Abstract
Glioblastoma multiforme (GBM) is a deadly primary brain tumor. Conditional cytotoxic/immune-stimulatory gene therapy (Ad-TK and Ad-Flt3L) elicits tumor regression and immunological memory in rodent GBM models. Since the majority of patients enrolled in clinical trials would exhibit adenovirus immunity, which could curtail transgene expression and therapeutic efficacy, we used high-capacity adenovirus vectors (HC-Ads) as a gene delivery platform. Herein, we describe for the first time a novel bicistronic HC-Ad driving constitutive expression of herpes simplex virus type 1 thymidine kinase (HSV1-TK) and inducible Tet-mediated expression of Flt3L within a single-vector platform. We achieved anti-GBM therapeutic efficacy with no overt toxicities using this bicistronic HC-Ad even in the presence of systemic Ad immunity. The bicistronic HC-Ad-TK/TetOn-Flt3L was delivered into intracranial gliomas in rats. Survival, vector biodistribution, neuropathology, systemic toxicity, and neurobehavioral deficits were assessed for up to 1 year posttreatment. Therapeutic efficacy was also assessed in animals preimmunized against Ads. We demonstrate therapeutic efficacy, with vector genomes being restricted to the brain injection site and an absence of overt toxicities. Importantly, antiadenoviral immunity did not inhibit therapeutic efficacy. These data represent the first report of a bicistronic vector platform driving the expression of two therapeutic transgenes, i.e., constitutive HSV1-TK and inducible Flt3L genes. Further, our data demonstrate no promoter interference and optimum gene delivery and expression from within this single-vector platform. Analysis of the efficacy, safety, and toxicity of this bicistronic HC-Ad vector in an animal model of GBM strongly supports further preclinical testing and downstream process development of HC-Ad-TK/TetOn-Flt3L for a future phase I clinical trial for GBM.
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults, affecting ∼18,000 new patients every year; its prognosis remains poor despite standard treatment with surgery, radiotherapy, and chemotherapy (temozolomide) (36-38, 44; J. C. Buckner, presented at the ASCO Annual Meeting, 2009). Complete resection is mostly impossible due to the highly infiltrative nature of this disease. Residual GBM cells remaining within the nonneoplastic brain parenchyma eventually lead to tumor recurrence that is resistant to conventional chemotherapy and radiotherapy, ultimately leading to the patient's death (44). Several dendritic cell vaccination strategies aiming to stimulate the patient's immune system to seek out and destroy residual brain tumor cells are currently under preclinical and clinical development and constitute a promising adjuvant treatment for GBM (18, 24, 42, 43, 45).
We have developed a novel immunotherapeutic approach for GBM using first-generation adenoviral vectors (Ads) to deliver a combination of therapeutic transgenes into the tumor mass (2, 7, 9, 12, 14, 17, 48) which is slated to begin phase I clinical testing this year. In our strategy, rather than vaccinating against tumor antigens, we aim to reconstruct an immune circuit that is absent from the normal brain. Our gene therapy strategy consists of the conditionally cytotoxic herpes simplex virus type 1 thymidine kinase (TK) (2, 12), which kills proliferating tumor cells in the presence of the prodrug ganciclovir (GCV), used in combination with human soluble Fms-like tyrosine kinase 3 ligand (Flt3L), which recruits bone marrow-derived dendritic cells (DCs) to the normal brain or brain tumor microenvironment in mice (11, 12) and rats (1, 13). We previously showed that codelivery of Ad-Flt3L and Ad-TK into the brain tumor milieu induces an anti-GBM-specific immune response (9, 12, 17, 27, 48), leading to long-term survival of rats bearing intracranial CNS1, 9L, and F98 tumors (2, 17, 27) and mice bearing intracranial GL26, GL261, and B16-F10 tumors (12, 48). In addition, the combination of Ad-Flt3L plus Ad-TK induces GBM-specific immunological memory that improves survival in intracranial multifocal and recurrent models of GBM in both rats and mice (9, 12, 17, 20, 22, 27).
First-generation adenoviral vectors have been used in clinical trials to deliver HSV1-TK (31). Ad-TK delivered into the margins of the tumor cavity after surgical resection of GBM was well tolerated in over 70 patients in six early clinical trials (19, 34). Publication of final results from a large, multicenter phase III trial involving 251 patients is awaited; preliminary analysis indicates small but statistically significant benefits from adding gene therapy to standard care. Final analysis will have to wait for the detailed publications (3, 16).
To provide stable, long-term therapeutic transgene expression, “gutless,” high-capacity adenoviral (HC-Ad) vectors have been engineered in which all viral encoding genes have been eliminated and replaced with inert, noncoding stuffer DNA sequences (26, 30). In the presence of an anti-adenoviral immune response, transgene expression in the brain from first-generation Ads is reduced, while expression from HC-Ad vectors remains stable for at least 1 year (4, 25, 28). Therefore, we engineered two HC-Ad vectors to either constitutively express TK (HC-Ad-TK) (21, 27) or express Flt3L under the control of the tightly regulatable mCMV-TetOn expression system (HC-Ad-TetOn-Flt3L) (6, 8, 27, 47). Intratumoral administration of HC-Ad-TK and HC-Ad-TetOn-Flt3L led to high levels of therapeutic efficacy, and the therapy was well tolerated, with no overt toxicity and no spread of vector genomes outside the injected brain hemisphere in treated Lewis rats bearing syngeneic intracranial brain tumors (27).
To reduce vector dose and facilitate GMP manufacturing of the clinical product, we engineered a novel, bicistronic HC-Ad vector that encodes, for the first time, both constitutively expressed HSV1-TK and inducible Flt3L from a single HC-Ad vector genome. Herein we demonstrate therapeutic efficacy of the bicistronic HC-Ad in naïve rats bearing intracranial tumors and also in tumor-bearing rats which had been exposed to adenoviruses and therefore exhibited anti-Ad immunity. The safety profile of this approach was assessed by evaluating the biodistribution of HC-Ad vector genomes, systemic and neurological toxicity, and behavioral abnormalities over the course of 1 year posttreatment. These data represent the first report of a bicistronic vector platform driving the expression of two therapeutic transgenes, i.e., constitutive HSV1-TK and inducible Flt3L. Further, our data demonstrate no promoter interference and optimum gene delivery and expression from within this single-vector platform. Analysis of the efficacy, safety, and toxicity of this bicistronic HC-Ad vector in an animal model of GBM strongly supports further preclinical testing and downstream process development of HC-Ad-TK/TetOn-Flt3L for a future phase I clinical trial for GBM.
MATERIALS AND METHODS
Engineering of HC-Ad plasmid.
To generate the HC-Ad vector genome plasmid, the previously described HC-Ad shuttle plasmid encoding tetracycline-inducible expression of Flt3L (pBS-MSC1m-mCMV-TetOn-TRE-Flt3L) (6, 17, 46, 47) was cut with AscI to excise the intact mCMV-TetOn-TRE-Flt3L expression cassette, which was then blunt-end cloned into NheI sites of pBS-MSC1m-hCMV-TK (6, 17, 21), an HC-Ad shuttle plasmid encoding TK under the control of the constitutively active human cytomegalovirus (CMV) promoter, to generate the final HC-Ad shuttle plasmid pBS-MCS1m-hCMV-TK/mCMV-TetOn-Flt3L (see Fig. S1 in the supplemental material). The previously described HC-Ad vector genome plasmid pSTK120.1 (47) was modified to accept the large bicistronic expression cassette by PmlI-mediated excision of an additional ∼6-kb stuffer sequence to generate the new HC-Ad genome plasmid pSTK120.2. The entire bicistronic expression cassette was excised from pBS-MCS1m-hCMV-TK/mCMV-TetOn-Flt3L with NotI and cloned into the EagI site of pSTK120.2 to generate the bicistronic HC-Ad vector genome plasmid pSTK120.2-hCMV-TK/mCMV-TetOn-Flt3L. Each constituent and junctions of the bicistronic expression cassette were analyzed by sequencing analysis at the UCLA Sequencing Core (see Fig. S2 in the supplemental material).
