Abstract
Recent studies indicate that sexual transmission of human immunodeficiency virus type 1 (HIV-1) generally results from productive infection by only one virus, a finding attributable to the mucosal barrier. Surprisingly, a recent study of injection drug users (IDUs) from St. Petersburg, Russia, also found most subjects to be acutely infected by a single virus. Here, we show by single-genome amplification and sequencing in a different IDU cohort that 60% of IDU subjects were infected by more than one virus, including one subject who was acutely infected by at least 16 viruses. Multivariant transmission was more common in IDUs than in heterosexuals (60% versus 19%; odds ratio, 6.14; 95% confidence interval [CI], 1.37 to 31.27; P = 0.008). These findings highlight the diversity in HIV-1 infection risks among different IDU cohorts and the challenges faced by vaccines in protecting against this mode of infection.
Elucidation of virus-host interactions during and immediately following the transmission event is one of the great challenges and opportunities in human immunodeficiency virus (HIV)/AIDS prevention research (14-16, 31, 34, 45). Recent innovations involving single-genome amplification (SGA), direct amplicon sequencing, and phylogenetic inference based on a model of random virus evolution (18-20, 43) have allowed for the identification of transmitted/founder viruses that actually cross from donor to recipient, leading to productive HIV type 1 (HIV-1) infection. Our laboratory and others have made the surprising finding that HIV-1 transmission results from productive infection by a single transmitted/founder virus (or virally infected cell) in ∼80% of HIV-infected heterosexuals and in ∼60% of HIV-infected men who have sex with men (MSM) (1, 13, 18, 24). These studies thus provided a precise quantitative estimate for the long-recognized genetic bottleneck in HIV-1 transmission (6, 11-13, 17, 25, 28, 30, 35, 38, 42, 47-49) and a plausible explanation for the low acquisition rate per coital act and for graded infection risks associated with different exposure routes and behaviors (15, 36).
In contrast to sexual transmission of HIV-1, virus transmission resulting from injection drug use has received relatively little attention (2, 3, 29, 42) despite the fact that injection drug use-associated transmission accounts for as many as 10% of new infections globally (26, 46). We hypothesized that SGA strategies developed for identifying transmitted/founder viruses following mucosal acquisition are applicable to deciphering transmission events following intravenous inoculation and that, due to the absence of a mucosal barrier, injection drug users (IDUs) exhibit a higher frequency of multiple-variant transmission and a wider range in numbers of transmitted viruses than do acutely infected heterosexual subjects. We obtained evidence in support of these hypotheses from the simian immunodeficiency virus (SIV)-Indian rhesus macaque infection model, where we showed that discrete low-diversity viral lineages emanating from single or multiple transmitted/founder viruses could be identified following intravenous inoculation and that the rectal mucosal barrier to infection was 2,000- to 20,000-fold greater than with intravenous inoculation (19). However, we also recognized potentially important differences between virus transmission in Indian rhesus macaques and virus transmission in humans that could complicate an IDU acquisition study. For example, in the SIV macaque model, the virus inocula can be well characterized genetically and the route and timing of virus exposure in relation to plasma sampling precisely defined, whereas in IDUs, the virus inoculum is generally undefined and the timing of virus infection only approximated based on clinical history and seroconversion testing (8). In addition, IDUs may have additional routes of potential virus acquisition due to concomitant sexual activity. Finally, there is a paucity of IDU cohorts for whom incident infection is monitored sufficiently frequently and clinical samples are collected often enough to allow for the identification and enumeration of transmitted/founder viruses. To address these special challenges, we proposed a pilot study of 10 IDU subjects designed to determine with 95% confidence if the proportion of multivariant transmissions in IDUs was more than 2-fold greater than the 20% frequency established for heterosexual transmission (1, 13, 18, 24). A secondary objective of the study was to determine whether the range in numbers of transmitted/founder viruses in IDUs exceeded the 1-to-6 range observed in heterosexuals (1, 13, 18, 24). To ensure comparability among the studies, we employed SGA-direct amplicon sequencing approaches, statistical methods, and power calculations identical to those that we had used previously to enumerate transmitted/founder viruses in heterosexual and MSM cohorts (1, 13, 18, 20, 24).
