Abstract
Effective HIV-specific T-cell immunity requires the ability to inhibit virus replication in the infected host, but the functional characteristics of cells able to mediate this effect are not well defined. Since Gag-specific CD8 T cells have repeatedly been associated with lower viremia, we examined the influence of Gag specificity on the ability of unstimulated CD8 T cells from chronically infected persons to inhibit virus replication in autologous CD4 T cells. Persons with broad (≥6; n = 13) or narrow (≤1; n = 13) Gag-specific responses, as assessed by gamma interferon enzyme-linked immunospot assay, were selected from 288 highly active antiretroviral therapy (HAART)-naive HIV-1 clade C-infected South Africans, matching groups for total magnitude of HIV-specific CD8 T-cell responses and CD4 T-cell counts. CD8 T cells from high Gag responders suppressed in vitro replication of a heterologous HIV strain in autologous CD4 cells more potently than did those from low Gag responders (P < 0.003) and were associated with lower viral loads in vivo (P < 0.002). As previously shown in subjects with low viremia, CD8 T cells from high Gag responders exhibited a more polyfunctional cytokine profile and a stronger ability to proliferate in response to HIV stimulation than did low Gag responders, which mainly exhibited monofunctional CD8 T-cell responses. Furthermore, increased polyfunctionality was significantly correlated with greater inhibition of viral replication in vitro. These data indicate that enhanced suppression of HIV replication is associated with broader targeting of Gag. We conclude that it is not the overall magnitude but rather the breadth, magnitude, and functional capacity of CD8 T-cell responses to certain conserved proteins, like Gag, which predict effective antiviral HIV-specific CD8 T-cell function.
Studies aimed at correlating overall quantitative differences in breadth or magnitude of the gamma interferon (IFN-γ)-positive HIV-specific CD8 T-cell response and plasma HIV viral loads have failed to show an association with control of viremia (2, 18). However, multiple studies (10, 12-15, 18, 22, 29) have shown that broadly directed and/or dominant HIV-specific CD8 T-cell responses against the Gag protein, as measured by IFN-γ enzyme-linked immunospot (ELISPOT) assay, are associated with lower viremia in chronic HIV-1 infection. In contrast, non-Gag-specific T-cell responses, as shown in some studies, did not contribute to immune control. Indeed, more broadly directed CD8 T-cell responses directed to the Env protein have been associated with elevated viremia (15). The functional mechanism underlying enhanced viral control by Gag-specific CD8 T-cell responses has not been determined.
One potential explanation for enhanced antiviral pressure by Gag-specific but not other virus-specific CD8 T-cell responses may be differences in the fitness cost associated with escape mutations within the highly conserved Gag protein compared to that of other viral proteins (5, 23, 27). Alternatively, the maturation phenotype and functional quality of HIV-specific CD8 T cells may be the more critical predictors of the effectiveness of a virus-specific response (1, 4, 7, 15, 20, 25). In addition to the secretion of IFN-γ, CD8 T cells exhibit a spectrum of additional antiviral functions, including cytolysis, cell proliferation, and production of cytokines and chemokines. The capacity of CD8 T cells to secrete multiple cytokines following stimulation with HIV peptides is also associated with long-term nonprogressive infection, although subsequent studies have argued that polyfunctionality may simply correlate with reduced antigen stimulation rather than being a direct mediator of viral control (4, 19, 28, 34). Increased expression of the negative immunoregulatory molecule PD-1 on HIV-specific CD8 T cells is associated with higher viral loads (8, 21, 30). Finally, high HIV-specific CD8 T-cell proliferative capacity is associated with lower HIV viral loads (9). However, a direct link between HIV-specific antiviral efficacy and any specific functional capacity has yet to be established.
Following the resolution of acute HIV-1 infection, HIV-specific CD8 T-cell responses reduce viral replication to a set point, which varies dramatically among individuals but is a strong predictor of the rate of HIV disease progression (17). It is therefore plausible that more potent antiviral CD8 T-cell responses, at set point, that are able to contain viral replication more aggressively may provide enhanced control of disease progression. However, to date, the majority of studies aimed at defining differences in the viral suppressive properties of protective HIV-1-specific CD8 T-cell responses have focused narrowly either on single-peptide-specific cytotoxic T lymphocyte (CTL) clones or cell lines (7) or on specific subpopulations of study subjects such as “elite” controllers (25). Studies examining the relationship between in vitro inhibition of viral replication over a broad range of viral loads and antigen specificities have not been performed. Furthermore, little work has focused on defining the antiviral properties of HIV-specific CD8 T-cell responses in clade C infection (33).
Thus, to address the potential role of antigen specificity in the antiviral properties of HIV-specific CD8 T-cell responses, we compared the phenotypic and functional characteristics of bulk CD8 T cells in a group of untreated chronically clade C-infected persons that broadly targeted Gag-specific responses (≥6 Gag-specific responses) to those of subjects that had very narrow or absent Gag-specific responses (≤1 Gag-specific response). Importantly, the two groups were selected such that total CD4 cell counts and total magnitude of HIV-specific CD8 T-cell responses by IFN-γ ELISPOT assay were matched. Our results confirm that, for the same level of CD4 cell count and overall magnitude of HIV-specific CD8 T-cell responses, subjects whose CD8 T-cell responses are dominantly and broadly directed against the Gag protein exhibit lower plasma viral loads than do subjects who target this protein less. Furthermore, we demonstrate that this enhanced viral control is associated with an enhanced ability of isolated CD8 T cells to inhibit replication of a heterologous HIV-1 strain in autologous CD4 cells in vitro, enhanced ability to proliferate in the presence of cognate antigen, and a more polyfunctional cytokine response, but not with a difference in the maturation status of HIV-specific CD8 T cells. These data indicate that the specificity of the CD8 T-cell response to HIV is important for viral control and that it is a distinct polyfunctional phenotype of CD8 T cells that is able to proliferate and secrete antiviral cytokines, which is indicative of effective antiviral CD8 T-cell function.
MATERIALS AND METHODS
Study subjects.
Individuals (n = 288) with self-reported untreated chronic HIV-1 clade C infection were recruited through three clinics in KwaZulu Natal Province, Durban, South Africa. Mean viral load and mean CD4 cell counts were 4.80 ± 0.87 RNA copies/ml (log10) and 329 ± 195 cells/μl, respectively. For further more-detailed functional analyses, we selected 26 individuals with a mean viral load of 4.78 ± 0.86 RNA copies/ml (log10) and mean CD4 counts of 356 ± 158 cells/μl. Informed consent was obtained from all participating individuals, and the study was approved by institutional review boards at the University of KwaZulu Natal and Massachusetts General Hospital.
HLA tissue typing.
High- and intermediate-resolution HLA class I typing was performed by sequence-specific PCR according to standard procedures (14). DNA was extracted from peripheral mononuclear cells (PBMCs) using a Puregene DNA isolation kit for blood (Gentra Systems).
