Abstract
Mycobacterium bovis bacillus Calmette-Guérin (BCG), which elicits a degree of protective immunity against tuberculosis, is the most widely used vaccine in the world. Due to its persistence and immunogenicity, BCG has been proposed as a vector for vaccines against other infections, including HIV-1. BCG has a very good safety record, although it can cause disseminated disease in immunocompromised individuals. Here, we constructed a recombinant BCG vector expressing HIV-1 clade A-derived immunogen HIVA using the recently described safer and more immunogenic BCG strain AERAS-401 as the parental mycobacterium. Using routine ex vivo T-cell assays, BCG.HIVA401 as a stand-alone vaccine induced undetectable and weak CD8 T-cell responses in BALB/c mice and rhesus macaques, respectively. However, when BCG.HIVA401 was used as a priming component in heterologous vaccination regimens together with recombinant modified vaccinia virus Ankara-vectored MVA.HIVA and ovine atadenovirus-vectored OAdV.HIVA vaccines, robust HIV-1-specific T-cell responses were elicited. These high-frequency T-cell responses were broadly directed and capable of proliferation in response to recall antigen. Furthermore, multiple antigen-specific T-cell clonotypes were efficiently recruited into the memory pool. These desirable features are thought to be associated with good control of HIV-1 infection. In addition, strong and persistent T-cell responses specific for the BCG-derived purified protein derivative (PPD) antigen were induced. This work is the first demonstration of immunogenicity for two novel vaccine vectors and the corresponding candidate HIV-1 vaccines BCG.HIVA401 and OAdV.HIVA in nonhuman primates. These results strongly support their further exploration.
Vaccine strategies must balance safety with immunogenicity. Recombinant attenuated subunit vaccines are generally regarded as safe, but not sufficiently immunogenic as stand-alone vaccines (17). Heterologous prime-boost regimens employing diverse attenuated viruses or bacteria as vectors delivering a common, often T cell-based, immunogen have been shown to induce stronger responses than multiple repeated dosings of the same vaccine modalities (19, 22, 39, 54). This is because heterologous regimens allow boosting of pathogen insert-specific responses while avoiding the accumulation of antivector immunity, which can significantly decrease vaccine “take” (1, 41). Results of the STEP study, which used a candidate single-vector human immunodeficiency virus type 1 (HIV-1) vaccine (6, 17, 41), have highlighted the need for novel alternative vaccine vectors and strategies. Such alternatives could complement the limited mainstream vectors and provide additional safety and immunogenicity through increased flexibility, for example, through the availability of personalized vaccination regimens based on preexisting immune status and/or responsiveness to vaccination.
Mycobacterium bovis bacillus Calmette-Guérin (BCG) remains the world's most widely used vaccine, with over three billion doses administered since its deployment in 1920s. It is the only licensed vaccine against tuberculosis and is administered at birth as part of the WHO Expanded Programme on Immunization (EPI). Due to its many attractive features, BCG or related mycobacterial vectors have also been explored in the context of vaccines against a number of infectious agents such as Leishmania, Borrelia burgdorferi, Streptococcus pneumoniae, Bordetella pertussis, malaria, cottontail rabbit papillomavirus, measles virus, and indeed human and simian immunodeficiency viruses (34). Many of these vaccines showed immunogenicity and protection in murine models, and some were also immunogenic in nonhuman primates (8, 56, 67, 68). In human adults, recombinant BCG (rBCG) vaccines alone failed to provide consistent protection against Lyme disease (13). In addition to adult applications, we have suggested the use of rBCG expressing an HIV-1-derived immunogen as the priming component of a vaccine platform against mother-to-child transmission of HIV-1 through infected breast milk (32), where it would be critical to elicit a protective HIV-1-specific response as soon as possible after birth.
To compare vectors and heterologous prime-boost regimens directly, we have advocated and pioneered the development of a panel of vaccine modalities delivering the same shared immunogen (18). Our first such model immunogen is called HIVA (21). This is a T-cell immunogen comprising HIV-1 consensus clade A Gag and a string of partially overlapping immunodominant CD8 T-cell epitopes originating from Gag, Pol, Nef, and Env, which has already been tested extensively in human volunteers (20). To facilitate iterative preclinical improvements of the HIVA vaccines, epitopes recognized by murine (58) and rhesus macaque (44) CD8 T cells were also incorporated. Furthermore, we have formulated HIVA into various vaccine modalities, including plasmid DNA (21), modified vaccinia virus Ankara (MVA) (21), human adenovirus serotype 5 (HAdV-5) (5), Semliki Forest virus replicons (18, 49), recombinant lysine auxotroph BCG strain Pasteur (32), and baculovirus-expressed and purified, bluetongue virus-derived chimeric NS1 tubules (37); the immunogenicity of these vectors has been compared directly and in heterologous combinations. More recently, we reported on the immunogenicity of a novel and promising vaccine vector derived from ovine atadenovirus type 7 (OAdV) (5); OAdV is the prototype member of the genus Atadenovirus, which is structurally and biologically distinct from Mastadenovirus (e.g., HAdV-5) (2, 50). Importantly, no immunity to OAdV has so far been detected in human sera (26). In mice, OAdV.HIVA induced strong polyfunctional HIVA-specific T cell responses with distinct kinetics from those induced by HAdV5.HIVA and displayed demonstrable single-dose efficacy against a surrogate virus challenge (5). OAdV is approved for use in a phase I human clinical trial (http://clinicaltrials.gov identifier no. NCT00625430). All of the vectors/modalities we explore are perceived to be safe and acceptable for use in humans.
Here, as a step toward translating our results into human volunteers, we constructed a novel vaccine designated BCG.HIVA401 vectored by AERAS-401, a Danish 1331 strain of BCG with improved immunogenicity and safety (57), and demonstrated priming of T cells to the HIVA transgene product in rhesus macaques. These BCG.HIVA401-primed HIV-1-specific CD4 and CD8 T-cell responses were readily boosted with MVA.HIVA and OAdV.HIVA vaccines to elicit broad and robust HIV-1-specific T cell responses.
MATERIALS AND METHODS
BCG.HIVA401 vaccine construction and preparation.
Escherichia coli strains were grown at 37°C on tryptic soy agar (EMD) or in tryptic soy broth (Teknova) with gentle agitation. Kanamycin was used at a final concentration of 50 μg/ml, and hygromycin was used at a final concentration of 150 μg/ml. The M. bovis endosomalytic rBCG strain AERAS-401 (57) and derivatives were cultured at 37°C on 7H10 agar (BD Biosciences) or in Middlebrook 7H9 medium supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC) enrichment (BD Biosciences) plus 0.05% (vol/vol) tyloxapol (Sigma). For antigen expression studies, strains were cultured in the above growth medium without OADC enrichment and supplemented with 3% glucose.
An expression cassette encoding the HIVA immunogen linked to the M. bovis Ag85B promoter and a 19-kDa signal peptide was generated by PCR from plasmid pJH222.HIVA (32), using primers 85BpromL4 (5′-GCGGATTAATACGGAAATGAGACGACTTTGCGCC-3′) and HIVAR4 (5′-GCGGATTAATAAGCTTCCTCTAGATGCATGCTCGAGCG-3′). The cassette was ligated into the integrative shuttle plasmid pCB02, which uses the mycobacteriophage L5 integrase (38), and electroporated into rBCG strain AERAS-401 as described previously (40). Integrants were selected on 7H10 plus hygromycin and confirmed by PCR; PCR-positive rBCG.HIVA401 isolates were grown in protein-free 7H9 medium and analyzed by Western blotting for HIVA expression. Briefly, cells were pelleted by centrifugation and culture supernatants were concentrated 100×. The resultant fractions were separated by discontinuous SDS-PAGE, transferred to polyvinylidene difluoride (PVDF) membranes, and probed with anti-Pk tag antibodies (Serotec).
Preparation of MVA.HIVA virus stock.
Construction of MVA.HIVA was described previously (21). Working vaccine stocks were grown in chicken embryo fibroblast cells using Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin/streptomycin, and glutamine, and then purified on a 36% sucrose cushion; the titer was determined, and the stocks were stored at −80°C until use.
Preparation of OAdV.HIVA virus stock.
