Abstract
Equine rhinitis A virus (ERAV) is genetically closely related to foot-and-mouth disease virus (FMDV), and both are now classified within the genus Aphthovirus of the family Picornaviridae. For disease security reasons, FMDV can be handled only in high-containment facilities, but these constraints do not apply to ERAV, making it an attractive alternative for the study of aphthovirus biology. Here, we show, using immunofluorescence, pharmacological agents, and dominant negative inhibitors, that ERAV entry occurs (as for FMDV) via clathrin-mediated endocytosis and acidification of early endosomes. This validates the use of ERAV as a model system to study the mechanism of cell entry by FMDV.
Equine rhinitis A virus (ERAV) belongs to the genus Aphthovirus of the family Picornaviridae (23) and is closely related to foot-and-mouth disease virus (FMDV) as they share physicochemical properties (18, 19), nucleotide sequence (15, 28, 34), and structural similarities (31). Picornaviruses are small nonenveloped RNA viruses, comprising a 30-nm-diameter capsid made of 60 copies of each of four capsid proteins, VP1 to VP4, which encapsidate a single-stranded RNA genome (7 to 8 kb) (24). The family has 12 genera and includes a number of important pathogens (e.g., poliovirus [PV], human rhinovirus [HRV], hepatitis A virus [HAV], etc.).
Despite extensive study of FMDV, there are still many aspects of aphthovirus biology, such as uncoating and genome delivery, that are yet to be elucidated. At acidic pHs, these viruses appear to simply dissociate into subunits during uncoating, and the mechanisms by which they deliver their genomes across a cellular membrane into the cytoplasm are poorly understood. In contrast, the enterovirus capsid remains intact throughout the infection process, and models have been proposed for the mechanism by which these viruses interact with the membrane and deliver their genomes into the cytoplasm. However, it has yet to be established how broadly applicable these models are for all picornaviruses (30).
We have recently shown that acid-induced capsid dissociation of ERAV proceeds via a transient intact empty particle, from which the RNA has been lost (31). This suggests a mechanism that coordinates genome release and delivery, as in the model for enteroviruses. To validate these studies in cell culture, we first wished to identify the endocytic route used by the virus and assess the role of acidification in the entry process. Picornaviruses utilize a variety of endocytic pathways. For example, FMDV and HRVs enter the cell via clathrin-mediated endocytosis and are subsequently delivered to the endosome. Here, they encounter an acidic pH, which is an indispensable step for a productive infection (2, 5, 12, 22). In contrast, echovirus 1 (enterovirus) uses the caveolin-dependent uptake of caveolae and delivery to caveosomes, where no change in pH is observed (16). PV entry is independent of clathrin- and caveolin-mediated endocytosis (6).
To elucidate the entry route of ERAV, we used a combination of methods that include immunofluorescence (IF) microscopy, pharmacological inhibitors of specific endocytosis pathways, and dominant negative proteins.
ERAV entry is rapid.
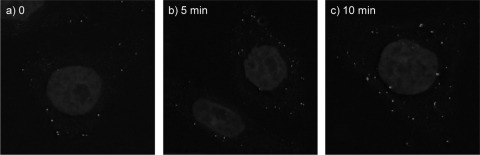
Purified ERAV was labeled with the fluorophore Cy2 (GE Healthcare) according to the manufacturer's instructions. The conjugation of a fluorophore to the viral capsid can interfere with receptor binding and internalization; we therefore titrated the ratio of virus/Cy2 used in labeling reactions and selected the virus/Cy2 ratio that gave a reasonable signal in IF without affecting virus infectivity. HeLa Ohio cells were grown in standard medium on glass coverslips (30-mm diameter; Agar Scientific). The cells were cooled at 4°C for 30 min before ERAV-Cy2 (multiplicity of infection [MOI] = 10) was adsorbed for 20 min at 4°C. This MOI was chosen to obtain a clear signal without overloading the cells with virus. Unattached virus was removed, and the cells were incubated in growth medium at 37°C for 0 to 10 min before fixing in 4% formaldehyde in phosphate-buffered saline (PBS). Images were acquired using a DeltaVision three-dimensional (3D) deconvoluting microscope. Figure 1 shows progressive movement of the virus from the cell surface to the interior within 10 min postinfection (pi). This rapid internalization is compatible with clathrin-dependent entry, as seen with FMDV (5), but not with caveolin-mediated entry.
