Abstract
The signal transducer and activator of transcription 5 (Stat5) plays a pivotal role in the proliferation, secretory differentiation, and survival of mammary epithelial cells. However, there is little information about Stat5 target genes that facilitate these biological processes. We provide here experimental evidence that the prolactin-mediated phosphorylation of Stat5 regulates the transcriptional activation of the Akt1 gene. Stat5 binds to consensus sequences within the Akt1 locus in a growth factor-dependent manner to initiate transcription of a unique Akt1 mRNA from a distinct promoter, which is only active in the mammary gland. Elevating the levels of active Akt1 restores the expression of cyclin D1 and proliferation of Jak2-deficient mammary epithelial cells, which provides evidence that Akt1 acts downstream of Jak/Stat signaling. The ligand-inducible expression of Stat5 in transgenic females mediates a sustained upregulation of Akt1 in mammary epithelial cells during the onset of postlactational involution. Stat5-expressing mammary glands exhibit a delay in involution despite induction of proapoptotic signaling events. Collectively, the results of the present study elucidate an underlying mechanism by which active Stat5 mediates evasion from apoptosis and self-sufficiency in growth signals.
Canonical Jak/Stat pathways relay extracellular polypeptide signals, i.e., hormones and cytokines, from transmembrane receptors to target gene promoters in the nucleus. Specific cell types respond to these signals by altering their growth properties and their physiological activities (1, 29, 45). In the developing mammary gland, the Janus kinase 2 (Jak2) is essential for the tyrosine phosphorylation and activation of the signal transducer and activator of transcription 5 (Stat5a and Stat5b) in response to prolactin signaling (47, 50). Developmental studies on genetically engineered mice that lack Jak2 and Stat5 conditionally at defined stages of mammogenesis demonstrated that these two signal transducers play a pivotal role in the proliferation of alveolar progenitors and the survival of epithelial cells that undergo secretory differentiation during late pregnancy and lactation (16, 50). In addition, it has been shown recently in mice that express Stat5a conditionally in a Stat5a/b knockout background that this transcription factor controls the development of luminal progenitors in the mammary gland (53).
Despite a wealth of knowledge about the involvement of Stat5 in mammogenesis and the transcriptional regulation of milk protein genes (32, 37, 49), there is little information about putative Stat5 target genes that facilitate the proliferation and survival of mammary epithelial cells. Previous reports linked prolactin signaling to the cell cycle machinery in an indirect manner via IGF-2 (9), and Stat5 may also directly enhance the transcriptional activation of the Cyclin D1 gene (10). Recent studies in our laboratory on Jak2-deficient mammary epithelial cells and their isogenic wild-type controls showed that the ablation of Jak2/Stat5 signaling instigated a decrease in the expression of total and phosphorylated levels of the serine-threonine kinase Akt1 (42). Akt1 is known to inhibit GSK3β, a kinase that phosphorylates cyclin D1 on threonine 286 and initiates its nuclear export and degradation (17). Consequently, the conditional deletion of Jak2 caused a reduction in the total levels of cyclin D1, which significantly slowed the proliferation of mammary epithelial cells in culture. This phenotypic abnormality could be restored through expression of a mutant form of cyclin D1 (T286A) that constitutively resides in the nucleus (42). Collectively, these previous reports may provide mechanisms of how Jak2/Stat5 signaling is tied to the cell cycle machinery during pregnancy when alveolar progenitors rapidly multiply. They do not, however, explain how this pathway contributes to the survival of nondividing, differentiated alveolar cells since Cyclin D1 expression is significantly reduced in these cells (33).
Among the three members of the Akt family, only Akt1 is upregulated in the mammary gland during pregnancy and lactation, and knockout studies have demonstrated that this particular serine-threonine protein kinase plays a role in the functional differentiation of the secretory epithelium and metabolic pathways that regulate milk synthesis (8, 35). In addition to its metabolic functions, Akt1 may also serve as a potent survival factor for secretory epithelial cells. Akt1 levels decline rapidly after weaning of the offspring, and Akt1 deficiency accelerates the postlactational involution of the mammary gland (35). Conversely, the continuous upregulation of Akt1 inhibits programmed cell death of terminally differentiated epithelial cells (4, 25, 46). Interestingly, a similar phenotype was reported in mice that overexpress a constitutively active prolactin receptor (PRLR) or a hyperactive Jak2/Stat5/6 fusion protein (20, 28), but a definitive association between Akt1 and Jak/Stat signaling remained elusive. Signaling through the PRLR has been shown to phosphorylate Akt1 via Src-mediated signal transduction to PI3 kinase but not through a Stat5-dependent mechanism in lymphoid and breast cancer cells (for references, refer to Clevenger et al. [13]). Since our recent study and that of Fresno Vara et al. (19) suggested that the functional ablation of Jak2 did not abolish the PRLR-induced activation of Src, it was surprising to note that Akt1 expression and activation was significantly reduced in Jak2 conditional knockout cells (42). We therefore reasoned that mammary epithelial cells might possess alternative mechanisms to regulate the expression of Akt1 in a Jak2/Stat5-dependent manner.
We provide here experimental evidence in vitro and in vivo that the PRLR-mediated activation of Stat5 regulates the transcriptional activation of the Akt1 gene. We identified a novel promoter in Akt1, which is specifically active in mammary epithelial cells. Stat5 was able to bind to recognition sites in proximity of a unique noncoding exon in a PRL-induced manner. Finally, we demonstrate that the conditional overexpression of Stat5 in secretory epithelial cells of the mammary gland caused a prolonged expression of Akt1 and a delay in postlactational involution despite the induction of upstream proapoptotic signaling events. Collectively, the results of the present study suggest that prolactin mediates cell survival through the transcriptional activation of the Akt1 gene via the Jak2/Stat5 signal transduction cascade. Subsequently, Akt1 can be phosphorylated by the PRLR and other growth factor receptors to exert its role in cell survival.
MATERIALS AND METHODS
Mouse models.
The generation of the Wap-rtTA knockin strain was published recently (15), and the cloning of the TetO-Stat5(S710F)Flag-IRES-luciferase transgene will be reported elsewhere (B. A. Creamer et al., unpublished data). To conditionally express Stat5, double-transgenic females were administered 2 mg of Dox/ml in their drinking water starting on lactation day 7. After performing bioluminescence imaging at day 10 of lactation as described previously (15), we sealed the left mammary glands to induce milk stasis. All glands were collected 1, 2, 3, and 5 days later. All animals used in the present study were treated humanely and in accordance with institutional guidelines and federal regulations.
Cell culture and expression vectors.
HC11 and primary mammary epithelial cells were cultured as described previously (42). For stable expression of the caPRLR and myr-Akt1 in HC11 cells, we generated retroviral vectors by cloning the corresponding cDNAs into pBabe-puro and pBabe-hygro, respectively. The construction of the pMSCV-Stat5(S710F) vector was described previously (38). After an overnight culture in growth factor-free medium, HC11 cells were treated with 10 nM PRL for 20 min to activate Stat5 signaling.
Immunoprecipitation and Western blot analysis.
The preparation of whole-cell extracts of clarified cell lysates and tissues homogenates, the source of all antibodies as well as the experimental procedures for immunoprecipitation and Western blot analysis were described in detail elsewhere (42). The anti-phospho-Stat5a/b (Y694/9) antibody was kindly provided by Hallgeir Rui (Thomas Jefferson University). The anti-Stat5a antiserum was a gift from Lothar Hennighausen (National Institutes of Health).
Histology, immunohistochemistry, and TUNEL assay.
Mammary gland tissues were collected and procured for histological examination as described previously (42). The immunohistochemical detection of pStat3 was performed using primary antibodies from Cell Signaling, Inc., and the detection of biotinylated secondary antibodies was performed using Vectastain Elite ABC kits from Vector Laboratories. TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) staining was carried out on histological sections using the in situ cell death detection kit (Roche Applied Sciences, Indianapolis, IN). Three representative areas (i.e., 1,000 nuclei) of each specimen were counted, and a paired Student t test using Prism (GraphPad Software, La Jolla, CA) was performed to calculate statistically significant differences in apoptotic indices.
mRNA expression analyses and 5′RACE (5′ rapid amplification of cDNA ends).
