Abstract
A20 possesses both deubiquitinase (DUB) and ubiquitin E3 ligase activities that are required for termination of Toll-like receptor (TLR) signaling leading to NF-κB activation and for blockage of tumor necrosis factor (TNF)-induced cytotoxicity and apoptosis. A20 is induced by the Epstein-Barr virus (EBV) oncoprotein LMP1. However, its dual ubiquitin-editing activities have not been investigated in the context of either EBV infection or IRF7 responses. Both A20 and IRF7 have oncogenic properties. We have recently shown that LMP1 activates IRF7 through K63-linked ubiquitination which requires RIP1 and TRAF6, but how this ubiquitination event is regulated has not been studied. Here, we show that A20 negatively regulates IRF7 transcriptional activity induced by LMP1. Deletion or mutation of A20 C-terminal zinc finger motifs had no effect on the inhibition of IRF7 activity, whereas DUB-deficient truncation or point mutation ablated the ability of A20 to inhibit IRF7. Correspondingly, the A20 N-terminal DUB domain, but not the C-terminal E3 ligase domain, interacts physically with IRF7. Transient expression of A20 reduced K63-linked ubiquitination of IRF7 in vivo, but an in vitro deubiquitination assay with purified constituents shows that IRF7 did not act as a substrate for A20 DUB activity. Moreover, A20 interacts with IRF7 endogenously in latently EBV-infected type 3 Raji cells, in which expression of both A20 and IRF7 is constitutively induced by the considerable level of endogenous LMP1. Knockdown of endogenous A20 in Raji cells by expression of A20 short hairpin RNA (shRNA) vectors increases endogenous IRF7 activity and ubiquitination, as well as the protein level of LMP1, a target of IRF7. Thus, A20 negatively regulates LMP1-stimulated IRF7 ubiquitination and activity in EBV latency, and its DUB activity is indispensable for this function. Finally, we discussed the regulation and function of IRFs in EBV latency.
Interferon (IFN) regulatory factor 7 (IRF7) is the central regulator of type I IFNs, which have 13 subtypes and constitute a highly pleiotropic cytokine family that participates not only in immune modulation but also in oncogenesis, cellular development, and homeostasis. Aberrant production of IFNs is associated with many types of diseases, such as cancers, immune disorders, and multiple sclerosis (8, 10, 21, 34, 45, 48, 60). IFNs exert their functions through induction of hundreds of IFN-inducible genes (ISGs) (48). IRF7 is expressed constitutively at low levels in lymphoid cells and can be induced to high levels in lymphocytes and other cell types by type I IFNs, lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-α), 12-O-tetradecanoylphonol-13-acetate (TPA), or virus infection, or by itself (41). Inactive IRF7 predominantly resides in the cytoplasm but translocates to the nucleus upon phosphorylation induced by microbial nucleic acids which are recognized by endolysosomal Toll-like receptors (TLR3, -7, -8, and -9), RIG-I-like receptors (RLRs), the DNA-dependent activator of IRFs (DAI) (4, 21, 36), the recently identified RNA polymerase III (1, 13), and NOD2 (47). A recent study has also shown that IRF7 is activated by TLR2 in inflammatory monocytes where TLR2 recognizes viral ligands (3).
Of special interest, IRF7 is induced as well as activated by the Epstein-Barr virus (EBV) oncoprotein LMP1, which is a constitutively active member of the TNF receptor (TNFR) superfamily. LMP1 is the only EBV protein that also has oncogenic potential in non-B cells. LMP1 exerts its functions by initiating both antiapoptotic and proliferative growth factor-like signals, which are complex, have diverse outcomes, and share signaling cascades with CD40 and a combination of TNFRI and TNFRII (30). LMP1 signaling is elaborately regulated. For example, the adaptor protein BS69 (25) and signal transducing adaptor protein-2 (STAP2) (26) block LMP1 activation of NF-κB. Also, LMP1 activation of IRF7 is suppressed by a naturally dominant negative IRF5 (40, 70), which is induced in EBV-infected B cells through both TLR7 (35) and LMP1 (16) signaling pathways.
Intriguingly, as with IRF7, the antiapoptotic factor A20 is also induced by LMP1 and therefore is also constitutively expressed in EBV-immortalized B cells (19, 29). Generally, A20 is constitutively expressed in only a few types of cells, including thymocytes, resting peripheral T lymphocytes, and the differentiated monocyte cell line THP-1. In addition to LMP1, A20 can be induced by many stimuli, such as poly(I-C), LPS, TNF-α, and viral infection, in a variety of cell types, such as fibroblasts, B lymphocytes, and NIH 3T3 cells (43). Two recent studies have identified the A20-encoding gene (TNFAIP3) as a tumor suppressor gene in some subtypes of non-Hodgkin lymphomas (22), Hodgkin lymphoma and primary mediastinal B-cell lymphoma in that somatic and clonal biallelic inactivation of TNFAIP3 is detected frequently in these lymphomas (51).
Ubiquitination is one of the most important regulatory mechanisms involved in a variety of biological processes, including immune responses (7). As with phosphorylation, ubiquitination is a reversible and dynamic process which is delicately controlled and adjusted by ubiquitin E3 ligases and deubiquitinating enzymes (DUBs) (7, 12, 15, 28, 46). Among the well-known DUBs, A20 has the ability to deubiquitinate K63-linked ubiquitination of RIP1 (66) and TRAF6 (9). A20 is of particular interest because it has dual functions in the modification of ubiquitination; in addition to the N-terminal OTU domain, which confers its DUB activity, its C-terminal Zn finger domain has E3 ligase activity which catalyzes K48-linked polyubiquitination of RIP in vitro (66). Since TRAF6 and RIP1 are important intermediates in TLR and TNFR signaling, both these ubiquitin-editing activities of A20 are required for termination of TLR signaling leading to IKK activation and blockage of TNF-induced cytotoxicity and apoptosis (9, 65, 66). Recently, it has been shown that A20 requires the ubiquitin E3 ligases Itch and RNF11 for its inhibition of the NF-κB signaling pathway in vivo. These proteins and others may associate with A20 in a ubiquitin-editing complex which assists A20 in determining substrate and Ub linkage specificity in vivo (56, 57). In addition, phosphorylation of A20 by IKKβ enhances its ability to inhibit NF-κB signaling (23).
A20 also interacts with IRF3 kinases NAK/TBK1 and IKKi/IKKɛ (49) and negatively regulates IRF3 transcriptional activity by inhibiting its dimerization, phosphorylation, and DNA binding activity (32). In this case, its ubiquitination-editing function may not be involved. A20, which interacts with TRAF2 and blocks CD40-mediated NF-κB activity, also blocks LMP1 CTAR2-mediated NF-κB and Jun N-terminal protein kinase (JNK) signaling through disruption of the TRAF2/TRADD complex (17, 20) and mediates LMP1 blockage of P53-induced apoptosis (19). However, its ubiquitin-editing functions in the EBV context have not been investigated.