We first surveyed investigators representing acute-infection cohorts in the United States, Canada, Russia, and China; only one cohort—the Montreal Primary HIV Infection Cohort (41)—had IDU clinical samples and clinical data available for study. The Montreal cohort of subjects with acute and early-stage HIV-1 infection was established in 1996 and recruits subjects from both academic and private medical centers throughout the city. Injection drug use is an important contributing factor to Montreal's HIV burden, with IDUs comprising approximately 20% of the city's AIDS cases and 35% of the cohort (21, 40, 41). A large proportion of Montreal's IDUs use injection cocaine, with 50 to 69% of subjects reporting cocaine as their injection drug of choice (4, 5, 9, 22, 23).
Subjects with documented serological evidence of recent HIV-1 infection and a concurrent history of injection drug use were selected for study. These individuals had few or no reported risk factors for sexual HIV-1 acquisition. Clinical history and laboratory tests of HIV-1 viremia and antibody seroconversion were used to determine the Fiebig clinical stage (8) and to estimate the date of infection (Table 1). One subject was determined to be in Fiebig stage III, one subject was in Fiebig stage IV, five subjects were in Fiebig stage V, and three subjects were in Fiebig stage VI. We performed SGA-direct amplicon sequencing on stored plasma samples and obtained a total of 391 3′ half-genomes (median, 25 per subject; range, 19 to 167). Nine of these sequences contained large deletions or were G-to-A hypermutated and were excluded from subsequent analysis. Sequences were aligned, visually inspected using the Highlighter tool (www.hiv.lanl.gov/content/sequence/HIGHLIGHT/highlighter.html), and analyzed by neighbor-joining (NJ) phylogenetic-tree construction. A composite NJ tree of full-length gp160 env sequences from all 10 subjects (Fig. 1A) revealed distinct patient-specific monophyletic lineages, each with high bootstrap support and separated from the others by a mean genetic distance of 10.79% (median, 11.29%; range, 3.00 to 13.42%). Maximum within-patient env gene diversity ranged from 0.23% to 3.34% (Table 1). Four subjects displayed distinctly lower within-patient maximum env diversities (0.23 to 0.49%) than the other six subjects (1.48% to 3.34%). The lower maximum env diversities in the former group are consistent with infection either by a single virus or by multiple closely related viruses, while the higher diversities can be explained only by transmission of more than one virus based on empirical observations (1, 13, 18, 24) and mathematical modeling (18, 20).
TABLE 1.
Subject demographics and HIV-1 envelope analysis results
| Subject identifier | Age (yr) | Sexa | Fiebig stage | Estimated no. of days postinfectionb | CD4 count | Plasma viral load (log) | No. of SGA amplicons | Diversity of env genes (%)c |
No. of transmitted/ founder viruses |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Interquartile range | Maximumd | Model predictione | Phylogenetic estimatef | ||||||||
| HDNDRPI034 | 47 | M | III | 29 | 240 | 7.88 | 163 | 1.07 | 0.55 | 3.34 | >1 | 16 |
| HDNDRPI029 | 18 | F | IV | 48 | 440 | 4.34 | 29 | 0.16 | 0.15 | 0.49 | 1 | 1 |
| HTM385 | 24 | M | V | 62 | 406 | 5.37 | 22 | 0.12 | 0.08 | 0.27 | 1 | 1 |
| CQLDR03 | 42 | M | V | 66 | NDg | 5.01 | 21 | 0.08 | 0.08 | 0.23 | 1 | 1 |
| HDNDRPI001 | 36 | M | V | 28 | 690 | 5.94 | 25 | 0.90 | 0.63 | 1.91 | >1 | 5 |
| HTM319 | 39 | M | V | 68 | 520 | 4.43 | 25 | 0.77 | 0.46 | 1.54 | >1 | 3 |
| HDNDRPI032 | 37 | M | V | 73 | 1,040 | 3.53 | 19 | 1.48 | 2.99 | 3.34 | >1 | 3 |
| ACTDM580208 | 39 | M | VI | 93 | 387 | 4.53 | 30 | 1.17 | 0.97 | 2.64 | >1 | 3 |
| ACT54869022 | 28 | M | VI | 68 | 723 | 3.43 | 27 | 0.07 | 0.04 | 0.24 | 1 | 1 |
| PSL024 | 46 | M | VI | 82 | 340 | 4.46 | 21 | 0.82 | 0.63 | 1.57 | >1 | 3 |
M, male; F, female.
Numbers of days postinfection were estimated on the basis of serological markers, clinical symptoms, or a history of a high-risk behavior leading to virus exposure.
Diversity measurements determined by PAUP* analysis.
