Abstract
Lentiviral vectors are promising vaccine vector candidates that have been tested extensively in preclinical models of infectious disease and cancer immunotherapy. They are also used in gene therapy clinical trials both for the ex vivo modification of cells and for direct in vivo injection. It is therefore critical to understand the mechanism(s) by which such vectors might stimulate the immune system. We evaluated the effect of lentiviral vectors on myeloid dendritic cells (DC), the main target of lentiviral transduction following subcutaneous immunization. The activation of DC cultures was independent of the lentiviral pseudotype but dependent on cell entry and reverse transcription. In vivo-transduced DC also displayed a mature phenotype, produced tumor necrosis factor alpha (TNF-α), and stimulated naive CD8+ T cells. The lentiviral activation of DC was Toll-like receptor (TLR) dependent, as it was inhibited in TRIF/MyD88 knockout (TRIF/MyD88−/−) DC. TLR3−/− or TLR7−/− DC were less activated, and reverse transcription was important for the activation of TLR7−/− DC. Moreover, lentivirally transduced DC lacking TLR3 or TLR7 had an impaired capacity to induce antigen-specific CD8+ T-cell responses. In conclusion, we demonstrated TLR-dependent DC activation by lentiviral vectors, explaining their immunogenicity. These data allow the rational development of strategies to manipulate the host's immune response to the transgene.
Anticancer immunotherapy is based on enhancing the immune response to tumor cells. To achieve this, it is of paramount importance to break the strong tolerogenic mechanisms that are in place due to the fact that tumor-associated antigens are frequently self-derived proteins. In addition, tumors can actively induce immune suppression. Fortunately, the idea of active immunotherapy against cancer has been invigorated by our growing knowledge of tumor immunology.
Tumor antigens are recognized by T cells, and the latter can mediate tumor cell lysis (5). Furthermore, antigen-presenting cells, in particular dendritic cells (DC), are the mediators of such immune responses (40). Therefore, DC loaded ex vivo with tumor antigens have been exploited as anticancer vaccines (42), and the in situ delivery of tumor antigens to DC is being evaluated. In this regard, the use of lentiviral vectors is promising. Several groups have demonstrated the in vivo transduction of DC (14, 19, 36, 44) and have shown with different tumor models that this results in strong antigen-specific cytotoxic T-lymphocyte (CTL) responses (11, 12, 14, 19, 29, 31, 32, 36, 39, 44). These responses are superior to those induced by ex vivo-transduced DC and result in delayed tumor outgrowth (12). These data indicate that lentiviral vectors or components present in the lentiviral preparation are immunogenic, leading to the delivery of the transgene as well as signals that activate strong adaptive immune responses.
Several groups have suggested a role for plasmacytoid DC (pDC) in lentivirus-mediated immunogenicity. Wild-type HIV-1 activates pDC through Toll-like receptor 7 (TLR7), which results in the production of type I interferon (IFN) (4, 15). Since recombinant lentiviral vectors are often derived from HIV-1, they may trigger similar innate immune responses after in vivo administration. Brown et al. previously demonstrated that type I IFN production upon lentiviral transduction was independent of the envelope pseudotype but dependent on cell entry (10). An in vitro challenge of antigen-presenting cells suggested that pDC are responsible for this response, with a role for TLR7 and TLR9. Furthermore, the presence of additional elements involved in triggering the innate host response was suggested, as TLR7/9 antagonists are not sufficient to prevent the type I IFN response (10).
Recently, it was reported that vesicular stomatitis virus glycoprotein (VSV.G)-pseudotyped lentiviral preparations contain tubulovesicular structures of cellular origin, carrying, among others, plasmid DNA used for lentivirus production. These structures triggered TLR9 in pDC, hence inducing type I IFN production (37).
However, it is generally considered that DC of myeloid origin are critical for inducing effective antitumor immune responses. Nonetheless, the adjuvant effect of lentiviral transduction on myeloid DC remains unclear. Studies with human DC demonstrated some activation, which was dose dependent (9, 17, 41), mediated by protein kinase R (9, 41) and possibly TLR (9). Thus far, there are no functional studies on the role of myeloid DC activation during immunization.
Here, we address whether and how lentiviral vectors activate mouse myeloid DC. We show that these DC are activated by lentiviral transduction in cell cultures and in vivo. This activation is dependent on viral entry and reverse transcription and involves TLR engagement and the triggering of proinflammatory signaling pathways. We have further demonstrated that TLR3 and TLR7 play a crucial role in the immunogenicity of the lentiviral vector.
MATERIALS AND METHODS
Cells and mice.
293T cells were grown in Dulbecco modified Eagle medium (DMEM; Gibco) supplemented with 10% fetal calf serum (FCS; Harlan), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco), and 2 mM l-glutamine (Gibco). OT-1 transgenic mice, in which CD8+ T cells express a T-cell receptor (TCR) specific for the SIINFEKL peptide of ovalbumin presented on H2-kb, were bred in-house. Mouse bone marrow-derived DC were prepared from C57BL/6, TRIF knockout (TRIF−/−) (47), MYD88−/− (26), TRIF/MyD88−/− (30), TLR3−/− (22), and TLR7−/− (21) mice, as previously described (7). C57BL/6 mice were purchased from Harlan and handled according to institutional guidelines. Bone marrow from TRIF−/− and MYD88−/− mice were provided by Jean Langhorne and Xuemei Wu, respectively (National Institute for Medical Research, Mill Hill, London, United Kingdom). TRIF/MyD88−/−, TLR3−/−, and TLR7−/− mice were a kind gift from Caetano Reis e Sousa (London Research Institute, London, United Kingdom). At day 7 of culture, DC were plated at a density of 1 × 106 cells per well in 1 ml of medium containing mouse granulocyte-macrophage colony-stimulating factor (GM-CSF) (20 ng/ml; Peprotech). These DC were either left untreated; matured with 100 ng/ml lipopolysaccharide (LPS) (Escherichia coli serotype O55:B5; Sigma-Aldrich), 12.5 μg/ml poly(I:C) (Sigma-Aldrich), or 2.5 μg/ml ssRNA40/Lyovec (Invivogen); or transduced with lentivectors as described below.
Lentiviral vector production, titration, and transduction of DC. (i) Plasmids.
The transfer vectors pHR′trip CMV GFP SIN (7), pHR′trip CMV luc2-Ires-tNGFR SIN (28), pHRSIN-CSGW, and pHRSIN-Thy1.1 (3) were previously described. For the construction of pDUAL-Thy1.1-IiOVA, the Thy1.1 insert (3) was cloned into the BamHI-NotI restriction sites to replace green fluorescent protein (GFP) in pDUAL-GFP-IiOVA (13). Both the multiply attenuated packaging plasmid pCMVΔR8.9 and the vesicular stomatitis virus glycoprotein (VSV.G)-encoding plasmid pMD.G were a kind gift from D. Trono (University of Geneva, Geneva, Switzerland). The mutations in the attachment sites and integrase of packaging plasmid pCMVΔR8.74 were described previously (2, 24). The mutations resulting in deficient reverse transcriptase were described previously (25, 33). The plasmid encoding the murine leukemia virus envelope 10A1 was a kind gift from Wolfgang Uckert (Max Delbrück Center for Molecular Medicine, Berlin, Germany).
