Abstract
Integrin α1β1 negatively regulates the generation of profibrotic reactive oxygen species (ROS) by inhibiting epidermal growth factor receptor (EGFR) activation; however, the mechanism by which it does this is unknown. In this study, we show that caveolin-1 (Cav-1), a scaffolding protein that binds integrins and controls growth factor receptor signaling, participates in integrin α1β1-mediated EGFR activation. Integrin α1-null mesangial cells (MCs) have reduced Cav-1 levels, and reexpression of the integrin α1 subunit increases Cav-1 levels, decreases EGFR activation, and reduces ROS production. Downregulation of Cav-1 in wild-type MCs increases EGFR phosphorylation and ROS synthesis, while overexpression of Cav-1 in the integrin α1-null MCs decreases EGFR-mediated ROS production. We further show that integrin α1-null MCs have increased levels of activated extracellular signal-regulated kinase (ERK), which leads to reduced activation of peroxisome proliferator-activated receptor γ (PPARγ), a transcription factor that positively regulates Cav-1 expression. Moreover, activation of PPARγ or inhibition of ERK increases Cav-1 levels in the integrin α1-null MCs. Finally, we show that glomeruli of integrin α1-null mice have reduced levels of Cav-1 and activated PPARγ but increased levels of phosphorylated EGFR both at baseline and following injury. Thus, integrin α1β1 negatively regulates EGFR activation by positively controlling Cav-1 levels, and the ERK/PPARγ axis plays a key role in regulating integrin α1β1-dependent Cav-1 expression and consequent EGFR-mediated ROS production.
Integrins are transmembrane receptors for extracellular matrix components and are composed of noncovalently bound α and β subunits. In mammals, 1 of 18 α subunits heterodimerizes with 1 of 8 β subunits to form 24 distinct integrins, each with specific but overlapping functions (4, 29). Integrins regulate cellular processes such as cell adhesion, differentiation (2), cell cycle progression (26), reactive oxygen species (ROS) production (10, 72), and extracellular matrix synthesis and remodeling (23). In addition, integrins interact with and regulate the activity of different growth factor receptors (3), and this cross talk coordinates biological processes by regulating downstream signaling pathways (8, 55, 58, 61). Integrin occupancy causes growth factor receptor autophosphorylation (44), and growth factor receptors and integrins associate following growth factor stimulation or integrin activation (5, 61, 73).
Caveolin-1 (Cav-1) is one of the three members of the caveolin family and is a structural protein involved in the formation of caveola-rich membrane domains (64). Caveolae are clearly defined, small, flask-shaped invaginations of the plasma membrane enriched in sphingolipids and cholesterol and are implicated in transcytosis, lipid metabolism, and receptor trafficking (35). Changes in plasma membrane localization and/or expression of Cav-1 can profoundly affect Cav-1-mediated functions. Different factors can regulate Cav-1 expression at both transcriptional and translational levels: transforming growth factor β (TGF-β) and epidermal growth factor (EGF), for example, negatively regulate Cav-1 expression (41, 68), while transcription factors such as FOXO or peroxisome proliferator-activated receptor gamma (PPARγ) positively control Cav-1 expression (7, 40, 67).
Cav-1 can control the activation and function of growth factor receptors (15). In this context, Cav-1 negatively regulates TGF-β-mediated signaling by promoting TGF-β receptor internalization and degradation (16). Moreover, Cav-1 positively controls the expression of insulin receptor substrate 1, a factor involved in cell proliferation and differentiation (9). Cav-1 also regulates the activation state of the EGF receptor (EGFR); however, whether this scaffolding protein serves as a positive or negative regulator of EGFR functions depends on the cell type. In some cells, increased plasma membrane expression or phosphorylation levels of Cav-1 correlate with decreased EGFR expression/activation and increased receptor internalization (37, 48, 49, 70), as seen in laryngeal squamous carcinoma cells where upregulation of Cav-1 leads to decreased EGFR-mitogen-activated protein kinase (MAPK) signaling and consequent cell growth inhibition (27). In contrast, in rat mesangial cells (MCs), Cav-1 promotes EGFR transactivation following mechanical stress (75); in tumor cells, oxidative stress leads to Cav-1 hyperphosphorylation with consequent EGFR internalization and prolonged receptor activation (34), and radiation induces Cav-1-EGFR association, nuclear EGFR transport, and DNA-protein kinase (PK)-mediated DNA repair (19).
In addition to regulating growth factor receptor-mediated functions, Cav-1 interacts with various integrins. These interactions can regulate Cav-1 redistribution and endocytosis, integrin endocytosis, and integrin-dependent signaling (20, 53). Cav-1 has been shown to mediate EGFR-promoted internalization of integrins (45), and induction of integrin α2β1 clustering triggers its redistribution from membrane rafts to caveolae and subsequent protein kinase C-mediated internalization (66). In endothelial cells, Cav-1 is essential for integrin αvβ3-mediated activation of PI3-K/Akt (56), and Cav-1-driven integrin β1 endocytosis is a critical step for regulating fibronectin turnover (57).
The collagen-binding receptor integrin α1β1 functions as a negative regulator of EGFR, and deletion of integrin α1β1 in MCs leads to increased ligand-independent EGFR phosphorylation, with a consequent increase in ROS production and collagen deposition (10). One reason for increased EGFR activation in integrin α1-null MCs is because integrin α1β1 is required for the recruitment and activation of T-cell protein tyrosine phosphatase (TCPTP), which leads to EGFR dephosphorylation (43). In this study, we determined whether Cav-1 might also cooperate with integrin α1β1 to negatively regulate EGFR activation, since Cav-1 interacts with integrins and can act as a negative regulator of EGFR activation/function (10, 27, 30, 37, 48, 69). We provide evidence that integrin α1-null MCs have reduced levels of total and plasma membrane-associated Cav-1, and reexpression of the integrin α1 subunit increases the levels of total and plasma membrane-associated Cav-1, decreases EGFR activation, and reduces ROS production. When Cav-1 is overexpressed in integrin α1-null MCs, it localizes to caveola-rich fractions and leads to decreased EGFR activation and ROS production. We show that the decreased level of Cav-1 in the integrin α1-null MCs is due to increased basal levels of activated extracellular signal-regulated kinase (ERK), which causes decreased nuclear translocation and activation of PPARγ, a transcription factor that positively regulates Cav-1 expression (7, 40, 62). Finally, we show that injured glomeruli from integrin α1-null mice have reduced levels of Cav-1 and nuclear PPARγ and concomitant increased levels of phosphorylated EGFR (pEGFR). All together, these results indicate that integrin α1β1 negatively regulates EGFR activation by controlling the levels of Cav-1 and that the ERK/PPARγ axis plays a key role in regulating integrin α1β1-dependent Cav-1 expression and consequent EGFR-mediated ROS production.
MATERIALS AND METHODS
Cell culture.
Primary MCs were isolated from wild-type and integrin α1-null SV129 mice or from wild-type and Cav-1-null C57BL/6 mice, as previously described (11), and used for experiments at passages 2 to 4. Immortalized MCs were isolated from wild-type and integrin α1-null mice, crossed onto the Immortomouse background as previously described (11), and propagated at 33°C in the presence of 100 IU/ml gamma interferon. For experiments, cells were cultured at 37°C without gamma interferon for at least 4 days before use, as this is the optimal time for immortalized MCs to acquire a phenotype similar to that of freshly isolated primary MCs. Populations of integrin α1-null MCs expressing the human integrin α1 cDNA (α1KO-Rec cells) were generated as previously described (10).
Antibodies.
Anti-human integrin α1 TS2/7 and MAB1973 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Chemicon (Temecula, CA), respectively. Anti-phosphorylated EGFR (anti-pY1173), anti-Cav-1, anti-PPARγ, anti-β-actin, anti-N-cadherin, and anti-poly(ADP-ribose) polymerase (anti-PARP) antibodies were purchased from Santa Cruz Biotechnology. Anti-ERK antibody was purchased from Cell Signaling (Beverly, MA), anti-mouse integrin β1 was obtained from Chemicon, and anti-EGFR antibody was purchased from Upstate Biotechnology (Lake Placid, NY).
ROS detection.
ROS production was measured with dihydrorhodamine as previously described (11). Briefly, 1.5 × 105 MCs were plated in six-well plates in Dulbecco's modified Eagle's medium (DMEM) containing 1% fetal calf serum (FCS). After 2 days, the cells were incubated in serum-free medium with or without the EGFR kinase inhibitor AG1478 (300 nM; Calbiochem). After 24 h, 2 μM dihydrorhodamine (Sigma) was added to the cells, and after 2 h the cells were trypsinized and the generation of fluorescent rhodamine 123 was analyzed by a FACScan. Three independent experiments were performed in triplicate.
Luciferase assays.