The model prediction of the maximum achievable env diversity 100 days after transmission is 0.60% (95% CI, 0.54 to 0.68%). Diversity values exceeding this range imply transmission and productive infection by more than one virus. Diversity values less than 0.54% can be explained by transmission of one virus or of multiple closely related viruses (18).
Model described in Keele et al. (18).
Minimum estimate of transmitted/founder viruses.
ND, not determined.
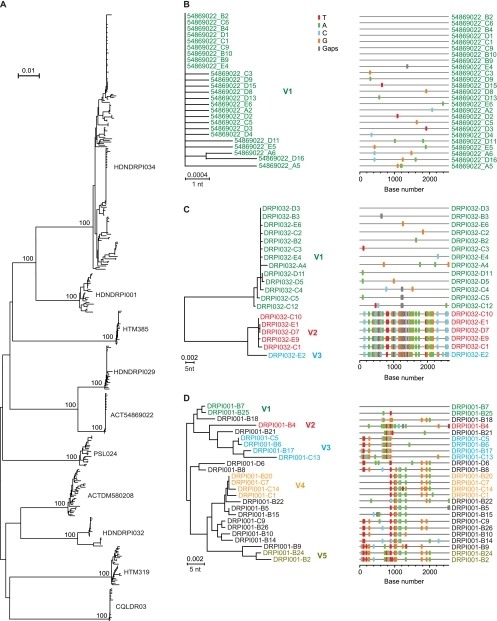
FIG. 1.
NJ trees and Highlighter plots of HIV-1 gp160 env sequences. (A) Composite tree of 382 gp160 env sequences from all study subjects. The numerals at the nodes indicate bootstrap values for which statistical support exceeded 70%. (B) Subject ACT54869022 sequences suggest productive infection by a single virus (V1). (C) Subject HDNDRPI032 sequences suggest productive infection by as many as three viruses. (D) Subject HDNDRPI001 sequences suggest productive infection by at least five viruses with extensive interlineage recombination. Sequences are color coded to indicate viral progeny from distinct transmitted/founder viruses. Recombinant virus sequences are depicted in black. Methods for SGA, sequencing, model analysis, Highlighter plotting, and identification of transmitted/founder virus lineages are described elsewhere (18, 20, 24, 44). The horizontal scale bars represent genetic distance. nt, nucleotide.
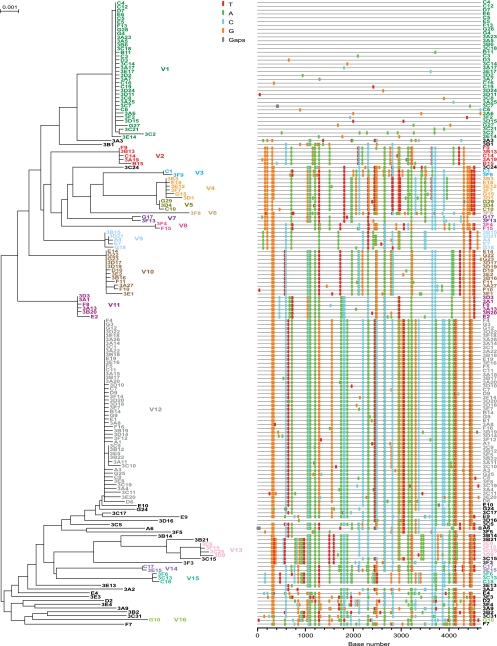
An example of productive clinical infection by a single virus is shown in phylogenetic tree and Highlighter plots from subject ACT54869022 (Fig. 1B). A similar phylogenetic pattern of single-variant transmission was found in 4 of 10 IDU subjects (Table 1). Examples of multivariant transmission are shown for subject HDNDRPI032, for whom there was evidence of infection by 3 transmitted/founder viruses (Fig. 1C) and for subject HDNDRPI001, for whom there was evidence of infection by at least 5 transmitted/founder viruses (Fig. 1D). One IDU subject, HDNDRPI034, had evidence of multivariant transmission to an extent not previously seen in any of 225 subjects who acquired their infection by mucosal routes (1, 13, 18, 24) or in any of 13 IDUs, as recently reported by Masharsky and colleagues (29). We greatly extended the depth of our analysis in this subject to include 163 3′ half-genome sequences in order to increase the sensitivity of detection of low-frequency viral variants. Power calculations indicated that a sample size of 163 sequences gave us a >95% probability of sampling minor variants comprising as little as 2% of the virus population. By this approach, we found evidence of productive infection by at least 16 genetically distinct viruses (Fig. 2). Fourteen of these could be identified unambiguously based on the presence of discrete low-diversity viral lineages, each consisting of between 2 and 48 sequences. Two additional unique viral sequences with long branch lengths (3F8 and G10) exhibited diversity that was sufficiently great to indicate a distinct transmission event as opposed to divergence from other transmitted/founder lineages (see the legend to Fig. 2). It is possible that still other unique sequences from this subject also represented transmitted/founder viruses, but we could not demonstrate this formally. We also could not determine if all 16 (or more) transmission events resulted from a single intravenous inoculation or from a series of inoculations separated by hours or days; however, it is likely that all transmitted viruses in this subject resulted from exposure to plasma from a single infected individual, since the maximum env diversity was only 3.34% (Fig. 1A). It is also likely that transmission occurred within a brief window of time, since the period from transmission to the end of Fiebig stage III is typically only about 25 days (95% CI, 22 to 37 days) (18, 20) and the diversity observed in all transmitted/founder viral lineages in subject HDNDRPI034 was exceedingly low, consistent with model predictions for subjects with very recent infections (18, 20).