(ii) Production and titration.
Viruses were produced by the transient transfection of 293T cells with transfer vector-, packaging-, and envelope-encoding plasmids. Transfection was performed by lipofection (39) or calcium phosphate precipitation (7). Supernatants were collected 48, 72, and 96 h later and pooled, and viral particles were 200-fold concentrated by ultracentrifugation. Viruses were resuspended in phosphate-buffered saline (PBS) containing 10 μg/ml protamine sulfate (LeoPharma) and kept at −80°C until use. Lentivectors were characterized as previously described (2, 7).
(iii) DC transduction.
On day 6, 106 DC were resuspended in 100 μl of infection cocktail, which contained lentiviral vectors (multiplicity of infection [MOI] of 15) and DC medium supplemented with 20 ng/ml murine GM-CSF (muGM-CSF). These DC were subsequently plated in a 24-well plate and left for 2 h at 37°C in 5% CO2. Subsequently, GM-CSF-containing DC medium was added to cultures of cells at a cell density of 106 DC/ml. Transductions were performed in the presence of 10 μg/ml protamine sulfate (LeoPharma); however, to ensure that the addition of this polycationic agent did not affect DC activation, we also performed several transductions without the addition of protamine sulfate.
Flow cytometry.
DC were analyzed by using anti-B7.1-biotin, anti-B7.2-biotin, anti-CD40-biotin (affinity purified and biotinylated in-house), anti-CD11c-allophycocyanin (APC), anti-H-2Kb-fluorescein isothiocyanate (FITC), and anti-Thy1.1-phycoerythrin (PE) (BD). Surface staining was performed as described previously (7). Biotinylated antibodies were detected with streptavidin-PE-Cy7 (BD). Cells were acquired by use of a FACSCalibur apparatus, and analysis was performed by using CellQuest software (BD).
ELISA.
Supernatants from DC cultures were harvested and screened in a sandwich enzyme-linked immunosorbent assay (ELISA) for the presence of interleukin-6 (IL-6), IL-10, IL-12p70, IL-23, tumor necrosis factor alpha (TNF-α) (eBioscience), or IFN-β (PBL Biomedical Laboratories) according to the manufacturers' instructions.
Allogeneic mixed-lymphocyte reaction (allo-MLR).
DC were cocultured at a stimulator-to-responder ratio of 1 to 100 with 2 × 105 BALB/c CD90+ spleen cells, which were sorted by using the VarioMACS technique (Miltenyi Biotec). After 4 days of incubation, cells were pulsed for 16 h with 1 μCi/well [3H]thymidine (Amersham). The incorporation of [3H]thymidine was measured by using a β-scintillation counter (Microbeta-Wallac).
In vivo tracking of transduced cells. (i) Injections.
Mice were injected subcutaneously in the footpad with 107 transducing units (TU) (20 μl) of firefly luciferase (luc2-Ires-tNGFR)-encoding lentivectors or in the tail base with 107 TU Thy1.1-encoding lentivectors or with PBS for negative controls (five mice per group).
(ii) In vivo bioluminescence imaging.
Imaging of firefly luciferase-transduced cells was performed 6, 24, and 48 h after injection. Anesthesia, intravenous injection of d-luciferin, and imaging were performed as described previously (28).
(iii) DC purification from lymph nodes.
Para-aortical and inguinal lymph nodes were harvested 2 days after mice were injected with Thy1.1-encoding lentivectors. These DC were incubated with collagenase (Worthington) and mashed into a single-cell suspension. Fc receptors were blocked before CD11c+ cells were selected by using magnetically activated cell sorting (MACS) beads (Miltenyi Biotec).
(iv) TNF-α production by isolated CD11c+ cells.
The isolated CD11c+ cells were plated into a flat-bottom 96-well plate at 2 × 105 CD11c+ cells/200 μl of DC medium. No additional stimuli were provided. Supernatants were collected 24 h after plating and analyzed for the presence of TNF-α by ELISA (eBioscience).
In vitro stimulation of OT-1.
DC were pulsed for 2 h with 10 μM H-2Kb-restricted peptide SIINFEKL (ovalbumin) or SVYDFFVWL (Trp2) (Thermo Electron Cooperation). After two wash steps, these DC were cocultured at a stimulator-to-responder ratio of 1 to 100 with 2 × 105 OT-1 spleen cells. Twenty-four hours later, the supernatants were tested for the presence of IFN-γ by ELISA (eBioscience). T-cell proliferation was measured as described above for allo-MLR.
Evaluation of proinflammatory signaling pathways.
To evaluate whether the lentiviral transduction of DC results in the activation of NF-κB and signaling via the IFN-β promoter, which is mediated by NF-κB, AP-1, and IFN regulatory factor (IRF), we applied a lentiviral reporter system. On day 4, DC were plated at 106 DC/ml of medium and transduced with lentiviral vectors encoding cherry fluorescent protein or GFP under the transcriptional control of NF-κB or the latter together with AP-1 and IRF3 (IFN-β promoter). On day 7, the reporter-modified DC were either left untreated (background signaling), stimulated with LPS, differently pseudotyped, or treated with reverse transcriptase-deficient lentiviral vectors as described above. Reporter expression was evaluated 4, 24, and 48 h later by confocal microscopy and flow cytometry.
Vaccination, pentamer staining, and in vivo CTL. (i) Vaccination.
Six- to eight-week-old mice (five mice per group) were injected subcutaneously in the tail base with 1 × 105 wild-type, TLR3−/−, or TLR7−/− DC, which were transduced with a fusion protein containing the first 80 amino acids of the invariant chain fused to ovalbumin (IiOVA)-Thy1.1-encoding lentiviral vectors. Mice injected with PBS served as controls.
(ii) Pentamer staining.
Blood cells collected from the tail vain were treated with red blood cell lysis buffer (Gentra Systems), washed twice in PBS, and subsequently incubated with 5 μl of PE-conjugated SIINFEKL/H2-Kb pentamer (Proimmune) for 10 min at room temperature.
The cells were washed and incubated on ice with APC-conjugated anti-CD8 (BD) for 15 min. Samples were washed and acquired with a FACSCalibur apparatus by using Cell-Quest software (BD). At least 300,000 events per sample were acquired.
(iii) In vivo CTL.
Spleen cells from naive mice were resuspended in Hanks balanced salt solution (HBSS) at 5 × 106 cells/ml and pulsed with SIINFEKL peptide at 10 μM for 2 h, after which they were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at 5 μM. These cells were mixed at a 1:1 ratio with nonpulsed cells that were labeled with CFSE at 0.5 μM. Specific lysis of the pulsed cells was analyzed 18 h later by flow cytometry of cells from the spleen. The percent killing was calculated as described previously (12).