Construction of OAdV.HIVA was described previously (5). OAdV.HIVA was grown on permissive CSL503 ovine fetal lung cells, and the titer was determined according to published procedures (3). In macaques, OAdV.HIVA is expected to be an attenuated nonreplicating virus as it is in all nonovine cell types that have been tested (2).
Peptides and preparation of peptide pools.
HIVA-derived 15-mer peptides overlapping by 11 amino acid residues were kindly provided by the International AIDS Vaccine Initiative and employed as described previously (46). Peptides were synthesized, purified by high-performance liquid chromatography and confirmed to be >80% pure using mass spectroscopy (Sigma-Genosys). Individual peptides were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) to yield a stock of 40 mg/ml and stored at −80°C. Peptides were either mixed into pools 1 to 4, each containing 20 to 23 individual peptides, corresponding to the Gag p24 and p17 regions of HIVA or combined into one pool of 90 peptides called pool 90. Pool 5 corresponded to the C-terminal polyepitope region of HIVA. Working pool stocks were prepared by combining 20 μl of each peptide and adding phosphate-buffered saline (PBS) to a final volume of 5 ml; stocks were then sterile filtered, aliquoted, and stored at 4°C for up to 1 week before use. Purified protein derivative (PPD) RT49 was purchased from Statens Serum Institute, Denmark, and used at a concentration of 20 μg/ml in assay wells.
Mouse immunizations and isolation of splenocytes.
Groups of 5 female BALB/c mice, 5 to 6 weeks of age, were injected with the following doses of vaccines: 106 CFU of BCG.HIVA401 intraperitoneally (i.p.), 106 PFU of MVA.HIVA intramuscularly (i.m.), and/or 107 infectious units (IU) of OAdV.HIVA i.m. The interval between BCG.HIVA401 prime and virus boosts was 12 weeks. Mice were sacrificed 4 weeks after the last immunization, the spleens were then removed and pressed individually through a cell strainer (Falcon) using a 2-ml syringe with a rubber plunger. The splenocytes were washed twice and suspended in 10 ml of lymphocyte medium (RPMI 1640 supplemented with 10% FBS, penicillin/streptomycin, 20 mM HEPES, and 15 mM 2-mercaptoethanol). All mouse procedures and care conformed strictly to United Kingdom Home Office Guidelines.
Measurement of supernatant IFN-γ by Bio-Plex.
To measure supernatant gamma interferon (IFN-γ), mouse splenocytes at 2 × 106 cells in 100 μl of R10 (RPMI 1640 supplemented with 10% FBS and penicillin/streptomycin) were added to each well of a 96-well round-bottomed plate (Costar), pulsed with the H-peptide at 2 μg/ml or medium alone and incubated at 37°C in 5% CO2 for 24 h. Plates were then spun at 330 × g for 5 min, and the supernatant was removed and stored at −80°C until use. Upon thawing, the supernatant samples were spun at 17,000 × g for 10 min and the cytokine assay was performed using a Bio-Plex mouse cytokine 23-Plex panel (1 × 96-well) kit (Bio-Rad) according to the manufacturer's protocol.
Rhesus macaques, vaccination, and isolation of PBMC.
Eight adult female Indian rhesus macaques (Macaca mulatta) from a United Kingdom breeding colony were housed and treated strictly in accordance with United Kingdom Home Office Guidelines. Four macaques per group were vaccinated with either BBMO or BBOM, where “B” represents 107 CFU of BCG.HIVA401 intradermally (i.d.), “M” represents 5 × 107 PFU of MVA.HIVA i.m., and “O” represents 1010 IU of OAdV.HIVA i.m. Vaccines were delivered at intervals of 4 weeks. Phlebotomy was performed from the femoral vein. Peripheral blood mononuclear cells (PBMC) were isolated using Lymphoprep cushion centrifugation (Nycomed Pharma) and either used fresh or cryopreserved in liquid nitrogen until use as described previously (30).
IFN-γ ELISPOT assay.
The frequencies of cells that released IFN-γ upon restimulation with HIVA-derived peptides or purified protein derivative (PPD) RT49 (Statens Serum Institute, Denmark) were assessed using an enzyme-linked immunospot (ELISPOT) assay. The procedures and reagents of a commercially available kit (Mabtech) were used throughout. Briefly, the released IFN-γ was captured by monoclonal antibody (MAb) GZ-4 immobilized on the bottom of the assay wells, visualized using a combination of the secondary MAb 7-B6-1 coupled to an enzyme and a chromogenic substrate (Bio-Rad), and quantified by spot counting using the AID ELISPOT reader system (Autoimmun Diagnostika). All assays were carried out in duplicate, and the background counts were subtracted from the experimental counts.
In vitro culture of macaque peripheral blood mononuclear cells.
Frozen PBMC were thawed and restimulated with 2 μg/ml peptides in R10 containing 500 U/ml human interleukin-2 (IL-2) and incubated in 5% CO2 at 37°C. On day 3, 500 U/ml IL-2 was added, 2 μg/ml of peptides was again added on day 7, and 500 U/ml IL-2 was added on day 10. On day 14, the cells were washed and analyzed in an IFN-γ ELISPOT assay.
CD4 cell depletion.
Magnetic bead (Dynal) depletion of CD4 cells was carried out according to the manufacturer's instructions. Briefly, no fewer than 107 PBMC were washed with PBS and spun slowly at 4°C for 30 min with the magnetic beads. Cells in the supernatant were harvested for analysis by IFN-γ ELISPOT assay.
CFSE proliferation assay.
Frozen PBMC were thawed, resuspended in R10, and incubated overnight at 37°C in 5% CO2. Cells were counted and placed at 2 × 106 in 96-well round-bottomed plates. Carboxyfluorescein succinimidyl ester (CFSE; Invitrogen) was diluted in PBS at 1:2,000; washed cells were labeled at this concentration for 8 min at 37°C and then washed again. Mock or peptide pool 90 was added at 2 μg/ml, and the cells were incubated in R10 with HEPES buffer at 37°C in 5% CO2 for 5 days. After incubation, cells were stained with anti-CD3-allophycocyanin (APC) (BD) and anti-CD8-PerCP (peridinin chlorophyll protein) (BD). At least 5,000 events were acquired for each condition using a FACS Calibur flow cytometer and analyzed with Summit (Dako) software. Results are expressed with the following formula: proliferation index (PI) = (sum of the cells in all generations)/(computed number of original parent cells theoretically present at the start of the experiment). Animal M7 provided sufficient cells for a positive concanavalin A (ConA; 5 μg/ml) control and negative (DMSO) control.
Flow cytometric cell sorting and phenotypic analysis.
Soluble fluorochrome-labeled CM9/Mamu-A*01-APC tetramers were produced and used as previously described previously (52). For phenotypic analyses of tetramer-reactive cells, the following directly conjugated MAbs were used: anti-CD3-H7-APC, anti-CD4-QD655, anti-CD8-Pacific Blue, anti-CD28-Texas Red-phycoerythrin (PE), anti-CD45RA-fluorescein isothiocyanate (FITC), and anti-CD95-Cy5-PE. Dead cells were excluded using LIVE/DEAD fixable Aqua dead cell stain kit (Invitrogen). Flow cytometric cell sorting was conducted with a modified FACSAria (BD) at 70 lb/in2; postsort purity was consistently >98%. In all cases, electronic compensation was performed with antibody-capture beads (BD) stained separately with individual MAbs present in the experimental samples.
Clonotype analysis.
Molecular analysis of all expressed T-cell receptor β locus (TRB) gene products in sorted tetramer-labeled CD8 T-cell populations was conducted using a template switch-anchored reverse transcription (RT)-PCR as described previously (12, 52). Sequences were aligned using IMGT nomenclature based on the extraction and characterization of macaque TR genes (15). Public sequences were assigned with reference to an extensive database derived from previous studies (29, 51, 52).
RESULTS
Construction and characterization of the next-generation BCG.HIVA401 vaccine.
