Abstract
Several host genes control retroviral replication and pathogenesis through the regulation of immune responses to viral antigens. The Rfv3 gene influences the persistence of viremia and production of virus-neutralizing antibodies in mice infected with Friend mouse retrovirus complex (FV). This locus has been mapped within a narrow segment of mouse chromosome 15 harboring the APOBEC3 and BAFF-R loci, both of which show functional polymorphisms among different strains of mice. The exon 5-lacking product of the APOBEC3 allele expressed in FV-resistant C57BL/6 (B6) mice directly restricts viral replication, and mice lacking the B6-derived APOBEC3 exhibit exaggerated pathology and reduced production of neutralizing antibodies. However, the mechanisms by which the polymorphisms at the APOBEC3 locus affect the production of neutralizing antibodies remain unclear. Here we show that the APOBEC3 genotypes do not directly affect the B-cell repertoire, and mice lacking B6-derived APOBEC3 still produce FV-neutralizing antibodies in the presence of primed T helper cells. Instead, higher viral loads at a very early stage of FV infection caused by either a lack of the B6-derived APOBEC3 or a lack of the wild-type BAFF-R resulted in slower production of neutralizing antibodies. Indeed, B cells were hyperactivated soon after infection in the APOBEC3- or BAFF-R-deficient mice. In contrast to mice deficient in the B6-derived APOBEC3, which cleared viremia by 4 weeks after FV infection, mice lacking the functional BAFF-R allele exhibited sustained viremia, indicating that the polymorphisms at the BAFF-R locus may better explain the Rfv3-defining phenotype of persistent viremia.
Several host genetic factors control retroviral replication and pathogenesis through the regulation of immune responses to viral antigens. The recovery from Friend virus 3 gene (Rfv3) was identified as a host gene locus that affects the persistence of viremia and development of virus-specific antibody (Ab) responses upon Friend virus (FV) infection (5). FV is the pathogenic retrovirus complex composed of replication-competent Friend murine leukemia virus (F-MuLV) and the defective spleen focus-forming virus (SFFV). The product of the SFFV env gene, gp55, forms a complex with the erythropoietin receptor and the short form of the hematopoietic-cell-specific receptor tyrosine kinase (STK), and this interaction induces the growth and terminal differentiation of erythroid progenitor cells, causing increased hematocrit values and massive splenomegaly. The resultant increase in targets of FV integration consequently causes the emergence of mono- or oligoclonal erythroleukemia through insertional activation of transcription factors or disruption of a tumor suppressor gene (15, 29, 34). Mice of the C57BL background possess mutations in the intron of the Stk gene and lack expression of the short-form STK, resulting in resistance to SFFV-induced splenomegaly (37). This host factor was first described as polymorphisms at the Fv2 locus, with the resistance allele found in C57BL mice being designated the recessive Fv2r (21).
The Rfv3 gene was originally described based on the segregation of FV-induced leukemia development from persistence of viremia (5). (B10.A × A/WySn)F1 and A/WySn mice both developed leukemia after FV infection due to their shared susceptible major histocompatibility complex (MHC) haplotype, H2a. However, despite the common trait of susceptibility to leukemia development, most of the (B10.A × A/WySn)F1 mice had cleared viremia by 30 to 50 days after FV inoculation, while the majority of A/WySn mice remained viremic. As one-half of the (B10.A × A/WySn)F1 × A/WySn backcross mice had cleared viremia by 30 to 50 days after FV inoculation, a single gene affecting the persistence of viremia was postulated and designated Rfv3 (5). When crosses of H2b mice were similarly analyzed, it was found that most of the A.BY mice remained viremic at 30 to 60 days after FV infection and developed leukemia, while (C57BL/10 × A.BY)F1 mice cleared viremia and showed resistance to leukemia development. Again, about one-half of the (C57BL/10 × A.BY)F1 × A.BY backcross mice remained viremic at 30 to 60 days postinfection, and a slightly larger fraction developed leukemia (4, 5). These results indicate that clearance of viremia in the presence of the C57BL-derived dominant Rfv3 genotype is a prerequisite for resistance against FV-induced leukemia development, and H2b mice can show a low incidence of leukemia provided that they possess the resistant Rfv3 genotype and clear viremia (4). Since BALB.B mice remained viremic at 30 to 50 days after FV infection (5), it has been postulated that C57BL/6 (B6) and C57BL/10 mice possess the dominant Rfv3r genotype, which confers early clearance of viremia, while A.BY, A/WySn, and BALB.B mice share the recessive Rfv3s genotype, associated with persistence of viremia irrespective of their H2 haplotypes (4). These Rfv3 genotypes were later associated with the production of anti-FV Abs capable of lysing FV-induced leukemia cells and a reduction in cell surface expression of FV antigens on spleen cells in Rfv3r mice (9), suggesting that the Rfv3 gene might regulate the production of anti-FV Abs. The location of the Rfv3 gene was narrowed down to a segment of mouse chromosome 15 by comparing levels of viremia at 30 days after FV infection in (B10.A × A/WySn)F1 × A/WySn backcross and (B10.A × A/WySn)F2 mice (12, 48). We took a separate approach and examined the titers of virus-neutralizing Abs in (B10.A × A/WySn)F1 × A/WySn backcross mice (16). By analyses of linkages between polymorphic markers and titers of FV-neutralizing Abs at postinoculation day (PID) 15, the Rfv3 locus was again mapped within a narrow segment of chromosome 15 (16). We further compared the expression levels, after FV infection, of all the candidate genes between the Rfv3r/s and Rfv3s/s mice (29), and polymorphisms at the apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like editing complex 3 locus (APOBEC3) have recently been associated with the levels of viremia or restricted replication of FV by two independent research groups (43, 49).
The major transcript of the APOBEC3 allele expressed in FV-resistant B6 mice lacks exon 5 (Δ5), and the product of this mA3b allele highly restricts FV replication both in vitro and in vivo (49). On the other hand, BALB/c and A strains of mice express the full-length and Δ5 transcripts from their mA3d allele; the translated products of both poorly inhibit the replication of an infectious F-MuLV clone. An additional allelic difference is that the expression levels of mA3b are significantly higher than those of the mA3d allele (36, 49). Thus, a lack of expression of the resistance-associated mA3b allele in gene-targeted mice and their hybrids resulted in 100- to 1,000-fold-higher levels of FV replication and higher hematocrit values (49). Additionally, reduced levels of virus-neutralizing Abs were observed in mice lacking the mA3b allele (43). However, the mechanisms by which the polymorphisms at the APOBEC3 locus, which encodes the intracellular cytidine deaminase protein, may affect the production of virus-neutralizing Abs remain unclear. Further, the relationship between the production of virus-neutralizing Abs and control of viremia also remains uncertain, as the reduction in the number of virus-producing cells can be observed prior to the detection of virus-neutralizing Abs in the serum of vaccinated animals (17, 27). The possible causative relationships between the production of virus-neutralizing Abs and clearance of viremia are important, especially because the original Rfv3 phenotypes were defined by the persistence of viremia at PIDs >30 (4, 5), while the polymorphisms in the genetic markers linked with the APOBEC3 locus affect serum titers of virus-neutralizing Abs at PID 15 (16).
Of note, A/WySn mice, which were used to identify Rfv3 as a single gene locus (4, 5), but not A/J mice are known to possess a natural mutation in the receptor for B-cell-activating factor belonging to the TNF family (BAFF-R), which results in reduced size of the peripheral B-cell compartment and attenuation of antigen-specific IgG responses (1, 51). The structural gene for this receptor, BAFF-R, is located in chromosome 15, only about 2 megabase pairs telomeric to the APOBEC3 locus. A/WySn mice harbor a 4.7-kb insertion in exon 3 of this gene (35, 51). Both differentiation and maturation phenotypes and functions of B cells in A/WySn mice are essentially similar to those observed in mice with a targeted disruption of the BAFF-R gene, in which both immature and mature peripheral B cells exhibit shorter half-lives (20, 39, 44, 45). As the genetic mapping of the Rfv3 locus has been performed by using A/WySn mice or their progeny obtained by crossing them with B10.A mice (12, 16, 48), there remains the possibility that the polymorphism at the BAFF-R locus in close linkage with the APOBEC3 genotypes may have affected the Rfv3 phenotypes. We report here that polymorphisms at the APOBEC3 and BAFF-R loci independently influence the production of virus-neutralizing Abs upon FV infection. We further propose a possible mechanism by which the APOBEC3 alleles affect F-MuLV-specific Ab responses.
MATERIALS AND METHODS
Mice and virus.
C57BL/6 CrSlc and A/J JmsSlc mice were purchased from Japan SLC, Inc., Hamamatsu, Japan. A/WySnJ and B6(Cg)-Tnfrsf13ctmMass/J (B6-BAFF-R−/−) mice were purchased from The Jackson Laboratory, Bar Harbor, ME. The B6-BAFF-R−/− mice homozygously carry a targeted disruption of the BAFF-R gene (44). The APOBEC3-deficient mice on a B6 background (B6-mA3−/−) have been described previously (26, 49). Mice 7 to 10 weeks old were used throughout the present study. All animals were housed and bred in the Experimental Animal Facilities at Kinki University School of Medicine under specific-pathogen-free conditions, and all animal experiments were conducted according to the guidelines of Kinki University. A stock of B-tropic FV complex without contamination of lactate dehydrogenase-elevating virus has been described previously (49). Replication-competent helper virus of the FV, F-MuLV, was purified from the culture supernatant of Mus dunni cells persistently infected with infectious molecular clone FB29 (46).