To assess the basal levels of PPARγ activation, MCs were simultaneously transfected using Effectene (Qiagen, Valencia, CA) with a Renilla luciferase-thymidine kinase plasmid (Promega, Madison, WI) and a firefly luciferase plasmid under a promoter containing a PPARγ response element derived from the PPARγ target acyl coenzyme A (acyl-CoA) oxidase gene (generous gift of Lijun Ma, Vanderbilt University) (31). Twenty-four hours after transfection, the cells were washed and incubated in the presence of 1% FCS with or without the MEK inhibitor PD98059 (0 to 30 μM). Twenty-four hours later, the cells were trypsinized and suspended in medium containing 1% fetal bovine serum (FBS) and 60 μM EnduRen live cell substrate (Promega, Madison, WI). After 3 h at 37°C, Renilla activity was measured in live cells using a TD-20/20 luminometer. The cells were then quickly pelleted and lysed, and the firefly luciferase activity was measured using the Luciferase Assay System (Promega, Madison, WI). Firefly luciferase units were normalized for Renilla luciferase activity to correct for differences in transfection efficiency. Four independent experiments were performed in triplicate.
Generation of MCs overexpressing Cav-1.
To overexpress Cav-1 in MCs, full-length human myc-tagged Cav-1 (myc-Cav-1; generous gift of Richard G. W. Anderson, University of Texas Southwestern Medical Center) was cloned into pcDNA4.1 and transfected into wild-type or integrin α1-null MCs using Effectene transfection reagent (Qiagen, Valencia, CA). Cells were selected with Zeocin (Invitrogen, Carlsbad, CA) at 200 to 400 μg/ml for 2 weeks, and Zeocin-resistant clones were isolated and screened by Western blotting for the expression of myc-tagged Cav-1. Three clones of each cell line expressing comparable levels of myc-Cav-1 were chosen for experiments.
Inhibition of Cav-1 expression by siRNA.
The murine Cav-1 sequences 5′-GGUGAUGAUGUCAUACAAATT-3′ (primer 1), 5′-GGAGAUUGACCUGGUCAACTT-3′ (primer 2), and 5′-CCAUCAAUUUGGAGACUAUTT-3′ (primer 3) were targeted for RNA interference. Double-stranded small interfering RNAs (siRNAs) were obtained from Ambion. Subconfluent populations of MCs were transfected with a mixture of 1 + 2 and 1 + 3 Cav-1 siRNAs (15 nM each) or glyceraldehyde-3-phosphate dehydrogenase siRNA (30 nM) as a control with the Effectene transfection reagent (Qiagen). After 72 h, cells were serum starved for 24 h and equal amounts of cell lysates (20 μg/lane) were analyzed by Western blotting for levels of Cav-1, ERK, pEGFR, and EGFR.
Treatment with kinase inhibitors and PPARγ ligands.
To determine the contribution of ERK in the regulation of Cav-1 expression, MCs were treated with the MEK inhibitor PD98059 (Calbiochem Corp., La Jolla, CA) for 24 h at a concentration of 20 μM in the absence of serum before Western blot analysis. To assess the role of PPARγ in the regulation of Cav-1 expression, wild-type and integrin α1-null MCs were treated with the PPARγ inhibitor GW9662 (4 to 16 nM; Tocris, MO) (38) and with the PPARγ agonist pioglitazone (2 to 4 μM; Takeda, Japan) (14, 40) in serum-free medium, respectively. Forty-eight hours later, the levels of total Cav-1 and nuclear PPARγ were analyzed by Western blot analysis as described below.
Western blot analysis.
To detect levels of Cav-1 or activated ERK, equal amounts of cell lysates (20 μg/lane) from serum-starved MCs were subjected to 10% SDS-PAGE and subsequently transferred to a nitrocellulose membrane and incubated with anti-Cav-1 or anti-phosphorylated ERK (anti-pERK) antibody. Immunoreactive proteins were visualized with an appropriate peroxidase-conjugated secondary antibody and an ECL kit. Equal loading was confirmed by reprobing the membranes with anti-ERK antibodies.
To assess the expression levels of Cav-1 in Triton-insoluble fractions of MCs, lysates were prepared as described previously (60). Briefly, MCs were scraped in lysis buffer (10 mM HEPES [pH 7.2], 1% Triton X-100, 100 mM NaCl, 2 mM EDTA, plus proteinase and phosphatase inhibitors), incubated at 4°C for 20 min, and centrifuged at 10,000 × g for 20 min. The pellet was subsequently dissolved in solubilization buffer (10 mM HEPES [pH 7.2], 1% SDS, 100 mM NaCl, 2 mM EDTA, plus proteinase and phosphatase inhibitors), briefly sonicated, incubated at 4°C for 20 min, and centrifuged at 10,000 × g for 20 min. The supernatant was collected and considered to be the Triton-insoluble fraction. Equal amounts of Triton-insoluble fractions (20 μg/lane) were analyzed by Western blotting for levels of Cav-1 as described above. Anti-N-cadherin antibodies were used to ensure the presence of this marker, primarily in detergent-insoluble fractions.
Nuclear protein preparation.
Nuclear proteins were extracted as previously reported (18). Serum-starved MCs were scraped off the plates and spun at 1,000 rpm for 5 min. Cell pellets were suspended in buffer A (20 mM HEPES [pH 7.9], 10 mM NaCl, 3 mM MgCl2, 0.2 mM EDTA, 1 mM dithiothreitol [DTT], 0.5% NP-40, 10% glycerol, proteinase and phosphatase inhibitors), incubated for 30 min at room temperature, and centrifuged at 1,000 rpm for 10 min. Upon removal of the supernatants (cytoplasmic extracts), the pellets were washed three times with buffer B (20 mM HEPES [pH 7.9], 0.2 mM EDTA, 1 mM DTT, 20% glycerol, proteinase and phosphatase inhibitors) and then suspended in buffer C (20 mM HEPES [pH 7.9], 400 mM NaCl, 0.2 mM EDTA, 1 mM DTT, 20% glycerol, proteinase and phosphatase inhibitors). Samples were incubated for 30 min at 4°C and then centrifuged at 13,000 rpm for 20 min. Supernatants were collected as nuclear extracts. Equal amounts of cytoplasmic and nuclear extracts were analyzed for levels of PPARγ by using Western blotting and anti-PPARγ antibody. Anti-ERK and anti-PARP (an RNA polymerase) antibodies were used to validate the purity of the cytoplasm and nuclear protein preparations, respectively.
Purification of caveolin-enriched fractions.
Caveolin-enriched membrane fractions were prepared as described previously (32). Serum-starved MCs were scraped off the plates and spun at 1,000 rpm for 5 min. Pellets were suspended in 2 ml Na2CO3 (500 mM, pH 11.0), homogenized using a Dounce homogenizer, and sonicated. The homogenate was then adjusted to 45% sucrose by the addition of 2 ml 90% sucrose in MES (morpholineethanesulfonic acid)-buffered saline (MBS) (25 mM MES [pH 6.5], 150 mM NaCl) and placed at the bottom of an ultracentrifuge tube. A 5 to 35% discontinuous sucrose gradient was formed by the addition of 4 ml 5% sucrose and 4 ml 35% sucrose, both in MBS containing 250 mM Na2CO3. After centrifugation at 100,000 × g for 16 to 20 h, 12 1-ml fractions were collected and equal volumes of each fraction were analyzed by Western blotting for levels of integrin α1 (anti-human integrin α1, MAB1973), integrin β1 (anti-mouse integrin β1), phosphorylated EGFR (anti-pY1173 antibody), EGFR, Cav-1, and ERK.
Models of glomerular injury.
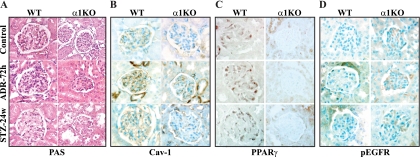
All experiments were performed according to institutional animal care guidelines. Wild-type and integrin α1-null BALB/c male mice (5 weeks old, ∼20 g body weight) received a single intravenous injection of doxorubicin (Adriamycin [ADR; Sigma, St. Louis, MO] at 10 mg/kg) or streptozotocin (STZ) (100 mg/kg; Sigma) as described previously (11, 74). Kidneys from 72-h-ADR-treated and 24-week-STZ-treated mice were used for analysis of Cav-1, PPARγ, and activated EGFR levels, as at these time points glomerular injury (characterized by mesangiolysis and glomerulosclerosis, respectively) is more severe in the integrin α1-null mice than in their wild-type counterparts (11, 74).
Immunofluorescence and immunohistochemistry.
To analyze Cav-1 localization, MCs were plated on chamber slides. After 3 days, the cells were serum starved for 24 h, fixed in 4% formaldehyde for 10 min, and permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) for 5 min. After the cells were blocked with 3% bovine serum albumin in PBS, they were incubated with anti-mouse Cav-1 together with anti-rabbit N-cadherin (both at 1:500), followed by the appropriate fluorescein isothiocyanate- or rhodamine isothiocyanate-conjugated secondary antibodies (Calbiochem). Slides were mounted with antifade mounting medium (Vectastain; Vector Labs) and analyzed under an epifluorescence microscope (Nikon).
Immunohistochemistry on paraffin kidney sections (5 μm) was done using anti-mouse Cav-1 (1:400), anti-pEGFR (1:50), or anti-mouse PPARγ (1:50), followed by appropriate horseradish peroxidase-conjugated secondary antibodies (1:200; Jackson Immunoresearch, West Grove, PA) and Sigma Fast diaminobenzidine chromogenic tablets (Sigma).