Construction of novel BCG strain AERAS-401 [BCG1331 ΔureC::ΩpfoA(G137Q)], the starting parental strain in this study, was described previously (57) and was based on the observation that AERAS-401 conferred better protection against model Mycobacterium tuberculosis challenge of mice than the commonly used BCG vaccine strain SSI-1331 (BCG1331). Briefly, AERAS-401 was generated by two modifications aimed at increasing strain immunogenicity. First, BCG1331was transformed with a variant form of perfringolysin O (pfoA), which is normally secreted by Clostridium perfringens and associated with lysis of the endosome compartment (33). The PfoA(G137Q) protein carries a single-amino-acid substitution, G137Q, which results in the loss of the pfoA toxicity to mammalian cells attributed to its short half-life in the host cell cytosol, yet retains the ability to mediate bacterial escape from a vacuole (33). This endosome lysis has also been shown to confer a general trend toward increased survival of immunodeficient SCID mice inoculated with AERAS-401 relative to BCG1331 (57), which is an important safety feature for vaccines that are to be deployed in populations with a high incidence of HIV-1. Second, the pfoA(G137Q) gene was inserted into and deleted the ureC locus, the product of which normally plays a role in the capacity of mycobacteria to block the acidification of the early phagosome. A trend toward enhanced efficacy was also observed for the ureC deletion (16, 25).
Here, to construct recombinant BCG expressing immunogen HIVA, the HIVA open reading frame (21) was linked to the M. bovis Ag85B promoter and oligonucleotide coding for 19-kDa protein signal peptide (Fig. 1A), as described previously (32); the whole HIVA expression cassette was then stably inserted into the attB chromosomal locus of parental AERAS-401 using an allelic exchange plasmid pCB02 (Fig. 1B) followed by removal of the cointegrated antibiotic marker used for initial recombinant selection. Thus, the resulting strain, designated BCG.HIVA401, was markerless, fully compatible with good laboratory practice and suitable for production of master seed bacterial stock for manufacture of a clinical vaccine lot. The HIVA protein was readily detectable in the bacterial pellet after total cell lysate protein separation by SDS-PAGE and staining with Coomassie brilliant blue (Fig. 1C). In addition, HIVA released into the culture supernatant was detected in Western blots using a MAb against the C-terminal Pk tag (Fig. 1D).
FIG. 1.
Expression of the HIVA immunogen from BCG.HIVA401. (A) A schematic representation of the HIVA insert shows the mycobacterial 85B antigen promoter, the 19-kDa signal peptide (SP), consensus clade A Gag p24 and p17 domains, and a string of partially overlapping epitopes (Epitopes). The polyepitope region contains epitopes derived from Gag, which are not present in the p24 and p17 domains, Pol, Nef, and Env (21). To facilitate the preclinical development of the HIVA vaccines, epitopes recognized by CD8 T cells from rhesus macaques in the context of Mamu-A*01 (red) and mice in the context of H-2Dd (blue), and MAb SV5-Pk (24) (black) were coupled to the C terminus. (B) Schematic depiction of E. coli-mycobacterium shuttle plasmid pCB02 with the following functional regions: res, γ-δ resolvase binding site; M13, sequence derived from phagemid M13; Ori, E. coli origin of replication; lacZ-α, LacZ-α complementation fragment, which facilitates blue/white screening in E. coli; Int, mycobacteriophage L5 integrase (38); and 19-kDa SP, 19-kDa protein signal peptide. (C) Coomassie brilliant blue-stained gel of the parental AERAS-401 and BCG.HIVA401 whole-cell pellet lysate proteins separated by SDS-PAGE. Mr markers are shown on the left, and HIVA is indicated (arrow). (D) Western blot of culture supernatants using anti-Pk tag MAb to detect the presence of the HIVA immunogen (arrow).
BCG.HIVA401 primes CD8 T-cell responses in mice.
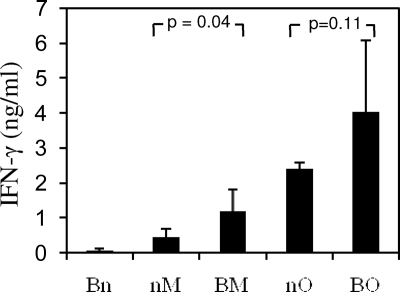
Before committing to more labor-intensive and expensive experiments in nonhuman primates, T-cell immunogenicity of the novel BCG.HIVA401 vaccine was confirmed in BALB/c mice. Animals were injected either with BCG.HIVA401 i.p. (B), MVA.HIVA i.m. (M) or OAdV.HIVA i.m. (O) vaccines alone or in simple BO or BM prime-boost combinations separated by an interval of 12 weeks. Mice were sacrificed 4 weeks after the boost. The immunogenicity readout was focused on the immunodominant H-2Dd-restricted epitope RGPGRAFVTI derived from HIV-1 Env (59), here designated H, which was added for this very purpose to the C terminus of HIVA (Fig. 1A). While no H-specific production of IFN-γ by immune splenocytes was detected after administration of BCG.HIVA401 alone, priming with this vaccine increased responses to heterologous boosts by MVA.HIVA and OAdV.HIVA relative to the viral vaccines administered alone (Fig. 2). These results concur with our published data on the immunogenicity of BCG.HIVA222 derived from the Pasteur strain in BALB/c mice (32) and confirm, albeit indirectly, an immunogenic effect of BCG.HIVA401 on murine CD8 T cells.
FIG. 2.
Vaccine immunogenicity in BALB/c mice. Groups of BALB/c mice were immunized as indicated at intervals of 12 weeks and sacrificed 4 weeks after the last immunization. Splenocyes were isolated and stimulated with peptide H or mock pulsed for 24 h. Secretion of IFN-γ into the supernatants was determined by using a Luminex assay. B, BCG.HIVA401; M, MVA.HIVA; O, OAdV.HIVA; n, no treatment. Results are shown as means ± standard deviations (SD) (n = 5). Statistical significance is indicated and was determined by Student's t test. (Analysis of variance was not appropriate as the groups had vastly different SD.)
BCG.HIV401 induces strong PPD-specific responses in macaques.
Next, the immunogenicity of BCG.HIVA401 was assessed in nonhuman primates. Eight rhesus macaques were divided into 2 groups of 4 and vaccinated using either BBMO or BBOM regimens delivering BCG.HIVA401 i.d. and both MVA.HIVA and OAdV.HIVA i.m. With the possible use of rBCG as a prime for infant vaccination against breast milk transmission in mind, the first three immunizations were spaced at 4-week intervals. This is an accelerated regimen compared to previous macaque schedules involving rBCG, which used intervals of 18 to 23 weeks between individual immunizations (8, 62). The first administration of 107 CFU of BCG.HIVA401 was reactogenic at the site of injection, but the lesions resolved spontaneously within 3 to 4 weeks. Adverse effects after the second dosing were very mild. As a measurement of BCG-specific immune responses and an indication of BCG.HIVA401 vaccine take, PBMC isolated at various time points during the immunization schedule were assessed for PPD reactivity in an IFN-γ ELISPOT assay. In all macaques, strong responses to PDD were detected; these peaked at a median of 4,134 (range, 3,465 to ∼4,500, which verges on the threshold of reliable counting) spot-forming units (SFU)/106 PBMC at around week 7 (i.e., 3 weeks after the second BCG.HIVA401 administration) and declined gradually thereafter (Fig. 3). BCG-specific responses were not boosted by either of the subsequent MVA.HIVA or OAdV.HIVA vaccines. Thus, the BCG.HIVA401 vaccine alone elicited a strong and boostable BCG-specific response in recipient macaques.
FIG. 3.
PPD-specific T-cell responses elicited by BCG.HIVA401. Two groups of 4 rhesus macaques were immunized using either BBMO (black) or BBOM (gray) regimen, where “B” represents BCG.HIVA401, “M” represents MVA.HIVA, and “O” represents OAdV.HIVA. Vaccination time points are indicated below the graph. Responses to BCG were determined in an ex vivo IFN-γ ELISPOT assay using PPD as the antigen. The panel shows median responses for the two groups after subtracting the mock-stimulated background. nd, not done. The peak responses reached an overall median of 4,134 (range, 3,465 to ∼4,500 at the threshold of reliable counting) SFU/106 PBMC at week 7.
BCG.HIV401 primes robust HIV-1-specific CD8 T-cell responses in rhesus macaques.
