Abstract
Natural simian immunodeficiency virus (SIV) infection in sooty mangabeys (SMs) typically does not result in AIDS, despite high-level viremia and significant depletion of mucosal CD4+ T cells. Here, we report the results of the first longitudinal study of a large cohort of SMs naturally infected with SIV (n = 78) housed at the Yerkes National Primate Research Center from which samples were obtained three times over a 5-year period. In this study, we observed (i) no signs of simian AIDS, (ii) stable SIV loads, (iii) a slow but progressive decline in CD4+ T-cell counts (from a mean of 1,067.0 cells/mm3 at time point 1 to 764.8 cells/mm3 at time point 3) and increases in the numbers of animals with CD4+ T-cell levels below 500 and 200 cells/mm3 (from 8 to 28 of 78 and from 1 to 4 of 78, respectively), (iv) progressive declines in percentages of naïve CD4+ and CD8+ T cells (from 37.7 to 24.8% and from 21.0 to 13.0%, respectively), and (v) stably low levels of activated/proliferating T cells as well as CD4+ CCR5+ T cells. Since the level of total CD4+ T cells and the fraction of naïve T cells in SIV-uninfected SMs also declined, it is possible that some of these observations are related to aging, as the SIV-infected animals were significantly older than the uninfected animals. In contrast to the decline in CD4+ T cell counts in individuals infected with human immunodeficiency virus (HIV), the decline in CD4+ T cell counts in SMs naturally infected with SIV over a 5-year period was not predicted by either plasma viremia or levels of T-cell activation. Taken together, these results confirm that natural SIV infection is nonprogressive from a clinical, virological, and immunological point of view and that stable levels of viremia associated with persistently low-level immune activation represent key differences from the natural course of HIV infection in humans.
Over the past few years, there has been renewed interest in understanding the immunology and virology of so-called natural simian immunodeficiency virus (SIV) infections in African nonhuman primate species (20, 41, 51). Natural SIV hosts include the chimpanzee (Pan troglodytes), the gorilla (Gorilla gorilla), the sooty mangabey (SM; Cercocebus atys), the mandrill (Mandrillus sphinx), African green monkeys (AGMs; Chlorocebus spp.), and numerous others (57). Of note, SIVs that infect two of these species, the chimpanzee-derived SIVcpz and the SM-derived SIVsmm, are the origins of the human immunodeficiency virus type 1 (HIV-1) and HIV-2 epidemics, respectively (7, 11). A striking feature of natural SIV hosts is that, in marked contrast to HIV-infected humans, they tend to have benign infections, with normal CD4+ T-cell counts in the majority of observed animals and progression to simian AIDS in only a few cases (44). The only known exception to this rule is the recent observation that chimpanzees naturally infected with SIVcpz in the Gombe forest of Tanzania manifest an ∼16-fold increase in mortality compared to uninfected apes (19). The mechanisms underlying the favorable outcome of SIV infection in monkey species such as SMs and AGMs have been the subject of intense studies but thus far have been incompletely elucidated. Since natural SIV infections are characterized by chronically high levels of virus replication occurring primarily in short-lived CD4+ T cells, it does not appear that natural SIV hosts remain healthy due to stronger or more effective adaptive immune responses to the virus or that the virus is intrinsically less cytopathic in these animals (9, 41, 51). In recent studies, more emphasis has been placed on other factors, such as the absence of chronic immune activation, more vigorous CD4+ T-cell regeneration, and lower levels of CCR5 expression on CD4+ T cells (40, 43, 52, 56). Elucidating the mechanisms responsible for the AIDS resistance of natural SIV hosts may provide important insight into the mechanisms of AIDS pathogenesis in HIV-infected humans.
Among the numerous natural SIV hosts, SMs have been extensively studied due to the existence of a large colony of animals naturally infected and uninfected with SIV at the Yerkes National Primate Research Center of Emory University, Atlanta, GA. In this body of experimental work, we and others have described in substantial detail the interaction between SIV and the SM immune system (1, 2, 6, 9, 13, 14, 25, 32, 38, 39, 43, 50, 52, 56). One concept that has emerged from a recent comparative study of the impact of SIV infection on the transcriptional profiles of SMs and rhesus macaques is that the acute phase of infection is far from being immunologically silent in SMs, with initial strong upregulation of immune activation and upregulation of type I interferon response genes that resolve approximately 4 to 6 weeks later (4). In addition, it has become apparent that natural SIV infection in SMs, while by and large nonprogressive, does in fact have an impact on the homeostasis of the CD4+ T-cell compartment. First, SIV-infected SMs manifest rapid, severe, and persistent depletion of mucosal CD4+ T cells (14). Second, small subsets of SMs both naturally and experimentally infected with SIV experience severe systemic CD4+ T-cell depletion (32, 56). Third, in at least one animal, the development of classical AIDS has been observed (23). Collectively, these observations prompted us to reevaluate whether natural SIV infection in SMs is truly nonpathogenic, as we have often stated in the past, or whether it is simply less pathogenic or less progressive. To clarify this point, we here report the results of the first longitudinal assessment of the virologic and immunologic statuses of SMs naturally infected with SIV in a study of 78 infected individuals as well as a group of 22 SIV-uninfected animals housed at the Yerkes Center over a period of 5 years (2004 to 2009). We confirmed that natural SIV infection in SMs is ultimately nonprogressive, as it did not result in AIDS in any of these 78 animals. In addition, we observed that the natural history of SIV infection in SMs is associated with a triad of (i) stable viral loads, (ii) slowly decreasing CD4+ T-cell counts, and (iii) persistently low-level immune activation. The fact that neither the viral load nor the level of immune activation predicts the magnitude of decline in CD4+ T-cell counts in SMs naturally infected with SIV identifies a clear immunologic difference between the infections in these animals and the progressive HIV and SIV infections in humans and macaques.