Synthetic HIV-1 peptides.
A panel of 410 overlapping peptides (18-mers with a 10-amino-acid overlap) spanning the 2001 C clade consensus sequence (15) was synthesized on an automated peptide synthesizer (MBS 396; Advanced ChemTech).
ELISPOT assay.
IFN-γ ELISPOT assays were performed as previously described (2). Briefly, isolated PBMCs were plated at a concentration of 50,000 to 100,000 cells per well in 96-well polyvinylidene plates (MAIP S45; Millipore) that had been precoated with 0.5 g/ml of anti-IFN-γ monoclonal antibody 1-DIK (Mabtech, Stockholm, Sweden). Peptides were added at a final concentration of 2 μg/ml. Four wells containing PBMCs and R10 medium alone were used as negative controls along with two positive controls containing phytohemagglutinin (PHA). Plates were incubated overnight at 37°C, 5% CO2 and developed as described previously (11). The numbers of spots per well were counted using an automated ELISPOT plate reader (AID EliSPOT reader system; Autoimmune Diagnostika GmbH, Strassberg, Germany), and the number of specific spot-forming cells (SFC), was calculated by subtracting the negative-control wells (mean plus 3 standard deviations). A total of 55 SFC/106 PBMC or greater after subtraction of background was considered positive.
Viral inhibition assay.
Inhibition of viral replication by isolated CD8 T cells was assessed in a previously established assay system (32, 33). Bulk CD8 T cells were isolated from previously frozen PBMCs by positive selection with anti-CD8 antibody-coated magnetic beads (Dynal) (25) and kept for 3 days in culture without adding any mitogens or cytokines. CD8 T-cell-depleted PBMCs were stimulated in interleukin-2 (IL-2; 50 U/ml)-containing medium with the bispecific anti-CD3:anti-CD8 monoclonal antibody, which selectively activates and expands CD4 T lymphocytes while simultaneously depleting all remaining CD8 T cells (31). These CD4+ lymphocytes were then infected at day 3 with an X4 tropic HIV-1 laboratory strain, NL4-3, at a multiplicity of infection (MOI) of 0.1 for 4 h at 37°C, washed twice, resuspended in medium, and plated at 1 × 105 cells per well onto a 96-well plate. To assess viral inhibition, effector cells then were added at a ratio of 1:1 in a total 200 μl of medium in the presence of IL-2 at 50 U/ml. At 2- to 3-day intervals, the cocultures were fed by removing and replacing one-half of the culture supernatant with fresh medium. The removed supernatant was cryopreserved for later p24 antigen quantification by a standard quantitative enzyme-linked immunosorbent assay (commercial kit from PerkinElmer Life Sciences, Inc.). Log inhibition units were calculated by subtracting log10 p24 values with CD8 T cells from log10 p24 values without CD8 T cells at the peak of inhibition between days 5 and 7. As controls, we measured the inhibitory capacity of CD8 T cells from 10 HIV-negative subjects, in whom no significant inhibition was detectable (mean log p24 inhibition, 0.05 ± 0.1 [0 to 0.26] pg/ml). Inhibition above the background (mean plus 3 standard deviations) was considered significant (0.35 log10 p24 [pg/ml]). Two additional subjects, who were initially screened, were excluded from the final analysis because growth of autologous virus in the uninfected CD4 T-cell control population was detectable, and therefore, the assays could not be interpreted.
Flow cytometric detection of antigen-induced intracellular cytokine secretion and maturation phenotype.
Pools of overlapping peptides spanning the HIV-1 clade C proteins Gag, Pol, Env, Nef, and the accessory and regulatory proteins (Acc/Reg) were used as antigens. Intracellular staining assays were carried out as described previously (14, 15). Briefly, frozen PBMCs were thawed and then incubated with 2 μg/ml of peptide; anti-CD28 and anti-CD49d antibodies (BD Biosciences) were added, as was anti-CD107a where indicated. Cells were incubated for 1 h at 37°C and 5% CO2, followed by an additional 5 h in the presence of a Golgi inhibitor (brefeldin A; Sigma). Cells were then stained with a dead cell dye (Live/Dead cell dye; Invitrogen Corporation) followed by staining with surface antibodies (CD3, CD8, CCR7, and CD45RA; BD Biosciences) at 4°C for 30 min. After two washes, cells were fixed and permeabilized using the Caltag cell fixation and permeabilization kit (Caltag, Burlingame, CA). Subsequently, intracellular antigen staining was performed using anti-IFN-γ and anti-IL-2 specific monoclonal antibodies (BD Biosciences). Cells were then washed and analyzed on an LSRII flow cytometer (BD Biosciences). Control conditions were established by the use of autologous unstimulated PBMCs, and PBMCs stimulated with phorbol-12-myristate-13-acetate-ionomycin (PMA/I) were used as positive controls.
Proliferation assay.
The proliferative capacity of HIV-specific CD8 T cells was evaluated by flow cytometry. Pools of overlapping peptides spanning the HIV-1 clade C proteins Gag, Pol, Env, Nef, and Acc/Reg were used as antigens. PBMCs were stained with 0.35 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Breda, Netherlands) for 7 min at 37°C and then stimulated for 7 days with 2 μg/ml peptide, PHA, or medium alone. After labeling with anti-CD8, anti-CD3, and anti-CD4 antibodies, PBMCs were fixed in 1% paraformaldehyde and analyzed on an LSRII flow cytometer (BD Biosciences).
Statistical analyses.
Unpaired t tests and Spearman's rank correlation tests were performed using GraphPad Prism version 4.0a. All tests were two-tailed, and P values of <0.05 were considered significant. Multiple-regression analysis was performed using SAS procedure PROC REG with option STEPWISE.
RESULTS
High Gag responders exhibit lower plasma viral loads.
CD8 T-cell responses directed to the HIV Gag protein have been associated with lower viremia (10, 12-15, 18, 22, 29); however, the mechanism for this control has not been identified. In order to better define the antiviral properties associated with HIV-specific CD8 T cells of differing specificities, we initially measured the relative targeting of Gag as well as the breadth of Gag-specific responses in PBMCs of 288 HIV-1 clade C-infected individuals, as described before (15), with an IFN-γ ELISPOT assay using overlapping peptides spanning all expressed HIV proteins.
In this cohort, the mean total magnitude of CD8 T-cell responses against the entire expressed HIV-1 proteome was 6,318 ± 5,634 (SFU/106 cells), whereas the mean total breadth to all proteins was 8.5 ± 5.6, similar to other studies performed on clade B infection (2). The total magnitude of Gag-specific CD8 T-cell responses ranged from 0 to 10,810 SFC/106 PBMCs, with a mean of 1,870 ± 1,997 SFC/106 PBMCs. The breadth of Gag responses ranged from 0 to 13 targeted Gag peptides, with a mean of 2.45 ± 2.35 (Fig. 1).
FIG. 1.

