Abstract
We have previously shown that rhesus macaques were partially protected against high-dose intravenous challenge with simian-human immunodeficiency virus SHIVSF162P4 following sequential immunization with alphavirus replicon particles (VRP) of a chimeric recombinant VEE/SIN alphavirus (derived from Venezuelan equine encephalitis virus [VEE] and the Sindbis virus [SIN]) encoding human immunodeficiency virus type 1 HIV-1SF162 gp140ΔV2 envelope (Env) and trimeric Env protein in MF59 adjuvant (R. Xu, I. K. Srivastava, C. E. Greer, I. Zarkikh, Z. Kraft, L. Kuller, J. M. Polo, S. W. Barnett, and L. Stamatatos, AIDS Res. Hum. Retroviruses 22:1022-1030, 2006). The protection did not require T-cell immune responses directed toward simian immunodeficiency virus (SIV) Gag. We extend those findings here to demonstrate antibody-mediated protection against mucosal challenge in macaques using prime-boost regimens incorporating both intramuscular and mucosal routes of delivery. The macaques in the vaccination groups were primed with VRP and then boosted with Env protein in MF59 adjuvant, or they were given VRP intramuscular immunizations alone and then challenged with SHIVSF162P4 (intrarectal challenge). The results demonstrated that these vaccines were able to effectively protect the macaques to different degrees against subsequent mucosal SHIV challenge, but most noteworthy, all macaques that received the intramuscular VRP prime plus Env protein boost were completely protected. A statistically significant association was observed between the titer of virus neutralizing and binding antibodies as well as the avidity of anti-Env antibodies measured prechallenge and protection from infection. These results highlight the merit of the alphavirus replicon vector prime plus Env protein boost vaccine approach for the induction of protective antibody responses and are of particular relevance to advancing our understanding of the potential correlates of immune protection against HIV infection at a relevant mucosal portal of entry.
After more than 25 years of human immunodeficiency virus (HIV) research, a prophylactic vaccine able to control or prevent the worldwide spread of HIV/AIDS remains an elusive goal. Recent results in Thailand with the recombinant canary pox (ALVAC-HIV, vCP1521; Sanofi-Pasteur) prime-gp120 (AIDSVAX B/E) protein boost vaccine approach give us hope that such a vaccine is achievable (45). Nevertheless, the results from this trial as well as the disappointing outcome of the Step Study trial (7, 29, 46) vividly highlight the need to better understand the immune correlates of protection and the immune responses engendered by the diverse new vaccine technologies currently under evaluation (13, 18, 20, 49). In the case of viral vectors, this is particularly critical, as the spectrum of immune responses elicited in animal models does not necessarily predict those eventually observed in human clinical trials and will require more thorough evaluations in order to identify the most predictive models. At the moment, nonhuman primate models, such as simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV) infection of macaques appear to be the most informative for guiding vaccine development (3, 24, 47, 55), and more rigorous application of these models has begun to yield new and encouraging insights into protective immunity (5, 19, 27, 56). Moreover, as most HIV transmissions occur through mucosal membranes, understanding the correlates of protection, following successful vaccinations, against mucosal challenge is of strong interest.
Alphaviruses are positive-sense single-stranded 11.5-kb RNA viruses in the Togaviridae family. They are relatively simple enveloped viruses of approximately 60-nm diameter that have a cytoplasmic RNA-based life cycle and mature at the plasma membranes of infected cells. Recombinant alphavirus replicon particles used for vaccine applications are composed of a replicon vector that encodes the viral replicases (nonstructural proteins [NSPs]) and the vaccine antigen of interest and two packaging vectors that encode the major viral structural proteins (capsid and glycoproteins E1 and E2) required for particle formation. The chimeric (VEE/SIN) alphavirus vector system used in this study was derived from Venezuelan equine encephalitis virus (VEE) and the Sindbis virus (SIN). The recombinant VEE, SIN, and Semliki viruses expressing SIV or HIV antigens as well as antigens from a diverse and growing list of pathogens have been evaluated extensively in animals by several groups (6, 15, 16, 17, 22, 32, 34, 35, 36, 38, 42, 44, 57, 58). The chimeric alphavirus replicon particles (VRP) used here were designed to combine the immune potency of the VEE replicon with the safety profile of the SIN structural proteins (38).
In previous studies, we showed that rhesus macaques could be protected against high-dose intravenous challenges with SHIVSF162P4 following sequential immunization with chimeric recombinant VRP encoding human immunodeficiency virus type 1 (HIV-1) SF162 gp140ΔV2 envelope (Env) and trimeric SF162 gp140ΔV2 Env in the MF59 adjuvant (57). We also showed the Env protein delivered with potent adjuvants (the LTK63 mucosal adjuvant and the MF59 adjuvant) using intramuscular (i.m.) or combined mucosal (intranasal [i.n.]) plus i.m. vaccine regimens provided complete protection against intravaginal (IVAG) challenge with SHIVSF162P4 (2). The current work extends these studies by investigating the immunogenicity and protective efficacy of recombinant VRP delivered either mucosally, by the i.n. or intrarectal (i.r.) route, or parenterally by the i.m. route as a vector system for priming humoral immune responses prior to mucosal i.r. SHIVSF162P4 challenge in the rhesus macaque model.
In these studies, the alphavirus vector priming immunizations are followed by sequential booster immunizations with a highly purified and well-characterized trimeric V2-deleted envelope glycoprotein delivered in MF59, an oil-in-water emulsion, as an adjuvant. The HIV-1 Env antigen used in both the recombinant alphavirus prime and protein boost was derived from the macrophage-tropic chemokine (C-C motif) receptor 5 (CCR5)-utilizing HIV-1SF162 strain, which closely matches the envelope of the SHIVSF162P4 used for the i.r. challenge. This vaccine challenge study design thus serves as a useful starting point to better understand the mechanisms of immune protection against a relevant challenge virus and also the route of challenge in an active immunization model. Despite accelerated efforts in our laboratory and many others to identify the next generation of Env immunogens, evaluations of the breadth of protection are reserved for ongoing and future studies.
MATERIALS AND METHODS
Alphavirus replicon particles, proteins, and adjuvants.
Two VEE/SIN VRP preparations were made, one encoding SIV GagPol and another encoding HIV-1 SF162 gp140ΔV2. VRP were generated by cotransfection of in vitro-transcribed RNA species, harvested as culture supernatants, and purified as described previously (16, 38). Replicon particle titers were determined by intracellular staining of expressed Env followed by overnight infection of BHK-21 cells with serial dilutions of particles and expressed as infectious units (IU) per ml.
The SIV GagPol plasmid DNA has been described previously (60). The Env protein was derived from the clade B CCR5-tropic strain HIV-1SF162. The o-gp140ΔV2 protein contained a 30-amino-acid deletion in the V2 loop region and was produced in stable CHO cell lines and then purified and characterized as previously described (52, 53).
Immunizations and challenge of rhesus macaques.
