Abstract
Chronic hepatitis C virus (HCV) infection is often associated with insulin resistance and hepatic steatosis. Insulin regulates gene expression of key enzymes in glucose and lipid metabolism by modulating the activity of specific Forkhead box transcriptional regulators (FoxO1 and FoxA2) via the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway in the liver. In this study, we observed that HCV infection of human hepatocytes impaired insulin-induced FoxO1 translocation from the nucleus to the cytoplasm and significantly reduced accumulation of FoxA2 in the nucleus. Phosphorylation of FoxO1 at Ser256, a downstream target for Akt, was inhibited in hepatocytes infected with HCV or expressing the core protein or full-length (FL) genome of HCV. Further, an interaction between FoxO1 and 14-3-3 protein, important for FoxO1 translocation, was inhibited in HCV core-expressing cells. Hepatocytes infected with HCV, expressing the core protein alone or polyprotein displayed an increased level of glucose-6-phosphatase (G6P) mRNA. On the other hand, microsomal triglycerol transfer protein (MTP) activity and apolipoprotein B (ApoB) secretion were significantly reduced in hepatocytes expressing HCV proteins. Together, these observations suggest that HCV infection or ectopic expression of the core protein either alone or together with other viral proteins from an FL gene construct differentially modulates FoxO1 and FoxA2 activation and affects insulin-induced metabolic gene regulation in human hepatocytes.
Chronic hepatitis C virus (HCV) infection is often associated with insulin resistance and hepatic steatosis (1, 5, 10, 28, 38). Insulin resistance is paradoxically associated with a reduced ability for insulin signaling to inhibit glucose production, whereas insulin-stimulated lipogenesis is enhanced in the liver. Insulin regulates gene expression of key enzymes in glucose and lipid metabolism by modulating the activity of specific Forkhead box transcriptional regulators (FoxO1 and FoxA2) in the liver. Insulin binds with receptors, activates Akt, and phosphorylates FoxO1. Akt-catalyzed phosphorylation of FoxO1 impairs its DNA binding ability with a concomitant inhibition of Fox-dependent gene expression (9). Phosphorylated FoxO1 may translocate from the nucleus to the cytoplasm, although the functional relevance of this translocation may not be fully related to localization of the protein (39). In the liver, FoxO1 mediates the expression of genes involved in both glucose and lipid metabolism (3, 24, 32, 34), while FoxA2 promotes lipid metabolism during fasting by triggering expression of the fatty acid oxidation program (41). FoxO1 has three serine/threonine residues that are potential targets for phosphorylation by serine/threonine kinase Akt, one of the downstream targets of phosphatidylinositol 3-kinase (PI3K), and plays an important role in mediating insulin effects. Phosphorylation of Ser256 at the C-terminal end of the DNA binding domain of FoxO1 is required for effective phosphorylation of Thr24 and Ser319, and phosphorylation at this site can impair DNA binding activity (43). The distribution of FoxO1 in insulin-responsive tissues and its regulation by insulin-stimulated Akt phosphorylation allow FoxO1 to mediate a variety of important metabolic functions (13). On the other hand, insulin inhibits FoxA2 through a mechanism that involves threonine phosphorylation at amino acid position 156 and possibly nuclear exclusion (42). Thus, insulin resistance may be mediated by the modulation of Forkhead box transcription factors, preventing optimal stimulation of the glucose-6-phosphatase (G6P) gene, triglyceride degradation, and fatty acid oxidation.
Hepatic glucose output is regulated by the G6P catalytic subunit (G6PC) and phosphoenolpyruvate carboxykinase (PEPCK) rate-limiting enzymes for gluconeogenesis and glucose release. Although several transcription factors have been shown to regulate gluconeogenesis, evidence is accumulating that in vivo shutdown of hepatic glucose output by insulin involves Akt-dependent phosphorylation of FoxO1, which controls the expression of G6P and PEPCK. On the other hand, FoxA2 activates genes involved in hepatic lipid metabolism. Activation of FoxA2 in the liver leads to increased oxidation and secretion of fatty acids in the form of triglycerols (TG). Very-low-density lipoprotein (VLDL) is secreted by hepatocytes in response to de novo synthesis of TG. The secretion and assembly of VLDL-associated triglycerides is regulated at various levels. The key enzyme controlling VLDL synthesis is the microsomal triglycerol transfer protein (MTP). VLDL synthesis may be regulated by FoxO1 and/or FoxA2 through the modulation of MTP expression. Functionally, MTP catalyzes the loading of lipids to the nascent apolipoprotein B (ApoB) in the endoplasmic reticulum. This stabilizes the newly synthesized ApoB and facilitates further processing, leading to its secretion. Reduction of MTP activity by inhibitors or gene knockouts effectively lowers the plasma lipoprotein level, whereas enforced expression of hepatic MTP in mice increases the plasma level of ApoB-containing lipoproteins (reviewed in reference 6). HCV may have an effect on MTP activity, resulting in fatty liver disease (30).
During chronic HCV infection, viral proteins may regulate the functions of FoxO1 and FoxA2, preventing optimal stimulation of normal metabolic functions of the liver. We have shown previously that HCV core protein modulates the insulin-signaling pathway by upregulating serine phosphorylation of insulin receptor substrate 1 (IRS-1) via Jun N-terminal protein kinase (JNK) and by altering Akt phosphorylation (7). However, we do not know how these observations functionally correlate with insulin resistance through the downstream mediators. A clear understanding of the downstream pathways in HCV-infected hepatocytes should provide a mechanistic clue for the development of HCV-induced insulin resistance. Therefore, in this study, we investigated the effect of HCV upon Akt modulation, FoxO1 and FoxA2 translocation and their activation status, and metabolic gene regulation of their associated functions.
MATERIALS AND METHODS
Transfection of hepatocytes and generation of cell culture-grown HCV.
Immortalized human hepatocytes (IHHs) were generated by transfection of HCV core plasmid DNA into primary human hepatocytes (8, 33). The IHHs displayed a weak level of HCV core protein expression, especially at the late-passage level (>20 passages) and were used for the generation of HCV genotype 1a. Huh7 cells were used separately for production of HCV genotype 2a. Huh7 cells were transfected with a plasmid DNA containing the HCV core or full-length (FL) gene under the control of a cytomegalovirus (CMV) promoter in a mammalian expression vector (pcDNA3-Core or pCI-neo-HCV FL, respectively), using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Stable colonies from transfected cells were selected, using neomycin as previously described (33). Mock-transfected cells were used in parallel as a control. HCV genotype 1a (clone H77) and genotype 2a (clone JFH1) were grown in human hepatocytes as previously described (19). Cell culture supernatant was filtered through a 0.45-μm-pore-size cellulose acetate membrane (Nalgene, Rochester, NY). HCV RNA was quantified by real-time PCR (in an ABI Prism 7000 real-time thermocycler), using HCV analyte-specific reagents ([ASRs] Abbott Molecular) (Department of Pathology, Saint Louis University). Virus growth was measured by a fluorescence focus-forming assay in focus forming units (FFUs)/ml, using a monoclonal antibody (HL1126) to HCV NS5a (genotype 2a) (kindly provided by Chen Liu, University of Florida) known to cross-react with HCV genotype 1a.