Immunofluorescence analysis of frozen kidney sections (5 μm) was done using anti-mouse Cav-1 (1:1,000), anti-pEGFR (1:50), or anti-mouse PPARγ (1:50), followed by appropriate fluorescein isothiocyanate- or rhodamine isothiocyanate-conjugated secondary antibodies (1:400). The number of glomerular cells with positive nuclear PPARγ staining was evaluated by manual counting and expressed as the average number of cells with nuclear PPARγ staining per glomerulus. The glomerular area occupied by Cav-1- or pEGFR-positive structures was evaluated using Scion Image analysis as previously described (50) and expressed as the percentage of area occupied by Cav-1- or pEGFR-positive structures. Three kidneys per genotype were analyzed for each treatment, and a total of 30 glomeruli per treatment were analyzed.
Statistical analysis.
The Student t test for comparisons between two groups and analysis of variance using Sigma-Stat software for statistical differences between multiple groups were used. A P value of ≤0.05 was considered statistically significant.
RESULTS
Reduced levels of Cav-1 in integrin α1-null MCs.
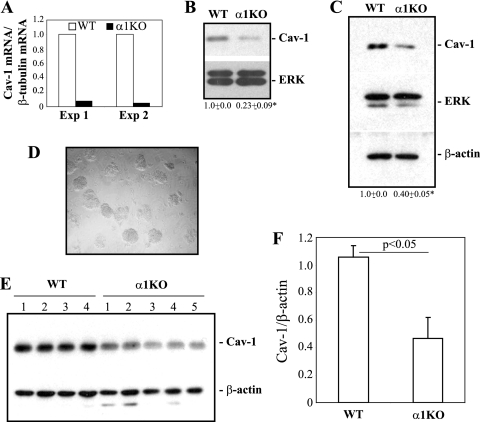
Based on the findings that (i) Cav-1 binding to tyrosine kinase receptors leads to downregulation of expression and/or activity of these signaling molecules (13, 35, 36), (ii) upregulation of Cav-1 in epithelial cells directly correlates with inhibition of EGFR activation (1, 47, 48, 69), and (iii) integrin α1-null MCs have increased basal levels of activated EGFR activation (10), we analyzed the basal levels of Cav-1 mRNA and protein in immortalized wild-type and integrin α1-null MCs. We show that integrin α1-null MCs have reduced levels of Cav-1 mRNA (Fig. 1 A) and protein (Fig. 1B) compared to their wild-type counterparts.
FIG. 1.
Reduced levels of Cav-1 in integrin α1-null MCs. (A) The levels of Cav-1 mRNA in the immortalized MCs indicated were analyzed by real-time PCR and normalized to β-tubulin message. The values are fold changes of Cav-1 mRNA relative to that of wild-type (WT) cells and represent two independent experiments. (B and C) Total cell lysates from the indicated immortalized (B) or primary (C) MCs (20 μg/lane) were analyzed for levels of Cav-1. Membranes were reincubated with anti-ERK and/or β-actin antibody to verify loading. Cav-1 and ERK bands were quantified by densitometry analysis, and the Cav-1 signal is expressed as a Cav-1/ERK ratio. Values are the means ± standard deviations (SDs) of results of three experiments and represent fold changes relative to WT cells. *, significant difference (P < 0.05) between WT and integrin α1KO cells. (D and E) Glomeruli (D) were isolated from 4 wild-type and 5 integrin α1-null mice and analyzed by Western blotting for basal levels of Cav-1 (E). (F) Cav-1 and β-actin bands were quantified by densitometry analysis, and the Cav-1 signal is expressed as described for panels B and C.
To confirm the generality and in vivo relevance of this finding, the levels of Cav-1 were analyzed in primary cultures of MCs as well as in isolated glomeruli obtained from wild-type and integrin α1-null mice. Significantly decreased levels of Cav-1 were evident in both primary MCs and glomeruli isolated from integrin α1-null mice (Fig. 1C to F). As both primary and immortalized integrin α1-null MCs have decreased Cav-1 levels compared to primary or immortalized wild-type cells, we utilized immortalized MCs for further studies, as they grow easily and can be manipulated genetically and biochemically.
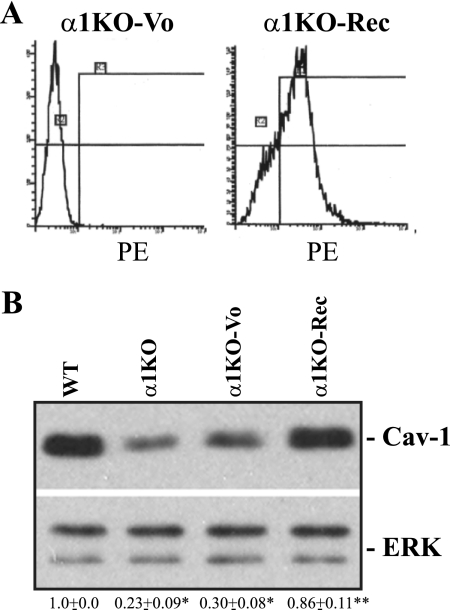
To determine whether the phenotype of integrin α1-null MCs was due to loss of α1β1, we reexpressed the human integrin α1 subunit into integrin α1-null cells (α1KO-Rec cells) (Fig. 2 A). Similar to wild-type cells, α1KO-Rec cells expressed more Cav-1 than integrin α1-null or vector-transfected integrin α1-null MCs (α1KO-Vo cells) (Fig. 2B). Next, we determined the localization of Cav-1 by performing double immunofluorescence staining on MCs with anti-Cav-1 and anti-N-cadherin antibodies. Whereas Cav-1 and N-cadherin were visualized primarily on the plasma membrane of wild-type and α1KO-Rec cells, no Cav-1 staining was evident on the plasma membrane of integrin α1-null cells (see Fig. S1A in the supplemental material). Triton-insoluble fractions confirmed significantly decreased levels of Cav-1 in fractions of integrin α1-null MCs compared to wild-type and α1KO-Rec cells (Fig. S1B). Thus, (i) integrin α1-null MCs have decreased total and plasma membrane-associated levels of Cav-1, and (ii) decreased levels of Cav-1 in integrin α1-null MCs are a direct consequence of loss of integrin α1β1.
FIG. 2.
Decreased levels of Cav-1 in integrin α1-null MCs are a direct consequence of the loss of integrin α1β1. (A) Integrin α1-null MCs were transfected with either the empty vector (α1KO-Vo) or the human integrin α1 subunit (α1KO-Rec) cDNA, and cell populations expressing the integrin α1 subunit were sorted with a fluorescence-activated cell sorter. PE, phycoerythrin. (B) Total cell lysates from the MCs indicated (20 μg/lane) were analyzed for levels of Cav-1. The levels of Cav-1 were quantified as described in the legend to Fig. 1. Values are the means ± SDs of results of three experiments and represent fold changes relative to WT cells. Differences between WT and α1KO or α1KO-Vo cells (*) and between α1KO-Vo and α1KO-Rec cells (**) were significant (P < 0.05).
Decreased levels of Cav-1 in integrin α1-null MCs correlates with increased caveola-associated EGFR.
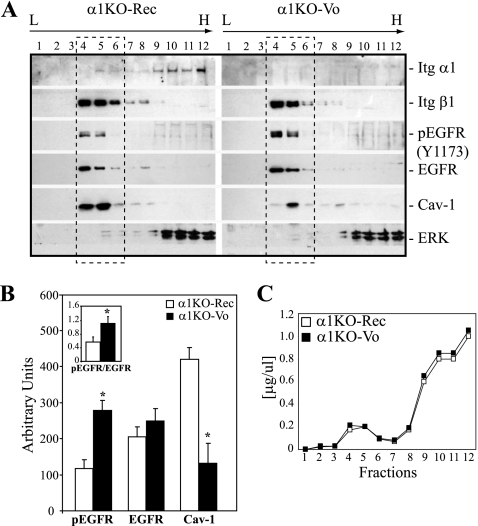
Since EGFR, Cav-1, and integrins reside in caveolae, we performed buoyant density cell fractionation to determine the presence and the levels of these three membrane-associated molecules in the same caveola-rich fractions. α1KO-Rec cells were used, as their expression of the human integrin α1 subunit can be easily detected by commercially available antibodies. As shown in Fig. 3 A, integrin α1 and β1 subunits, as well as EGFR, were found in the lighter caveola-rich fractions (fractions 4 to 6; confirmed by immunoblotting with Cav-1). Significantly decreased levels of total Cav-1 were found in fractions 4 to 6 of α1KO-Vo cells (Fig. 3A and B). Importantly, the levels of Cav-1 in fractions 4 to 6 of α1KO-Vo MCs were inversely correlated to those of activated EGFR (Fig. 3A and B). Figure 3C shows comparable protein contents in fractions collected from α1KO-Rec and α1KO-Vo cells. Thus, integrin α1-null MCs have reduced caveola-bound Cav-1 that might account for the increased levels of membrane-associated and activated EGFR.
FIG. 3.