MATERIALS AND METHODS
Animals.
Seventy-eight SMs naturally infected with SIV and 22 uninfected SMs from the colony housed at the Yerkes National Primate Research Center of Emory University were included in this longitudinal study. Blood samples were collected three times, once between 2004 and 2005 (time point 1 [TP1]), once between 2006 and 2007 (TP2), and once between 2008 and 2009 (TP3). Eighty-two healthy, SIV-uninfected rhesus macaques were also included in this study. All animals were maintained in accordance with National Institutes of Health guidelines. For uninfected animals, negative results from PCR analyses of plasma samples for the presence of SIV and negative HIV-2 serology results confirmed the absence of SIV infection.
Viral load.
SIVsmm loads in plasma samples were measured by real-time PCR as described previously (52, 56).
Flow cytometry and immunophenotyping analyses of peripheral blood lymphocytes.
Immunophenotyping of whole-blood samples was performed according to standard procedures using multicolor flow cytometry and monoclonal antibodies (MAbs) that were originally designed for humans or macaques and have been found to be cross-reactive in SMs. CD4+ and CD8+ T cells were identified using anti-CD3 Alexa Fluor 700 (clone SP34-2; BD Pharmingen), anti-CD4 peridinin chlorophyll protein (PerCP)-Cy5.5 (clone L200; BD Pharmingen), and anti-CD8 Pacific Blue (clone RPA-T8; BD Pharmingen). Memory T-cell subsets were identified using anti-CD28 phycoerythrin (PE)-Cy7 (clone CD28.2; eBioscience) and anti-CD95 PE-Cy5 (clone DX2; BD Pharmingen). Anti-CCR5 allophycocyanin (clone 3A9; BD Pharmingen) and anti-Ki67 fluorescein isothiocyanate (clone B56; BD Pharmingen) were also used for T-cell immunophenotyping. Ki67 staining was performed intracellularly after the BD Pharmingen Cytofix/CytoPerm kit was used for fixation and permeabilization of samples first surface stained with appropriate antibodies. Flow cytometric acquisition and analysis of samples were performed with at least 10,000 events collected by an LSRII flow cytometer driven by FACSDiva software. High-resolution analysis of the acquired data was performed using Flow Jo software (Tree Star).
Statistical analysis.
Spearman correlations were used to assess relationships between two different parameters for infected SMs at a given time point. In longitudinal comparisons in which the interest was in assessing differences only between TP1 and TP3, two-tailed paired t tests were conducted. Random-effect models were used to assess trends across the three time points, with Tukey's adjustment for multiple comparisons. These models are similar to analysis of variance (ANOVA) models but allow for incomplete/nonbalanced data, as well as the inherent within-animal correlation of longitudinal study results. Variables were transformed, as necessary, to meet distributional assumptions of the statistical tests. All analyses used an overall α value of 0.05. Statistics were calculated using GraphPad Prism 4.0c (GraphPad Software) for Macintosh and SAS 9.2 (SAS Inc.) for Windows.
RESULTS
Study design and animal population.
The colony of SMs naturally infected with SIV and uninfected SMs housed at the Yerkes National Primate Research Center has been the subject of several virologic and immunologic studies (1, 2, 6, 9, 13, 14, 25, 32, 38, 39, 43, 50, 52, 56), including a large cross-sectional survey of 110 animals that revealed the existence of a small subset of SIV-infected SMs with low CD4+ T-cell counts but no signs of disease progression (56). However, none of the published studies have provided a longitudinal assessment of the immunologic features of natural SIV infection in SMs over a prolonged period of time. To address this specific issue, we have now conducted a longitudinal study involving a total of 78 SIV-infected and 22 uninfected SMs with analysis of data from three time points spanning a 5-year period (between 2004 and 2009). Of note, none of the 78 SIV-infected SMs that were included in this study developed any signs of simian AIDS between 2004 and 2009, thus confirming the generally benign nature of the infection.
SMs naturally infected with SIV show stable levels of viremia.
In HIV-infected humans, the main markers of disease progression are viral load and CD4+ T-cell count, with a large number of studies showing that the natural history of infection is associated with increasing levels of viremia and declining CD4+ T-cell counts (29, 30, 55). To investigate whether and to what extent natural SIV infection in SMs may recapitulate these features of HIV infection, we first longitudinally measured plasma SIVsmm viremia in our cohort of animals naturally infected with SIV. As shown in Fig. 1A, the levels of viremia in SMs naturally infected with SIV were stable over time, with no significant differences between TP1 and TP3 observed (averages, 169,417 SIV RNA copies/ml of plasma and 120,207 copies/ml of plasma, respectively; P value, not significant). Figure 1B shows the longitudinal trend in viremia for each individual animal. The observation of a stable level of viremia during the natural history of SIV infection in SMs confirms a difference from pathogenic HIV-1 infection in humans (1).
FIG. 1.
SMs naturally infected with SIV show stable levels of viremia. (A) Viral loads in 78 SMs naturally infected with SIV were measured as numbers of SIV RNA copies per milliliter of plasma. Samples from animals were obtained at three time points, between 2004 and 2005 (TP1), 2006 and 2007 (TP2), and 2008 and 2009 (TP3). Triangles represent results for individual animals, and horizontal lines indicate means. (B) Viral load trends across three time points for each individual animal are shown by connecting lines.