Assays for plasma viral load.
Mice were bled from the buccal vein with an 18-gauge needle under ether anesthesia, and the plasma was separated and stored at −30°C until use. Serial 3-fold dilutions of plasma were loaded in the presence of 4 μg/ml Polybrene (Sigma-Aldrich Corp., St. Louis, MO) on monolayers of Mus dunni cells that had been seeded at 1.0 × 104 cells per well of 24-well plates on the previous day in RPMI 1640 supplement with 10% fetal bovine serum (FBS). Two days later, the cells were washed twice with phosphate-buffered balanced salt solution (PBBS) and fixed with methanol. F-MuLV-infected cell foci were stained with monoclonal Ab (MAb) 720 (41), which detects the F-MuLV env gene product, and visualized by using biotinylated anti-mouse immunoglobulin (Ig) Ab and avidin-biotinylated peroxidase complex (Vector Laboratories, Burlingame, CA) as described previously (17, 27, 41). The foci were counted under a magnifier, and the F-MuLV titers of plasma samples were calculated as focus-forming units (FFU) per ml.
Assays for virus-neutralizing Abs.
The in vitro assays for quantitative measurement of F-MuLV-neutralizing Abs have been described elsewhere in detail (17, 27, 47). Mice were bled, and the sera were separated and heat inactivated at 56°C for 30 min. As demonstrated previously (19), this heating procedure inactivates most of the remaining infectivity due to viremia, if there is any, in serum samples, which we also confirmed (data not shown). Serial 2-fold dilutions of sera were mixed with 100 FFU of the F-MuLV stock virus. The virus/antibody mixtures were added onto cultured Mus dunni cells, and F-MuLV-infected cell foci were counted as described in the protocol for the plasma viremia assay. The virus mixed with PBBS was added to control wells. Neutralizing titers were calculated as the logarithm of maximum dilutions that gave a reduction in the number of F-MuLV-infected cell foci to <25% of those in the control wells. IgG titers were determined by treating each serum with 2-mercaptoethanol (17).
Northern blot analyses and quantitative real-time PCR assays.
Northern blot analyses and quantitative real-time PCR assays were performed with total RNA prepared from various mouse tissues or cells to determine APOBEC3 mRNA expression as described previously using the same primers and probes (49). CD19+ B cells and CD3+ T cells were separated from the spleens of B6 mice by using MAb-conjugated magnetic microbeads (Miltenyi Biotec GmbH, Germany) according to the manufacturer's instructions. For the Northern blot analysis, 2 micrograms of total RNA was separated in a 1% formaldehyde-agarose gel and visualized by ethidium bromide staining. The mRNA encoding β-actin was used as an internal control. For the quantitative real-time PCR assays, the expression levels of mouse GAPDH mRNA were used as an internal control. The ΔCT values were determined by subtracting the average threshold cycle (CT) value for GAPDH from the average CT value for APOBEC3. The relative expression levels of the APOBEC3 mRNA in each cell population were then determined as ΔΔCT values relative to the ΔCT values obtained with the whole spleen cells.
Immunization and enzyme-linked immunosorbent assay (ELISA).
Mice were injected intraperitoneally with 100 μg of 2,4-dinitrophenylated (DNP) ovalbumin (DNP-OVA; Biosearch Technologies, Novato, CA) in alum and boosted once with 50 μg of DNP-OVA without alum at an interval of 10 days. The mice were bled before or at 7 days after the last immunization, and the sera were separated. T-cell-independent responses were elicited by intraperitoneal injection of 100 μg DNP-Ficoll (Biosearch Technologies). The serum Abs were captured on plates coated with DNP-conjugated keyhole limpet hemocyanin; they were then detected by incubation with peroxidase-conjugated rabbit anti-mouse IgM (μ-chain specific) or anti-mouse IgG (γ-chain specific), both purchased from Zymed Laboratories (San Francisco, CA), followed by an incubation with benzidine as a chromogen (Sigma-Aldrich). Relative Ab titers were determined by comparing values of optical density at 405 nm (OD405) with those obtained with an appropriate dilution of standard mouse IgM or IgG captured onto goat anti-mouse Ig-coated plates.
Peptide synthesis and immunization.
Peptide i, representing a single T-helper-cell epitope encoded by the F-MuLV env gene (gp70 residues 462 to 479) (27), was purchased from Operon Biotechnologies (Tokyo, Japan). For immunization, the lyophilized peptide was dissolved in PBBS and emulsified with an equal volume of complete Freund's adjuvant (CFA; Difco Laboratories, Detroit, MI). Mice were immunized once subcutaneously in the abdominal wall with multiple split doses for a total of 100 μl of the emulsion containing 10 μg of the peptide. Three weeks later, they were challenged with FV complex as described previously (17, 27). The control mice were given the same amount of CFA emulsified with PBBS. FV-induced pathology was assessed by monitoring hematocrit values, and spleen weights were measured at 77 days after FV infection.
Flow cytometry.
Flow cytometric analyses of cell surface markers were performed as described elsewhere (17, 47). Spleen cell suspensions were stained with the following MAbs conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), PerCP-Cy5.5, allophycocyanin, or biotin: anti-mouse CD19, anti-mouse CD69, anti-mouse TER-119, anti-mouse IgD (δ-chain specific), and anti-mouse IgM (μ-chain specific), all purchased from BD Biosciences. F-MuLV-infected cells were detected with biotinylated MAb 720 as described previously (17). Data were acquired with a FACSCalibur (BD Biosciences, Franklin Lakes, NJ) and were analyzed with CELLQuest Pro software.
Genotyping.
Mouse genomic DNA was prepared from the tails as described previously (16). BAFF-R genotypes were determined by PCR analyses using the oligonucleotide primers 5′-CACCATGGGCGCCAGGAGACTC-3′ (for both B6- and A/WySn-derived alleles) and 5′-GAAGTCCACAAGCCCAGTAGAGAT-3′ (for the B6-derived allele) or 5′-ACGTTCACGGGAAAAACAGAGT-3′ (for the A/WySn-derived allele). For determination of the APOBEC3 genotypes, a genomic region harboring intron 4 of APOBEC3, in which there are sequence polymorphisms between the two strains of mice (36), was amplified using the primers 5′-AATTTAAAAAGTGTTGGAAGAAG-3′ and 5′-CTGCCCTCCACGCAGAACCTC-3′ and sequenced.
Statistics.
One-way analysis of variance (ANOVA) with the Bonferroni post hoc test was performed for the comparison of multiple groups using Prism software (GraphPad Software, Inc., San Diego, CA), and individual significance in the difference was calculated by two-tailed Student's or Welch's t test depending on whether variances of compared groups were regarded as equal or not. Correlation coefficients (R) were also calculated using the Prism software.
RESULTS
APOBEC3 genotypes affect the kinetics of virus-neutralizing Ab production and early levels of plasma viremia.
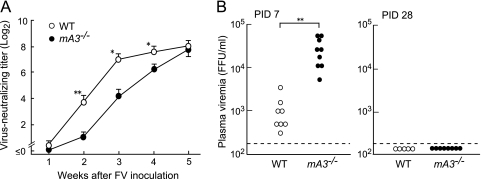
To evaluate the possible effects of the APOBEC3 polymorphisms on anti-FV B-cell responses, we first analyzed the kinetics of neutralizing Ab production and changes in plasma levels of viremia following FV inoculation in B6 mice possessing the disrupted APOBEC3 gene (mA3−/−) and their wild-type (WT) counterparts. FV-neutralizing Ab titers in the mA3−/− mice were significantly lower at 2 weeks postinfection than those in the WT mice, and this difference in Ab titers persisted throughout the 4 weeks of FV infection (Fig. 1 A). However, Ab titers in the mA3−/− and WT mice became comparable at 5 weeks postinfection. Virus-neutralizing Abs detected in both groups of B6 mice were almost exclusively IgG. Thus, mA3−/− mice showed a significant delay in the initiation of virus-neutralizing Ab production. Plasma viral loads at PID 7 in the mA3−/− mice were on average 18-fold higher than those in the control WT mice; however, at PID 28 no viremia was detectable in both groups (Fig. 1B), in contrast to the previously observed persistence of viremia in Rfv3s/s mice at PIDs >30 (4, 5). These findings indicate that the B6-derived, resistance-associated mA3b allele not only restricts FV replication but also facilitates the production of FV-neutralizing Abs, which is consistent with previous findings (43, 49), except that FV-neutralizing Abs can be produced, albeit more slowly, in the absence of mA3b.
FIG. 1.
Kinetics of virus-neutralizing Ab production and levels of plasma viremia in B6 mice lacking the mA3b allele upon FV infection. The mA3−/− and WT B6 mice were inoculated with 10,000 spleen focus-forming units (SFFU) of FV. (A) Sera were collected weekly for 5 weeks, and titers of Abs capable of neutralizing an infectious molecular clone of F-MuLV were measured by focal immunoassay. Neutralizing titers were determined as serum dilutions causing a 75% reduction in the infectivity. Each data point represents the mean titer of IgG plus IgM calculated with 5 to 9 separate serum samples ± standard error of the mean (SEM). One-way ANOVA for repeated measures with Bonferroni post hoc tests was used to calculate P values (*, P < 0.05; **, P < 0.005). (B) Levels of plasma viremia were measured at PIDs 7 and 28. Each dot represents the level of FV viremia for an individual mouse as detected by focal immunoassay using Mus dunni cells. The limit of detection was 200 FFU/ml. **, P < 0.005 by Student's t test.