FIG. 1.
ERAV internalization time course. ERAV-Cy2 was bound to cells in the cold, and unbound virus was washed away before infection initiated at physiological temperature. Cells were fixed at 0, 5, and 10 min pi (panels a, b, and c, respectively).
ERAV colocalizes with markers of clathrin-dependent endocytosis.
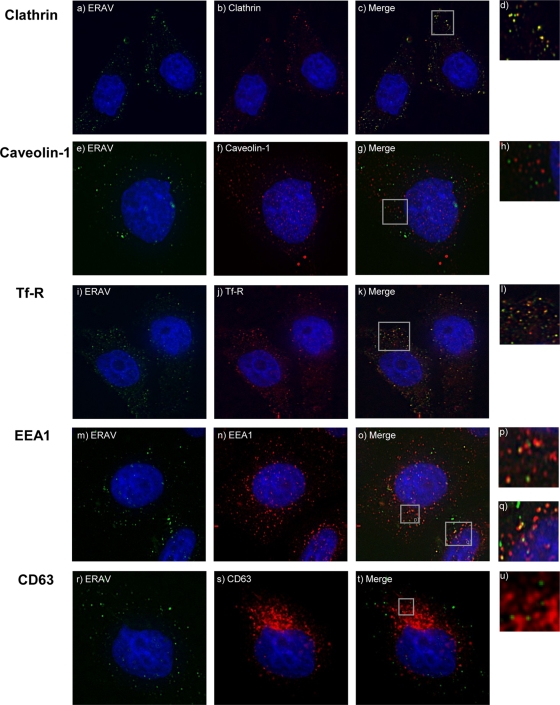
An established method for identification of the route of viral entry is IF colocalization of virus with markers of known endocytosis pathways. HeLa Ohio cells were precooled and infected with ERAV-Cy2 as described above. Cells were then incubated in growth medium at 37°C for 0 min, 5, 10, 20, or 30 min before being fixed in 4% formaldehyde in PBS. Cells were permeabilized with 0.1% Triton X-100 (Sigma) in PBS for 15 min and blocked with 3% fish skin gelatin (Sigma) in PBS for 60 min. Antibodies against the following cellular proteins were used: clathrin (Abcam), caveolin-1 (Santa Cruz), transferrin receptor (Tfr-R; Abcam), early endosomal marker 1 (EEA1; Abcam), and CD63 (Abcam). Secondary antibodies conjugated to Alexa-594 (Invitrogen) were used, and images were acquired using a DeltaVision 3D deconvoluting microscope. We used fluorescently tagged transferrin, which is internalized alongside its receptor via clathrin-dependent endocytosis (9), to confirm the staining patterns observed with clathrin, Tfr-R, and EEA1 antibodies (data not shown). We also attempted to confirm the staining pattern for caveolin-1 with cholera toxin B (CTB), which is mainly internalized in a caveolin-dependent manner (27, 29). However, we were not able to visualize CTB, presumably because HeLa cells have been found to express low levels of CTB receptor (11) (T. Jackson, personal communication). ERAV colocalized with clathrin 10 min pi (Fig. 2), suggesting that it enters the cell via clathrin-dependent endocytosis. This was confirmed by colocalization with markers of early endosomes Tfr-R and EEA1 at 20 min pi (Fig. 2). In contrast, the virus did not colocalize with caveolin-1, a marker of caveola-mediated endocytosis (Fig. 2). No colocalization was observed with CD63, a marker of late endosomes and lysosomes (Fig. 2), possibly due to rapid degradation in these compartments.
FIG. 2.