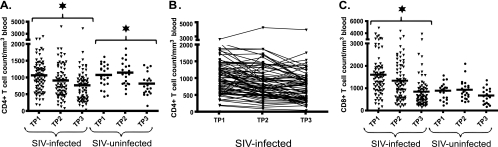
Natural SIV infection in SMs is associated with a moderate but significant decline in CD4+ T-cell count.
To then longitudinally assess the effect of natural SIV infection on the counts of peripheral CD4+ T cells in SMs, we measured these cells in our cohort of animals. Perhaps unexpectedly, our analysis revealed that SMs naturally infected with SIV manifested significant decline in CD4+ T-cell counts over time, from an average ± standard deviation of 1,067 ± 57 cells/mm3 at TP1 to 765 ± 64 cells/mm3 at TP3 (P < 0.001) (Fig. 2A). Of note, we observed that the number of animals with CD4+ T-cell counts below 500 cells/mm3 increased from 8 (10.3%) at TP1 to 28 (35.9%) at TP3. The same degree of increase in the total number of SIV-infected SMs with CD4+ T-cell counts below 200 cells/mm3, from 1 (1.3%) at TP1 to 4 (5.1%) at TP3, was found. Interestingly, we also observed a similar decline in the CD4+ T-cell counts in SIV-uninfected SMs, from an average of 1,076 ± 73 cells/mm3 at TP1 to 818 ± 76 cells/mm3 at TP3 (P < 0.001). This effect was most evident between TP2 and TP3 (Fig. 2A) and is consistent with a potential effect of aging in reducing the levels of circulating CD4+ T cells. As the SIV-infected SMs were significantly older than their SIV-uninfected counterparts (17.2 ± 0.5 years of age versus 12.4 ± 0.7 years [P < 0.0001; unpaired t test]), the more rapid loss of CD4+ T cells in the infected animals may be explained partially by the additive effect of aging. Of note, the effect of age on CD4+ T cell count is not unique to SMs, as we found significant inverse correlation between CD4+ T cell counts and age in a cross-sectional survey of 82 healthy, SIV-uninfected rhesus macaques (P = 0.020) (data not shown). Our analysis also indicated that SIV-infected SMs showed a significant decline (P < 0.001) in the average CD8+ T-cell count, from 1,598 ± 108 cells/mm3 at TP1 to 843 ± 79 cells/mm3 at TP3 (Fig. 2C), and that this decline was significantly steeper than that in SIV-uninfected SMs (P < 0.05; linear mixed-effects models with Tukey's adjustment for multiple comparisons). This effect of SIV infection on the CD8+ T-cell counts in SMs may be complex, as the levels we observed at TP3 are consistent with those found in uninfected animals at all time points, thus suggesting that the observed decline in SIV-infected SMs may represent normalization of a CD8+ T-cell expansion previously induced by the infection.
FIG. 2.
Natural SIV infection in SMs is associated with moderate but significant decline in CD4+ T-cell counts. (A and B) CD4+ T-cell counts (A) and their longitudinal trends (B) for individual animals. (C) CD8+ T-cell counts in 78 SMs naturally infected with SIV (▾) and 22 SIV-uninfected SMs (•) were determined at three time points. Asterisks indicate P values of <0.001, as determined by linear mixed-effects models with Tukey's adjustments for multiple comparisons.
Taken together, these results reveal for the first time that natural SIV infection in SMs is associated with a moderate but significant decline in CD4+ T-cell counts over time. However, since a similar decline in CD4+ T-cell counts in SIV-uninfected animals over the same time period was observed, it seems unlikely that natural SIV infection in SMs has any significant negative impact on CD4+ T-cell homeostasis.
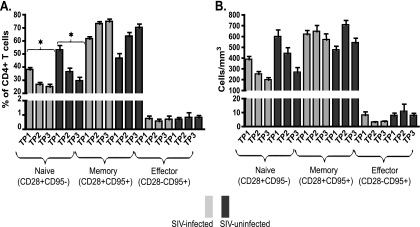
SMs naturally infected with SIV show a progressive decline in naïve T cells with corresponding increases in memory and effector T cells.
Pathogenic HIV infection in humans and SIV infection in macaques are associated with specific changes in the relative proportions of naïve, memory, and effector T cells (5, 16, 28, 35, 46). To investigate how natural SIV infection in SMs impacts these T-cell subpopulations, we longitudinally measured the fractions of CD4+ and CD8+ T cells expressing the naïve (CD28+ CD95−), memory (CD28+ CD95+), and effector (CD28− CD95+) phenotypes (47, 56). Note that this definition of memory cells includes both CD62L+ CCR7+ “central” memory T cells (Tcm) and CD62L− CCR7− “effector” memory T cells (Tem). Since the measurement of Tcm and Tem was conducted only at the second and third time points, we used the levels of total memory T cells in our analysis. As depicted in Fig. 3A, both SIV-infected and uninfected SMs experienced significant decreases in the percentages of naïve CD4+ T cells and concomitant increases in the percentages of circulating memory CD4+ T cells, with no change in the percentages of effector CD4+ T cells. As depicted in Fig. 3B, absolute counts of these CD4+ T-cell subsets showed that natural SIV infection in SMs was associated with declines in numbers of both naïve and effector CD4+ T cells, with constant numbers of memory CD4+ T cells. A similar trend of decline in the fraction of naïve cells and relative expansion of memory cells within the CD8+ T-cell pool was also observed (data not shown). Since the relative declines in naïve CD4+ T cell counts in SIV-infected and uninfected animals were of similar magnitudes, it is difficult to determine to what extent the decline in infected animals was caused by the infection as opposed to being simply related to aging.