Total breadth of IFN-γ-positive Gag-specific CD8 T cells (mean, 2.45 ± 2.35) in a cohort of 288 HIV-1 clade C-infected Zulu/Xhosa, as measured by ELISPOT assay with overlapping peptides (n = 410) to all expressed proteins. Subjects were selected from the upper box (dotted line), representing high Gag responders, and the lower box (dashed line), representing low Gag responders, based on sample availability.
From this cohort, we next selected two groups of individuals based on the overall breadth of HIV-specific CD8 T-cell responses: those with broadly directed responses against Gag, and individuals with narrow/no Gag-specific CD8 T-cell responses. The groups included 13 individuals with ≥6 Gag-specific responses (top 10%, mean breadth, 7.76 ± 2.2; mean magnitude, 3,342 ± 1,162 SFC/106 PBMCs) and 13 individuals with ≤1 response against Gag (mean breadth, 0.77 ± 0.43; mean magnitude, 1,161 ± 1,294 SFC/106 PBMCs), therefore differing significantly in Gag-specific breadth and magnitude.
These groups were selected such that they were not significantly different based on two additional variables: mean total CD4 counts (intended to avoid the selection of individuals with differential disease progression) and total magnitude of HIV-specific CD8 T-cell responses. Thus, high Gag responders had a mean of 395.8 ± 113.7 CD4 T cells/μl, and low Gag responders had a mean of 316.5 ± 189 CD4 T cells/μl (Fig. 2A) (P = 0.207). Furthermore, high Gag responders exhibited a mean total magnitude of HIV-specific CD8 T-cell responses of 11,927 ± 4,587 SFU/106 PBMCs, and low Gag responders had a mean of 9,956 ± 3,302 spot-forming units (SFU)/106 PBMCs (Fig. 2B) (P = 0.22). The relative contributions of Gag-specific responses to the total magnitude of HIV-specific responses were 31% ± 14% in the high Gag responders and 11.5% ± 12% in the low Gag responders (P < 0.001).
FIG. 2.
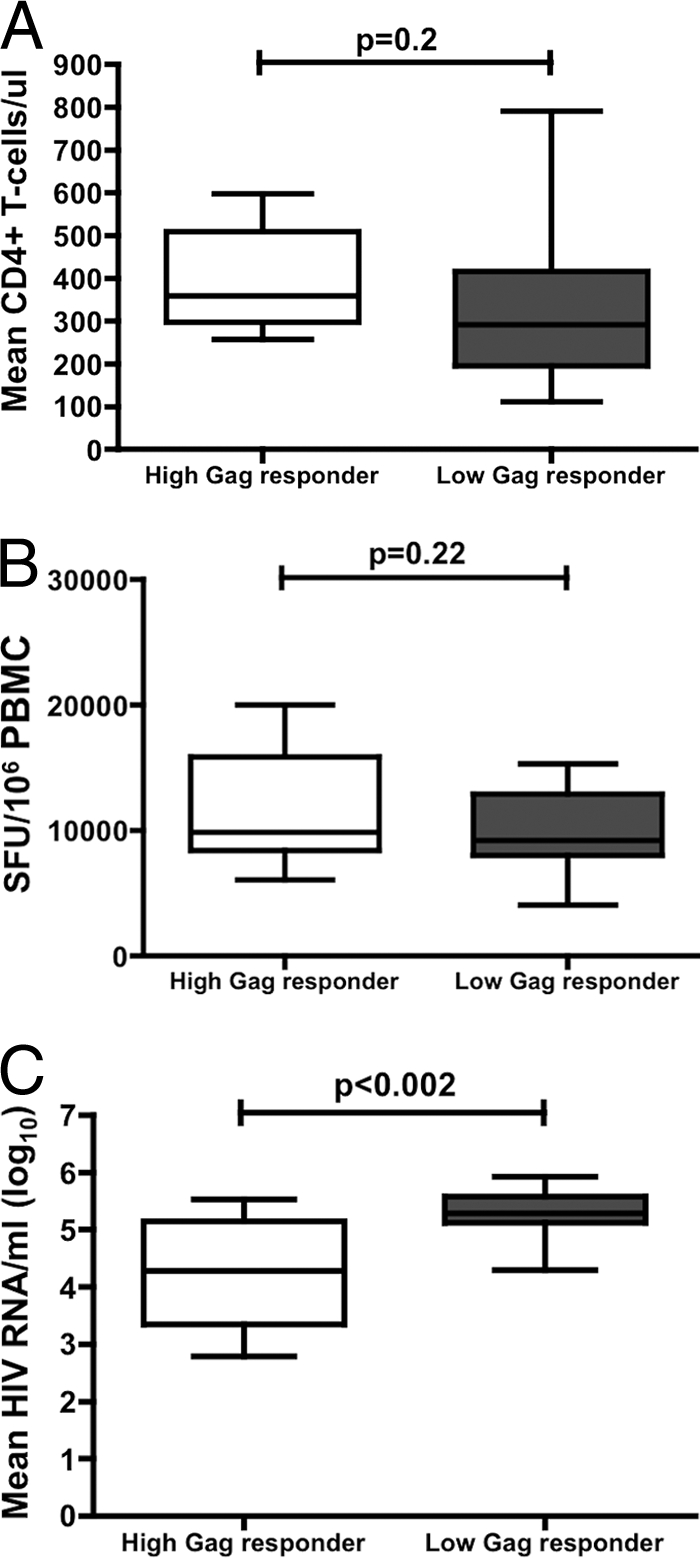
Characteristics of high (n = 13) and low (n = 13) Gag responders. (A) The mean total CD4 T-cell counts were measured by flow cytometry (B) HIV-specific CD8 T-cell responses in isolated PBMCs measured by IFN-γ ELISPOT assay in high and low Gag-responders. (C) The total magnitude of plasma HIV-1 RNA measured by PCR.
Consistent with previous observations of clade C virus infection (15), we observed a significant difference in mean HIV RNA levels between high and low Gag responders (mean log viral loads [number of copies/ml] were 4.27 ± 0.92 and 5.29 ± 0.42, respectively; P < 0.002) (Fig. 2C), thus confirming an association between breadth of Gag-specific CD8 T-cell responses and lower plasma viral loads in the two selected groups, despite comparable CD4 T-cell counts and comparable overall magnitude of HIV-specific CD8 T-cell responses.
CD8 T cells from high Gag responders can potently suppress HIV replication in vitro.
We next investigated whether individuals matched for CD4 count and total magnitude of IFN-γ ELISPOT responses, but who differed in breadth of Gag-specific responses, varied in their ability to suppress viral replication in vitro. For these experiments, we used a previously described method (7, 25) which allows one to measure the ability of unstimulated and unexpanded bulk CD8 T cells from HIV-infected persons to inhibit virus replication in autologous CD4 T cells in vitro. The assay replicates specific steps of antigen processing and presentation known to influence recognition of infected cells by HIV-specific CTLs that are not assessed in standard IFN-γ ELISPOT assays. Although outgrowth of autologous virus in CD4+ cells can be a confounding factor in these chronically infected individuals, we were able to use controlled inoculums of a primary HIV isolate to infect these CD4 T cells, as autologous virus outgrowth was undetectable before day 7 in culture (data not shown), thereby allowing us to measure the ability of CD8 T cells to inhibit a heterologous virus, in this case a well-characterized clade B primary isolate.