Reagents.
Commercially available antibodies to FoxA2, 14-3-3 protein (Santa Cruz Biotechnology, Inc., CA), phospho-FoxO1 (Ser256), FoxO1, phospho-Akt (Ser473, Thr308), and total Akt (Cell Signaling Technology, Danvers, MA), as well as horseradish peroxidase (HRP)-conjugated antibody to actin and UCN-01 (Sigma-Aldrich, St. Louis, MO), were procured. A-443654 compound was kindly provided by Vincent L. Giranda (Abbott Laboratories, Abbott Park, IL).
Fluorescence and confocal microscopy.
IHHs were transfected with FoxO1-green fluorescent protein (FoxO1-GFP) plasmid DNA (kindly provided by Domenico Accili, Columbia University, NY). Thirty-six hours after being transfected, cells were serum starved overnight and then were incubated at 37°C for 3 h in the presence or absence of insulin (100 nM). Alternatively, IHHs or Huh7 cells were infected at a multiplicity of infection (MOI) of 0.1 with HCV genotype 1a (H77) or genotype 2a (JFH1) (2). FoxO1-GFP was introduced by transfection, and cells were treated with insulin or left untreated. Mock-transfected or mock-infected cells were similarly treated as controls for comparison. Cells were fixed with formaldehyde (4%), stained for NS5A with mouse monoclonal antibody and a secondary antibody conjugated with Alexa Flour 598 (Molecular Probes, CA), and treated with ProLong Gold antifade reagent with DAPI ([4′6-diamidino-2-phenylindole] Invitrogen, Carlsbad, CA) for fluorescence or confocal microscopy (Olympus FV1000).
Huh7 cells were transiently transfected with HCV JFH1 transcripts for virus production. Cells were fixed after 48 h and incubated with a mouse monoclonal antibody to FoxA2 for endogenous-protein labeling. Cells were also stained with a rabbit antibody to HCV core protein and a secondary antibody conjugated with fluorochrome (anti-mouse Alexa Fluor 488 or anti-rabbit Alexa Fluor 568; Molecular Probes, Carlsbad, CA). Fluorescences were merged digitally to monitor colocalization.
Coimmunoprecipitation assay.
Huh7 cells were transfected with HCV core plasmid DNA, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Empty vector DNA was used in parallel as a negative control. Cells were serum starved overnight, treated with 100 nM insulin for 3 h, and lysed with TNTG buffer (30 mM Tris [pH 8.0], 150 mM NaCl, 1% Triton X-100, 10% glycerol, and a cocktail of protease inhibitors). After sonication, cell debris was removed by centrifugation. Clear cell lysates were mixed with antibody to FoxO1 (Cell Signaling) or 14-3-3 (Santa Cruz) and protein-G-Sepharose beads for 14 h. The beads were washed with TNTG buffer and subjected to SDS-PAGE under reducing conditions for Western blot analysis to detect 14-3-3, FoxO1, and HCV core proteins.
Treatment with Akt inhibitors.
Cells were serum starved in Dulbecco's modified Eagle medium (DMEM) for 16 h and treated with UCN-01 (2.5 μM) and/or A-443654 (1.0 μM) for 30 min. Cells were washed with phosphate-buffered saline (PBS) and treated with insulin (100 nM) for 3 h. Untreated cells were treated similarly as controls for comparison.
Nuclear extraction and Western blot analysis.
Cells were processed to separate nuclear and cytoplasmic fractions, using a commercially available kit (Chemicon International, Inc., Temecula, CA). Fractions were mixed with 2× SDS-PAGE sample-reducing buffer and heated to 95°C for 5 min, and proteins were resolved by SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane and blocked with 3% nonfat dry milk. The membrane was incubated with a primary antibody, followed by a secondary antibody coupled to horseradish peroxidase, to detect protein bands by using chemiluminescence (Amersham, Piscataway, NJ). Cellular actin was detected, using a specific antibody, for comparisons of the protein load in each lane.
Real-time PCR analysis for G6PC and MTP mRNA levels.
Cellular RNA was isolated by using a commercially available kit (Purescript; Gentra Systems, MN) according to the supplier's protocol. cDNA synthesis was carried out by using random hexamers (Invitrogen, Carlsbad, CA) and ThermoScript II RNase H reverse transcriptase. The mRNA level of G6PC was determined by real-time PCR (Applied Biosystems, Foster City, CA) by using specific oligonucleotide primers (assay identification number, Hs 00609178 m1 G6PC; Applied Biosystems). Results were normalized for GAPDH (glyceraldehyde-3-phosphate dehydrogenase). All reactions were performed in triplicate in an ABI Prism 7700 analyzer in a 25-μl final volume. The MTP gene expression level was also analyzed by real-time PCR, using primers as described previously (18).
MTP activity assay.
MTP activity was analyzed using a commercially available kit (Roar Biomedical, NY) according to the supplier's protocol. Briefly, 100 μg of protein was combined with 4 μl each of suspensions of donor and acceptor particles. The change in fluorescence intensity was monitored after 1 h of incubation at 37°C at an excitation wavelength of 465 nm and to an emission wavelength of 535 nm. To determine blank values, 5 μl of vesicles were added to 95 μl of homogenization buffer. Total fluorescence of donor vesicles was determined by adding 95 μl of isopropanol to 5 μl of vesicles. MTP activity (percent transfer) was calculated by the following equation: percent transfer = samplefluorescence units − blankfluorescence units/(totalfluorescence units − blankfluorescence units) × 100. Specific activity was expressed as the mean percent transfer/mg of total proteins/hour of triplicate quantifications.
ApoB ELISA.
Total human ApoB from hepatocyte culture fluid was quantitated by solid-phase capture sandwich enzyme-linked immunosorbent assay (ELISA), using a commercially available kit (ALerCHEK, Inc., Portland, ME). Briefly, 4 × 105 cells were seeded on a 6-well plate in DMEM containing 10% fetal bovine serum. Cells were washed after 12 h, and medium without serum was added. After 24 h, cells were treated with insulin or not treated for 12 h, and culture medium was used to estimate the level of ApoB by ELISA.
Statistical analysis.
Statistical differences were analyzed by paired Student's t test by Microsoft Excel, and a P value of <0.05 was regarded as statistically significant.
RESULTS
Insulin-induced FoxO1 translocation is impaired in HCV-infected hepatocytes.