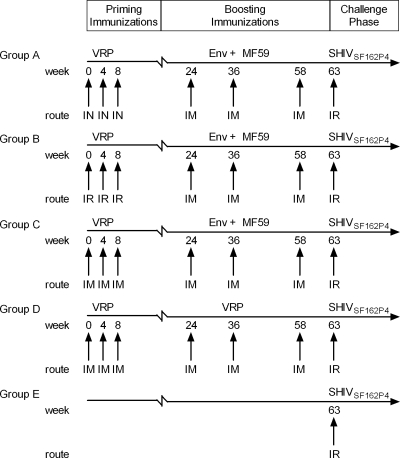
All animals used in this study were housed at the California National Primate Research Center and were cared for in accordance with established guidelines and the experimental procedures performed under approval from the respective Institutional Animal Care and Use Committees. A total of 30 male rhesus macaques of Indian origin were divided into 5 groups (groups A to E) of 6 animals each. All animals in groups A to D received a total of 3 priming immunizations, consisting of VRP encoding both SIVmac239 GagPol and HIV-1SF162 Env (1 × 108 IU each) at weeks 0, 4, and 8. Group A was primed i.n., group B was primed i.r., and groups C and D were primed i.m. (quadriceps). Groups A to C were then boosted with 100 μg SF162 o-gp140ΔV2 protein formulated in the MF59 adjuvant at weeks 24, 36, and 58. Monkeys in group D were boosted at the same time as monkeys in groups A to C, but they received VRP encoding Env (1 × 108 IU each) instead. All i.n. and i.r. vaccinations with VRP were performed with a dose of 1 × 108 IU each. Group E was the naïve control group.
Five weeks following the final boost (week 63), all animals were challenged with the subtype B SHIVSF162P4 (1,800 50% tissue culture infective doses [TCID50]) via the i.r. route. The SHIVSF162P4 challenge stock was obtained from Advanced BioScience Laboratories, Inc. (Kensington, MD) and has been described previously (40). All immunizations and sample collections were performed on anesthetized macaques. The time intervals between immunizations and challenge are shown in Fig. 1. Sera were collected from whole-blood samples and stored at −20°C. At 2 weeks after 3 and 6 immunizations, rectal and vaginal lavage samples (RL and VL samples) were collected and frozen immediately on dry ice and stored at −80°C. Plasma was analyzed for viral RNA by a branched-DNA (bDNA) assay (Bayer Corporation, Emeryville, CA).
FIG. 1.
Experimental design. Four groups (six rhesus macaques in each group) were immunized following a prime-boost regimen (groups A to D), and one group received no immunizations (group E). Vaccinated animals received 3 priming immunizations with 2 different VRP preparations (GagPol and Env) at weeks 0, 4, and 8 by either the intranasal (IN), intrarectal (IR), or intramuscular (IM) route. At weeks 24, 36, and 58, animals were immunized i.m. with either Env protein in MF59 adjuvant (groups A to C) or VRP (group D). All groups were challenged i.r. at week 63 with SHIVSF162P4.
Envelope-specific antibody titers.
Total and linear Env (gp140)-specific antibody (Ab) titers, as well as epitope-specific serum antibody titers, were determined by standard enzyme-linked immunosorbent assay (ELISA) protocols as previously described (50, 54). Responses against V1 and V3 were measured against the corresponding V1 and V3 peptides (V1, KNATNTKSSNWKEMDRGEIK; V3, CTRKSITIGPGRAFYC). The peptides were synthesized by Sigma-Genosys (The Woodlands, TX). Antibody avidity index determination was performed using an ammonium thiocyanate (NH4SCN) displacement ELISA as described elsewhere (54). Rectal and vaginal samples were assayed for anti-o-gp140 total IgG and IgA titers using a Europium-based ELISA as described previously (2).
HIV NAb assays.
Virus neutralization was assessed using molecularly cloned pseudoviruses and a luciferase reporter gene assay in TZM-bl cells (Tranzyme, Inc., Durham, NC) as described previously (25, 30). Briefly, a total of 200 TCID50 pseudovirus was added to each diluted serum sample in a well and incubated at 37°C for 1 h. Following incubation, cells in DEAE-dextran-containing medium were added to each well (10,000 cells/well) and incubated for 48 h at 37°C. The final concentration of DEAE-dextran was 10 μg/ml. A single round of infection of HIV-1 Env pseudoviruses were prepared by cotransfection of 293T cells with an envelope expression plasmid containing a full-length gp160 env gene along with an env-deficient HIV-1 backbone vector (pSG3Δenv), using TransIT-LT1 transfection reagent (Mirus Bio Corp., Madison, WI) as previously reported (26). After 48 h, the cell culture supernatant containing the pseudovirus was filtered through a 0.45-μm filter. Neutralizing activity was measured as the reduction in luciferase gene expression. The percent reduction in relative luminescence units (RLU) was calculated relative to the RLU in the presence of preimmunization serum. Neutralizing antibody (NAb) titers against HIV-1SF162 were determined using 3-fold serially diluted serum samples. The breadth of neutralizing antibodies in sera was assessed at a serum dilution of 1:15.
Peptide epitope mapping of serum neutralizing activity.
Mapping of neutralizing epitopes was performed by means of peptide inhibition using the TZM-bl assay with a few modifications. Diluted serum samples were preincubated with the corresponding peptides (V1 and/or V3) at 10 μg/ml for 1 h at 37°C prior to the addition of virus for the neutralization assay. The same peptides corresponding to the V1 and V3 regions of the SF162 Env that were used for ELISA were also used for neutralizing epitope mapping. The dilution of serum used was that corresponding to the dilution yielding a 70% reduction in virus infection (ID70).
RIBA.
Macaque serum samples collected prior to and 2, 4, and 11 weeks following challenge were tested for the presence of anti-SIV Gag antibodies using HIV-1/HIV-2 RIBA kit (Novartis, Emeryville, CA). The seroconversion to Gag was used as a surrogate assay to determine whether animals were infected postchallenge as previously reported (10).
Statistical analyses.
Comparisons between multiple groups was carried out using analysis of variance (ANOVA). A two-sided Wilcoxon rank sum analysis was used to test for differences between immunization groups. The Mann-Whitney test was used to test for differences in humoral responses between protected and infected groups (as shown in Fig. 5). For all comparisons, a two-sided P < 0.05 was considered statistically significant.
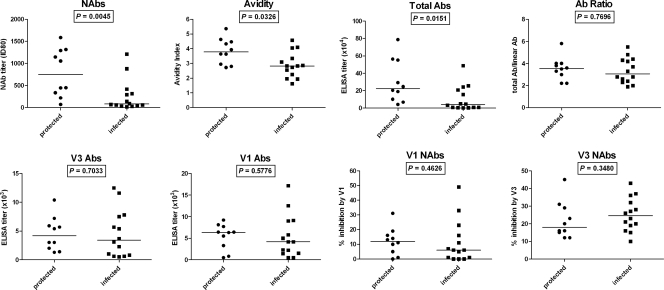
FIG. 5.
Antibodies with high neutralizing capacity, binding titers, and avidity prechallenge are associated with protection from SHIV infection. The animals were divided into protected and infected groups on the basis of the peak viral load (<2.7 and ≥4.0, respectively). Significant differences between groups were observed with respect to NAb titer, total Ab titer, and avidity. Avidity, total Abs, and Ab ratio are as described in footnotes to Table 1 (footnote c, a, and b, respectively). V1 and V3 NAbs are as described in the legend to Fig. 3. P values were obtained by the Mann-Whitney test.
RESULTS
Immunization of rhesus macaques with an alphavirus prime plus Env protein boost elicits robust serum antibody responses.