The APOBEC3 transcripts are preferentially expressed in B cells, but Ab production in response to nonviral antigens is unaffected in APOBEC3-deficient mice.
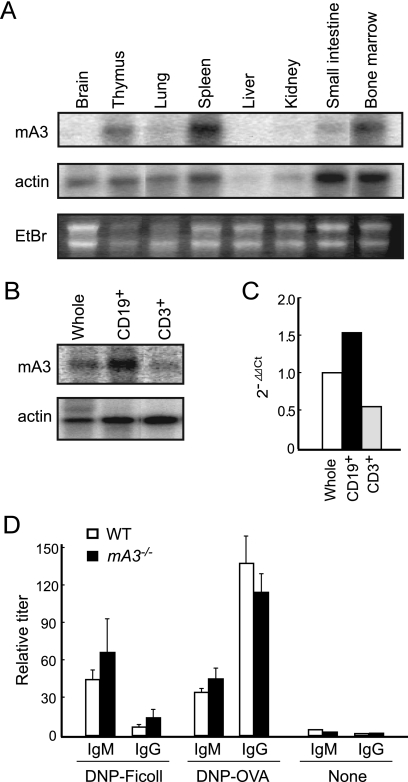
To address the mechanism by which the B6-derived APOBEC3 facilitates the production of virus-neutralizing Abs, we investigated the expression patterns of the APOBEC3 gene in B6 mice. The APOBEC3 transcripts were detected in abundance in the spleen and bone marrow (Fig. 2 A). Further, we found that B cells more highly expressed the APOBEC3 mRNA than T cells (Fig. 2B and C), possibly indicating a selective function of this enzyme in Ab formation.
FIG. 2.
Expression of APOBEC3 mRNA in B cells and humoral immune responses against nonviral antigens in APOBEC3-deficient mice. (A and B) Expression levels of APOBEC3 mRNA in the indicated tissues (A) or cells (B) prepared from B6 mice, as analyzed by Northern blotting. CD19+ or CD3+ cells were separated from the spleens of B6 mice by magnetic cell sorting. The RNA in agarose gels was visualized by ethidium bromide (EtBr) staining, and the β-actin gene was used as an internal control. (C) Expression levels of APOBEC3 mRNA analyzed by quantitative real-time PCR assays. The relative expression levels of APOBEC3 mRNA in each cell population were determined as ΔΔCT relative to the values obtained with the whole spleen cells. Means for each cell type are shown. The experiments were performed twice with essentially identical results. (D) Antibody responses to nonviral antigens in APOBEC3-deficient B6 mice. mA3−/− and WT B6 mice were injected intraperitoneally (i.p.) with 100 μg of DNP-OVA in alum and boosted 10 days later with 50 μg of DNP-OVA without alum. T-cell-independent responses were elicited by i.p. injection of 100 μg DNP-Ficoll. Anti-DNP Ab titers of either IgM or IgG class in the sera before and at 7 days after the last immunization were measured by ELISA. The data shown here are the mean values of relative Ab titers determined with 4 to 6 separate serum samples and SEM.
The above finding led us to examine the possible effects of APOBEC3 genotypes on the formation of B-cell repertoires, since the activation-induced cytidine deaminase (AID), one of the APOBEC family enzymes, is selectively expressed in B cells and functions as an essential initiator of both Ab class switching and somatic hypermutation (33). Thus, we first asked if a lack of the mA3b allele affects the production and class switching of Abs against nonviral antigens. The mA3−/− and WT B6 mice were immunized with DNP-OVA, a T-cell-dependent antigen, or DNP-Ficoll, a T-cell-independent antigen, and their anti-DNP Ab titers in sera were analyzed. As shown in Fig. 2D, mA3−/− mice responded to both antigens with specific IgM and IgG levels that were not different from those in the WT B6 mice. Thus, it is unlikely that APOBEC3 is directly required for the generation of antigen-specific B-cell repertoires and/or the differentiation of B cells into Ab-producing cells.
Efficient production of FV-neutralizing Abs from mA3b-lacking B cells in the presence of viral-antigen-primed CD4+ T cells.
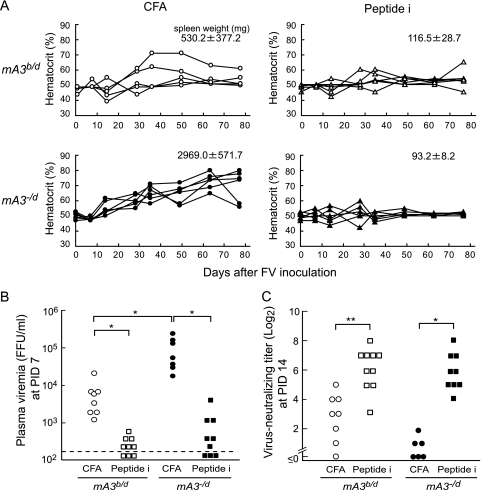
We next asked if the mA3b-lacking B cells are selectively impaired in the recognition of FV antigens and/or differentiation into plasma cells capable of secreting virus-specific Abs. Our previous studies showed that a single immunization with an 18-mer peptide (peptide i) harboring a single CD4+ T-cell epitope identified within the F-MuLV env gene product confers protective immunity to FV infection in susceptible H2a/b mice (17, 27). To examine if the presence of the resistance-associated mA3b allele is an absolute requirement for the production of FV-neutralizing Abs, F1 hybrids between the mA3−/− or WT B6 and A/WySn mice that possessed the mA3−/d or mA3b/d genotype, respectively, were generated and immunized with peptide i, and the production of FV-neutralizing Abs and disease development following FV infection were analyzed. Consistent with our previous findings (49), nonimmunized mA3−/d mice exhibited higher hematocrit values, sustained after FV infection, and higher spleen weights at PID 77 than control mA3b/d mice (Fig. 3 A), confirming that the B6-derived APOBEC3 can partially restrict FV-induced disease development. However, when the mA3−/d mice were immunized with peptide i prior to infection, FV-induced pathologies became barely detectable. Concomitantly, the levels of plasma viremia at PID 7 in the peptide-immunized mA3−/d mice were very low and were comparable to those observed in immunized mA3b/d mice, although the levels of viremia in nonimmunized mA3−/d mice were much higher than those in the nonimmunized WT mice (Fig. 3B). Of note, virus-neutralizing Ab titers at PID 14 in the immunized mA3−/d mice were as high as those in immunized mA3b/d mice (Fig. 3C). As the presence of virus-neutralizing Abs is a prerequisite for the prevention of FV-induced-disease development (4, 17), these results indicate that B cells lacking the resistance-associated mA3b allele are nevertheless able to produce levels of FV-neutralizing Abs that are sufficient to prevent the development of virus-induced pathologies in the presence of CD4+ T cells primed with the viral antigen. Thus, mouse APOBEC3 does not appear to directly control the amounts and/or specificities of FV-neutralizing Abs, and the B-cell repertoire responsible for the recognition of FV-neutralizing epitopes develops in the absence of mA3b.
FIG. 3.
Virus-neutralizing Ab responses in (B6 × A/WySn)F1 mice possessing or lacking the mA3b allele. (B6-mA3b/b × A/WySn)F1 (mA3b/d) and (B6-mA3−/− × A/WySn)F1 (mA3−/d) mice were immunized with 10 μg of peptide i emulsified with an equal volume of CFA or with CFA alone as a control, followed by infection with 1,500 SFFU FV. (A) Their hematocrit values were monitored following FV infection, and spleen weights (means ± SEM) were measured at PID 77. Each data point shows an actual hematocrit value detected from an individual mouse. (B and C) Plasma viral loads at PID 7 (B) and virus-neutralizing Ab titers of IgG plus IgM classes in the sera at PID 14 (C) were determined for each experimental group of mice. Each dot represents the actual value obtained from an individual mouse. The detection limit of plasma viremia was 200 FFU/ml. *, P < 0.05; **, P < 0.005 (by t test).
Reduced production of neutralizing Abs in the mA3b-deficient mice upon F-MuLV infection.