FIG. 2.
NJ tree and Highlighter plot of HIV-1 3′ half-genome sequences from subject HDNDRPI034. Sequences emanating from 16 transmitted/founder viruses are color coded. Fourteen transmitted/founder viral lineages comprised of 2 or more identical or nearly identical sequences could be readily distinguished from recombinant sequences (depicted in black), which invariably appeared as unique sequences containing interspersed segments shared with other transmitted/founder virus lineages. The two sequences with the longest branch lengths (3F8 and G10) were interpreted to represent rare progeny of discrete transmitted/founder viruses because their unique polymorphisms far exceeded the maximum diversity estimated to occur in the first 30 days of infection (0.22%; CI, 0.15 to 0.31%) (18) and far exceeded the diversity observed within the other transmitted/founder virus lineages. The horizontal scale bar represents genetic distance.
Lastly, we compared the multiplicity of HIV-1 transmission in the Montreal IDU subjects with that of non-IDU subjects for whom identical SGA methods had been employed. In this combined-cohort analysis, we found the frequency of multiple-variant transmission in heterosexuals to be 19% (34 of 175) and in MSM 38% (19 of 50) (Table 2) (24). The current study was powered to detect a >2-fold difference in multivariant transmission between IDUs and heterosexual subjects; in fact, we observed a 3-fold-higher frequency of multiple-variant transmission in Montreal IDUs (6 of 10 subjects [60%]) than in heterosexuals (odds ratio, 6.14; 95% CI, 1.37 to 31.27; Fisher exact test, P = 0.008) and a 1.5-fold-higher frequency in Montreal IDUs than in MSM (odds ratio, 2.41; 95% CI, 0.50 to 13.20; P = 0.294, not significant). In addition, we found that the range of numbers of transmitted/founder viruses was greater in IDUs (range, 1 to 16 viruses; median, 3) than in either heterosexuals (range, 1 to 6 viruses; median, 1) or MSM (range, 1 to 10 viruses; median, 1). The finding of larger numbers of transmitted/founder viruses in IDUs was not simply the result of more intensive sampling, since the numbers of sequences analyzed in all studies were comparable. Moreover, it is notable that in studies reported elsewhere, we sampled as many as 239 sequences by SGA or as many as 500,000 sequences by 454 pyrosequencing from four acutely infected MSM subjects and in each case found evidence of productive clinical infection by only a single virus (24; W. Fischer, B. Keele, G. Shaw, and B. Korber, unpublished). These results thus suggest that IDUs may be infected by more viruses and by a greater range of viruses than is the case following mucosal transmission. On this count, our findings differ from those reported by Masharsky and coworkers for an IDU cohort from St. Petersburg, Russia (29). Their study found a low frequency of multiple virus transmissions (31%), not significantly different from that of acutely infected heterosexuals, and a low number of transmitted/founder viruses (range, 1 to 3 viruses; median, 1). Because the SGA methods employed in both studies were identical, the numbers of sequences analyzed per subject were comparable (median of 25 sequences in Montreal versus 33 in St. Petersburg), and because the discriminating power of the SGA-direct sequencing method was sufficient to distinguish transmitted/founder viruses differing by as few as 3 nucleotides, or <0.1% of nucleotides (Fig. 2, compare lineages V4 and V5), it is unlikely that differences in the genetic diversity of HIV-1 in the two IDU populations explain the differences in findings between the two studies. Instead, we suspect that the explanation lies in the small cohort sizes (10 versus 13 subjects) and the particular risk behaviors of the IDUs in each cohort. The Russian cohort is heavily weighted toward heroine use, whereas the Montreal cohort is weighted toward injection cocaine use, the latter being associated with more frequent drug administration and the attendant infection risks of needle sharing (4).