Adenoviral vectors.
The rescue, amplification, and quality control of the HC-Ad vector HC-Ad-TK/TetOn-Flt3L and the first-generation adenoviral vector Ad-0, used for immunization, were performed as described by us previously (6, 29, 47).
In vitro characterization of HC-Ad vectors.
CNS1 cells and primary astrocytes were seeded (25,000/well) and infected 24 h later with the HC-Ad at a multiplicity of infection of 2,000 viral particles (vp)/cell. Transgene expression was assessed by enzyme-linked immunosorbent assay (ELISA) (R&D Systems) and immunocytochemistry (ICC) as described by us previously (1, 12, 27). TK cytotoxicity was assessed by flow-cytometric analysis of propidium iodide-annexin V-stained cells (9, 12, 15, 27).
Rat brain tumor model.
A total of 4,500 rat GBM CNS1 cells (3 μl) were stereotactically implanted in the right striatum of syngeneic Lewis rats (220 to 250 g; Harlan, Indianapolis, IN) as described previously (5, 9, 27). Six days later, rats received an intratumoral injection of either 5 × 109 vp HC-Ad or saline. The total volume delivered was 3 μl (delivered in three locations ventral of the dura: 5.5, 5.0, and 4.5 mm) into the tumor mass. Starting 2 days before HC-Ad administration, rats were fed doxycycline (DOX)-containing chow ad libitum as described previously (27). Twenty-four hours after treatment, rats received GCV (25 mg/kg, intraperitoneally), twice daily for 10 days. All experimental procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Preimmunization studies.
Animals were systemically preimmunized with Ad-0 two weeks before injection of CNS1 tumors as described by us previously (27). Blood was collected by retro-orbital bleeding during implantation of CNS1 tumors, and circulating levels of anti-adenovirus neutralizing antibodies were assessed as described before (21). Six days after tumor implantation, rats received intratumoral treatment with the bicistronic HC-Ad as described above.
Biodistribution of vector genomes and quantification of Flt3L transgene copies in the brain.
Analysis of biodistribution of vector genomes was performed using quantitative PCR (qPCR) at 5 days, 30 days, 6 months, and 1 year posttreatment as described previously (27, 32, 33). Total DNA was purified from the brain hemispheres ipsilateral and contralateral to the brain tumor injection site, cerebellum, brain stem, spleen, liver, testes, gut, lung, heart, cervical draining lymph nodes, kidney, and lumbar spinal cord and used for the quantitation of vector genomes and Flt3L transgene copies. Vector genomes and Flt3L transgene copies are shown as the number of vector genomes/25 mg of tissue. Results are based on five rats per group.
Neuropathological analysis.
Neuropathological analysis was performed at 5 days, 30 days, 6 months, or 1 year posttreatment as described previously (9, 21, 27).
Assessment of Flt3L expression in brain tissue.
Analysis of Flt3L expression in the brain of treated animals was performed 5 days and 30 days posttreatment using an Flt3L-specific ELISA as described previously (13, 27).
Analysis of blood biochemistry.
At 5 days, 30 days, 6 months, and 1 year posttreatment, blood was collected and a comprehensive panel of serum chemistry and hematologic parameters was performed by Antech Diagnostics (Irvine, CA). Blood from naïve, age-matched animals was used to establish reference values (27). The median, minimum, and maximum values for each parameter are shown.
Behavioral analysis.
The long-term behavioral impact of intratumoral delivery of HC-Ad was evaluated 1 year posttreatment by assessing amphetamine-induced rotational behavior, asymmetry abnormalities in forelimb use, and spontaneous motor and rearing behavior as described previously (20, 27). Naïve, age-matched Lewis rats were used as controls.
Statistical analysis.
Sample sizes were calculated to detect differences between groups with a power of 80% at a 0.05 significance level using PASS 2008 (power and sample size software; NCSS, Kaysville, UT). Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's posttest or two-tailed Student's t test (NCSS). Kaplan-Meier survival curves were analyzed using the Mantel log-rank test (GraphPad Prism version 3.00; GraphPad Software, San Diego CA). P values of less than 0.05 were used to determine the null hypothesis to be invalid. The statistical tests used are indicated in the figure legends.
RESULTS
In vitro characterization of HC-Ad-TK/TetOn-Flt3L: glioma-specific conditional cytotoxicity and regulated release of Flt3L.
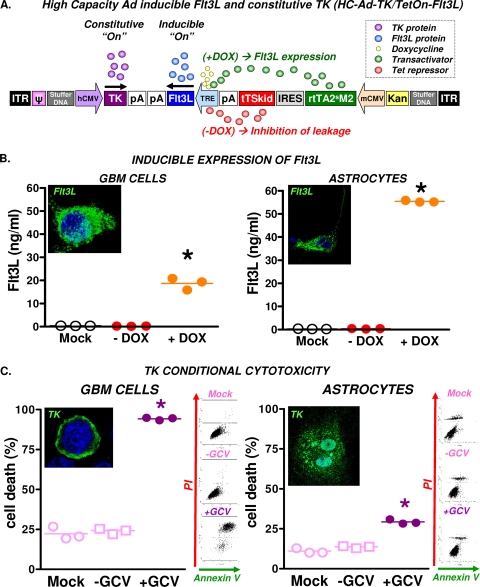
Characterization of HC-Ad-TK/TetOn-Flt3L was performed in vitro in rat CNS1 GBM cells and in primary astrocyte cultures. Infection with HC-Ad-TK/TetOn-Flt3L (Fig. 1A) led to tightly regulated Flt3L release in both GBM cells and astrocytes, with negligible release in the OFF state (without DOX) (Fig. 1B). Flt3L expression was also confirmed by immunofluorescence in the presence of DOX. Expression of TK in HC-Ad-infected cells was visualized by immunofluorescence in both GBM cells and astrocytes (Fig. 1C). However, cytotoxic effects were observed only in the presence of the prodrug GCV in CNS1 glioma cells. Low levels of cell death were observed in HC-Ad-infected primary astrocytes cultured in the presence of GCV (Fig. 1C). This is likely due to low levels of cell proliferation in astrocyte cultures, which would make these cells susceptible to the cytotoxic effects of TK+GCV.
FIG. 1.
Structure and in vitro characterization of therapeutic bicistronic HC-Ad. (A) Structure and transcriptional regulation of HC-Ad-TK/TetOn-Flt3L. (B) CNS1 GBM cells and rat astrocytes in primary culture were infected with HC-Ad-TK/TetOn-Flt3L with or without the inducer doxycycline (Dox). Flt3L expression was assessed by immunofluorescence and ELISA. *, P < 0.05 versus mock and infected controls, by one-way ANOVA followed by Tukey's test. (C) CNS1 GBM cells and astrocytes in primary culture were infected with the bicistronic HC-Ad and incubated with or without the prodrug GCV. TK expression was assessed by immunofluorescence, and cell death was determined by flow-cytometric analysis of annexin V/propidium iodide-stained cells as shown in representative dot plots. *, P < 0.05 versus mock and infected controls, by one-way ANOVA followed by Tukey's test.