Decreased caveola-associated Cav-1 in integrin α1-null MCs correlates with increased caveola-associated EGFR. (A) Cav-1-enriched membrane fractions were prepared from the cells indicated using detergent-free buffers and sucrose gradient centrifugation (see Materials and Methods). Twelve fractions were collected, and equal volumes were subjected to Western blotting using the antibodies indicated. Note that cells lacking integrin α1β1 (α1KO-Vo) have more phosphorylated EGFR and less Cav-1 in the light caveola-rich fractions (fractions 4 to 6). Itg, integrin. (B) Densitometry analysis of the proteins indicated was performed for fractions 4 to 6. Values are the means ± SDs of results of three experiments and are expressed as arbitrary units. The insert represents the ratio of pEGFR to EGFR in α1KO-Vo and α1KO-Rec cells. *, significant difference (P < 0.05) between α1KO-Vo and α1KO-Rec cells. (C) Profile of the protein concentration of the 12 fractions described for panel A, showing comparable levels of protein content in each fraction.
Downregulation of Cav-1 in wild-type MCs leads to increased EGFR activation.
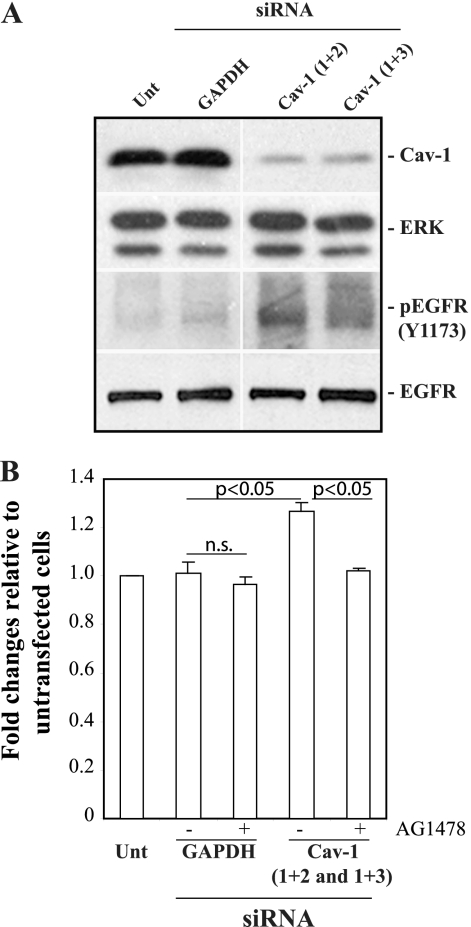
To further confirm the inverse relationship between Cav-1 and EGFR activation, the levels of Cav-1 were downregulated by siRNA in wild-type MCs. Cav-1 siRNA-treated wild-type cells showed decreased total Cav-1 levels, with consequent increased basal levels of activated EGFR (Fig. 4 A). Since we showed that the levels of activated EGFR in MCs directly correlate to ROS production (10), we evaluated the levels of ROS in control and Cav-1 siRNA-treated MCs. Increased levels of ROS were observed in wild-type MCs following downregulation of Cav-1 expression (Fig. 4B). Importantly, the increased ROS levels in cells treated with siRNA Cav-1 reverted to the levels of untreated wild-type cells upon treatment with the EGFR kinase inhibitor AG1478, suggesting that the increase in ROS after Cav-1 suppression is an EGFR-dependent event (Fig. 4B).
FIG. 4.
Downregulation of Cav-1 in wild-type MCs leads to increased EGFR activation and ROS production. (A) Cell lysates (20 μg/lane) of serum-starved MCs either untransfected (Unt) or transfected for 72 h with GAPDH (glyceraldehyde-3-phosphate dehydrogenase) siRNAs or Cav-1 siRNA (1 + 2 and 1 + 3 represent the mixture of siRNAs used, as described in Materials and Methods) were analyzed by Western blotting with anti-Cav-1, anti-ERK, anti-pY1173, and anti-EGFR antibodies. The results of one representative experiment of three independent experiments are shown. (B) MCs were transfected with the siRNA indicated or left untransfected. After 2 days, the cells were incubated in serum-free medium with or without the EGFR kinase inhibitor AG1478 (300 nM). After 24 h, 2 μM dihydrorhodamine was added to the wells and ROS generation was evaluated. Values represent the means ± SDs of results of three independent experiments performed in triplicate and are expressed as fold changes relative to untransfected cells. n.s., not significant.
To confirm the validity of the siRNA finding, the levels of activated EGFR and basal ROS were analyzed in primary cultures of MCs isolated from Cav-1-null mice. These MCs also showed higher levels of activated EGFR and ROS than primary wild-type MCs (see Fig. S2 in the supplemental material). Thus, Cav-1 controls the activation state and the function of EGFR in MCs.
Integrin α1β1 regulates the expression but not the localization of Cav-1.
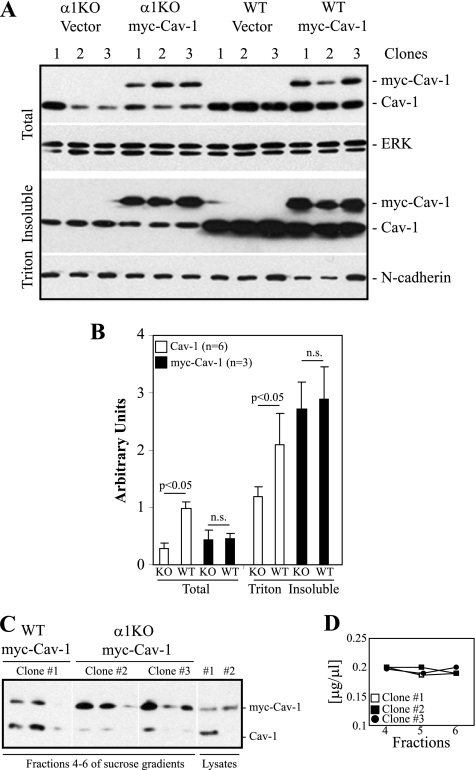
As most of the Cav-1 in wild-type MCs is found in Triton-insoluble fractions (see Fig. S1B in the supplemental material) and is plasma membrane associated (Fig. S1A), we hypothesized that integrin α1β1 might play a key role in regulating both the levels and the plasma membrane localization of Cav-1. To test this, we generated clones of wild-type and integrin α1-null MCs expressing comparable total levels of Cav-1 tagged to myc (myc-Cav-1) (Fig. 5 A, top, and Fig. 5B [three clones per genotype are shown]). Interestingly, comparable levels of myc-Cav-1 were observed in Triton-insoluble fractions derived from myc-Cav-1-transfected wild-type and integrin α1-null MCs (Fig. 5A, bottom, and Fig. 5B). Similarly, comparable levels of myc-Cav-1 were detected in lighter caveola-rich fractions derived from transfected wild-type (Fig. 5C, clone 1) and integrin α1-null (Fig. 5C, clones 2 and 3) MCs. Figure 5D shows comparable protein contents in fractions collected from the various clones analyzed. These results strongly suggest that integrin α1β1 controls the levels of Cav-1 but not the localization of this scaffolding protein to caveolae.
FIG. 5.
Integrin α1β1 is not required for the localization of Cav-1 to caveolae. (A) Wild-type and integrin α1KO MCs were transfected with an empty vector (Vector) or with a construct carrying the Cav-1 cDNA tagged to myc (myc-Cav-1). (A) Total cell lysates and Triton-insoluble fractions of 3 vector-expressing or 3 myc-Cav-1-expressing clones were analyzed by Western blotting with anti-Cav-1, anti-ERK, and anti-N-cadherin antibodies. (B) Endogenous Cav-1 (6 samples/genotype) as well as myc-Cav-1 (3 samples/genotype) and ERK (for total fractions) or N-cadherin (for Triton-insoluble fractions) bands were quantified by densitometry analysis, and the Cav-1 signal was expressed as described in the legend to Fig. 1. Note that comparable high levels of myc-Cav-1 are found in the Triton-insoluble fractions of both WT and α1KO myc-Cav-1-expressing cells. (C) Cav-1-enriched membrane fractions were prepared as described in the legend to Fig. 3. Analysis of fractions 4 to 6 revealed comparable levels of caveola-associated myc-Cav-1 in all myc-Cav-1-expressing clones evaluated (clone 1 is shown for WT myc-Cav-1-expressing cells, while clones 2 and 3 are shown for α1KO myc-Cav-1-expressing cells). (D) Protein profile of fractions 4 to 6 showing comparable protein contents for all the clones analyzed.
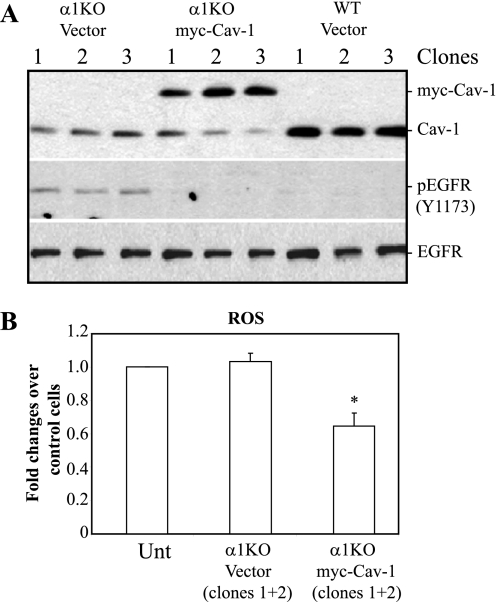
To confirm the functionality of increased levels of membrane-associated myc-Cav-1 in the integrin α1-null MCs, the levels of activated EGFR and ROS were analyzed. As shown in Fig. 6, clones of integrin α1-null cells expressing myc-Cav-1 (3 clones shown) showed decreased levels of activated EGFR (Fig. 6A) and reduced ROS production (Fig. 6B) compared to vector control-transfected cells, confirming the functionality of myc-Cav-1 in integrin α1-null MCs.