We have presented extensive evidence that LMP1 constitutively induces and activates IRF7 in EBV latency programs and have elucidated many aspects of the mechanisms leading to these important events (24, 38, 73, 74). As an important participator in both immune responses and EBV latency, IRF7 activity must be delicately modulated at different levels in these important physiological events. We have previously identified IRF5, which is also highly expressed in EBV latency 3 as dominant negative mutants (35, 40, 70), and shown that it negatively regulates IRF7 activity in the EBV context (40). Of interest, we have recently shown that ubiquitination mediates LMP1 activation of IRF7 and that RIP1 and TRAF6 are required for the activation (24, 38). However, how this ubiquitination event is regulated is not clear. For this purpose, we focus here on A20, as A20, like IRF7, is also constitutively induced by LMP1 and therefore, together with IRF7 and LMP1, is expressed at high levels in EBV type 3 latency (19, 29). Moreover, A20 is an important DUB that targets different substrates in different biological contexts, such as oncogenesis and immune responses (61, 62). We show here that A20 negatively regulates LMP1-stimulated IRF7 ubiquitination and activity in EBV latency and that its DUB activity is required for this inhibition. As the biological consequences, we show that knockdown of A20 expression results in an increase in endogenous IRF7 ubiquitination and activity as well as in the protein level of its target, LMP1, in P3HR1 cells.
MATERIALS AND METHODS
Cell lines.
Raji cells are a Burkitt's lymphoma B-cell line with type 3 EBV latency. HEK 293 cells are derived from human kidney epithelial cells. 293-TLR3, 293-TLR7, and 293-TLR4/CD14/MD2 stable cell lines were from Invivogen. B lymphocytes were maintained in RPMI 1640 plus 10% fetal bovine serum (FBS) plus antibiotics, and epithelial cells are maintained in Dulbecco's modified Eagle's medium (DMEM) plus 10% FBS plus antibiotics.
Constructs.
Flag-A20 and its mutants were gifts from and have been previously described by Rongtuan Lin, Ingrid Wertz, and Karen O'Rourke (32, 66). Myc-IRF7 and its mutants were constructed by subcloning IRF7 cDNA into pCMV-Tag3 (Stratagene). Two pSuper-shA20 constructs and controls were gifts from Peter Storz; insert sequences were previously described (61). LMP1, IRF7, IFN-α4-Luc, and Ub constructs were described in our previous studies (24, 38).
TLR ligands.
LPS (a ligand for TLR4) from Salmonella enterica serovar Typhimurium and the TLR3 ligand poly(inosine-cytosine) (poly[I-C]) were purchased from Sigma, and the TLR7 ligand Gardiquimod was purchased from Invivogen. Concentrations used were 100 μg/ml for poly(I-C), 10 μg/ml for LPS, and 0.5 μg/ml for Gardiquimod.
Luciferase reporter assays and data analysis.
Cells were transfected with expression plasmids as indicated in the figure legends, together with IFN-α4p-Luc and Renilla luciferase as internal transfection control. Empty vector was used to equalize the total amounts of DNA in all transfections. Eighteen hours later, cells were treated with TLR ligands for 6 h before collection. Luciferase activity was measured with equal amounts (20% of total for each sample) of protein lysates in an Lmax luminometer (Molecular Devices Corp.) with the use of a dual luciferase assay kit (Promega). Results are the averages ± the standard errors (SE) of duplicates for each sample. Results obtained consistently from at least three independent experiments are shown. The ability of the vector controls to activate IFN-α4p-Luc was set to 1.
Immunoprecipitation (IP).
To obtain endogenous Raji cell proteins, cells were lysed in NP-40 lysis buffer (10 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 5 mM dithiothreitol [DTT], 0.2 mM Na orthovanadate, and complete protease inhibitors). Half a milligram of total protein was incubated with 1 μg of mixed A20 antibodies, including goat polyclonal clone A15 (Santa Cruz), mouse monoclonal clone E5-1619 (BD Pharmingen), rabbit polyclonal clone H100 (Santa Cruz), and mouse monoclonal clone 8E8.38 (Santa Cruz) or rabbit and mouse IRF7 antibodies (Santa Cruz) overnight at 4°C. Protein A/G beads (Santa Cruz) were added and incubated for 1 h at 4°C. Precipitates were washed five times with NP-40 buffer.
For transiently expressed proteins, 293 cells in 100-mm dishes were transfected with 1 μg Flag-A20 or its mutants together with 1 μg Myc-IRF7, 0.5 μg LMP1, and 0.5 μg hemagglutinin (HA)-Ub or HA-K63Ub. Other epitope tags were used as indicated in the figures. Two days after transfection, cells were lysed in NP-40 lysis buffer and immunoprecipitated with 1 μg mouse monoclonal anti-FLAG M2 (Sigma), anti-HA F-7 (Santa Cruz), anti-Myc 9E10 (Santa Cruz), or anti-IRF7 G-8 (Santa Cruz). For detection of IRF7, a second IP was performed with anti-IRF7 after denaturing the immunoprecipitated proteins in 0.5% SDS at 95°C for 5 min. Western blot analysis was performed with anti-mouse κ chain-horseradish peroxidase (HRP) secondary antibody (Southern Biotech Inc.).
In vivo deubiquitination assay.
For in vivo deubiquitination assays, 293 cells in six-well plates were transfected with 0.5 μg Myc-K63Ub, 0.5 μg HA-LMP1, 1.0 μg pcDNA3-IRF7, and 0.5 μg Flag-A20 or its mutants with the use of the Effectene transfection reagent (Invitrogen) or Fugene transfection reagent (Roche). Cells were harvested after 36 h. Total proteins were used for IP, and 5% was used for input controls. Lysates for IP were diluted with 1 ml NP-40 buffer, and IP was performed with 2 μg anti-IRF7 G-8 and protein A/G-Sepharose beads overnight. Beads were washed four times in NP-40 buffer and denatured in 50 μl of 1% SDS and then immunoprecipitated a second time with 1 μg anti-IRF7 G-8 and protein A/G beads overnight. Beads were washed four times with NP-40 buffer before Western blotting with anti-Myc.
Raji and P3HR1 cells were transfected with pSuper-shA20 vectors with the use of Amaxa Nucleofector kit V, and transfection efficiency was monitored by cotransfected green fluorescent protein (GFP) plasmids and reached 70%. Two days after transfection, cells were harvested, and 1 mg total protein was subjected to double IP with anti-IRF7 G-8 as described above. For luciferase assay, 2 μg IFN-α4-Luc and 1 μg Renilla luciferase were cotransfected. Dual luciferase assay was performed 2 days later.
RESULTS
A20 negatively regulates IRF7 transcriptional activity.