ERAV colocalization with endocytosis markers. Cells were infected with Cy2-labeled ERAV for 0 to 30 min before being visualized as described in the text. Antibodies against endocytosis markers were used in combination with secondary antibodies conjugated to Alexa-594 (Invitrogen). Cell nuclei were stained with Hoechst. Images were acquired using a DeltaVision deconvoluting microscope. (a) ERAV-Cy2 (green). (b) Clathrin (red) at 10 min pi. (c) Merge of panels a and b, showing colocalization (yellow) of ERAV and clathrin. (e to t) Cells fixed at 20 min pi with ERAV-Cy2 (green) and caveolin-1, Tfr-R, EEA1, or CD63 (red). (g) Overlay of panels e and f, showing ERAV (green) and caveolin-1 (red). (k and o) Colocalization (yellow) of ERAV with Tfr-R or EEA1, respectively. Panel k is a merge of panels i and j, and panel o is a merge of panels m and n. (t) Overlay of panels r and s, showing ERAV (green) and CD63 (red). Panels d, h, l, p, q, and u are enlargements from the Merge panels.
ERAV internalization and replication are prevented by a dominant negative inhibitor of clathrin-mediated endocytosis.
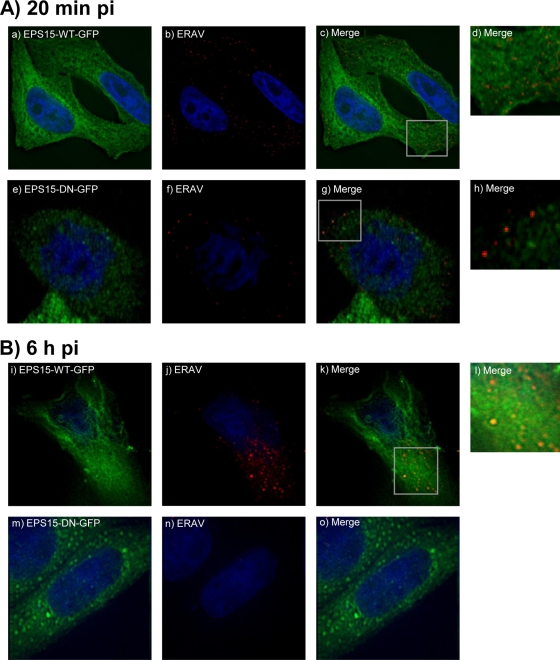
Eps15 participates in clathrin-mediated endocytosis via its interaction with the clathrin adaptor protein 2 (AP2) (4, 26). Expression of the C terminus of Eps15 acts as a dominant negative inhibitor of clathrin-mediated endocytosis by preventing native Eps15 from binding to AP2 (3, 8). HeLa Ohio cells grown on coverslips were transfected with plasmids expressing either the wild-type form of Eps15 tagged with green fluorescent protein (Eps15-WT-GFP) or the dominant negative form pEps15-95/295-GFP (Eps15-DN-GFP) (3) by using Lipofectamine (Invitrogen). ERAV (MOI = 10) was allowed to enter the cells for 20 min, by which time the virus has colocalized with endosomal markers (see above), and for 6 h, by which time replication and synthesis of new viral proteins are well established. The cells were washed, fixed, permeabilized, and blocked as described above. ERAV was detected with antibodies purified from convalescent horse serum (kindly provided by the Animal Health Trust) by using protein G and an antihorse rhodamine (Abcam). At 20 min pi in cells transfected with wild-type Eps15, ERAV was detected mainly in the cytoplasm (Fig. 3A). In contrast, in cells transfected with the dominant negative form of Eps15, virus was observed predominantly at the cell surface. This suggests that ERAV internalization is dependent on functional Eps15 and therefore on a functional clathrin-mediated pathway. When the cells were fixed at 6 h pi, high levels of ERAV proteins, indicative of replication, were detected in cells expressing the wild-type form of Eps15 but not in cells expressing the dominant negative form of Eps15 (Fig. 3B). This suggests that it is not only internalization but also initiation of a productive infection cycle that is dependent on functional Eps15 and therefore on a functional clathrin-mediated pathway.
FIG. 3.