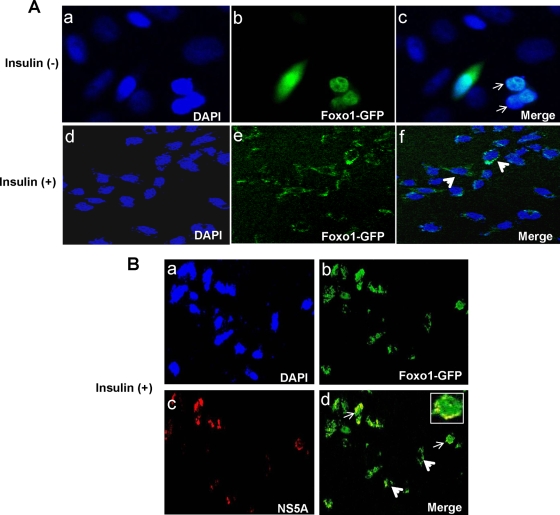
We examined the effect of HCV infection on IHHs or Huh7 cells via insulin-induced FoxO1 translocation from the nucleus to the cytoplasm. Mock-infected hepatocytes were treated similarly for comparison. For this experiment, a FoxO1-GFP clone was transfected into hepatocytes, and after 48 h, cells were serum starved for 2 h and then stimulated with 100 nM insulin for 3 h. Hepatocytes without insulin treatment were used similarly as controls. The majority of the mock-infected hepatocytes displayed FoxO1-GFP localization in the nucleus in the absence of insulin treatment (Fig. 1A and C). In contrast, insulin-stimulated mock-infected hepatocytes strongly shifted localization of FoxO1-GFP from the nucleus to the cytoplasm. In a parallel experiment, IHHs harboring HCV genotype 1a or Huh7 harboring HCV genotype 2a were transfected with FoxO1-GFP to examine FoxO1 translocation following insulin treatment. Nucleo-cytoplasmic localization of FoxO1 was observed in about 30 to 40% of HCV-infected hepatocytes displaying NS5A expression after insulin treatment. The results from representative experiments are shown in Fig. 1B and D. However, hepatocytes that did not display HCV NS5A expression exhibited a clear cytoplasmic localization of FoxO1. Thus, our results suggest that HCV infection of human hepatocytes impairs insulin-induced FoxO1 translocation from the nucleus to the cytoplasm. An earlier report suggested that insulin inhibition of transcription stimulated by FoxO1 is not solely due to nuclear exclusion (39). We further examined whether the phosphorylation status of FoxO1 correlates with its activation status in subsequent studies.
FIG. 1.
HCV infection impairs insulin-induced translocation of FoxO1 from the nucleus to the cytoplasm. (A) Mock-infected IHHs were transfected with FoxO1-GFP, fixed, and stained with DAPI. Confocal microscopy suggests that FoxO1 is located primarily in the nucleus. Merged green and blue images are shown by arrows. Insulin treatment translocated FoxO1 from the nucleus to the cytoplasm in most of the hepatocytes, as shown by the arrows. (C) Similar results were observed with Huh7 cells. (B) IHHs were infected with HCV genotype 1a and transfected with FoxO1-GFP, fixed, and stained with DAPI. Hepatocytes were stained with a monoclonal antibody to NS5A and a secondary antibody conjugated with Alexa Fluor 598. Hepatocytes displayed localization of the viral protein as red in the cytoplasms of infected cells. Confocal microscopy displayed the nucleo-cytoplasmic localization of FoxO1 as green in a number of HCV-infected cells following insulin treatment (indicated by arrows), while the cytoplasms of cells not stained for viral protein had a distinct green fluorescence (indicated by arrowhead). The inset image exhibits merged red and green fluorescence at a higher magnification. (D) Similar observations were noted with Huh7 cells infected with HCV genotype 2a.
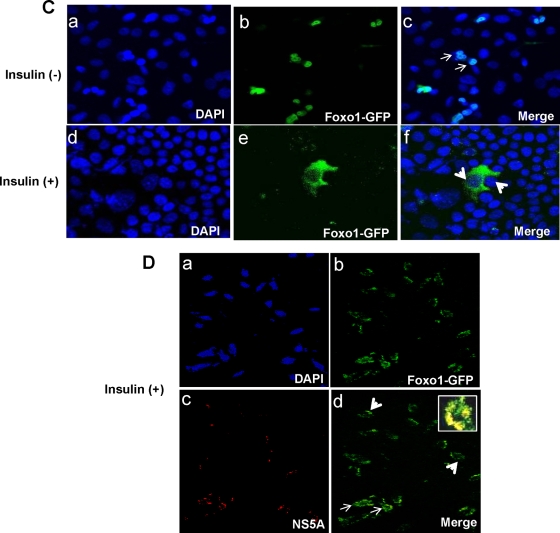
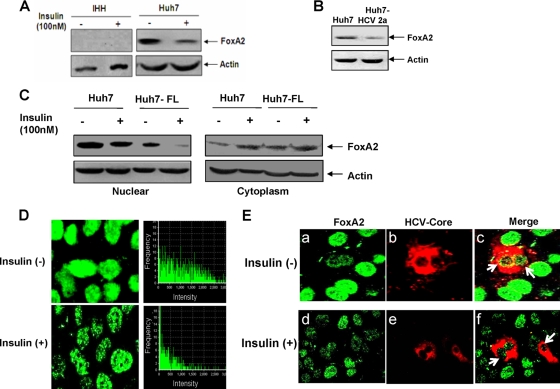
HCV-infected hepatocytes reduce nuclear accumulation of FoxA2.
Insulin stimulation induces phosphorylation of a conserved Thr156 residue specific for FoxA2. This phosphorylation site plays a crucial role in reducing the level of or in the disappearance of nuclear FoxA2, thereby inhibiting its ability to activate the transcription of target genes in the nucleus. IHHs do not display FoxA2 expression either by immunofluorescence (data not shown) or by Western blot analysis (Fig. 2A), which could be due to immortalization from the introduction of the HCV core gene into primary human hepatocytes (33). On the other hand, the level of FoxA2 decreases upon insulin treatment of Huh7 cells. Western blot analysis of Huh7 cells infected with HCV genotype 2a also suggested a reduction in FoxA2 expression compared to that of mock-infected control Huh7 cells (Fig. 2B). These results suggest that HCV infection reduces FoxA2 in Huh7 cells, similar to what was observed upon insulin treatment of uninfected hepatocytes. The effect of insulin on FoxA2 distribution in the nucleus and cytoplasm in control and HCV protein-expressing Huh7 cells as determined in a cell fractionation study is shown in Fig. 2C. Incubation of hepatocytes with 100 nM insulin resulted in lower levels of FoxA2 in the nuclear fraction and increased localization in the cytoplasm. An immunofluorescence study suggested that insulin treatment significantly reduces nuclear FoxA2 levels in control Huh7 cells (Fig. 2D). To understand the effect of HCV infection on nuclear localization of FoxA2, Huh7 cells were infected with HCV genotype 2a (clone JFH1), and after 5 days, cells were stained for detection of endogenous-FoxA2 and -HCV core proteins by immunofluorescence (Fig. 2E). Interestingly, HCV core-expressing cells displayed a reduced level of FoxA2 accumulation in the nucleus, but uninfected cells had a much higher level of FoxA2 accumulation in the nucleus. Insulin treatment of HCV-infected cells did not alter the reduction of FoxA2 accumulation in HCV core-expressing cells, while uninfected cells displayed a loss of FoxA2 accumulation in the nucleus. Together, our results suggest that HCV infection of hepatocytes reduces FoxA2 levels in the nucleus even in the absence of insulin treatment.
FIG. 2.