Total RNA was extracted from flash-frozen tissues and cell pellets using standard guanidinium thiocyanate-phenol-chloroform extraction. A Super-Script II kit from Invitrogen with oligo(dT) primers was used to perform the first-strand synthesis according to the manufacturer's protocol. Quantitative detection of Akt1 transcripts was performed using the TaqMan Mastermix (Applied Biosystems, Foster City, CA) with the addition of Akt1-, Cish-, and Gapdh-specific primers and probe sets (nm_Mm00437443_m1, Mm00515488_m1, and 4352339E). Detection of Akt1m and Gapdh was performed using iQ SYBR green Supermix (Bio-Rad, Hercules, CA), and primer sequences are available from the authors upon request. The quantitative PCRs (qPCRs) were carried out in triplicate in a CFX96 real-time PCR detection system (Bio-Rad). The expression values obtained were normalized against Gapdh via a standard curve generated by serial dilution of pooled lactating mammary gland cDNA.
RNA ligase-mediated RACE (RLM-RACE) was performed according to the manufacturer's instructions (Applied Biosystems).
ChIP assay.
Chromatin immunoprecipitation (ChIP) was performed according to a protocol by LeBaron et al. (30). Input and bound DNA was detected quantitatively by real-time PCR using specific primers to amplify Stat5-binding sites in Akt1 and Wap. Primer sequences will be provided upon request. The ChIP assay to assess the binding of Stat3 to the p55α and p50α promoters was described previously (2). Arbitrary concentrations of the amplified products were determined against a serial dilution of genomic DNA. Assay background was determined by detecting nonspecific DNA amplicons using a Gapdh primer set. In addition, the enrichment was normalized against amplicons from immunoprecipitations using an isotype-matched control IgG.
RESULTS
PRLR-mediated activation of Stat5 enhances the transcriptional activation of Akt1.
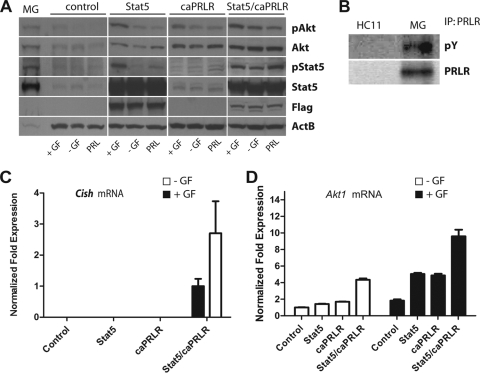
Since Jak2 deficiency resulted in decreased levels of total and phosphorylated Akt1 (42), we examined whether PRLR signaling through Jak2/Stat5 was able to regulate the expression and activation of Akt1 in normal mammary epithelial cells (MECs). Similar to a previous report (5), the treatment of HC11 cells with PRL alone had almost none or only a marginal effect on the expression and activation of Akt1 compared to a combination of growth factors that contained fetal bovine serum, epidermal growth factor (EGF), and insulin (Fig. 1A, control). This is not surprising since the steady-state levels of Stat5 and the PRLR are significantly lower in cultured MECs compared to mammary epithelial cells in their innate environment in vivo (Fig. 1A, MG, and Fig. 1B). We therefore expressed a hyperactive form of Stat5 (Stat5S710F) or a constitutively active prolactin receptor (caPRLR) in HC11 cells, and both were sufficient to elevate the total levels of Akt1 in these cells (Fig. 1A, middle panels). Introducing a hyperactive PRLR signaling pathway by coexpressing both Stat5 and caPRLR resulted in a significant increase in the expression and phosphorylation of Akt1 even in the absence of all growth factors (Fig. 1A, right panel). This finding supports our previous observation in Jak2-deficient MECs that PRLR signaling through the Jak2/Stat5 pathway regulates the expression and activation of Akt1. In addition, the significant upregulation of Cish, a known transcriptional target of Stat5 in mammary epithelial cells, confirmed that exogenous Stat5 was persistently activated by the caPRLR in these cells that express high levels of Akt1 (Fig. 1C).
FIG. 1.
Expression of Akt1 is regulated by Jak2/Stat5 signaling in mammary epithelial cells. (A) Western blot analysis using immortalized mammary epithelial cells (HC11; control) that express Stat5a, a constitutively active prolactin receptor (caPRLR), or both. Cells were cultured in the absence or presence of growth factors (GF), i.e., insulin, EGF, and serum. Alternatively, cells were treated for 20 min with PRL 24 h after the removal of all growth factors (MG, lactating mammary gland control). (B) Immunoprecipitation (IP)/Western blot analysis to compare the expression and tyrosine phosphorylation levels of the prolactin receptor (PRLR) in normal HC11 cells and in the lactating mammary gland (MG). (C) Quantitative real-time RT-PCR analysis to assess the relative expression of Cish mRNA in HC11 cells that express Stat5a, caPRLR, or both. Cish is a known transcriptional target of active Stat5 and negative regulator of Jak2/Stat5 signaling, which explains its higher expression in cells with active PRLR/Stat5 signaling in the absence of additional growth factors (normalized expression of cells with active PRLR/Stat5 that were maintained in the presence of GF was set to 1). (D) Quantitative real-time RT-PCR analysis to determine the relative expression of Akt1 mRNA in HC11 cells expressing exogenous Stat5a, caPRLR, or both (normalized expression of −GF control was set to 1). All expression values shown in panels C and D were normalized to Gapdh. Bars indicate the standard error (SE).
Next, we examined whether Stat5 plays a role in the transcriptional activation of the Akt1 gene. Similar to the Western blot results, coexpression of Stat5 and caPRLR resulted in significantly elevated levels of Akt1 mRNA as determined by quantitative RT-PCR (qRT-PCR) (Fig. 1D). Interestingly, there was a clear synergistic effect of the caPRLR and exogenous Stat5 in association with other growth factors in controlling the transcriptional regulation of the Akt1 gene (Fig. 1D, solid bars). We will demonstrate later that these growth factors seem to enhance binding of Stat5 to regulatory elements within the Akt1 locus.
PRLR signaling facilitates the transcriptional activation of Akt1 from a mammary gland-specific promoter.
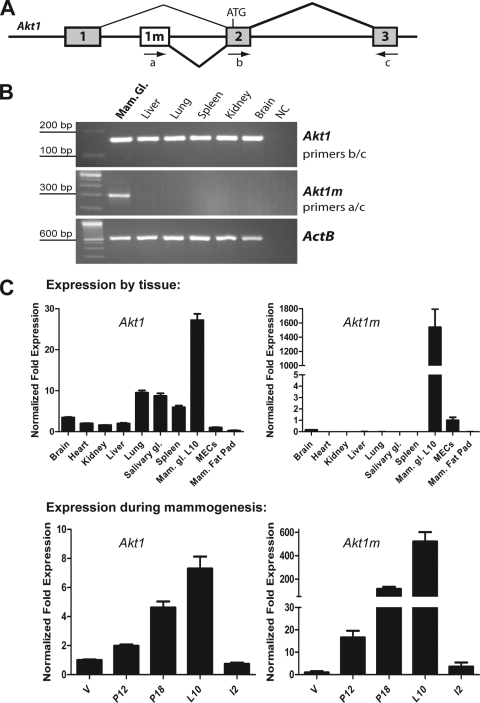
The Akt1 gene is ubiquitously expressed, and its encoded serine-threonine protein kinase plays a critical role in the proliferation, survival, and metabolism of numerous cell types. In mice, the transcriptional activation of Akt1 was reported to utilize GC-rich regulatory elements, which is typical for housekeeping gene promoters (6). Since it was unclear how the transcriptional activation of this gene could be regulated by Stat5, we performed a sequence analysis of the Akt1 gene encompassing the first three exons, including several kilobases upstream of the untranslated first exon. The initial analysis revealed that Akt1 possesses at least five optimal binding sites for Stat5 homodimers (TTCYNRGAA) (48). One consensus site was ∼1.3 kb upstream of exon 1, and all other binding sites were located at an even greater distance within introns 1 and 2. To verify the commonly known transcriptional start site, we performed a 5′RACE on RNA derived from the mammary gland of a lactating female. Unexpectedly, the cloning and sequence analysis of the nested PCR product revealed a novel 5′ exon of Akt1, exon 1m (Fig. 2A), which is located only 1.3 kb upstream of exon 2 (i.e., the first coding exon). Since only the novel exon 1m preceded the Akt1 coding sequence, we concluded that this mRNA transcript originated from distinct regulatory elements more than 1.4 kb downstream of the previously described promoter of Akt1. After generating PCR primers that specifically recognize the novel transcript (primers a/c; Fig. 2A), we performed a reverse transcription-PCR (RT-PCR) analysis on a panel of selected organs from a lactating female. A primer set within the coding region of Akt1 (exons 2/3, primers b/c) was used as a control to detect other transcripts that originate from alternative sites including the ubiquitously expressed promoter. Although Stat5a and Stat5b possess essential functions in a number of selected tissues such as spleen and liver, it was surprising to note that the novel exon 1m was only expressed in the lactating mammary gland (Fig. 2B). The qRT-PCR analysis revealed that secretory mammary epithelial cells have, by far, the highest levels of Akt1 compared to all other organs, including an epithelium-free mammary fat pad or primary MECs in culture (Fig. 2C). Interestingly, the expression of the novel Akt1m transcript was significantly lower in primary MECs that were cultured, and it appeared to be specifically expressed in the epithelium of the functionally differentiated mammary gland and not in stromal cells (i.e., the cleared fat pad). The analysis of expression profiles during mammogenesis demonstrated that the total levels of Akt1 mRNA gradually increased during pregnancy and lactation and sharply declined 48 h after weaning the offspring. In particular, the Akt1m transcript appeared to be under strict control of lactogenic hormones as it increased significantly from the virgin gland throughout pregnancy and lactation. The upregulation of the Akt1m transcript mirrors almost precisely the PRLR-induced activation of Stat5 during these stages of mammary gland development. This is comparable to the transcriptional activation of milk protein genes that are tightly controlled by Stat5.