FIG. 3.
Naïve, memory, and effector CD4+ T-cell dynamics in SMs naturally infected with SIV. Percentages (A) and absolute counts (B) of naïve, memory, and effector CD4+ T cells in 78 SIV-infected and 22 SIV-uninfected SMs at three time points were determined. Asterisks indicate P values of <0.001, as determined by linear mixed-effects models with Tukey's adjustments for multiple comparisons.
Taken together, these data highlight a major difference from pathogenic SIVmac infection in rhesus macaques, which is associated with selective depletion of memory CD4+ T cells in peripheral blood (35, 46).
SMs naturally infected with SIV maintain persistently low levels of T-cell proliferation.
While pathogenic HIV and SIV infections in humans and macaques are associated with a state of chronic, generalized immune activation (53), natural SIV infection in SMs is characteristically associated with low levels of immune activation during the chronic phase (6, 15, 21, 36, 42, 48, 52, 56, 60). In this longitudinal study, we sought to determine whether natural SIV infection in SMs is associated with any sign of increasing immune activation over time. To this end, we measured the percentages of CD4+ and CD8+ T cells expressing the proliferation marker Ki67 that is typically expressed on activated cells (49). As shown in Fig. 4, we found no significant change between TP1 and TP3 in the percentages of proliferating Ki67+ CD4+ and CD8+ T cells (3.1% of CD4+ Ki67+ T cells at TP1 and 3.9% at TP3 and 3.3% of CD8+ Ki67+ T cells at TP1 and 3.5% at TP3). This result indicates that persistently low-level immune activation is a key feature of the chronic phase of natural SIV infection in SMs and that the moderate decline in CD4+ T-cell counts observed in these animals does not appear to trigger any homeostasis-driven increase in CD4+ T-cell proliferation.
FIG. 4.
SMs naturally infected with SIV maintain persistently low levels of T-cell proliferation. Percentages of CD4+ Ki67+ T cells (A) and CD8+ Ki67+ T cells (B) in 78 SMs naturally infected with SIV (▾) and 22 SIV-uninfected SMs (•) were measured at three time points.
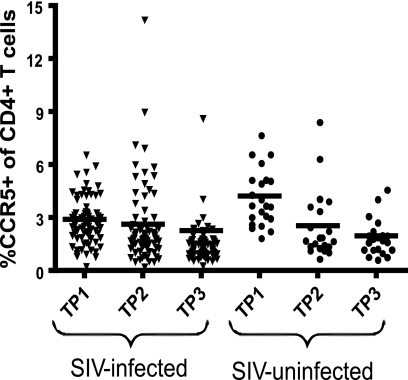
SMs naturally infected with SIV maintain low but stable levels of CD4+ CCR5+ T cells.
Most strains of HIV and SIV use CCR5 as a coreceptor for virus entry and thus preferentially infect CD4+ CCR5+ T cells, which are rapidly depleted in the effector lymphoid sites of mucosal tissues (5, 14, 22, 26, 28, 45, 46, 58). However, the in vivo dynamics of the fraction of CD4+ CCR5+ T cells within the total pool of circulating CD4+ T cells appears to be more complex, with the fractions of these cells observed in HIV-infected humans and in SIV-infected rhesus macaques of Chinese origin (8, 33, 37) higher than those observed in SIV-infected rhesus macaques of Indian origin (27). As shown in Fig. 5, the percentages of CD4+ CCR5+ T cells in our cohort of SMs naturally infected with SIV (2.9% at TP1, 2.6% at TP2, and 2.3% at TP3) remained relatively stable over time, thus indicating a limited impact on the pool of circulating CD4+ CCR5+ T cells in this model of nonprogressive SIV infection.
FIG. 5.
SMs naturally infected with SIV maintain low but stable levels of CD4+ CCR5+ T cells. The percentages of CD4+ CCR5+ T cells in 78 SMs naturally infected with SIV (▾) and 22 SIV-uninfected SMs (•) were measured at three time points.
Neither the level of viremia nor the magnitude of T-cell proliferation predicts the magnitude of decline in CD4+ T-cell counts in SIV-infected SMs.
The finding that natural SIV infection in SMs is associated with moderate but significant decline in peripheral blood CD4+ T-cell counts prompted us to investigate what factors could predict the tempo of this decline. In HIV-infected humans, the main predictors of decline in CD4+ T-cell counts are the viral load (29, 30) and the level of immune activation (12, 17, 24, 54). To determine if a similar relationship is present in SMs naturally infected with SIV, we performed a series of analyses of correlations between the change in CD4+ T-cell counts from TP1 to TP3 (hereinafter referred to as ΔCD4) and the viral load, the levels of proliferating (i.e., Ki67+) CD4+ and CD8+ T cells, and the fraction of memory (CD95+ CD28+) CD4+ T cells at TP1. As shown in Fig. 6, these analyses revealed that ΔCD4 is not correlated with the viral load, the percentages of proliferating CD4+ and CD8+ T cells, or the percentage of memory CD4+ T cells as measured at TP1. In all, these results indicate that, in contrast to the decline in CD4+ T-cell counts observed in HIV-infected humans, the decline in CD4+ T-cell counts observed in SMs naturally infected with SIV does not appear to be consistently predicted by the levels of viremia or immune activation.