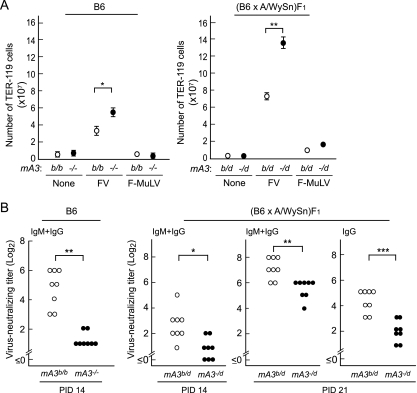
Infection with the FV complex induces the growth and terminal differentiation of erythroid progenitor cells in mice possessing the Fv2s allele. Therefore, it is possible that the skewed differentiation of myeloid cells into the erythroid compartment and/or physical derangements of cell-to-cell interactions in the spleen and bone marrow may impede the immune cell cooperation that is required for the production of virus-neutralizing Abs. In fact, due to the expression of SFFV gp55, the numbers of TER-119+ erythroid cells in the spleen were massively increased in the Fv2r/s (B6 × A/WySn)F1 mice (Fig. 3A and 4 A). To eliminate the possible effect of erythroid cell proliferation on anti-FV immune responses, we utilized Fv2r/r B6 mice and infection with SFFV-free F-MuLV. Infection of B6 mice with FV complex induced a slight increase in the number of TER-119+ erythroid cells, and this was significantly exacerbated in the absence of the mA3b (Fig. 4A). Similar leakiness of the Fv2-associated resistance to FV-induced erythroid progenitor cell proliferation has been observed in B6 mice lacking T cells (11). On the other hand, infection with F-MuLV alone did not induce the growth of erythroid progenitor cells in either parental Fv2r/r or F1 hybrid Fv2r/s mice regardless of the presence or absence of the mA3b allele (Fig. 4A). Nevertheless, when infected with this nonpathogenic F-MuLV, the mice lacking the mA3b allele exhibited significantly reduced production of neutralizing Abs compared with control mice harboring the mA3b allele (Fig. 4B). Of note, the production of the IgG class of virus-neutralizing Abs was markedly delayed in the FV-susceptible (B6 × A/WySn)F1 mice in the absence of the mA3b allele, suggesting that a lack of the mA3b allele influences not only the production but also the class switching of virus-neutralizing Abs in the absence of splenomegaly (Fig. 4B). These results indicate that the reduced production of virus-neutralizing Abs observed in the mA3b-lacking mice is not due to the derangement of immune responses secondary to the exuberant growth of erythroid cells.
FIG. 4.
Reduced levels of neutralizing Abs in mice lacking the mA3b allele following infection with nonpathogenic F-MuLV. (A) The mA3−/− and the WT mA3b/b B6 mice were infected with FV (10,000 SFFU) or F-MuLV (10,000 FFU), and (B6-mA3−/− × A/WySn)F1 (mA3−/d) and the control (mA3b/d) mice were infected with 300 units of each virus. The numbers of TER-119+ erythroid cells in the spleen at PID 10 were determined by fluorescence-activated cell sorter (FACS) analysis. Each dot represents the mean number of TER-119+ cells calculated with 5 to 7 mice, and error bars indicate SEM. (B) Virus-neutralizing Ab titers following F-MuLV infection. Ab titers were analyzed at PID 14 in the B6 mice, while the neutralizing titers in the F1 hybrid mice were compared at PIDs 14 and 21. Titers of IgG Abs at PID 21 were determined by treating each serum with 2-mercaptoethanol. Each dot represents the actual Ab titer obtained from an individual mouse. *, P < 0.05; **, P < 0.005; ***, P < 0.0005 (by t test).
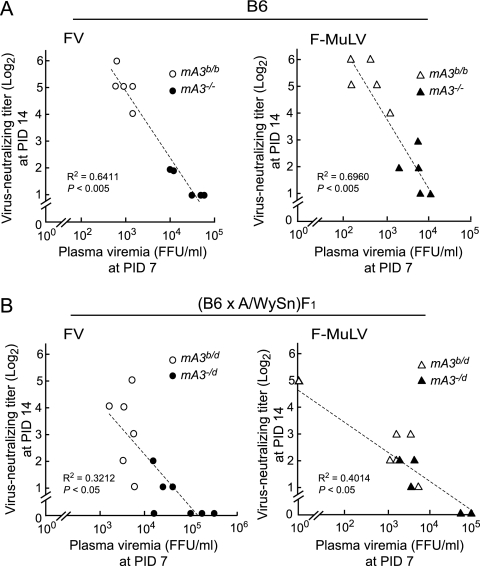
Inverse correlations between levels of viremia at PID 7 and virus-neutralizing Ab titers at PID 14.
As infection with F-MuLV alone in the absence of splenomegaly caused delayed production of virus-neutralizing Abs in mice lacking the mA3b allele, we next explored if the replication of F-MuLV alone directly deranges the immune responses. Interestingly, when levels of viremia and titers of virus-neutralizing Abs were compared, we found a strong inverse correlation between viral loads at PID 7 and virus-neutralizing Ab titers at PID 14 in B6 mice possessing or lacking the mA3b allele, regardless of whether the virus inoculated was FV complex or F-MuLV alone (Fig. 5 A). Similar results were also observed in (B6 × A/WySn)F1 mice (Fig. 5B). These results suggest that effective early restriction of viral replication in the presence of B6-derived APOBEC3 might lead to a later improvement in virus-neutralizing Ab production. This notion is also consistent with the results shown in Fig. 3, where early control of viremia by peptide immunization resulted in higher titers of neutralizing Abs in the absence of the mA3b. In other words, higher levels of neutralizing Abs might result from, rather than cause, the restriction of F-MuLV replication in the presence of the mA3b allele.
FIG. 5.
Inverse correlations between plasma viral loads at PID 7 and neutralizing Ab titers at PID 14. (A) mA3−/− and WT mA3b/b B6 mice were infected with 10,000 units of either FV or F-MuLV. (B) mA3−/d and control mA3b/d F1 hybrid mice were infected with 300 units of either virus. Each dot represents the actual level of plasma viremia and neutralizing Ab titer observed in an individual mouse.
Higher viral loads in the absence of B6-derived APOBEC3 correlate with altered B-cell phenotypes.
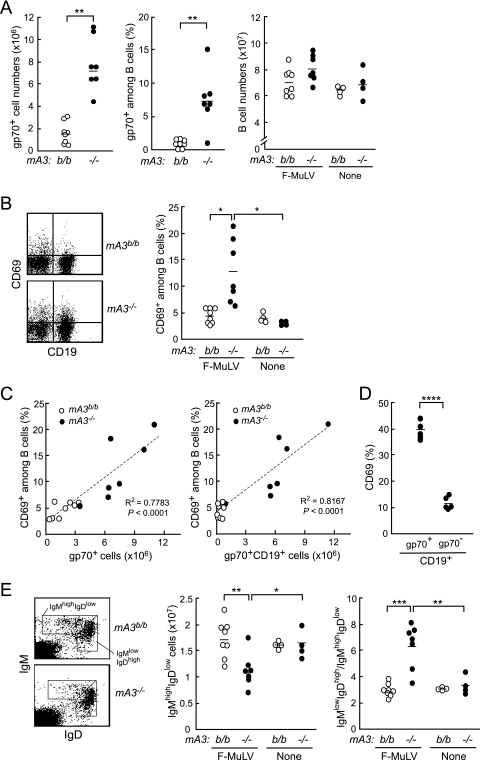
To evaluate the possible effects of F-MuLV infection on B-cell functions, we next analyzed possible alterations in the numbers and phenotypes of B cells in the spleens of mA3−/− B6 mice upon F-MuLV infection. The numbers of virus-infected cells expressing the F-MuLV gp70 were remarkably higher in the mA3−/− mice than in the WT counterparts at 10 days after F-MuLV infection (Fig. 6 A), in agreement with previous data showing increased numbers of virus-producing cells in the mA3b-lacking mice (49). Furthermore, we found significantly higher percentages of gp70+ cells among splenic B cells in the mA3−/− mice than in the WT mice; around 10% of the splenic B cells from mA3−/− mice were gp70 positive, while very small fractions of B cells were gp70+ in the WT mice (Fig. 6A). The total numbers of B cells detected as CD19+ cells showed no significant differences between the mA3−/− and control B6 mice both before and at 10 days after F-MuLV infection (Fig. 6A). We next analyzed the expression of an activation marker on B cells and proportions of B-cell subpopulations in the spleen. Percentages of CD69-positive cells among splenic B cells were significantly higher in the mA3b-deficient mice than in the WT mice after F-MuLV infection (Fig. 6B). Of note, the percentages of CD69+ cells among splenic B cells directly correlated with the numbers of F-MuLV-infected splenic cells or of infected B cells, and the percentages of CD69+ cells were higher among infected B cells than among gp70-negative B cells in mA3−/− mice (Fig. 6C and D), indicating that F-MuLV-infected B cells are preferentially activated. We further found that the absolute numbers of IgMhigh IgDlow transitional B cells decreased, whereas the ratios of IgMlow IgDhigh mature cells to IgMhigh IgDlow transitional cells increased, in the mA3−/− mice following F-MuLV infection (Fig. 6E). Importantly, there were no significant changes in the numbers of B-cell populations in the WT mice upon F-MuLV infection (Fig. 6E). We also examined if F-MuLV infection influences B-lineage cell populations in the bone marrow, but there were no significant changes regardless of the mA3 genotypes (data not shown). Taken together, the above data indicate that the rate of F-MuLV infection is directly associated with the levels of B-cell activation and proportions of B-cell subpopulations in the spleen, which may ultimately affect the production of virus-neutralizing Abs.
FIG. 6.
Changes in phenotypes of peripheral B cells in the mA3−/− B6 mice following F-MuLV infection. Spleen cells were prepared from mA3−/− or WT B6 mice at 10 days after F-MuLV infection (10,000 SFFU). (A) Comparisons of the numbers of CD19+ B cells and gp70+ cells and the percentages of gp70+ cells among B cells in the spleen between the mA3−/− and WT mice. (B) CD69 expression on the surfaces of splenic B cells. A representative pattern of CD69 expression on CD19+ B cells in the spleen (left) and the percentages of CD69+ cells among B cells before and 10 days after F-MuLV infection (right) are shown. (C) Correlations between the percentages of CD69+ cells among B cells and the numbers of gp70+ splenic cells or gp70+ splenic B cells at PID 10. (D) Percentages of CD69+ cells among gp70-positive or gp70-negative splenic B cells in the mA3−/− mice at PID 10. (E) Cell surface expression of IgM and IgD on splenocytes. Representative dot plots (left), the numbers of IgMhigh IgDlow cells (middle), and the ratios of IgMlow IgDhigh cells to IgMhigh IgDlow cells (right) are shown. In the graphs in panels A, B, D, and E, each dot represents the actual value for the indicated cell population in a mouse, and horizontal bars represent the mean values for each group. *, P < 0.05; **, P < 0.005; ***, P = 0.0001; ****, P < 0.00005 (by t test).