In most of these chronically infected subjects, inhibition of heterologous virus replication was observed, whereas this was not the case in uninfected controls. CD8 T cells from infected individuals with broad Gag-specific CD8 T-cell responses suppressed viral replication more potently than did CD8 T cells from the comparison group with low Gag responses (mean log p24 inhibition [pg/ml], 1.38 ± 0.74 versus 0.49 ± 0.60; P < 0.003) (Fig. 3), providing clear evidence for the pivotal role of CD8 T cells targeting Gag, rather than total magnitude of HIV-specific CD8 T cells, in controlling viral replication in vitro. In contrast, the amount of viral inhibition in vitro did not correlate with total mean CD4 counts or mean plasma HIV RNA levels (Spearman's r = 0.06 and −0.19, respectively; both not significant).
FIG. 3.
(A) Representative results from three high Gag responders and four low Gag responders, showing inhibition of viral replication over 7 days of culture. Open squares represent CD4 T cells infected with NL4-3; closed circles represent CD4/CD8 cocultures; open circles represent CD4 T cells without superinfection with NL4-3. (B) Capacity of CD8 T cells from high Gag responders (n = 13) versus low Gag responders (n = 13) to inhibit replication of HIV-1 wild-type virus in autologous CD4 T cells. Log10 p24 antigen concentration was measured in supernatants taken from cocultures of CD4 T cells infected with a reference strain of HIV-1 (NL4-3) to which autologous isolated CD8 T cells were added. Values shown are from day 5 or 7, depending on which day showed peak differences in p24 inhibition. Lines represent mean values ± standard deviation (SD).
We also wished to determine whether Gag-independent breadth alone would account for better control of viral replication in vitro. We therefore evaluated the relationship between breadth of all HIV-specific responses in the group with ≤1 Gag response with their capacity to inhibit viral replication, but we were unable to detect a correlation (Spearman's r = 0.26; P = 0.39), arguing against a protein-independent association between breadth and inhibition of viral replication. Together, these data indicate that circulating HIV-specific CD8 T cells can markedly inhibit replication of a heterologous HIV strain in primary lymphocytes in vitro and that the broad Gag-specific responses are central in this regard, in an assay that is sensitive to critical steps in antigen processing/presentation.
Enhanced inhibitory CD8 T-cell function in high Gag responders is observed in the presence and absence of protective HLA alleles.
Particular HLA class I alleles are associated with enhanced control of viremia, while others are associated with rapid disease progression (14). To investigate whether elevated antiviral activity among the high Gag responders was simply associated with an enrichment of protective HLA class I alleles, we compared both groups for the presence of HLA alleles B*57, B*5801, and B*8101, as these alleles are significantly associated with lower pVL in subtype C infection after correction for HLA linkage disequilibrium (14). Three individuals from the high-Gag-responder group and four subjects from the low-Gag-responder group expressed one of the alleles associated with protection, which strongly suggests that it is the targeting of Gag rather than the expression of a protective allele which associates with enhanced antiviral activity of CD8 T cells in vitro (see Table S1 in the supplemental material).
HIV-specific CD8 T cells from high Gag responders exhibit a more polyfunctional cytokine profile.
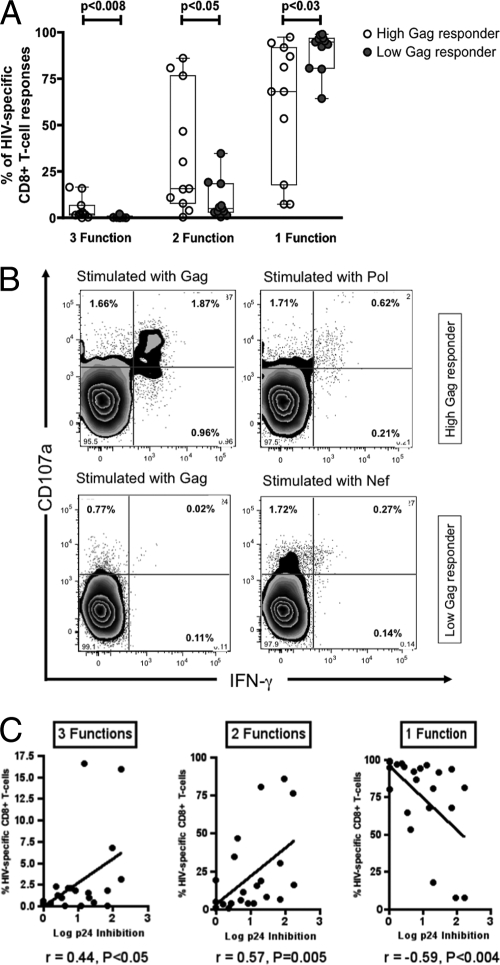
It has been shown that the frequency and proportion of the HIV-specific T-cell responses with highest functionality are inversely correlated with viral load in subjects with progressive HIV infection (4). These results suggest that the quality, and not quantity, of HIV-specific CD8 T cells is a stronger predictor of protection from disease progression. To investigate a potential association between functionality and Gag specificity in this untreated clade C virus-infected cohort, we measured intracellular cytokine secretion of CD8 T cells by flow cytometry upon stimulation with pools of HIV-1 peptides representing the HIV proteins Gag, Pol, Env, Nef, and Acc/Reg. There were no differences in the hierarchies of IFN-γ and IL-2 secretion between the two groups, with IFN-γ secreted more frequently than IL-2. However, individuals with high Gag responses showed a trend toward lower levels of CD107a expression (1.64% versus 4.94%; P = 0.08). The latter might reflect a reduced activation/stimulation status in high Gag responders due to lower levels of plasma HIV RNA in these individuals (3). Yet, when comparing the cumulative polyfunctionality of the HIV-specific CD8 T-cell response upon stimulation with HIV peptide pools, high Gag responders exhibited significantly more 3-function (percentage of CD8 T cells, 4.75 ± 5.95% versus 0.63 ± 0.62%; P < 0.008) and 2-function responses (percentage of CD8 T cells, 34.03 ± 32.87% versus 9.24 ± 10.54%; P < 0.05) than did low Gag responders, suggesting that these individuals have a qualitatively different HIV-specific CD8 T-cell response from that of low Gag responders (Fig. 4A and B). Interestingly, low Gag responders exhibited significantly more monofunctional CD8 T-cell responses, as a potential sign of relative exhaustion (percentage of HIV-specific CD8 T cells, 90.12 ± 10.71% versus 61.22 ± 34.97%; P < 0.03) (28). These data show that high Gag responders exhibit more polyfunctional responses than do low/narrow Gag responders.