FIG. 6.
Decreased EGFR activation and ROS production in integrin α1-null MCs overexpressing myc-Cav-1. (A) Total cell lysates (20 μg/ml) of the cells indicated (3 clones shown) were analyzed by Western blotting with anti-Cav-1, anti-pY1173, and anti-EGFR antibodies. Note that α1KO myc-Cav-1-expressing cells show reduced EGFR activation compared to cells transfected with vector only. (B) The MCs indicated were plated in six-well plates at a density of 1.5 × 105 cells/well in DMEM containing 1% FCS. After 2 days, 2 μM dihydrorhodamine was added to the wells and ROS generation was evaluated. Values represent the means ± SDs of results of one experiment performed in triplicate and are expressed as fold changes relative to ROS generated by untransfected (Unt) α1KO cells.
Integrin α1β1 regulates Cav-1 levels via the ERK/PPARγ axis.
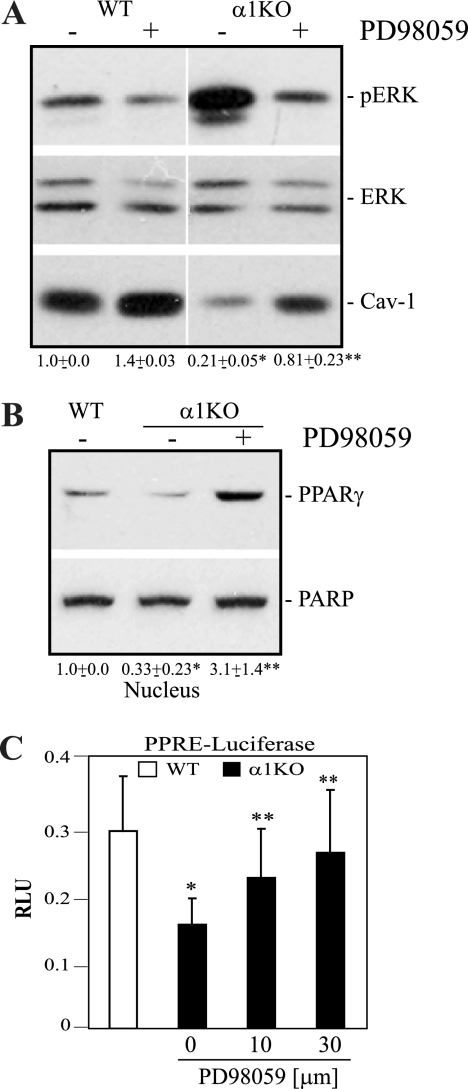
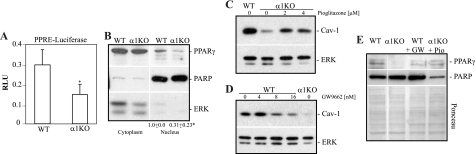
We next determined the pathways whereby integrin α1β1 controls Cav-1 levels. PPARγ was recently identified as a transcription factor that positively regulates Cav-1 expression (7, 40, 62). Moreover, activated ERK negatively controls PPARγ activity either by inhibiting phosphorylation of this transcription factor or by preventing its nuclear localization (6). Since we previously showed that integrin α1-null MCs have increased basal levels of activated ERK (12) (see Fig. 8A), we determined whether there is a possible link between decreased PPARγ activity and increased ERK activation in the integrin α1-null MCs. We initially analyzed the basal nuclear levels and activation state of PPARγ in wild-type and integrin α1-null MCs. Using a PPARγ-selective luciferase reporter assay, we detected a significant reduction in basal levels of PPARγ activity in the integrin α1-null cells compared to that in their wild-type counterparts (Fig. 7 A), which directly correlated with a significant decrease in nuclear localization of this transcription factor (Fig. 7B). To further corroborate the loss of PPARγ activity with decreased Cav-1 levels in the integrin α1-null MCs, we treated integrin α1-null cells with the PPARγ agonist pioglitazone and wild-type cells with the PPARγ inhibitor GW9662. Treatment of integrin α1-null cells with pioglitazone resulted in increased Cav-1 levels and nuclear PPARγ localization (Fig. 7C and E), while G9662-treated wild-type cells showed reduced Cav-1 levels and nuclear PAPRγ localization (Fig. 7D and E). Thus, loss of integrin α1β1 leads to decreased nuclear localization and activity of PPARγ and consequent decreased Cav-1 levels.
FIG. 8.
Increased ERK activation in the integrin α1-null MCs is responsible for decreased PPARγ activity and Cav-1 levels. (A and B) The cells indicated were either left untreated or treated with 20 μM PD98059. Twenty-four hours later, total cell lysates (A) or nuclear extracts (B) were analyzed by Western blotting for levels of pERK, ERK, Cav-1, PPARγ, and PARP. Values in panels A (Cav-1/ERK) and B (PPARγ/PARP) are the means ± SDs of results of three independent experiments and represent changes relative to WT cells. Differences between wild-type and α1KO cells (*) or untreated and PD98059-treated α1KO cells (**) were significant, with a P of <0.05. (C) Cells were cotransfected with the luciferase constructs described in the legend to Fig. 7 and incubated 24 h later with or without PD98059 at the concentrations indicated. Twenty-four hours later, luciferase activity was evaluated and normalized as described in the legend to Fig. 7. The asterisks are as described for panels A and B. RLU, relative luciferase units.
FIG. 7.
Decreased basal PPARγ activity in integrin α1-null MCs is responsible for decreased levels of Cav-1. (A) MCs were cotransfected with a Renilla luciferase-thymidine kinase plasmid and a firefly luciferase plasmid under a promoter containing a PPARγ response element (PPRE). Twenty-four hours after transfection, luciferase activity was evaluated as described in Materials and Methods. Firefly luciferase units were normalized for Renilla luciferase activity to correct for differences in transfection efficiency. Values are the means ± SDs of results of four independent experiments performed in triplicate. *, significant differences (P < 0.05) between wild-type and integrin α1KO cells. (B) Cytoplasmic and nuclear extracts of wild-type and integrin α1-null MCs were analyzed by Western blotting for levels of PPARγ. Equal loading and purity of the preparations were verified by incubating the membranes with anti-ERK or anti-PARP antibodies. PPARγ and PARP bands in nuclear fractions were quantified by densitometry analysis, and the PPARγ signal is expressed as the PPARγ/PARP ratio. Values are the means ± SDs of results of three experiments and represent changes relative to wild-type cells. The asterisk is as described for panel A. (C and D) Wild-type and integrin α1-null MCs were treated with either pioglitazone (Pio) (C) or GW9662 (GW) (D) at the concentrations indicated. Twenty-four hours later, total cell lysates were analyzed by Western blotting for levels of Cav-1 and ERK. (E) The cells indicated were treated with either pioglitazone (4 μM) or GW9662 (16 nM) as described above, and nuclear extracts were analyzed by Western blotting for levels of PPARγ. Equal loading was verified by Ponceau staining rather than immunoblotting for PARP, as PPARγ activation leads to PARP cleavage and downregulation (24, 51). Vertical lines indicate a repositioned gel lane.
To determine whether the decreased activity of PPARγ was due to increased ERK activation, integrin α1-null cells were treated with the MEK inhibitors UO126 (not shown) or PD98059 (Fig. 8) and the levels of total Cav-1 as well as nuclear PPARγ were analyzed. PD98059 treatment resulted in decreased basal ERK activation (Fig. 8A), increased Cav-1 levels (Fig. 8A), and concomitant nuclear localization of PPARγ (Fig. 8B). Treatment with PD98059 also resulted in increased basal PPARγ activity (Fig. 8C), strongly indicating that increased ERK activation in the integrin α1-null MCs is responsible for reduced nuclear PPARγ localization and activity as well as reduced Cav-1 expression.
Reduced levels of Cav-1 and increased levels of activated EGFR in glomeruli of integrin α1-null mice.
We confirmed the in vivo relevance of our in vitro finding by analyzing the expression levels of Cav-1, nuclear PPARγ, and activated EGFR in the glomeruli of wild-type and integrin α1-null mice in two models of glomerular injury (Fig. 9 A). Immunohistochemistry performed on paraffin kidney sections revealed reduced levels of Cav-1 and nuclear PPARγ with concomitant increased levels of phosphorylated EGFR in the glomeruli of integrin α1-null mice both at baseline and following renal injury (Fig. 9B to D), and these findings correlate with the increased glomerulosclerosis and ROS production previously reported in injured integrin α1-null mice (11, 74). Immunofluorescence analysis of frozen kidney sections was also performed to validate and better quantify the immunohistochemistry data. As shown in Fig. S3 in the supplemental material, analysis of frozen kidney sections showed a significant reduction in the levels of Cav-1 and nuclear PPARγ, with concomitant significantly increased levels of pEGFR in glomeruli of integrin α1-null mice both at baseline and following injury. Thus, these in vivo findings, which parallel our in vitro observations, suggest that lack of integrin α1β1 expression decreases PPARγ-dependent Cav-1 expression, with consequent increased EGFR activation, ROS production, and fibrosis.