Intratumoral delivery of HC-Ad-TK/TetOn-Flt3L mediates long-term survival regardless of anti-Ad immunization status.
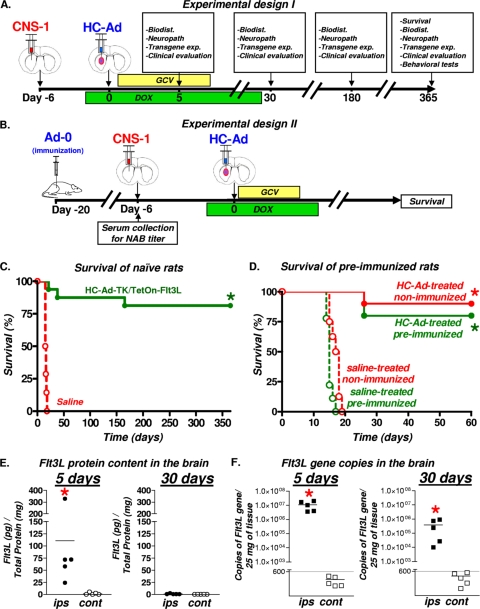
Using Flt3L and TK encoded on two separate HC-Ad vectors, we showed previously that up 5 × 109 vp of each HC-Ad vector can be safely administered via intratumoral injection into tumor-bearing rats with high levels of therapeutic efficacy and negligible levels of toxicity (27). Thus, we selected the maximum tolerated dose (5 × 109 vp of HC-Ad) for this study to assess the efficacy, neuropathology, biodistribution, and behavioral abnormalities associated with intratumoral delivery of HC-Ad-TK/TetOn-Flt3L (Fig. 2A and B). Rats bearing intracranial CNS1 tumors received an intratumoral injection of 5 × 109 vp of the therapeutic HC-Ad vector or saline. GCV was administered twice daily for 10 days. Rats received DOX-containing chow for 4 weeks. Intratumoral delivery of 5 × 109 vp of therapeutic HC-Ad vector led to tumor regression and survival for at least 1 year in ∼75% of rats (Fig. 2C). Since most patients undergoing gene therapy clinical trials will exhibit evidence of previous exposure to adenovirus (10), we assessed the efficacy of HC-Ad-TK/TetOn-Flt3L in a preclinical setting that models this likely clinical scenario. Lewis rats were systemically immunized with a first-generation Ad vector without a transgene (Ad-0). Successful immunization against adenovirus was confirmed using an anti-Ad neutralizing-antibody assay (data not shown) (4, 21, 27, 41, 47). Preimmunized animals were implanted with CNS1 cells and then treated with HC-Ad (Fig. 2B). HC-Ad treatment led to more than ∼75% survival in immunized rats for at least 2 months (Fig. 2D). Based on our previous experience with the CNS1 tumor model, it is very rare for animals to succumb to tumor burden beyond 50 days posttreatment; furthermore, this time frame is sufficient to assess anti-GBM immunological memory (2, 9, 17, 20-22, 27).
FIG. 2.
Efficacy and Flt3L expression after intratumoral administration of therapeutic bicistronic HC-Ad. (A) HC-Ad-TK/TetOn-Flt3L (16/group) or saline (14/group) was delivered intratumorally into 6-day intracranial CNS1 tumors. (B) For preimmunization studies, animals were preimmunized with either saline or Ad-0. Two weeks later, animals were implanted with CNS1 tumor cells and then treated 6 days later with an intratumoral injection of either saline or HC-Ad (8 to 10/group). (C) Kaplan-Meier survival curves of naïve tumor-bearing rats treated with bicistronic therapeutic HC-Ad. *, P < 0.05 versus saline (log-rank test). (D) Kaplan-Meier survival curves of preimmunized animals treated with bicistronic therapeutic HC-Ad. *, P < 0.05 versus saline (log-rank test). (E) Levels of Flt3L protein in brain hemispheres ipsilateral (ips) and contralateral (cont) to the bicistronic HC-Ad injection site were assessed by ELISA at 5 and 30 days after treatment (5/group). *, P < 0.05 versus the contralateral value (Student's t test). (F) DNA was isolated from both brain hemispheres (5/group), and Flt3L transgene copies were quantified by qPCR at 5 and 30 days after delivery of bicistronic HC-Ad. *, P < 0.05 versus the contralateral value (Student's t test).
Flt3L protein expression and copies of Flt3L transgene decrease within 30 days of HC-Ad delivery.
The levels of Flt3L protein and transgene copies were determined in the brain 5 and 30 days after intratumoral administration of the bicistronic HC-Ad in naïve tumor-bearing rats. Analysis of Flt3L protein content in the brain by ELISA revealed that Flt3L is present only in the brain hemisphere ipsilateral to the injection at 5 days posttreatment, although one animal displayed higher levels of Flt3L protein in the HC-Ad-treated brain hemisphere than the other four treated animals. Statistical analysis reveals that levels of Flt3L in the brain hemisphere ipsilateral to the injection would remain significantly elevated if this animal were to be considered an outlier and consequently removed from the data analysis. However, we believe that inclusion of all individual rats in the data analysis more accurately reflects the true biological effects and consequences of HC-Ad-TK/TetOn-Flt3L treatment of test subjects bearing syngeneic brain tumors, and this animal was therefore included in the data presentation and statistical analysis. Flt3L protein was undetectable in the brain at day 30 (Fig. 2E). Flt3L protein was not found in the contralateral brain hemisphere at either time point. We also quantified the copies of the Flt3L transgene using real-time quantitative PCR (qPCR). There was an ∼50-fold decrease in the levels of Flt3L transgene in the injected brain hemisphere between 5 days and 30 days after HC-Ad injection. Copies of the Flt3L transgene were below detectable limits in the contralateral brain hemisphere at both 5 days and 30 days after HC-Ad injection (Fig. 2F).
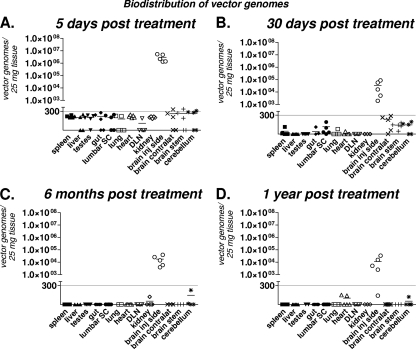
Biodistribution of bicistronic HC-Ad vector genomes is restricted to the injected brain hemisphere at all time points tested.
We assessed the biodistribution of HC-Ad vector genomes in the central nervous system and in peripheral organs 5 days, 30 days, 6 months, and 1 year after treatment using qPCR analysis (27, 32). Bicistronic HC-Ad genomes were restricted to the injected brain hemisphere at all time points tested (Fig. 3). A decrease in HC-Ad vector genomes was detected between day 5 and day 30 after HC-Ad delivery, which is in agreement with the decline in the levels of Flt3L transgene copies and protein observed during the same period (Fig. 2F). Importantly, HC-Ad vector genomes were below detectable limits in all peripheral organs, including the liver, as well as other regions of the central nervous system (CNS) at all time points (Fig. 3), indicating the high safety profile of intratumoral delivery of a bicistronic HC-Ad vector.
FIG. 3.