HCV reduces accumulation of FoxA2 in the nucleus. (A) Western blots showing FoxA2 status in insulin-treated and untreated hepatocytes. Blots were reprobed with an antibody to actin to ascertain the level of protein load in each lane. (B) Western blots showing a reduction in the level of FoxA2 in HCV genotype 2a-infected Huh7 cells. Blots were reprobed with an antibody to actin for comparison of protein load. (C) Distribution patterns of FoxA2 in nuclear and cytoplasmic fractions of Huh7 cells expressing HCV-FL in insulin-treated and untreated cells are shown. (D) Huh7 cells were treated with insulin (100 nM) or untreated, and FoxA2 was stained with a specific antibody and a secondary antibody conjugated to Alexa Fluor 488 (green). FoxA2 was retained exclusively in the nucleus in the absence of insulin. Cells treated with insulin displayed a reduced level of FoxA2 accumulation in the nucleus. Fluorescence frequency intensities (green) from randomly selected fields determined by using FluoView software (Olympus) are shown. (E) Huh7 cells were infected with HCV genotype 2a. Cells were treated with insulin 5 days postinfection or left untreated and were similarly stained for FoxA2 (green) (a and d) and HCV core (red) (b and e). Cells expressing HCV core (red) displayed a reduced level of FoxA2 in the nucleus (green); a merged photograph is indicated by arrowheads (c). Insulin treatment also reduced the level of FoxA2 in the nuclei of uninfected hepatocytes (f).
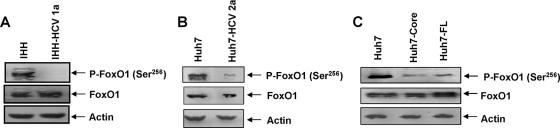
HCV-infected hepatocytes inhibit FoxO1 Ser256 phosphorylation.
Ser256 is important, as it is located within a basic region of the DNA binding domain, and phosphorylation at this site impairs DNA binding activity for Akt-mediated modulation. To understand the mechanism for impairment of FoxO1 translocation during insulin resistance in HCV-infected hepatocytes, we examined the status of Ser256 phosphorylation by Western blot analysis. Infection of IHHs with HCV genotype 1a significantly reduced the status of FoxO1 Ser256 phosphorylation, although the total FoxO1 level remained similar (Fig. 3A). A similar effect was observed with HCV genotype 2a-infected Huh7 cells (Fig. 3B). Huh7 cells stably transfected with the HCV core or FL gene also reduced the status of FoxO1 Ser256 phosphorylation compared to that of control Huh7 cells (Fig. 3C). Thus, our results demonstrate that the presence of HCV core either alone or together with another protein or proteins inhibits Ser256 phosphorylation of FoxO1 in hepatocytes and may contribute to interference with FoxO1 transcriptional activity.
FIG. 3.
Hepatocytes expressing HCV proteins inhibit FoxO1 Ser256 phosphorylation. Lysates from IHHs infected with HCV genotype 1a (A) or Huh7 cells infected with HCV genotype 2a (B) were analyzed for phosphorylated FoxO1 Ser256 [P-FoxO1(Ser256)] and total FoxO1 status and were compared with mock-infected control cells by Western blot analysis, using specific antibodies. (C) HCV core- or FL-transfected Huh7 cells were similarly analyzed for FoxO1 status. Blots were reprobed with an antibody to actin to ascertain the level of protein load in each lane.
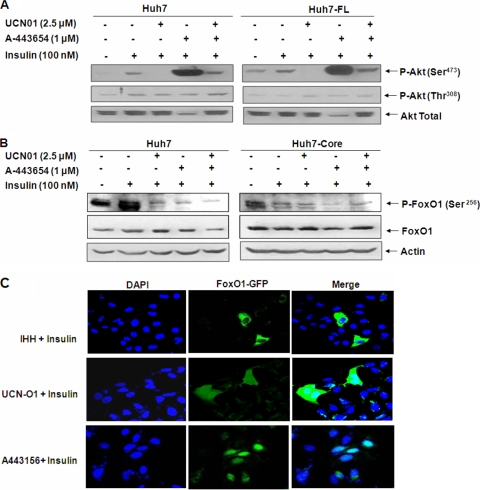
Altered Akt Ser473 phosphorylation in HCV protein-expressing hepatocytes does not affect FoxO1 Ser256 phosphorylation.
An earlier report suggested that phosphorylation of both Ser473 and Thr308 residues in Akt is required for complete Akt activation (23). Moreover, the specific phosphorylation site dictates the specificity of Akt function. For example, Thr308 phosphorylation of Akt is necessary for increased glucose uptake, whereas Ser473 phosphorylation is not (21, 40). In our previous study (7), we observed that the phosphorylation status of Akt Ser473 is upregulated in HCV core-expressing cells. We asked whether an increase in Ser473 phosphorylation had an effect on FoxO1 translocation. It has been reported that A-443654 specifically increases Akt Ser473 phosphorylation (14), whereas UCN-01 is a phosphoinositide-dependent kinase (PDK) inhibitor and can dephosphorylate Akt by inhibiting phosphorylation at either the Thr308 or Ser473 residue, depending upon the cell type (21, 35). To examine the effect of phosphorylation modulators on Akt following HCV polyprotein expression, we treated control and Huh7 cells stably transfected with the HCV FL gene with insulin in the presence or absence of phosphorylation modulators. Western blot analysis was performed to determine the status of Ser473 and Thr308 phosphorylation, using specific antibodies (Fig. 4A). Our results suggest that UCN-01 treatment alone or together with A-443654 reduces the status of insulin-induced Akt Ser473 phosphorylation in control as well as HCV FL-expressing cells but does not alter the status of Thr308 phosphorylation. On the other hand, A-443654 treatment specifically upregulated the status of Ser473 phosphorylation and reduced total Akt in control and HCV polyprotein-expressing Huh7 cells. Similar observations were noted with Huh7 cells stably transfected with HCV FL.
FIG. 4.
FoxO1 phosphorylation in HCV protein-expressing hepatocytes is insensitive to Akt inhibitors. (A) Akt phosphorylation status in hepatocytes expressing the HCV FL gene in the presence of phosphorylation inhibitors. Blots were reprobed with an antibody to actin to ascertain the level of protein load in each lane. (B) Western blots showing the Ser256 phosphorylation status of P-FoxO1 and total FoxO1 in insulin-treated control and HCV core-transfected cell lysates following incubation with UCN-O1 and/or A443654 inhibitors. Blots were reprobed with an antibody to actin to ascertain the level of protein load in each lane. (C) Insulin-treated IHHs displaying translocation of GFP-tagged FoxO1 from the nucleus to the cytoplasm and Akt phosphorylation inhibitors preventing cytoplasmic translocation of GFP-tagged FoxO1. The panels on the right show merged images of DAPI-stained and GFP-expressing hepatocytes.