FIG. 9.
Decreased Cav-1 and nuclear PPARγ with increased phosphorylated EGFR levels in the glomeruli of injured integrin α1-null mice. (A) Histological images of glomeruli from wild-type and integrin α1-null mice untreated (control) or treated with doxorubicin (Adriamycin [ADR]) or streptozotocin (STZ) for 72 h or 24 weeks, respectively. (B to D) Cav-1, PPARγ, and pEGFR staining of kidney sections from wild-type and integrin α1-null mice at baseline or after ADR or STZ treatment. Increased pEGFR staining with concomitant decreased Cav-1 and nuclear PPARγ staining was observed primarily in the kidneys of integrin α1-null mice at baseline and persisted in injured mice. Original magnification, ×600.
DISCUSSION
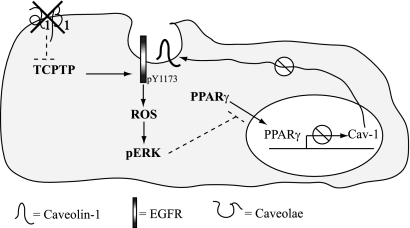
Integrin α1β1 acts as a negative regulator of EGFR-mediated signaling. We and others previously showed that this collagen receptor negatively regulates EGFR activation by binding and activating the phosphatase TCPTP (10, 43). In this paper, we provide evidence that Cav-1 represents another mechanism whereby integrin α1β1 controls EGFR-mediated signaling. In particular, we show that integrin α1β1 negatively regulates EGFR-mediated ROS production by acting as a positive regulator of Cav-1 expression, although it is dispensable for its plasma membrane localization. Based on our previous study (10) and the present findings, we propose that (i) in the absence of integrin α1β1, loss of TCPTP recruitment/activation leads to increased basal levels of EGFR activation (10), (ii) increased EGFR activation leads to upregulated ROS production and ERK activation (10), (iii) increased ERK activation leads to decreased nuclear translocation and activation of PPARγ, and (iv) reduced PPARγ activation leads to decreased Cav-1 levels and further upregulation of EGFR activation (see Fig. 10 for details). Thus, integrin α1β1 can negatively regulate the EGFR/ROS axis in MCs by activating TCPTP and modulating Cav-1 expression in an ERK/PPARγ-dependent manner.
FIG. 10.
Regulation of Cav-1 levels by integrin α1β1. Lack of integrin α1β1 results in loss of TCPTP recruitment with increased basal EGFR activation and ROS production. This leads to increased ERK activation with concomitant decreased nuclear PPARγ localization and activity. This in turn leads to decreased Cav-1 levels and further increased EGFR activation and ROS production.
The role of the EGF/EGFR axis in renal injury is controversial. After ischemic injury or toxic nephropathy, renal EGFR expression increases and exogenously administered EGFR ligands accelerate the recovery of tubular function (28). In addition, waved-2 mice, which express a functionally hypomorphic EGFR (42), have reduced renal regenerative capacity following acute toxic tubule damage (71). On the other hand, orpk mice (which develop renal cysts) crossed with the waved-2 mice have decreased EGFR activation and tubular cyst formation (52). Mice overexpressing a dominant negative EGFR in the proximal tubules are resistant to injury following subtotal nephrectomy or renal ischemia (63). Moreover, the EGFR kinase inhibitor gefitinib reduces glomerular collagen expression and the decline of renal function in hypertensive rodents (22). Finally, our data show that (i) increased basal EGFR activation in integrin α1-null MCs contributes to upregulated ROS production (10), (ii) increased expression of activated EGFR is observed in the glomeruli of injured integrin α1-null mice (present study), and (iii) downregulation of EGFR activation by Cav-1 overexpression leads to decreased ROS production (present study) and supports the idea that activation of EGFR in MCs of the glomeruli contributes to renal injury.
The contribution of Cav-1 to the regulation of EGFR activation is also controversial. In some studies, the plasma membrane localization and phosphorylation of Cav-1 is inversely correlated with the levels and activation of EGFR (27, 30, 37, 48, 69); however, in other studies, Cav-1 has been shown to play a direct role in regulating the activation state of EGFR, either by enhancing the level of this growth factor receptor at the plasma membrane or by facilitating its translocation to the nucleus (19, 34, 75). In this study, we show that Cav-1 acts as a negative regulator of EGFR-mediated signaling in integrin α1-null MCs.
The finding that overexpression of Cav-1 in integrin α1-null MCs is sufficient to decrease EGFR activation and consequent production of profibrotic ROS strongly suggests that Cav-1 acts as an antifibrotic factor in MCs. This agrees with the observation that Cav-1 is a negative modulator of TGF-β-mediated signaling (39), and patients with systemic sclerosis or idiopathic pulmonary fibrosis show decreased expression of Cav-1 levels (17). Moreover, mice lacking Cav-1 have a decreased life span due to pulmonary collagen fibril deposition and lung fibrosis (46). Consistent with this, mice lacking integrin α1β1 show decreased levels of glomerular Cav-1 expression both at baseline and following injury (present study) as well as increased levels of activated EGFR (present study) and collagen synthesis (11, 23, 74). All together, these data suggest that the antifibrotic actions of integrin α1β1 are mediated in part by positively regulating Cav-1 levels and decreasing the levels of activated EGFR.
The expression levels of Cav-1 can be regulated in multiple ways. Growth factors such as TGF-β and EGF negatively regulate Cav-1 expression at both transcriptional and translation levels (41, 68), while transcription factors such as FOXO or PPARs positively control Cav-1 expression (7, 40, 67). We present the first evidence that the collagen-binding receptor integrin α1β1 controls Cav-1 expression at the transcriptional level but does not regulate its localization to caveola-rich fractions.
We identified PPARγ as a key mediator in the integrin α1β1/Cav-1 axis. Similar to Cav-1 and integrin α1β1, PPARγ can be also classified as an antifibrotic molecule. This nuclear receptor prevents fibrosis primarily by inhibiting TGF-β-mediated signaling, and PPARγ ligands have been shown to inhibit TGF-β-stimulated profibrotic differentiation of lung fibroblasts and reduce lung scarring in animal models of fibrosis (59). In addition, the PPARγ ligand troglitazone prevents TGF-β-mediated collagen synthesis in keloid fibroblasts (76) and reduces renal interstitial fibrosis via inhibition of TGF-β signaling (33). Consistent with these data, fibroblasts lacking PPARγ show increased Smad signaling and consequent collagen expression (25). Our in vivo finding that glomeruli of integrin α1-null mice have decreased PPARγ expression concomitant with decreased Cav-1 levels and increased glomerulosclerosis further indicates that PPARγ acts as an antifibrotic factor by positively regulating the levels of Cav-1. Furthermore, PPARγ ligands can induce Cav-1 expression in tumor cells and macrophages (7, 62), as well as in MCs (present study).
A key regulator of PPARγ activity is ERK, which has been shown to negatively regulate PPARγ-mediated transcriptional functions either by inhibiting PPARγ phosphorylation or by preventing its nuclear translocation (6). ERK has been shown to negatively regulate Cav-1 expression, although its effects are species specific, as it downregulates Cav-1 levels and phosphorylation in rodent (21, 54) but not in human (54) cells. Although Cav-1 levels do not appear to be regulated by ERK in humans, a plausible link between Cav-1 levels and ERK activation in human cells/tissues is provided by the finding that lung fibroblasts from scleroderma patients show reduced expression of Cav-1 but increased levels of activated ERK (65). Interestingly, downregulation of Cav-1 levels in normal fibroblasts leads to increased ERK activation (65). Moreover, decreased levels of Cav-1 are evident in patients with idiopathic pulmonary fibrosis, and overexpression of Cav-1 in human pulmonary fibroblasts prevents TGF-β-mediated ERK activation (68). These findings in humans could also explain the increased basal levels of ERK activation observed in integrin α1-null MCs, which also express reduced Cav-1 levels. Importantly, we now provide evidence that inhibition of ERK activation in integrin α1-null MCs increases both PPARγ nuclear translocation and Cav-1 levels. This finding not only confirms that ERK can act as a negative regulator of Cav-1 but also provides a mechanism whereby ERK controls the transcriptional levels of this scaffolding protein. More importantly, we show that integrin α1β1 utilizes the ERK/PPARγ pathway to positively regulate Cav-1 expression, thus modulating the functions of this scaffolding protein.
Supplementary Material
Acknowledgments
This work was supported by a Merit Review award from the Department of Veterans Affairs (A.P., R.Z., and R.C.H.), grant 2P01DK065123 (A.P. and R.Z.), grants DK075594 and DK65123 (R.Z.), the AHA Established Investigator Award (R.Z.), and the O'Brien Center grant P30DK79341-01 (A.P., R.Z., R.C.H., and M.-Z.Z.).
We thank Cathy Alford at the Department of Veterans Affairs for help with the flow cytometric analysis.
Footnotes
Published ahead of print on 5 April 2010.