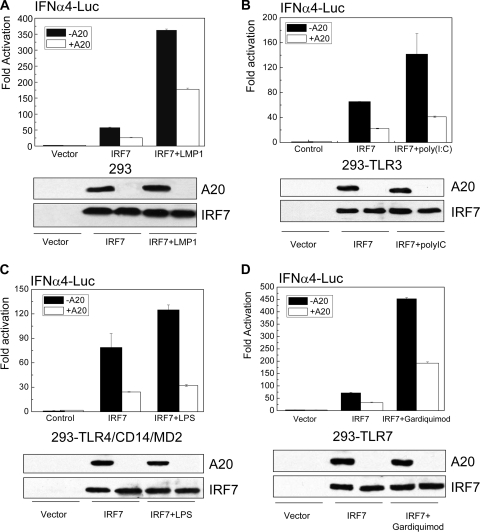
IRF7 is now known to be activated by endolysosomal TLRs, particularly by TLR7, RLRs, DAI, and EBV LMP1 (2, 21, 63). Although TLR4 is believed not to activate IRF7 in vivo, treatment of 293-TLR4/MD2 stable cells with LPS, a ligand for TLR4, did activate IRF7-mediated transcription (18). To explore if A20 can affect IRF7 transcriptional activity stimulated by LMP1, TLR3, TLR4, or TLR7, we first performed promoter-reporter assays with the use of IFN-α4-luciferase (Luc) promoter-reporter construct. Renilla luciferase expression construct was used as the internal control. 293 cells were transfected with IRF7 and LMP1 with or without A20; 293-TLR3 cells were transfected with IRF7 and treated with the TLR3 ligand poly(I-C); 293-TLR4/CD14/MD2 cells were transfected with IRF7 and treated with the TLR4 ligand LPS; and 293-TLR7 cells were transfected with IRF7 and treated with the TLR7 ligand Gardiquimod. Firefly and Renilla luciferase activities were measured with a dual luciferase assay kit (Promega).
As shown in Fig. 1, A20 significantly inhibits IRF7 activity stimulated by all the signaling pathways examined. These pathways use distinct mechanisms and components for IRF7 activation. In LMP1 and TLR7 signaling pathways, TRAF6 but not TRAF3 is required, and RIP1 is also required for LMP1 activation of IRF7, whereas in TLR3 and TLR4 signaling pathways, neither TRAF6 nor RIP1 is required for IRF7 activation, and TRAF3 is required instead of TRAF6. TRAF6 and RIP1 are two known targets for A20 DUB activity. Moreover, our recent studies have shown that IRF7 ubiquitination is required for its activation by LMP1 (24, 38). Thus, it is likely that A20 targets IRF7 itself rather than these signaling components, including RIP1 and TRAF6, for negative regulation of IRF7 activity.
FIG. 1.
A20 inhibits IRF7 transcriptional activity. 293 cells in 24-well plates were transfected with 0.1 μg IRF7, 0.1 μg A20, 0.02 μg LMP1, 50 ng IFN-α4-Luc, and 20 ng Renilla luciferase. Cells were treated with TLR ligands at concentrations indicated in Materials and Methods for 6 h before harvest for measurement of luciferase activity. Results are representative of at least three independent experiments. Results are the averages ± SE of duplicates for each sample. Western blots show the expression levels of transfected IRF7 and A20 in the tested samples. (A) A20 inhibits LMP1-promoted IRF7 activity. (B) A20 inhibits IRF7 activity stimulated by poly(I-C)/TLR3. (C) A20 inhibits IRF7 activity stimulated by LPS/TLR4. (D) A20 inhibits IRF7 activity stimulated by Gardiquimod/TLR7.
A20 and IRF7 associate endogenously in Raji cells.
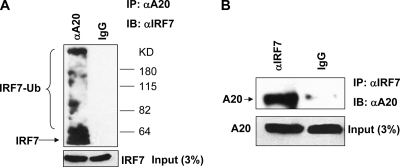
To test our hypothesis that A20 DUB activity targets IRF7, we first checked if endogenous A20 and IRF7 interact in Raji cells, a type 3 latently infected Burkitt's lymphoma cell line that expresses high levels of both A20 and IRF7 due to the considerable levels of LMP1 expressed (33, 64). A mixture of A20 antibodies specifically coimmunoprecipitated A20 with IRF7 protein (Fig. 2A). Interestingly, besides the regular size of IRF7, A20 antibodies also coimmunoprecipitated IRF7 with a pattern of higher molecular weights, which may represent ubiquitinated IRF7, as shown in our recent studies (24, 38), implying that A20 may prefer to interact with ubiquitinated IRF7. Results from reverse IP with IRF7 antibody further confirm the interaction between the proteins (Fig. 2B). These results indicate that endogenous IRF7 and A20 interact in EBV-transformed cells.
FIG. 2.
IRF7 interacts with A20 endogenously in EBV-transformed Raji cells. One milligram of total protein from Raji cells was subjected to IP with A20 or IRF7 antibodies. (A) IP with a mixture of A20 antibodies, and Western blot analysis with the mouse monoclonal IRF7 antibody G-8. (B) IP with a mixture of IRF7 antibodies, and Western blot analysis with the mouse monoclonal A20 antibody E5-1619.
A20 N terminus is responsible for interaction with and regulation of IRF7.
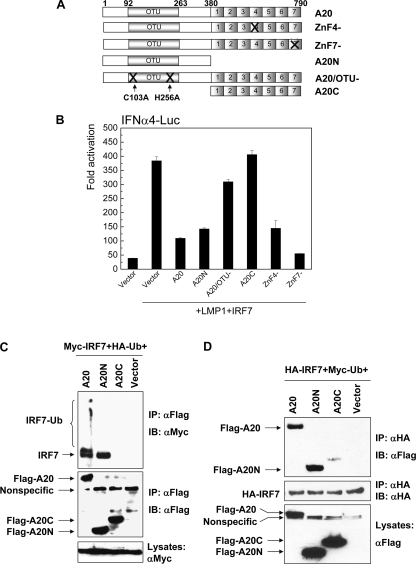
Since the DUB activity of A20 is attributed to its N-terminal OTU domain, we investigated whether the N terminus of A20 is responsible for interaction with and inhibition of IRF7. For this purpose, we used two Flag-A20 truncated mutants, Flag-A20N, which has only the N terminus, and Flag-A20C, which has only the C terminus, and some point mutants, including ZnF4− (mutation in zinc finger 4), ZnF7−, and the DUB-deficient mutant A20/OTU− [A20(C103A/H256A)], for promoter-reporter assays (Fig. 3A). Results show that Flag-A20 and Flag-A20N inhibit LMP1-stimulated IRF7 transcriptional activity, but Flag-A20C did not have detectable effect. In the C-terminal Zn finger domain, ZnF4 confers A20 E3 ligase activity for RIP1 ubiquitination (66), and ZnF7 is required for inhibition of IRF3 (32). However, neither ZnF4− nor ZnF7− impaired A20's ability to inhibit IRF7, and the DUB-deficient point mutant A20/OTU− did not significantly impair A20 inhibition of IRF7 either. These data demonstrate that the N-terminal OTU domain and its DUB activity, but not the C-terminal E3 ligase domain, are required for inhibition of IRF7 (Fig. 3B).