Insulin-induced Ser473/Thr308 Akt phosphorylation is an important molecular event in the regulation of cellular metabolism. We have recently shown that hepatocytes infected with HCV upregulate Akt Ser473 phosphorylation, without affecting Thr308 phosphorylation, in the presence of insulin (7). Since UCN-O1 and A-443654 have opposite effects on Akt Ser473 phosphorylation in Huh7 cells, we determined the effect of the status of Akt Ser473 phosphorylation on FoxO1 regulation, using these two inhibitors. To examine the effect of Akt modulation on FoxO1 phosphorylation, we treated mock-transfected control or HCV core-expressing hepatocytes either separately or together with UCN-01 and A-443654. Western blot analysis suggested that treatment with both inhibitors significantly reduced insulin-induced FoxO1 phosphorylation in control Huh7 cells (Fig. 4B). On the other hand, HCV core protein expression in hepatocytes reduced the status of FoxO1 phosphorylation upon insulin treatment, and it was not further affected upon treatment with the inhibitors. These results suggest that modulation of Akt serine phosphorylation does not affect the phosphorylation status of FoxO1.
To examine the effect of Akt inhibitors on FoxO1 translocation, we transfected FoxO1-GFP plasmid into IHHs and treated them after 48 h of transfection with the Akt inhibitors. The inhibitors prevented insulin-induced FoxO1 translocation from the nucleus to the cytoplasm (Fig. 4C). This observation indicated that FoxO1 is retained in the nucleus even after modulation of Akt Ser473phosphorylation, which may be due to reduced FoxO1 phosphorylation by the inhibitors.
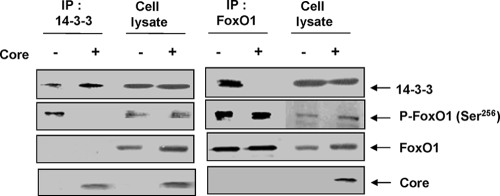
HCV core protein inhibits insulin-induced association of 14-3-3 and phospho-FoxO1 in vivo.
FoxO1 translocation also depends on 14-3-3 protein, which binds to phospho-Ser/Thr motifs important for translocation (43). HCV core protein can bind with 14-3-3 (4, 22) and may play a role similar to that of HIV Vpr protein in the regulation of FoxO1 (20). Insulin induces phosphorylation of a specific Ser256 residue of FoxO1 protein via activation of Akt. This phosphorylation is known to create a binding site for 14-3-3 and the subsequent translocation of the complex into the cytoplasm of hepatocytes for the abolition of transcriptional activation (43). HCV core protein plays an important role in regulating hepatocyte growth, senescence, and differentiation through its interaction with 14-3-3 protein (4). Thus, we wanted to know whether an interaction between HCV core and 14-3-3 has an effect on FoxO1 regulation. Our coimmunoprecipitation analysis suggested that HCV core protein expression inhibits interaction of the phosphorylated form of FoxO1 with 14-3-3 (Fig. 5). Precipitation of 14-3-3 by a specific antibody from cell lysates coprecipitated phosphorylated FoxO1 in control cells. However, we could not detect phosphorylated FoxO1 in core-expressing cells immunoprecipitated with 14-3-3 antibody (Fig. 5, left panel, lane 2). In a reciprocal experiment, we immunoprecipitated cell lysates with FoxO1 antibody and could not detect 14-3-3 in HCV core-expressing cells. Together, our results suggest that interaction of core and 14-3-3 interrupts the 14-3-3-P-FoxO1 association, thus impairing cytoplasmic translocation.
FIG. 5.
HCV core protein inhibits insulin-induced association of 14-3-3 and FoxO1 proteins. Huh7 cells were transfected with a HCV core construct and immunoprecipitated endogenous 14-3-3, P-FoxO1, or HCV core proteins by specific antibodies. Immunoprecipitates (IP) were analyzed by Western blotting for detection of coprecipitating proteins.
HCV-infected hepatocytes differentially modulate G6P and MTP gene expression.
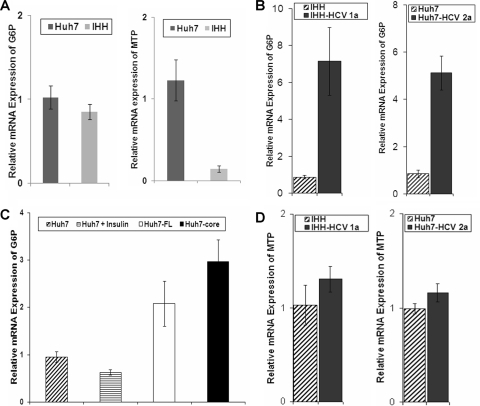
Gluconeogenesis is regulated mainly at the transcriptional level by FoxO1 through the activation of genes, including G6P. On the other hand, MTP is reported to be regulated by FoxO1 and/or FoxA2. Since Huh7 cells and IHHs displayed different levels of FoxO1 and FoxA2 expression, we first examined the basal mRNA expression of G6P and MTP by real-time PCR for each of these cell lines. Our results indicate that basal G6P mRNA expression is comparable in IHHs and Huh7 cells; however, MTP gene expression is significantly reduced in IHHs compared to that in Huh7 cells (Fig. 6A). Further, our results indicate that G6P mRNA expression is significantly increased upon HCV genotype 1a infection compared to that in uninfected control IHHs (Fig. 6B). A significant upregulation in G6P mRNA expression was also observed in HCV polyprotein- or core-expressing cells compared to that in the controls (Fig. 6C). Similar results were also observed following HCV genotype 2a infection of Huh7 cells. Thus, our results indicate that HCV infection causes upregulation of the G6P gene compared to that in the mock-infected hepatocytes.
FIG. 6.
HCV-infected hepatocytes differentially modulate G6P and MTP mRNA expression. (A) Real-time PCR was performed to determine the mRNA status of G6P and MTP. GAPDH was used as a housekeeping gene for endogenous control. (B and C) G6P expression in IHHs infected with HCV genotype 1a or Huh7 cells infected with HCV genotype 2a (B) and in HCV polyprotein- or core-expressing cells (C) compared to that in uninfected Huh7 cells used as controls. P values for the numbers of control and experimental hepatocytes in panels B and C that were infected with HCV or that express core or FL HCV indicate a significant difference (P < 0.05). (D) MTP expression in hepatocytes infected with HCV genotype 1a or 2a as determined by real-time PCR. Triplicate sets of experiments were performed, and relative expression levels were estimated for each set of experiments. The basal value from parental control hepatocytes (IHHs or Huh7 cells) was arbitrarily set at 1, and standard deviations are represented as error bars.
The assembly of VLDL particles in the liver is catalyzed by MTP. FoxA2 promotes VLDL synthesis and secretion primarily by increasing MTP gene expression (41). To investigate the effect of HCV infection upon MTP gene regulation, real-time PCR analysis was performed. The results suggest no significant change in MTP gene expression in HCV genotype 1a- or 2a-infected hepatocytes compared to that in uninfected control cells (Fig. 6D). Therefore, our results suggest that HCV infection in hepatocytes can differentially modulate metabolic gene expression.