Polymorphism at the BAFF-R locus influences the persistence of viremia.
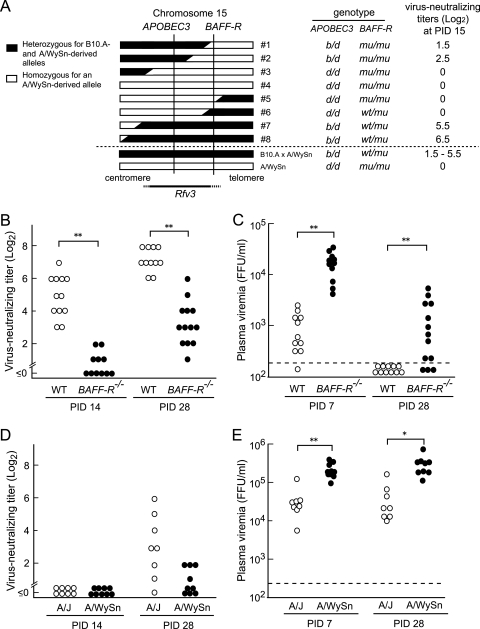
Although the mA3−/− B6 mice showed higher levels of viremia than the WT mice at PID 7, they had cleared viremia by 4 weeks postinfection (Fig. 1B). This is apparently inconsistent with the previously observed phenotype of the Rfv3s/s mice, which continued to be viremic at >30 days following FV inoculation (5, 12). These observations led us to postulate that another locus within the mapped region on chromosome 15 might affect the persistence of viremia. In fact, the previous studies for the genetic mapping of the Rfv3 locus were performed using A/WySn mice or their progeny obtained by crossing them with B10.A mice (5, 12, 16). Strain A/WySn is known to possess a natural mutation in the gene encoding BAFF-R, and it should be noted that the BAFF-R gene is located very close to the APOBEC3 locus on chromosome 15 (16, 29). Therefore, we first reexamined our data on the genetic mapping of Rfv3 performed using (B10.A × A/WySn)F1 × A/WySn backcross mice (16). Among the mice that possessed a chromosomal recombination within or near the putative Rfv3 locus, two mice (mice 1 and 2) in which both alleles of BAFF-R were derived from A/WySn mice (BAFF-Rmu) showed lower titers of virus-neutralizing Abs upon FV infection than those (mice 7 and 8) harboring a single allele of the B6-derived, wild-type BAFF-R (BAFF-Rwt/mu), even though all four mice were heterozygous for APOBEC3 (mA3b/d) (Fig. 7 A). To evaluate the possible effect of BAFF-R genotypes on virus-neutralizing Ab production more decisively, we next used B6 mice harboring the disrupted BAFF-R gene and analyzed their virus-neutralizing Ab titers and plasma viral loads following FV infection. As expected, the BAFF-R−/− B6 mice showed lower titers of virus-neutralizing Abs at PID 14 and higher levels of plasma viremia at PID 7 than the WT counterparts (Fig. 7B and C) despite their mA3b/b genotype. More importantly, unlike the mA3−/−, BAFF-Rwt/wt mice, which had cleared viremia by PID 28 (Fig. 1B), 10 of the 13 FV-infected BAFF-R−/− mice were still viremic even at 28 days postinfection, while all 11 of the infected WT mice had cleared viremia (P = 0.00015, Fisher's exact test). Although BAFF-R−/− B6 mice possessed virus-neutralizing Abs at PID 28, their titers were significantly lower than those in the WT B6 mice and comparable to the levels of neutralizing titers in B6-mA3−/− mice at PID 21 (Fig. 1A and 7B). These results from the analysis using the gene-disrupted B6 mice indicate that the polymorphism at the BAFF-R locus, rather than that at APOBEC3, better explains the persistence of viremia at later stages of FV infection, the originally described Rfv3 phenotype.
FIG. 7.
Effects of polymorphisms at BAFF-R on FV-neutralizing Ab production and plasma viremia. (A) Linkage mapping of the Rfv3 gene by using (B10.A × A/WySn) × A/WySn backcross mice (16). The genotypes of APOBEC3 (d, A/WySn derived; b, B10.A derived) and BAFF-R (mu, A/WySn derived; wt, B10.A derived) for eight mice that possessed a recombination at the indicated region in chromosome 15 were determined by genome sequencing and PCR-based analyses, respectively,. The neutralizing Ab titers of these mice infected with 150 SFFU FV are shown at the right. (B and C) BAFF-R−/− and WT B6 mice were infected with 10,000 SFFU FV, and their plasma levels of viremia and virus-neutralizing Ab titers were analyzed by the same methods as described for Fig. 1. (D and E) A/WySn and A/J mice were infected with 1,500 SFFU FV, and the neutralizing Ab titers at PID 14 and PID 28 and plasma viral loads at PID 7 and PID 28 were analyzed. *, P < 0.005; **, P < 0.0005 (by t test).
We also analyzed the levels of plasma viremia and neutralizing Ab titers in A/J mice possessing the wild-type BAFF-R gene and compared them with those in BAFF-R-mutated A/WySn mice. A/J mice showed significantly lower viral loads both at PID 7 and PID 28 and higher neutralizing Ab titers at PID 28 than A/WySn mice (Fig. 7D), although both A strains continued to be viremic at PID 28, probably due to their lack of the mA3b allele and resistant MHC haplotype, H2b. In fact, it has been shown that the duration of viremia is prolonged (6) and Ab responses are delayed (28) in H2a mice in comparison with H2b mice even in the Rfv3r/s (B10 × A)F1 background. These results, taken together, show that, regardless of H2 haplotypes, a lack of the wild-type BAFF-R resulted in higher levels of viremia at PID 28.
Changes in B-cell phenotypes in BAFF-R−/− B6 mice upon F-MuLV infection.
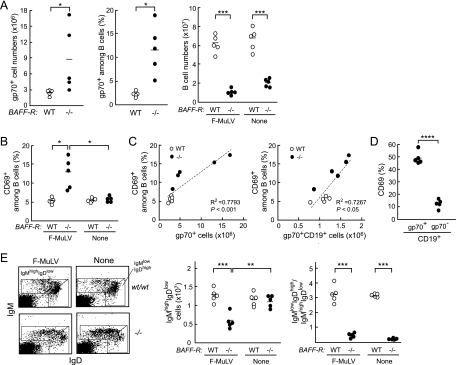
We next asked if the BAFF-R−/− B6 mice, like mA3−/− B6 mice, show alterations in activation and maturation phenotypes of B cells in the spleen after F-MuLV infection. As expected, both the numbers of gp70+ cells in the spleen and the percentages of gp70+ cells among splenic B cells were significantly higher in the BAFF-R−/− B6 mice at PID 10 than in the WT mice (Fig. 8 A). Consistent with previous reports (1, 39, 51), both absolute numbers of mature B cells and ratios of IgMlow IgDhigh mature cells to IgMhigh IgDlow transitional cells were remarkably lower in the spleens of BAFF-R−/− B6 mice than in those of WT mice irrespective of F-MuLV infection (Fig. 8A and E). Following F-MuLV infection, the numbers of IgMhigh IgDlow transitional B cells significantly decreased in comparison with those prior to infection in the BAFF-R−/− mice (Fig. 8E), as was observed in the mA3−/− mice (Fig. 6E). Furthermore, the percentages of CD69+ cells among splenic B cells in the BAFF-R−/− mice were markedly higher at 10 days after F-MuLV infection than the percentages of those in the WT mice, and strong correlations between the percentages of CD69+ cells among splenic B cells and the numbers of infected splenic cells or of infected splenic B cells were observed (Fig. 8B and C). In addition, higher percentages of gp70-positive B cells than of gp70-negative B cells were CD69 positive (Fig. 8D). These alterations in numbers and phenotypes of splenic B cells upon F-MuLV infection in the BAFF-R−/− mice were strikingly similar to those observed in the mA3−/− B6 mice (Fig. 6), indicating that higher viral loads developed either in the absence of mA3b or in the absence of wild-type BAFF-R commonly result in the altered phenotypes of splenic B cells.
FIG. 8.
Changes in phenotypes of peripheral B cells in the BAFF-R−/− B6 mice following F-MuLV infection. Spleen cells were prepared from BAFF-R−/− or WT B6 mice at 10 days after F-MuLV infection (10,000 SFFU). (A) Comparisons of the numbers of CD19+ B cells and gp70+ cells and percentages of gp70+ cells among B cells in the spleen between the BAFF-R−/− and WT mice. (B) Percentages of CD69+ cells among B cells before and 10 days after F-MuLV infection. (C) Correlations between the percentages of CD69+ cells among splenic B cells and the numbers of gp70+ splenic cells (left) or gp70+ splenic B cells (right) at PID 10. (D) Percentages of CD69+ cells among gp70-positive or gp70-negative splenic B cells at PID 10 in the BAFF-R−/− mice. (E) Cell surface expression of IgM and IgD on splenocytes. Representative dot plots (left), the numbers of IgMhigh IgDlow cells (middle), and the ratios of IgMlow IgDhigh cells to IgMhigh IgDlow cells (right) are shown. In the graphs in panels A, B, D, and E, each dot represents the actual value for the indicated cell population in a mouse, and horizontal bars represent the mean values for each group. *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.00005 (by t test).