FIG. 3.
The A20 N terminus is responsible for interaction with and regulation of IRF7. (A) Scheme of expression plasmids of A20 and its mutants used in this study. (B) The A20 N terminus and its DUB activity are required for LMP1-stimulated IRF7 activity. Transfection of 293 cells and dual luciferase assay were performed as described in the legend for Fig. 1. (C and D) The A20 N terminus is responsible for interaction with IRF7. 293 cells in six-well plates were transfected with 1 μg Flag-A20 or its mutants, 1 μg Myc-IRF7 or HA-IRF7, and 0.5 μg HA-Ub or Myc-Ub. Reciprocal IP was performed with lysates collected 48 h after transfection. Western blot analysis was performed with corresponding antibodies as indicated.
Correspondingly, reciprocal IP results show that Flag-A20 and Flag-A20N interact with Myc-IRF7 but Flag-A20C did not (Fig. 3C and D). In the sample transfected with Flag-A20, Myc-IRF7, and HA-Ub, Western blotting detects significant ubiquitinated Myc-IRF7 after IP with Flag antibody, further indicating that A20 prefers to interact with ubiquitinated IRF7 (Fig. 3C). This conclusion is also supported by the fact that IRF7 failed to interact with A20 in the absence of cotransfected ubiquitin under our experimental conditions (data not shown). However, these ubiquitinated Myc-IRF7 proteins were not detected in the sample transfected with Flag-A20N instead of Flag-A20. This result may be explained by the fact that in our system, Flag-A20N, in addition to Flag-A20C, is consistently expressed at much higher levels than is Flag-A20 although they are in the same vector, and therefore, ubiquitinated IRF7 may be nondetectable in the presence of the higher level of DUB activity of Flag-A20N. In fact, ubiquitinated IRF7 is not always ready to be simultaneously detected with antibodies for detection of regular IRF7. In most cases, it can be detected only with antibodies for detection of ubiquitination. These results demonstrate that the A20 N terminus, but not the C terminus, is responsible for its interaction with IRF7 and negative regulation of IRF7 activity.
A20 DUB activity is required for reduction of LMP1-stimulated IRF7 ubiquitination.
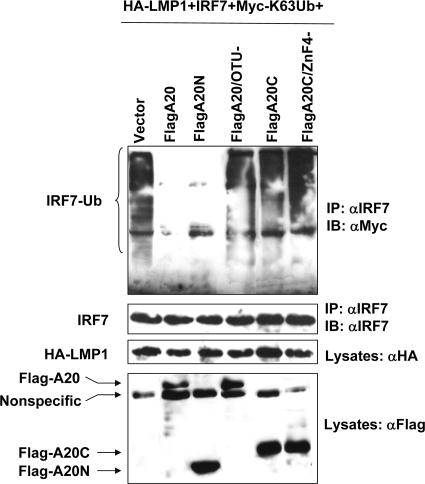
Furthermore, we employed transient expression to check if the A20 DUB domain and its DUB activity are required to regulate IRF7 ubiquitination stimulated by LMP1. We transfected 293 cells with expression plasmids as indicated in Fig. 4. Double IP under denaturing conditions was performed as detailed in Materials and Methods. Results show that, in the presence of Flag-A20 or Flag-A20N, LMP1-stimulated ubiquitination of IRF7 is dramatically reduced, whereas the DUB-deficient mutant Flag-A20/OTU− did not have this effect. Similar to Flag-A20/OTU−, Flag-A20C and its mutant ZnF4− did not have the ability to inhibit IRF7 ubiquitination (Fig. 4). These results confirm that the A20 N terminus and its DUB activity are responsible for inhibition of LMP1-promoted IRF7 ubiquitination.
FIG. 4.
A20 DUB activity is required for reduction of LMP1-stimulated IRF7 ubiquitination. 293 cells in six-well plates were transfected with plasmids as indicated. Cells were harvested 48 h after transfection for double IP with anti-IRF7. Proteins were subjected to Western blot analysis with anti-Myc or anti-IRF7. Cell lysates (5% input) were probed with anti-HA for LMP1 and anti-Flag for A20 and its mutants.
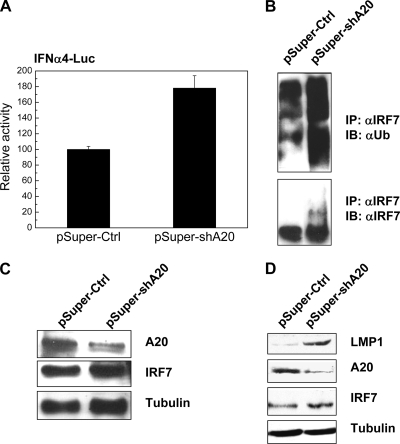
Suppression of A20 level in Raji cells increases endogenous IRF7 ubiquitination and activity.
Since A20 DUB activity is required for inhibition of IRF7, we next checked if A20 affects endogenous IRF7 ubiquitination and activity in EBV latency. We have previously shown that IRF7 is highly induced, ubiquitinated, and activated endogenously by LMP1 in EBV latency 3 (24, 38). Raji cells were transfected with two previously published A20 short hairpin RNA (shRNA) vectors (61), or with vector control. Knockdown efficiency was evaluated by Western blot analysis (Fig. 5C). The cells were then transfected with IFN-α4-Luc and Renilla constructs, and endogenous IRF7 activity was measured 24 h later. Knockdown of expression of A20 results in a significant increase in endogenous IRF7 activity (Fig. 5A). Correspondingly, endogenous IRF7 ubiquitination is significantly enhanced (Fig. 5B). Notably, knockdown of A20 did not result in significant change in the expression level of IRF7 protein (Fig. 5C). These results indicate that, in EBV latency, A20 negatively regulates endogenous IRF7 activity and ubiquitination.
FIG. 5.
Expression of shA20 in Raji cells increases endogenous ubiquitination and activity of IRF7. (A to C). Type 3 latently EBV-infected cells (Raji) were infected with shA20 expression vectors. For luciferase assay, 2 μg IFN-α4-Luc and 1 μg Renilla luciferase were cotransfected. After 2 days, cells were collected for double IP or for luciferase assay. (A) Expression of shA20 (a mix of shA20-1 and shA20-2) increases endogenous IRF7 activity. (B) Expression of shA20 enhances endogenous ubiquitination of IRF7. (C) Endogenous A20 level is reduced in cells expressing shA20. (D) P3HR1 cells were transfected with the A20 shRNA constructs. Forty-eight hours later, cell lysates were used for Western blotting with LMP1 (clone CS1-4, Dako), A20, IRF7, and tubulin antibodies.