Statistical analysis.
Where applicable, we carried out a Kruskal-Wallis test. Differences were considered significant at a P value of <0.05. Statistical significance is indicated in the figures.
RESULTS
Activation of DC by lentiviral vectors is dependent on cell entry and reverse transcription.
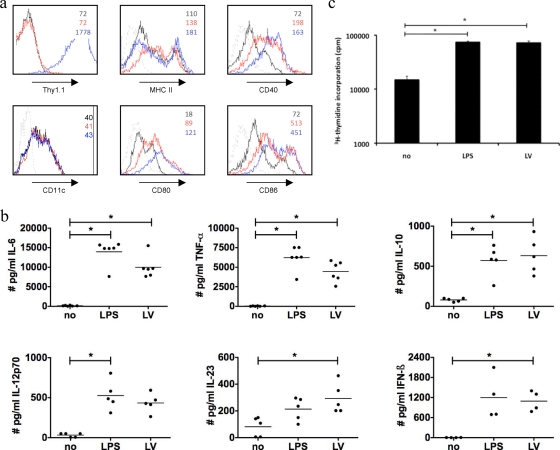
In our previous work we evaluated lentiviral vectors as a tool to modify mouse DC ex vivo. We demonstrated that lentivirally transduced DC (MOI of 15) that were additionally treated with LPS for 24 h had characteristics similar to those of LPS-activated nontransduced DC (7). When we recently compared lentivirally transduced DC (no additional LPS) to immature DC, we noticed that the former DC were activated. As DC play a pivotal role in detecting pathogens (27), we decided to further examine the activation of DC by lentiviral vectors in vitro. Immature DC and LPS-stimulated DC (24 h, mature) served as controls. On average, 79% ± 10% (n = 5) of DC were transgenes 24 h after transduction. These lentivirally transduced DC showed levels of expression of antigen-presenting and costimulatory molecules (Fig. 1a) as well as several cytokines (Fig. 1b) similar to those of LPS-activated DC. Moreover, they showed similar allogeneic T-cell-stimulatory capacities (Fig. 1c). DC activation was sustained until 72 h posttransduction. We further demonstrated that the activation was not due to a contamination of the viral preparation with endotoxins (<0.1 endotoxin units [EU]/μl) and that it was independent of the lentiviral vector production, processing, or transduction protocol (data not shown).
FIG. 1.
Activation of DC following in vitro lentiviral transduction. At day 7 of culture, DC were plated at 106 DC/ml medium. These DC were not manipulated (no), incubated with 100 ng/ml LPS for 24 h (LPS), or transduced with lentiviral vectors encoding Thy1.1 at an MOI of 15 (LV) (n = 5). (a) Flow cytometric analysis performed 24 h after transduction. The expression of CD11c, Thy1.1, CD40, CD80, CD86, and MHC class II on the immature (black), mature (red), and transduced (blue) DC was evaluated. The gray line represents isotype control staining. The numbers in the graph represent the mean fluorescence intensities (MFI). Data from one representative experiment out of five are shown. (b) Levels of cytokines in supernatants of immature (no), mature (LPS), and transduced (LV) DC. Each experiment is represented by a dot, and the horizontal line indicates the mean. These DC were used as stimulators in a mixed-lymphocyte reaction at a stimulator-to-responder ratio of 1 to 100. The proliferation of allogeneic CD90+ T cells was evaluated 4 days after the start of the coculture as a measure for DC functionality. (c) Proliferation was measured by [3H]thymidine incorporation. The data shown are representative of data from one experiment out of five independent experiments.
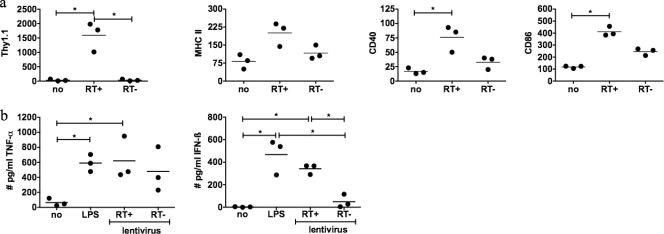
Subsequently, we determined which element—cell entry, viral envelope, and/or virus activity—is mediating the DC activation. Therefore, DC were incubated with heat-inactivated lentiviral vectors, lentiviral vectors pseudotyped with a murine leukemia virus glycoprotein (10A1) or the vesicular stomatitis virus glycoprotein (VSV.G), or lentiviral vectors containing an inactive reverse transcriptase or integrase mutant. The incubation of DC with heat-inactivated lentiviral vectors did not result in transgene-positive cells or DC activation (data not shown). We further observed that both vector pseudotypes as well as the integrase-deficient lentiviral vectors activated DC (data not shown). Interestingly, DC transduced with reverse transcriptase-deficient lentiviral vectors displayed less phenotypical activation (Fig. 2a). More importantly, these DC produced high levels of TNF-α, whereas they were impaired in their ability to produce IFN-β (Fig. 2b). This was not unexpected considering that IFN-β production is mediated by TRIF, a signal adaptor molecule downstream of TLR3 (and TLR4). Since TLR3 recognizes double-stranded nucleic acids, a lentiviral life cycle intermediate that is formed after reverse transcription, we would not expect high levels of IFN-β. These data indicate that vector components can activate DC but that full activation is dependent on viral reverse transcription activity.
FIG. 2.
Activation of DC is partially dependent on reverse transcription. Manipulation of day 7 DC was performed as described in the legend of Fig. 1. Lentiviral vectors contained either an active reverse transcriptase (RT+) or inactive reverse transcriptase (RT−) (equal amount of p24). (a) Graphs representing the mean fluorescent intensity of the evaluated markers as obtained by flow cytometric analysis. The expression of Thy1.1, CD40, CD86, and MHC class II on immature DC (no), LPS-activated DC (LPS), and active and inactive reverse transcriptase-transduced DC was evaluated. The numbers in the graph represent the MFI of the CD11c+ population. (b) Production of TNF-α and IFN-β by the respective DC. Each experiment is represented by a dot. The horizontal lines show the means.
In vivo-transduced DC have a mature phenotype and function.
We previously demonstrated that the subcutaneous administration of lentiviral vectors results in transgene-positive cells in the draining lymph node (12). It was further demonstrated that these transgene-positive cells are, among others, DC (14, 18). Here, we wanted to compare in vivo-transduced DC found in the lymph node to those of control mice in terms of activation.