DISCUSSION
Infections with poorly cytopathic or noncytopathic viruses, including lymphocytic choriomeningitis virus (LCMV), hepatitis C virus, and human immunodeficiency virus (HIV), typically result in delayed formation of protective neutralizing Abs (3, 7, 42), while acutely cytopathic viruses are generally controlled by early production of virus-neutralizing Abs (52). Given that passive transfer of neutralizing Abs provides protection against chimeric simian-human immunodeficiency virus (SHIV) infection in macaque models (2, 13), understanding the mechanisms by which the production of neutralizing Abs is delayed in noncytopathic viral infections is important when considering successful vaccines to prevent and regulate persistent human viral infections, including that of HIV.
Friend virus provides a useful experimental model of persistent infection with a noncytopathic virus. We and others have shown that the neutralizing Ab responses are important for both natural resistance to FV infection and effective vaccine-induced protection against FV (4, 8, 17, 24). The present study has demonstrated that the polymorphisms at the host loci APOBEC3 and BAFF-R, each of which exhibits totally different physiological functions by encoding an intracellular cytidine deaminase or B-cell surface receptor for a survival factor, respectively, nevertheless affect the efficacies of virus-specific Ab responses against the retrovirus through strikingly similar mechanisms. We previously showed that polymorphisms at the APOBEC3 locus directly influence both F-MuLV replication in vitro and the development of FV-induced pathogenesis in vivo; the APOBEC3 isoform highly expressed in the FV-resistant B6 strain of mice strongly inhibited virus replication and the development of FV-induced erythroid cell expansion (49). In the present study, we initially focused on the relationships between the APOBEC3 genotypes and the kinetics of the production of virus-neutralizing Abs. Our findings indicated that the presence of the B6-derived mA3b allele is associated with rapid induction and class switching of F-MuLV-neutralizing Abs. As for the mechanisms by which the polymorphic APOBEC3 affects neutralizing Ab production, FV-induced growth of erythroid progenitor cells, which is more exuberant in the absence of the mA3b allele, is unrelated to reduced Ab production, since mice lacking the mA3b allele exhibited low neutralizing titers even upon infection with nonpathogenic F-MuLV (Fig. 4). Further, it is also unlikely that APOBEC3, as a DNA mutator, directly modifies B-cell repertoires, since we have found unaffected production and class switching of hapten-specific Abs following immunization with the T-independent and T-dependent forms of the antigen even in mA3b-defficient B6 mice (Fig. 2). We also detected the early production of virus-neutralizing Abs from the mA3b-lacking B cells upon priming of T helper cells (Fig. 3).
Intriguingly, we found a strong inverse correlation between the plasma viral loads at PID 7 and titers of neutralizing Abs at 2 weeks postinfection when both mA3b-possessing and -lacking mice were analyzed as a group upon inoculation with either FV or F-MuLV (Fig. 5). The overall inverse correlation between the plasma levels of viremia at PID 7 and neutralizing Ab titers at PID 14 is not caused by the possible blockage of otherwise detectable neutralizing Abs by circulating viral particles, as serum samples were heat inactivated prior to the Ab assays to reduce the remaining infectivity due to viremia, if there was any, and neutralizing Abs were detectable even in the presence of high levels of viremia. In fact, in BAFF-R−/− B6 and A/J mice, significant titers of neutralizing Abs, comparable to the levels of those detected in WT B6 mice at PID 14, were detected in the presence of levels of viremia that were as high as or even higher than those in mA3−/− B6 mice at PID 7 (Fig. 7). Therefore, the possible presence of viremia does not explain the lack of detectable neutralizing titers at PID 14. Thus, these findings suggest that the levels of viral replication soon after FV infection affect the subsequent production of virus-specific Abs, rather than that virus-neutralizing Abs restrict the replication of the virus in the early phase. These notions are also consistent with the effect of T-helper-cell priming on the early production of virus-neutralizing Abs in the absence of mA3b (Fig. 3), as the numbers of virus-producing cells were drastically decreased 7 days after FV infection (27), even in the absence of B cells (17), in mice immunized with peptide i. Although further experiments are required, F-MuLV infection alone appears to impair virus-specific B-cell immune responses during the very early phase, which in turn results in the delayed and reduced production of virus-neutralizing Abs. In this context, in HIV-infected viremic individuals, both the cellular and humoral arms of the immune responses are unable to control the virus infection (10), and numerous studies have shown that B-cell dysfunction represents a central feature of HIV type 1 (HIV-1) infection (18, 30). The defective CD4+ T-cell help may account for the observed B-cell abnormalities in HIV-1-infected individuals (54); however, since impaired B-cell functions have been observed early during HIV-1 infection, preceding the functional defects in CD4+ T-cell activities (25, 50), the above HIV-induced B-cell dysfunction might be intrinsic. Infections with other viruses of a poorly cytopathic or noncytopathic nature, including hepatitis C virus and LCMV, also result in functional defects of B cells (3, 7, 14, 42).
As a possible link between early F-MuLV viremia and subsequent B-cell dysfunction, we found increased proportions of cells expressing the activation marker CD69 among splenic B cells in the mA3−/− mice compared to the WT mice after infection (Fig. 6). Further, the proportions of CD69-positive cells were in correlation with the numbers of virus-infected cells in the spleen. In HIV-infected individuals without long-term highly active antiretroviral therapy (HAART), a wide range of B-cell alterations, including increased polyclonal activation and enhanced expression of activation markers, have also been observed (7, 22, 23). Likewise, F-MuLV may directly induce hyperactivation and dysfunction of B cells. In addition, we also found increased ratios of mature to transitional B cells in the spleens of the APOBEC3-deficient B6 mice following F-MuLV infection. Transitional B cells, following F-MuLV infection, may rapidly differentiate into mature cells, undergo apoptosis, or change their location within the lymphoid organs. In this regard, a similar reduction in the number of transitional B cells was also observed upon F-MuLV infection in BAFF-R−/− mice (Fig. 8E), in which peripheral maturation of B cells is severely hampered (1, 20, 39, 51). Thus, it is more likely that transitional B cells are not differentiating into mature cells but are probably undergoing apoptosis in FV-infected mice. Indeed, HIV-infected individuals also exhibit abnormalities in B-cell subpopulations in their peripheral blood, including an increase in immature/transitional CD10+ B cells and expansion by B cells with the CD27-negative exhausted phenotype (22, 31). Decreased survival of B cells has also been reported in both HIV-1 and simian immunodeficiency virus (SIV) infections (32, 38, 53). These abnormal phenotypes of B cells are likely to contribute to various facets of B-cell dysfunctions observed in several virus infections, including those currently demonstrated in F-MuLV infection. Consistent with this, earlier polyclonal hypergammaglobulinemia upon LCMV infection was correlated with delayed initiation of virus-neutralizing Ab responses (40). As B cells are definitively infected with F-MuLV in the absence of the mA3b allele (Fig. 6) and mouse APOBEC3 is expressed at higher levels in B cells than in T cells (Fig. 2), it is tempting to speculate that F-MuLV infection of B cells may directly impair their differentiation into Ab-secreting cells and/or the survival of antigen-stimulated peripheral B cells in the absence of the resistant APOBEC3 genotype.
In addition to the effects of the APOBEC3 polymorphisms on Ab responses, we have also shown here that the polymorphisms at the BAFF-R gene located close to the APOBEC3 locus on chromosome 15 influence the production of neutralizing Abs and the levels of plasma viremia in FV-infected mice. Among the progeny of (B10.A × A/WySn)F1 mice backcrossed to A/WySn mice, the ones harboring only the A/WySn-derived BAFF-R mutant allele showed lower titers of virus-neutralizing Abs than those with a B10.A-derived allele. More importantly, most of the B6 mice lacking the functional BAFF-R allele possessed very low or undetectable levels of virus-neutralizing Abs at PID 14 and remained viremic at 28 days after infection with FV (Fig. 7); the latter phenotype differs from that of the mA3−/− mice and mimics the susceptible Rfv3 phenotype originally observed in A/WySn mice and one-half of the (B10.A × A/WySn) × A/WySn backcross mice (5). In fact, in comparison with A/J mice that possess the wild-type BAFF-R gene, BAFF-R-mutant A/WySn mice showed extreme delays in neutralizing Ab production and much higher levels of viremia at PID 28 (Fig. 7). Thus, it appears that the Rfv3 phenotypes defined by earlier neutralizing Ab production result from polymorphisms at both the BAFF-R and APOBEC3 loci; on the other hand, the Rfv3 phenotypes defined by persistent high-level viremia are predominantly influenced by the BAFF-R genotype (Table 1 ). It should be noted that the product of the mutant BAFF-R allele derived from A/WySn mice can interfere with the functions of the wild-type BAFF-R, and thus BAFF-Rmu and WT BAFF-R alleles are codominant (20, 39). Therefore, although (B6-mA3−/− × A/WySn)F1 mice showed viremia in the absence of the mA3b allele, this was observed in the condition of a reduced level of functional BAFF-R.
TABLE 1.