TABLE 2.
Multiplicity of HIV-1 infection in IDU, heterosexual, and MSM subjects
| Cohort | Reference | Virus subtype | Total no. of subjects | Single-variant transmission |
Multiple-variant transmission |
P value | Odds ratio | 95% CI | Median | Range | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of subjects | % of total | No. of subjects | % of total | |||||||||
| Heterosexuals | Keele et al. (18) | B | 79 | 65 | 82.30 | 14 | 17.70 | 1 | 1-4 | |||
| Abrahams et al. (1) | C | 69 | 54 | 78.30 | 15 | 21.70 | 1 | 1-5 | ||||
| Haaland et al. (13) | A or C | 27 | 22 | 81.50 | 5 | 18.50 | 1 | 1-6 | ||||
| Total | 175 | 141 | 80.60 | 34 | 19.40 | 0.008a | 6.14 | 1.37-31.27 | 1 | 1-6 | ||
| MSM | Keele et al. (18) | B | 22 | 13 | 59.10 | 9 | 40.90 | 1 | 1-6 | |||
| Li et al. (24) | B | 28 | 18 | 64.30 | 10 | 35.70 | 1 | 1-10 | ||||
| Total | 50 | 31 | 62.00 | 19 | 38.00 | 0.294b | 2.41 | 0.50-13.20 | 1 | 1-10 | ||
| IDUs | Bar | B | 10 | 4 | 40.00 | 6 | 60.00 | 3 | 1-16 | |||
Fisher's exact test of multiple-variant transmission in heterosexuals versus in IDUs.
Fisher's exact test of multiple-variant transmission in MSM versus in IDUs.
The results from the present study indicate that transmission of HIV-1 to IDUs can be associated with a high frequency of multiple-variant transmission and a broad range in the numbers of transmitted viruses. This wide variation in the multiplicity of HIV-1 infection in IDUs is likely due to the absence of a mucosal barrier to virus transmission (12, 19) and differences in the virus inocula (27, 29, 32, 39). The findings substantiate concerns raised in recent HIV-1 vaccine efficacy trials that different vaccine candidates may be more efficacious in preventing infection by some exposure routes than by others (7, 10, 33, 37). They further suggest that biological comparisons of molecularly cloned transmitted/founder viruses responsible for vaginal, rectal, penile, and intravenous infection could facilitate a mechanistic understanding of HIV-1 transmission and vaccine prevention (24, 44).
Nucleotide sequence accession numbers.
HIV-1 sequences were deposited in GenBank under accession numbers GU562001 to GU562291 and GU938194 to GU938295. See also www.hiv.lanl.gov/content/sequence/HIV/USER_ALIGNMENTS/Bar.
Acknowledgments
We thank the study participants; M. Legault, D. Rouleau, R. LeBlanc, R. Thomas, B. Trottier, P. Cote, J. Baril, B. Brenner, M. Wainberg, and the clinical staff affiliated with the Montreal Primary HIV-1 Infection Cohort; core research facilities of the Centers for HIV/AIDS Research at UAB; P. Hraber, R. Swanstrom, and J. Anderson for helpful discussions and sharing of data prior to publication; and J. White for manuscript preparation.
This work was supported by the Center for HIV/AIDS Vaccine Immunology (CHAVI), the Canadian Institutes of Health Research, Fonds de la Recherché en Santé du Quebec, and by grants and contracts from the National Institutes of Health (AI67854, AI27767, and HHSN266200400088C) and the Bill & Melinda Gates Foundation (grant 37874).