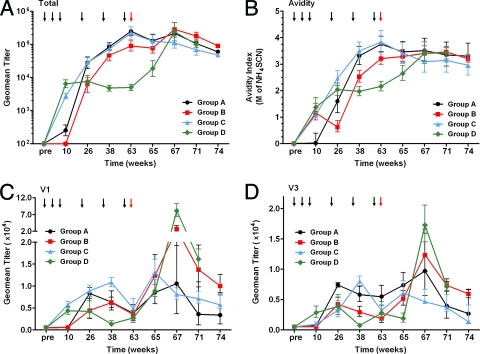
Groups of animals were immunized with two recombinant alphavirus replicon particles (VRP) preparations, one expressing SIV GagPol and the other expressing Env from HIV-1SF162, alone or in combination with booster immunizations with trimeric SF162 o-gp140ΔV2 in MF59 adjuvant as described in Materials and Methods and shown in Fig. 1. Env-specific serum antibody titers were measured at regular intervals throughout the course of immunization during the entire study (Fig. 2). Both groups that received the VRP via the i.m. route (groups C and D) elicited the highest titers of total Env-specific Abs as determined by ELISA using trimeric gp140 protein following the first three immunizations (week 10) (Fig. 2A). Both groups had significantly higher titers than those obtained following i.n. immunizations, which were an order of magnitude lower (P = 0.0050). i.r. administration of VRP did not result in a measurable level of Env-specific total serum Abs. Following the three boosting immunizations (week 63), the anti-Env Ab titers of animals boosted with homologous SF162 Env protein in MF59 adjuvant increased significantly, reaching levels 10- to 100-fold higher than those observed following VRP priming, while boosting with VRP did not increase titers by week 63 (group D). All three groups that received Env protein boosting immunizations exhibited titers that were significantly higher than the group boosted with VRP (P < 0.005). Groups A (i.n.) and C (i.m.) had the highest titers following boosting, which were indistinguishable from one another (P = 0.9372).
FIG. 2.
Antibody responses following immunization and challenge. (A) Group geometric mean (Geomean) SF162 envelope-specific ELISA titers. (B) Avidity of HIV-1 strain SF162 envelope-specific antibodies. The molar concentration of NH4SCN required for displacement of 50% Ab is shown. (C and D) Linear V1 (C) and V3 (D) antibody responses as measured by peptide ELISA. The animals were immunized as described in the legend to Fig. 1. Black arrows indicate when the animals were immunized, and red arrows indicate when the animals were challenged with SHIV. The values are means ± standard deviations (error bars).
We also monitored the levels of IgG and IgA in both rectal and vaginal lavage samples of the animals 2 weeks following the third immunization with VRP and 2 weeks after the final boosting immunization. Of note, although IgA (but not IgG) was detected in rectal lavage samples, no o-gp140-specific IgA or IgG titers were detectable at this site. Moreover, although both IgA and IgG were found in vaginal lavage samples, o-gp140-specific IgA or IgG titers were not detected (data not shown).
To further evaluate the relative contributions of Abs directed to conformational Env epitopes compared to linear Env epitopes, in addition to measuring binding Abs to trimeric gp140 as described above, we also measured serum Ab titers directed against reduced and denatured SF162 gp140ΔV2 protein by ELISA. Similar kinetics were observed for each group for these specific Abs against the linear epitopes as found above when measuring total Ab (data not shown). To determine whether there was any difference in the quality of the antigen-binding sites of the Abs elicited through the different immunization regimens, we examined the ratios of total Abs to those recognizing linear epitopes. Following VRP priming, the average Ab ratio was below 1 (0.3 to 0.7) for all groups regardless of the immunization route, indicative of Abs with more linear specificity and/or Abs with relatively low titers. After the first i.m. Env protein boost, the ratio increased in the groups primed i.n. and i.r. to 1.2 and 1.9, respectively. These ratios remained relatively unchanged following the second protein boost but increased to 2.9 and 3.8 following the third protein boost. Interestingly, the Ab ratio did not change in group C (i.m. primed with VRP and i.m. boosted with Env and MF59) even following the first two protein boosts (0.3) and was similar to that found for in animals given two VRP boosts (0.4). However, following the third protein boost, the ratio increased to 4.1 in the group primed i.m.
To assess the role of Abs specific to epitopes in variable loops, ELISA was carried out using peptides corresponding to the V1 and V3 regions of the SF162 Env (Fig. 2C and D); the immunogens did not contain intact V2 loops, so this was not measured. Similar to the findings above, i.m. priming with VRP elicited the highest levels of V1-specific Abs (P = 0.0048 for group C versus group A; P = 0.0037 for group C versus group B). V3-specific Abs were detected in each animal in group D and one animal from group C, but none of the other animals had detectable level of anti-V3 Abs at this time. Boosting with Env protein increased the levels of these Abs in all groups, while additional immunizations with VRP had no effect on the level of these Abs. However, the groups boosted with Env protein displayed levels of V1- and V3-specific Abs that decreased over time such that by week 63 all groups had indistinguishable levels of V1-specific Abs (P > 0.05) and only group A had significantly higher anti-V3 Ab titers than the other groups (P < 0.05). The titers of the V1- and V3-specific Abs were similar by the day of challenge but comprised of a greater percentage of Ab specificity in group D, since this group had the lowest titers overall. This may be attributable to differences in epitope presentation by the Env antigen delivered by VRP as opposed to purified protein in the MF59 adjuvant. This needs to be further explored in future studies.
High avidity and high titers of virus neutralizing antibodies were elicited in groups given i.m. or i.n. VRP prime plus i.m. Env protein boost.
To better understand the functional role that anti-Env specific Abs may play in providing protection from SHIV challenge, Env-specific Ab avidities and virus neutralizing titers were evaluated. Following three priming VRP immunizations by the i.m. or i.r. route, Abs of modest avidity were induced, while the group A animals given priming VRP immunization by the i.n. route displayed very low avidity (Fig. 2, week 10). All groups showed increased Ab avidities after subsequent boosting immunization through week 63 including the group boosted with VRP. Nevertheless, the groups boosted with Env protein plus adjuvant were better able to maintain higher Ab avidities until the day of challenge than the group boosted with VRP (P < 0.05).
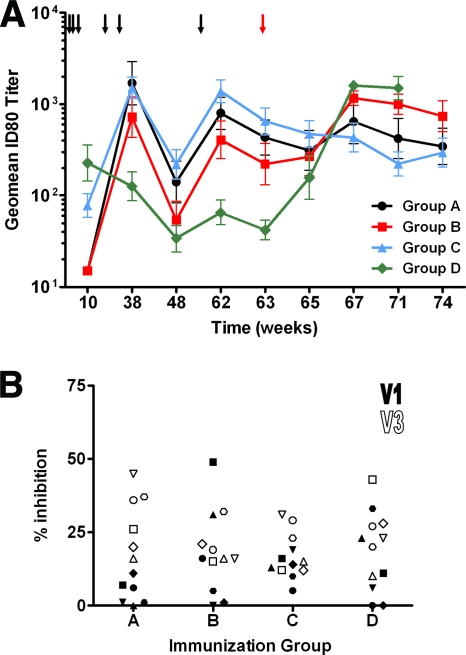
The SF162 neutralizing antibody (NAb) titers were measured following the priming immunizations. Priming via the i.m. route yielded the highest neutralizing titers, while i.n. or i.r. administration resulted in Abs with very low neutralizing capacity (Fig. 3A). Interestingly, although i.r. priming yielded serum Abs with similar avidity to those obtained through i.m. priming, these Abs did not neutralize the SF162 virus even at the lowest dilution tested. Following boosting, animals receiving Env protein displayed an increase in the ID80 titers, but the titers on the day of challenge (week 63) were slightly lower than those observed at week 38, i.e., following two boosting immunizations. In contrast to the increase in Ab avidity that was observed for the animals boosted with VRP, ID80 titers decreased from week 10 to week 38 as well as from week 38 to week 63. Similar to that observed for Ab avidity, all three protein-boosted groups had significantly higher NAb titers than the VRP-boosted group (P < 0.05), but they were not significantly different from each other (P > 0.05). In addition, sera from 1 week prior to challenge (week 62) were tested for the capability to neutralize a small panel of clade B isolates. We observed only modest titers against SS1196 by sera from groups A and C (average ID50 titers of 55 and 29, respectively) (data not shown).