Genotypes of FV resistance loci and Rfv3-related phenotypes observed in the present study in different strains of mice
| Mouse straina | Genotype of: |
Viremiab (FFU/ml) (SEM) at PID: |
No of mice with virus-neutralizing Absc/total no. tested at PID: |
|||||
|---|---|---|---|---|---|---|---|---|
| Fv2 | H2 | APOBEC3 | BAFF-R | 7 | 28 | 7 | 28 | |
| C57BL/6 (B6) | r/r | b/b | b/b | wt/wt | 1,341 (319) | <133 | 21/22 | 17/17 |
| B6-mA3−/− | r/r | b/b | −/− | wt/wt | 31,920 (7,200) | <133 | 0/9 | 10/10 |
| B6-BAFF-R−/− | r/r | b/b | b/b | −/− | 16,309 (2,490) | 2,458 (923) | 0/11 | 9/13 |
| A/WySn | s/s | a/a | d/d | mu/mu | 239,978 (38,611) | 353,544 (67,086) | 0/9 | 0/9 |
| A/J | s/s | a/a | d/d | wt/wt | 36,438 (11,585) | 42,000 (16,479) | 0/8 | 5/8 |
| (B6 × A/WySn)F1 | r/s | b/a | b/d | wt/mu | 5,250 (1,729) | 139 (6) | 9/12 | 10/10 |
| (B6-mA3−/− × A/WySn)F1 | r/s | b/a | −/d | wt/mu | 79,157 (25,757) | 7,328 (4,731) | 0/10 | 12/12 |
Homozygous H2b mice were infected with 10,000 SFFU of FV; H2b/a and H2a/a mice were infected with 1,500 SFFU of FV. All mice used are Fv1b/b.
Average levels of plasma viremia calculated with 8 to 22 mice.
Numbers of mice with a neutralizing Ab titer of ≥23.
It is intriguing that the mA3b-deficient mice and mice lacking the WT BAFF-R showed almost identical changes in B-cell subpopulations after F-MuLV infection (Fig. 6 and 8). In fact, IgMhigh IgDlow transitional B cells decreased significantly at PID 10 in both mA3−/− and BAFF-R−/− B6 mice. As APOBEC3 is preferentially expressed in B cells (Fig. 2) and a lack of APOBEC3 resulted in significantly higher proportions of B cells that were infected and activated (Fig. 6), it is reasonable to postulate that in mice possessing the highly functional mA3b allele APOBEC3 restricts F-MuLV replication in B cells and thereby preserves the functionality of B cells that produce virus-neutralizing Abs. However, in the case of BAFF-R, it is unlikely that this receptor is directly involved in the replication of F-MuLV in B cells. As BAFF-R-lacking B cells are short-lived (39, 45), the reduced survival of possibly F-MuLV-infected transitional B cells would result in reduced, rather than increased, levels of F-MuLV replication in BAFF-R−/− mice, even if transitional cells might be the preferential target of F-MuLV infection. Thus, the more likely explanation would be that some functions of mature B cells, most probably Ab production in response to F-MuLV antigens, are required for early containment of F-MuLV replication, and a lack of this early immune restriction in BAFF-R−/− mice results in more-rapid and extensive replication of F-MuLV in B cells, which in turn causes the excessive B-cell activation, disappearance of transitional B cells, and delayed production of neutralizing Abs. In this regard, it should be noted that the levels of plasma viremia were much lower in WT mice than in mice lacking functional BAFF-R even at 7 days after FV inoculation (Fig. 7), when virus-neutralizing Abs were not detectable in the serum (Fig. 1) (17, 27). Further, A/J mice possessed significantly lower levels of viremia than A/WySn mice at PID 7, even though virus-neutralizing Abs were still undetectable a week later, at PID 14 (Fig. 7D and E). These findings indicate that B-cell-associated immune responses may also function by 7 days postinfection in restricting FV replication. Since we have previously detected serum Abs capable of binding to the surfaces of FV-infected cells at PID 7 (17), the nonneutralizing anti-FV Abs may be involved in the restriction of FV replication observed at PID 7. Alternatively, neutralizing Abs locally active in the spleen and bone marrow, titers of which are below the level of detection in the sera, may restrict cell-to-cell FV propagation.
Finally, although levels of viremia were similarly high at PID 7 in the mA3b- and BAFF-R-deficient mice, the titers of virus-neutralizing Abs gradually increased and viremia was cleared by PID 28 in mA3−/− mice in the presence of the wild-type BAFF-R (Fig. 1 and Table 1). Therefore, in addition to similar degrees of B-cell hyperactivation and presumable dysregulation of peripheral maturation of Ab-producing cells secondary to nonrestricted viral replication in the absence of the mA3b or wild-type BAFF-R allele, the lack of functional BAFF-R itself does contribute to the further delays in the control of viremia after PID 7. In fact, when BAFF-R is functional, virus-neutralizing Abs can be produced later even in the absence of mA3b and viremia can be reduced, as observed in A/J mice (Fig. 7). Although the presence or absence of viremia at PID 28 was also affected by genotypes at mA3 and H2, as well as BAFF-R, loci in different strains of mice (Table 1), it is clear that genotypes at the BAFF-R locus alone produce an large difference in levels of viremia at PID 28, while the influence of the mA3 genotypes on viremia at PID 28 can be observed only in the presence of the codominant BAFF-Rmu allele. Thus, it is likely that the Rfv3 phenotypes originally defined by persistence of viremia at PIDs >30 might be largely attributable to the effect of BAFF-R mutation in A/WySn mice.
Acknowledgments
We thank M. Sakamoto for technical assistance and J. B. Dowell for critical reading and correction of the manuscript.
This work was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, including the High-Tech Research Center project, and those from the Ministry of Health, Labor and Welfare of Japan for research on HIV/AIDS.
Footnotes
Published ahead of print on 7 April 2010.
REFERENCES
- 1.Amanna, I. J., J. P. Dingwall, and C. E. Hayes. 2003. Enforced bcl-xL gene expression restored splenic B lymphocyte development in BAFF-R mutant mice. J. Immunol. 170:4593-4600. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 3.Battegay, M., D. Moskophidis, H. Waldner, M. A. Brundler, W. P. Fung-Leung, T. W. Mak, H. Hengartner, and R. M. Zinkernagel. 1993. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J. Immunol. 151:5408-5415. [PubMed] [Google Scholar]
- 4.Chesebro, B., M. Miyazawa, and W. J. Britt. 1990. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu. Rev. Immunol. 8:477-499. [DOI] [PubMed] [Google Scholar]
- 5.Chesebro, B., and K. Wehrly. 1979. Identification of a non-H-2 gene (Rfv-3) influencing recovery from viremia and leukemia induced by Friend virus complex. Proc. Natl. Acad. Sci. U. S. A. 76:425-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro, B., and K. Wehrly. 1976. Studies on the role of the host immune response in recovery from Friend virus leukemia. I. Antiviral and antileukemia cell antibodies. J. Exp. Med. 143:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Milito, A., A. Nilsson, K. Titanji, R. Thorstensson, E. Reizenstein, M. Narita, S. Grutzmeier, A. Sonnerborg, and F. Chiodi. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180-2186. [DOI] [PubMed] [Google Scholar]
- 8.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat. Med. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 9.Doig, D., and B. Chesebro. 1979. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J. Exp. Med. 150:10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 11.Hasenkrug, K. J. 1999. Lymphocyte deficiencies increase susceptibility to Friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J. Virol. 73:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasenkrug, K. J., A. Valenzuela, V. A. Letts, J. Nishio, B. Chesebro, and W. N. Frankel. 1995. Chromosome mapping of Rfv3, a host resistance gene to Friend murine retrovirus. J. Virol. 69:2617-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessell, A. J., P. Poignard, M. Hunter, L. Hangartner, D. M. Tehrani, W. K. Bleeker, P. W. Parren, P. A. Marx, and D. R. Burton. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15:951-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunziker, L., M. Recher, A. J. Macpherson, A. Ciurea, S. Freigang, H. Hengartner, and R. M. Zinkernagel. 2003. Hypergammaglobulinemia and autoantibody induction mechanisms in viral infections. Nat. Immunol. 4:343-349. [DOI] [PubMed] [Google Scholar]
- 15.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1-42. [DOI] [PubMed] [Google Scholar]
- 16.Kanari, Y., M. Clerici, H. Abe, H. Kawabata, D. Trabattoni, S. L. Caputo, F. Mazzotta, H. Fujisawa, A. Niwa, C. Ishihara, Y. A. Takei, and M. Miyazawa. 2005. Genotypes at chromosome 22q12-13 are associated with HIV-1-exposed but uninfected status in Italians. AIDS 19:1015-1024. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata, H., A. Niwa, S. Tsuji-Kawahara, H. Uenishi, N. Iwanami, H. Matsukuma, H. Abe, N. Tabata, H. Matsumura, and M. Miyazawa. 2006. Peptide-induced immune protection of CD8+ T cell-deficient mice against Friend retrovirus-induced disease. Int. Immunol. 18:183-198. [DOI] [PubMed] [Google Scholar]