FIG. 2.
A mammary gland-specific transcript of Akt1 originates from a distinct promoter. (A) Genomic structure of the mouse Akt1 gene; exon 1 represents the commonly known 5′-untranslated region of the mature mRNA. A novel noncoding exon (1m), which originates from downstream regulatory elements, was identified by using 5′RACE in a mammary gland-derived mRNA pool. Arrows indicate the location of RT-PCR primers that were used in panel B. (B) RT-PCR to determine the tissue-specific transcriptional activation of the newly identified exon 1m. PCR primers that amplify the coding region of Akt1 (exons 2 and 3), as well as beta-actin (ActB), were used as controls. (C) The upper panels show the results of quantitative real-time RT-PCR to determine Akt1 and Akt1m mRNA levels in tissues from a lactating female, as well as an epithelium-free mammary fat pad in comparison to cultured primary mammary epithelial cells (MECs, their relative expression level set to 1). The lower panels show Akt1 and Akt1m mRNA expression levels at various stages of mammary gland development (V, virgin; P12, pregnancy day 12; L10, lactation day 10; I2, involution day 2). The relative expression levels of the virgin gland were set to 1. In all four panels, expression was normalized to Gapdh. Bars represent the SE.
Stat5 binds to consensus sites within Akt1 and enhances the transcriptional activation of Akt1m.
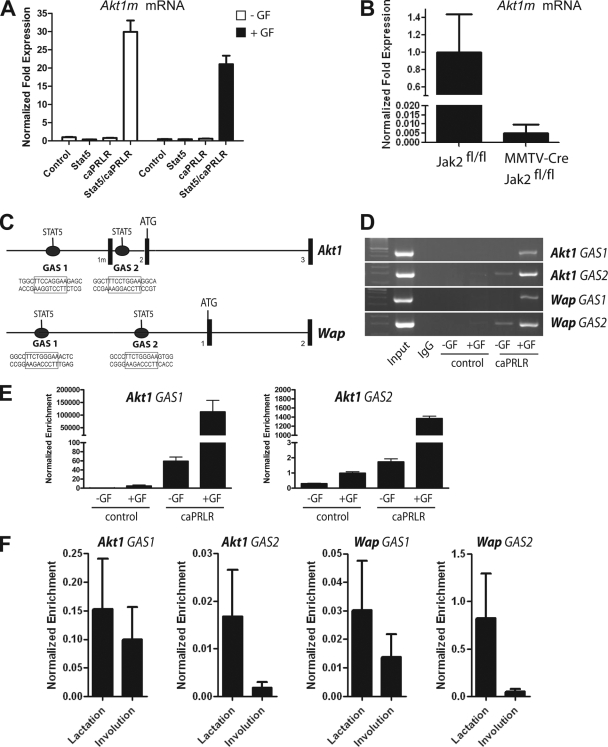
To verify that the prolactin-induced activation of Stat5 enhances the transcription of the mammary-specific Akt1 mRNA (Akt1m), we performed a qRT-PCR assay on the various HC11 cell lines that express Stat5 and the constitutively active PRLR (Fig. 3A). The results show that, regardless whether or not the cells were grown in the presence of other growth factors, the Akt1m transcript substantially increased after the constitutive activation of Stat5. To confirm these findings in vivo, we examined the relative expression of the Akt1m transcript in the mammary glands of mice that are conditionally deficient in Jak2 (MMTV-Cre Jak2fl/fl) and their Jak2-expressing control (Jak2fl/fl). We had chosen day 12.5 of gestation for this analysis, since alveolar progenitors multiply at this particular stage of mammary gland development and express elevated levels of active Stat5 in wild-type mice, whereas the pregnancy-induced activation Stat5 is significantly reduced in Jak2-deficient females (50). Also, the phenotypic differences between Jak2 conditional knockout females and their controls are not as diverse during midpregnancy as postpartum females. As illustrated in Fig. 3B, the lack of active Stat5 in response to Jak2 deficiency resulted in a significantly reduced expression of the newly identified, mammary-specific Akt1 transcript. Collectively, the examination of the expression of the Akt1m mRNA in response to increased PRLR/Stat5 signaling in cultured cells, as well as the analysis of this transcript in the Jak2-deficient mammary gland, clearly demonstrates that PRLR signaling through Jak2 and Stat5 is the main determinant for regulating the expression of the Akt1m transcript from a distinct mammary-gland specific promoter.
FIG. 3.
Stat5 binds to consensus sites within Akt1 and greatly enhances the transcriptional activation of Akt1m. (A) Quantitative real-time RT-PCR analysis to assess the activation of the Akt1m transcript in HC11 cells expressing Stat5, caPRLR, or both. Cells were maintained in the presence or absence of growth factors (GF); normalized expression of the −GF control was set to 1. (B) qRT-PCR analysis to assess the transcriptional activation of Akt1m in the mammary gland of a Jak2-deficient female (MMTV-Cre Jak2fl/fl) and her wild-type littermate control (Jak2fl/fl) at day 12.5 of gestation. All expression values shown in panels A and B were normalized to Gapdh. Bars represent the SE. (C) Location of putative binding sites for Stat5 homodimers (TTCYNRGAA) within the 5′ region of the Akt1 locus and the Wap gene. (D and E) PCR and quantitative real-time PCR analysis on chromatin that was bound to endogenous Stat5 and coprecipitated with an antibody against Stat5a (ChIP). HC11 cells with or without exogenous caPRLR were maintained in the presence or absence of growth factors (GF). The promoter of Wap, which is a known Stat5 target gene, was used as a positive control for this ChIP assay. (F) Stat5 ChIP combined with qPCR analysis to assess the binding of Stat5 to interferon gamma activation sites (GAS) within the promoters of Akt1 and Wap in mammary tissues of females at day 10 of lactation and day 2 of involution. Quantitative real-time values of amplified Stat5 consensus sites within Akt1 and Wap shown in panels E and F were normalized against precipitated DNA using an IgG control antibody, as well as unspecific amplicons from nonconsensus binding regions. Bars represent the SE.