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Abrahams, M.-R., J. A. Anderson, E. E. Giorgi, C. Seoighe, K. Mlisana, L.-H. Ping, G. S. Athreya, F. K. Treurnicht, B. F. Keele, N. Wood, J. F. Salazar-Gonzalez, T. Bhattacharya, H. Chu, I. Hoffman, S. Galvin, C. Mapanje, P. Kazembe, R. Thebus, S. Fiscus, W. Hide, M. S. Cohen, S. A. Karim, B. F. Haynes, G. M. Shaw, B. H. Hahn, B. T. Korber, R. Swanstrom, and C. Williamson. 2009. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J. Virol. 83:3556-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aceijas, C., G. V. Stimson, M. Hickman, and T. Rhodes. 2004. Global overview of injecting drug use and HIV infection among injecting drug users. AIDS 18:2295-2303. [DOI] [PubMed] [Google Scholar]
- 3.Baggaley, R. F., M. C. Boily, R. G. White, and M. Alary. 2006. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS 20:805-812. [DOI] [PubMed] [Google Scholar]
- 4.Brogly, S. B., J. Bruneau, J. Vincelette, F. Lamothe, and E. L. Franco. 2000. Risk behaviour change and HIV infection among injection drug users in Montreal. AIDS 14:2575-2582. [DOI] [PubMed] [Google Scholar]
- 5.Bruneau, J., F. Lamothe, E. Franco, N. Lachance, M. Desy, J. Soto, and J. Vincelette. 1997. High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am. J. Epidemiol. 146:994-1002. [DOI] [PubMed] [Google Scholar]
- 6.Derdeyn, C. A., J. M. Decker, F. Bibollet-Ruche, J. L. Mokili, M. Muldoon, S. A. Denham, M. L. Heil, F. Kasolo, R. Musonda, B. H. Hahn, G. M. Shaw, B. T. Korber, S. Allen, and E. Hunter. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019-2022. [DOI] [PubMed] [Google Scholar]
- 7.Dolin, R. 2009. HIV vaccine trial results—an opening for further research. N. Engl. J. Med. 361:2279-2280. [DOI] [PubMed] [Google Scholar]
- 8.Fiebig, E. W., D. J. Wright, B. D. Rawal, P. E. Garrett, R. T. Schumacher, L. Peddada, C. Heldebrant, R. Smith, A. Conrad, S. H. Kleinman, and M. P. Busch. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871-1879. [DOI] [PubMed] [Google Scholar]
- 9.Fischer, B., J. Rehm, S. Brissette, S. Brochu, J. Bruneau, N. El-Guebaly, L. Noel, M. Tyndall, C. Wild, P. Mun, and D. Baliunas. 2005. Illicit opioid use in Canada: comparing social, health, and drug use characteristics of untreated users in five cities (OPICAN study). J. Urban Health 82:250-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, N. M., D. N. Forthal, C. D. Harro, F. N. Judson, K. H. Mayer, and M. F. Para. 2005. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J. Infect. Dis. 191:654-665. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb, G. S., L. Heath, D. C. Nickle, K. G. Wong, S. E. Leach, B. Jacobs, S. Gezahegne, A. B. van 't Wout, L. P. Jacobson, J. B. Margolick, and J. I. Mullins. 2008. HIV-1 variation before seroconversion in men who have sex with men: analysis of acute/early HIV infection in the multicenter AIDS cohort study. J. Infect. Dis. 197:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenier, J. L., C. J. Miller, D. Lü, P. J. Dailey, F. X. Lu, K. J. Kunstman, S. M. Wolinsky, and M. L. Marthas. 2001. Route of simian immunodeficiency virus inoculation determines the complexity but not the identity of viral variant populations that infect rhesus macaques. J. Virol. 75:3753-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haaland, R. E., P. A. Hawkins, J. Salazar-Gonzalez, A. Johnson, A. Tichacek, E. Karita, O. Manigart, J. Mulenga, B. F. Keele, G. M. Shaw, B. H. Hahn, S. A. Allen, C. A. Derdeyn, and E. Hunter. 2009. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 5:e1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haase, A. T. 2005. Perils at mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5:783-792. [DOI] [PubMed] [Google Scholar]
- 15.Hladik, F., and M. J. McElrath. 2008. Setting the stage: host invasion by HIV. Nat. Rev. Immunol. 8:447-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hladik, F., P. Sakchalathorn, L. Ballweber, G. Lentz, M. Fialkow, D. Eschenbach, and M. J. McElrath. 2007. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearney, M., F. Maldarelli, W. Shao, J. B. Margolick, E. S. Daar, J. W. Mellors, V. Rao, J. M. Coffin, and S. Palmer. 2009. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J. Virol. 83:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L. H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keele, B. F., H. Li, G. H. Learn, P. Hraber, E. E. Giorgi, T. Grayson, C. Sun, Y. Chen, W. W. Yeh, N. L. Letvin, J. R. Mascola, G. J. Nabel, B. F. Haynes, T. Bhattacharya, A. S. Perelson, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, H. Y., E. E. Giorgi, B. F. Keele, B. Gaschen, G. S. Athreya, J. F. Salazar-Gonzalez, K. T. Pham, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, B. H. Hahn, G. M. Shaw, B. T. Korber, T. Bhattacharya, and A. S. Perelson. 2009. Modeling sequence evolution in acute HIV-1 infection. J. Theor. Biol. 261:341-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legault, M., R. Thomas, B. Trottier, D. Rouleau, J. Baril, P. Cote, C. Tremblay, R. LeBlanc, A. Dascal, and J. Routy. 2008. Abstr. 17th Annu. Can. Conf. HIV/AIDS Res., Montreal, Quebec, Canada, abstr. 227.