FIG. 3.
Neutralizing antibody responses. (A) Neutralization of HIV-1 strain SF162 by sera from immunized rhesus macaques and macaques postchallenge. Sera taken following the third VRP prime, the second and third booster immunizations, and the SHIV challenge were assayed in TZM-bl cells for neutralizing activity against SF162. Individual serum samples were assayed, and the geometric mean (Geomean) 80% (ID80) neutralization titer is shown. The values are means ± standard errors of means (error bars). The lowest dilution tested was 1:15. The black and red arrows indicate when the animals were immunized and challenged, respectively. (B) Peptide mapping of serum neutralizing epitopes. The percent inhibition of SF162 neutralization in the presence of 10 μg/ml of the indicated peptide is shown. Inhibition in the presence of V1 (filled) or V3 (open) peptides is shown. Each symbol shows the value for an individual animal in a group. Neutralization was carried out at a serum dilution corresponding to the ID70 value.
A significant fraction of the neutralizing activity of SF162-specific antibodies is directed at the V3 and V1 regions.
Epitope mapping was carried out to determine whether variations in the NAb epitope recognition patterns of the sera influenced neutralization potency. In addition, variations in Ab specificity could occur due to the route of immunization as well as the method used in the boosting phase. Sera obtained 1 week prior to challenge, week 62, were used for this purpose. Two peptides were utilized in this study. One peptide corresponded to the loop of the V1 region (V1), while the other (V3) was composed of the residues located immediately prior to and following the GPGR motif of the V3 loop (see Materials and Methods). Inhibition of SF162 neutralization by the V3 peptide was observed to a similar degree for all animals (Fig. 3B) (P > 0.05). Similarly, no difference in V3 specificity was observed when boosting with Env protein or VRP. In general, all immunization regimens elicited V1 NAb levels that were lower than those found for V3. As observed with V3, the boosting regimen did not seem to have an effect on the level of NAbs with V1 specificity. However, the NAb levels directed against V1 in the i.n. and i.m. primed groups were different (P = 0.0304), being higher in the i.m. group.
Immunization with alphavirus replicon particle prime-protein boost regimens protects rhesus macaques against intrarectal SHIV challenge.
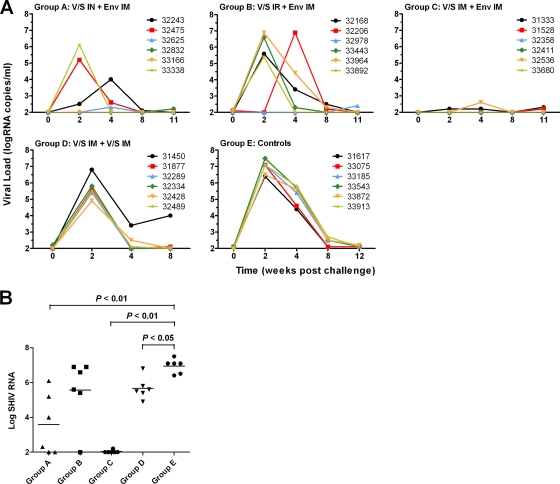
Plasma viral loads of immunized and naïve control animals were monitored prior to and following SHIVSF162P4 challenge (Fig. 4A). Overall, there was a significant difference in the peak viral loads in all groups (P < 0.005, ANOVA). The peak viral loads for all naïve animals (group E) occurred 2 weeks following challenge. Virus levels gradually decreased to below the level of detection (100 RNA copies/ml) by 8 to 12 weeks following challenge in group E animals (Fig. 4A). Similarly, all animals that received six VRP immunizations without Env protein boosting (group D) were infected, and the peak level of virus was observed at week 2. However, the peak viral load of group D was reduced compared to the control group (P < 0.05) (Fig. 4B). Additionally, all six animals in this group (group D) had reduced virus levels by week 4 compared to the control animals, although the viral load increased in one animal again by week 8. Immunization using the i.r. VRP prime-i.m. Env boost regimen (group B) resulted in the protection of 1 out of 6 animals, but overall, the peak viral load in this group was not significantly reduced compared to the control group (P > 0.05). Immunization via the i.n. prime-i.m. boost regimen (group A) resulted in an initial undetectable viral load in two animals following challenge, but each of these animals became infected by week 11 postchallenge. Another animal in this group exhibited a very low level of virus at week 4. The remaining three animals were clearly infected, although the peak viral load in one of these animals was delayed (week 4). The peak viral load in this animal was more than 2 log units lower than that determined for the control animals. Overall, peak viral loads were significantly reduced for this group (group A) compared to naïve animals (P < 0.01) (Fig. 4B). The vaccine regimen conferring the best protection was i.m. VRP prime followed by i.m. Env boost (group C). In this group, all six animals were protected with a few animals displaying very low levels of virus near the limit of detection of the assay. Peak viral loads were significantly reduced in group C compared to the naïve group (P < 0.01), group B (P < 0.05), and group D (P < 0.01) but not group A (P > 0.05) (Fig. 4B).
FIG. 4.
VRP prime-protein boost vaccine regimen provided protection from SHIV infection. (A) The rhesus macaques were immunized with VRP encoding both SIVmac239 GagPol and HIV-1SF162 Env via the intranasal (IN), intrarectal (IR), or intramuscular (IM) route and boosted with Env or VRP encoding Env by the intramuscular route. The plasma viral load of individual animals was measured via a branched-DNA assay. The limit of detection for this assay was 100 copies/ml. (B) The peak viral load values (either 2 or 4 weeks following challenge) are indicated for individual animals, with the group means represented by horizontal lines. Significant reductions in group peak viral load compared to the control group were observed for the groups receiving VRP i.n. or i.m. P values for the means of the groups were determined by two-sided Wilcoxon rank sum analysis.
In all cases, when the plasma virus load measurements were found to be at or near the limit of detection using the bDNA assay, the absence of active SHIV infection was confirmed by the lack of seroconversion to SIV p27 Gag antigen using the RIBA HIV-1/HIV-2 assay (Table 1). Further analysis by DNA PCR was not performed.
TABLE 1.