Next, we performed a Stat5 ChIP assay on HC11 cells expressing the constitutively active PRLR and their untransfected controls to verify that endogenous Stat5 binds directly to consensus sites within the Akt1 gene in a PRLR-inducible manner. For this analysis, we focused on two Stat5 consensus sites, one upstream of the ubiquitously expressed exon 1 and a second site located in intron 1, which is in close proximity (300 bp) to the mammary-specific exon 1m (Fig. 3C). As a positive control for this assay, we amplified DNA regions around Stat5 consensus sites within the Whey acidic protein locus (Wap), which is known to be under stringent transcriptional control of active Stat5 (37). Although Mukhopadhyay et al. (39) reported the presence of only one interferon gamma activation site within the promoter of the rat Wap gene, we found at least two optimal binding sites for Stat5, 581 bp and 1.3 kb upstream of the first exon of the mouse Wap locus (Fig. 3C). Collectively, this experiment revealed that Stat5 was able to bind to all consensus sites within Akt1 and Wap when cells were grown in the presence of growth factors, and the binding was significantly enhanced in cells expressing the constitutively active PRLR (Fig. 3D and E). Although the PRLR-mediated activation of endogenous Stat5 appeared to be sufficient for the enrichment of this transcription factor on the promoters of Akt1 and Wap, the presence of additional growth factors seemed to significantly enhance or stabilize the binding of Stat5 on these consensus sites of both genes. This might explain why we observed a synergistic affect of constitutive PRLR signaling with other growth factors in controlling the transcriptional activation of Akt1 (Fig. 1D). To verify the binding of Stat5 to consensus sites within the Akt1 and Wap loci in vivo, we performed a Stat5 ChIP assay combined with a quantitative PCR analysis on tissues of wild-type mice during lactation and at day 2 of involution. The first stage of involution on the second day following milk stasis is characterized by a significant reduction in the phosphorylation of Stat5, the activation of Stat3, and the expression of proapoptotic factors in mammary epithelial cells. This initial phase of mammary gland remodeling is reversible, and the mammary gland still expresses abundant levels of total Stat5 (31). Hence, we performed the ChIP assay at two developmental stages that exhibit relatively equal amounts of epithelial tissues that significantly differ in the levels of Stat5 phosphorylation. The results of this analysis (Fig. 1F) clearly demonstrate that the dephosphorylation of Stat5 in response to milk stasis during involution dissociates this transcription factor from its binding sites within regulatory elements of Akt1, as well as Wap. In conclusion, the results of the in vitro and in vivo Stat5 binding studies clearly suggest that the transcriptional activation of Akt1 in mammary epithelial cells is directly mediated by the PRLR-induced activation and binding of Stat5 to its consensus sites within the Akt1 promoter. The dissociation of inactive Stat5 from the Akt1 promoter during the initial phase of involution results in a sharp decline in the transcriptional activation of this gene (Fig. 2C, lower panels).
Expression of Akt1 accelerates the proliferation of Jak2-deficient mammary epithelial cells.
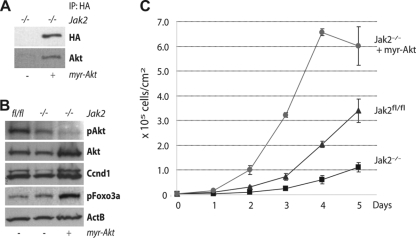
After identifying Stat5 as a regulator for the transcriptional activation of the Akt1 gene, we intended to examine next whether Akt1 functions downstream of Jak2 and Stat5 in a biological context. In vitro and in vivo, the Cre-mediated deletion of Jak2 decelerates the numeric expansion of MECs due to reduced levels of cyclin D1 (42). In culture, Jak2-deficient MECs resumed normal proliferation when exogenous, constitutively nuclear cyclin D1 was expressed. The novel finding that Akt1 is a direct transcriptional target of Stat5 corresponds well to our previously proposed model, which suggests that the Jak2/Stat5 pathway regulates the levels of the cyclin D1 protein by altering the expression and functionality of Akt1 and GSK-3β. If this is correct, then reinstating the expression of active Akt1 might elevate the level of the cyclin D1 protein and thereby accelerate the proliferation of Jak2-deficient cells. To experimentally test this idea, we generated Jak2-deficient MECs expressing a hemagglutinin (HA)-tagged, myristoylated (myr) form of Akt1 (Fig. 4A). As postulated, a gain-of-function of Akt1 was entirely sufficient to increase the expression of cyclin D1 (Fig. 4B), and consequently, Jak2-deficient MECs that express myr-Akt1 exhibited a marked increase in proliferation that even exceeded the growth rate of Jak2-expressing control cells due to excess levels of cyclin D1 (Fig. 4C). In conclusion, the findings of this initial study provided the first experimental evidence that Akt1 functions downstream of the Jak2/Stat5 signaling pathway, and this serine-threonine protein kinase acts as a functional link between Stat5 and the cell cycle machinery by regulating the levels of cyclin D1.
FIG. 4.
Expression of myristolated Akt1 increases cyclin D1 levels and accelerates the proliferation of Jak2-deficient MECs. (A) Immunoprecipitation (IP) and Western blot analysis to verify the expression of HA-tagged myr-Akt1 in MECs that lack Jak2. (B) Western blot analysis to assess the expression of Akt1 and cyclin D1 (Ccnd1) in MECs lacking Jak2 with or without exogenous Akt1, as well as isogenic Jak2-expressing control cells. Phosphorylated Foxo3a was used as another positive control to verify that exogenous Akt1 is functional in Jak2-deficient cells. Beta-actin (ActB) was used as a loading control. (C) Viable cell count over a 5-day period to determine growth rates of Jak2-deficient MECs with or without expression of exogenous Akt1. All time points were analyzed in triplicate. Bars represent the SE.
The ligand-inducible overexpression of Stat5 inhibits postlactational involution through sustained expression of Akt1.
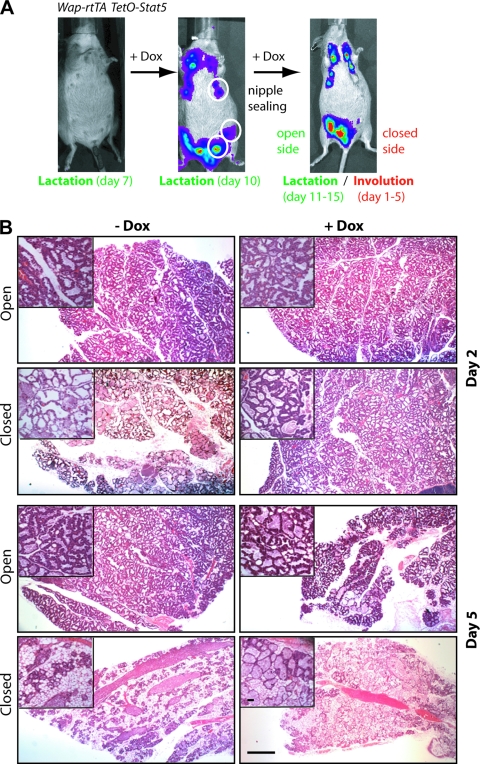
The generation and analysis of Jak2 and Stat5 conditional knockout mice revealed that this signaling pathway is not only required for the proliferation of alveolar progenitors, but it is equally important for the survival of their differentiating descendants (16, 50). To address whether an increase in expression and activation of Stat5 mediates epithelial cell survival through transcriptional activation of Akt1 in vivo, we generated transgenic mice that overexpress a Flag-tagged, hyperactive mutant of Stat5 (S710F) in a doxycycline (Dox)-controlled manner [TetO-Stat5(S710F)-IRES-luciferase]. The spatial expression of Stat5 in secretory mammary epithelial cells was accomplished by crossing TetO-Stat5 transgenic mice with a novel knock-in strain generated in our laboratory that expresses the reverse tetracycline-controlled transactivator (rtTA) under regulation of the endogenous Wap gene promoter (WAP-rtTA) (15). The inclusion of a luciferase reporter into the bicistronic TetO-Stat5 transgene allowed us to monitor the Dox-inducible expression of Stat5 in lactating females using bioluminescence imaging (Fig. 5A). After confirming the correct temporal and spatial expression of exogenous Stat5 in individual mice, we sealed the left mammary glands 3, 4, and 5 at day 10 of lactation to induce milk stasis, programmed cell death, and remodeling in these particular glands despite high circulating levels of PRL. The closed glands, along with the open controls, were collected on days 1, 2, 3, and 5 following sealing for histological and biochemical analyses. The initial evaluation of hematoxylin and eosin (H&E)-stained sections of the number 4 mammary glands revealed that milk-stasis-induced remodeling was significantly delayed in the closed glands of Dox-treated females at day 5 (Fig. 5B). Compared to the equivalent (i.e., sealed) tissues in untreated females (−Dox) that exhibited extensive remodeling within 5 days after sealing (i.e., loss of epithelial cells and adipocyte expansion), the integrity and size of secretory alveoli was largely maintained in the Stat5-expressing epithelium of the closed glands. The lactating mammary glands (i.e., open glands) expressing exogenous Stat5 (+Dox) and their controls (−Dox) did not exhibit any phenotypic differences.
FIG. 5.