Induction of HIV-1-specific responses was initially determined in IFN-γ ELISPOT assays using peptide pools 1 to 4, comprising 15-mers overlapping by 11 amino acids spanning the Gag p24/p17 region, and pool 5, corresponding to the polyepitope domain of HIVA (47); these are the same peptides that were used in previously published macaque and clinical studies (14, 20, 30, 63). Pool 5 contained the immunodominant Mamu-A*01-restricted epitope CTPYDINQM derived from simian immunodeficiency virus (SIV) Gag (CM9; residues 181 to 189) (44); macaques M3, M5, M6, and M8 were Mamu-A*01+. Following the first BCG.HIVA401 immunization, small but definite HIV-1-specific responses were detected in the region of 60 SFU/106 PBMC; these increased to a mean of 258 SFU/106 PBMC after the second dose of BCG.HIVA401 (Fig. 4A). Macaques M1 to M4 and M5 to M8 then received MO and OM heretologous virus vaccine boosts, respectively. HIV-1-specific T-cell responses increased only modestly following the first recombinant virus administration. However, the second viral boost resulted in a substantial enhancement of the HIV-1-specific T-cell response, which reached mean frequencies of 1,305 and 1,194 SFU/106 PBMC for the BBMO and BBOM groups, respectively. Overall, there were no notable differences between the two regimens, although one time point following each of the first and second heterologous boosts reached a statistically significant difference (P = 0.02) in favor of OAdV.HIVA (Fig. 4A). These data suggest that BCG.HIVA410 primes T cell responses that can be boosted to robust levels by heterologous virus-vectored vaccines.
FIG. 4.
HIV-1-specific T-cell responses elicited by HIVA. Macaques M1 to M4 and M5 to M8 were vaccinated using the BBMO or BBOM regimen, respectively, where “B” represents BCG.HIVA401, “M” represents MVA.HIVA, and “O” represents OAdV.HIVA, at the time points indicated. HIV-1-specific T-cell responses were measured in an ex vivo IFN-γ ELISPOT assay using HIVA-derived peptide pools. Pool-stimulated responses are shown after subtracting the mock-stimulated background. nd, not done due to lack of sample availability. Panel A shows the sum of HIV-1-specific responses induced by the BBMO (black) and BBOM (gray) regimens. The results are shown as mean ± SD (n = 4). Asterisks indicate statistically significant (P = 0.02) difference determined using Student's t test. Panel B shows vaccine-elicited T-cell responses in individual rhesus macaques to individual peptide pools. Pools 1 to 4 (four grades of gray) span the HIV-1 Gag p24 and p17 regions of HIVA; pool 5 (black) corresponds to the polyepitope string and contains the Mamu-A*01-restricted epitope CM9. ¶, Mamu-A*01+ animals.
Both BBMO and BBOM regimens induce broadly specific anti-HIV-1 responses.
The observation that vaccine-induced T-cell responses to several of the 5 HIVA-derived peptide pools provided the first indication that multiple epitopes were targeted (Fig. 4B). To substantiate this, HIVA-specific T-cell responses were deconvoluted at three time points to define specificity at the level of individual 15-mer peptides. For the peak responses (week 16), these peptides were employed in an ex vivo IFN-γ ELISPOT assay. To increase sensitivity, PBMC isolated after BB (week 7) and BBMO/BBOM (week 17) vaccinations were first expanded in vitro prior to individual peptide stimulation as described previously (14). These analyses demonstrated that the BBMO/BBOM vaccine regimens induced predominantly HIV-1 Gag-specific T cells that recognized a median of 4 (range of 1 to 7) individual epitopes (Table 1). The protective Mamu-A*01 allele conferred no advantage or disadvantage in terms of either the magnitude or the breadth of the T-cell response. Thus, the HIVA vaccines used in the BBMO/BBOM regimens elicited T-cell responses specific for multiple HIV-1 epitopes.
TABLE 1.
The breadth of HIVA vaccine-induced T-cell responses
| Macaque | T-cell response toa: |
|||||
|---|---|---|---|---|---|---|
| BB at wk 7 (cultured) |
BBMO/BBOM |
|||||
| wk 16 (ex vivo) |
wk 17 (cultured) |
|||||
| Peptide no.b | Sequence | Peptide no.b | Sequence | Peptide no.b | Sequence | |
| M1 | 97-98 | KRWIIFRDYVDRFYKTLRA | 21 | RLHPVHAGPIPPGQM-8 | 5 | RTLNAWVKVIEEKAF |
| 99 | YVDRFYKTLRAIFQS | 33 | YKRWIILGLNKIVRM | 31 | PPIPVGDIYKRWIIL | |
| 80-81 | CVHQRIDVKDTKEALDKIE-8 | 95-96 | PIPVGEIYKRWIIFRDYVD | |||
| 97 | KRWIIFRDYVDRFYK-8 | |||||
| 119-120 | HGVYHPDIVIYQYMDDLTP | |||||
| M2 | 76-77 | TSEELKSLFNTVATLYCVH | 21 | RLHPVHAGPIPPGQM-8 | ||
| 86-87 | SKQKTQQAAADTQSSSKVS | 68 | SRELERFALNPSLLE | |||
| 77-78 | LKSLFNTVATLYCVHQRID | |||||
| 80-81 | CVHQRIDVKDTKEALDKIE-8 | |||||
| M3c | 7-8 | VIEEKAFSPEVIPMFSALS-8 | 33-34 | YKRWIILGLNKIVRMYSPV | ||
| 125-126 | YPLACTPYDINQMLRGPGR-8 | 69-70 | ERFALNPSLLETAEGCQQI | |||
| M4 | 16 | HQAAMQMLKDTINEE-8 | 33-34 | YKRWIILGLNKIVRMYSPV | ||
| 38 | ILDIRQGPKEPFRDY | 41-42 | RDYVDRFFKTLRAEQATQE | |||
| 66-67 | RLKHLVWASRELERFALNP | 95-96 | PIPVGEIYKRWIIFRDYVD | |||
| 94 | LEFPPIPVGEIYKRW-8 | 97-98 | KRWIIFRDYVDRFYKTLRA | |||
| 106-107 | ERYLKDQQLLTVYYGVPVW | |||||
| 110-111 | PVWKRPQVPLRPMTYKAVD | |||||
| 120 | HPDIVIYQYMDDLTP | |||||
| M5c | 7-8 | VIEEKAFSPEVIPMFSALS-8 | 35-36 | LNKIVRMYSPVSILDIRQG | ||
| 16 | HQAAMQMLKDTINEE-8 | 41-42 | RDYVDRFFKTLRAEQATQE | |||
| 21 | RLHPVHAGPIPPGQM | 45-46 | TQEVKNWMTETLLVQNANP | |||
| 57 | HKARVLGTGARASVL-8 | 113-114 | MTYKAVDLSHFLKEKGGLI | |||
| 106-107 | ERYLKDQQLLTVYYGVPVW-8 | |||||
| 125-126 | YPLACTPYDINQMLRGPGR-8 | |||||
| M6c | 35-36 | LNKIVRMYSPVSILDIRQG | 7-8 | VIEEKAFSPEVIPMFSALS | 31-32 | PPIPVGDIYKRWIILGLNK |
| 68-69 | SRELERFALNPSLLETAEG | 16 | HQAAMQMLKDTINEE | 37-38 | SPVSILDIRQGPKEPFRDY | |
| 57 | HKARVLGTGARASVL | 39-40 | RQGPKEPFRDYVDRFFKTL | |||
| 110-111 | PVWKRPQVPLRPMTYKAVD | 95-96 | PIPVGEIYKRWIIFRDYVD | |||
| 120 | HPDIVIYQYMDDLTP | 125-126 | YPLACTPYDINQMLRGPG | |||
| M7 | 57 | HKARVLGTGARASVL-8 | 15-16 | IVGGHQAAMQMLKDTINEE | ||
| 110-111 | PVWKRPQVPLRPMTYKAVD-8 | 31-32 | PPIPVGDIYKRWIILIVRM | |||
| 39-40 | RQGPKEPFRDYVDRFFKTL | |||||
| 69-70 | ERFALNPSLLETAEGCQQI | |||||
| 107-108 | KDQQLLTVYYGVPVWKRPQ | |||||
| 119-120 | HGVYHPDIVIYQYMDDLTP | |||||
| M8c | 29-30 | QIGWMTSNPPIPVGDIYKR | 7-8 | VIEEKAFSPEVIPMFSALS | ||
| 16 | HQAAMQMLKDTINEE | |||||
| 20 | AEWDRLHPVHAGPIP | |||||
| 57 | HKARVLGTGARASVL | |||||
| 66-67 | RLKHLVWASRELERFALNP | |||||
| 68 | SRELERFALNPSLLE | |||||
| 125-126 | YPLACTPYDINQMLRGPGR | |||||
B, BCG.HIVA401; M, MVA.HIVA; and O, OAdV.HIVA. Underlined portions of sequences were previously identified Mamu-A*01-restricted epitopes (30, 44). An “8” at the end of the peptide sequence indicates that a positive restimulation was obtained with CD4 cell-depleted PBMC.