Prechallenge (week 63) HIV-1 SF162-specific humoral responses and postchallenge virologic outcome
| Group and animal | Total Ab titera (×104) | Ab ratiob | Avidity (M)c | NAb titer (ID80) | Viral loadd (log) | RIBA resulte |
|---|---|---|---|---|---|---|
| Group A | ||||||
| 32243 | 25.7 | 1.9 | 4.05 | 1,213 | 4.0 (wk 4) | + |
| 32475 | 20.8 | 2.6 | 3.35 | 314 | 5.2 | + |
| 32625 | 6.7 | 3.0 | 2.80 | 70 | 2.3 (wk 4) | − |
| 32832 | 56.4 | 2.2 | 4.46 | 1,316 | <2.0 | − |
| 33166 | 24.6 | 3.3 | 3.63 | 450 | <2.0 | − |
| 33338 | 48.8 | 4.4 | 4.57 | 418 | 6.1 | + |
| Group B | ||||||
| 32168 | 4.9 | 3.7 | 3.10 | 138 | 5.6 | + |
| 32206 | 15.5 | 2.3 | 2.83 | 881 | 6.9 (wk 4) | + |
| 32978 | 18.9 | 2.2 | 3.93 | 1,056 | <2.0 | − |
| 33443 | 3.3 | 4.2 | 2.74 | 55 | 6.6 | + |
| 33694 | 4.6 | 5.5 | 2.83 | 57 | 6.9 | + |
| 33892 | 24.4 | 4.8 | 4.09 | 292 | 5.4 | + |
| Group C | ||||||
| 31333 | 10.1 | 4.0 | 4.64 | 445 | 2.2 | − |
| 31528 | 20.4 | 3.8 | 2.95 | 334 | <2.0 | − |
| 32358 | 29.2 | 4.0 | 5.36 | 1,145 | <2.0 | − |
| 32411 | 4.2 | 3.6 | 2.72 | 219 | <2.0 | − |
| 32536 | 55.1 | 3.5 | 3.64 | 1,291 | 2.6 (wk 4) | − |
| 33680 | 78.7 | 5.8 | 4.33 | 1,585 | <2.0 | − |
| Group D | ||||||
| 31450 | 0.2 | 2.8 | 1.63 | <15 | 6.8 | ND |
| 31877 | 0.8 | 2.0 | 2.87 | 91 | 5.6 | ND |
| 32289 | 0.8 | 2.4 | 1.94 | 69 | 5.4 | ND |
| 32334 | 0.4 | 4.3 | 2.25 | 18 | 5.8 | ND |
| 32428 | 0.8 | 3.3 | 2.55 | 33 | 4.9 | ND |
| 32489 | 0.3 | 2.8 | 1.96 | 36 | 5.5 | ND |
Total SF162 o-gp140ΔV2 antibody titers determined by ELISA.
Ab ratio = total Ab titer/linear Ab titer.
Molar concentration of NH4SCN required for displacement of 50% Ab.
Peak viral load with the corresponding time indicated if it was not week 2.
Gag-specific Ab detection using RIBA assay for sera from weeks 2, 4, and 11. ND, not done.
Humoral responses following SHIV challenge.
Ab responses were also monitored from the day of challenge (week 63) to 11 weeks postchallenge (week 74). Group C animals controlled infection, and as a result, the levels of binding Abs in these animals continually decreased following challenge (Fig. 2A). Interestingly though, the levels of V1 and V3 Abs in this group increased 2 weeks following challenge before gradually decreasing (Fig. 2C and D). Ab avidity and neutralizing titers also decreased following challenge (Fig. 2 and 3A), suggesting that sufficient levels of high-avidity Abs were present in these animals, which helped control viremia during the acute phase of infection as reflected by the lack of anamnestic response in these animals. In contrast, the remaining three immunization groups exhibited an increase in both binding and NAb production in response to increases in the viral load (Fig. 3A and 4). The binding Ab levels of these groups increased between 2 and 4 weeks postchallenge (Fig. 2). Groups A and B, which had relatively high-avidity Abs at the day of challenge, displayed a small increase in avidity by week 67. In contrast, group D animals, which had significantly lower Ab avidity than the other groups at week 63, exhibited a large increase in avidity following challenge (Fig. 2B). In a similar manner, the ID80 NAb titer for group D increased by over an order of magnitude following challenge, while smaller increases were observed for groups A and B (Fig. 3A).
Virus-neutralizing antibody titer and avidity prechallenge are predictors of the outcome of virus challenge.
To determine whether certain features of the Ab response to vaccination played a role in protection against the SHIV challenge, the relationship of each of the serologic parameters measured to protection was examined (Fig. 5). Animals from the vaccinated groups (groups A to D) were categorized into two groups, protected and infected. The protected group consisted of animals with peak viral loads of <2.7 (n = 10), while the infected group animals had peak viral loads of ≥4.0 (n = 14). Based on this analysis, statistically significant differences were observed between the two groups with respect to binding Abs, avidity, and NAb titer. The titer of SF162 NAbs was found to be the most predictive with respect to protection from SHIV infection. The protected group had significantly higher homologous ID80 neutralizing titers than those in the infected group (P = 0.0045, Mann-Whitney test). Similarly, animals with higher-avidity Abs on the day of challenge were more likely to be protected (P = 0.0326, Mann-Whitney test). The level of binding Abs on the day of challenge was also significantly higher in animals that were protected from infection (P = 0.0151, Mann-Whitney test). However, the ratio of total binding Abs to linear binding Abs was not found to be related to protection (P = 0.7696, Mann-Whitney test). The levels of V1 and V3 Abs, whether binding or neutralizing, also did not appear to be associated with protection (P > 0.1 for all groups, Mann-Whitney test; Fig. 5, bottom).
DISCUSSION
To date, studies of recombinant viral vectors and nucleic acid-based approaches for HIV vaccines have resulted in a relatively comprehensive examination of the utility of these platforms to induce protective CD8+ T-cell immunity in animal and human subjects. The rationale for this emphasis on T-cell-focused vaccine approaches using vectors stemmed, in part, from the substantial challenges in engineering effective HIV envelope antigens for incorporation into a broadly protective vaccine. In addition, there was also considerable optimism that new gene delivery technologies would be able to elicit the required T-cell responses shown to control virus load during natural HIV infection. Indeed, recent excitement has been generated surrounding new studies in which next generation T-cell vaccine approaches have begun to show significant impact on acute and set point viremia after robust mucosal (19) or intravenous (5) homologous SIV challenges, and in one case against a mucosal heterologous SIV challenge (56).
Nevertheless, clinical results with the first generation of T-cell-focused vaccines tested in humans have shown that, while these approaches may be useful additions to an effective HIV vaccine, they are challenging because of the enormous genetic diversity of HIV and many unknowns surrounding the required breadth and depth of the host immune responses required, the contribution of host genetics, and unanticipated effects of the vaccine vectors on these responses (7).
In contrast, while not entirely unexplored, there has been less attention in recent years to the value of the viral vector vaccine delivery method in eliciting protective humoral immune responses. In the present study, like those performed earlier by ourselves and others, we used prime-boost regimens employing viral vector primes combined with Env protein boosts with the goal of eliciting the humoral and cell-mediated immune responses required for protection (1, 28, 33, 39). Since the first studies reported by Shiu-Lok Hu and colleagues reported as early as 1992 (23) using this type of regimen, there have been strong indications that a vector prime-protein boost regimen could elicit protective immunity in nonhuman primate challenge models for HIV (33, 37, 41, 57).
Importantly, the same general vaccine concept of vector prime plus Env protein boost yielded the first ever positive HIV vaccine efficacy results in Thailand whereby a prime-boost regimen of Sanofi-Pasteur's recombinant HIV canary pox (ALVAC-HIV) vaccine combined with VaxGen's bivalent monomeric gp120 (AIDSVAX B/E) vaccines delivered in the alum adjuvant was shown to protect 31% of vaccinated individuals in that region in a modified intent-to-treat (mITT) analysis of the data collected over a 3-year observation period postvaccination (45). The immune mechanisms underlying the observed protection are not yet clear, but the possible role of Ab-mediated responses represents an intense area of investigation.