To define the biological consequence of negative regulation of IRF7 ubiquitination by A20, we assessed the change in endogenous level of the LMP1 protein in EBV-transformed P3HR1 cells after expression of A20 shRNAs. The P3HR1 cell line was chosen because the genome of this cell line lacks EBNA2, which is the potent inducer of LMP1, and therefore these cells express very low levels of LMP1, which is induced by the low level of endogenous IRF7 (39). Thus, these cells are useful to evaluate the physiological effect of IRF7 on LMP1 in EBV latency (39, 40). P3HR1 cells were transfected with A20 shRNA constructs as described above, and endogenous levels of LMP1, A20, and IRF7 were evaluated. As shown in Fig. 5D, when the endogenous A20 level was reduced by A20 shRNAs, the endogenous level of LMP1 was elevated. These results indicate that A20 can negatively regulate IRF7 induction of LMP1.
Together, these results from endogenous systems show that A20 negatively regulates LMP1-promoted IRF7 ubiquitination and activity and, correspondingly, negatively regulates IRF7 biological function in EBV latency.
DISCUSSION
Regulation of IRF7 transcriptional activity by A20.
Activation of IRF7 is essential for its many functions as a transcription factor. Thus, fine-tuning of its activity at different levels is important for IRF7 to execute its functions in distinct biological contexts. We have shown that IRF5, which was later shown as dominant negative mutants in EBV latency (70), negatively regulates LMP1-promoted IRF7 activity (40). Recently, we have shown that LMP1 promotes K63-linked ubiquitination of IRF7, which mediates its activation by LMP1 (24, 38). In this study, we aimed to study how this ubiquitination event is modulated. We show through several approaches that A20 inhibits the ubiquitination of IRF7 and that its activity is stimulated by LMP1. Analysis with A20 mutants indicates that A20 N-terminal DUB activity is responsible for its effect on IRF7. Thus, A20 is the first identified DUB which regulates IRF7 ubiquitination. However, we have yet to succeed in showing that IRF7 is a substrate for A20 in an in vitro system, probably because A20 requires other cellular proteins for its DUB function. In support of this possibility, recent studies have shown that E3 Itch and the RING finger protein RNF11 are required for A20 termination of NF-κB activation in TNFR and TLR signaling pathways (56, 57).
In RLR signaling pathways, A20 blocks RIG-I-mediated activation of IRF3 (32). In TLR signaling pathways, evidence for A20 blockage of IFN promoter activity is controversial. A20 was shown to strongly inhibit IFN promoter activity stimulated by TLR3 or Sendai or Newcastle disease virus infection (49, 65). However, a later study showed that A20 weakly inhibited ISRE promoter activity stimulated by TLR3 (32), and another study using small interfering RNA (siRNA) screening showed that A20 siRNA had no detectable effect on IFN-α4 promoter activity stimulated by TLR3 signaling in 293-TLR3 stable cells (27). The latter result may be explained by the fact that A20 is expressed only in a limited range of cell types and is almost not detectable in 293 cells, and IRF7, the major activator of the IFN-α4 promoter, is also barely detectable in 293 cells. Therefore, use of A20 siRNA to test A20's effect on IFN-α4 promoter activity stimulated by endogenous IRF7 was not sensitive enough in these cells. Here, using transiently expressed A20 and IRF7, we show that A20 inhibits IRF7 transactivation of the IFN-α4 promoter in response to treatment with TLR3, TLR4, or TLR7 ligand. Furthermore, we for the first time show that A20 modulates IRF7 transcriptional activity stimulated by EBV LMP1.
Regulation of IRF-mediated IFN promoter activity by A20 is likely accomplished through different mechanisms. In TLR signaling, A20 targets TRIF (65) as well as interacts with IKKɛ/TBK1 kinases (49) and therefore blocks IRF3 activation. In RIG-I signaling, A20 was believed to act upstream of IKKɛ/TBK1 to block IRF3 activation (32). In all these cases, A20 DUB activity was not required. Instead, A20 C-terminal zinc finger 7 (ZnF7) is required for the IRF3 inhibition in RIG-I responses (32). In contrast, our studies show that the A20 N-terminal DUB domain and DUB activity, but neither the C-terminal ZnF domain nor E3 ligase activity, are required for inhibition of IRF7. Furthermore, A20 is shown to interact directly with IRF7 and thereby reduce IRF7 ubiquitination stimulated by LMP1. Although we also show that A20 inhibits IRF7 transcriptional activity stimulated by TLR ligands, whether different mechanisms are used needs to be clarified further.
Our results strongly support that A20 directly removes K63 Ub chains from IRF7. On the other hand, A20 blocks NF-κB activation in TLR and TNFR signaling pathways through targeting TRAF6 and RIP for degradation (9, 61, 66). Since TRAF6 and RIP are required for LMP1 activation of IRF7, regulation of stability of these two proteins may also contribute to the regulation of IRF7 ubiquitination by A20. To address this possibility in the future, an IRF7 mutant, which cannot bind to A20 but retains activation activity, will be very helpful, although it may not be able to create it. However, given the fact that the C-terminal E3 ligase activity, which is required for RIP degradation in TLR signaling (9), is not required for A20 inhibition of IRF7, whether TRAF6 and RIP are also targeted by A20 in LMP1 signaling needs to be investigated.
Herpesviruses have evolved multiple strategies to manipulate the cellular ubiquitin system (53-55). For example, herpes simplex virus type 1 (HSV-1) and Kaposi's sarcoma-associated herpesvirus (KSHV) encode ubiquitin E3 ligases ICP0, and MIR1 and MIR2, respectively, which modulate host immune responses (14, 31, 55). Although EBV has not been found to encode an E3 ligase, it encodes BPLF1 with DUB activity and two others, BSLF1 and BXLF1, which were predicted to have DUB activity (59). We have recently shown that BPLF1 DUB activity targets EBV ribonucleotide reductase and reduces its activity (67). EBV also employs many cellular DUBs to modulate intracellular signaling. For example, EBV EBNA1 antigen recruits USP7 (HAUSP) to disrupt promyelocytic leukemia nuclear bodies (58), and EBV upregulates ubiquitin C-terminal hydrolase 1 (UCHL1) to stabilize β-catenin in type 3 latency and to potentially regulate principal oncogenic pathways (5, 6, 52). Here, we show that A20, a protein with DUB activity induced by LMP1, regulates IRF7 activity promoted by LMP1 and, as a consequence, regulates the LMP1 level in EBV latency through its DUB activity. Therefore, A20 may regulate IRF7 oncogenic function in the EBV context, as discussed below (Fig. 6).