- 18.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 19.Lelie, P. N., H. W. Reesink, and C. J. Lucas. 1987. Inactivation of 12 viruses by heating steps applied during manufacture of a hepatitis B vaccine. J. Med. Virol. 23:297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lentz, V. M., M. P. Cancro, F. E. Nashold, and C. E. Hayes. 1996. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J. Immunol. 157:598-606. [PubMed] [Google Scholar]
- 21.Lilly, F. 1970. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J. Natl. Cancer Inst. 45:163-169. [PubMed] [Google Scholar]
- 22.Malaspina, A., S. Moir, J. Ho, W. Wang, M. L. Howell, M. A. O'Shea, G. A. Roby, C. A. Rehm, J. M. Mican, T. W. Chun, and A. S. Fauci. 2006. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc. Natl. Acad. Sci. U. S. A. 103:2262-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez-Maza, O., E. Crabb, R. T. Mitsuyasu, J. L. Fahey, and J. V. Giorgi. 1987. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 138:3720-3724. [PubMed] [Google Scholar]
- 24.Messer, R. J., U. Dittmer, K. E. Peterson, and K. J. Hasenkrug. 2004. Essential role for virus-neutralizing antibodies in sterilizing immunity against Friend retrovirus infection. Proc. Natl. Acad. Sci. U. S. A. 101:12260-12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miedema, F., A. J. Petit, F. G. Terpstra, J. K. Schattenkerk, F. de Wolf, B. J. Al, M. Roos, J. M. Lange, S. A. Danner, J. Goudsmit, and P. T. A. Schellekens 1988. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4 + T helper cell depletion occurs. J. Clin. Invest. 82:1908-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikl, M. C., I. N. Watt, M. Lu, W. Reik, S. L. Davies, M. S. Neuberger, and C. Rada. 2005. Mice deficient in APOBEC2 and APOBEC3. Mol. Cell. Biol. 25:7270-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazawa, M., R. Fujisawa, C. Ishihara, Y. A. Takei, T. Shimizu, H. Uenishi, H. Yamagishi, and K. Kuribayashi. 1995. Immunization with a single T helper cell epitope abrogates Friend virus-induced early erythroid proliferation and prevents late leukemia development. J. Immunol. 155:748-758. [PubMed] [Google Scholar]
- 28.Miyazawa, M., J. Nishio, K. Wehrly, and B. Chesebro. 1992. Influence of MHC genes on spontaneous recovery from Friend retrovirus-induced leukemia. J. Immunol. 148:644-647. [PubMed] [Google Scholar]
- 29.Miyazawa, M., S. Tsuji-Kawahara, and Y. Kanari. 2008. Host genetic factors that control immune responses to retrovirus infections. Vaccine 26:2981-2996. [DOI] [PubMed] [Google Scholar]
- 30.Moir, S., and A. S. Fauci. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9:235-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moir, S., J. Ho, A. Malaspina, W. Wang, A. C. DiPoto, M. A. O'Shea, G. Roby, S. Kottilil, J. Arthos, M. A. Proschan, T. W. Chun, and A. S. Fauci. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205:1797-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moir, S., A. Malaspina, O. K. Pickeral, E. T. Donoghue, J. Vasquez, N. J. Miller, S. R. Krishnan, M. A. Planta, J. F. Turney, J. S. Justement, S. Kottilil, M. Dybul, J. M. Mican, C. Kovacs, T. W. Chun, C. E. Birse, and A. S. Fauci. 2004. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J. Exp. Med. 200:587-599. [PubMed] [Google Scholar]
- 33.Muramatsu, M., K. Kinoshita, S. Fagarasan, S. Yamada, Y. Shinkai, and T. Honjo. 2000. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102:553-563. [DOI] [PubMed] [Google Scholar]
- 34.Ney, P. A., and A. D. D'Andrea. 2000. Friend erythroleukemia revisited. Blood 96:3675-3680. [PubMed] [Google Scholar]
- 35.Ng, L. G., A. P. Sutherland, R. Newton, F. Qian, T. G. Cachero, M. L. Scott, J. S. Thompson, J. Wheway, T. Chtanova, J. Groom, I. J. Sutton, C. Xin, S. G. Tangye, S. L. Kalled, F. Mackay, and C. R. Mackay. 2004. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J. Immunol. 173:807-817. [DOI] [PubMed] [Google Scholar]
- 36.Okeoma, C. M., J. Petersen, and S. R. Ross. 2009. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J. Virol. 83:3029-3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persons, D. A., R. F. Paulson, M. R. Loyd, M. T. Herley, S. M. Bodner, A. Bernstein, P. H. Correll, and P. A. Ney. 1999. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat. Genet. 23:159-165. [DOI] [PubMed] [Google Scholar]
- 38.Peruchon, S., N. Chaoul, C. Burelout, B. Delache, P. Brochard, P. Laurent, F. Cognasse, S. Prevot, O. Garraud, R. Le Grand, and Y. Richard. 2009. Tissue-specific B-cell dysfunction and generalized memory B-cell loss during acute SIV infection. PLoS One 4:e5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman, Z. S., S. P. Rao, S. L. Kalled, and T. Manser. 2003. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J. Exp. Med. 198:1157-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Recher, M., K. S. Lang, A. Navarini, L. Hunziker, P. A. Lang, K. Fink, S. Freigang, P. Georgiev, L. Hangartner, R. Zellweger, A. Bergthaler, A. N. Hegazy, B. Eschli, A. Theocharides, L. T. Jeker, D. Merkler, B. Odermatt, M. Hersberger, H. Hengartner, and R. M. Zinkernagel. 2007. Extralymphatic virus sanctuaries as a consequence of potent T-cell activation. Nat. Med. 13:1316-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robertson, M. N., M. Miyazawa, S. Mori, B. Caughey, L. H. Evans, S. F. Hayes, and B. Chesebro. 1991. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and western blotting. J. Virol. Methods 34:255-271. [DOI] [PubMed] [Google Scholar]
- 42.Rosa, D., G. Saletti, E. De Gregorio, F. Zorat, C. Comar, U. D'Oro, S. Nuti, M. Houghton, V. Barnaba, G. Pozzato, and S. Abrignani. 2005. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc. Natl. Acad. Sci. U. S. A. 102:18544-18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santiago, M. L., M. Montano, R. Benitez, R. J. Messer, W. Yonemoto, B. Chesebro, K. J. Hasenkrug, and W. C. Greene. 2008. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science 321:1343-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sasaki, Y., S. Casola, J. L. Kutok, K. Rajewsky, and M. Schmidt-Supprian. 2004. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 173:2245-2252. [DOI] [PubMed] [Google Scholar]
- 45.Shulga-Morskaya, S., M. Dobles, M. E. Walsh, L. G. Ng, F. MacKay, S. P. Rao, S. L. Kalled, and M. L. Scott. 2004. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J. Immunol. 173:2331-2341. [DOI] [PubMed] [Google Scholar]
- 46.Sitbon, M., B. Sola, L. Evans, J. Nishio, S. F. Hayes, K. Nathanson, C. F. Garon, and B. Chesebro. 1986. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell 47:851-859. [DOI] [PubMed] [Google Scholar]
- 47.Sugahara, D., S. Tsuji-Kawahara, and M. Miyazawa. 2004. Identification of a protective CD4+ T-cell epitope in p15gag of Friend murine leukemia virus and role of the MA protein targeting the plasma membrane in immunogenicity. J. Virol. 78:6322-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Super, H. J., K. J. Hasenkrug, S. Simmons, D. M. Brooks, R. Konzek, K. D. Sarge, R. I. Morimoto, N. A. Jenkins, D. J. Gilbert, N. G. Copeland, W. Frankel, and B. Chesebro. 1999. Fine mapping of the Friend retrovirus resistance gene, Rfv3, on mouse chromosome 15. J. Virol. 73:7848-7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeda, E., S. Tsuji-Kawahara, M. Sakamoto, M. A. Langlois, M. S. Neuberger, C. Rada, and M. Miyazawa. 2008. Mouse APOBEC3 restricts Friend leukemia virus infection and pathogenesis in vivo. J. Virol. 82:10998-11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terpstra, F. G., B. J. Al, M. T. Roos, F. De Wolf, J. Goudsmit, P. T. Schellekens, and F. Miedema. 1989. Longitudinal study of leukocyte functions in homosexual men seroconverted for HIV: rapid and persistent loss of B cell function after HIV infection. Eur. J. Immunol. 19:667-673. [DOI] [PubMed] [Google Scholar]
- 51.Thompson, J. S., S. A. Bixler, F. Qian, K. Vora, M. L. Scott, T. G. Cachero, C. Hession, P. Schneider, I. D. Sizing, C. Mullen, K. Strauch, M. Zafari, C. D. Benjamin, J. Tschopp, J. L. Browning, and C. Ambrose. 2001. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science 293:2108-2111. [DOI] [PubMed] [Google Scholar]
- 52.Thomsen, A. R., A. Nansen, C. Andersen, J. Johansen, O. Marker, and J. P. Christensen. 1997. Cooperation of B cells and T cells is required for survival of mice infected with vesicular stomatitis virus. Int. Immunol. 9:1757-1766. [DOI] [PubMed] [Google Scholar]
- 53.Titanji, K., F. Chiodi, R. Bellocco, D. Schepis, L. Osorio, C. Tassandin, G. Tambussi, S. Grutzmeier, L. Lopalco, and A. De Milito. 2005. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS 19:1947-1955. [DOI] [PubMed] [Google Scholar]
- 54.Wolthers, K. C., S. A. Otto, S. M. Lens, R. A. Van Lier, F. Miedema, and L. Meyaard. 1997. Functional B cell abnormalities in HIV type 1 infection: role of CD40L and CD70. AIDS Res. Hum. Retroviruses 13:1023-1029. [DOI] [PubMed] [Google Scholar]