FIG. 6.
Neither the level of viremia nor the magnitude of T-cell proliferation predicts the tempo of decline in CD4+ T-cell counts in SIV-infected SMs. Scatter plots depict the relationships between ΔCD4 and the viral load (A), the percentage of CD4+ Ki67+ T cells (B), the percentage of CD8+ Ki67+ T cells (C), and the percentage of memory (CD28+ CD95+) CD4+ T cells (D) at TP1 for 78 SMs naturally infected with SIV.
DISCUSSION
Over the past several years, we and others have intensively studied the virologic and immunologic features of natural SIV infection in SMs. These studies led to some basic conclusions regarding this model of infection that can be summarized as follows: (i) the infection is usually not associated with progression to simian AIDS, (ii) the set-point level of viremia typically ranges between 104 and 106 SIV RNA copies/ml of plasma, (iii) 80 to 90% of animals maintain CD4+ T-cell counts comparable to those observed in uninfected SMs, (iv) the level of T-cell activation is significantly lower than those detected in humans and macaques with pathogenic HIV and SIV infections, respectively, (v) the breadth and magnitude of antiviral cellular immune responses are similar to, if not lesser than, those found in pathogenic HIV and SIV infections, and (vi) the infection is associated with a short in vivo life span of productively infected cells (41, 51). Of note, all these studies were conducted either as cross-sectional analyses of naturally infected animals (9, 18, 25, 52, 56, 59) or as longitudinal analyses of animals experimentally infected with SIV (10, 14, 25, 31, 32, 34, 50). Here, we report the results of a longitudinal virologic and immunologic analysis of a cohort of 78 SMs naturally infected with SIV and 22 uninfected SMs from which samples were obtained at three different time points over a 5-year period. To the best of our knowledge, this work represents the first comprehensive longitudinal assessment of SIV infection in the large group of naturally infected animals housed at the Yerkes Center.
The main finding of this study is that the natural history of SIVsmm infection in SMs is characterized by a triad of stable viral loads, slowly declining CD4+ T-cell counts, and persistently low levels of immune activation. These results identify a previously unrecognized difference from the natural history of HIV infection in humans, in which declining CD4+ T-cell counts are associated with increasing levels of both viremia and immune activation. Of note, a decline in CD4+ T-cell counts in uninfected SMs had a level of significance similar to that in uninfected SMs, suggesting that, at least in part, the loss of CD4+ T cells in SIV-infected SMs may be simply an effect of aging. It should also be noted that the interpretation of these results is somewhat complicated by the fact that in this study the SMs naturally infected with SIV were significantly older than the uninfected ones (17.2 ± 0.5 years of age versus 12.4 ± 0.7 years of age [P < 0.0001; unpaired t test]). Thus, it is difficult to directly compare the effects of aging on CD4+ T-cell counts in these two groups of SIV-infected and uninfected animals. Previous cross-sectional studies of SIV-infected SMs revealed lower CD4+ T-cell counts than those in uninfected animals (18, 52). However, these results should be approached with caution as the cross-sectional design of these studies precludes direct comparisons with the longitudinal analysis in our present study.
The fact that SMs naturally infected with SIV tend to significantly deplete their pool of peripheral CD4+ T cells over time is not entirely unexpected, as several previous studies indicated that this model of nonprogressive infection can be associated with perturbations of CD4+ T-cell homeostasis. First, a previous study from our group showed that a subset of 5 to 10% of SMs naturally infected with SIV may experience profound CD4+ T-cell depletion, and in two cases the CD4+ T-cell count dropped below 50 cells/mm3 (56). Second, a virus with expanded coreceptor tropism emerged in a group of experimentally SIV-infected SMs, which subsequently manifested extreme CD4+ T-cell depletion in both blood and mucosal tissues (32). Third, a study of the levels of CD4+ T cells in mucosal tissues (those of the rectum and lung) in SIV-infected SMs revealed that these cells are depleted in the majority of animals (14). Collectively, the results of these studies indicate that, in SMs, SIV infection is not immunologically silent but rather has a clearly discernible effect on the host immune system, particularly in determining the size of the pool of mucosal (and, to a lesser extent, circulating) CD4+ T cells. While this effect is predominantly subclinical, with animals typically living an apparently normal life span in captivity, we suggest that this infection is better defined as nonprogressive rather than nonpathogenic. The recent finding that chimpanzees naturally infected with SIVcpz have an ∼16-fold increase in mortality compared to uninfected animals (19) emphasizes how the clinical spectrum of SIV infection in African nonhuman primates is far from being fully appreciated.
The observation of a slow but progressive decline in CD4+ T-cell counts in SMs naturally infected with SIV must be reconciled with the fact that these animals do not appear to show any increased morbidity or mortality. One possibility is that this CD4+ T-cell depletion is so slow that the SMs simply die of other causes (i.e., natural death) prior to developing clinically significant immunodeficiency. Of note, we previously observed that SIV infections in SMs do not progress to AIDS even when the animals experience severe systemic and mucosal CD4+ T-cell depletion (32, 56). This puzzling finding may be explained by postulating that the immune system of SMs has evolved to be less dependent on the function of CD4+ T cells, with other cell types providing immunological help in vivo. Another possibility is that at some point in the infection, T helper cells in SMs stop expressing the CD4 molecule in a pattern similar to what has been described recently for African green monkeys (3). A third potential explanation is that in a “resting,” non-chronically activated immune environment, the need for CD4+ T-cell help is much smaller and that even very low levels of CD4+ T cells are sufficient to avoid AIDS. Further studies are needed to fully elucidate the role of CD4+ T cells in SMs as well as other natural SIV hosts.