Effects of inhibition of clathrin-mediated endocytosis on ERAV entry. (A) Detection of ERAV (red) 20 min pi (b and f) of cells expressing wild-type Eps15 (Eps15-WT-GFP) (green) (a) or dominant negative Eps15 (Eps15-DN-GFP) (green) (e), which inhibits clathrin-mediated endocytosis. ERAV is located predominantly in the cytoplasm in cells expressing wild-type Eps15 (c, overlay of a and b). Panel d is an enlargement from panel c. ERAV is located predominantly at the cell surface in cells expressing dominant negative Eps15 (g, overlay of panels e and f). Panel h is an enlargement from panel g. (B) Panels i to o show cells fixed at 6 h pi. Panel k is a merge of panels i (Eps15-WT-GFP [green]) and j (ERAV [red]). The large ERAV signal in the cytoplasm is indicative of replication. Panel o is a merge of panels m (Eps15-DN-GFP [green]) and n (ERAV [red]). The lack of ERAV signal in the cytoplasm suggests lack of replication.
Productive infection by ERAV is dependent on clathrin-mediated endocytosis.
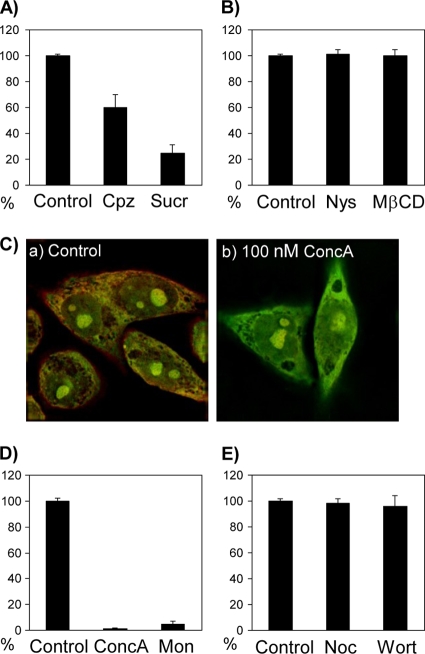
ERAV, like most picornaviruses, has a high particle/infectious unit ratio (24), and it is important to confirm results obtained by IF with methods that specifically identify entry of infectious particles. Therefore, we analyzed the effect on ERAV infection of inhibitors of either clathrin- or caveolin-mediated endocytosis. Two inhibitors of clathrin-dependent endocytosis were used. Sucrose causes clathrin-coated pits to disappear and induces abnormal clathrin polymerization into empty cages (13); chlorpromazine also causes loss of coated pits from the plasma membrane and induces clathrin-coated cages to assemble on endosomal membranes (32). The working concentrations of these compounds were first selected using fluorescently labeled transferrin, which is internalized via clathrin-dependent endocytosis (9). Transferrin-Alexa-594 (Invitrogen) was added to HeLa Ohio cells after pretreatment for 30 min with increasing concentrations of chlorpromazine (10, 15, 20, and 50 μM) or sucrose (0.2, 0.4, and 0.6 M). The minimal concentrations that inhibited transferrin uptake, and therefore endocytosis, without signs of cytotoxicity (15 μM for chlorpromazine and 0.4 M for sucrose) (data not shown) were used in the following virus inhibition assays. Confluent HeLa Ohio cells in six-well plates were treated for 60 min with medium containing chlorpromazine or sucrose. ERAV (20 to 30 PFU) in standard medium (containing 2% fetal bovine serum [FBS] and chlorpromazine or sucrose) was allowed to attach and enter the cells for 60 min at 37°C. Cell-bound, noninternalized virus was removed with an acidic wash (citric acid, pH 3.0) for 2 min on ice. The cells were then covered with growth medium containing 0.6% agarose (Melford). After 3 days, the plates were fixed and stained with staining buffer (0.5% [wt/vol] crystal violet in 10% [vol/vol] methanol, 5% formaldehyde [wt/vol] in PBS). The number of virus plaques was expressed as a percentage of the no-drug control. Both clathrin inhibitory compounds resulted in a reduced number of ERAV plaques compared to the untreated cells; chlorpromazine or sucrose treatment resulted in 40% and 85% reductions, respectively (Fig. 4A). No reduction in the number of plaques was observed if the inhibitors were added 3 h pi (data not shown), confirming that they affected ERAV specifically at the entry stage. To investigate alternative routes for ERAV entry, we also used two reagents known to inhibit caveola-mediated endocytosis by interfering with the lipid components of caveolae. Nystatin, a cholesterol-complexing agent, and methyl-β-cyclodextrin (MβCD), which stimulates cholesterol efflux from the cell membrane, were used in plaque assays as described above at 25 μM and 7.5 mM, respectively. Neither nystatin nor MβCD reduced the number of plaques compared to untreated controls (Fig. 4B), suggesting that ERAV entry does not require caveola-mediated endocytosis.