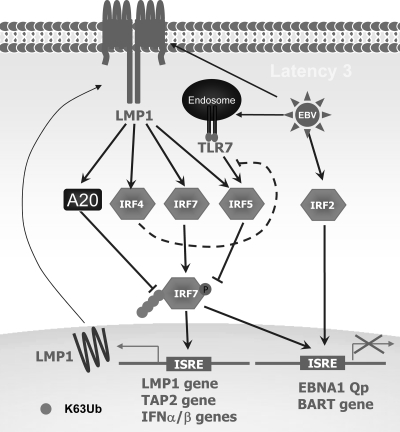
FIG. 6.
Regulation and function of IRFs in EBV latency. The only three IRFs with oncogenic properties, IRF7 (39, 73), -2 (72), and -4 (35, 69), as well as IRF5 (35, 40, 70), are all expressed at much higher levels in EBV type 3 latency. Both IRF4 (35, 69) and IRF7 (73) are induced by EBV LMP1, and IRF5 is induced by both TLR7 and LMP1 (16, 35), whereas IRF2 is induced through an unknown mechanism and negatively regulates the EBNA1 Q promoter in latency 3 (72). Moreover, IRF7 and likely IRF4 are activated by LMP1 (24, 38, 69). The activated IRF7 induces expression of LMP1, TAP2 and type I IFNs (39, 68, 74) but negatively regulates the EBV EBNA1 Q promoter and BART gene P1 promoter (11, 71). However, IRF5 (40) as well as A20 that is also induced by LMP1 (19, 29) negatively regulates LMP1-promoted IRF7 activity in EBV latency 3. In addition, like its role in antiviral responses (37), IRF4 may negatively regulate TLR7 activation of IRF5 in the EBV context.
Regulation and function of IRFs in EBV latency.
EBV latency has an intimate association with IRFs, as several of them, including three IRFs characterized as oncogenic, IRF7 (39, 73), -2 (72), and -4 (35, 69), as well as IRF5 (35, 40, 70), are all expressed at much higher levels in EBV-transformed B cells with type 3 latency, which is associated with posttransplant disorders and AIDS-associated central nervous system lymphomas in immunosuppressed patients (44). Interestingly, both IRF4 (35, 69) and IRF7 (73) are induced by LMP1, and IRF5 is induced by both TLR7 and LMP1 (16, 35), whereas IRF2 is induced through an unknown mechanism and was reported not to be induced by LMP1 (73). Moreover, IRF7 and likely IRF4 are activated by LMP1 (24, 38, 69) (Fig. 6). While IRF2, -4, and -7 are believed to be positive regulators in EBV oncogenesis, IRF5 expressed in the EBV context has at least two naturally occurring variants, a truncation mutant, V12 (35), and a point mutant, IRF5(A68P) (70), both of which function as dominant negative mutants, and in fact, we have shown that IRF5 in EBV-infected B cells negatively regulates LMP1-promoted IRF7 activity (40). IRF2 is constitutively expressed at a low level in type 1 latency (42, 50). In contrast, it is expressed at a higher level and represses the Q promoter in type 3 latency (72). In addition, given that IRF4 is a negative regulator of TLR7 activation of IRF5 in antiviral responses (37), it may have similar function in the EBV context.
Despite these observations, the roles of IRFs in EBV oncogenesis are poorly understood. As a family of transcription factors, IRFs are presumed to contribute to EBV oncogenesis through regulation of a spectrum of oncogenes or apoptosis-related genes. However, until now, only a few targets have been identified for IRF7, including the EBNA1 Q promoter (71), LMP1 (39), BamH I-A rightward transcript (BART) P1 promoter (11), cellular transporter associated with antigen processing (TAP2) (74), and type I IFNs (68) (Fig. 6). We are now performing microarray analysis to profile IRF7-regulated cellular genes in EBV latency 3. This work will address the critical role of IRF7 in EBV latency.
In addition to IRF5, which negatively regulates IRF7 activity in EBV latency (40), we now identify A20, which is also induced by LMP1 (19, 29), as a distinct negative regulator for IRF7, indicating that IRF7 is complexly modulated in the EBV context. Although both IRF5 and A20 negatively regulate IRF7 activity and counteract LMP1's effect on IRF7, they use different mechanisms; IRF5 acts as a dominant negative mutant, while A20 acts at the level of regulatory ubiquitination. In addition, we believe that other cellular proteins contribute to the delicate balance of EBV latency through regulation of IRF7 at different levels, including transcriptional and posttranslational levels, and we are conducting proteomic screening coupled with mass spectrometry to profile IRF7-interacting proteins in EBV latency 3. Further functional analysis of these proteins will not only be important for understanding the fine modulation of EBV latency, but will also help in expanding future work on IRF7-mediated innate immune responses.
Acknowledgments
We express our gratitude to Gang Liu, Rongtuan Lin, Ingrid Wertz, Karen O'Rourke, and Peter Storz for providing reagents for this study. We thank Julia Shackelford for helpful discussion.
This research was supported by a grant from the NIH (R21-AI042371).
Footnotes
Published ahead of print on 14 April 2010.