Biodistribution of bicistronic HC-Ad vector genomes in tumor-bearing animals. HC-Ad vector genomes were quantified in the tissues indicated at 5 days (A), 30 days (B), 6 months (C), and 1 year (D) after intratumoral injection of bicistronic HC-Ad into naïve, tumor-bearing rats. The solid line indicates the detection limit.
Administration of bicistronic HC-Ad vector does not lead to neurological or systemic side effects.
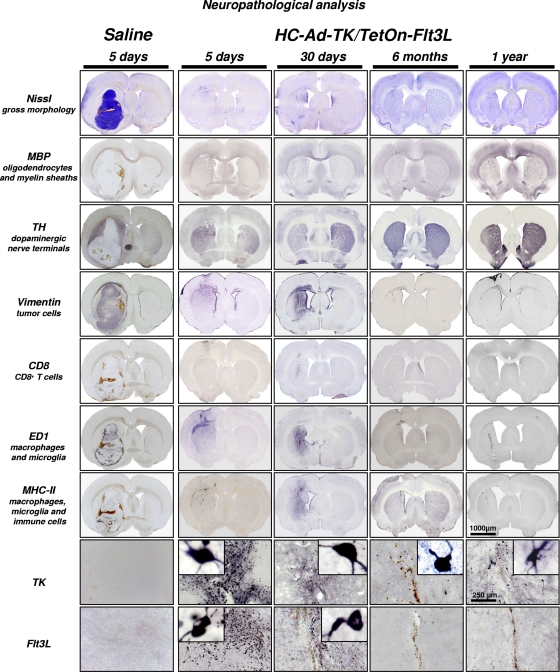
To assess the potential detrimental inflammatory consequences of HC-Ad-TK/TetOn-Flt3L-mediated brain tumor regression on the architecture of the brain, we performed an extensive neuropathological analysis of brain sections at 5 days, 30 days, 6 months, and 1 year after intratumor HC-Ad delivery. Representative images are shown in Fig. 4. Nissl staining revealed a rapid elimination of tumor burden within 5 days of HC-Ad delivery. Myelin basic protein (MBP) and tyrosine hydroxylase (TH) immunoreactivity indicated rapid restoration of brain architecture, even at this early time point. Profuse infiltration of immune cells was localized within the tumor-bearing hemisphere, with a sustained presence of macrophages and major histocompatibility complex II (MHCII)-positive immune cells observed at 30 days, which was ameliorated by 6 months. A complete restoration of the normal brain architecture was observed at the 6-month and 1-year time points, with the exception of minor ventriculomegaly in the brain hemisphere that harbored the tumor. Residual macrophages were localized in the scar left after tumor regression at 1 year posttherapy.
FIG. 4.
Neuropathological analysis of brains at 5 days, 30 days, 6 months, and 1 year after intratumoral administration of bicistronic HC-Ad to naïve, tumor-bearing Lewis rats. Images of TK and Flt3L immunoreactivity are higher-magnification images from the brain hemisphere ipsilateral to the HC-Ad injection. TH, tyrosine hydroxylase; MBP, myelin basic protein; MHCII, major histocompatibility complex II.
Immunocytochemical characterization of transgene expression revealed abundant cells expressing TK and Flt3L within the tumor mass 5 days after the treatment (Fig. 4). The presence of transgene-expressing cells declined as the tumor regressed, and by 6 months and 1 year, only a few positive cells, most probably nonneoplastic cells transduced with the bicistronic HC-Ad, remained surrounding the scar area. The long-term expression of TK in normal brain cells further highlights the idea that conditional cytotoxicity of TK in combination with GCV does not kill normal brain cells and is a safe cytotoxic approach for brain cancer. The long-term safety of TK (plus GCV) in the normal brain is supported by our previous work in which naïve, non-tumor-bearing animals injected with adenoviral vectors encoding TK (plus GCV) did not exhibit any evidence of neurotoxicity at 7 days or 60 days postinjection (9), and the long-term survivors treated with an adenovirus encoding TK (plus GCV) displayed evidence of TK immunoreactivity in the normal brain 3 months postdelivery with no indication of pathology in the TK-immunoreactive cells (15).
In order to detect any possible systemic side effects, serum chemistry and blood cell counts were assessed in the blood of tumor-bearing rats 5 days, 30 days, 6 months, and 1 year posttreatment (Table 1; also, see Tables S1 and S2 in the supplemental material). Analysis of biochemical laboratory parameters indicated normal liver and renal function; values of aspartate transaminase (AST), alanine aminotransferase (ALT), bilirubin, urea, and creatinine were within the normal range of age-matched naïve animals at all time points tested. Red and white cell counts in the treated animals were also within normal ranges, indicating that Flt3L expression in the brain tumor does not substantially alter the levels of circulating immune cells.
TABLE 1.
Clinical and hematological parameters of tumor-bearing animals at 5 days, 30 days, and 1 year posttreatment
| Clinical and hematological parametera | 5 days |
1 month |
1 year |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naïve |
Saline |
HC-Ad |
Naive |
HC-Ad |
Naive |
HC-Ad |
||||||||
| Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | Median | Range | |
| AST (U/liter) | 109.0 | 93-233 | 131.5 | 83-260 | 113.0 | 90-227 | 231.0 | 79-333 | 78.5 | 47-227 | 67.0 | 62-77 | 78.0 | 58-93 |
| ALT (U/liter) | 47.0 | 42-82 | 46.5 | 31-57 | 34.0 | 31-46 | 68.5 | 38-80 | 29.0 | 23-46 | 40.0 | 37-52 | 36.5 | 30-47 |