FIG. 4.
Effects of pharmacological inhibitors of clathrin- and caveolin-dependent endocytosis and endosomal acidification on ERAV infectivity. (A) HeLa Ohio cells were treated with inhibitors of clathrin-dependent endocytosis and then infected with ERAV. The number of virus plaques generated in the presence of the inhibitors was expressed as a percentage of the no-drug control. ERAV infectivity was inhibited 40% by chlorpromazine (Cpz; 15 μM) and 85% by sucrose (Sucr; 0.4 M). (B) Effects of inhibition of caveolin-dependent endocytosis on ERAV. Neither nystatin (Nys) nor MβCD reduced the number of plaques compared to untreated controls. (C) Cells were treated with concanamycin A (ConcA) (b) or dimethyl sulfoxide (DMSO) alone (a) for 30 min at 37°C followed by visualization of endosomal acidification by AO fluorescence. In mock-treated cells, the acidic vesicles appear orange due to accumulation of AO, while in concanamycin A-treated cells, no red/orange color is detected, confirming inhibition of endosomal acidification (see the text). (D) Reduction of ERAV infectivity by inhibition of endosomal acidification with concanamycin A (100 nm) or monensin (Mon; 25 μM). (E) Progression from early to late endosomes is not necessary for ERAV infectivity since nocodazole (Noc; 20 μM) or wortmannin (Wort; 25 nM) did not reduce plaque numbers.
ERAV infection is dependent upon endosomal acidification.
Concanamycin A was used as an inhibitor of endosomal acidification in order to assess the role of pH in ERAV infection (10). The inhibitory effect of concanamycin A was confirmed by monitoring endosomal pH with the fluorophore acridine orange (AO; Invitrogen). AO is a weak base that becomes concentrated in acidic endosomes and exhibits a concentration-dependent shift from green (low concentration) to red (high concentration) fluorescence (35). HeLa Ohio cells on coverslips were incubated for 30 min in the presence or absence of concanamycin A (50, 100, and 200 nM). AO (0.5 μg/ml) was added for 15 min before the cells were visualized by fluorescence microscopy and red and green signals overlaid. In the absence of concanamycin A, acidic vesicles appeared orange because AO was trapped within the endosomes and its concentration increased (Fig. 4C). When concanamycin A was used at 100 nM (optimal concentration), no red/orange staining was detected, suggesting that endosomal acidification was inhibited (Fig. 4C). The effect of concanamycin A (100 nM) was then assessed on ERAV infection in virus inhibition assays, as described above. No plaques were seen in the presence of concanamycin A, suggesting that acidification is essential for the entry process (Fig. 4D). To confirm this result, we used monensin, a carboxylic ionophore that intercalates in the endosomal membrane and exchanges cytoplasmic K+ for protons, thereby increasing endosomal pH (17). In the presence of monensin (25 μM), ERAV infection was inhibited by 95% (Fig. 4D).
To identify more precisely the pH necessary for productive infection, we inhibited the maturation of early (pH 6.5) endosomes to late (pH 5.5) endosomes. This was achieved by inducing depolymerization of microtubules by exposure of cells to 20 μM nocodazole (1) or by inhibition of phosphoinositide 3-kinase signaling with 25 nM wortmannin (33). Neither drug reduced the number of plaques compared to the untreated controls (Fig. 4E) in infectious assays as described above, suggesting that maturation from early to late endosome is not necessary for ERAV infection. It is also worth noting that microtubules are involved in caveolin-dependent endocytosis. Therefore, the lack of an inhibitory effect of nocodazole on ERAV suggests that its entry is not dependent on caveolar endocytosis, thus supporting our general conclusions.