Doxycycline (Dox)-controlled overexpression of hyperactive Stat5a delays mammary gland involution in vivo. (A) Experimental design. Double transgenic Wap-rtTA/TetO-Stat5(S710F) mice were analyzed for ligand-inducible coexpression of luciferase using bioluminescence imaging (IVIS200) prior to treatment with Dox on lactation day 7 (left panel). After confirming the correct temporal and spatial activation of the TetO-Stat5 transgene in Dox-treated females on lactation day 10, we sealed the nipples of the left mammary glands 3, 4, and 5 to induce milk stasis (middle panel). All sealed (closed) mammary glands and their lactating (open) controls were collected between the first and fifth days after closing the nipples (right panel). (B) Comparative histological analysis of H&E-stained sections of mammary glands of Wap-rtTA/TetO-Stat5(S710F) double-transgenic females. These involuting (closed) mammary glands and their lactating (open) intra-individual control tissues were examined 2 and 5 days after sealing the nipples. Bars represent 500 μm (50 μm in the insets).
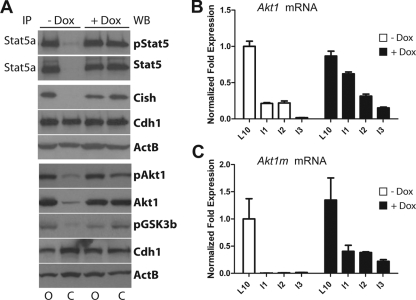
Next, we performed Western blot analyses to verify that the delay in involution in the Dox-treated females was a consequence of the expression of exogenous Stat5. As expected, the expression of endogenous Stat5 (i.e., total and active) declined rapidly in the sealed glands of untreated (−Dox) control mice (Fig. 6A). In contrast, high levels of total and phosphorylated Stat5 were still present on the second day following sealing in the involuting mammary glands of Dox-treated females. The continuous expression of Cish on day 2 after sealing indicated that Stat5 was transcriptionally active in these tissues. More importantly, total and phosphorylated levels of Akt1, as well as phosphorylated GSK3β, were still upregulated in the presence of active Stat5. In contrast, the rapid deactivation of endogenous Stat5 in response to milk stasis in the closed glands of untreated control females resulted in a significant downregulation of total Akt1. This was not due to a loss of epithelial cells since, at this developmental stage, the secretory epithelium remained largely intact, and the levels of E-cadherin (Cdh1) did not change (Fig. 6A). Subsequently, we performed a qRT-PCR analysis on tissues of Dox-treated mice and their controls over a period of 3 days after sealing to determine whether the loss of transcriptionally active Stat5 had an impact on the expression of Akt1 (Fig. 6B and C). This line of investigation clearly showed that Akt1 transcription sharply declined in the closed glands of untreated (−Dox) control females, and this result was identical to a normal weaning process (Fig. 2C, lower panel, I2 bar). In contrast, the expression of exogenous Stat5 (+Dox) resulted in a prolonged transcriptional activation of Akt1. In these latter glands, Akt1 expression declined more gradually, possibly due to a slow but steady transcriptional downregulation of the Wap-rtTA locus, which, like Akt1, is under the control of Stat5. Interestingly, the Akt1m transcript, which originates from the newly identified promoter, appeared to be under very stringent control of active Stat5 (Fig. 6C). Its expression was no longer detected when Stat5 became dephosphorylated and downregulated within the first 24 to 48 h of milk stasis. In contrast, the prolonged presence of exogenous, hyperactive Stat5 was sufficient to enhance the expression of the Akt1m transcript. The level of Akt1m expression remained at nearly 30 to 40% in the involuting glands expressing Stat5 3 days after sealing the nipples compared to the expression of this transcript in the corresponding lactating control glands of an untreated female.
FIG. 6.
Doxycycline (Dox)-controlled overexpression of hyperactive Stat5a leads to sustained transcription of Akt1. (A) Immunoprecipitation (IP)/Western blot analysis to assess the expression of active Stat5 on the second day after sealing the nipples (lanes C, closed) of lactating Wap-rtTA/TetO-Stat5(S710F) double-transgenic females that were treated with Dox and their untreated controls. The lactating (lanes O, open) glands of the same animals served as intraindividual controls. The protein levels of Cish, as well as total and phosphorylated Akt1, were determined by conventional immunoblotting. Beta-actin (ActB) was used as a general loading control, and unchanged expression levels of E-cadherin (Cadh1) indicate equal amounts of epithelial cells in all samples prior to programmed cell death and remodeling. (B and C) Quantitative real-time PCR analysis to assess the relative expression levels of Akt1 and Akt1m mRNA in involuting mammary glands between 1 and 3 days after sealing the nipples (I1 to I3) of Dox-treated females and their untreated controls. These relative expression values were compared to lactating control tissues of open glands (L10). The expression values of lactating tissues of untreated mice, which are represented by open bars, were set to 1.0. All expression values were normalized to Gapdh. Bars represent the SE.
In summary, the generation and analysis of females expressing exogenous levels of Stat5 in a ligand-controlled manner clearly confirmed that Akt1 is a target of this transcription factor in the developing mammary gland. The results of the present study also demonstrate that this pathway is biologically relevant since it contributes to a prolonged survival of secretory epithelial cells.
Gain-of-function of Stat5 promotes epithelial cell survival despite induction of proapoptotic signals during early involution.
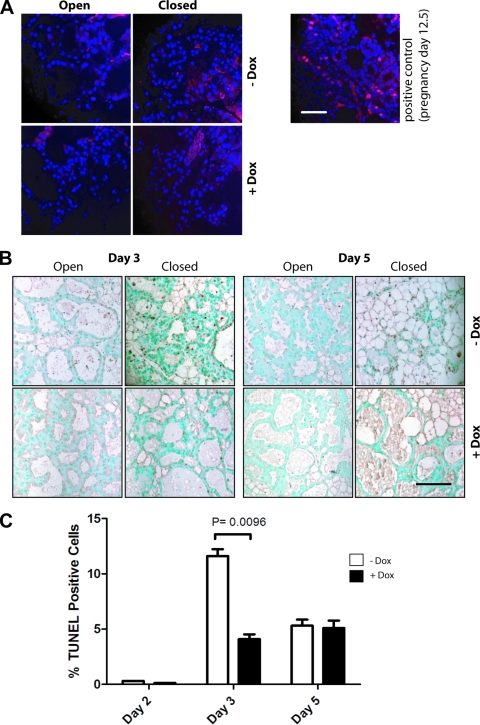
The involution of the mammary gland occurs in two distinct phases (31, 34). During the first phase, the mammary gland initiates programmed cell death through downregulation of survival factors and activation of proapoptotic signals. The second phase of involution describes the actual remodeling of the mammary gland, i.e., the loss of the secretory epithelium, which becomes noticeable in the murine gland after the third day of milk stasis. We performed immunostaining for Ki67 and a TUNEL assay on involuting mammary glands of Dox-treated Wap-rtTA/TetO-Stat5 double-transgenic females and their untreated (−Dox) controls to assess whether the delay in remodeling in Stat5-expressing females is a consequence of abnormal cell proliferation or a delay in apoptosis. Neither the lactating glands nor the involuting tissues of both experimental groups contained Ki67-positive cells (Fig. 7A), suggesting that Stat5 does not initiate aberrant proliferation in the differentiated epithelium. As expected, a surge of apoptotic cells was observed in the closed glands of untreated females 3 days after sealing the nipples (Fig. 7B and C), and the remodeling of these glands was near completion by day 5 of involution. In contrast, involuting glands of mice expressing exogenous Stat5 exhibited a significantly lower number of apoptotic cells on day 3 after sealing. The lumina were still filled with milk on day 5 of involution, and the rate of apoptosis remained low. In conclusion, gain-of-function of Stat5 during the first phase of involution promotes cell survival and delays the onset of remodeling.
FIG. 7.
Gain of function of Stat5 does not initiate aberrant proliferation of differentiated epithelial cells but promotes their survival during involution. (A) Immunostaining for Ki67 (red) on involuting mammary glands of Dox-treated Wap-rtTA/TetO-Stat5 double-transgenic females and their untreated (−Dox) controls. A mammary gland from a female at day 12.5 of gestation was used as a positive control. Sections were counterstained with DAPI (blue). The bar represents 50 μm. (B) TUNEL analysis of apoptotic nuclei was performed on histological sections from lactating (open) and involuting mammary glands (closed) of doxycycline (Dox)-treated Wap-rtTA/TetO-Stat5(S710F) females and their untreated controls on day 3 and day 5 after sealing the nipples of the left glands. Sections were counterstained with methyl green. The bar represents 50 μm. (C) Relative number of TUNEL-positive cells on days 2, 3, and 5 after sealing the nipples. One-thousand cells from three representative areas of the sections were counted for each time point. Bars represent the SE, and the P value was calculated by using a paired Student t test.