Peptides were 15-mers overlapping by 11 amino acids. Where two peptides are shown, both stimulated IFN-γ responses, but the minimal epitope was not identified and so the whole 19-amino-acid region is shown.
Mamu-A*01+ rhesus macaques.
HIVA elicits T-cell responses that readily proliferate and are oligoclonal.
Proliferation in response to recall antigens is the key feature of immunological memory. We therefore examined the ability of HIVA vaccine-induced T-cell responses to undergo antigen-specific proliferation in vitro. Both the BBMO and BBOM vaccine regimens elicited HIV-1-specific T-cells that readily proliferated when stimulated with pool 90 (mix of pools 1 to 4) peptides (Fig. 5). There was no statistically significant difference in the proliferative capacity of the HIV-1-specific T cells induced by the two regimens.
FIG. 5.

Proliferative capacity of HIVA vaccine-induced HIV-1-specific T cells. PBMC were isolated after either the BBMO (black) or BBOM (gray) regimen, where “B” represents BCG.HIVA401, “M” represents MVA.HIVA, and “O” represents OAdV.HIVA, at week 17 and assessed for their proliferation upon antigenic reexposure following stimulation with pool 90 (pools 1 to 4) peptides or mock stimulation. Events were gated on CD3+ CD8+ cells, and the number of cells showing CFSE dilution following peptide pool restimulation was determined on dot plots with CD8 on the y axis. Representative examples of CFSE dot plots for animal M7 are shown (top). The proliferation group data are presented as mean ± SD (n = 4 and 3; with macaque M5 not tested due to lack of sample availability) calculated after mock background subtraction.
It is established in the pathogenic SIVmac239 challenge model that CD8 T-cell responses to the Mamu-A*01-restricted SIV Gag-derived epitope CM9 are protective (7, 23, 45). Furthermore, the early mobilization of public clonotypes within this response has been identified as a molecular signature of protection that predicts post-primary set point virus load (51). We therefore determined the patterns of CM9-specific CD8 T cell clonotype recruitment in two Mamu-A*01+ macaques (M3 and M5) following the BBMO and BBOM regimens (week 18), respectively, using a template-switch-anchored RT-PCR that amplifies all expressed TRB gene products without bias. In addition, we conducted a polychromatic flow cytometric analysis of CM9-specific CD8 T-cell phenotype. The CD3+ CD4− CD8+ CD95+ T cells that bound the CM9/Mamu-A*01 tetramer in macaque M3 were predominantly CD28− CD45RA−. In contrast, the corresponding cells in macaque M5 displayed a heterogeneous phenotype (Fig. 6A). In terms of TCR usage, the CM9-specific CD8 T-cell population in macaque M3 exhibited an oligoclonal and highly skewed repertoire, whereas the corresponding population in macaque M5 was more diverse and contained two public clonotypes (Fig. 6B). These data demonstrate directly the effective recruitment of CM9-specific CD8 T-cell clonotypes into several compartments of the vaccine-induced memory pool.
FIG. 6.
Immunophenotype and clonal composition of CM9-specific CD8 T-cell populations. (A) The phenotypic profiles, as defined by expression of CD28 and CD45RA, of CM9/Mamu-A*01 tetramer-reactive CD8 T cells are shown for macaques M3 and M5 following BBMO or BBOM vaccination (week 18), respectively, where “B” represents BCG.HIVA401, “M” represents MVA.HIVA, and “O” represents OAdV.HIVA. Plots are gated on singlet, live, CD3+ CD4− CD8+ CD95+ cells. The CM9/Mamu-A*01 tetramer-positive events, with their percentages indicated in the top right corner, are shown as red dots superimposed on density plots showing the phenotype of all gated cells. (B) T-cell receptor β (TRB) CDR3 amino acid sequences, TRB variable (TRBV), and TRB joining (TRBJ) usage and the relative frequency (Freq.) of CD8 T cell clonotypes specific for the CM9 epitope are shown for macaques M3 and M5 at week 18. Colored boxes in the CDR3 sequence column indicate public clonotypes. Public clonotypes were defined on the basis of TRB amino acid sequences that were present in more than one macaque with reference to an extensive database derived from previous studies (29, 51, 52).
DISCUSSION
There is an increasing body of data to indicate that priming with rBCG in heterologous regimens that boost with attenuated viral or protein modalities represents an effective strategy for the induction of passenger immunogen-specific T-cell responses (4, 8, 9, 32, 42, 56). Here, by using a more immunogenic and safer rBCG (57) in prime-boost regimens with rMVA and novel rOAdV (5) vaccines, we demonstrated (i) enhanced T-cell induction in mice by using regimens that incorporated BCG.HIVA401 priming (with BCG.HIVA401 priming for increased responses not formally observed in rhesus macaques); (ii) the induction in rhesus macaques of high-frequency HIV-1-specific T-cell responses, which recognized multiple HIV-1 Gag-derived epitopes; and (iii) the effective recruitment in rhesus macaques of insert-specific T-cell clonotypes into diverse compartments of the memory pool. These are desirable features of HIV-1 vaccine-induced T cells.
This is the first immunogenicity study of BCG.HIVA401 and, indeed, of the parental AERAS-401 BCG strain in nonhuman primates. BCG.HIVA401 alone induced strong T-cell responses to PPD, yet only weak responses specific for the HIV-1 transgene product HIVA (Fig. 3 and 4). This might represent a positive feature of BCG-vectored vaccine usage during the priming phase of more complex regimens because the nature of priming events can dramatically influence the differentiation and fate of the elicited CD8 T cells (60). Indeed, it was reported previously that recombinant mycobacterium-induced T lymphocytes that were skewed toward durable antigen-specific memory CD8 T cells and rapidly expanded by heterologous virus-vectored vaccines sharing the same immunogen (28, 60). This quality memory induction is possibly a consequence of the strong CD4 T cell help that mycobacteria elicit (43, 61). Similarly, despite the induction of specific T cells at or below the limit of detection, DNA priming increased the consistency of the subsequent MVA.HIVA boost to 100% in a group of 8 human volunteers (14).
The MO and OM boosts yielded T-cell responses of similar overall frequencies. The first viral boost resulted in only a small augmentation of HIV-1-specific T-cell responses, possibly due to the accelerated regimen. Thus, after 4 weeks, mycobacteria might still persist at high levels, thereby driving a cytokine storm and ongoing activation of innate responses that could decrease vaccine take. Nevertheless, the second viral vaccination delivered a substantial T-cell boost and induced broadly targeted responses capable of rapid expansion upon antigenic reexposure. Small, but significant differences were detected between the two heterologous boosts in macaques, with OAdV.HIVA inducing higher frequencies of HIV-1-specific T cells than MVA.HIVA (Fig. 4A). Greater T cell induction by OAdV.HIVA over MVA.HIVA was also detected in mice (Fig. 2). The overall T-cell immunogenicity of our two regimens is similar to a recently published regimen consisting of two rBCG primes boosted by an rHAdV-5 vaccine (8). Clearly, further experiments are necessary to optimize the timing, dosage, and administration routes of the BCG.HIVA401, MVA.HIVA, and OAdV.HIVA vaccination regimen. However, the availability of other HIVA vaccine modalities provides the opportunity for further augmentation of vaccine-induced HIV-1-specific T-cell frequencies (53a).