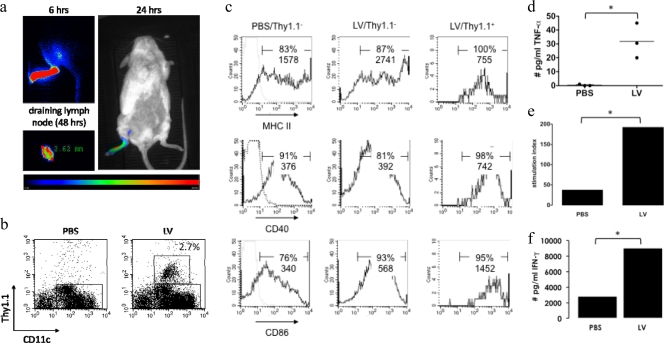
To determine the time point for the evaluation of lentivirally transduced and migrated DC in the lymph node, we tracked transduced cells by administering lentiviral vectors encoding firefly luciferase into the footpad (107 TU/20 μl). We then monitored transduced DC by in vivo bioluminescence imaging 6, 24, and 48 h later, because this technique was previously used to track transduced DC (28). Light emission was observed at the site of injection and at the draining lymph node region at all evaluated time points. After the last imaging, the popliteal lymph node was isolated and imaged for 2 min, demonstrating the presence of firefly luciferase-expressing cells (Fig. 3a).
FIG. 3.
Tracking and evaluation of in vivo-transduced cells. (a) In vivo bioluminescence imaging was performed 6, 24, and 48 h after footpad injection of 107 TU of lentiviral vectors encoding firefly luciferase. (b) Thirty-six hours after the subcutaneous administration of lentivectors encoding Thy1.1, lymph nodes were isolated, and CD11c+ cells were sorted and stained for Thy1.1 (n = 3). (c) Isolated cells were stained for CD40, CD86, and MHC class II. Cells were gated as CD11c+/Thy1.1− or CD11c+/Thy1.1+. The graphs in b and c are representative of data from three independent experiments and show the percentages and MFI of marker-positive cells. (d) Production of TNF-α by the DC. Each experiment is represented as a dot, and the horizontal line shows the mean. The CD11c+ cells were loaded with the MHC class I-restricted ovalbumin peptide SIINFEKL or an irrelevant peptide (Trp2 [SVYDFFVWL]) and cultured at a stimulator-to-responder ratio of 1 to 100 with ovalbumin TCR-transgenic CD8+ T cells (n = 2). (e and f) Proliferation of T cells (e) and production of IFN-γ (f) by these T cells. The results are representative of data from two independent experiments.
To specifically analyze the phenotype of in vivo-transduced DC, we subcutaneously administered Thy1.1-encoding lentiviral vectors or PBS (control) to Thy1.2 mice. Lymph nodes were removed 36 h later, and DC were sorted. Cells were stained for Thy1.1 and activation markers. On average, 2.5% ± 0.6% (n = 3) of lymph node DC from lentivirus-immunized mice stained positive for Thy1.1 (Fig. 3b). These DC showed an enhanced expression of CD40 and CD86 compared to Thy1.1-negative DC, which in turn were more activated than DC from control mice (Fig. 3c). Curiously, a higher percentage of Thy1.1-positive DC stained positive for major histocompatibility complex (MHC) class II than did control DC. It must be noted that these DC had a lower level of MHC class II expression (mean fluorescence intensity [MFI]), an observation that was also made upon the in vitro transduction of DC (Fig. 1a).
However, this finding was not unexpected, as MHC class II molecules are retained intracellularly and are exposed to the cell surface upon maturation without de novo synthesis (38). In fact, MHC class II transcription is inhibited upon maturation (23, 46). Therefore, we would expect an increase in the number of MHC class II-positive DC but not the amount of MHC class II upon strong activation.
We further demonstrated the production of TNF-α by the DC obtained from lentiviral vector-immunized mice but not control DC (Fig. 3d). Finally, we demonstrated that DC from lentiviral vector-immunized mice were more potent in the antigen-specific stimulation of OT-1 T cells (proliferation and production of IFN-γ) than DC from mice injected with PBS (Fig. 3e). These data suggest that the activation observed in vitro is not an artifact but is relevant in vivo and is a first explanation of the lentiviral vector potency as a therapeutic cancer vaccine.
Lentiviral transduction of DC results in signaling via proinflammatory pathways.
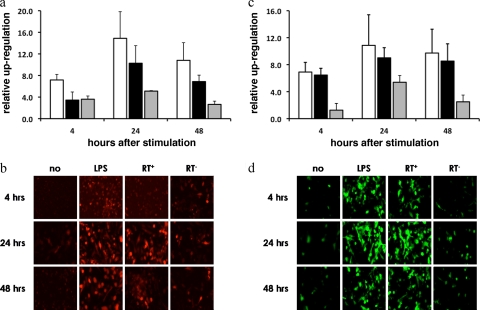
To confirm that the lentiviral transduction of DC resulted in the recognition of viral components and, as such, triggers signaling pathways linked to DC activity (8), we assessed the activation of an NF-κB-dependent promoter and an IFN-β promoter upon transduction. It is well established that the latter's activity is mediated by NF-κB, AP-1, and IRF. Day 4 DC were lentivirally transduced to contain a cherry fluorescent protein or GFP reporter gene under the transcriptional control of the NF-κB-dependent promoter or IFN-β promoter, respectively. On day 7, the reporter-modified DC were either left untreated, stimulated with LPS, differently pseudotyped, or treated with reverse transcriptase-deficient lentiviral vectors. Reporter expression was evaluated 4, 24, and 48 h later by confocal microscopy and flow cytometry. NF-κB, AP-1, and IRF signaling was clearly observed in transduced cells irrespective of the pseudotype (data not shown) and followed a pattern similar to that of upregulation upon LPS stimulation, showing activity as soon as 4 h posttransduction (Fig. 4). The fact that we observed reporter expression at this early time point suggests that the transcription factor activity is triggered upon the recognition of viral components and is not a result of secondary signaling events.
FIG. 4.
Lentiviral transduction of DC results in the upregulation of NF-κB, AP-1, and IRF. At day 4, DC were plated at 106 DC/ml medium and transduced with lentiviral vectors encoding cherry fluorescent protein or GFP under the transcriptional control of NF-κB or the latter together with AP-1 and IRF3 (IFN-β promoter) (n = 3). (a and c) Graphs representing the flow cytometry data of a representative experiment demonstrating the relative upregulation of reporter expression (fold increase compared to background) driven by the NF-κB and the IFN-β promoters, respectively. Stimulation with LPS (white bars), active reverse transcriptase (black bars), and inactive reverse transcriptase (gray bars) lentiviral vectors is depicted. (b and d) Confocal images representative of the data obtained with the NF-κB (b) and IFN-β (d) promoters.
Interestingly, reverse transcription was shown to be essential for the strong upregulation of transcription factors (Fig. 4), confirming the critical role for reverse transcriptase activity to maximally upregulate stimulatory molecules and the production of IFN-β (Fig. 2).