Among the diverse viral vector systems currently under evaluation for the next generation of recombinant vaccines, alphavirus replicon-based systems appear to compare favorably to the field due to their simple genome structure, nonnuclear localization, high level of expression yet lack of virus spread, adjuvant effects, ease of manipulation, wide tropism for host cells, and resistance to the effects of preexisting antivector immune responses. Most importantly, recombinant alphaviruses provide a platform that has been shown to elicit protective Ab responses against many diverse pathogens in preclinical models (15, 22, 36, 44, 48). In one key example, protection of both juvenile and infant rhesus macaques against measles virus was achieved after a single intradermal dose of recombinant VEE/SIN VRP expressing measles virus hemagglutinin (35). In these studies, antigen-specific CD4+ T-cell responses and long-lived protective levels of virus NAbs induced by the alphavirus-vectored vaccine were critical for protection. The immune responses elicited and the levels of protection observed resembled those of highly effective live inactivated measles virus vaccine and differed dramatically from the weak immune responses and disease exacerbation observed using formalin-inactivated vaccines.
For HIV, although the nonhuman primate challenge models currently in use have their limitations, protection against infection and disease progression in these models yet provides an important tool for the assessment and comparison of different vaccine approaches (31). In the studies described here, vaccines were delivered to rhesus macaques that were subsequently challenged by SHIVSF162P4 by the intrarectal route (40). Two recombinant VRP preparations encoding SIVmac239 GagPol and HIVSF162 were used to immunize macaques using regimens that employed either mucosal (i.r. or i.n.) priming or systemic (i.m.) priming in combination with multiple booster immunizations with SF162 gp40ΔV2 protein in MF59 adjuvant. The results showed complete protection of all animals that received i.m. immunizations with the combined VRP prime-Env protein boost regimen, while only a subset of animals primed by mucosal routes showed any protection, and none of the macaques that received recombinant VRP alone were protected. VRP vaccines similar to those used here were previously shown to elicit both humoral and cellular immune responses when delivered parenterally to small animals and macaques (16, 17, 38) and protection of macaques as measured by significant reductions in acute-phase virus load following intravenous homologous SHIVSF162 challenge (57). The present work extends the latter result to demonstrate the complete protective efficacy of the recombinant VRP prime plus Env protein boost approach in the face of a relevant mucosal challenge with a closely related virus, SHIV. The observed protection showed a statistically significant association with the titers of virus NAbs and binding Abs as well as high-avidity Abs in prechallenge sera. These findings are consistent with previous results that showed Ab-mediated protection against SHIV challenge in macaques immunized with recombinant adenovirus primes with VRP or Env protein boosts (6) and the results of others showing an inverse correlation between Ab avidity after immunization and reduced peak viral load (59). The protection seen here did not appear to be associated with the titers of V1- or V3-directed Ab responses. In addition, while the possible protective role of other Ab-mediated effector functions, such as antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cell-mediated viral inhibition (ADCVI), is suggested by other studies of active and passive immunization (6, 11, 12, 14, 21), the contributions of these Ab-mediated mechanisms were not evaluated here.
In the face of the current debate as to whether mucosal vaccination or immune responses play a role in protection against mucosal infection, our data suggest that local (rectal) IgA responses did not seem to be required to protect against rectal challenge with SHIV. It is important to note that while HIV enters through mucosal portals, such as the rectal and vaginal mucosa, it subsequently traverses the mucosa and spreads systemically. Indeed, in this study, as in most studies by others, we measured only the plasma viral load, which does not reflect the local viral load in the rectal mucosa. This was due to our desire to preserve the integrity of the rectal mucosal milieu. In this regard, it has been shown that while intestinal IgA does not seem to play a role in acute responses against retrovirus, it is important for memory responses against reinfection with the same virus (51). Moreover, it is well established that both oral live attenuated and inactivated poliovirus-based vaccines protect against poliomyelitis, even though the latter does not result in intestinal IgA responses. Like HIV-1, poliovirus enters the host through the mucosa and spreads systemically. It can be argued that because the virus exerts its disease systemically, the systemic route of vaccination is also effective.
While the levels of IgG in intestinal lumen are low, as reflected in the rectal lavage samples, because of strong protease activity, this does not reflect the amount of IgG secreted by plasma cells in the intestinal lamina propria plasma cells (8). Hence, due to the study design, we were unable to measure the number of antigen-specific lamina propria IgG-secreting cells that might have played a role in virus neutralization after virus entry into the lamina propria through the epithelial layer. Moreover, while the protection observed here occurred most prominently following parenteral immunizations, it remains to be seen whether more effective mucosal deliveries would provide additional benefit against increasingly robust virus challenges.
In this study, local or systemic anti-Gag cellular responses were not measured; while previous studies indicated that the modest levels of Gag-specific T-cell responses in the periphery were elicited following a VRP prime, these were not essential for protection following intravenous challenge (57). This would be an important direction for future studies employing novel mucosal deliveries of vaccine, relevant mucosal immunologic and virologic readouts, and heterologous SHIV mucosal challenges.
Finally, these results support the importance of Ab-mediated protection in the setting of active immunization against HIV, consistent with previous results of vaccinations with adjuvant Env proteins showing complete protection against intravaginal challenge (2). A formidable challenge, namely, how to identify improved Env antigens for incorporation into this vaccine platform in order to expand the breadth of immune protection, remains.
Acknowledgments
This work was supported by U.S. Public Health Service grants U51RR00169 from the National Center for Research Resources and U19 AI51596, NIH IPCAVD grant 1 U19 AI51596, and HIV DDT contract N01-AI-50007 and HIVRAD grant 5 P01AI066287-03 from NIAID, NIH.
We also thank Ping Shi for statistical analysis and Christian Mandl and Rino Rappuoli for their ongoing support of these studies.
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Barnett, S. W., J. M. Klinger, B. Doe, C. M. Walker, L. Hansen, A. M. Duliege, and F. M. Sinangil. 1998. Prime-boost immunization strategies against HIV. AIDS Res. Hum. Retroviruses 14(Suppl. 3):S299-S309. [PubMed] [Google Scholar]
- 2.Barnett, S. W., I. K. Srivastava, E. Kan, F. Zhou, A. Goodsell, A. D. Cristillo, M. G. Ferrai, D. E. Weiss, N. L. Letvin, D. Montefiori, R. Pal, and M. Vajdy. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS 22:339-348. [DOI] [PubMed] [Google Scholar]