REFERENCES
- 1.Ablasser, A., F. Bauernfeind, G. Hartmann, E. Latz, K. A. Fitzgerald, and V. Hornung. 2009. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 10:1065-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balachandran, S., T. Venkataraman, P. B. Fisher, and G. N. Barber. 2007. Fas-associated death domain-containing protein-mediated antiviral innate immune signaling involves the regulation of IRF7. J. Immunol. 178:2429-2439. [DOI] [PubMed] [Google Scholar]
- 3.Barbalat, R., L. Lau, R. M. Locksley, and G. M. Barton. 2009. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10:1200-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barral, P. M., D. Sarkar, Z. Z. Su, G. N. Barber, R. DeSalle, V. R. Racaniello, and P. B. Fisher. 2009. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol. Ther. 124:219-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bheda, A., J. Shackelford, and J. S. Pagano. 2009. Expression and functional studies of ubiquitin C-terminal hydrolase L1 regulated genes. PLoS One 4:e6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bheda, A., W. Yue, A. Gullapalli, C. Whitehurst, R. Liu, J. S. Pagano, and J. Shackelford. 2009. Positive reciprocal regulation of ubiquitin C-terminal hydrolase L1 and beta-catenin/TCF signaling. PLoS One 4:e5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhoj, V. G., and Z. J. Chen. 2009. Ubiquitylation in innate and adaptive immunity. Nature 458:430-437. [DOI] [PubMed] [Google Scholar]
- 8.Bonjardim, C. A., P. C. P. Ferreira, and E. G. Kroon. 2009. Interferons: signaling, antiviral and viral evasion. Immunol. Lett. 122:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boone, D. L., E. E. Turer, E. G. Lee, R. C. Ahmad, M. T. Wheeler, C. Tsui, P. Hurley, M. Chien, S. Chai, O. Hitotsumatsu, E. McNally, C. Pickart, and A. Ma. 2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5:1052-1060. [DOI] [PubMed] [Google Scholar]
- 10.Borden, E. C., G. C. Sen, G. Uze, R. H. Silverman, R. M. Ransohoff, G. R. Foster, and G. R. Stark. 2007. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6:975-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, H., J. Huang, F. Y. Wu, G. Liao, L. Hutt-Fletcher, and S. D. Hayward. 2005. Regulation of expression of the Epstein-Barr virus BamHI-A rightward transcripts. J. Virol. 79:1724-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, Z. J., and L. J. Sun. 2009. Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33:275-286. [DOI] [PubMed] [Google Scholar]
- 13.Chiu, Y. H., J. B. MacMillan, and Z. J. Chen. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daubeuf, S., D. Singh, Y. Tan, H. Liu, H. J. Federoff, W. J. Bowers, and K. Tolba. 2009. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood 113:3264-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshaies, R. J., and C. A. P. Joazeiro. 2009. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78:399-434. [DOI] [PubMed] [Google Scholar]
- 16.Dirmeier, U., R. Hoffmann, E. Kilger, U. Schultheiss, C. Briseno, O. Gires, A. Kieser, D. Eick, B. Sugden, and W. Hammerschmidt. 2005. Latent membrane protein 1 of Epstein-Barr virus coordinately regulates proliferation with control of apoptosis. Oncogene 24:1711-1717. [DOI] [PubMed] [Google Scholar]
- 17.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-kappaB pathway involving TNF receptor-associated factors. Oncogene 14:2899-2916. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald, K. A., D. C. Rowe, B. J. Barnes, D. R. Caffrey, A. Visintin, E. Latz, B. Monks, P. M. Pitha, and D. T. Golenbock. 2003. LPS-TLR4 signaling to IRF3/7 and NF-κB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198:1043-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1996. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J. Virol. 70:8653-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fries, K. L., W. E. Miller, and N. Raab-Traub. 1999. The A20 protein interacts with the Epstein-Barr virus latent membrane protein 1 (LMP1) and alters the LMP1/TRAF1/TRADD complex. Virology 264:159-166. [DOI] [PubMed] [Google Scholar]
- 21.Honda, K., and T. Taniguchi. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644-658. [DOI] [PubMed] [Google Scholar]
- 22.Honma, K., S. Tsuzuki, M. Nakagawa, H. Tagawa, S. Nakamura, Y. Morishima, and M. Seto. 2009. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood 114:2467-2475. [DOI] [PubMed] [Google Scholar]
- 23.Hutti, J. E., B. E. Turk, J. M. Asara, A. Ma, L. C. Cantley, and D. W. Abbott. 2007. IKKβ phosphorylates the K63 deubiquitinase A20 to cause feedback inhibition of the NF-κB pathway. Mol. Cell. Biol. 27:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huye, L. E., S. Ning, M. Kelliher, and J. S. Pagano. 2007. IRF7 is activated by a viral oncoprotein through RIP-dependent ubiquitination. Mol. Cell. Biol. 27:2910-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda, O., Y. Sekine, A. Mizushima, K. Oritani, T. Yasui, M. Fujimuro, R. Muromoto, A. Nanbo, and T. Matsuda. 2009. BS69 negatively regulates the canonical NF-kappaB activation induced by Epstein-Barr virus-derived LMP1. FEBS Lett. 583:1567-1574. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda, O., Y. Sekine, T. Yasui, K. Oritani, K. Sugiyma, R. Muromoto, N. Ohbayashi, A. Yoshimura, and T. Matsuda. 2008. STAP-2 negatively regulates both canonical and noncanonical NF-κB activation induced by Epstein-Barr virus-derived latent membrane protein 1. Mol. Cell. Biol. 28:5027-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayagaki, N., Q. Phung, S. Chan, R. Chaudhari, C. Quan, K. M. O'Rourke, M. Eby, E. Pietras, G. Cheng, J. F. Bazan, Z. Zhang, D. Arnott, and V. M. Dixit. 2007. DUBA: a deubiquitinase that regulates type I interferon production. Science 318:1628-1632. [DOI] [PubMed] [Google Scholar]
- 28.Komander, D., M. J. Clague, and S. Urbe. 2009. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10:550-563. [DOI] [PubMed] [Google Scholar]
- 29.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 267:24157-24160. [PubMed] [Google Scholar]
- 30.Li, H. P., and Y. S. Chang. 2003. Epstein-Barr virus latent membrane protein 1: structure and functions. J. Biomed. Sci. 10:490-504. [DOI] [PubMed] [Google Scholar]
- 31.Liang, C., J. S. Lee, and J. U. Jung. 2008. Immune evasion in Kaposi's sarcoma-associated herpes virus associated oncogenesis. Semin. Cancer Biol. 18:423-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, R., L. Yang, P. Nakhaei, Q. Sun, E. Sharif-Askari, I. Julkunen, and J. Hiscott. 2006. Negative regulation of the retinoic acid-inducible gene I-induced antiviral state by the ubiquitin-editing protein A20. J. Biol. Chem. 281:2095-2103. [DOI] [PubMed] [Google Scholar]
- 33.Lu, F., A. Weidmer, C. G. Liu, S. Volinia, C. M. Croce, and P. M. Lieberman. 2008. Epstein-Barr virus-induced miR-155 attenuates NF-κB signaling and stabilizes latent virus persistence. J. Virol. 82:10436-10443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshak-Rothstein, A. 2006. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6:823-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin, H. J., J. M. Lee, D. Walls, and S. D. Hayward. 2007. Manipulation of the TLR7 signaling pathway by Epstein-Barr virus. J. Virol. 81:9748-9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakhaei, P., P. Genin, A. Civas, and J. Hiscott. 2009. RIG-I-like receptors: sensing and responding to RNA virus infection. Semin. Immunol. 21:215-222. [DOI] [PubMed] [Google Scholar]