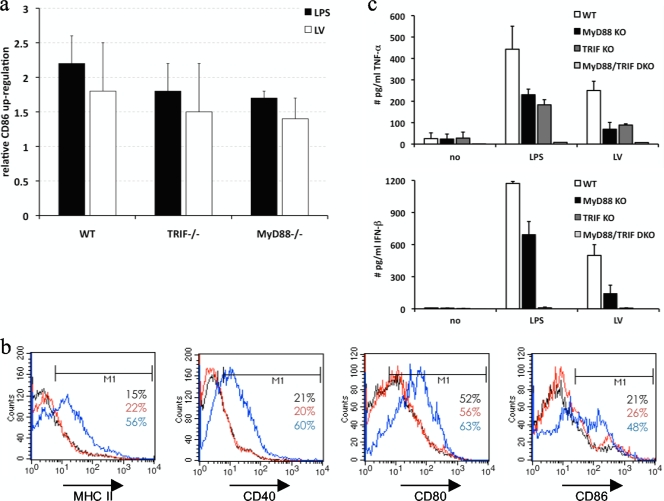
Activation of DC by lentiviral vectors is dependent on TRIF and MyD88.
The DC maturation and activation of proinflammatory signaling pathways indicated that lentiviral vectors were detected by pattern recognition receptors on myeloid DC. In fact, we previously demonstrated with a 293T reporter assay that lentiviral vectors can trigger TLR (9).
To assess the contribution of TLR to lentivirus-mediated DC maturation, we transduced DC deficient for the adaptor proteins TRIF and/or MyD88, since all TLR use at least one of these for signal transduction. The upregulation of maturation markers was observed for wild-type, TRIF−/−, or MyD88−/− DC upon LPS or lentiviral stimulation (Fig. 5a). In contrast, TRIF/MyD88−/− DC were not matured with LPS or upon lentiviral transduction, although these DC were matured with TNF-α, a TLR-independent stimulus (Fig. 5b). This finding indicates that TLR signaling strongly contributed to DC maturation. Moreover, we observed TNF-α production upon the lentiviral transduction of wild-type, TRIF−/−, and MyD88−/− DC but not TRIF/MyD88−/− DC (Fig. 5c). The secretion of IFN-β was observed for wild-type and MyD88−/− DC but was abrogated in TRIF−/− and TRIF/MyD88−/− DC (Fig. 5c). This was expected since TRIF is an essential adaptor for IFN-β induction. The lack of TRIF/MyD88−/− DC activation demonstrates a critical role for TLR in lentivirus-mediated DC activation.
FIG. 5.
DC activation by lentiviral vectors is dependent on both TRIF and MyD88. DC of wild-type, TRIF−/−, MyD88−/−, or TRIF/MyD88−/− mice were manipulated as described in the legend of Fig. 1 (n = 3). (a) Graph showing the upregulation of CD86 upon stimulation with LPS (black bars) or transduction with lentiviral vectors (white bars) of wild-type (WT), TRIF−/−, and MyD88−/− DC. The data shown are representative of data from three independent experiments. (b) Flow cytometry graphs representing TRIF/MyD88−/− DC, which were not stimulated (black lines) or stimulated with lentiviral vectors (red lines) or TNF-α (blue lines). The percentage of marker-positive cells is indicated. The data are representative of data from three independent experiments. (c) Graphs showing a summary of the TNF-α and IFN-β secretion by wild-type (white bars), MyD88−/− (black bars), TRIF−/− (dark gray bars), and TRIF/MyD88−/− (light gray bars) DC (n = 3). KO, knockout; DKO, double knockout.
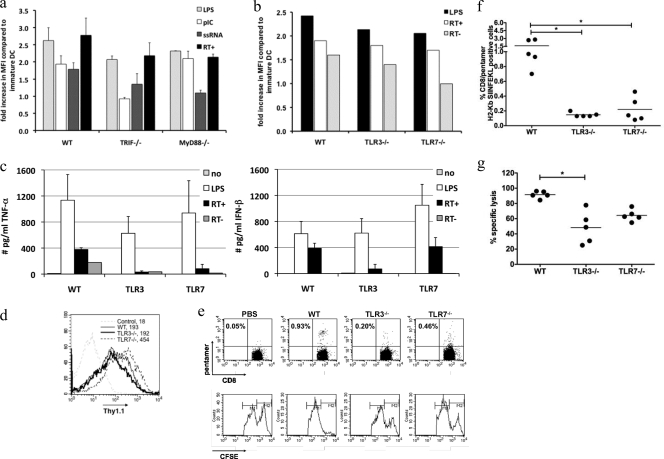
Lentiviral DC activation is TLR3 and TLR7 mediated and explains their immunogenicity.
We hypothesized that two of the most likely TLR candidates for the recognition of lentiviral vectors by DC are TLR3, which is involved in IFN-β induction, and TLR7, which is involved in proinflammatory cytokine production. To address this issue, we first stimulated wild-type, MyD88−/−, and TRIF−/− DC with lentiviral vectors and, as a comparison, poly(I:C), a well-known TLR3 agonist, or ssRNA40/Lyovec, a commonly applied TLR7 trigger (n = 2). Phenotypical analysis revealed that wild-type DC were activated by all stimuli, which was expected, since these DC contain the involved TLR and adaptor molecules. As shown previously, LPS induced the activation of MyD88−/− and TRIF−/−, demonstrating that these DC can efficiently mature. MyD88−/− DC did not respond to ssRNA40/Lyovec (TLR7), whereas stimulation with poly(I:C) (TLR3) resulted in activation similar to that with stimulation with lentiviral vectors. TRIF−/− DC did not mature with poly(I:C) but responded to ssRNA40/Lyovec similarly to lentiviral vectors. These data are a first hint that both TLR3 and TLR7 are involved in the observed lentivirus-mediated activation (Fig. 6a).
FIG. 6.
TLR3 and TLR7 are involved in the activation of DC by lentiviral vectors. DC of wild-type, MyD88−/−, TRIF−/−, TLR3−/−, and TLR7−/− mice were transduced as described in the text. As a comparison, MyD88−/− and TRIF−/− DC were stimulated with poly(I:C) (pIC) or ssRNA40/Lyovec (ssRNA). Flow cytometry evaluating surface marker expression was performed 24 h after manipulation. (a) Graph showing the upregulation of CD86 upon stimulation with LPS (light gray bars), poly(I:C) (white bars), and ssRNA40/Lyovec (dark gray bars) or transduction with lentiviral vectors (black bars) of wild-type, MyD88−/−, and TRIF−/− DC. The data shown represent the means ± standard deviations from two independent experiments. (b) Graph showing the upregulation of CD86 upon stimulation with LPS (black bars) and transduction with lentiviral vectors (white bars) and reverse transcriptase mutant lentiviral vectors (gray bar) of wild-type, TLR3−/−, and TLR7−/− DC. The data shown are representative of data from three independent experiments. (c) Graphs showing the secretion of TNF-α and IFN-β by wild-type, TLR3−/−, and TLR7−/− DC. The data are a summary of the collected data. (d) Flow cytometry graphs representing the Thy1.1 positivity of wild-type, TLR3−/−, and TLR7−/− DC transduced with lentiviral vectors expressing IiOVA and Thy1.1. (e) Representative graphs from pentamer staining (top) and histograms from the in vivo CTL assay (bottom). (f and g) Graphs showing the percentage of CD8/pentamer H-2Kb SIINFEKL-positive cells (f) and percent specific lysis (g) in wild-type, TLR3−/−, and TLR7−/− DC. Each dot represents the data for one mouse, and the number of mice per group was 5. The horizontal lines indicate the means.