In this study, we sought to identify predictors of the magnitude of decline in CD4+ T-cell counts in SMs naturally infected with SIV by performing a series of statistical analyses in which we investigated potential correlations between parameters measured at TP1 of our longitudinal analysis and ΔCD4. Interestingly, we found that neither the viral load nor the level of immune activation (measured as the fractions of CD4+ Ki67+ and CD8+ Ki67+ T cells, as well as the fraction of memory CD4+ T cells) predicts the magnitude of decline in CD4+ T-cell counts. Similarly, we did not find evidence for an effect of baseline total CD4+ T-cell counts or naïve CD4+ T-cell counts (data not shown). Based on these results, we favor the possibility that the depletion of CD4+ T cells in SMs naturally infected with SIV may reflect insufficient regeneration of these cells that is not directly related to the amount of virus replication or the prevailing level of immune activation.
While this study is clearly descriptive in nature, we believe that it is nonetheless of interest as it is the first ever conducted with such a large group of nonhuman primates naturally infected with SIV over an extended period of observation. In particular, differences between the results described here and findings from studies of the natural history of HIV infection in humans provide clues as to the pathogenic mechanisms that are likely to be central to the development of clinically significant immunodeficiency after infection with a primate lentivirus. Further follow-up with the colony of SMs naturally infected with SIV housed at the Yerkes National Primate Research Center will provide additional clarification of the natural history of this model of nonprogressive infection.
Acknowledgments
We thank Stephanie Ehnert, Elizabeth Strobert, and all the animal care and veterinary staff at the Yerkes National Primate Research Center and the Virology Core of the Emory Center for AIDS Research (CFAR).
This work was supported by NIH grants AI-66998 and AI-76174 (to G.S.) and RR-00165 (to the Yerkes National Primate Research Center).
Footnotes
Published ahead of print on 24 March 2010.
REFERENCES
- 1.Apetrei, C., R. Gautam, B. Sumpter, A. C. Carter, T. Gaufin, S. I. Staprans, J. Else, M. Barnes, R. Cao, Jr., S. Garg, J. M. Milush, D. L. Sodora, I. Pandrea, and G. Silvestri. 2007. Virus subtype-specific features of natural simian immunodeficiency virus SIVsmm infection in sooty mangabeys. J. Virol. 81:7913-7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. P., G. Silvestri, J. T. Safrit, B. Sumpter, N. Kozyr, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2007. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. J. Immunol. 178:8002-8012. [DOI] [PubMed] [Google Scholar]
- 3.Beaumier, C. M., L. D. Harris, S. Goldstein, N. R. Klatt, S. Whitted, J. McGinty, C. Apetrei, I. Pandrea, V. M. Hirsch, and J. M. Brenchley. 2009. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat. Med. 15:879-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosinger, S. E., Q. Li, S. N. Gordon, N. R. Klatt, L. Duan, L. Xu, N. Francella, A. Sidahmed, A. J. Smith, E. M. Cramer, M. Zeng, D. Masopust, J. V. Carlis, L. Ran, T. H. Vanderford, M. Paiardini, R. B. Isett, D. A. Baldwin, J. G. Else, S. I. Staprans, G. Silvestri, A. T. Haase, and D. J. Kelvin. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J. Clin. Invest. 119:3556-3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Z., P. Telfier, A. Gettie, P. Reed, L. Zhang, D. D. Ho, and P. A. Marx. 1996. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J. Virol. 70:3617-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Roda Husman, A. M., H. Blaak, M. Brouwer, and H. Schuitemaker. 1999. CC chemokine receptor 5 cell-surface expression in relation to CC chemokine receptor 5 genotype and the clinical course of HIV-1 infection. J. Immunol. 163:4597-4603. [PubMed] [Google Scholar]
- 9.Dunham, R., P. Pagliardini, S. Gordon, B. Sumpter, J. Engram, A. Moanna, M. Paiardini, J. N. Mandl, B. Lawson, S. Garg, H. M. McClure, Y. X. Xu, C. Ibegbu, K. Easley, N. Katz, I. Pandrea, C. Apetrei, D. L. Sodora, S. I. Staprans, M. B. Feinberg, and G. Silvestri. 2006. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes, J. D., S. N. Gordon, M. Zeng, A. M. Chahroudi, R. M. Dunham, S. I. Staprans, C. S. Reilly, G. Silvestri, and A. T. Haase. 2008. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J. Immunol. 180:6798-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, F., E. Bailes, D. L. Robertson, Y. Chen, C. M. Rodenburg, S. F. Michael, L. B. Cummins, L. O. Arthur, M. Peeters, G. M. Shaw, P. M. Sharp, and B. H. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:436-441. [DOI] [PubMed] [Google Scholar]