FIG. 4.
(A) Ability of HIV-specific CD8 T cells from high Gag responders (n = 11) versus low Gag responders (n = 11) to secrete IFN-γ and IL-2 and to degranulate upon stimulation with HIV peptide pools. Monofunctional cells typically included CD8 T cells that degranulated (upregulated CD107a expression), and double-positive cells included those that degranulated and secreted IFN-γ, while the triple-positive cells also were primed to secrete IL-2. (B) Representative results from a high Gag and a low Gag responder showing CD107a expression and IFN-γ secretion after stimulation with HIV peptide pools (Gag, Pol, and Env). The high Gag responder exhibits type 2 functional responses after stimulation with Gag and Pol, whereas the low Gag responder reacts just to the Env peptide pool with CD107a expression. (C) Correlation between inhibitory capacity and polyfunctionality of HIV-specific CD8 T cells. For type 3 functions, Spearman's r = 0.44 and P < 0.05; for type 2 functions, Spearman's r = 0.57 and P = 0.005; for type 1 function, Spearman's r = −0.59 and P < 0.004.
Interestingly, the frequency of CD8 T cells with 3 plus 2 functions in each individual was significantly correlated with the capacity of bulk CD8 T cells to inhibit viral replication in vitro (Spearman's r = 0.44 and 0.57, respectively; P < 0.05 and P = 0.005, respectively) (Fig. 4C). In contrast, the frequency of monofunctional T cells was inversely correlated with the inhibitory capacity of bulk CD8 T cells (Spearman's r = −0.59; P < 0.004) (Fig. 4C). These data strongly suggest that polyfunctional T-cell responses retain the capacity to inhibit viral replication, whereas potentially exhausted monofunctional responses are a predictor of ineffective CD8 T-cell control over viral replication.
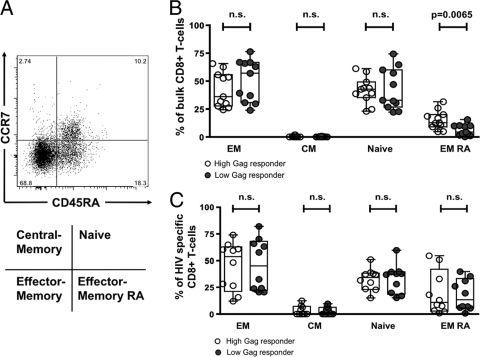
Bulk CD8 T cells from high Gag responders show increased frequencies of terminally differentiated effector CD8 T cells.
It has been suggested that the inability of virus-specific CD8 T cells to fully mature into effector cells may be associated with the failure to control viremia in chronic HIV-1 infection (6). A recent report showed that fully differentiated HIV-1-specific effector CD8 T cells were more frequently detectable in individuals that control HIV spontaneously compared to normal progressors (1). In particular the differentiation from effector memory cells to terminally differentiated effector cells may be interrupted in chronic progressive HIV-1 infection (6, 26). We therefore compared the frequencies of terminally differentiated effector cells among our 2 populations of subjects, allowing us to examine these relationships over a wide range of viral loads. As a first step we examined maturation phenotype, based on CCR7 and CD45RA expression (Fig. 5A) in unstimulated bulk CD8 T cells from 10 high Gag and 10 low Gag responders. High Gag-responders exhibited a significantly higher frequency of fully differentiated effector CD8 T cells (CD45RA+/CCR7−) in bulk CD8 T-cell compared to low Gag responders (Fig. 5B) (15.22 ± 8.39 versus 6.29 ± 4.95% of CD8 T cells, respectively; P = 0.0065). However, no difference was observed when comparing the frequencies of effector memory (CD45RA−/CCR7−), central memory (CD45RA−/CCR7+), and naïve (CD45RA+/CCR7+) T cells among the two patient populations (Fig. 5B). Thus, elevated levels of terminally differentiated effector CD8 T cells, but not other memory subsets, are correlated with enhanced control of viral replication.
FIG. 5.
(A) Representative flow plot with gating strategy. (B and C) Phenotype of bulk CD8 T cells (B) and HIV-specific CD8 T cells (C) comparing high Gag responders (n = 10) versus low Gag responders (n = 10). EM, effector memory (CD45RA−/CCR7−); CM, central memory (CD45RA−/CCR7+); naïve (CD45RA+/CCR7+); EM RA, effector memory RA (CD45RA+/CCR7−). n.s., not significant.
In order to further investigate whether the increased frequency of fully differentiated effector CD8 T cells was associated with potent cytolytic HIV-specific CD8 T cells, we used five pools of overlapping peptides corresponding to the individual HIV-1 proteins Gag, Pol, Env, and Nef, as well as the Acc/Reg pool, as antigenic stimuli for detection of CD107a expression, a marker of effector cell degranulation, in the same individuals as described above. In order to compare the distributions of maturation phenotypes of all HIV-specific CD8 T cells of each given individual, we calculated the mean percentage of CD8 T cells for each maturation type (effector memory, central memory, naïve, and effector memory RA). However, no difference was detectable in the differentiation status of HIV-1-specific CD8 T cells between high Gag and low Gag responders (Fig. 5C). We then investigated whether Gag-specific CD8 T cells in high Gag responders exhibit a distinct differentiation status compared to HIV-specific CD8 T cells in low Gag responders. Yet again, no differences in the differentiation status of Gag-specific CD8 T-cell responses were observed between the two groups (Fig. 5C). Thus, overall, although high Gag responders show increased levels of terminally differentiated CD8 T cells in bulk populations, no difference was observable in the maturation status of total HIV protein-specific populations, arguing against an association between differentiation status and ability of CD8 T cells to control HIV infection in these two groups.
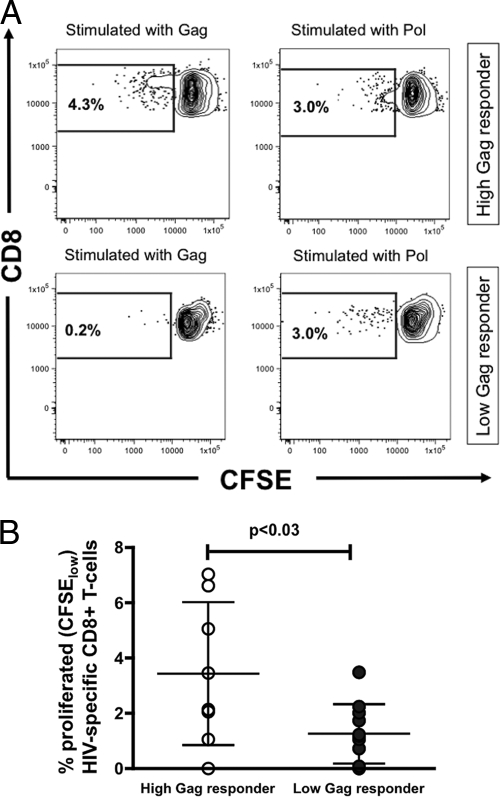
HIV-specific CD8 T cells from high Gag responders proliferate more robustly.