To further assess the role of TLR3 and TLR7 in lentivirus-induced DC activation, we transduced TLR3−/− and TLR7−/− DC. Interestingly, both TLR−/− DC upregulated CD86 (Fig. 6b) as well as the other evaluated markers (data not shown) to an extent similar to that of wild-type DC after lentiviral transduction. Curiously, maturation was abrogated when TLR7−/− DC were transduced with reverse transcriptase-deficient lentivectors (Fig. 6b). This demonstrates the necessity of lentivector life cycle intermediates to possibly activate TLR3. A role for TLR3 was further supported by the lack of high TNF-α and IFN-β levels in TLR3−/− cultures (Fig. 6c). Moreover, a role for TLR7 was suggested, since TLR7−/− DC produced only low levels of TNF-α, although they were able to produce IFN-β (Fig. 6c).
Several groups reported the synergy between TLR3 and TLR7 signaling in DC activation (35) and the subsequent induction of immune responses (45). To assess whether TLR3 and TLR7 are involved in lentiviral vector immunogenicity, we immunized C57BL/6 mice with wild-type, TLR3−/−, and TLR7−/− DC, which were lentivirally transduced to encode a model transgene containing ovalbumin epitopes. The expansion of ovalbumin-specific CD8+ T cells upon immunization with wild-type transduced DC was evident as assessed by pentamer staining (pentamer H2-Kb-restricted peptide SIINFEKL). In contrast, this expansion was impaired in mice immunized with either TLR3−/− or TLR7−/− DC transduced to a similar extent with the same lentiviral vector (Fig. 6d, e, and f).
To confirm these results on a functional level, we further performed an in vivo lysis assay, demonstrating the maximal lysis of target cells in mice immunized with wild-type DC (Fig. 6e and f). Moreover, we demonstrated that immunization with the respective TLR−/− DC does result in the induction of CTL but to a lesser extent than with wild-type DC (Fig. 6e and g). Interestingly, as already suggested by the results of the pentamer staining, we observed that TLR7−/− DC performed better than TLR3−/− DC, suggesting that TLR3 plays a more pronounced role in DC activation (Fig. 6e and g).
These data demonstrate the involvement of both TLR3 and TLR7 in the activation of DC upon lentiviral transduction. Moreover, this combined TLR activation is shown to be of importance for the induction of antigen-specific CD8+ T-cell responses.
DISCUSSION
In the present study we investigated the effect of lentiviral transduction on myeloid DC, both in culture and in vivo, demonstrating their activation. This idea was instigated by the observation that tumor antigen-encoding lentiviral vectors are a successful off-the-shelf antitumor vaccine (11, 12, 14, 19, 29, 31, 32, 36, 39, 44), even though they are devoid of viral genes and therefore considered to be nonimmunogenic (6). Whereas several studies have addressed the contribution of pDC to the observed immune response (10, 37), none of them assessed whether myeloid DC are involved and, if so, which mechanisms are at the basis of the immune response. Nevertheless, it is the latter DC type that is believed to induce potent antitumor T-cell responses (8). Here, we demonstrate in vitro and in vivo that myeloid DC transduced with lentiviral vectors are activated. Furthermore, we show a critical role for reverse transcription in lentivirus-mediated DC activation. More importantly, we have demonstrated that this DC activation is critically dependent on TLR, in particular TLR3 and TLR7, and that this combined TLR triggering strongly contributes to lentiviral vector immunogenicity.
Several groups have reported the lentiviral transduction of mouse bone marrow-derived DC. In those reports, it was shown that lentiviral transduction did not hamper the subsequent maturation of these DC with strong stimuli, such as inflammatory cytokines, LPS, or CpG oligonucleotides (7, 14, 34, 48). Only He et al. investigated whether the lentiviral transduction of DC in itself resulted in DC activation (20). In contrast to our data, He et al. did not observe enhanced CD86 expression or IL-12 secretion. However, it must be noted that the transductions were performed at day 2 of culture (DC precursors), whereas we performed the transductions at day 7 (differentiated DC), and that this difference in timing could account for the observed difference in activation (20). Importantly, our data are in agreement with data reported previously for the activation of human monocyte-derived DC by lentiviral vectors (9, 41). A dose-dependent activation of human DC was demonstrated upon lentiviral transduction. Moreover, it was shown previously in a reporter assay that lentiviral vectors can engage TLR (9). Although the latter was not directly assessed with DC due to technical hurdles, it demonstrates the value of our current findings.
Previously, it was shown that the direct administration of lentiviral vectors results in the transient production of type I IFN and that pDC are partially responsible for this cytokine production (10). However, the role of myeloid DC was not addressed. We demonstrate that myeloid DC can produce IFN-β upon lentiviral transduction. Moreover, using different protocols and differently pseudotyped vectors, we demonstrated that type I IFN production by myeloid DC was, in contrast to pDC, independent of contaminants such as tubulovesicular structures present in VSV.G-pseudotyped lentiviral preparations (37). Interestingly, using deficient reverse transcription mutants, we demonstrated that IFN-β production was dependent on reverse transcription, possibly by double-stranded intermediates and their sensing receptor(s).
Besides type I IFN production, it was shown previously that in vivo lentivector administration results in the production of proinflammatory cytokines (43, 44). It was suggested that transduced CD11c+ DC were responsible. We now demonstrate that in vivo-transduced DC isolated from mice vaccinated with lentivectors produce TNF-α. Importantly, in agreement with the above-mentioned studies, we demonstrated the necessity for cell entry and viral replication but not integration for full DC activation. The need for viral reverse transcription was true for phenotypical activation and TNF-α production and, moreover, was critical for the IFN-β response, which was almost absent upon transduction with reverse transcription-deficient lentiviral vectors. This need for reverse transcription activity points toward an important role for double-stranded nucleic acid-sensing receptors within the myeloid DC.