Optimized rBCG is a very attractive priming component for adult prophylactic HIV-1 vaccines. We have argued that rBCG is also a logical means of preventing HIV-1 transmission from infected mothers to breastfeeding infants (32), because BCG is an integral component of the EPI given to infants at birth or upon the first contact with a health care worker and is followed by other EPI vaccines between 4 and 6 weeks later, depending on the national guidelines. In immunocompromised individuals, BCG can cause disseminated disease and is, therefore, not recommended for HIV-1-infected infants. Nevertheless, because of the risk of tuberculosis in impoverished countries, WHO guidelines do recommend BCG vaccination for healthy asymptomatic babies of unknown HIV-1 status (64). Thus, rBCG expressing an HIV-1 immunogen would serve as a dual-priming platform for vaccines against M. tuberculosis (42) and HIV-1. In this respect, the demonstrably increased safety in SCID mice of AERAS-401 relative to its parental BCG strain SSI-1331 (57) is an important reassurance for such an approach. Here, we have tested the immunogenicity of BBMO and BBOM regimens delivered 4 weeks apart with the view that it is critical in infants to elicit protective HIV-1-specific responses as soon after birth as possible to prevent breast milk transmission, although in infants, only one dose of rBCG would be used. While robust anti-HIV-1 T-cell responses were induced by the 4-wk-gap regimen, these might be higher still if more commonly used intervals between individual doses were employed (8, 62).
Ovine atadenovirus (OAdV) is a novel and underexplored vaccine vector, which is distinct from the more commonly used mastadenoviruses (2, 50). Here, we demonstrated that OAdV is capable of antigen presentation for the induction of effective CD8 and CD4 T cell responses in heterologous prime-boost regimens. In addition, OAdV avoids the problems and possible risks associated with preexisting immunity to HAdV vectors, such as HAdV-5, in target populations. This work, which is the first demonstration of the safety and T-cell immunogenicity of OAdV in nonhuman primates, justifies its further development as a vaccine vector for HIV-1, other infections, and cancer.
The HIVA construct (21) has been an extremely useful model immunogen both for clinical (14, 20, 65) and preclinical (30, 31, 36, 48) vaccine development. The epitopes recognized in mice and Mamu-A*01+ rhesus macaques continue to be mapped, and the corresponding CD8 T-cell responses are being characterized in greater detail. However, there is no appropriate challenge available for the vaccinated animals because HIVA is designed for humans and is, therefore, derived from HIV-1 antigens; HIV-1 does not replicate in rhesus macaques. As for human efficacy, broad Gag-specific responses have been associated with good control of HIV-1 replication in chronically infected patients (27, 35, 53), responses to HIVA have been readily detected in exposed uninfected children in Kenya (55), and it has been demonstrated that HIVA boosts specific CD8 and CD4 T cell responses effectively in patients infected with a variety of HIV-1 clades (10, 11, 66). Thus, HIVA as a Gag-based immunogen concurs at several levels with the emerging correlates of T-cell-mediated control of HIV-1 replication.
In conclusion, although patients have benefited from highly active antiretroviral treatment, the HIV-1 pandemic continues unabated and an effective vaccine remains the best solution for halting the spread of this virus in resource-poor areas. New, safer, and more immunogenic vaccine vectors in combination are necessary for the definition of an optimal strategy. Here, we tested for the first time in nonhuman primates a unique vector combination of two novel vaccines based on modified BCG and sheep atadenovirus and demonstrated their potential utility for further HIV-1 vaccine development.
Acknowledgments
This work was supported by the Medical Research Council (United Kingdom); the Australian Research Council (ARC; DP0452362); the Spanish Research Council; the Foundation for Research and Prevention of AIDS in Spain (FIPSE 36338/02); the Fundación Mutua Madrileña de Automóviles (second call for proposals); Fundacio BCN SIDA 2002; and the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health (United States). D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow, T.H. is a Jenner Institute Investigator, V.V. is an ARC Future Fellow, and M.F.Q. is a Marie Curie International Outgoing Research Fellow.
We thank Linda Lockett and Jan Shaw of CSIRO Molecular and Health Technologies for producing purified OAdV.HIVA and for assistance with statistical analysis, respectively, and Warren Kitchen and his team at the Defense Science and Technology Laboratory (United Kingdom) for excellent animal care and handling.
The authors have no conflict of interest, except for G.W.B., who is an inventor of the ovine atadenovirus vector system and Chief Scientific Officer of Biotech Equity Partners Pty., Ltd., which has licensed the vector from the CSIRO.
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 2.Both, G. W. 2004. Ovine atadenovirus: a review of its biology, biosafety profile and application as a gene delivery vector. Immunol. Cell Biol. 82:189-195. [DOI] [PubMed] [Google Scholar]
- 3.Both, G. W., F. Cameron, A. Collins, L. J. Lockett, and J. Shaw. 2007. Production and release testing of ovine atadenovirus vectors. Methods Mol. Med. 130:69-90. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, L., Y. A. Skeiky, M. R. Alderson, Y. Lobet, W. Dalemans, O. C. Turner, R. J. Basaraba, A. A. Izzo, T. M. Lasco, P. L. Chapman, S. G. Reed, and I. M. Orme. 2004. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect. Immun. 72:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridgeman, A., Y. Roshorm, L. J. Lockett, Z. Z. Xu, R. Hopkins, J. Shaw, G. W. Both, and T. Hanke. 2010. Ovine atadenovirus, a novel and highly immunogenic vector in prime-boost studies of a candidate HIV-1 vaccine. Vaccine 28:474-483. [DOI] [PubMed] [Google Scholar]
- 6.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayabyab, M. J., B. Korioth-Schmitz, Y. Sun, A. Carville, H. Balachandran, A. Miura, K. R. Carlson, A. P. Buzby, B. F. Haynes, W. R. Jacobs, and N. L. Letvin. 2009. Recombinant Mycobacterium bovis BCG prime-recombinant adenovirus boost vaccination in rhesus monkeys elicits robust polyfunctional simian immunodeficiency virus-specific T-cell responses. J. Virol. 83:5505-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chege, G. K., R. Thomas, E. G. Shephard, A. Meyers, W. Bourn, C. Williamson, J. Maclean, C. M. Gray, E. P. Rybicki, and A. L. Williamson. 2009. A prime-boost immunisation regimen using recombinant BCG and Pr55(gag) virus-like particle vaccines based on HIV type 1 subtype C successfully elicits Gag-specific responses in baboons. Vaccine 27:4857-4866. [DOI] [PubMed] [Google Scholar]
- 10.Dorrell, L., H. Yang, A. K. Iversen, C. Conlon, A. Suttill, M. Lancaster, T. Dong, I. Cebere, A. Edwards, S. Rowland-Jones, T. Hanke, and A. J. McMichael. 2005. Therapeutic immunization of highly active antiretroviral therapy-treated HIV-1-infected patients: safety and immunogenicity of an HIV-1 gag/poly-epitope DNA vaccine. AIDS 19:1321-1323. [DOI] [PubMed] [Google Scholar]
- 11.Dorrell, L., H. Yang, B. Ondondo, T. Dong, K. di Gleria, A. Suttill, C. Conlon, D. Brown, P. Williams, P. Bowness, N. Goonetilleke, T. Rostron, S. Rowland-Jones, T. Hanke, and A. J. McMichael. 2006. Expansion and diversification of HIV-1-specific T cells following immunisation of HIV-1-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 gag vaccine. J. Virol. 80:4705-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douek, D. C., M. R. Betts, J. M. Brenchley, B. J. Hill, D. R. Ambrozak, K. L. Ngai, N. J. Karandikar, J. P. Casazza, and R. A. Koup. 2002. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J. Immunol. 168:3099-3104. [DOI] [PubMed] [Google Scholar]