Previous studies (9, 20) have shown that increased proliferative capacity of CD8 T cells is associated with long-term control of plasma HIV RNA. Therefore, to investigate whether differences exist between the proliferative capacities of high and low Gag responders, the proliferative capacities of CD8 T cells were compared among the two groups following stimulation with clade C HIV peptide pools (Fig. 6A and B). In this chronically infected cohort with comparable CD4 T-cell levels, high Gag responders proliferated more extensively following stimulation (see Fig. S1 in the supplemental material) than did CD8 T cells from individuals with low Gag responses (percentage of CFSElow CD8 T cells, 3.43 ± 2.35% versus 1.25 ± 1.07%; P < 0.03). The observation that the proliferation in high Gag responders was overall driven by Gag-specific responses supports their general functional superiority despite the limited numbers of subjects examined.
FIG. 6.
(A) Representative results from a high Gag responder and a low Gag responder showing proliferated (CFSElow) CD8 T cells after stimulation with HIV peptide pools (Gag and Pol). CD8 T cells from the high Gag responders proliferated after stimulation with Gag and Pol, whereas CD8 T cells from the low Gag responder reacted just to the Pol peptide pool with proliferation. (B) Proliferative capacity of HIV-specific CD8 T cells from high Gag responders (n = 8) versus low Gag responders (n = 11) upon stimulation with HIV peptide pools, as measured by CFSE intensity by flow cytometry. Lines represent mean values ± SD.
Total breadth and viral suppressive capacity of HIV-specific CD8 T-cell responses predict levels of plasma viral load.
We next examined the relationship between the functional properties of CD8 T cells and plasma viral loads. In particular, we were interested in identifying which marker would predict plasma HIV RNA levels the best. We therefore performed a multiple-regression model analysis including total and Gag-specific breadth, total and Gag-specific magnitude, polyfunctionality, proliferation and virus suppressive capacity of CD8 T cells, and plasma viral load. The analysis was performed on data from 16 subjects, for whom data were available for all variables. Both total breadth (including Gag breadth) and p24 inhibition represented the 2 variables that predicted viral load control significantly (P < 0.001 and P = 0.03, respectively), while the other variables were not significant predictors. The fact that antiviral capacity of CD8 T cells is associated with the breadth of Gag responses but also predicts plasma viral loads strongly supports the crucial role of Gag-specific CD8 T cells in viral control.
DISCUSSION
The HIV Gag protein is considered to be a critical antigen for inclusion in most CD8 T-cell-based vaccine candidates, because it is relatively conserved and, more importantly, because of the fact that the breadth of the CD8 T-cell response to Gag correlates strongly with lower plasma viral load (15, 16). However, relatively little is known about the underlying mechanism for this association. Thus, to begin to tease out the underlying mechanism accounting for Gag-specific CD8 T-cell control of viral replication, we performed in-depth CD8 T-cell functional studies of 26 subjects that either had broad Gag responses or had one or less Gag response from a cohort of 288 chronically HIV-1 clade C-infected patients. Furthermore, to minimize other potential confounding variables in this analysis, subjects were selected such that there were no statistically significant differences between the groups in terms of total magnitude of HIV-specific CD8 T-cell responses or total peripheral CD4 cell counts. Due to the critical dependency of viral load on Gag breadth, we were unable to identify subjects with differential breadth but similar viral load in our cohort. These subjects are likely rare or would potentially include subjects that have escaped from all Gag responses.
Using these matched patient groups, we were then able to examine the functional properties of isolated bulk CD8 T-cell populations from these individuals and, in particular, compare their abilities to inhibit HIV replication in vitro. We hypothesized that individuals that generate broad Gag responses, which are associated with enhanced control of HIV viral replication in vivo (15), mount more potent antiviral CD8 T-cell activity in vitro than do persons with narrow or no Gag responses. To verify this hypothesis, we compared the functional properties of unstimulated bulk CD8 T cells to suppress HIV-1 replication in autologous CD4 T cells in vitro.
Our data indicate that CD8 T cells from high Gag responders display a significantly stronger ability to suppress HIV replication in autologous CD4 T cells in an in vitro assay than do subjects with narrow or no Gag-specific responses, although both groups exhibit similar ranges of total HIV-specific CD8 T cells. This finding supports the conclusion that Gag-specific CD8 T cells are more effective in eliminating HIV-infected target cells and that it is the specificity rather than total magnitude of HIV-specific responses that is important for viral control. These observations may be explained by the fact that the remarkably conserved viral capsid protein is processed immediately after viral entry and that early presentation of Gag epitopes on infected cells facilitates targeting and lysis through cytotoxic T lymphocytes (CD8 T cells) specific for Gag epitopes before viral spread occurs (24). Additionally, breadth alone, excluding Gag responses, did not account for enhanced control of viral replication, supporting the notion that Gag-specific CD8 T-cell responses are critical for immune control. Although we were not able to examine whether particular Gag responses were associated with enhanced viral control or whether Gag responses function as a continuous variable, where incremental addition of these responses may lead to gradual increases in control over viral replication, it is plausible that having a narrow but well-focused Gag response would be equally potent in suppressing viral replication. In order to answer this important question, it would have been necessary to study a much larger number of subjects than was possible for the detailed analyses performed. However, the protective HLA class I alleles HLA B*57, B*5801, and B*8101 that target Gag in an immunodominant manner did not differ significantly in their distributions among the two groups (see Table S1 in the supplemental material), suggesting that specificity for this early highly conserved protein, rather than the particular HLA allele-restricted responses, dictates superior antiviral control.
In addition to enhanced antiviral function in vitro, CD8 T cells from high Gag responders displayed an overall more polyfunctional cytokine profile upon stimulation with HIV peptide pools, with significantly more 2- and 3-function T cells than observed in low Gag responders, whereas the latter exhibited significantly more monofunctional CD8 T-cell responses. Furthermore, the strength of the polyfunctional cytokine profile was directly correlated with the extent to which CD8 T cells mediated viral suppression in vitro. Consistent with this observation, individuals with high proportions of monofunctional CD8 T cells suppressed viral replication poorly in vitro. Although polyfunctional CD8 T-cell responses have been documented to be enriched in subjects with long-term nonprogressive infection (4), a recent study argues that the functional profile of an HIV-specific CD8 T cell is largely determined by the duration and intensity of antigenic exposure and is therefore primarily a consequence of the level of viremia (28). Thus, to eliminate the confounding effect of differential rates of disease progression on changes in T-cell function, study subjects were matched for CD4 T-cell counts, likely normalizing for the stage of disease progression and therefore for immune exhaustion (8). As had been previously shown, the high-Gag-responder group exhibited lower mean plasma virus levels for the same CD4 T-cell count, and therefore, we cannot exclude a potential effect of the reduced total antigen load on CD8 T-cell function, potentially suggesting that high Gag responders, with lower viral loads, have a more intact CD8 T-cell compartment that is able to respond rapidly and aggressively to autologous infected CD4 T cells. The association between polyfunctionality and viral suppression in vitro might therefore be different in individuals in whom responses other than Gag-specific CD8 T-cell responses are dominating. Conversely, the fact that subjects are matched for the magnitude of their HIV-specific CD8 T-cell response for the same CD4 T-cell count may reflect intrinsically superior antiviral capacity of Gag-specific CD8 T-cell responses in viral control.