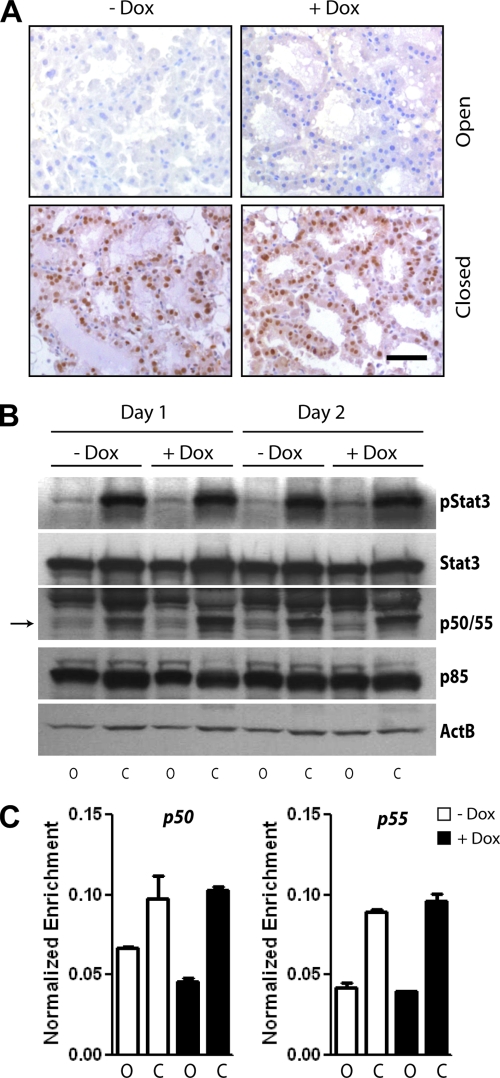
Based on the results from the TUNEL assay, we subsequently assessed whether exogenous levels of Stat5 interfere with the induction of proapoptotic signals during the first phase of involution. Specifically, the Tyr705 phosphorylation of Stat3 is known to herald the initiation of postlactational involution, and the functional ablation of this STAT family member has been reported to delay apoptosis (11, 23, 31). As shown in Fig. 8A, the gain of function of Stat5 did not affect the activation and nuclear localization of Stat3, and the total as well as phosphorylated levels of this signal transducer did not change in the closed mammary glands despite expression of hyperactive Stat5 (Fig. 8B). Abell et al. (2) reported recently that active Stat3 induces the transcription of short phosphatidylinositol 3-kinase (PI3 kinase) subunits, p50α and p55α, during the onset of involution. Stat3 was equally able to bind to consensus sites within regulatory regions of these genes (Fig. 8C), and the presence of similar levels of p50α and p55α protein in the sealed glands of both experimental groups (Fig. 8B) clearly suggests that Stat3 is transcriptionally active regardless of Stat5 overexpression. Collectively, the present study shows that gain of function of Stat5 promotes cell survival despite a parallel activation of Stat3 and the Stat3-mediated expression of the short PI3 kinase subunits in secretory MECs.
FIG. 8.
Proapoptotic signaling events are initiated despite Stat5-mediated sustained cell survival. (A) Immunohistochemistry to determine the activation and nuclear localization of Stat3 in Stat5-expressing, involuting tissues (+Dox, closed), and their controls 48 h after sealing the nipples. Slides were counterstained with hematoxylin. The bar represents 50 μm. (B) Western blot analysis to quantitatively assess the expression and activation of Stat3, as well as the Stat3-induced expression of the p50α/p55α PI3K subunits during the first and second days after sealing the nipples (lanes C, closed) of lactating Wap-rtTA/TetO-Stat5(S710F) double-transgenic females that were treated with Dox and their untreated controls. The lactating (lanes O, open) glands of the same animals served as intra-individual controls. (C) Stat3 ChIP assay combined with qPCR analysis to assess the binding of this transcription factor to the promoters of p50α and p55α in lactating and involuting mammary tissues of Wap-rtTA/TetO-Stat5(S710F) double-transgenic females that were treated with Dox (+Dox) and their untreated controls (−Dox). Tissues were collected on the second day after sealing the nipples (columns C, closed), and the lactating (columns O, open) glands of the same animals served as intra-individual controls. qPCR values of amplified Stat3 consensus sites within p55α and p55α were normalized against precipitated DNA using an IgG control antibody, as well as unspecific amplicons from nonconsensus binding regions, bars represent the SE. Note that activation of Stat3 and expression of p50/p55, which herald the onset of apoptosis beginning at 24 h after milk stasis, occur independently of Stat5-mediated suppression of programmed cell death.
DISCUSSION
The results of the present study suggest that Stat5, which is highly active during pregnancy and lactation in response to PRLR signaling, greatly increases the transcription of the Akt1 gene. Stat5 binds to two or more consensus sequences within the Akt1 locus in a growth factor-dependent manner, and it enhances the transcription of a unique Akt1 mRNA (Akt1m) from a distinct promoter. Interestingly, the transcriptional activation of Akt1m intensifies significantly during pregnancy and lactation, which is comparable to expression patterns of major milk protein genes. Since we were unable to assess the relative expression of the Akt1 transcript that specifically originates from the ubiquitously active promoter due to a very high GC content in exon 1, we cannot currently exclude the possibility that pStat5 may, at least to some extent, also enhance the activation of the constitutively active promoter. The considerable increase in metabolism in the mammary gland during pregnancy probably demands excess levels of Akt1, but this kinase also ensures the integrity and survival of the stressed secretory epithelium during lactation. In a normal mammary gland, the transcriptional activation of Akt1, in particular the transcription from the novel promoter, decreases sharply during the onset of involution when Stat5 is rapidly inactivated in response to milk stasis. Our in vivo experiments demonstrate that exogenous levels of transcriptionally active Stat5 counteract the downregulation of Akt1 during involution. Active Stat5 mediates the survival of secretory epithelial cells, and the continuous expression of this transcription factor at the onset of the postlactational involution period is sufficient to inhibit programmed cell death despite the initiation of proapoptotic signaling events mediated by active Stat3.
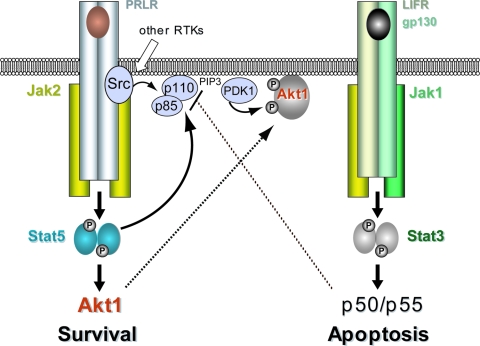
It is evident from previous studies (22, 52) and this report that a coordinated postlactational remodeling of the mammary gland requires a downregulation of survival factors such as Stat5 and Akt1, as well as the activation of proapoptotic signals, including Stat3. The extended survival of terminally differentiated cells during involution in our Stat5 gain-of-function model is likely caused by the Stat5-mediated sustained expression of Akt1. This conclusion is supported by the fact that females expressing Stat5 conditionally in the mammary gland share striking phenotypic similarities with transgenic mice that express mutant, myristoylated, or wild-type Akt1 under regulation of the MMTV-LTR (4, 25, 46). In two of these reports, it was also shown that expression of hyperactive or wild-type Akt1 is sufficient to promote an extended survival of the functionally differentiated mammary epithelium despite milk stasis-induced phosphorylation of Stat3 (4, 46). Similar to these models, we demonstrated in the present study that Stat3 is being activated and translocated into the nuclei of mammary epithelial cells that are being protected from postlactational remodeling due to sustained expression of active Stat5. Abell et al. (3) suggested that Stat3 modifies the activity of the p110 catalytic subunit of PI3K through a switch in the expression of p85α and p50α/p55α regulatory subunits. Although the significance of these shorter PI3K subunits during mammogenesis remains to be examined using p50α/p55α double-knockout mice (12), we can demonstrate in the present study that prolonged cell survival in response to gain-of-function of Stat5 occurs despite the Stat3-mediated transcriptional activation of p50α/p55α. The expression of these shorter regulatory subunits of PI3K also does not change on the level of the protein in the presence of active Stat5. Since we propose here that Stat5 regulates the expression of Akt1 on the transcriptional level (Fig. 9), it is not quite clear at this point how this transcription factor is able to override the proposed proapoptotic function of activated Stat3, as well as p50α and p55α. In addition to its classical role as a transcription factor, phosphorylated Stat5 might possess cytoplasmic functions that involve binding to adapter proteins and subunits of PI3 kinase (44). We demonstrated recently that phosphorylated Stat5 is able to bind to the SH2 domain-containing p85α subunit of PI3K in the lactating mammary gland (42), and active Stat5 may therefore directly modify the activity of the PI3 kinase (Fig. 9). The significance of this observation for cell survival and the activation of Akt1 are subjects of current investigations.