- 12.Giorgi, J. V., R. H. Lyles, J. L. Matud, T. E. Yamashita, J. W. Mellors, L. E. Hultin, B. D. Jamieson, J. B. Margolick, C. R. Rinaldo, Jr., J. P. Phair, and R. Detels. 2002. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 29:346-355. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, S. N., R. M. Dunham, J. C. Engram, J. Estes, Z. Wang, N. R. Klatt, M. Paiardini, I. V. Pandrea, C. Apetrei, D. L. Sodora, H. Y. Lee, A. T. Haase, M. D. Miller, A. Kaur, S. I. Staprans, A. S. Perelson, M. B. Feinberg, and G. Silvestri. 2008. Short-lived infected cells support virus replication in sooty mangabeys naturally infected with simian immunodeficiency virus: implications for AIDS pathogenesis. J. Virol. 82:3725-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon, S. N., N. R. Klatt, S. E. Bosinger, J. M. Brenchley, J. M. Milush, J. C. Engram, R. M. Dunham, M. Paiardini, S. Klucking, A. Danesh, E. A. Strobert, C. Apetrei, I. V. Pandrea, D. Kelvin, D. C. Douek, S. I. Staprans, D. L. Sodora, and G. Silvestri. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 179:3026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gougeon, M. L., H. Lecoeur, F. Boudet, E. Ledru, S. Marzabal, S. Boullier, R. Roue, S. Nagata, and J. Heeney. 1997. Lack of chronic immune activation in HIV-infected chimpanzees correlates with the resistance of T cells to Fas/Apo-1 (CD95)-induced apoptosis and preservation of a T helper 1 phenotype. J. Immunol. 158:2964-2976. [PubMed] [Google Scholar]
- 16.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881-1888. [DOI] [PubMed] [Google Scholar]
- 18.Kaur, A., M. Di Mascio, A. Barabasz, M. Rosenzweig, H. M. McClure, A. S. Perelson, R. M. Ribeiro, and R. P. Johnson. 2008. Dynamics of T- and B-lymphocyte turnover in a natural host of simian immunodeficiency virus. J. Virol. 82:1084-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keele, B. F., J. H. Jones, K. A. Terio, J. D. Estes, R. S. Rudicell, M. L. Wilson, Y. Li, G. H. Learn, T. M. Beasley, J. Schumacher-Stankey, E. Wroblewski, A. Mosser, J. Raphael, S. Kamenya, E. V. Lonsdorf, D. A. Travis, T. Mlengeya, M. J. Kinsel, J. G. Else, G. Silvestri, J. Goodall, P. M. Sharp, G. M. Shaw, A. E. Pusey, and B. H. Hahn. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460:515-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhoff, F. 2009. Is the high virulence of HIV-1 an unfortunate coincidence of primate lentiviral evolution? Nat. Rev. Microbiol. 7:467-476. [DOI] [PubMed] [Google Scholar]
- 21.Kornfeld, C., M. J. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J. F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 115:1082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 23.Ling, B., C. Apetrei, I. Pandrea, R. S. Veazey, A. A. Lackner, B. Gormus, and P. A. Marx. 2004. Classic AIDS in a sooty mangabey after an 18-year natural infection. J. Virol. 78:8902-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, Z., W. G. Cumberland, L. E. Hultin, H. E. Prince, R. Detels, and J. V. Giorgi. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83-92. [DOI] [PubMed] [Google Scholar]
- 25.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Klucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 14:1077-1087. [DOI] [PubMed] [Google Scholar]
- 26.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 27.Mattapallil, J. J., N. L. Letvin, and M. Roederer. 2004. T-cell dynamics during acute SIV infection. AIDS 18:13-23. [DOI] [PubMed] [Google Scholar]
- 28.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellors, J. W., A. Munoz, J. V. Giorgi, J. B. Margolick, C. J. Tassoni, P. Gupta, L. A. Kingsley, J. A. Todd, A. J. Saah, R. Detels, J. P. Phair, and C. R. Rinaldo, Jr. 1997. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann. Intern. Med. 126:946-954. [DOI] [PubMed] [Google Scholar]
- 30.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 31.Meythaler, M., A. Martinot, Z. Wang, S. Pryputniewicz, M. Kasheta, B. Ling, P. A. Marx, S. O'Neil, and A. Kaur. 2009. Differential CD4+ T-lymphocyte apoptosis and bystander T-cell activation in rhesus macaques and sooty mangabeys during acute simian immunodeficiency virus infection. J. Virol. 83:572-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milush, J. M., J. D. Reeves, S. N. Gordon, D. Zhou, A. Muthukumar, D. A. Kosub, E. Chacko, L. D. Giavedoni, C. C. Ibegbu, K. S. Cole, J. L. Miamidian, M. Paiardini, A. P. Barry, S. I. Staprans, G. Silvestri, and D. L. Sodora. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J. Immunol. 179:3047-3056. [DOI] [PubMed] [Google Scholar]
- 33.Monceaux, V., L. Viollet, F. Petit, M. C. Cumont, G. R. Kaufmann, A. M. Aubertin, B. Hurtrel, G. Silvestri, and J. Estaquier. 2007. CD4+ CCR5+ T-cell dynamics during simian immunodeficiency virus infection of Chinese rhesus macaques. J. Virol. 81:13865-13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthukumar, A., D. Zhou, M. Paiardini, A. P. Barry, K. S. Cole, H. M. McClure, S. I. Staprans, G. Silvestri, and D. L. Sodora. 2005. Timely triggering of homeostatic mechanisms involved in the regulation of T-cell levels in SIVsm-infected sooty mangabeys. Blood 106:3839-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okoye, A., M. Meier-Schellersheim, J. M. Brenchley, S. I. Hagen, J. M. Walker, M. Rohankhedkar, R. Lum, J. B. Edgar, S. L. Planer, A. Legasse, A. W. Sylwester, M. Piatak, Jr., J. D. Lifson, V. C. Maino, D. L. Sodora, D. C. Douek, M. K. Axthelm, Z. Grossman, and L. J. Picker. 2007. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J. Exp. Med. 204:2171-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onanga, R., S. Souquiere, M. Makuwa, A. Mouinga-Ondeme, F. Simon, C. Apetrei, and P. Roques. 2006. Primary simian immunodeficiency virus SIVmnd-2 infection in mandrills (Mandrillus sphinx). J. Virol. 80:3301-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrowski, M. A., S. J. Justement, A. Catanzaro, C. A. Hallahan, L. A. Ehler, S. B. Mizell, P. N. Kumar, J. A. Mican, T. W. Chun, and A. S. Fauci. 1998. Expression of chemokine receptors CXCR4 and CCR5 in HIV-1-infected and uninfected individuals. J. Immunol. 161:3195-3201. [PubMed] [Google Scholar]