- 22.Leri, F., J. Bruneau, and J. Stewart. 2003. Understanding polydrug use: review of heroin and cocaine co-use. Addiction 98:7-22. [DOI] [PubMed] [Google Scholar]
- 23.Leri, F., J. Stewart, A. Tremblay, and J. Bruneau. 2004. Heroin and cocaine co-use in a group of injection drug users in Montreal. J. Psychiatry Neurosci. 29:40-47. [PMC free article] [PubMed] [Google Scholar]
- 24.Li, H., K. J. Bar, S. Wang, J. M. Decker, Y. Chen, C. Sun, J. F. Salazar-Gonzalez, M. G. Salazar, G. H. Learn, C. J. Morgan, J. E. Schumacher, P. Hraber, E. E. Giorgi, T. Bhattacharya, B. T. Korber, A. S. Perelson, J. Eron, M. S. Cohen, C. B. Hicks, B. F. Haynes, M. Markowitz, B. F. Keele, B. H. Hahn, and G. M. Shaw. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog., in press. [DOI] [PMC free article] [PubMed]
- 25.Long, E. M., H. L. Martin, Jr., J. K. Kreiss, S. M. Rainwater, L. Lavreys, D. J. Jackson, J. Rakwar, K. Mandaliya, and J. Overbaugh. 2000. Gender differences in HIV-1 diversity at time of infection. Nat. Med. 6:71-75. [DOI] [PubMed] [Google Scholar]
- 26.Lu, L., M. Jia, Y. Ma, L. Yang, Z. Chen, D. D. Ho, Y. Jiang, and L. Zhang. 2008. The changing face of HIV in China. Nature 455:609-611. [DOI] [PubMed] [Google Scholar]
- 27.Ma, Z.-M., M. Stone, M. Piatak, Jr., B. Schweighardt, N. L. Haigwood, D. Montefiori, J. D. Lifson, M. P. Busch, and C. J. Miller. 2009. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J. Virol. 83:3288-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis, L., and R. Shattock. 2006. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nat. Rev. Microbiol. 4:312-317. [DOI] [PubMed] [Google Scholar]
- 29.Masharsky, A. E., E. N. Dukhovlinova, S. V. Verevochkin, O. V. Toussova, R. V. Skochilov, J. A. Anderson, I. Hoffman, M. S. Cohen, R. Swanstrom, and A. P. Kozlov. A significant transmission bottleneck among newly and recently HIV-1 infected injection drug users in St. Petersburg, Russia. J. Infect. Dis., in press. [DOI] [PubMed]
- 30.Miller, C. J., Q. Li, K. Abel, E.-Y. Kim, Z.-M. Ma, S. Wietgrefe, L. La Franco-Scheuch, L. Compton, L. Duan, M. D. Shore, M. Zupancic, M. Busch, J. Carlis, S. Wolinsky, and A. T. Haase. 2005. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 79:9217-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore, J. P., S. G. Kitchen, P. Pugach, and J. A. Zack. 2004. The CCR5 and CXCR4 coreceptors—central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retroviruses 20:111-126. [DOI] [PubMed] [Google Scholar]
- 32.Munch, J., E. Rucker, L. Standker, K. Adermann, C. Goffinet, M. Schindler, S. Wildum, R. Chinnadurai, D. Rajan, A. Specht, G. Gimenez-Gallego, P. C. Sanchez, D. M. Fowler, A. Koulov, J. W. Kelly, W. Mothes, J. C. Grivel, L. Margolis, O. T. Keppler, W. G. Forssmann, and F. Kirchhoff. 2007. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 131:1059-1071. [DOI] [PubMed] [Google Scholar]
- 33.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 34.Pope, M., and A. T. Haase. 2003. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat. Med. 9:847-852. [DOI] [PubMed] [Google Scholar]