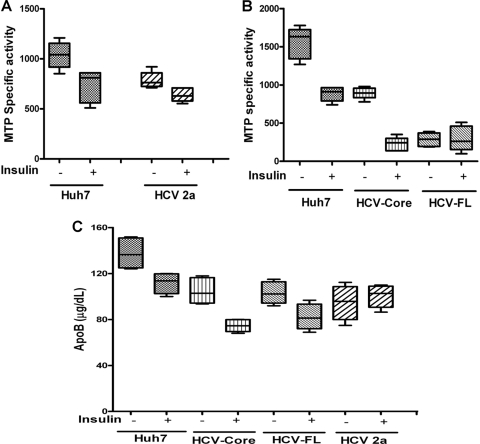
HCV infection or viral protein expression in hepatocytes reduces MTP activity and ApoB secretion.
MTP catalyzes the loading of lipids to the nascent ApoB in the endoplasmic reticulum. This stabilizes the newly synthesized ApoB and facilitates further processing, leading to its secretion. Reduction of MTP activity by inhibitors effectively lowers the plasma lipoprotein level. We examined the status of MTP and ApoB in HCV genotype 2a-infected Huh7 cells and in stable transfectants of Huh7 cells expressing HCV core or FL from genotype 1a. Results were compared with that of the parental control. HCV infection or protein expression in Huh7 cells significantly reduced MTP activity compared to that in the control Huh7 cells, and insulin treatment further reduced MTP activity to a significant level (Fig. 7A and B). Interestingly, HCV FL-expressing cells displayed a higher level of reduction in MTP activity, even in the absence of insulin treatment. Further, we observed that the MTP mRNA expression level (Fig. 6D) does not correlate with MTP functional activity (Fig. 7A and B). This could be due to the short half-life associated with MTP, as previously observed by other investigators (25). Examination of the culture supernatants determined that, as expected, there was a higher level of ApoB secretion in the control cells than in insulin-treated cells (Fig. 7C). Culture supernatants from HCV protein-expressing cells exhibited a higher level of ApoB reduction at the basal level and a further-reduced level of ApoB secretion upon insulin treatment. We did not include IHHs in MTP-related assays, as the real-time PCR data already indicated a massive repression of the MTP mRNA level compared to that of Huh7 cells (Fig. 6A). Thus, our results from Huh7 cells implicated impaired assembly of VLDL, as is often observed clinically in HCV patients with liver steatosis (31).
FIG. 7.
HCV infection or protein expression in hepatocytes reduces MTP activity and ApoB secretion. (A) MTP transfer activity in HCV genotype 2a-infected Huh7 cells and mock-infected controls was measured by a fluorescent assay and expressed as specific activity (mean percent transfer/mg total proteins/h of triplicate quantifications). (B) Similarly, MTP activity was determined in control, HCV core-, and HCV FL-expressing Huh7 cells. (C) ApoB secretion was analyzed in the supernatants of hepatocytes treated with serum-free medium alone or with medium containing insulin (100 nM) for 24 h. Results from three independent experiments are shown. Boxes indicate upper and lower quartiles; horizontal lines inside boxes indicate median values. The P values for the differences in ApoB secretion between control, HCV genotype 2a-infected cells (P < 0.05), HCV core-expressing cells (P < 0.01), and HCV FL-expressing cells (P < 0.05) were statistically significant.
DISCUSSION
Our observations from the present study suggest that HCV infection or ectopic expression of the core protein either alone or together with other viral proteins from an FL gene construct differentially modulates FoxO1 and FoxA2 activation and affects insulin-induced metabolic gene regulation in human hepatocytes. In this study, we used IHHs generated by the immortalization of primary human hepatocytes following introduction of the HCV core gene from genotype 1a (33). IHHs displayed core mRNA expression detectable by real-time (RT)-PCR and the presence of a very low level of the core protein in Western blots (8). Interestingly, IHHs exhibited properties similar to those of normal hepatocytes upon insulin treatment, while the same cell line displayed distinct properties upon production of ectopic HCV protein(s). This difference could be due to the HCV protein expression level in hepatocytes. We also included Huh7 cells in our study for infection with HCV genotype 2a or expression of viral protein(s) to eliminate any unknown shortcomings of IHHs.
In this study, we have shown that HCV impairs insulin-induced FoxO1 translocation from the nucleus to the cytoplasm and reduces nuclear accumulation of FoxA2. FoxO1 or FoxA2 modulation might be responsible for a significant upregulation of the G6P gene and a reduction in MTP activity during HCV infection. Since FoxO1 and FoxA2 are possible targets of Akt, two pharmacological inhibitors, A-443654 and UCN-01, were used to perturb Akt signaling and verify the effect of Akt Ser473 phosphorylation upon the downstream insulin-signaling pathway. Our results suggest that modulation of the status of Akt Ser473phosphorylation by the inhibitors in control hepatocytes reduced FoxO1 phosphorylation. However, this modulation had no significant effect upon insulin-induced FoxO1 phosphorylation in HCV protein-expressing hepatocytes.
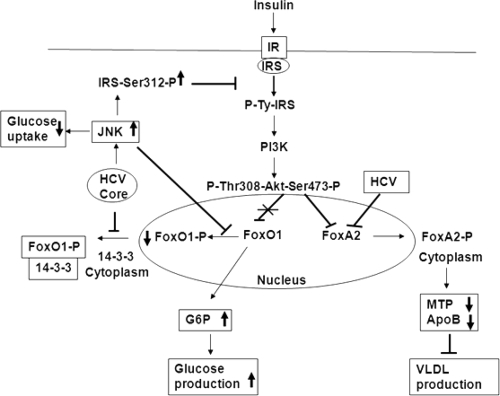
FoxO1 activation depends on 14-3-3 protein, which binds to phospho-Ser/Thr motifs (43). Phosphorylation of Ser256 on FoxO1 serves as a high-affinity binding site for 14-3-3 protein, inhibits access of FoxO1 to DNA, and stimulates its translocation from the nucleus to the cytoplasm. Thus, the interaction of 14-3-3 with phosphorylated FoxO1 may be an important step in insulin's ability to exert an inhibitory action. Interestingly, HCV core protein binds with 14-3-3 (4, 22) and may play a role in FoxO1 regulation similar to that of HIV Vpr protein (20). FoxO1 activity may also be regulated by JNK or JNK-dependent phosphorylation of 14-3-3 protein (reviewed in reference 15), thus contributing to nuclear localization. Indeed, our immunoprecipitation results (Fig. 5) suggest that an association between HCV core and 14-3-3 proteins may interrupt phospho-FoxO1 binding with 14-3-3 protein and may impair translocation of FoxO1 from the nucleus to the cytoplasm (Fig. 8).
FIG. 8.
Schematic presentation of the signaling pathway induced by HCV for metabolic regulation. Based on our previous and current observations, we propose that HCV may interrupt at different nodes of the metabolic signaling pathway for insulin resistance and lipid metabolism. Core protein activates JNK and upregulates IRS Ser312 phosphorylation, thus inhibiting glucose uptake (7). On the other hand, core protein inhibits phospho-FoxO1 and 14-3-3 protein interaction and thus may impair FoxO1 translocation from the nucleus to the cytoplasm, or activated JNK may have a role in the regulation of FoxO1 phosphorylation (15). This leads to upregulation of G6P in the nucleus and enables an increase in glucose synthesis. HCV inactivates FoxA2 by increasing cytoplasmic translocation, leading to lower levels of MTP activity and ApoB secretion. Bold arrow pointing upward (↑) represent activation, downward-pointing arrows (↓) represent repression, and blunt arrows (⊥) represent blockade of signaling.