- 38.Paiardini, M., B. Cervasi, J. C. Engram, S. N. Gordon, N. R. Klatt, A. Muthukumar, J. Else, R. S. Mittler, S. I. Staprans, D. L. Sodora, and G. Silvestri. 2009. Bone marrow-based homeostatic proliferation of mature T cells in nonhuman primates: implications for AIDS pathogenesis. Blood 113:612-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paiardini, M., B. Cervasi, B. Sumpter, H. M. McClure, D. L. Sodora, M. Magnani, S. I. Staprans, G. Piedimonte, and G. Silvestri. 2006. Perturbations of cell cycle control in T cells contribute to the different outcomes of simian immunodeficiency virus infection in rhesus macaques and sooty mangabeys. J. Virol. 80:634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paiardini, M., J. Hoffman, B. Cervasi, A. M. Ortiz, F. Stroud, G. Silvestri, and M. E. Wilson. 2009. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav. Immun. 23:286-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paiardini, M., I. Pandrea, C. Apetrei, and G. Silvestri. 2009. Lessons learned from the natural hosts of HIV-related viruses. Annu. Rev. Med. 60:485-495. [DOI] [PubMed] [Google Scholar]
- 42.Pandrea, I., C. Apetrei, J. Dufour, N. Dillon, J. Barbercheck, M. Metzger, B. Jacquelin, R. Bohm, P. A. Marx, F. Barre-Sinoussi, V. M. Hirsch, M. C. Muller-Trutwin, A. A. Lackner, and R. S. Veazey. 2006. Simian immunodeficiency virus SIVagm.sab infection of Caribbean African green monkeys: a new model for the study of SIV pathogenesis in natural hosts. J. Virol. 80:4858-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandrea, I., C. Apetrei, S. Gordon, J. Barbercheck, J. Dufour, R. Bohm, B. Sumpter, P. Roques, P. A. Marx, V. M. Hirsch, A. Kaur, A. A. Lackner, R. S. Veazey, and G. Silvestri. 2007. Paucity of CD4+ CCR5+ T cells is a typical feature of natural SIV hosts. Blood 109:1069-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pandrea, I., D. L. Sodora, G. Silvestri, and C. Apetrei. 2008. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 29:419-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandrea, I. V., R. Gautam, R. M. Ribeiro, J. M. Brenchley, I. F. Butler, M. Pattison, T. Rasmussen, P. A. Marx, G. Silvestri, A. A. Lackner, A. S. Perelson, D. C. Douek, R. S. Veazey, and C. Apetrei. 2007. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J. Immunol. 179:3035-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2002. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 48.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagnier, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 72:3872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scholzen, T., and J. Gerdes. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311-322. [DOI] [PubMed] [Google Scholar]
- 50.Silvestri, G., A. Fedanov, S. Germon, N. Kozyr, W. J. Kaiser, D. A. Garber, H. McClure, M. B. Feinberg, and S. I. Staprans. 2005. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 79:4043-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silvestri, G., M. Paiardini, I. Pandrea, M. M. Lederman, and D. L. Sodora. 2007. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Invest. 117:3148-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 53.Sodora, D. L., and G. Silvestri. 2008. Immune activation and AIDS pathogenesis. AIDS 22:439-446. [DOI] [PubMed] [Google Scholar]
- 54.Sousa, A. E., J. Carneiro, M. Meier-Schellersheim, Z. Grossman, and R. M. Victorino. 2002. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J. Immunol. 169:3400-3406. [DOI] [PubMed] [Google Scholar]
- 55.Stein, D. S., J. A. Korvick, and S. H. Vermund. 1992. CD4+ lymphocyte cell enumeration for prediction of clinical course of human immunodeficiency virus disease: a review. J. Infect. Dis. 165:352-363. [DOI] [PubMed] [Google Scholar]
- 56.Sumpter, B., R. Dunham, S. Gordon, J. Engram, M. Hennessy, A. Kinter, M. Paiardini, B. Cervasi, N. Klatt, H. McClure, J. M. Milush, S. Staprans, D. L. Sodora, and G. Silvestri. 2007. Correlates of preserved CD4+ T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J. Immunol. 178:1680-1691. [DOI] [PubMed] [Google Scholar]
- 57.VandeWoude, S., and C. Apetrei. 2006. Going wild: lessons from naturally occurring T-lymphotropic lentiviruses. Clin. Microbiol. Rev. 19:728-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Z., B. Metcalf, R. M. Ribeiro, H. McClure, and A. Kaur. 2006. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J. Virol. 80:2771-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss, L., N. Haeffner-Cavaillon, M. Laude, J. Gilquin, and M. D. Kazatchkine. 1989. HIV infection is associated with the spontaneous production of interleukin-1 (IL-1) in vivo and with an abnormal release of IL-1 alpha in vitro. AIDS 3:695-699. [DOI] [PubMed] [Google Scholar]