- 13.Edelman, R., K. Palmer, K. G. Russ, H. P. Secrest, J. A. Becker, S. A. Bodison, J. G. Perry, A. R. Sills, A. G. Barbour, C. J. Luke, M. S. Hanson, C. K. Stover, J. E. Burlein, G. P. Bansal, E. M. Connor, and S. Koenig. 1999. Safety and immunogenicity of recombinant bacille Calmette-Guerin (rBCG) expressing Borrelia burgdorferi outer-surface protein A (OspA) lipoprotein in adult volunteers: a candidate Lyme disease vaccine. Vaccine 17:904-919. [DOI] [PubMed] [Google Scholar]
- 14.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, N. Mahmoud, I. Cebere, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, P. Fast, L. Dorrell, T. Hanke, and A. McMichael. 2006. Prime-boost vaccination with recombinant DNA and MVA expressing HIV-1 clade A gag and immunodominant CTL epitopes induces multifunctional HIV-1-specific T cells in healthy subjects. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenaway, H. Y., M. Kurniawan, D. A. Price, D. C. Douek, M. P. Davenport, and V. Venturi. 2009. Extraction and characterization of the rhesus macaque T-cell receptor beta-chain genes. Immunol. Cell Biol. 87:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grode, L., P. Seiler, S. Baumann, J. Hess, V. Brinkmann, A. Nasser Eddine, P. Mann, C. Goosmann, S. Bandermann, D. Smith, G. J. Bancroft, J. M. Reyrat, D. van Soolingen, B. Raupach, and S. H. Kaufmann. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Invest. 115:2472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke, T. 2008. STEP trial and HIV-1 vaccines inducing T-cell responses. Expert Rev. Vaccines 7:303-309. [DOI] [PubMed] [Google Scholar]
- 18.Hanke, T., C. Barnfield, E. G.-T. Wee, L. Ågren, R. V. Samuel, N. Larke, and P. Liljeström. 2003. Construction and immunogenicity in a prime-boost regimen of a Semliki Forest virus-vectored experimental HIV clade A vaccine. J. Gen. Virol. 84:361-368. [DOI] [PubMed] [Google Scholar]
- 19.Hanke, T., T. J. Blanchard, J. Schneider, C. M. Hannan, M. Becker, S. C. Gilbert, A. V. S. Hill, G. L. Smith, and A. McMichael. 1998. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine 16:439-445. [DOI] [PubMed] [Google Scholar]
- 20.Hanke, T., N. Goonetilleke, A. J. McMichael, and L. Dorrell. 2007. Clinical experience with plasmid DNA- and modified vaccinia vaccine Ankara (MVA)-vectored HIV-1 clade A vaccine inducing T cells. J. Gen. Virol. 88:1-12. [DOI] [PubMed] [Google Scholar]
- 21.Hanke, T., and A. J. McMichael. 2000. Design and construction of an experimental HIV-1 vaccine for a year-2000 clinical trial in Kenya. Nat. Med. 6:951-955. [DOI] [PubMed] [Google Scholar]
- 22.Hanke, T., V. C. Neumann, T. J. Blanchard, M. Becker, P. Sweeney, A. V. S. Hill, G. L. Smith, and A. McMichael. 1999. Effective induction of HIV-specific CTL by multi-epitope DNA using a gene gun in a combined vaccination regime. Vaccine 17:589-596. [DOI] [PubMed] [Google Scholar]
- 23.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, A. S. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanke, T., P. Szawlowski, and R. E. Randall. 1992. Construction of solid matrix-antibody-antigen complexes containing simian immunodeficiency virus p27 using tag-specific monoclonal antibody and tag-linked antigen. J. Gen. Virol. 73:653-660. [DOI] [PubMed] [Google Scholar]
- 25.Hess, J., D. Miko, A. Catic, V. Lehmensiek, D. G. Russell, and S. H. Kaufmann. 1998. Mycobacterium bovis Bacille Calmette-Guerin strains secreting listeriolysin of Listeria monocytogenes. Proc. Natl. Acad. Sci. U. S. A. 95:5299-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 73:6930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honeyborne, I., A. Prendergast, F. Pereyra, A. Leslie, H. Crawford, R. Payne, S. Reddy, K. Bishop, E. Moodley, K. Nair, M. van der Stok, N. McCarthy, C. M. Rousseau, M. Addo, J. I. Mullins, C. Brander, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2007. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J. Virol. 81:3667-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovav, A. H., M. J. Cayabyab, M. W. Panas, S. Santra, J. Greenland, R. Geiben, B. F. Haynes, W. R. Jacobs, Jr., and N. L. Letvin. 2007. Rapid memory CD8+ T-lymphocyte induction through priming with recombinant Mycobacterium smegmatis. J. Virol. 81:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hryniewicz, A., D. A. Price, M. Moniuszko, A. Boasso, Y. Edghill-Spano, S. M. West, D. Venzon, M. Vaccari, W. P. Tsai, F. Tryniszewska, J. Nacsa, F. Villinger, A. A. Ansari, C. J. Trindade, M. Morre, D. Brooks, P. Arlen, H. J. Brown, C. M. Kitchen, J. A. Zack, D. C. Douck, G. M. Shearer, M. G. Lewis, R. A. Koup, and G. Franchini. 2007. Interleukin 15 but not interleukin abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251 infected macaques. J. Immunol. 178:3492-3504. [DOI] [PubMed] [Google Scholar]
- 30.Im, E.-J., K. di Gleria, A. J. McMichael, and T. Hanke. 2006. Induction of long-lasting multi-specific CD8+ T cells by a 4-component DNA-MVA/HIVA-RENTA candidate HIV-1 vaccine in rhesus macaques. Eur. J. Immunol. 36:2574-2584. [DOI] [PubMed] [Google Scholar]
- 31.Im, E. J., and T. Hanke. 2007. Short communication: preclinical evaluation of candidate HIV type 1 vaccines in inbred strains and an outbred stock of mice. AIDS Res. Hum. Retroviruses 23:857-862. [DOI] [PubMed] [Google Scholar]
- 32.Im, E. J., N. Saubi, G. Virgili, C. Sander, D. Teoh, J. M. Gatell, H. McShane, J. Joseph, and T. Hanke. 2007. Vaccine platform for prevention of tuberculosis and mother-to-child transmission of human immunodeficiency virus type 1 through breastfeeding. J. Virol. 81:9408-9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, S., K. Preiter, and D. A. Portnoy. 1996. Conversion of an extracellular cytolysin into a phagosome-specific lysin which supports the growth of an intracellular pathogen. Mol. Microbiol. 21:1219-1225. [DOI] [PubMed] [Google Scholar]
- 34.Joseph, J., N. Saubi, E. Pezzat, and J. M. Gatell. 2006. Progress towards an HIV vaccine based on recombinant bacillus Calmette-Guerin: failures and challenges. Expert Rev. Vaccines 5:827-838. [DOI] [PubMed] [Google Scholar]
- 35.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 36.Larke, N., E.-J. Im, R. Wagner, C. Williamson, A.-L. Williamson, A. J. McMichael, and T. Hanke. 2007. Combined single-clade candidate HIV-1 vaccines induce T cell responses limited by multiple forms of in vivo immune interference. Eur. J. Immunol. 37:566-577. [DOI] [PubMed] [Google Scholar]
- 37.Larke, N., A. Murphy, C. Wirblich, D. Teoh, M. J. Estcourt, A. J. McMichael, P. Roy, and T. Hanke. 2005. Induction of human immunodeficiency virus type 1-specific T cells by a bluetongue virus tubule-vectored vaccine prime-recombinant modified virus Ankara boost regimen. J. Virol. 79:14822-14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, M. H., L. Pascopella, W. R. Jacobs, Jr., and G. F. Hatfull. 1991. Site-specific integration of mycobacteriophage L5: integration-proficient vectors for Mycobacterium smegmatis, Mycobacterium tuberculosis, and bacille Calmette-Guerin. Proc. Natl. Acad. Sci. U. S. A. 88:3111-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, S., M. Rodrigues, D. Rodriguez, J. R. Rodriguez, M. Esteban, P. Palese, S. R. Nussenzweig, and F. Zavala. 1993. Priming with recombinant influenza virus followed by administration of recombinant vaccinia virus induces CD8+ T-cell-mediated protective immunity against malaria. Proc. Natl. Acad. Sci. U. S. A. 90:5214-5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lugosi, L., W. Jacobs, and B. R. Bloom. 1989. Transformation of BCG with plasmid DNA. Acta Leprol. 7(Suppl. 1):256-257. [PubMed] [Google Scholar]
