry. 2009. The order Herpesvirales. Arch. Virol. 154:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Regge, N., H. J. Nauwynck, K. Geenen, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, T. C. Mettenleiter, and H. W. Favoreel. 2006. Alphaherpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 174:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diefenbach, R. J., M. Miranda-Saksena, M. W. Douglas, and A. L. Cunningham. 2008. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 18:35-51. [DOI] [PubMed] [Google Scholar]
- 12.Döhner, K., K. Radtke, S. Schmidt, and B. Sodeik. 2006. Eclipse phase of herpes simplex virus type 1 infection: efficient dynein-mediated capsid transport without the small capsid protein VP26. J. Virol. 80:8211-8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 14.Feierbach, B., M. Bisher, J. Goodhouse, and L. W. Enquist. 2007. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. J. Virol. 81:6846-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granzow, H., B. G. Klupp, W. Fuchs, J. Veits, N. Osterrieder, and T. C. Mettenleiter. 2001. Egress of alphaherpesviruses: comparative ultrastructural study. J. Virol. 75:3675-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan, A. S., and A. E. Vatter. 1959. A comparison of herpes simplex and pseudorabies viruses. Virology 7:394-407. [DOI] [PubMed] [Google Scholar]
- 19.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2000. Primary envelopment of pseudorabies virus at the nuclear membrane requires the UL34 gene product. J. Virol. 74:10063-10073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaVail, J. H., A. N. Tauscher, A. Sucher, O. Harrabi, and R. Brandimarti. 2007. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience 146:974-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaVail, J. H., A. N. Tauscher, J. W. Hicks, O. Harrabi, G. T. Melroe, and D. M. Knipe. 2005. Genetic and molecular in vivo analysis of herpes simplex virus assembly in murine visual system neurons. J. Virol. 79:11142-11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leestma, J. E., M. B. Bornstein, R. D. Sheppard, and L. A. Feldman. 1969. Ultrastructural aspects of herpes simplex virus infection in organized cultures of mammalian nervous tissue. Lab. Invest. 20:70-78. [PubMed] [Google Scholar]
- 23.Lyman, M. G., B. Feierbach, D. Curanovic, M. Bisher, and L. W. Enquist. 2007. Pseudorabies virus Us9 directs axonal sorting of viral capsids. J. Virol. 81:11363-11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyman, M. G., C. D. Kemp, M. P. Taylor, and L. W. Enquist. 2009. Comparison of the pseudorabies virus Us9 protein with homologs from other veterinary and human alphaherpesviruses. J. Virol. 83:6978-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyman, M. G., D. Curanovic, and L. W. Enquist. 2008. Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts. PLoS Pathog. 4:e1000065. doi: 10.1371/journal.ppat.1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mettenleiter, T. C. 2008. Pseudorabies virus, p. 341-351. In B. W. J. Mahy and M. Van Regenmortel (ed.), Encyclopedia of virology, 3rd ed., vol. 5. Elsevier, Oxford, United Kingdom. [Google Scholar]
- 27.Mettenleiter, T. C. 2003. Pathogenesis of neurotropic herpesviruses: role of viral glycoproteins in neuroinvasion and transneuronal spread. Virus Res. 92:197-206. [DOI] [PubMed] [Google Scholar]
- 28.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2009. Herpesvirus assembly: an update. Virus Res. 143:222-234. [DOI] [PubMed] [Google Scholar]
- 29.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda-Saksena, M., R. A. Boadle, A. Aggarwal, B. Tijono, F. J. Rixon, R. J. Diefenbach, and A. L. Cunningham. 2009. Herpes simplex virus utilizes the large secretory vesicle pathway for anterograde transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. J. Virol. 83:3187-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peeters, B., W. N. de, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. U. S. A. 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radtke, K., K. Döhner, and B. Sodeik. 2006. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell Microbiol. 8:387-400. [DOI] [PubMed] [Google Scholar]
- 36.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 80:3592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder, A., B. Bruun, H. M. Browne, and D. C. Johnson. 2007. A herpes simplex virus gD-YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J. Virol. 81:8337-8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder, A., K. Polcicova, and D. C. Johnson. 2008. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J. Virol. 82:10613-10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder, A., T. W. Wisner, and D. C. Johnson. 2006. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 80:11165-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 42.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alphaherpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 44.Yamada, K. M., B. S. Spooner, and N. K. Wessells. 1971. Ultrastructure and function of growth cones and axons of cultured nerve cells. J. Cell Biol. 49:614-635. [DOI] [PMC free article] [PubMed] [Google Scholar]