The experiments carried out with TRIF−/− DC further emphasize the need for such a receptor. The absence of TRIF, an adaptor molecule employed solely by the double-stranded RNA sensor TLR3 (with the exception of TLR4) (1), does not result in the production of IFN-β and, as such, suggests a role for TLR3. The experiments carried out with TRIF/MyD88−/− DC demonstrate that the observed DC activation is most likely dependent on TLR but not cytosolic pathogen recognition receptors. Since lentiviral vectors have potential ligands for TLR3 as well as TLR7 (1), the receptors mediating type I IFN and proinflammatory cytokine production, respectively, we further examined the role of both TLR in the observed DC activation. A role for TLR7 was demonstrated since TLR7−/− DC transduction resulted in low levels of proinflammatory cytokine production. These observations are in line with data from studies of pDC, where wild-type (4, 15) and recombinant (10, 37) lentiviral vectors were shown to interact with TLR7. Here, we show that TLR7 is also involved in the activation of myeloid DC. Importantly, the transduction of TLR7−/− DC did not affect IFN-β production, suggesting the existence of an additional trigger. Moreover, we showed that the latter was critically dependent on reverse transcription and, thus, the formation of double-stranded life cycle intermediates, since no activation whatsoever was observed upon transduction with deficient reverse transcriptase lentiviral vectors. For pDC, it was previously shown that TLR9, which is located in the endosomes and engaged by CpG DNA, is involved in pDC activation by VSV.G-pseudotyped lentiviral vectors (1, 10). However, TLR9 is not expressed in myeloid DC (1), and a more likely candidate is TLR3, which can interact with virally derived double-stranded RNA. Our previous results, i.e., the necessity for reverse transcription and TRIF for the production of IFN-β, already suggested that TLR3 might be the missing link. The transduction of TLR3−/− DC confirmed this hypothesis. The TLR3−/− DC produced low to undetectable amounts of TNF-α and IFN-β, emphasizing the need for TLR3 for full DC activation. In conclusion, TLR3 signaling and TLR7 signaling via TRIF and MyD88, respectively, are critical for the activation of myeloid DC by lentivectors.
Importantly, it was demonstrated previously that the simultaneous engagement of different TLR on DC, among which are TLR7 and TLR3, results in the production of type I IFN and the activation of the NF-κB pathway (16). These results are similar to what we observed upon lentiviral transduction. Moreover, it was demonstrated previously by similar studies with DC and TLR agonists that this combined stimulation evokes a sustained activation of DC and enhances effector T-cell functions (35, 45). We confirmed these data showing that lentivirally transduced TLR3−/− or TLR7−/− DC do induce an immune response to the model antigen ovalbumin but to a lesser extent than wild-type DC, which are simultaneously activated by TLR3 and TLR7.
In conclusion, we have presented evidence that recombinant lentiviral vectors initiate adaptive immune responses to the delivered transgene through a TLR-dependent activation of myeloid DC. These data have important implications for the use of lentiviral vectors as a treatment modality for cancer and infectious diseases as well as autoimmunity and allow the rational design of strategies to enhance or suppress immune activation, respectively.
Acknowledgments
K.B. is a postdoctoral fellow and M.K. is an aspirant of the Research Foundation-Flanders (FWO). Their research is further funded by the Interuniversity Attraction Poles Program, Belgian State, Belgian Science Policy. D.E. is supported by an Arthritis Research Campaign Career Development fellowship, and K.K. is supported by an MRC studentship.
We thank Carlo Heirman and Joeri Pen (Medical School of the Vrije Universiteit Brussel) for their help with cloning and the generation of bone marrow-derived DC, respectively. Furthermore, we thank Neil Rogers and Caetano Reis e Sousa (London Research Institute) for the kind gift of C57BL/6 MyD88/TRIF−/−, TLR3−/−, and TLR7−/− mice.
Footnotes
Published ahead of print on 17 March 2010.
REFERENCES
- 1.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 2.Apolonia, L., S. N. Waddington, C. Fernandes, N. J. Ward, G. Bouma, M. P. Blundell, A. J. Thrasher, M. K. Collins, and N. J. Philpott. 2007. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 15:1947-1954. [DOI] [PubMed] [Google Scholar]
- 3.Arce, F., H. M. Rowe, B. Chain, L. Lopes, and M. K. Collins. 2009. Lentiviral vectors transduce proliferating dendritic cell precursors leading to persistent antigen presentation and immunization. Mol. Ther. 17:1643-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. J. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Invest. 115:3265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon, T., P. G. Coulie, B. J. Van den Eynde, and P. van der Bruggen. 2006. Human T cell responses against melanoma. Annu. Rev. Immunol. 24:175-208. [DOI] [PubMed] [Google Scholar]
- 6.Breckpot, K., J. L. Aerts, and K. Thielemans. 2007. Lentiviral vectors for cancer immunotherapy: transforming infectious particles into therapeutics. Gene Ther. 14:847-862. [DOI] [PubMed] [Google Scholar]
- 7.Breckpot, K., M. Dullaers, A. Bonehill, S. van Meirvenne, C. Heirman, C. de Greef, P. van der Bruggen, and K. Thielemans. 2003. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 5:654-667. [DOI] [PubMed] [Google Scholar]
- 8.Breckpot, K., and D. Escors. 2009. Dendritic cells for active anti-cancer immunotherapy: targeting activation pathways through genetic modification. Endocr. Metab. Immune Disord. Drug Targets 9:328-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breckpot, K., P. Emeagi, M. Dullaers, A. Michiels, C. Heirman, and K. Thielemans. 2007. Activation of immature monocyte-derived dendritic cells after transduction with high doses of lentiviral vectors. Hum. Gene Ther. 18:536-546. [DOI] [PubMed] [Google Scholar]
- 10.Brown, B. D., G. Sitia, A. Annoni, E. Hauben, L. Sergi Sergi, A. Zingale, M. G. Roncarolo, L. G. Guidotti, and L. Naldini. 2007. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood 109:2797-2805. [DOI] [PubMed] [Google Scholar]
- 11.Chapatte, L., S. Colombetti, J. C. Cerottini, and F. Levy. 2006. Efficient induction of tumor antigen-specific CD8+ memory T cells by recombinant lentivectors. Cancer Res. 66:1155-1160. [DOI] [PubMed] [Google Scholar]
- 12.Dullaers, M., S. Van Meirvenne, C. Heirman, L. Straetman, A. Bonehill, J. L. Aerts, K. Thielemans, and K. Breckpot. 2006. Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther. 13:630-640. [DOI] [PubMed] [Google Scholar]
- 13.Escors, D., L. Lopes, R. Lin, J. Hiscott, S. Akira, R. J. Davis, and M. K. Collins. 2008. Targeting dendritic cell signaling to regulate the response to immunization. Blood 111:3050-3061. [DOI] [PubMed] [Google Scholar]