| Total bilirubin (mg/dl) | 0.1 | 0.1-0.1 | 0.2 | 0.1-0.9 | 0.3 | 0.3-0.4 | 0.1 | 0-0.1 | 0.1 | 0.1-0.4 | 0.1 | 0.1-0.1 | 0.1 | 0.1-0.1 |
| AP (U/liter) | 338.0 | 279-349 | 162.5 | 102-369 | 246.0 | 198-319 | 332.5 | 305-362 | 248.0 | 129-333 | 213.0 | 191-268 | 183.5 | 144-229 |
| Protein | 5.7 | 5.4-5.8 | 6.2 | 5.6-6.8 | 6.2 | 6-6.4 | 6.0 | 5.6-6.3 | 5.8 | 5.4-6.4 | 6.2 | 5.9-6.3 | 6.1 | 5.7-6.5 |
| Albumin | 3.5 | 3.2-3.6 | 3.0 | 2.6-3.3 | 3.1 | 3.1-3.2 | 3.3 | 2.9-3.5 | 3.0 | 2.8-3.2 | 3.1 | 2.9-3.2 | 3.0 | 2.9-3.2 |
| Globulin | 2.2 | 2.2-2.2 | 3.2 | 2.7-3.5 | 3.1 | 2.9-3.2 | 2.7 | 2.6-2.8 | 2.8 | 2.6-3.2 | 3.1 | 2.9-3.2 | 3.1 | 2.8-3.3 |
| Cholesterol (mg/dl) | 78.0 | 78-83 | 94.0 | 70-155 | 88.5 | 86-103 | 110.5 | 76-119 | 77.0 | 68-103 | 100.5 | 93-104 | 100.0 | 81-115 |
| BUN (mg/dl) | 21.0 | 20-22 | 21.0 | 14-39 | 24.5 | 22-25 | 23.0 | 20-33 | 22.0 | 16-27 | 20.5 | 18-23 | 17.0 | 13-18 |
| Creatinine (mg/dl) | 0.5 | 0.3-0.5 | 0.5 | 0.3-0.9 | 0.5 | 0.4-0.5 | 0.5 | 0.4-0.7 | 0.4 | 0.3-0.7 | 0.3 | 0.3-0.4 | 0.3 | 0.3-0.5 |
| BUN/creatinine ratio | 42.0 | 66.7-44 | 47.3 | 23.3-68 | 50.0 | 44-60 | 52.0 | 38.3-57.5 | 54.5 | 34-66.7 | 60.0 | 52.5-73.3 | 47.5 | 34-60 |
| Phosporus (mg/dl) | 13.9 | 9.8-15.4 | 14.7 | 11.8-21.4 | 18.2 | 13.6-22.7 | 18.5 | 9.5-25 | 10.6 | 9.4-22.7 | 6.8 | 6-8 | 7.1 | 6.5-11.6 |
| Calcium (mg/dl) | 9.6 | 9.2-9.9 | 10.1 | 7.8-12 | 10.3 | 9.3-11.4 | 9.0 | 8.4-9.5 | 10.2 | 9.3-11.4 | 10.2 | 9.8-10.2 | 9.8 | 9.6-12 |
| Glucose (mg/dl) | 196.0 | 160-275 | 220.0 | 84-385 | 140.5 | 82-182 | 143.0 | 106-194 | 188.0 | 82-399 | 187.5 | 143-206 | 206.0 | 169-366 |
| Sodium (mmol/liter) | 146.0 | 144-147 | 142.5 | 135-152 | 144.0 | 143-147 | 135.0 | 131-142 | 142.0 | 140-147 | 140.5 | 140-143 | 140.5 | 138-143 |
| Potassium (mmol/liter) | 11.8 | 11.3-12.9 | 12.0 | 7.8-18.9 | 11.5 | 9.7-12.6 | 20.6 | 9-27.5 | 8.2 | 7.1-12.6 | 8.9 | 7.7-9.4 | 8.4 | 7.2-9.8 |
| Chloride (mmol/liter) | 104.0 | 102-105 | 99.0 | 92-105 | 101.0 | 98-102 | 101.0 | 96-102 | 101.5 | 97-103 | 103.0 | 100-103 | 101.5 | 98-103 |
| CPK | 407.0 | 373-2,606 | 607.5 | 25-4,143 | 620.0 | 326-11,391 | 8,407.5 | 524-13,263 | 306.5 | 79-11,391 | 175.0 | 149-858 | 252.0 | 75-328 |
| White blood cells (103/μl) | 6.8 | 6.3-7 | 6.1 | 4.5-8.9 | 4.6 | 4.1-5.5 | 4.8 | 3.8-6.6 | 5.7 | 4.1-7.1 | 3.8 | 2.7-4.5 | 4.2 | 3.1-4.9 |
| Red blood cells (106/μl) | 8.2 | 7.6-9 | 8.9 | 8.3-10.1 | 8.5 | 7.9-8.8 | 8.6 | 7.7-9.1 | 8.8 | 7.9-9.3 | 8.7 | 7.8-8.9 | 9.0 | 8.6-9.3 |
| HGB (g/dl) | 14.8 | 13.6-15.9 | 14.8 | 14-16.6 | 14.1 | 13.1-15.2 | 13.8 | 12.7-14.7 | 14.6 | 13.1-15.3 | 14.1 | 13.3-14.4 | 14.8 | 14-15.5 |
| Mean corpuscular vol (fl) | 62.0 | 60-62 | 53.0 | 50-60 | 53.0 | 52-57 | 52.5 | 51-55 | 53.0 | 52-57 | 51.5 | 51-54 | 52.0 | 50-54 |
| Mean corpuscular hemoglobin (pg) | 17.8 | 17.7-18 | 16.4 | 15.9-17.2 | 16.8 | 16.5-17.3 | 16.2 | 15.9-16.5 | 16.6 | 16.1-17.3 | 16.3 | 15.8-17.1 | 16.4 | 16.1-16.9 |
| Neutrophils (103/μl) | 31.0 | 28-31 | 18.0 | 8-33 | 15.5 | 13-18 | 18.0 | 16-30 | 17.0 | 10-21 | 16.5 | 14-22 | 25.0 | 18-32 |
| Absolute neutrophils | 1,960.0 | 1,953-2,108 | 977.0 | 480-2,848 | 754.0 | 585-782 | 936.0 | 646-1,500 | 888.5 | 580-1,420 | 629.0 | 378-880 | 948.0 | 720-1,568 |
| Lymphocytes (103/μl) | 66.0 | 65-69 | 79.0 | 63-89 | 81.5 | 80-84 | 79.0 | 66-82 | 80.0 | 76-88 | 81.5 | 75-83 | 71.5 | 65-79 |
| Absolute lymphocytes | 4,488.0 | 4,095-4,830 | 4,781.0 | 3,690-5,874 | 3,730.0 | 3,280-4,565 | 3,476.0 | 3,002-5,412 | 4,565.0 | 3,280-5,538 | 3,035.5 | 2,241-3,690 | 3,063.5 | 2,139-3,408 |
| Mononuclear cells/μl | 2.0 | 2-2 | 2.0 | 1-3 | 2.0 | 1-2 | 1.0 | 1-1 | 1.0 | 1-3 | 1.5 | 1-2 | 2.0 | 1-2 |
| Absolute monocytes | 136.0 | 126-140 | 119.0 | 46-204 | 91.0 | 41-110 | 48.0 | 38-66 | 68.5 | 41-141 | 64.0 | 38-106 | 78.0 | 43-98 |
| Eosinophils/μl | 1.0 | 1-1 | 1.0 | 0-3 | 0.0 | 0-1 | 1.0 | 1-2 | 1.0 | 0-2 | 1.0 | 1-1 | 0.0 | 0-1 |
| Absolute eosinophils | 68.0 | 63-70 | 56.0 | 0-183 | 0.0 | 0-41 | 62.0 | 42-100 | 56.5 | 0-112 | 37.5 | 27-45 | 0.0 | 0-48 |
| Basophils/μl | 0.0 | 0-1 | 0.0 | 0-1 | 1.0 | 0-1 | 1.0 | 1-1 | 0.0 | 0-1 | 0.0 | 0-0 | 1.0 | 1-1 |
| Absolute basophils | 0.0 | 0-63 | 0.0 | 0-64 | 45.5 | 0-55 | 44.0 | 38-50 | 0.0 | 0-59 | 0.0 | 0-0 | 41.5 | 31-49 |
| Platelet count (103/μl) | 706.0 | 672-783 | 776.0 | 589-1,016 | 631.5 | 571-763 | 695.0 | 601-725 | 711.0 | 571-842 | 525.5 | 516-582 | 558.5 | 492-709 |
BUN, blood urea nitrogen; CPK, creatine phosphokinase; HGB, hemoglobin.
Behavioral consequences of tumor regression induced by treatment with bicistronic HC-Ad vector.