Supplemental material for this article may be found at http://mcb.asm.org/.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Abulrob, A., S. Giuseppin, M. F. Andrade, A. McDermid, M. Moreno, and D. Stanimirovic. 2004. Interactions of EGFR and caveolin-1 in human glioblastoma cells: evidence that tyrosine phosphorylation regulates EGFR association with caveolae. Oncogene 23:6967-6979. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. C., and F. M. Watt. 1993. Regulation of development and differentiation by the extracellular matrix. Development 117:1183-1198. [DOI] [PubMed] [Google Scholar]
- 3.Alam, N., H. L. Goel, M. J. Zarif, J. E. Butterfield, H. M. Perkins, B. G. Sansoucy, T. K. Sawyer, and L. R. Languino. 2007. The integrin-growth factor receptor duet. J. Cell. Physiol. 213:649-653. [DOI] [PubMed] [Google Scholar]
- 4.Askari, J. A., P. A. Buckley, A. P. Mould, and M. J. Humphries. 2009. Linking integrin conformation to function. J. Cell Sci. 122:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron, W., S. J. Shattil, and C. ffrench-Constant. 2002. The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins. EMBO J. 21:1957-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgermeister, E., and R. Seger. 2007. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle 6:1539-1548. [DOI] [PubMed] [Google Scholar]
- 7.Burgermeister, E., L. Tencer, and M. Liscovitch. 2003. Peroxisome proliferator-activated receptor-gamma upregulates caveolin-1 and caveolin-2 expression in human carcinoma cells. Oncogene 22:3888-3900. [DOI] [PubMed] [Google Scholar]
- 8.Byzova, T. V., C. K. Goldman, N. Pampori, K. A. Thomas, A. Bett, S. J. Shattil, and E. F. Plow. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851-860. [PubMed] [Google Scholar]
- 9.Chen, J., F. Capozza, A. Wu, T. Deangelis, H. Sun, M. Lisanti, and R. Baserga. 2008. Regulation of insulin receptor substrate-1 expression levels by caveolin-1. J. Cell. Physiol. 217:281-289. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X., T. D. Abair, M. R. Ibanez, Y. Su, M. R. Frey, R. S. Dise, D. B. Polk, A. B. Singh, R. C. Harris, R. Zent, and A. Pozzi. 2007. Integrin α1β1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol. Cell. Biol. 27:3313-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, X., G. Moeckel, J. D. Morrow, D. Cosgrove, R. C. Harris, A. B. Fogo, R. Zent, and A. Pozzi. 2004. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am. J. Pathol. 165:617-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosgrove, D., D. T. Meehan, D. Delimont, A. Pozzi, X. Chen, K. D. Rodgers, R. M. Tempero, M. Zallocchi, and V. H. Rao. 2008. Integrin alpha1beta1 regulates matrix metalloproteinases via P38 mitogen-activated protein kinase in mesangial cells: implications for Alport syndrome. Am. J. Pathol. 172:761-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couet, J., M. Sargiacomo, and M. P. Lisanti. 1997. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 272:30429-30438. [DOI] [PubMed] [Google Scholar]
- 14.Dasu, M. R., S. Park, S. Devaraj, and I. Jialal. 2009. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology 150:3457-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Laurentiis, A., L. Donovan, and A. Arcaro. 2007. Lipid rafts and caveolae in signaling by growth factor receptors. Open Biochem. J. 1:12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Galdo, F., M. P. Lisanti, and S. A. Jimenez. 2008. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr. Opin. Rheumatol. 20:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Galdo, F., F. Sotgia, C. J. de Almeida, J. F. Jasmin, M. Musick, M. P. Lisanti, and S. A. Jimenez. 2008. Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheum. 58:2854-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittmann, K., C. Mayer, R. Kehlbach, and H. P. Rodemann. 2008. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Mol. Cancer 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Echarri, A., O. Muriel, and M. A. Del Pozo. 2007. Intracellular trafficking of raft/caveolae domains: insights from integrin signaling. Semin. Cell Dev. Biol. 18:627-637. [DOI] [PubMed] [Google Scholar]
- 21.Engelman, J. A., X. L. Zhang, B. Razani, R. G. Pestell, and M. P. Lisanti. 1999. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase A signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J. Biol. Chem. 274:32333-32341. [DOI] [PubMed] [Google Scholar]
- 22.Francois, H., S. Placier, M. Flamant, P. L. Tharaux, D. Chansel, J. C. Dussaule, and C. Chatziantoniou. 2004. Prevention of renal vascular and glomerular fibrosis by epidermal growth factor receptor inhibition. FASEB J. 18:926-928. [DOI] [PubMed] [Google Scholar]
- 23.Gardner, H., A. Broberg, A. Pozzi, M. Laato, and J. Heino. 1999. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J. Cell Sci. 112:263-272. [DOI] [PubMed] [Google Scholar]
- 24.Genovese, T., S. Cuzzocrea, R. Di Paola, E. Mazzon, C. Mastruzzo, P. Catalano, M. Sortino, N. Crimi, A. P. Caputi, C. Thiemermann, and C. Vancheri. 2005. Effect of rosiglitazone and 15-deoxy-delta12,14-prostaglandin J2 on bleomycin-induced lung injury. Eur. Respir. J. 25:225-234. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh, A. K., J. Wei, M. Wu, and J. Varga. 2008. Constitutive Smad signaling and Smad-dependent collagen gene expression in mouse embryonic fibroblasts lacking peroxisome proliferator-activated receptor-gamma. Biochem. Biophys. Res. Commun. 374:231-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 27.Gu, D., H. Li, Z. Wang, Q. Chen, J. Jiang, and H. Zhu. 2007. Caveolin-1 inhibits the growth of human laryngeal squamous cell carcinoma and down regulates EGFR-MAPKs signaling pathway. Laryngoscope 117:1782-1789. [DOI] [PubMed] [Google Scholar]
- 28.Harris, R. C., E. Chung, and R. J. Coffey. 2003. EGF receptor ligands. Exp. Cell Res. 284:2-13. [DOI] [PubMed] [Google Scholar]
- 29.Hynes, R. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 30.Jiang, L. Q., X. Feng, W. Zhou, P. G. Knyazev, A. Ullrich, and Z. Chen. 2006. Csk-binding protein (Cbp) negatively regulates epidermal growth factor-induced cell transformation by controlling Src activation. Oncogene 25:5495-5506. [DOI] [PubMed] [Google Scholar]
- 31.Kanjanabuch, T., L. J. Ma, J. Chen, A. Pozzi, Y. Guan, P. Mundel, and A. B. Fogo. 2007. PPAR-gamma agonist protects podocytes from injury. Kidney Int. 71:1232-1239. [DOI] [PubMed] [Google Scholar]
- 32.Kawabe, J., S. Okumura, M. C. Lee, J. Sadoshima, and Y. Ishikawa. 2004. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 286:H1845-H1852. [DOI] [PubMed] [Google Scholar]
- 33.Kawai, T., T. Masaki, S. Doi, T. Arakawa, Y. Yokoyama, T. Doi, N. Kohno, and N. Yorioka. 2009. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab. Invest. 89:47-58. [DOI] [PubMed] [Google Scholar]
- 34.Khan, E. M., J. M. Heidinger, M. Levy, M. P. Lisanti, T. Ravid, and T. Goldkorn. 2006. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1-dependent perinuclear trafficking. J. Biol. Chem. 281:14486-14493. [DOI] [PubMed] [Google Scholar]
- 35.Krajewska, W. M., and I. Maslowska. 2004. Caveolins: structure and function in signal transduction. Cell. Mol. Biol. Lett. 9:195-220. [PubMed] [Google Scholar]
- 36.Kwon, H., and Y. Pak. 2010. Prolonged tyrosine kinase activation of insulin receptor by pY27-calveolin-2. Biochem. Biophys. Res. Commun. 391:49-54. [DOI] [PubMed] [Google Scholar]
- 37.Lajoie, P., E. A. Partridge, G. Guay, J. G. Goetz, J. Pawling, A. Lagana, B. Joshi, J. W. Dennis, and I. R. Nabi. 2007. Plasma membrane domain organization regulates EGFR signaling in tumor cells. Eur. J. Cell Biol. 179:341-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leesnitzer, L. M., D. J. Parks, R. K. Bledsoe, J. E. Cobb, J. L. Collins, T. G. Consler, R. G. Davis, E. A. Hull-Ryde, J. M. Lenhard, L. Patel, K. D. Plunket, J. L. Shenk, J. B. Stimmel, C. Therapontos, T. M. Willson, and S. G. Blanchard. 2002. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41:6640-6650. [DOI] [PubMed] [Google Scholar]
- 39.Le Saux, C. J., K. Teeters, S. K. Miyasato, P. R. Hoffmann, O. Bollt, V. Douet, R. V. Shohet, D. H. Broide, and E. K. Tam. 2008. Down-regulation of caveolin-1, an inhibitor of transforming growth factor-beta signaling, in acute allergen-induced airway remodeling. J. Biol. Chem. 283:5760-5768. [DOI] [PubMed] [Google Scholar]
- 40.Llaverias, G., M. Vazquez-Carrera, R. M. Sanchez, V. Noe, C. J. Ciudad, J. C. Laguna, and M. Alegret. 2004. Rosiglitazone upregulates caveolin-1 expression in THP-1 cells through a PPAR-dependent mechanism. J. Lipid Res. 45:2015-2024. [DOI] [PubMed] [Google Scholar]