- 14.Esslinger, C., L. Chapatte, D. Finke, I. Miconnet, P. Guillaume, F. Levy, and H. R. MacDonald. 2003. In vivo administration of a lentiviral vaccine targets DCs and induces efficient CD8(+) T cell responses. J. Clin. Invest. 111:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier, G., M. Humbert, F. Deauvieau, M. Scuiller, J. Hiscott, E. E. Bates, G. Trinchieri, C. Caux, and P. Garrone. 2005. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J. Exp. Med. 201:1435-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber, A., J. Kan-Mitchell, K. L. Kuhen, T. Mukai, and F. Wong-Staal. 2000. Dendritic cells transduced by multiply deleted HIV-1 vectors exhibit normal phenotypes and functions and elicit an HIV-specific cytotoxic T-lymphocyte response in vitro. Blood 96:1327-1333. [PubMed] [Google Scholar]
- 18.He, Y., and L. D. Falo. 2006. Induction of T cell immunity by cutaneous genetic immunization with recombinant lentivector. Immunol. Res. 36:101-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He, Y., J. Zhang, C. Donahue, and L. D. Falo, Jr. 2006. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity 24:643-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, Y., J. Zhang, Z. Mi, P. Robbins, and L. D. Falo, Jr. 2005. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J. Immunol. 174:3808-3817. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 22.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. U. S. A. 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kämpgen, E., N. Koch, F. Koch, P. Stoger, C. Heufler, G. Schuler, and N. Romani. 1991. Class II major histocompatibility complex molecules of murine dendritic cells: synthesis, sialylation of invariant chain, and antigen processing capacity are down-regulated upon culture. Proc. Natl. Acad. Sci. U. S. A. 88:3014-3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karwacz, K., S. Mukherjee, L. Apolonia, M. P. Blundell, G. Bouma, D. Escors, M. K. Collins, and A. J. Thrasher. 2009. Nonintegrating lentivector vaccines stimulate prolonged T-cell and antibody responses and are effective in tumor therapy. J. Virol. 83:3094-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushik, N., N. Rege, P. N. Yadav, S. G. Sarafianos, M. J. Modak, and V. N. Pandey. 1996. Biochemical analysis of catalytically crucial aspartate mutants of human immunodeficiency virus type 1 reverse transcriptase. Biochemistry 35:11536-11546. [DOI] [PubMed] [Google Scholar]
- 26.Kawai, T., O. Adachi, T. Ogawa, K. Takeda, and S. Akira. 1999. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11:115-122. [DOI] [PubMed] [Google Scholar]
- 27.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 28.Keyaerts, M., J. Verschueren, T. J. Bos, L. O. Tchouate-Gainkam, C. Peleman, K. Breckpot, C. Vanhove, V. Caveliers, A. Bossuyt, and T. Lahoutte. 2008. Dynamic bioluminescence imaging for quantitative tumour burden assessment using IV or IP administration of D-luciferin: effect on intensity, time kinetics and repeatability of photon emission. Eur. J. Nucl. Med. Mol. Imaging 35:999-1007. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. H., N. Majumder, H. Lin, S. Watkins, L. D. Falo, Jr., and Z. You. 2005. Induction of therapeutic antitumor immunity by in vivo administration of a lentiviral vaccine. Hum. Gene Ther. 16:1255-1266. [DOI] [PubMed] [Google Scholar]
- 30.Koga, R., S. Hamano, H. Kuwata, K. Atarashi, M. Ogawa, H. Hisaeda, M. Yamamoto, S. Akira, K. Himeno, M. Matsumoto, and K. Takeda. 2006. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J. Immunol. 177:7059-7066. [DOI] [PubMed] [Google Scholar]
- 31.Loisel-Meyer, S., T. Felizardo, J. Mariotti, M. E. Mossoba, J. E. Foley, R. Kammerer, N. Mizue, R. Keefe, J. A. McCart, W. Zimmermann, B. Dropulic, D. H. Fowler, and J. A. Medin. 2009. Potent induction of B- and T-cell immunity against human carcinoembryonic antigen-expressing tumors in human carcinoembryonic antigen transgenic mice mediated by direct lentivector injection. Mol. Cancer Ther. 8:692-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes, L., M. Dewannieux, U. Gileadi, R. Bailey, Y. Ikeda, C. Whittaker, M. P. Collin, V. Cerundolo, M. Tomihari, K. Ariizumi, and M. K. Collins. 2008. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J. Virol. 82:86-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe, D. M., V. Parmar, S. D. Kemp, and B. A. Larder. 1991. Mutational analysis of two conserved sequence motifs in HIV-1 reverse transcriptase. FEBS Lett. 282:231-234. [DOI] [PubMed] [Google Scholar]
- 34.Metharom, P., K. A. Ellem, C. Schmidt, and M. Q. Wei. 2001. Lentiviral vector-mediated tyrosinase-related protein 2 gene transfer to dendritic cells for the therapy of melanoma. Hum. Gene Ther. 12:2203-2213. [DOI] [PubMed] [Google Scholar]
- 35.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmowski, M. J., L. Lopes, Y. Ikeda, M. Salio, V. Cerundolo, and M. K. Collins. 2004. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J. Immunol. 172:1582-1587. [DOI] [PubMed] [Google Scholar]
- 37.Pichlmair, A., S. S. Diebold, S. Gschmeissner, Y. Takeuchi, Y. Ikeda, M. K. Collins, and C. Reis e Sousa. 2007. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J. Virol. 81:539-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierre, P., S. J. Turley, E. Gatti, M. Hull, J. Meltzer, A. Mirza, K. Inaba, R. M. Steinman, and I. Mellman. 1997. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature 388:787-792. [DOI] [PubMed] [Google Scholar]
- 39.Rowe, H. M., L. Lopes, Y. Ikeda, R. Bailey, I. Barde, M. Zenke, B. M. Chain, and M. K. Collins. 2006. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol. Ther. 13:310-319. [DOI] [PubMed] [Google Scholar]
- 40.Steinman, R. M., and J. Banchereau. 2007. Taking dendritic cells into medicine. Nature 449:419-426. [DOI] [PubMed] [Google Scholar]
- 41.Tan, P. H., S. C. Beutelspacher, S. A. Xue, Y. H. Wang, P. Mitchell, J. C. McAlister, D. F. Larkin, M. O. McClure, H. J. Stauss, M. A. Ritter, G. Lombardi, and A. J. George. 2005. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood 105:3824-3832. [DOI] [PubMed] [Google Scholar]
- 42.Tuyaerts, S., J. L. Aerts, J. Corthals, B. Neyns, C. Heirman, K. Breckpot, K. Thielemans, and A. Bonehill. 2007. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol. Immunother. 56:1513-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandendriessche, T., L. Thorrez, A. Acosta-Sanchez, I. Petrus, L. Wang, L. Ma, L. De Waele, Y. Iwasaki, V. Gillijns, J. M. Wilson, D. Collen, and M. K. Chuah. 2007. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J. Thromb. Haemost. 5:16-24. [DOI] [PubMed] [Google Scholar]
- 44.VandenDriessche, T., L. Thorrez, L. Naldini, A. Follenzi, L. Moons, Z. Berneman, D. Collen, and M. K. Chuah. 2002. Lentiviral vectors containing the human immunodeficiency virus type-1 central polypurine tract can efficiently transduce nondividing hepatocytes and antigen-presenting cells in vivo. Blood 100:813-822. [DOI] [PubMed] [Google Scholar]
- 45.Warger, T., P. Osterloh, G. Rechtsteiner, M. Fassbender, V. Heib, B. Schmid, E. Schmitt, H. Schild, and M. P. Radsak. 2006. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood 108:544-550. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, N. S., D. El-Sukkari, and J. A. Villadangos. 2004. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 103:2187-2195. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 48.Zarei, S., F. Leuba, J. F. Arrighi, C. Hauser, and V. Piguet. 2002. Transduction of dendritic cells by antigen-encoding lentiviral vectors permits antigen processing and MHC class I-dependent presentation. J. Allergy Clin. Immunol. 109:988-994. [DOI] [PubMed] [Google Scholar]