Exposure to acidic pH in the endosome is thought to be the trigger for genome release during FMDV infection (5, 14, 21), and both FMDV and ERAV dissociate under acidic conditions into pentameric subunits with release of RNA (7, 31). However, it is difficult to envision how the RNA is protected within the endosomal lumen following its release from the dissociated capsid and how it is transferred across the endosomal membrane. Earlier studies on FMDV described the formation of empty capsid particles lacking RNA (25), and we have recently shown that dissociation of ERAV proceeds via a transiently stable icosahedral empty particle from which the RNA has been ejected (31). We speculate that an uncoating intermediate particle may associate with the endosomal membrane to facilitate transfer of the RNA into the cytoplasm of the host cell, perhaps via a pore formed by VP4. This may protect the RNA from damaging exposure to the luminal contents of the vesicle, as would occur if the particle dissociated directly into its component subunits.
In summary, we have shown that productive infection of cells by ERAV is dependent on clathrin-mediated endocytosis and endosomal acidification, as is the case for FMDV (5, 14, 20). The requirement for acidification during infection is in agreement with recent studies describing the in vitro uncoating of ERAV, and these findings further strengthen the relevance of ERAV as a model for studying FMDV cell entry.
Acknowledgments
We thank Janet Daley and the Animal Health Trust, Newmarket, United Kingdom, for providing anti-ERAV serum.
This work was funded by the Biotechnology and Biological Sciences Research Council, United Kingdom, and the Medical Research Council, United Kingdom.
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Aniento, F., N. Emans, G. Griffiths, and J. Gruenberg. 1993. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 123:1373-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, M. Huttinger, D. Blaas, and R. Fuchs. 2001. Inhibition of clathrin-dependent endocytosis has multiple effects on human rhinovirus serotype 2 cell entry. J. Biol. Chem. 276:3952-3962. [DOI] [PubMed] [Google Scholar]
- 3.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112(Pt. 9):1303-1311. [DOI] [PubMed] [Google Scholar]
- 4.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berryman, S., S. Clark, P. Monaghan, and T. Jackson. 2005. Early events in integrin αvβ6-mediated cell entry of foot-and-mouth disease virus. J. Virol. 79:8519-8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandenburg, B., L. Y. Lee, M. Lakadamyali, M. J. Rust, X. Zhuang, and J. M. Hogle. 2007. Imaging poliovirus entry in live cells. PLoS Biol. 5:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burroughs, J. N., D. J. Rowlands, D. V. Sangar, P. Talbot, and F. Brown. 1971. Further evidence for multiple proteins in the foot-and-mouth disease virus particle. J. Gen. Virol. 13:73-84. [DOI] [PubMed] [Google Scholar]
- 8.Carbone, R., S. Fre, G. Iannolo, F. Belleudi, P. Mancini, P. G. Pelicci, M. R. Torrisi, and P. P. Di Fiore. 1997. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 57:5498-5504. [PubMed] [Google Scholar]
- 9.Dautry-Varsat, A. 1986. Receptor-mediated endocytosis: the intracellular journey of transferrin and its receptor. Biochimie 68:375-381. [DOI] [PubMed] [Google Scholar]
- 10.Dröse, S., and K. Altendorf. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Fishman, P. H., R. M. Bradley, J. Moss, and V. C. Manganiello. 1978. Effect of serum on ganglioside uptake and choleragen responsiveness of transformed mouse fibroblasts. J. Lipid Res. 19:77-81. [PubMed] [Google Scholar]