FIG. 9.
Divergent roles of Stat5 and Stat3 in the survival and death of secretory mammary epithelial cells in vivo.
Upon deregulated expression and activation, Jak2 and Stat5 may facilitate two important hallmarks of cancer, i.e., evasion from apoptosis and self-sufficiency in growth signals (21). The notion that a hyperactive Jak2/Stat5 pathway plays a role in neoplastic transformation is supported by the observation that a subset of human breast cancers contain nuclear localized, tyrosine phosphorylated Stat5 (14). In addition, it has recently been reported that gain of function of Stat5 promotes the occurrence of differentiated papillary adenocarcinomas in mice depending on the reproductive status of the females (18, 27). Although a full-term pregnancy early in life is commonly known to reduce the incidence in breast cancer in postmenopausal women, each gestation also increases temporarily (i.e., for a couple of years following pregnancy) the likelihood for developing breast cancer (51). Since Stat5 is a key mediator of pregnancy-induced growth signals and a potent suppressor of apoptosis, it is likely that this transcription factor plays a pivotal role in pregnancy-associated breast cancers. Stat5 and its upstream Janus kinase 2 might therefore serve as suitable targets for breast cancer prevention. In support of this assumption, several reports have shown that lowering the expression of Stat5 by deleting the Stat5a gene delays mammary tumorigenesis in various cancer models (24, 41). In addition, we have demonstrated recently that the deletion of Jak2, and therefore the functional ablation of Stat5, protects against ErbB2-induced mammary cancer in mice (43). Since Stat5 regulates the expression of Akt1, as well as the functionality of cyclin D1 in mammary epithelial cells, the protective effect of Jak2 deficiency on mammary cancer initiation might be mediated by both or either one of these downstream targets, as previous studies indicate (7, 36, 54).
Despite its suggested role in cancer initiation, the continuous activation of Stat5 in human tumors was associated with a favorable prognosis (40). This observation in humans, as well as the histopathology in transgenic mice, might suggest that active Stat5 mediates the maintenance of a more differentiated tumor phenotype. It is tempting to speculate that Akt1 is a main downstream target of the biological role of Stat5 in both cancer initiation and the suppression of metastasis. In fact, Hutchinson et al. (26) have observed that, although expression of Akt1 accelerated ErbB2-associated mammary tumorigenesis, this kinase suppressed cancer invasion and metastasis. Our newly generated mouse model that allows expression of Stat5 in a ligand-inducible manner will be a suitable genetic tool to assess whether Stat5 regulates the expression of Akt1 in preneoplastic and cancerous lesions and whether the ablation of Stat5 during various stages of carcinogenesis affects tumor progression.
Acknowledgments
This study was supported by the Public Health Service grant CA117930 from the National Cancer Institute. Additional financial support provided to K.-U.W. by the Nebraska Cancer and Smoking Disease Research Program (NE DHHS LB506 2009-45) was imperative to finance the maintenance of the mouse models. R.M. was supported by the Austrian Science Fund (FWF) grant SFB F28. B.A.C. and J.W.S. received graduate fellowships through the UNMC Cancer Research Training Program (CA009476) and a Program of Excellence Graduate Assistantship through the UNMC Graduate Studies Office. K.S. received a postdoctoral fellowship from the Susan G. Komen Breast Cancer Foundation (PDF0600835).
The sorting of viable cells was carried out at the UNMC Cell Analysis Facility (Charles A. Kuszynski), and we thank the members of the UNMC Mouse Genome Engineering Core Facility, Don Harms and Judith Stribley, for the pronuclear injection of the TetO-Stat5 construct. We also thank Anita Jennings from the UNMC Molecular Phenotyping Core Facility for her help labeling apoptotic cells in mammary tissue sections. The myr-Akt1 cDNA was kindly provided by Jorge Martin-Perez (Instituto de Investigaciones Biomedicas A. Sols, Madrid, Spain), and the caPRLR was gift from Isabelle Gourdou (INRA, Jouy-en-Josas Cedex, France).
Footnotes
Published ahead of print on 12 April 2010.
REFERENCES
- 1.Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. [DOI] [PubMed] [Google Scholar]
- 2.Abell, K., A. Bilancio, R. W. Clarkson, P. G. Tiffen, A. I. Altaparmakov, T. G. Burdon, T. Asano, B. Vanhaesebroeck, and C. J. Watson. 2005. Stat3-induced apoptosis requires a molecular switch in PI(3)K subunit composition. Nat. Cell Biol. 7:392-398. [DOI] [PubMed] [Google Scholar]
- 3.Abell, K., and C. J. Watson. 2005. The Jak/Stat pathway: a novel way to regulate PI3K activity. Cell Cycle 4:897-900. [DOI] [PubMed] [Google Scholar]
- 4.Ackler, S., S. Ahmad, C. Tobias, M. D. Johnson, and R. I. Glazer. 2002. Delayed mammary gland involution in MMTV-AKT1 transgenic mice. Oncogene 21:198-206. [DOI] [PubMed] [Google Scholar]
- 5.Bailey, J. P., K. M. Nieport, M. P. Herbst, S. Srivastava, R. A. Serra, and N. D. Horseman. 2004. Prolactin and transforming growth factor-beta signaling exert opposing effects on mammary gland morphogenesis, involution, and the Akt-forkhead pathway. Mol. Endocrinol. 18:1171-1184. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa, A., T. F. Franke, M. E. Gonzalez-Portal, K. Datta, T. Taguchi, J. Gardner, J. Q. Cheng, J. R. Testa, and P. N. Tsichlis. 1993. Structure, expression and chromosomal mapping of c-Akt: relationship to v-Akt and its implications. Oncogene 8:745-754. [PubMed] [Google Scholar]
- 7.Bowe, D. B., N. J. Kenney, Y. Adereth, and I. G. Maroulakou. 2002. Suppression of Neu-induced mammary tumor growth in cyclin D1 deficient mice is compensated for by cyclin E. Oncogene 21:291-298. [DOI] [PubMed] [Google Scholar]
- 8.Boxer, R. B., D. B. Stairs, K. D. Dugan, K. L. Notarfrancesco, C. P. Portocarrero, B. A. Keister, G. K. Belka, H. Cho, J. C. Rathmell, C. B. Thompson, M. J. Birnbaum, and L. A. Chodosh. 2006. Isoform-specific requirement for Akt1 in the developmental regulation of cellular metabolism during lactation. Cell Metab. 4:475-490. [DOI] [PubMed] [Google Scholar]
- 9.Brisken, C., A. Ayyannan, C. Nguyen, A. Heineman, F. Reinhardt, J. Tan, S. K. Dey, G. P. Dotto, R. A. Weinberg, and T. Jan. 2002. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev. Cell 3:877-887. [DOI] [PubMed] [Google Scholar]
- 10.Brockman, J. L., M. D. Schroeder, and L. A. Schuler. 2002. PRL activates the cyclin D1 promoter via the Jak2/Stat pathway. Mol. Endocrinol. 16:774-784. [DOI] [PubMed] [Google Scholar]
- 11.Chapman, R. S., P. C. Lourenco, E. Tonner, D. J. Flint, S. Selbert, K. Takeda, S. Akira, A. R. Clarke, and C. J. Watson. 1999. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. 13:2604-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, D., F. Mauvais-Jarvis, M. Bluher, S. J. Fisher, A. Jozsi, L. J. Goodyear, K. Ueki, and C. R. Kahn. 2004. p50alpha/p55alpha phosphoinositide 3-kinase knockout mice exhibit enhanced insulin sensitivity. Mol. Cell. Biol. 24:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clevenger, C. V., P. A. Furth, S. E. Hankinson, and L. A. Schuler. 2003. The role of prolactin in mammary carcinoma. Endocrinol. Rev. 24:1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cotarla, I., S. Ren, Y. Zhang, E. Gehan, B. Singh, and P. A. Furth. 2004. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. Int. J. Cancer 108:665-671. [DOI] [PubMed] [Google Scholar]
- 15.Creamer, B. A., A. A. Triplett, and K. U. Wagner. 2009. Longitudinal analysis of mammogenesis using a novel tetracycline-inducible mouse model and in vivo imaging. Genesis 47:234-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui, Y., G. Riedlinger, K. Miyoshi, W. Tang, C. Li, C. X. Deng, G. W. Robinson, and L. Hennighausen. 2004. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24:8037-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eilon, T., B. Groner, and I. Barash. 2007. Tumors caused by overexpression and forced activation of Stat5 in mammary epithelial cells of transgenic mice are parity-dependent and developed in aged, postestropausal females. Int. J. Cancer 121:1892-1902. [DOI] [PubMed] [Google Scholar]