- 3.Baroncelli, S., D. R. Negri, Z. Michelini, and A. Cara. 2008. Macaca mulatta, fascicularis and nemestrina in AIDS vaccine development. Expert Rev. Vaccines 7:1419-1434. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. U. S. A. 97:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., J. Liu, D. M. Lynch, K. L. O'Brien, A. La Porte, N. L. Simmons, A. M. Riggs, S. Clark, P. Abbink, D. C. Montefiori, G. Landucci, D. N. Forthal, S. G. Self, A. Carville, K. Mansfield, and J. Goudsmit. 2009. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J. Virol. 83:9584-9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogers, W. M., D. Davis, I. Baak, E. Kan, S. Hofman, Y. Sun, D. Mortier, Y. Lian, H. Oostermeijer, Z. Fagrouch, R. Dubbes, M. van der Maas, P. Mooij, G. Koopman, E. Verschoor, J. P. Langedijk, J. Zhao, E. Brocca-Cofano, M. Robert-Guroff, I. Srivastava, S. Barnett, and J. L. Heeney. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology 382:217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, M. N. Robertson, and the Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman, A. L., J. Mestecky, A. Moukarzel, and M. E. Ament. 1995. Intestinal immune function is unaffected by parenteral nutrition in man. J. Am. Coll. Nutr. 14:656-661. [DOI] [PubMed] [Google Scholar]
- 9.Buckner, C., L. G. Gines, C. J. Saunders, L. Vojtech, I. Srivastava, A. Gettie, R. Bohm, J. Blanchard, S. W. Barnett, J. T. Safrit, and L. Stamatatos. 2004. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology 320:167-180. [DOI] [PubMed] [Google Scholar]
- 10.Cherpelis, S., X. Jin, A. Gettie, D. D. Ho, S. W. Barnett, I. Shrivastava, and L. Stamatatos. 2001. DNA-immunization with a V2 deleted HIV-1 envelope elicits protective antibodies in macaques. Immunol. Lett. 79:47-55. [DOI] [PubMed] [Google Scholar]
- 11.Florese, R. H., T. Demberg, P. Xiao, L. Kuller, K. Larsen, L. E. Summers, D. Venzon, A. Cafaro, B. Ensoli, and M. Robert-Guroff. 2009. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J. Immunol. 182:3718-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forthal, D. N., G. Landucci, K. S. Cole, M. Marthas, J. C. Becerra, and K. Van Rompay. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 80:9217-9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedrich, T. C., and D. I. Watkins. 2008. Wanted: correlates of vaccine-induced protection against simian immunodeficiency virus. Curr. Opin. HIV AIDS 3:393-398. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Román, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 174:2185-2189. [DOI] [PubMed] [Google Scholar]
- 15.Greer, C. E., F. Zhou, H. S. Legg, Z. Tang, S. Perri, B. A. Sloan, J. zur Megede, Y. Uematsu, M. Vajdy, and J. M. Polo. 2007. A chimeric alphavirus RNA replicon gene-based vaccine for human parainfluenza virus type 3 induces protective immunity against intranasal virus challenge. Vaccine 25:481-489. [DOI] [PubMed] [Google Scholar]
- 16.Gupta, S., R. Janani, Q. Bin, P. Luciw, C. Greer, S. Perri, H. Legg, J. Donnelly, S. Barnett, D. O'Hagan, J. M. Polo, and M. Vajdy. 2005. Characterization of human immunodeficiency virus Gag-specific gamma interferon-expressing cells following protective mucosal immunization with alphavirus replicon particles. J. Virol. 79:7135-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, S., F. Zhou, C. E. Greer, H. Legg, T. Tang, P. Luciw, J. zur Megede, S. W. Barnett, J. J. Donnelly, D. T. O'Hagan, J. M. Polo, and M. Vajdy. 2006. Antibody responses against HIV in rhesus macaques following combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles. AIDS Res. Hum. Retroviruses 22:993-997. [DOI] [PubMed] [Google Scholar]
- 18.Gurunathan, S., R. E. Habib, L. Baglyos, C. Meric, S. Plotkin, B. Dodet, L. Corey, and J. Tartaglia. 2009. Use of predictive markers of HIV disease progression in vaccine trials. Vaccine 27:1997-2015. [DOI] [PubMed] [Google Scholar]
- 19.Hansen, S. G., C. Vieville, N. Whizin, L. Coyne-Johnson, D. C. Siess, D. D. Drummond, A. W. Legasse, M. K. Axthelm, K. Oswald, C. M. Trubey, M. Piatak, Jr., J. D. Lifson, J. A. Nelson, M. A. Jarvis, and L. J. Picker. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haut, L. H., and H. C. Ertl. 2009. Obstacles to the successful development of an efficacious T cell-inducing HIV-1 vaccine. J. Leukoc. Biol. 86:779-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hessell, A. J., P. Poignard, M. Hunter, L. Hangartner, D. M. Tehrani, W. K. Bleeker, P. W. Parren, P. A. Marx, and D. R. Burton. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper, J. W., A. M. Ferro, J. W. Golden, P. Silvera, J. Dudek, K. Alterson, M. Custer, B. Rivers, J. Morris, G. Owens, J. F. Smith, and K. I. Kamrud. 2009. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine 28:494-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, S. L., K. Abrams, G. N. Barber, P. Moran, J. M. Zarling, A. J. Langlois, L. Kuller, W. R. Morton, and R. E. Benveniste. 1992. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science 255:456-459. [DOI] [PubMed] [Google Scholar]
- 24.Kramer, V. G., N. B. Siddappa, and R. M. Ruprecht. 2007. Passive immunization as tool to identify protective HIV-1 Env epitopes. Curr. HIV Res. 5:642-655. [DOI] [PubMed] [Google Scholar]
- 25.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian, Y., I. Srivastava, V. R. Gómez-Román, J. Zur Megede, Y. Sun, E. Kan, S. Hilt, S. Engelbrecht, S. Himathongkham, P. A. Luciw, G. Otten, J. B. Ulmer, J. J. Donnelly, D. Rabussay, D. Montefiori, E. J. van Rensburg, and S. W. Barnett. 2005. Evaluation of envelope vaccines derived from the South African subtype C human immunodeficiency virus type 1 TV1 strain. J. Virol. 79:13338-13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubeck, M. D., R. Natuk, M. Myagkikh, N. Kalyan, K. Aldrich, F. Sinangil, S. Alipanah, S. C. Murthy, P. K. Chanda, S. M. Nigida, Jr., P. D. Markham, S. Zolla-Pazner, K. Steimer, M. Wade, M. S. Reitz, Jr., L. O. Arthur, S. Mizutani, A. Davis, P. P. Hung, R. C. Gallo, J. Eichberg, and M. Robert-Guroff. 1997. Long-term protection of chimpanzees against high-dose HIV-1 challenge induced by immunization. Nat. Med. 3:651-658. [DOI] [PubMed] [Google Scholar]
- 29.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, D. R. Casimiro, and the Step Study Protocol Team. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p. 12.11.11-12.11.15. In R. Coico (ed.), Current protocols in immunology. John Wiley & Sons, New York, NY. [DOI] [PubMed]
- 31.Morgan, C., M. Marthas, C. Miller, A. Duerr, C. Cheng-Mayer, R. Desrosiers, J. Flores, N. Haigwood, S. L. Hu, R. P. Johnson, J. Lifson, D. Montefiori, J. Moore, M. Robert-Guroff, H. Robinson, S. Self, and L. Corey. 2008. The use of nonhuman primate models in HIV vaccine development. PLoS Med. 5:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mossman, S. P., F. Bex, P. Berglund, J. Arthos, S. P. O'Neil, D. Riley, D. H. Maul, C. Bruck, P. Momin, A. Burny, P. N. Fultz, J. I. Mullins, P. Liljestrom, and E. A. Hoover. 1996. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J. Virol. 70:1953-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myagkikh, M., S. Alipanah, P. D. Markham, J. Tartaglia, E. Paoletti, R. C. Gallo, G. Franchini, and M. Robert-Guroff. 1996. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res. Hum. Retroviruses 12:985-992. [DOI] [PubMed] [Google Scholar]