Despite this superior functional profile of CD8 T cells in high Gag responders, no major differences in the differentiation status of CD8 T cells were observable by the expression of CD45RA and CCR7. Although high Gag responders exhibited a slightly higher frequency of terminally differentiated effector memory cells in their bulk population, this effect was lost upon closer examination of the bulk HIV-specific CD8 T-cell responses. Since HIV-specific CD8 T-cell responses were identified as all CD8 T cells that responded to stimulation with peptide pools with an upregulation of CD107a, we cannot rule out the possibility that certain epitope-specific CD8 T-cell responses may display a more differentiated effector phenotype, as has been shown for long-term nonprogressors (1). However, the fact that the maturation statuses of both bulk and HIV-responsive CD8 T cells were not different among the groups suggests that differences in viral load did not play a major role in modulating the antiviral potency of T-cell-mediated in vitro suppression of viral replication, but rather that the specificity of these responses was key for this activity.
Consistent with previous reports describing the protective role of CD8 T-cell proliferative capacity in association with enhanced control of HIV-1 clade C infection (9), we observed that CD8 T-cell responses from high Gag responders were able to proliferate more robustly upon stimulation with HIV peptide pools than low Gag responders. Previous studies have focused on proliferation of tetramer-positive cells, rather than on the entire breadth of responses to all HIV proteins, thereby rendering it difficult to determine whether global proliferation to a particular viral target may be associated with differential control of viral replication in vivo. Using peptide pools, we were able to perform a more comprehensive analysis of the two groups matched for total magnitude of responses and show significant differences in proliferation to specific peptide pools. As expected for these studies performed in persons with chronic infection, the overall percentage of cells able to proliferate to HIV proteins was small but clearly linked to the breadth of Gag-specific responses. The ability of CD8 T cells to expand upon antigen exposure seems to be a key function in controlling infections, and preservation of this capacity in high Gag responders might explain in part their more potent viral suppressive activity in vitro.
The inhibition assays employed a single laboratory strain, thus permitting comparison of the levels of inhibition among the individuals included in this study. Given the high degree of conservation in the Gag gene product, it is unlikely that the level of inhibition would be significantly altered using autologous Gag variants. Interestingly, although study subjects with low Gag responses targeted additional HIV gene products, including other conserved proteins, like Pol, CD8 T cells from these individuals control viral replication poorly in vitro, suggesting that despite the presence of other HIV-specific CD8 T-cell responses, Gag-specific responses exhibit the highest degree of antiviral control in this in vitro assay. However, further studies are warranted to dissect the antiviral capacity of other T-cell responses that may target other conserved genes. As the high Gag responders necessarily also displayed higher levels of Gag-specific CD8 T cells, it is plausible that breadth alone did not account for better viral control in vitro but that a certain threshold of magnitude of CD8 T-cell responses is necessary to mediate enhanced viral suppression. However, our results clearly demonstrate that it is not the magnitude of HIV-specific CD8 T-cell responses overall which confers viral control but rather Gag-specific breadth and magnitude, which in combination lead to more potent antiviral activity.
Interestingly, despite the fact that CD8 T cells from the majority of persons were able to suppress replication of heterologous virus, at least modestly, in autologous CD4 T cells, we observed no correlation between the inhibitory capacity of CD8 T cells and plasma viral loads. The disconnect between CD8 inhibitory capacity and viral loads may be explained in part by the limited number of patients we were able to examine who only showed a tendency toward an association (data not shown). However, it is plausible that the lack of correlation between inhibitory capacity and in vivo replication may be related to epitope differences in the autologous virus that is targeted by these CD8 T cells that may not be represented in the laboratory strain used in these assays.
In conclusion, we detect distinct differences in CD8 T-cell function between individuals with chronic HIV-1 clade C infection, associated with the breadth and magnitude of Gag-specific responses, despite the lack of significant differences in total CD4 count and total magnitude of HIV-specific immune responses. The data are consistent with the hypothesis that a broad Gag-specific CD8 T-cell response results in lower viral set point, potentially due to more potent inhibition of viral replication, which in turn is associated with greater polyfunctionality of the CD8 T-cell responses. The data are also consistent with the hypothesis that due to their inherently elevated polyfunctional capacity, Gag-specific CD8 T-cell responses may be able to inhibit viral replication more efficiently, therefore resulting in lower viral set points. However, distinguishing whether the Gag responses alone were responsible for robust containment of viral replication or whether the total T-cell population in a high Gag responder may be more functional and therefore also contribute to control of viral replication in vitro and in vivo would necessitate much more careful within-subject comparisons of viral replication inhibition by Gag- and non-Gag-specific CD8 T cells and within-subject comparisons of polyfunctionalities of Gag- and non-Gag-specific CD8 T-cell responses. Overall, our data strongly suggest that the breadth and magnitude of the Gag response are critical determinants of highly functional CD8 T-cell responses that are able to suppress viral replication more robustly both in vitro and in vivo, providing a functional correlate between breadth of Gag responses and viral load. These data indicate that specificity, rather than the total magnitude, of memory CD8 T-cell responses is the critical feature of an effective antiviral response, strongly suggesting that vaccine approaches that can induce breadth and magnitude against Gag may provide more robust control of viral replication postinfection.
Supplementary Material
Acknowledgments
B.D.W. received funding from grants RO1 AI30914 and AI28568, the Bill and Melinda Gates Foundation, Mark and Lisa Schwartz Foundation, DDCF, and CFAR. P.J.G. is funded by the Wellcome Trust and grant AI046995. T.N. holds the South African DST/NRF Chair in Systems Biology of HIV/AIDS.