- 35.Poss, M., H. L. Martin, J. K. Kreiss, L. Granville, B. Chohan, P. Nyange, K. Mandaliya, and J. Overbaugh. 1995. Diversity in virus populations from genital secretions and peripheral blood from women recently infected with human immunodeficiency virus type 1. J. Virol. 69:8118-8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powers, K. A., C. Poole, A. E. Pettifor, and M. S. Cohen. 2008. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect. Dis. 8:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rerks-Ngarm, S., P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J. G. McNeil, D. P. Francis, D. Stablein, D. L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M. L. Robb, N. L. Michael, P. Kunasol, and J. H. Kim. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 38.Ritola, K., C. D. Pilcher, S. A. Fiscus, N. G. Hoffman, J. A. E. Nelson, K. M. Kitrinos, C. B. Hicks, J. J. Eron, Jr., and R. Swanstrom. 2004. Multiple V1/V2 env variants are frequently present during primary infection with human immunodeficiency virus type 1. J. Virol. 78:11208-11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roan, N. R., and W. C. Greene. 2007. A seminal finding for understanding HIV transmission. Cell 131:1044-1046. [DOI] [PubMed] [Google Scholar]
- 40.Routy, J. P., N. Machouf, M. D. Edwardes, B. G. Brenner, R. Thomas, B. Trottier, D. Rouleau, C. L. Tremblay, P. Cote, J. G. Baril, R. S. Remis, R. P. Sekaly, and M. A. Wainberg. 2004. Factors associated with a decrease in the prevalence of drug resistance in newly HIV-1 infected individuals in Montreal. AIDS 18:2305-2312. [DOI] [PubMed] [Google Scholar]
- 41.Routy, J. P., P. Vanhems, D. Rouleau, C. Tsoukas, E. Lefebvre, P. Cote, R. LeBlanc, B. Conway, M. Alary, J. Bruneau, and R. P. Sekaly. 2000. Comparison of clinical features of acute HIV-1 infection in patients infected sexually or through injection drug use. The Investigators of the Quebec Primary HIV Infection Study. J. Acquir. Immune Defic. Syndr. 24:425-432. [DOI] [PubMed] [Google Scholar]
- 42.Sagar, M., E. Kirkegaard, E. M. Long, C. Celum, S. Buchbinder, E. S. Daar, and J. Overbaugh. 2004. Human immunodeficiency virus type 1 (HIV-1) diversity at time of infection is not restricted to certain risk groups or specific HIV-1 subtypes. J. Virol. 78:7279-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar-Gonzalez, J. F., E. Bailes, K. T. Pham, M. G. Salazar, M. B. Guffey, B. F. Keele, C. A. Derdeyn, P. Farmer, E. Hunter, S. Allen, O. Manigart, J. Mulenga, J. A. Anderson, R. Swanstrom, B. F. Haynes, G. S. Athreya, B. T. Korber, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar-Gonzalez, J. F., M. G. Salazar, B. F. Keele, G. H. Learn, E. E. Giorgi, H. Li, J. M. Decker, S. Wang, J. Baalwa, M. H. Kraus, N. F. Parrish, K. S. Shaw, M. B. Guffey, K. J. Bar, K. L. Davis, C. Ochsenbauer-Jambor, J. C. Kappes, M. S. Saag, M. S. Cohen, J. Mulenga, C. A. Derdeyn, S. Allen, E. Hunter, M. Markowitz, P. Hraber, A. S. Perelson, T. Bhattacharya, B. F. Haynes, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 46.UNAIDS. 2008. Report on the global AIDS epidemic. UNAIDS, Geneva, Switzerland.
- 47.Wolfs, T. F., G. Zwart, M. Bakker, and J. Goudsmit. 1992. HIV-1 genomic RNA diversification following sexual and parenteral virus transmission. Virology 189:103-110. [DOI] [PubMed] [Google Scholar]
- 48.Wolinsky, S. M., C. M. Wike, B. T. Korber, C. Hutto, W. P. Parks, L. L. Rosenblum, K. J. Kunstman, M. R. Furtado, and J. L. Munoz. 1992. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science 255:1134-1137. [DOI] [PubMed] [Google Scholar]
- 49.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]