Studies with hepatoma cells suggest that FoxO1 and FoxA2 possess the ability to regulate transcription of reporter genes containing an insulin response element (IRE) from the G6P, PEPCK, and MTP promoter in an insulin-dependent manner (18, 27). We have observed different levels of FoxO1 and FoxA2 protein expression in IHHs and Huh7 cells. FoxO1 was expressed at higher levels in both IHHs and Huh7 cells, and FoxA2 remained undetectable in IHHs. In Huh7 cells expressing core protein, we observed a reduced level of FoxA2 expression in the nucleus. This result corroborates with our data obtained from nuclear extraction experiments followed by Western blot analysis (Fig. 2C). The lack of a highly specific phospho-FoxA2 antibody makes it difficult to ascertain whether upregulation of phosphorylation is responsible for the undetectable status of FoxA2 in the nucleus. Further studies are in progress to understand the mechanism for FoxA2 downregulation by HCV core protein in IHHs. Next, we investigated the relative contributions of FoxO1 and FoxA2 on the regulation of G6P and MTP in HCV-infected cells. Basal mRNA expression of G6P was similar in IHHs and Huh7 cells. However, MTP mRNA expression was drastically reduced in IHHs compared to that in Huh7 cells. Since IHHs did not exhibit FoxA2 expression, this transcription factor might be important for MTP expression in hepatocytes (41). Our observations suggesting a differential effect on metabolic gene expression by HCV are in agreement with the results for hepatic gene expression in acutely infected chimpanzees (37).
Patients with nonalcoholic fatty liver disease (NAFLD) typically have increased serum levels of triglycerides, cholesterol, and ApoB, whereas hypobetalipoproteinemia is often associated with liver steatosis in HCV-infected patients (31, 36) and is indicative of impaired VLDL assembly. Apolipoproteins are important for the virus life cycle, and secretion of infectious HCV may require MTP activity, ApoB, and ApoE. However, the specific roles of these cellular factors in the HCV life cycle is yet to be clearly understood (11, 12, 16, 17, 29). On the other hand, free lipoproteins may regulate the rate of liver cell infection by competing with the virus for the same receptor (26). Therefore, a reduced lipoprotein level may be beneficial for HCV infection. This is further supported by the fact that HCV load inversely correlates with ApoB concentration (31). Our experimental observations also suggest that HCV protein expression in hepatocytes reduces MTP activity and ApoB secretion (Fig. 7 and 8), implicating impaired assembly of VLDL, as observed clinically in HCV-infected patients with liver steatosis (30, 31).
We have previously shown that HCV core protein activates JNK and upregulates IRS-Ser312 phosphorylation, leading to reduced glucose uptake (7). The presence of the PI3K inhibitor (LY294002) did not increase glucose uptake in core-expressing hepatocytes, implying that Akt may not be involved in this functional activity. In the present study, we have shown that HCV does not impair Akt kinase activity but inhibits FoxO1 phosphorylation, while HCV infection reduces FoxA2 expression in the nucleus. In addition, an interaction of the HCV core protein may interrupt 14-3-3 and phospho-FoxO1 binding, impairing FoxO1 translocation from the nucleus to the cytoplasm. As reported earlier, HCV core protein-induced activation of JNK may have a role in the regulation of FoxO1 (15). On the other hand, HCV appears to play a role in the inactivation of MTP, possibly by inactivating FoxA2. Reduction of MTP activity by hepatocytes expressing HCV core or polyprotein also decreased ApoB secretion and may contribute to the impaired production of VLDL, thereby facilitating fatty liver disease (Fig. 8). Taken together, our results suggest that FoxO1 and FoxA2 are differentially modulated by HCV and exhibit distinct effects upon G6P expression, MTP activity, and ApoB secretion. Targeting these interactions may be helpful in controlling HCV-induced metabolic deregulation.
Acknowledgments
We thank Domenico Accili for providing FoxO1-GFP plasmid, Vincent L. Giranda for compound A-443654, and Chen Liu for monoclonal antibody to HCV NS5A. We appreciate help from Leonard E. Grosso, Department of Pathology, Saint Louis University, with the analysis of HCV genome copy number and thank Lin Cowick for preparation of the manuscript.
This work was supported by research grants DK80812 (R.R.) and DK80817 (R.B.R.) from the National Institutes of Health.
Footnotes
Published ahead of print on 31 March 2010.
REFERENCES
- 1.Adinolfi, L. E., M. Gambardella, A. Andreana, M. F. Tripodi, R. Utili, and G. Ruggiero. 2001. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology 33:1358-1364. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Goughoulte, M., T. Kanda, K. Meyer, J. S. Ryerse, R. B. Ray, and R. Ray. 2008. Hepatitis C virus genotype 1a growth and induction of autophagy. J. Virol. 82:2241-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altomonte, J., A. Richter, S. Harbaran, J. Suriawinata, J. Nakae, S. N. Thung, M. Meseck, D. Accili, and H. Dong. 2003. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 285:E718-E728. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, H., J. Hayashi, M. Moriyama, Y. Arakawa, and O. Hino. 2000. Hepatitis C virus core protein interacts with 14-3-3 protein and activates the kinase Raf-1. J. Virol. 74:1736-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asselah, T., L. Rubbia-Brandt, P. Marcellin, and F. Negro. 2006. Steatosis in chronic hepatitis C: why does it really matter? Gut 55:123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Au, W. S., H. F. Kung, and M. C. Lin. 2003. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes 52:1073-1080. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee, S., K. Saito, M. Ait-Goughoulte, K. Meyer, R. B. Ray, and R. Ray. 2008. Hepatitis C virus core protein upregulates serine phosphorylation of insulin receptor substrate-1 and impairs the downstream akt/protein kinase B signaling pathway for insulin resistance. J. Virol. 82:2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu, A., K. Meyer, R. B. Ray, and R. Ray. 2002. Hepatitis C virus core protein is necessary for the maintenance of immortalized human hepatocytes. Virology 298:53-62. [DOI] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-869. [DOI] [PubMed] [Google Scholar]
- 10.Castéra, L., C. Hézode, F. Roudot-Thoraval, A. Bastie, E. S. Zafrani, J. M. Pawlotsky, and D. Dhumeaux. 2003. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut 52:288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, K. S., J. Jiang, Z. Cai, and G. Luo. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 81:13783-13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gastaminza, P., G. Cheng, S. Wieland, J. Zhong, W. Liao, and F. V. Chisari. 2008. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 82:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross, D. N., A. P. van den Heuvel, and M. J. Birnbaum. 2008. The role of FoxO in the regulation of metabolism. Oncogene 27:2320-2336. [DOI] [PubMed] [Google Scholar]