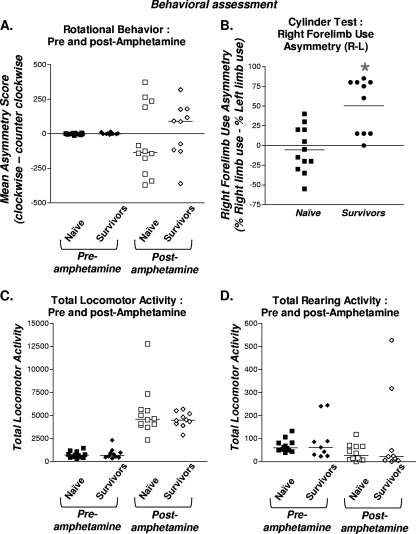
To determine whether intratumoral delivery of HC-Ad-TK/TetOn-Flt3L induces chronic neurological deficits, the long-term survivors underwent a panel of neurobehavioral testing 1 year after treatment. Analysis of amphetamine-induced rotational behavior, total locomotor activity, and rearing activity did not reveal any behavioral abnormalities compared to age-matched naïve controls (Fig. 5A, C, and D). Abnormalities in forelimb use asymmetry were detected in HC-Ad-treated long-term survivors after 1 year. However, we previously showed that differences in forelimb use asymmetry do not appear to be HC-Ad dose dependent (27) and are, thus, likely a residual consequence of regression of a very large intracranial brain tumor mass (Fig. 5B).
FIG. 5.
Behavioral assessment of long-term survivors 1 year after treatment with bicistronic HC-Ad. Behavioral assessment was performed before and after amphetamine treatment in long-term survivors 1 year after intratumoral administration of bicistronic HC-Ad into naïve, tumor-bearing rats. Naïve, age-matched rats were used as controls. (A) Rotational behavior. (B) Right-forelimb-use asymmetry. (C) Spontaneous motor behavior. (D) Rearing behavior.
DISCUSSION
Immunotherapies constitute a promising approach for the treatment of GBM (2, 7, 12, 14, 17, 43) due to the diffuse and infiltrative nature of the disease (44). Using first-generation adenoviral vectors as gene delivery vehicles, we have pioneered a cytotoxic/immune-stimulatory approach combining delivery of TK and Flt3L therapeutic genes directly to the tumor mass. We have previously demonstrated the therapeutic efficacy of this approach in rat and mouse syngeneic intracranial models of brain cancer (2, 9, 12, 17, 20, 22, 27, 48). The molecular mechanism of action of our treatment involves Flt3L-mediated recruitment of DCs into the GBM microenvironment; DCs phagocytose endogenous brain tumor antigens released in response to tumor cells' killing induced by Ad-TK (+GCV) (12). In addition, gene therapy-induced cell death releases HMGB1, a TLR2 agonist which is necessary for the activation of DCs and the subsequent activation of a systemic anti-GBM immune response (12). DCs loaded in situ with brain tumor antigens then migrate to the draining lymph node, where they present tumor antigen to naïve T cells, thus inducing a brain tumor-specific immune response (9, 12) and generating immunological memory that protects against recurrent brain tumors (12, 20, 27). A multicenter, dose-escalating phase I clinical trial testing this therapeutic approach in patients with recurrent high-grade glioma is scheduled to commence this year.
We recently published a study demonstrating therapeutic efficacy and a high safety profile of two separate high-capacity, gutless adenoviral vectors, each encoding either Flt3L or TK, to treat rats bearing intracranial brain tumors (27). Due to the complete deletion of all viral coding genes, HC-Ads do not express any viral antigens, and thus, cells infected with HC-Ads are not targeted by the anti-adenoviral immune response. This facilitates long-term transgene expression in the brain, even in the presence of a systemic anti-adenoviral immune response (4, 21, 27, 40, 41, 47). Another advantage of the HC-Ad vector platform is their ability to encode large, complex expression cassettes, such as the inducible TetOn expression cassette (27, 47). Due to the limited insert capacity of first-generation adenoviral vectors (Ads), it is impossible to package these large expression cassettes into viable vectors.
In this study, we tested the hypothesis that a single HC-Ad vector encoding constitutively expressed TK and inducible expression of Flt3L would be efficacious and safe, even in the presence of anti-Ad immunity, as could be present in humans undergoing clinical trials (10). The GBM model used, i.e., CNS1 cells implanted into the striatum of Lewis rats, develops into large macroscopic tumors that exhibit many of the histopathological features displayed in human GBM (5). Although the degree of invasion encountered in this model is modest and this could be considered a limitation of the model, due to its reproducibility, high penetrance, and development in an immune-competent host, it remains one of the most valuable models in which to test novel therapeutic modalities for GBM. We have previously established that the 5 × 109 vp dose of HC-Ad is the maximum tolerated dose that can be safely administered in the CNS1 brain tumor model without adverse side effects (27). At the time of vector delivery, the tumor is a large macroscopic mass; thus, the ratio of vector particles versus CNS1 cells is significantly lower. In the clinical scenario, we propose to deliver the therapeutic HC-Ad vector into the margins of the tumor bed, thus significantly increasing the ratio of HC-Ad viral particles to tumor cells.
Our results demonstrate the high therapeutic efficacy and corresponding reduction of the tumor mass within 30 days of treatment in >75% of rats. Tumor regression occurred concomitantly with a reduction in the copies of HC-Ad vector genomes and the content of Flt3L protein in the brain. These data suggest that tumor cells transduced with HC-Ad vectors are rapidly eliminated upon treatment. The absence of Flt3L protein in the brain could also be partially attributed to the cessation of DOX administration, which turns off Flt3L expression, 2 days before the Flt3L protein was measured. These results highlight the tight regulation of our TetOn switch and are congruent with our previously published results on the kinetics of DOX regulation of transgene expression in vivo (47).
The major concern for encoding both cytotoxic (TK) and immune-stimulatory transgenes (Flt3L) on a single vector is that TK-mediated killing of transduced cells could prematurely diminish the immune stimulatory effects of Flt3L, inducing the recruitment of DCs to the brain tumor. However, similar levels of therapeutic efficacy and elimination of HC-Ad genomes were observed in our previous preclinical study using Flt3L and TK encoded on two separate HC-Ad vectors (27), suggesting that encoding both cytotoxic and immune stimulatory transgenes on a single HC-Ad vector is a feasible and efficacious therapeutic approach in both naïve rats and animals systemically immunized against adenovirus. The preservation of brain architecture, the absence of severe long-term behavioral deficits 1 year after treatment, and the absence of significant chronic inflammation in the brain provide strong evidence of the safety of this bicistronic approach. While we did not assess neuropathology and serum chemistry in HC-Ad-treated preimmunized animals, HC-Ad vectors are completely devoid of all viral protein-encoding genes and thus have a safe immunological profile. Therefore, we do not expect any differences in the neuropathology or serum chemistry of these animals, and our previous studies of HC-Ads delivered into the normal brain (4, 40, 41) and into a CNS1-derived brain tumor (21) indicate this to be the case. Importantly, our data showing the restriction of HC-Ad vector genomes to the injected brain hemisphere demonstrate that the bicistronic HC-Ad vector does not diffuse to other regions of the CNS or peripheral organs. This is in agreement with our previous study using two HC-Ad vectors (27) and also in accordance with recent preclinical studies assessing the safety and biodistribution of first-generation and oncolytic adenoviral vectors following intracranial injection in naïve rodents (23, 35).
In addition to the advantages to downstream process development and GMP manufacture, the incorporation of both Flt3L and TK into a single HC-Ad vector would enhance the safety of this approach by reducing the total viral load by half compared to the two-vector approach. As the maximum amount of vector that can be injected is limited by vector dose-mediated toxicity, our strategy maximizes therapeutic transgene expression per total viral load, thus reducing the inflammatory impact on the brain. It is important to note that we did not observe any evidence of chronic inflammation in the brains of tumor-bearing animals treated with the bicistronic HC-Ad in our study (Fig. 4), suggesting that the maximum tolerated dose of this vector could be further escalated. Also, a recent study demonstrated that large-scale preparation of HC-Ad vectors can be produced using adherent cells in cell factories, a technology which is easily translatable to the production of clinical-grade HC-Ads for human clinical trials (39). In summary, the efficacy and safety of using a bicistronic HC-Ad vector in clinically relevant animal models strongly support the further development of HC-Ad-TK/TetOn-Flt3L as a second-generation delivery platform for its future implementation in phase I clinical trials for GBM.