- 19.Fresno Vara, J. A., M. V. Carretero, H. Geronimo, K. Ballmer-Hofer, and J. Martin-Perez. 2000. Stimulation of c-Src by prolactin is independent of Jak2. Biochem. J. 345(Pt. 1):17-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourdou, I., J. Paly, C. Hue-Beauvais, L. Pessemesse, J. Clark, and J. Djiane. 2004. Expression by transgenesis of a constitutively active mutant form of the prolactin receptor induces premature abnormal development of the mouse mammary gland and lactation failure. Biol. Reprod. 70:718-728. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 22.Hennighausen, L., and G. W. Robinson. 2005. Information networks in the mammary gland. Nat. Rev. Mol. Cell. Biol. 6:715-725. [DOI] [PubMed] [Google Scholar]
- 23.Humphreys, R. C., B. Bierie, L. Zhao, R. Raz, D. Levy, and L. Hennighausen. 2002. Deletion of Stat3 blocks mammary gland involution and extends functional competence of the secretory epithelium in the absence of lactogenic stimuli. Endocrinology 143:3641-3650. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys, R. C., and L. Hennighausen. 1999. Signal transducer and activator of transcription 5a influences mammary epithelial cell survival and tumorigenesis. Cell Growth Differ. 10:685-694. [PubMed] [Google Scholar]
- 25.Hutchinson, J., J. Jin, R. D. Cardiff, J. R. Woodgett, and W. J. Muller. 2001. Activation of Akt (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol. Cell. Biol. 21:2203-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson, J. N., J. Jin, R. D. Cardiff, J. R. Woodgett, and W. J. Muller. 2004. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 64:3171-3178. [DOI] [PubMed] [Google Scholar]
- 27.Iavnilovitch, E., R. D. Cardiff, B. Groner, and I. Barash. 2004. Deregulation of Stat5 expression and activation causes mammary tumors in transgenic mice. Int. J. Cancer 112:607-619. [DOI] [PubMed] [Google Scholar]
- 28.Iavnilovitch, E., B. Groner, and I. Barash. 2002. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol. Cancer Res. 1:32-47. [PubMed] [Google Scholar]
- 29.Ihle, J. N. 2001. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13:211-217. [DOI] [PubMed] [Google Scholar]
- 30.LeBaron, M. J., J. Xie, and H. Rui. 2005. Evaluation of genome-wide chromatin library of Stat5 binding sites in human breast cancer. Mol. Cancer 4:6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, M., X. Liu, G. Robinson, U. Bar-Peled, K. U. Wagner, W. S. Young, L. Hennighausen, and P. A. Furth. 1997. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc. Natl. Acad. Sci. U. S. A. 94:3425-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, S., and J. M. Rosen. 1995. Nuclear factor I and mammary gland factor (STAT5) play a critical role in regulating rat whey acidic protein gene expression in transgenic mice. Mol. Cell. Biol. 15:2063-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, D. I., M. D. Lessie, A. B. Gladden, C. H. Bassing, K. U. Wagner, and J. A. Diehl. 2008. Disruption of cyclin D1 nuclear export and proteolysis accelerates mammary carcinogenesis. Oncogene 27:1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lund, L. R., J. Romer, N. Thomasset, H. Solberg, C. Pyke, M. J. Bissell, K. Dano, and Z. Werb. 1996. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 122:181-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maroulakou, I. G., W. Oemler, S. P. Naber, I. Klebba, C. Kuperwasser, and P. N. Tsichlis. 2008. Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J. Cell Physiol. 217:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maroulakou, I. G., W. Oemler, S. P. Naber, and P. N. Tsichlis. 2007. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 67:167-177. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi, K., J. M. Shillingford, G. H. Smith, S. L. Grimm, K. U. Wagner, T. Oka, J. M. Rosen, G. W. Robinson, and L. Hennighausen. 2001. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 155:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriggl, R., V. Sexl, L. Kenner, C. Duntsch, K. Stangl, S. Gingras, A. Hoffmeyer, A. Bauer, R. Piekorz, D. Wang, K. D. Bunting, E. F. Wagner, K. Sonneck, P. Valent, J. N. Ihle, and H. Beug. 2005. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell 7:87-99. [DOI] [PubMed] [Google Scholar]
- 39.Mukhopadhyay, S. S., S. L. Wyszomierski, R. M. Gronostajski, and J. M. Rosen. 2001. Differential interactions of specific nuclear factor I isoforms with the glucocorticoid receptor and STAT5 in the cooperative regulation of WAP gene transcription. Mol. Cell. Biol. 21:6859-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nevalainen, M. T., J. Xie, J. Torhorst, L. Bubendorf, P. Haas, J. Kononen, G. Sauter, and H. Rui. 2004. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J. Clin. Oncol. 22:2053-2060. [DOI] [PubMed] [Google Scholar]
- 41.Ren, S., H. R. Cai, M. Li, and P. A. Furth. 2002. Loss of Stat5a delays mammary cancer progression in a mouse model. Oncogene 21:4335-4339. [DOI] [PubMed] [Google Scholar]
- 42.Sakamoto, K., B. A. Creamer, A. A. Triplett, and K. U. Wagner. 2007. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol. Endocrinol. 21:1877-1892. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto, K., W. C. Lin, A. A. Triplett, and K. U. Wagner. 2009. Targeting Janus kinase 2 in Her2/neu-expressing mammary cancer: implications for cancer prevention and therapy. Cancer Res. 69:6642-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos, S. C., V. Lacronique, I. Bouchaert, R. Monni, O. Bernard, S. Gisselbrecht, and F. Gouilleux. 2001. Constitutively active STAT5 variants induce growth and survival of hematopoietic cells through a PI 3-kinase/Akt dependent pathway. Oncogene 20:2080-2090. [DOI] [PubMed] [Google Scholar]
- 45.Schindler, C., and J. E. Darnell. 1995. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu. Rev. Biochem. 64:621-651. [DOI] [PubMed] [Google Scholar]
- 46.Schwertfeger, K. L., M. M. Richert, and S. M. Anderson. 2001. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol. Endocrinol. 15:867-881. [DOI] [PubMed] [Google Scholar]
- 47.Shillingford, J. M., K. Miyoshi, G. W. Robinson, S. L. Grimm, J. M. Rosen, H. Neubauer, K. Pfeffer, and L. Hennighausen. 2002. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 16:563-570. [DOI] [PubMed] [Google Scholar]
- 48.Soldaini, E., S. John, S. Moro, J. Bollenbacher, U. Schindler, and W. J. Leonard. 2000. DNA binding site selection of dimeric and tetrameric Stat5 proteins reveals a large repertoire of divergent tetrameric Stat5a binding sites. Mol. Cell. Biol. 20:389-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stocklin, E., M. Wissler, F. Gouilleux, and B. Groner. 1996. Functional interactions between Stat5 and the glucocorticoid receptor. Nature 383:726-728. [DOI] [PubMed] [Google Scholar]
- 50.Wagner, K. U., A. Krempler, A. A. Triplett, Y. Qi, N. M. George, J. Zhu, and H. Rui. 2004. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in Jak2 conditional knockout mice. Mol. Cell. Biol. 24:5510-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner, K. U., and G. H. Smith. 2005. Pregnancy and stem cell behavior. J. Mammary Gland Biol. Neoplasia 10:25-36. [DOI] [PubMed] [Google Scholar]
- 52.Watson, C. J., and K. Neoh. 2008. The Stat family of transcription factors have diverse roles in mammary gland development. Semin. Cell Dev. Biol. 19:401-406. [DOI] [PubMed] [Google Scholar]
- 53.Yamaji, D., R. Na, Y. Feuermann, S. Pechhold, W. Chen, G. W. Robinson, and L. Hennighausen. 2009. Development of mammary luminal progenitor cells is controlled by the transcription factor STAT5A. Genes Dev. 23:2382-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017-1021. [DOI] [PubMed] [Google Scholar]