- 41.Lu, Z., S. Ghosh, Z. Wang, and T. Hunter. 2003. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4:499-515. [DOI] [PubMed] [Google Scholar]
- 42.Luetteke, N. C., H. K. Phillips, T. H. Qiu, N. G. Copeland, H. S. Earp, N. A. Jenkins, and D. C. Lee. 1994. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 8:399-413. [DOI] [PubMed] [Google Scholar]
- 43.Mattila, E., T. Pellinen, J. Nevo, K. Vuoriluoto, A. Arjonen, and J. Ivaska. 2005. Negative regulation of EGFR signaling through integrin-alpha1beta1-mediated activation of protein tyrosine phosphatase TCPTP. Nat. Cell Biol. 7:78-85. [DOI] [PubMed] [Google Scholar]
- 44.Moro, L., M. Venturino, C. Bozzo, L. Silengo, F. Altruda, L. Beguinot, G. Tarone, and P. Defilippi. 1998. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 17:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ning, Y., T. Buranda, and L. G. Hudson. 2007. Activated epidermal growth factor receptor induces integrin alpha2 internalization via caveolae/raft-dependent endocytic pathway. J. Biol. Chem. 282:6380-6387. [DOI] [PubMed] [Google Scholar]
- 46.Park, D. S., A. W. Cohen, P. G. Frank, B. Razani, H. Lee, T. M. Williams, M. Chandra, J. Shirani, A. P. De Souza, B. Tang, L. A. Jelicks, S. M. Factor, L. M. Weiss, H. B. Tanowitz, and M. P. Lisanti. 2003. Caveolin-1 null (-/-) mice show dramatic reductions in life span. Biochemistry 42:15124-15131. [DOI] [PubMed] [Google Scholar]
- 47.Park, S. C. 2002. Functional recovery of senescent cells through restoration of receptor-mediated endocytosis. Mech. Ageing Dev. 123:917-926. [DOI] [PubMed] [Google Scholar]
- 48.Park, S. S., J. E. Kim, Y. A. Kim, Y. C. Kim, and S. W. Kim. 2005. Caveolin-1 is down-regulated and inversely correlated with HER2 and EGFR expression status in invasive ductal carcinoma of the breast. Histopathology 47:625-630. [DOI] [PubMed] [Google Scholar]
- 49.Park, W. Y., J. S. Park, K. A. Cho, D. I. Kim, Y. G. Ko, J. S. Seo, and S. C. Park. 2000. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J. Biol. Chem. 275:20847-20852. [DOI] [PubMed] [Google Scholar]
- 50.Pozzi, A., P. E. Moberg, L. A. Miles, S. Wagner, P. Soloway, and H. A. Gardner. 2000. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc. Natl. Acad. Sci. U. S. A. 97:2202-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qiao, L., Y. Dai, Q. Gu, K. W. Chan, J. Ma, H. Y. Lan, B. Zou, C. Rocken, M. P. Ebert, and B. C. Wong. 2008. Loss of XIAP sensitizes colon cancer cells to PPARgamma independent antitumor effects of troglitazone and 15-PGJ2. Cancer Lett. 268:260-271. [DOI] [PubMed] [Google Scholar]
- 52.Richards, W. G., W. E. Sweeney, B. K. Yoder, J. E. Wilkinson, R. P. Woychik, and E. D. Avner. 1998. Epidermal growth factor receptor activity mediates renal cyst formation in polycystic kidney disease. J. Clin. Invest. 101:935-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salanueva, I. J., A. Cerezo, M. C. Guadamillas, and M. A. del Pozo. 2007. Integrin regulation of caveolin function. J. Cell. Mol. Med. 11:969-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasai, K., K. Kakumoto, H. Hanafusa, and T. Akagi. 2007. The Ras-MAPK pathway downregulates Caveolin-1 in rodent fibroblast but not in human fibroblasts: implications in the resistance to oncogene-mediated transformation. Oncogene 26:449-455. [DOI] [PubMed] [Google Scholar]
- 55.Schneller, E. S., K. Vuori, and E. Ruoslahti. 1997. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. EMBO J. 16:5600-5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sedding, D. G., J. Hermsen, U. Seay, O. Eickelberg, W. Kummer, C. Schwencke, R. H. Strasser, H. Tillmanns, and R. C. Braun-Dullaeus. 2005. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ. Res. 96:635-642. [DOI] [PubMed] [Google Scholar]
- 57.Shi, F., and J. Sottile. 2008. Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J. Cell Sci. 121:2360-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sieg, D. J., C. R. Hauck, D. Ilic, C. K. Klingbeil, E. Schaefer, C. H. Damsky, and D. D. Schlaepfer. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249-256. [DOI] [PubMed] [Google Scholar]
- 59.Sime, P. J. 2008. The antifibrogenic potential of PPARgamma ligands in pulmonary fibrosis. J. Investig. Med. 56:534-538. [DOI] [PubMed] [Google Scholar]
- 60.Singh, A. B., and R. C. Harris. 2004. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J. Biol. Chem. 279:3543-3552. [DOI] [PubMed] [Google Scholar]
- 61.Soldi, R., S. Mitola, M. Strasly, P. Defilippi, G. Tarone, and F. Bussolino. 1999. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 18:882-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tencer, L., E. Burgermeister, M. P. Ebert, and M. Liscovitch. 2008. Rosiglitazone induces caveolin-1 by PPARgamma-dependent and PPRE-independent mechanisms: the role of EGF receptor signaling and its effect on cancer cell drug resistance. Anticancer Res. 28:895-906. [PubMed] [Google Scholar]
- 63.Terzi, F., M. Burtin, M. Hekmati, P. Federici, G. Grimber, P. Briand, and G. Friedlander. 2000. Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J. Clin. Invest. 106:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomas, C. M., and E. J. Smart. 2008. Caveolae structure and function. J. Cell. Mol. Med. 12:796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tourkina, E., P. Gooz, J. Pannu, M. Bonner, D. Scholz, S. Hacker, R. M. Silver, M. Trojanowska, and S. Hoffman. 2005. Opposing effects of protein kinase Calpha and protein kinase Cepsilon on collagen expression by human lung fibroblasts are mediated via MEK/ERK and caveolin-1 signaling. J. Biol. Chem. 280:13879-13887. [DOI] [PubMed] [Google Scholar]
- 66.Upla, P., V. Marjomaki, P. Kankaanpaa, J. Ivaska, T. Hyypia, F. G. Van Der Goot, and J. Heino. 2004. Clustering induces a lateral redistribution of alpha 2 beta 1 integrin from membrane rafts to caveolae and subsequent protein kinase C-dependent internalization. Mol. Biol. Cell 15:625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van den Heuvel, A. P., A. Schulze, and B. M. Burgering. 2005. Direct control of caveolin-1 expression by FOXO transcription factors. Biochem. J. 385:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, X. M., Y. Zhang, H. P. Kim, Z. Zhou, C. A. Feghali-Bostwick, F. Liu, E. Ifedigbo, X. Xu, T. D. Oury, N. Kaminski, and A. M. Choi. 2006. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J. Exp. Med. 203:2895-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, X. Q., P. Sun, and A. S. Paller. 2002. Ganglioside induces caveolin-1 redistribution and interaction with the epidermal growth factor receptor. J. Biol. Chem. 277:47028-47034. [DOI] [PubMed] [Google Scholar]
- 70.Wang, X. Q., Q. Yan, P. Sun, J. W. Liu, L. Go, S. M. McDaniel, and A. S. Paller. 2007. Suppression of epidermal growth factor receptor signaling by protein kinase C-alpha activation requires CD82, caveolin-1, and ganglioside. Cancer Res. 67:9986-9995. [DOI] [PubMed] [Google Scholar]
- 71.Wang, Z., J. K. Chen, S. W. Wang, G. Moeckel, and R. C. Harris. 2003. Importance of functional EGF receptors in recovery from acute nephrotoxic injury. J. Am. Soc. Nephrol. 14:3147-3154. [DOI] [PubMed] [Google Scholar]
- 72.Werner, E., and Z. Werb. 2002. Integrins engage mitochondrial function for signal transduction by a mechanism dependent on Rho GTPases. Eur. J. Cell Biol. 158:357-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodard, A. S., G. Garcia-Cardena, M. Leong, J. A. Madri, W. C. Sessa, and L. R. Languino. 1998. The synergistic activity of alphavbeta3 integrin and PDGF receptor increases cell migration. J. Cell Sci. 111:469-478. [DOI] [PubMed] [Google Scholar]
- 74.Zent, R., X. Yan, Y. Su, B. G. Hudson, D. B. Borza, G. W. Moeckel, Z. Qi, Y. Sado, M. D. Breyer, P. Voziyan, and A. Pozzi. 2006. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int. 70:460-470. [DOI] [PubMed] [Google Scholar]
- 75.Zhang, B., F. Peng, D. Wu, A. J. Ingram, B. Gao, and J. C. Krepinsky. 2007. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell. Signal. 19:1690-1700. [DOI] [PubMed] [Google Scholar]
- 76.Zhang, G. Y., C. G. Yi, X. Li, B. Ma, Z. J. Li, X. L. Chen, S. Z. Guo, and W. Y. Gao. 2009. Troglitazone suppresses transforming growth factor-beta1-induced collagen type I expression in keloid fibroblasts. Br. J. Dermatol. 160:762-770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.