Supplementary Material
Acknowledgments
We thank S. Melmed, L. Fine, and Mark Greene for their support and John Young and his staff, Department of Comparative Medicine, CSMC. We also thank Jaime DyBuncio, Nhi Bui, Maricel Gozo, Maksim Khayznikov, Kathy Lawrence, and Ali Zadmehr for technical assistance and Nicholas Sanderson for the primary astrocytes.
This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) grant U01 NS052465. The brain tumor program in our institute is funded by NIH/NINDS grant R01 NS057711 and R21 NS054143 to M.G.C., by NIH/NINDS grants R01 NS054193 and R01 NS42893 to P.R.L. by The Bram and Elaine Goldsmith and the Medallions Group Endowed Chairs in Gene Therapeutics to P.R.L. and M.G.C., respectively, by The Drown Foundation, by The Linda Tallen & David Paul Kane Foundation Annual Fellowship, and by the Board of Governors at CSMC. M.C. is supported by NIH/NINDS F32 NS058156.
We have no conflicts of interest.
Footnotes
Published ahead of print on 7 April 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ali, S., J. F. Curtin, J. M. Zirger, W. Xiong, G. D. King, C. Barcia, C. Liu, M. Puntel, S. Goverdhana, P. R. Lowenstein, and M. G. Castro. 2004. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol. Ther. 10:1071-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali, S., G. D. King, J. F. Curtin, M. Candolfi, W. Xiong, C. Liu, M. Puntel, Q. Cheng, J. Prieto, A. Ribas, J. Kupiec-Weglinski, N. van Rooijen, H. Lassmann, P. R. Lowenstein, and M. G. Castro. 2005. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 65:7194-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ark Therapeutics Group. 7 April 2009, posting date. Cerepro phase III trial update. http://www.drugs.com/clinical_trials/cerepro-phase-iii-trial-update-6970.html.
- 4.Barcia, C., M. Jimenez-Dalmaroni, K. M. Kroeger, M. Puntel, A. J. Rapaport, D. Larocque, G. D. King, S. A. Johnson, C. Liu, W. Xiong, M. Candolfi, S. Mondkar, P. Ng, D. Palmer, M. G. Castro, and P. R. Lowenstein. 2007. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: implications for clinical trials. Mol. Ther. 15:2154-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Candolfi, M., J. F. Curtin, W. S. Nichols, A. G. Muhammad, G. D. King, G. E. Pluhar, E. A. McNiel, J. R. Ohlfest, A. B. Freese, P. F. Moore, J. Lerner, P. R. Lowenstein, and M. G. Castro. 2007. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J. Neurooncol. 85:133-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candolfi, M., J. F. Curtin, W. D. Xiong, K. M. Kroeger, C. Liu, A. Rentsendorj, H. Agadjanian, L. Medina-Kauwe, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2006. Effective high-capacity gutless adenoviral vectors mediate transgene expression in human glioma cells. Mol. Ther. 14:371-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Candolfi, M., K. M. Kroeger, A. K. Muhammad, K. Yagiz, C. Farrokhi, R. N. Pechnick, P. R. Lowenstein, and M. G. Castro. 2009. Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr. Gene Ther. 9:409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Candolfi, M., G. E. Pluhar, K. Kroeger, M. Puntel, J. Curtin, C. Barcia, A. K. Muhammad, W. Xiong, C. Liu, S. Mondkar, W. Kuoy, T. Kang, E. A. McNeil, A. B. Freese, J. R. Ohlfest, P. Moore, D. Palmer, P. Ng, J. D. Young, P. R. Lowenstein, and M. G. Castro. 2007. Optimization of adenoviral vector-mediated transgene expression in the canine brain in vivo, and in canine glioma cells in vitro. Neuro Oncol. 9:245-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candolfi, M., K. Yagiz, D. Foulad, G. E. Alzadeh, M. Tesarfreund, A. K. Muhammad, M. Puntel, K. M. Kroeger, C. Liu, S. Lee, J. F. Curtin, G. D. King, J. Lerner, K. Sato, Y. Mineharu, W. Xiong, P. R. Lowenstein, and M. G. Castro. 2009. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin. Cancer Res. 15:4401-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 11.Curtin, J. F., M. Candolfi, T. M. Fakhouri, C. Liu, A. Alden, M. Edwards, P. R. Lowenstein, and M. G. Castro. 2008. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One 3:e1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtin, J., N. Liu, M. Candolfi, W. Xiong, A. Assi, K. Yagiz, M. Edwards, K. Michelsen, K. Kroeger, C. Liu, A. Muhammad, M. Clark, M. Arditi, B. Comin-Anduix, A. Ribas, P. Lowenstein, and M. Castro. 2009. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 6:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtin, J. F., G. D. King, C. Barcia, C. Liu, F. X. Hubert, C. Guillonneau, R. Josien, I. Anegon, P. R. Lowenstein, and M. G. Castro. 2006. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J. Immunol. 176:3566-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtin, J. F., G. D. King, M. Candolfi, R. B. Greeno, K. M. Kroeger, P. R. Lowenstein, and M. G. Castro. 2005. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr. Top. Med. Chem. 5:1151-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewey, R. A., G. Morrissey, C. M. Cowsill, D. Stone, F. Bolognani, N. J. Dodd, T. D. Southgate, D. Klatzmann, H. Lassmann, M. G. Castro, and P. R. Lowenstein. 1999. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 5:1256-1263. [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency. 17 December 2009, posting date. Questions and answers on the recommendation for the refusal of the marketing authorisation for Cerepro (sitimagene ceradenovec). http://www.ema.europa.eu/pdfs/human/opinion/Cerepro_Q&A_828428en.pdf.