- 12.Grunert, H. P., K. U. Wolf, K. D. Langner, D. Sawitzky, K. O. Habermehl, and H. Zeichhardt. 1997. Internalization of human rhinovirus 14 into HeLa and ICAM-1-transfected BHK cells. Med. Microbiol. Immunol. 186:1-9. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, S. H., K. Sandvig, and B. van Deurs. 1993. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns, H. L., S. Berryman, P. Monaghan, G. J. Belsham, and T. Jackson. 2009. A dominant negative mutant of rab5 inhibits infection of cells by foot-and-mouth disease virus: implications for virus entry. J. Virol. 83:6247-6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, F., G. F. Browning, M. J. Studdert, and B. S. Crabb. 1996. Equine rhinovirus 1 is more closely related to foot-and-mouth disease virus than to other picornaviruses. Proc. Natl. Acad. Sci. U. S. A. 93:990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marjomäki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55:663-700. [DOI] [PubMed] [Google Scholar]
- 18.Newman, J. F., D. J. Rowlands, and F. Brown. 1973. A physico-chemical sub-grouping of the mammalian picornaviruses. J. Gen. Virol. 18:171-180. [DOI] [PubMed] [Google Scholar]
- 19.Newman, J. F., D. J. Rowlands, F. Brown, D. Goodridge, R. Burrows, and F. Steck. 1977. Physicochemical characterization of two serologically unrelated equine rhinoviruses. Intervirology 8:145-154. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell, V., M. Larocco, and B. Baxt. 2008. Heparan sulfate-binding foot-and-mouth disease virus enters cells via caveola-mediated endocytosis. J. Virol. 82:9075-9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Donnell, V., M. LaRocco, H. Duque, and B. Baxt. 2005. Analysis of foot-and-mouth disease virus internalization events in cultured cells. J. Virol. 79:8506-8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prchla, E., E. Kuechler, D. Blaas, and R. Fuchs. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pringle, C. R., and M. A. Mayo. 1999. Virus taxonomy at the 11th International Congress of Virology, Sydney, Australia, 1999. Arch. Virol. 144:2065-2070. [DOI] [PubMed] [Google Scholar]
- 24.Racaniello, V. R. 2007. Picornaviridae: the viruses and their replication, p. 685-722. In Fields virology, vol. 1. Lippincott, Williams, and Wilkins, Philadelphia, PA. [Google Scholar]
- 25.Rowlands, D. J., D. V. Sangar, and F. Brown. 1975. A comparative chemical and serological study of the full and empty particles of foot-and-mouth disease virus. J. Gen. Virol. 26:227-238. [DOI] [PubMed] [Google Scholar]
- 26.Salcini, A. E., H. Chen, G. Iannolo, P. De Camilli, and P. P. Di Fiore. 1999. Epidermal growth factor pathway substrate 15, Eps15. Int. J. Biochem. Cell Biol. 31:805-809. [DOI] [PubMed] [Google Scholar]
- 27.Singh, R. D., V. Puri, J. T. Valiyaveettil, D. L. Marks, R. Bittman, and R. E. Pagano. 2003. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14:3254-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Studdert, M. J., and L. J. Gleeson. 1978. Isolation and characterisation of an equine rhinovirus. Zentralbl. Veterinarmed. B 25:225-237. [DOI] [PubMed] [Google Scholar]
- 29.Torgersen, M. L., G. Skretting, B. van Deurs, and K. Sandvig. 2001. Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114:3737-3747. [DOI] [PubMed] [Google Scholar]
- 30.Tuthill, T. J., E. Groppelli, J. M. Hogle, and D. J. Rowlands. Picornavirus cell entry. Cell entry of non-enveloped viruses. Curr. Top. Microbiol. Immunol., in press.
- 31.Tuthill, T. J., K. Harlos, T. S. Walter, N. J. Knowles, E. Groppelli, D. J. Rowlands, D. I. Stuart, and E. E. Fry. 2009. Equine rhinitis A virus and its low pH empty particle: clues towards an aphthovirus entry mechanism? PLoS Pathog. 5:e1000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wipf, P., and R. J. Halter. 2005. Chemistry and biology of wortmannin. Org. Biomol. Chem. 3:2053-2061. [DOI] [PubMed] [Google Scholar]
- 34.Wutz, G., H. Auer, N. Nowotny, B. Grosse, T. Skern, and E. Kuechler. 1996. Equine rhinovirus serotypes 1 and 2: relationship to each other and to aphthoviruses and cardioviruses. J. Gen. Virol. 77(Pt. 8):1719-1730. [DOI] [PubMed] [Google Scholar]
- 35.Zelenin, A. V. 1993. AO as a probe for molecular and cell biology, p. 83-89. In W. Mason (ed.), Fluorescent and luminescent probes for biological activity. Academic Press, London, United Kingdom.