- 34.Otten, G., M. Schaefer, C. Greer, M. Calderon-Cacia, D. Coit, J. Kazzaz, A. Medina-Selby, M. Selby, M. Singh, M. Ugozzoli, J. zur Megede, S. W. Barnett, D. O'Hagan, J. Donnelly, and J. Ulmer. 2003. Induction of broad and potent anti-human immunodeficiency virus immune responses in rhesus macaques by priming with a DNA vaccine and boosting with protein-adsorbed polylactide coglycolide microparticles. J. Virol. 77:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan, C.-H., C. E. Greer, D. Hauer, H. S. Legg, E.-Y. Lee, J. Bergen, B. Lau, R. J. Adams, J. M. Polo, and D. E. Griffin. 3 February 2010. A chimeric alphavirus replicon particle vaccine expressing the hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques from measles. J. Virol. doi: 10.1128/JVI.01566-09. [DOI] [PMC free article] [PubMed]
- 36.Pan, C. H., N. Nair, R. J. Adams, M. C. Zink, E. Y. Lee, F. P. Polack, M. Singh, D. T. O'Hagan, and D. E. Griffin. 2008. Dose-dependent protection against or exacerbation of disease by a polylactide glycolide microparticle-adsorbed, alphavirus-based measles virus DNA vaccine in rhesus macaques. Clin. Vaccine Immunol. 15:697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 78:2212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perri, S., C. E. Greer, K. Thudium, B. Doe, H. Legg, H. Liu, R. E. Romero, Z. Tang, Q. Bin, T. W. Dubensky, Jr., M. Vajdy, G. R. Otten, and J. M. Polo. 2003. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 77:10394-10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pialoux, G., J. L. Excler, Y. Riviere, G. Gonzalez-Canali, V. Feuillie, P. Coulaud, J. C. Gluckman, T. J. Matthews, B. Meignier, M. P. Kieny, et al. 1995. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI). The AGIS Group, and l'Agence Nationale de Recherche sur le SIDA. AIDS Res. Hum. Retroviruses 11:373-381. [DOI] [PubMed] [Google Scholar]
- 40.Polacino, P., K. Larsen, L. Galmin, J. Suschak, Z. Kraft, L. Stamatatos, D. Anderson, S. W. Barnett, R. Pal, K. Bost, A. H. Bandivdekar, C. J. Miller, and S. L. Hu. 2008. Differential pathogenicity of SHIV infection in pig-tailed and rhesus macaques. J. Med. Primatol. 37(Suppl. 2):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polacino, P., V. Stallard, D. C. Montefiori, C. R. Brown, B. A. Richardson, W. R. Morton, R. E. Benveniste, and S. L. Hu. 1999. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J. Virol. 73:3134-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Polo, J. M., J. P. Gardner, Y. Ji, B. A. Belli, D. A. Driver, S. Sherrill, S. Perri, M. A. Liu, and T. W. Dubensky, Jr. 2000. Alphavirus DNA and particle replicons for vaccines and gene therapy. Dev. Biol. (Basel) 104:181-185. [PubMed] [Google Scholar]
- 43.Putkonen, P., M. Quesada-Rolander, A. C. Leandersson, S. Schwartz, R. Thorstensson, K. Okuda, B. Wahren, and J. Hinkula. 1998. Immune responses but no protection against SHIV by gene-gun delivery of HIV-1 DNA followed by recombinant subunit protein boosts. Virology 250:293-301. [DOI] [PubMed] [Google Scholar]
- 44.Reap, E. A., J. Morris, S. A. Dryga, M. Maughan, T. Talarico, R. E. Esch, S. Negri, B. Burnett, A. Graham, R. A. Olmsted, and J. D. Chulay. 2007. Development and preclinical evaluation of an alphavirus replicon particle vaccine for cytomegalovirus. Vaccine 25:7441-7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rerks-Ngarm, S., P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J. G. McNeil, D. P. Francis, D. Stablein, D. L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M. L. Robb, N. L. Michael, P. Kunasol, and J. H. Kim. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 46.Robb, M. L. 2008. Failure of the Merck HIV vaccine: an uncertain step forward. Lancet 372:1857-1858. [DOI] [PubMed] [Google Scholar]
- 47.Sato, S., and W. Johnson. 2007. Antibody-mediated neutralization and simian immunodeficiency virus models of HIV/AIDS. Curr. HIV Res. 5:594-607. [DOI] [PubMed] [Google Scholar]
- 48.Saxena, S., S. S. Dahiya, A. A. Sonwane, C. L. Patel, M. Saini, A. Rai, and P. K. Gupta. 2008. A Sindbis virus replicon-based DNA vaccine encoding the rabies virus glycoprotein elicits immune responses and complete protection in mice from lethal challenge. Vaccine 26:6592-6601. [DOI] [PubMed] [Google Scholar]
- 49.Sekaly, R. P. 2008. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J. Exp. Med. 205:7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma, V. A., E. Kan, Y. Sun, Y. Lian, J. Cisto, V. Frasca, S. Hilt, L. Stamatatos, J. J. Donnelly, J. B. Ulmer, S. W. Barnett, and I. K. Srivastava. 2006. Structural characteristics correlate with immune responses induced by HIV envelope glycoprotein vaccines. Virology 352:131-144. [DOI] [PubMed] [Google Scholar]
- 51.Silvey, K. J., A. B. Hutchings, M. Vajdy, M. M. Petzke, and M. R. Neutra. 2001. Role of immunoglobulin A in protection against reovirus entry into murine Peyer's patches. J. Virol. 75:10870-10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava, I. K., E. Kan, Y. Sun, V. A. Sharma, J. Cisto, B. Burke, Y. Lian, S. Hilt, Z. Biron, K. Hartog, L. Stamatatos, R. Diaz-Avalos, R. H. Cheng, J. B. Ulmer, and S. W. Barnett. 2008. Comparative evaluation of trimeric envelope glycoproteins derived from subtype C and B HIV-1 R5 isolates. Virology 372:273-290. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava, I. K., L. Stamatatos, E. Kan, M. Vajdy, Y. Lian, S. Hilt, L. Martin, C. Vita, P. Zhu, K. H. Roux, L. Vojtech, D. C. Montefiori, J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2003. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J. Virol. 77:11244-11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J. Virol. 76:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valentine, L. E., and D. I. Watkins. 2008. Relevance of studying T cell responses in SIV-infected rhesus macaques. Trends Microbiol. 16:605-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson, N. A., B. F. Keele, J. S. Reed, S. M. Piaskowski, C. E. MacNair, A. J. Bett, X. Liang, F. Wang, E. Thoryk, G. J. Heidecker, M. P. Citron, L. Huang, J. Lin, S. Vitelli, C. D. Ahn, M. Kaizu, N. J. Maness, M. R. Reynolds, T. C. Friedrich, J. T. Loffredo, E. G. Rakasz, S. Erickson, D. B. Allison, M. Piatak, Jr., J. D. Lifson, J. W. Shiver, D. R. Casimiro, G. M. Shaw, B. H. Hahn, and D. I. Watkins. 2009. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J. Virol. 83:6508-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu, R., I. K. Srivastava, C. E. Greer, I. Zarkikh, Z. Kraft, L. Kuller, J. M. Polo, S. W. Barnett, and L. Stamatatos. 2006. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res. Hum. Retroviruses 22:1022-1030. [DOI] [PubMed] [Google Scholar]
- 58.Xu, R., I. K. Srivastava, L. Kuller, I. Zarkikh, Z. Kraft, Z. Fagrouch, N. L. Letvin, J. L. Heeney, S. W. Barnett, and L. Stamatatos. 2006. Immunization with HIV-1 SF162-derived envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology 349:276-289. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, J., L. Lai, R. R. Amara, D. C. Montefiori, F. Villinger, L. Chennareddi, L. S. Wyatt, B. Moss, and H. L. Robinson. 2009. Preclinical studies of human immunodeficiency virus/AIDS vaccines: inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J. Virol. 83:4102-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.zur Megede, J., B. Sanders-Beer, P. Silvera, D. Golightly, A. Bowlsbey, D. Hebblewaite, D. Sites, L. Nieves-Duran, R. Srivastava, G. R. Otten, D. Rabussay, L. Zhang, J. B. Ulmer, S. W. Barnett, and J. J. Donnelly. 2008. A therapeutic SIV DNA vaccine elicits T-cell immune responses, but no sustained control of viremia in SIVmac239-infected rhesus macaques. AIDS Res. Hum. Retroviruses 24:1103-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]