Footnotes
Published ahead of print on 24 March 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Addo, M. M., R. Draenert, A. Rathod, C. L. Verrill, B. T. Davis, R. T. Gandhi, G. K. Robbins, N. O. Basgoz, D. R. Stone, D. E. Cohen, M. N. Johnston, T. Flynn, A. G. Wurcel, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2007. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PLoS One 2:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brumme, Z. L., C. J. Brumme, J. Carlson, H. Streeck, M. John, Q. Eichbaum, B. L. Block, B. Baker, C. Kadie, M. Markowitz, H. Jessen, A. D. Kelleher, E. Rosenberg, J. Kaldor, Y. Yuki, M. Carrington, T. M. Allen, S. Mallal, M. Altfeld, D. Heckerman, and B. D. Walker. 2008. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J. Virol. 82:9216-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., A. Piechocka-Trocha, T. Miura, M. A. Brockman, B. D. Julg, B. M. Baker, A. C. Rothchild, B. L. Block, A. Schneidewind, T. Koibuchi, F. Pereyra, T. M. Allen, and B. D. Walker. 2009. Differential neutralization of human immunodeficiency virus (HIV) replication in autologous CD4 T cells by HIV-specific cytotoxic T lymphocytes. J. Virol. 83:3138-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Day, C. L., P. Kiepiela, A. J. Leslie, M. van der Stok, K. Nair, N. Ismail, I. Honeyborne, H. Crawford, H. M. Coovadia, P. J. Goulder, B. D. Walker, and P. Klenerman. 2007. Proliferative capacity of epitope-specific CD8 T-cell responses is inversely related to viral load in chronic human immunodeficiency virus type 1 infection. J. Virol. 81:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. StJohn, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldmacher, C., J. R. Currier, E. Herrmann, A. Haule, E. Kuta, F. McCutchan, L. Njovu, S. Geis, O. Hoffmann, L. Maboko, C. Williamson, D. Birx, A. Meyerhans, J. Cox, and M. Hoelscher. 2007. CD8 T-cell recognition of multiple epitopes within specific Gag regions is associated with maintenance of a low steady-state viremia in human immunodeficiency virus type 1-seropositive patients. J. Virol. 81:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honeyborne, I., A. Prendergast, F. Pereyra, A. Leslie, H. Crawford, R. Payne, S. Reddy, K. Bishop, E. Moodley, K. Nair, M. van der Stok, N. McCarthy, C. M. Rousseau, M. Addo, J. I. Mullins, C. Brander, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2007. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J. Virol. 81:3667-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769-775. [DOI] [PubMed] [Google Scholar]
- 15.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 16.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyles, R. H., A. Munoz, T. E. Yamashita, H. Bazmi, R. Detels, C. R. Rinaldo, J. B. Margolick, J. P. Phair, and J. W. Mellors. 2000. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J. Infect. Dis. 181:872-880. [DOI] [PubMed] [Google Scholar]
- 18.Masemola, A., T. Mashishi, G. Khoury, P. Mohube, P. Mokgotho, E. Vardas, M. Colvin, L. Zijenah, D. Katzenstein, R. Musonda, S. Allen, N. Kumwenda, T. Taha, G. Gray, J. McIntyre, S. A. Karim, H. W. Sheppard, and C. M. Gray. 2004. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. J. Virol. 78:3233-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 20.Migueles, S. A., C. M. Osborne, C. Royce, A. A. Compton, R. P. Joshi, K. A. Weeks, J. E. Rood, A. M. Berkley, J. B. Sacha, N. A. Cogliano-Shutta, M. Lloyd, G. Roby, R. Kwan, M. McLaughlin, S. Stallings, C. Rehm, M. A. O'Shea, J. Mican, B. Z. Packard, A. Komoriya, S. Palmer, A. P. Wiegand, F. Maldarelli, J. M. Coffin, J. W. Mellors, C. W. Hallahan, D. A. Follman, and M. Connors. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramduth, D., P. Chetty, N. C. Mngquandaniso, N. Nene, J. D. Harlow, I. Honeyborne, N. Ntumba, S. Gappoo, C. Henry, P. Jeena, M. M. Addo, M. Altfeld, C. Brander, C. Day, H. Coovadia, P. Kiepiela, P. Goulder, and B. Walker. 2005. Differential immunogenicity of HIV-1 clade C proteins in eliciting CD8+ and CD4+ cell responses. J. Infect. Dis. 192:1588-1596. [DOI] [PubMed] [Google Scholar]
- 23.Rousseau, C. M., M. G. Daniels, J. M. Carlson, C. Kadie, H. Crawford, A. Prendergast, P. Matthews, R. Payne, M. Rolland, D. N. Raugi, B. S. Maust, G. H. Learn, D. C. Nickle, H. Coovadia, T. Ndung'u, N. Frahm, C. Brander, B. D. Walker, P. J. Goulder, T. Bhattacharya, D. E. Heckerman, B. T. Korber, and J. I. Mullins. 2008. HLA class I-driven evolution of human immunodeficiency virus type 1 subtype c proteome: immune escape and viral load. J. Virol. 82:6434-6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacha, J. B., C. Chung, E. G. Rakasz, S. P. Spencer, A. K. Jonas, A. T. Bean, W. Lee, B. J. Burwitz, J. J. Stephany, J. T. Loffredo, D. B. Allison, S. Adnan, A. Hoji, N. A. Wilson, T. C. Friedrich, J. D. Lifson, O. O. Yang, and D. I. Watkins. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 178:2746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sáez-Cirión, A., C. Lacabaratz, O. Lambotte, P. Versmisse, A. Urrutia, F. Boufassa, F. Barre-Sinoussi, J. F. Delfraissy, M. Sinet, G. Pancino, and A. Venet. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 27.Schneidewind, A., M. A. Brockman, R. Yang, R. I. Adam, B. Li, S. Le Gall, C. R. Rinaldo, S. L. Craggs, R. L. Allgaier, K. A. Power, T. Kuntzen, C. S. Tung, M. X. LaBute, S. M. Mueller, T. Harrer, A. J. McMichael, P. J. Goulder, C. Aiken, C. Brander, A. D. Kelleher, and T. M. Allen. 2007. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J. Virol. 81:12382-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streeck, H., Z. L. Brumme, M. Anastario, K. W. Cohen, J. S. Jolin, A. Meier, C. J. Brumme, E. S. Rosenberg, G. Alter, T. M. Allen, B. D. Walker, and M. Altfeld. 2008. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS Med. 5:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streeck, H., M. Lichterfeld, G. Alter, A. Meier, N. Teigen, B. Yassine-Diab, H. K. Sidhu, S. Little, A. Kelleher, J. P. Routy, E. S. Rosenberg, R. P. Sekaly, B. D. Walker, and M. Altfeld. 2007. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 81:7725-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 31.Wilson, C. C., J. T. Wong, D. D. Girard, D. P. Merrill, M. Dynan, D. D. An, S. A. Kalams, R. P. Johnson, M. S. Hirsch, R. T. D'Aquila, et al. 1995. Ex vivo expansion of CD4 lymphocytes from human immunodeficiency virus type 1-infected persons in the presence of combination antiretroviral agents. J. Infect. Dis. 172:88-96. [DOI] [PubMed] [Google Scholar]
- 32.Yang, O. O., S. A. Kalams, M. Rosenzweig, A. Trocha, N. Jones, M. Koziel, B. D. Walker, and R. P. Johnson. 1996. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J. Virol. 70:5799-5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. U. S. A. 102:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.