- 17.Ghulam Muhammad, A. K., M. Candolfi, G. D. King, K. Yagiz, D. Foulad, Y. Mineharu, K. M. Kroeger, K. A. Treuer, W. S. Nichols, N. S. Sanderson, J. Yang, M. Khayznikov, N. Van Rooijen, P. R. Lowenstein, and M. G. Castro. 2009. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin. Cancer Res. 15:6113-6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimberger, A. B., and J. H. Sampson. 2009. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin. Biol. Ther. 9:1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Immonen, A., M. Vapalahti, K. Tyynela, H. Hurskainen, A. Sandmair, R. Vanninen, G. Langford, N. Murray, and S. Yla-Herttuala. 2004. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol. Ther. 10:967-972. [DOI] [PubMed] [Google Scholar]
- 20.King, G. D., K. M. Kroeger, C. J. Bresee, M. Candolfi, C. Liu, C. M. Manalo, A. M. Muhammad, R. J. Pechnick, P. R. Lowenstein, and M. G. Castro. 2008. Flt3L in combination with HSV1-TK mediated gene therapy reverses brain tumor induced behavioral deficits. Mol. Ther. 16:682-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King, G. D., A. G. Muhammad, W. Xiong, K. M. Kroeger, M. Puntel, D. Larocque, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2008. High-Capacity adenoviral vector-mediated anti-glioma gene therapy in the presence of systemic anti-adenovirus immunity. J. Virol. 82:4680-4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King, G. D., A. K. M. Muhammad, J. F. Curtin, C. Barcia, M. Puntel, C. Liu, S. B. Honig, M. Candolfi, S. Mondkar, P. R. Lowenstein, and M. G. Castro. 2008. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro-oncology 10:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langford, G., A. Dayan, S. Yla-Herttuala, and D. Eckland. 2009. A preclinical assessment of the safety and biodistribution of an adenoviral vector containing the herpes simplex virus thymidine kinase gene (Cerepro) after intracerebral administration. J. Gene Med. 11:468-476. [DOI] [PubMed] [Google Scholar]
- 24.Liau, L. M., R. M. Prins, S. M. Kiertscher, S. K. Odesa, T. J. Kremen, A. J. Giovannone, J. W. Lin, D. J. Chute, P. S. Mischel, T. F. Cloughesy, and M. D. Roth. 2005. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 11:5515-5525. [DOI] [PubMed] [Google Scholar]
- 25.Maione, D., C. Della Rocca, P. Giannetti, R. D'Arrigo, L. Liberatoscioli, L. L. Franlin, V. Sandig, G. Ciliberto, N. La Monica, and R. Savino. 2001. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. U. S. A. 98:5986-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morral, N., W. O'Neal, K. Rice, M. Leland, J. Kaplan, P. A. Piedra, H. Zhou, R. J. Parks, R. Velji, E. Aguilar-Cordova, S. Wadsworth, F. L. Graham, S. Kochanek, K. D. Carey, and A. L. Beaudet. 1999. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. U. S. A. 96:12816-12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muhammad, A. K., M. Puntel, M. Candolfi, A. Salem, K. Yagiz, C. Farrokhi, K. M. Kroeger, W. Xiong, J. F. Curtin, C. Liu, K. Lawrence, N. S. Bondale, J. Lerner, G. J. Baker, D. P. Foulad, R. N., D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 17 February 2010. Study of the efficacy, biodistribution and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin. Pharmacol. Ther. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 28.O'Neal, W. K., H. Zhou, N. Morral, C. Langston, R. J. Parks, F. L. Graham, S. Kochanek, and A. L. Beaudet. 2000. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol. Med. 6:179-195. [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer, D. J., and P. Ng. 2008. Methods for the production of helper-dependent adenoviral vectors. Methods Mol. Biol. 433:33-53. [DOI] [PubMed] [Google Scholar]
- 30.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. U. S. A. 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulkkanen, K. J., and S. Yla-Herttuala. 2005. Gene therapy for malignant glioma: current clinical status. Mol. Ther. 12:585-598. [DOI] [PubMed] [Google Scholar]
- 32.Puntel, M., J. F. Curtin, J. M. Zirger, A. K. Muhammad, W. Xiong, C. Liu, J. Hu, K. M. Kroeger, P. Czer, S. Sciascia, S. Mondkar, P. R. Lowenstein, and M. G. Castro. 2006. Quantification of high-capacity helper-dependent adenoviral vector genomes in vitro and in vivo, using quantitative TaqMan real-time polymerase chain reaction. Hum. Gene Ther. 17:531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puntel, M., K. M. Kroeger, N. R. Sanderson, C. E. Thomas, M. G. Castro, and P. R. Lowenstein. 2010. Gene transfer into rat brain using adenoviral vectors. Curr. Protoc. Neurosci. 50:4.24.1-4.24.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sathornsumetee, S., and J. N. Rich. 2008. Designer therapies for glioblastoma multiforme. Ann. N. Y. Acad. Sci. 1142:108-132. [DOI] [PubMed] [Google Scholar]
- 35.Sonabend, A. M., I. V. Ulasov, Y. Han, C. E. Rolle, S. Nandi, D. Cao, M. A. Tyler, and M. S. Lesniak. 2009. Biodistribution of an oncolytic adenovirus after intracranial injection in permissive animals: a comparative study of Syrian hamsters and cotton rats. Cancer Gene Ther. 16:362-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stupp, R., M. E. Hegi, M. J. van den Bent, W. P. Mason, M. Weller, R. O. Mirimanoff, and J. G. Cairncross. 2006. Changing paradigms-an update on the multidisciplinary management of malignant glioma. Oncologist 11:165-180. [DOI] [PubMed] [Google Scholar]
- 37.Stupp, R., A. F. Hottinger, M. J. van den Bent, P. Y. Dietrich, and A. A. Brandes. 2008. Frequently asked questions in the medical management of high-grade glioma: a short guide with practical answers. Ann. Oncol 19(Suppl. 7):vii209-vii216. [DOI] [PubMed] [Google Scholar]
- 38.Stupp, R., W. P. Mason, M. J. van den Bent, M. Weller, B. Fisher, M. J. Taphoorn, K. Belanger, A. A. Brandes, C. Marosi, U. Bogdahn, J. Curschmann, R. C. Janzer, S. K. Ludwin, T. Gorlia, A. Allgeier, D. Lacombe, J. G. Cairncross, E. Eisenhauer, and R. O. Mirimanoff. 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352:987-996. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, M., R. Cela, C. Clarke, T. K. Bertin, S. Mourino, and B. Lee. 2010. Large-scale production of high-quality helper-dependent adenoviral vectors using adherent cells in cell factories. Hum. Gene Ther. 21:120-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2000. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. U. S. A. 97:7482-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas, C. E., G. Schiedner, S. Kochanek, M. G. Castro, and P. R. Lowenstein. 2001. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 12:839-846. [DOI] [PubMed] [Google Scholar]
- 42.Walker, D. G., R. Laherty, F. H. Tomlinson, T. Chuah, and C. Schmidt. 2008. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: potential interaction with adjuvant chemotherapy. J. Clin. Neurosci. 15:114-121. [DOI] [PubMed] [Google Scholar]
- 43.Wei, J., G. DeAngulo, W. Sun, S. F. Hussain, H. Vasquez, J. Jordan, J. Weinberg, J. Wolff, N. Koshkina, and A. B. Heimberger. 2009. Topotecan enhances immune clearance of gliomas. Cancer Immunol. Immunother 58:259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen, P. Y., and S. Kesari. 2008. Malignant gliomas in adults. N. Engl. J. Med. 359:492-507. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler, C. J., K. L. Black, G. Liu, M. Mazer, X. X. Zhang, S. Pepkowitz, D. Goldfinger, H. Ng, D. Irvin, and J. S. Yu. 2008. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 68:5955-5964. [DOI] [PubMed] [Google Scholar]
- 46.Xiong, W., M. Candolfi, K. M. Kroeger, M. Puntel, S. Mondkar, D. Larocque, C. Liu, J. F. Curtin, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2008. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol. Ther. 16:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong, W., S. Goverdhana, S. A. Sciascia, M. Candolfi, J. M. Zirger, C. Barcia, J. F. Curtin, G. D. King, G. Jaita, C. Liu, K. Kroeger, H. Agadjanian, L. Medina-Kauwe, D. Palmer, P. Ng, P. R. Lowenstein, and M. G. Castro. 2006. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J. Virol. 80:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang, J., N. Sanderson, K. Wawrowski, M. Puntel, M. Castro, and P. Lowenstein. 2010. Kupfer-type immunological synapse characteristics do not predict anti-brain tumor cytolytic T-cell function in vivo. Proc. Natl. Acad. Sci. U. S. A. 107:4716-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.