- 14.Han, E. K., J. D. Leverson, T. McGonigal, O. J. Shah, K. W. Woods, T. Hunter, V. L. Giranda, and Y. Luo. 2007. Akt inhibitor A-443654 induces rapid Akt Ser-473 phosphorylation independent of mTORC1 inhibition. Oncogene 26:5655-5661. [DOI] [PubMed] [Google Scholar]
- 15.Huang, H., and D. J. Tindall. 2007. Dynamic FoxO transcription factors. J. Cell Sci. 120:2479-2487. [DOI] [PubMed] [Google Scholar]
- 16.Huang, H., F. Sun, D. M. Owen, W. Li, Y. Chen, M. Gale, Jr., and J. Ye. 2007. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. U. S. A. 104:5848-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, J., and G. Luo. 2009. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J. Virol. 83:12680-12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamagate, A., S. Qu, G. Perdomo, D. Su, D. H. Kim, S. Slusher, M. Meseck, and H. H. Dong. 2008. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118:2347-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda, T., A. Basu, R. Steele, T. Wakita, J. S. Ryerse, R. Ray, and R. B. Ray. 2006. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 80:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kino, T., M. U. De Martino, E. Charmandari, T. Ichijo, T. Outas, and G. P. Chrousos. 2005. HIV-1 accessory protein Vpr inhibits the effect of insulin on the Foxo subfamily of forkhead transcription factors by interfering with their binding to 14-3-3 proteins: potential clinical implications regarding the insulin resistance of HIV-1-infected patients. Diabetes 54:23-31. [DOI] [PubMed] [Google Scholar]
- 21.Kondapaka, S. B., M. Zarnowski, D. R. Yver, E. A. Sausville, and S. W. Cushman. 2004. 7-hydroxystaurosporine (UCN-01) inhibition of Akt Thr308 but not Ser473 phosphorylation: a basis for decreased insulin-stimulated glucose transport. Clin. Cancer Res. 10:7192-7198. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S. K., S. O. Park, C. O. Joe, and Y. S. Kim. 2007. Interaction of HCV core protein with 14-3-3ɛ protein releases Bax to activate apoptosis. Biochem. Biophy. Res. Commun. 352:756-762. [DOI] [PubMed] [Google Scholar]
- 23.Manning, B. D., and L. C. Cantley. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto, M., S. Han, T. Kitamura, and D. Accili. 2006. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J. Clin. Invest. 116:2464-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirandola, S., S. Realdon, J. Iqbal, M. Gerotto, F. Dal Pero, G. Bortoletto, M. Marcolongo, A. Vario, C. Datz, M. M. Hussain, and A. Alberti. 2006. Liver microsomal triglyceride transfer protein is involved in hepatitis C liver Steatosis. Gastroenterology 130:1661-1669. [DOI] [PubMed] [Google Scholar]
- 26.Monazahian, M., I. Böhme, S. Bonk, A. Koch, C. Scholz, S. Grethe, and R. Thomssen. 1999. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J. Med. Virol. 57:223-229. [DOI] [PubMed] [Google Scholar]
- 27.Nakae, J., T. Kitamura, D. L. Silver, and D. Accili. 2001. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Invest. 108:1359-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohata, K., K. Hamasaki, K. Toriyama, K. Matsumoto, A. Saeki, K. Yanagi, S. Abiru, Y. Nakagawa, M. Shigeno, S. Miyazoe, T. Ichikawa, H. Ishikawa, K. Nakao, and K. Eguchi. 2003. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer 97:3036-3043. [DOI] [PubMed] [Google Scholar]
- 29.Owen, D. M., H. Huang, J. Ye, and M. Gale, Jr. 2009. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology 394:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perlemuter, G., A. Sabile, P. Letteron, G. Vona, A. Topilco, Y. Chrétien, K. Koike, D. Pessayre, J. Chapman, G. Barba, and C. Bréchot. 2002. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 16:185-194. [DOI] [PubMed] [Google Scholar]
- 31.Petit, J. M., M. Benichou, L. Duvillard, V. Jooste, J. B. Bour, A. Minello, B. Verges, J. M. Brun, P. Gambert, and P. Hillon. 2003. Hepatitis C virus-associated hypobetalipoproteinemia is correlated with plasma viral load, steatosis, and liver fibrosis. Am. J. Gastroenterol. 98:1150-1154. [DOI] [PubMed] [Google Scholar]
- 32.Puigserver, P., J. Rhee, J. Donovan, C. J. Walkey, J. C. Yoon, F. Oriente, Y. Kitamura, J. Altomonte, H. Dong, D. Accili, and B. M. Spiegelman. 2003. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature 423:550-555. [DOI] [PubMed] [Google Scholar]
- 33.Ray, R. B., K. Meyer, and R. Ray. 2000. Hepatitis C virus core protein promotes immortalization of primary human hepatocytes. Virology 271:197-204. [DOI] [PubMed] [Google Scholar]
- 34.Samuel, V. T., C. S. Choi, T. G. Phillips, A. J. Romanelli, J. G. Geisler, S. Bhanot, R. McKay, B. Monia, J. R. Shutter, R. A. Lindberg, G. I. Shulman, and M. M. Veniant. 2006. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55:2042-2050. [DOI] [PubMed] [Google Scholar]
- 35.Sato, S., N. Fujita, and T. Tsuruo. 2002. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine). Oncogene 21:1727-1738. [DOI] [PubMed] [Google Scholar]
- 36.Serfaty, L., T. Andreani, P. Giral, N. Carbonell, O. Chazouillères, and R. Poupon. 2001. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J. Hepatol. 34:428-434. [DOI] [PubMed] [Google Scholar]
- 37.Su, A. I., J. P. Pezacki, L. Wodicka, A. D. Brideau, L. Supekova, R. Thimme, S. Wieland, J. Bukh, R. H. Purcell, P. G. Schultz, and F. V. Chisari. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. U. S. A. 99:15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tazawa, J., M. Maeda, M. Nakagawa, H. Ohbayashi, F. Kusano, M. Yamane, Y. Sakai, and K. Suzuki. 2002. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig. Dis. Sci. 47:710-715. [DOI] [PubMed] [Google Scholar]
- 39.Tsai, W. C., N. Bhattacharyya, L. Y. Han, J. A. Hanover, and M. M. Rechler. 2003. Insulin inhibition of transcription stimulated by the forkhead protein Foxo1 is not solely due to nuclear exclusion. Endocrinology 144:5615-5622. [DOI] [PubMed] [Google Scholar]
- 40.Virkamäki, A., K. Ueki, and C. R. Kahn. 1999. Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Invest. 103:931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfrum, C., and M. Stoffel. 2006. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 3:99-110. [DOI] [PubMed] [Google Scholar]
- 42.Wolfrum, C., E. Asilmaz, E. Luca, J. M. Friedman, and M. Stoffel. 2004. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature 432:1027-1032. [DOI] [PubMed] [Google Scholar]
- 43.Zhao, X., L. Gan, H. Pan, D. Kan, M. Majeski, S. A. Adam, and T. G. Unterman. 2004. Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem. J. 378:839-849. [DOI] [PMC free article] [PubMed] [Google Scholar]