- 41.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 43.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 44.Miller, M. D., H. Yamamoto, A. L. Hughes, D. I. Watkins, and N. L. Letvin. 1991. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147:320-329. [PubMed] [Google Scholar]
- 45.Moniuszko, M., D. Bogdan, R. Pal, D. Venzon, L. Stevceva, J. Nacsa, E. Tryniszewska, Y. Edghill-Smith, S. M. Wolinsky, and G. Franchini. 2005. Correlation between viral RNA levels but not immune responses in plasma and tissues of macaques with long-standing SIVmac251 infection. Virology 333:159-168. [DOI] [PubMed] [Google Scholar]
- 46.Mwau, M., I. Cebere, J. Sutton, P. Chikoti, N. Winstone, E. G.-T. Wee, T. Beattie, Y.-H. Chen, L. Dorrel, H. McShane, C. Schmidt, M. Brooks, S. Patel, J. Roberts, C. Conlon, S. Rowland-Jones, J. Bwayo, A. J. McMichael, and T. Hanke. 2004. An HIV-1 clade A vaccine in clinical trials: stimulation of HIV-specific T cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J. Gen. Virol. 85:911-919. [DOI] [PubMed] [Google Scholar]
- 47.Mwau, M., A. J. McMichael, and T. Hanke. 2002. Design and validation of an ELISPOT assay for use in clinical trials of candidate HIV vaccines. AIDS Res. Hum. Retroviruses 18:611-618. [DOI] [PubMed] [Google Scholar]
- 48.Nkolola, J. P., E. G.-T. Wee, E.-J. Im, C. P. Jewell, N. Chen, X.-N. Xu, A. J. McMichael, and T. Hanke. 2004. Engineering RENTA, a DNA prime-MVA boost HIV vaccine tailored for Eastern and Central Africa. Gene Ther. 11:1068-1080. [DOI] [PubMed] [Google Scholar]
- 49.Nordstrom, E. K., M. N. Forsell, C. Barnfield, E. Bonin, T. Hanke, M. Sundstrom, G. B. Karlsson, and P. Liljestrom. 2005. Enhanced immunogenicity using an alphavirus replicon DNA vaccine against human immunodeficiency virus type 1. J. Gen. Virol. 86:349-354. [DOI] [PubMed] [Google Scholar]
- 50.Pantelic, R. S., L. J. Lockett, R. Rothnagel, B. Hankamer, and G. W. Both. 2008. Cryoelectron microscopy map of Atadenovirus reveals cross-genus structural differences from human adenovirus. J. Virol. 82:7346-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Price, D. A., T. E. Asher, N. A. Wilson, M. C. Nason, J. M. Brenchley, I. S. Metzler, V. Venturi, E. Gostick, P. K. Chattopadhyay, M. Roederer, M. P. Davenport, D. I. Watkins, and D. C. Douek. 2009. Public clonotype usage identifies protective Gag-specific CD8+ T cell responses in SIV infection. J. Exp. Med. 206:923-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price, D. A., S. M. West, M. R. Betts, L. E. Ruff, J. M. Brenchley, D. R. Ambrozak, Y. Edghill-Smith, M. J. Kuroda, D. Bogdan, K. Kunstman, N. L. Letvin, G. Franchini, S. M. Wolinsky, R. A. Koup, and D. C. Douek. 2004. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21:793-803. [DOI] [PubMed] [Google Scholar]
- 53.Rolland, M., D. Heckerman, W. Deng, C. M. Rousseau, H. Coovadia, K. Bishop, P. J. Goulder, B. D. Walker, C. Brander, and J. I. Mullins. 2008. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One 3:e1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Rosario, M., A. Bridgeman, E. D. Quakkelaar, M. F. Quigley, B. J. Hill, M. L. Knudsen, V. Ammendola, K. Ljungberg, N. Borthwick, E.-J. Im, A. J. McMichael, J. W. Drijfhout, H. Y. Greenaway, V. Venturi, D. C. Douek, S. Colloca, P. Liljeström, A. Nicosia, D. A. Price, C. J. M. Melief, and T. Hanke. Long peptides induce polyfunctional T cells against conserved regions of HIV with superior breadth to single-gene vaccines in macaques. Eur. J. Immunol., in press. [DOI] [PubMed]
- 54.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. S. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 55.Slyker, J. A., B. L. Lohman, D. A. Mbori-Ngacha, M. Reilly, E. G. Wee, T. Dong, A. J. McMichael, S. L. Rowland-Jones, T. Hanke, and G. John-Stewart. 2005. Modified vaccinia Ankara expressing HIVA antigen stimulates HIV-1-specific CD8 T cells in ELISpot assays of HIV-1 exposed infants. Vaccine 23:4711-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Someya, K., D. Cecilia, Y. Ami, T. Nakasone, K. Matsuo, S. Burda, H. Yamamoto, N. Yoshino, M. Kaizu, S. Ando, K. Okuda, S. Zolla-Pazner, S. Yamazaki, N. Yamamoto, and M. Honda. 2005. Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guerin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif. J. Virol. 79:1452-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, R., Y. A. Skeiky, A. Izzo, V. Dheenadhayalan, Z. Imam, E. Penn, K. Stagliano, S. Haddock, S. Mueller, J. Fulkerson, C. Scanga, A. Grover, S. C. Derrick, S. Morris, D. M. Hone, M. A. Horwitz, S. H. Kaufmann, and J. C. Sadoff. 2009. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 27:4412-4423. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi, H., J. Cohen, A. Hosmalin, K. B. Cease, R. Houghten, J. L. Cornette, C. DeLisi, B. Moss, R. N. Germain, and J. A. Berzofsky. 1988. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 85:3105-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi, H., Y. Nakagawa, K. Yokomuro, and J. A. Berzofsky. 1993. Induction of CD8+ cytotoxic T lymphocytes by immunization with syngeneic irradiated HIV-1 envelope derived peptide-pulsed dendritic cells. Int. Immunol. 5:849-857. [DOI] [PubMed] [Google Scholar]
- 60.van Faassen, H., M. Saldanha, D. Gilbertson, R. Dudani, L. Krishnan, and S. Sad. 2005. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J. Immunol. 174:5341-5350. [DOI] [PubMed] [Google Scholar]
- 61.van Stipdonk, M. J., G. Hardenberg, M. S. Bijker, E. E. Lemmens, N. M. Droin, D. R. Green, and S. P. Schoenberger. 2003. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 4:361-365. [DOI] [PubMed] [Google Scholar]
- 62.Verreck, F. A., R. A. Vervenne, I. Kondova, K. W. van Kralingen, E. J. Remarque, G. Braskamp, N. M. van der Werff, A. Kersbergen, T. H. Ottenhoff, P. J. Heidt, S. C. Gilbert, B. Gicquel, A. V. Hill, C. Martin, H. McShane, and A. W. Thomas. 2009. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS One 4:e5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wee, E. G.-T., S. Patel, A. J. McMichael, and T. Hanke. 2002. A DNA/MVA-based candidate HIV vaccine for Kenya induces multi-specific T cell responses in rhesus macaques. J. Gen. Virol. 83:75-80. [DOI] [PubMed] [Google Scholar]
- 64.WHO. 2007. Application of revised BCG recommendations. Wkly. Epidemiol. Rec. 82:195-196. [Google Scholar]
- 65.Winstone, N., A. Guimarães-Walker, J. Roberts, D. Brown, V. Loach, N. Goonetilleke, T. Hanke, and A. J. McMichael. 2009. Increased detection of proliferating, polyfunctional, HIV-1-specific T cells in DNA-MVA vaccinated human volunteers by cultured IFN-γ ELISPOT assay. Eur. J. Immunol. 39:975-985. [DOI] [PubMed] [Google Scholar]
- 66.Yang, H., T. Dong, E. Turnbull, S. Ranasinghe, B. Ondondo, N. Goonetilleke, N. Winstone, K. di Gleria, P. Bowness, C. Conlon, P. Borrow, T. Hanke, A. McMichael, and L. Dorrell. 2007. Broad TCR usage in functional HIV-1-specific CD8+ T cell expansions driven by vaccination during highly active antiretroviral therapy. J. Immunol. 179:597-606. [DOI] [PubMed] [Google Scholar]
- 67.Yasutomi, Y., S. Koenig, S. S. Haun, C. K. Stover, R. K. Jackson, P. Conard, A. J. Conley, E. A. Emini, T. R. Fuerst, and N. L. Letvin. 1993. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 150:3101-3107. [PubMed] [Google Scholar]
- 68.Yu, J. S., J. W. Peacock, S. Vanleeuwen, T. Hsu, W. R. Jacobs, Jr., M. J. Cayabyab, N. L. Letvin, R. Frothingham, H. F. Staats, H. X. Liao, and B. F. Haynes. 2006. Generation of mucosal anti-human immunodeficiency virus type 1 T-cell responses by recombinant Mycobacterium smegmatis. Clin. Vaccine Immunol. 13:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]