- 37.Negishi, H., Y. Ohba, H. Yanai, A. Takaoka, K. Honma, K. Yui, T. Matsuyama, T. Taniguchi, and K. Honda. 2005. Negative regulation of Toll-like-receptor signaling by IRF4. Proc. Natl. Acad. Sci. U. S. A. 102:15989-15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning, S., A. D. Campos, B. Darnay, G. Bentz, and J. S. Pagano. 2008. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the tumor necrosis factor receptor family member latent membrane protein 1. Mol. Cell. Biol. 28:6536-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ning, S., A. M. Hahn, L. E. Huye, and P. S. Joseph. 2003. Interferon regulatory factor 7 regulates expression of Epstein-Barr virus latent membrane protein 1: a regulatory circuit. J. Virol. 77:9359-9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ning, S., L. E. Huye, and J. S. Pagano. 2005. Interferon regulatory factor 5 represses expression of the Epstein-Barr virus oncoprotein LMP1: braking of the IRF7/LMP1 regulatory circuit. J. Virol. 79:11671-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ning, S., L. E. Huye, and J. S. Pagano. 2005. Regulation of the transcriptional activity of the IRF7 promoter by a pathway independent of interferon signaling. J. Biol. Chem. 285:12262-12270. [DOI] [PubMed] [Google Scholar]
- 42.Nonkwelo, C., I. K. Ruf, and J. Sample. 1997. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J. Virol. 71:6887-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Opipari, A. W., Jr., M. S. Boguski, and V. M. Dixit. 1990. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J. Biol. Chem. 265:14705-14708. [PubMed] [Google Scholar]
- 44.Pagano, J. S. 2002. Viruses and lymphomas. N. Engl. J. Med. 347:78-79. [DOI] [PubMed] [Google Scholar]
- 45.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 46.Rotin, D., and S. Kumar. 2009. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10:398-409. [DOI] [PubMed] [Google Scholar]
- 47.Sabbah, A., T. H. Chang, R. Harnack, V. Frohlich, K. Tominaga, P. H. Dube, Y. Xiang, and S. Bose. 2009. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10:1073-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadler, A. J., and B. R. G. Williams. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8:559-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saitoh, T., M. Yamamoto, M. Miyagishi, K. Taira, M. Nakanishi, T. Fujita, S. Akira, N. Yamamoto, and S. Yamaoka. 2005. A20 is a negative regulator of IFN regulatory factor 3 signaling. J. Immunol. 174:1507-1512. [DOI] [PubMed] [Google Scholar]
- 50.Schaefer, B. C., E. Paulson, J. L. Strominger, and S. H. Speck. 1997. Constitutive activation of Epstein-Barr virus (EBV) nuclear antigen 1 gene transcription by IRF1 and IRF2 during restricted EBV latency. Mol. Cell. Biol. 17:873-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmitz, R., M. L. Hansmann, V. Bohle, J. I. Martin-Subero, S. Hartmann, G. Mechtersheimer, W. Klapper, I. Vater, M. Giefing, S. Gesk, J. Stanelle, R. Siebert, and R. Kuppers. 2009. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J. Exp. Med. 206:981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shackelford, J., C. Maier, and J. S. Pagano. 2003. Epstein-Barr virus activates beta-catenin in type III latently infected B lymphocyte lines: association with deubiquitinating enzymes. Proc. Natl. Acad. Sci. U. S. A. 100:15572-15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shackelford, J., and J. S. Pagano. 2004. Tumor viruses and cell signaling pathways: deubiquitination versus ubiquitination. Mol. Cell. Biol. 24:5089-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shackelford, J., and J. S. Pagano. 2005. Targeting of host-cell ubiquitin pathways by viruses. Essays Biochem. 41:139-156. [DOI] [PubMed] [Google Scholar]
- 55.Shackelford, J., and J. S. Pagano. 2007. Role of the ubiquitin system and tumor viruses in AIDS-related cancer. BMC Biochem. 8:S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shembade, N., N. S. Harhaj, K. Parvatiyar, N. G. Copeland, N. A. Jenkins, L. E. Matesic, and E. W. Harhaj. 2008. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat. Immunol. 9:254-262. [DOI] [PubMed] [Google Scholar]
- 57.Shembade, N., K. Parvatiyar, N. S. Harhaj, and E. W. Harhaj. 2009. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NFκB signalling. EMBO J. 28:513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sivachandran, N., F. Sarkari, and L. Frappier. 2008. Epstein-Barr nuclear antigen 1 contributes to nasopharyngeal carcinoma through disruption of PML nuclear bodies. PLoS Pathog. 4:e1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sompallae, R., S. Gastaldello, S. Hildebrand, N. Zinin, G. Hassink, K. Lindsten, J. Haas, B. Persson, and M. G. Masucci. 2008. Epstein-Barr virus encodes three bona fide ubiquitin-specific proteases. J. Virol. 82:10477-10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stetson, D. B., and R. Medzhitov. 2006. Type I interferons in host defense. Immunity 25:373-381. [DOI] [PubMed] [Google Scholar]
- 61.Storz, P., H. Doppler, C. Ferran, S. T. Grey, and A. Toker. 2005. Functional dichotomy of A20 in apoptotic and necrotic cell death. Biochem. J. 387:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun, S. C. 2008. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 8:501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448:501-505. [DOI] [PubMed] [Google Scholar]
- 64.Thornburg, N. J., W. Kulwichit, R. H. Edwards, K. H. Y. Shair, K. M. Bendt, and N. Raab-Traub. 2006. LMP1 signaling and activation of NF-κB in LMP1 transgenic mice. Oncogene 25:288-297. [DOI] [PubMed] [Google Scholar]
- 65.Wang, Y. Y., L. Li, K. J. Han, Z. Zhai, and H. B. Shu. 2004. A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-κB and ISRE and IFN-β promoter. FEBS Lett. 576:86-90. [DOI] [PubMed] [Google Scholar]
- 66.Wertz, I. E., K. M. O'Rourke, H. Zhou, M. Eby, L. Aravind, S. Seshagiri, P. Wu, C. Wiesmann, R. Baker, D. L. Boone, A. Ma, E. V. Koonin, and V. M. Dixit. 2004. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430:694-699. [DOI] [PubMed] [Google Scholar]
- 67.Whitehurst, C. B., S. Ning, G. Bentz, F. Dufour, E. Gershburg, J. Shackelford, Y. Langelier, and J. S. Pagano. 2009. The Epstein-Barr virus (EBV) deubiquitinating enzyme BPLF1 reduces EBV ribonucleotide reductase activity. J. Virol. 83:4345-4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu, D., K. Brumm, and L. Zhang. 2006. The latent membrane protein 1 of Epstein-Barr virus (EBV) primes EBV latency cells for type I interferon production. J. Biol. Chem. 281:9163-9169. [DOI] [PubMed] [Google Scholar]
- 69.Xu, D., L. Zhao, L. Del Valle, J. Miklossy, and L. Zhang. 2008. Interferon regulatory factors 4 is involved in Epstein-Barr virus-mediated transformation of human B lymphocytes. J. Virol. 82:6251-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang, L., T. Zhao, X. Shi, P. Nakhaei, Y. Wang, Q. Sun, J. Hiscott, and R. Lin. 2009. Functional analysis of a dominant negative mutation of interferon regulatory factor 5. PLoS One 4:e5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, L., and J. S. Pagano. 1997. IRF7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol. Cell. Biol. 17:5748-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang, L., and J. S. Pagano. 1999. Interferon regulatory factor 2 represses the Epstein-Barr virus BamHI Q latency promoter in type III latency. Mol. Cell. Biol. 19:3216-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang, L., and J. S. Pagano. 2000. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J. Virol. 74:1061-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang, L., and J. S. Pagano. 2001. Interferon regulatory factor 7 mediates activation of Tap-2 by Epstein-Barr virus latent membrane protein 1. J. Virol. 75:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]