Abstract
Multiple sclerosis (MS) is a human CNS autoimmune demyelinating disease. Epidemiological evidence has suggested a role for virus infection in the initiation and/or exacerbation of MS. Theiler’s murine encephalomyelitis virus (TMEV)- induced demyelinating disease serves as a relevant mouse model for MS. TMEV- infected mice develop a demyelinating disease with clinical symptoms beginning around 35 days post infection which is associated with development of myelin- specific, PLP139–151, CD4+ T cell responses. Viruses have been suggested to initiate autoimmune disease through bystander activation of immune cells or through bystander damage to tissue during infection. We examined the effect of the innate immune response on development of autoimmune demyelinating disease by altering the innate immune response through administration of innate immune cytokines, IFNα or IFNβ, or antiserum against the type I interferons during the innate immune response to TMEV. Administration of IFNβ, but not IFNα, to TMEV- infected mice led to reduced myelin-specific CD4+ T cell responses and reduced demyelinating disease which was associated with decreased immune cell infiltration into the CNS and increased expression of IL-10 in the CNS. Conversely, administration of antiserum to IFNβ led to a more severe demyelinating disease. In addition, administration of polyI:C, which is an innate immune agonist, to TMEV- infected mice during the innate immune response resulted in decreased myelin-specific CD4+ T cell responses and reduced demyelinating disease. These results demonstrate that activating or enhancing the innate immune response can reduce the subsequent initiation and progression of the autoimmune response and demyelinating disease.
Keywords: Autoimmunity, Viral, Cytokines, Rodent, Neuroimmunology
Introduction
Multiple sclerosis (MS) is a demyelinating disease affecting humans that is associated with an inflammatory autoimmune response in the central nervous system (CNS). Theiler’s murine encephalomyelitis virus (TMEV)- induced demyelinating disease (IDD) serves as a mouse model of MS. TMEV infection of susceptible SJL mice results in a persistent infection of the CNS. Clinical signs of demyelinating disease first appear around 35–40 days post infection and continue as a chronic progressive disease characterized by ascending hind-limb paralysis. TMEV –IDD is associated with development of myelin- specific CD4+ T cell responses via the process of epitope spreading. The initial autoimmune CD4+ T cell response is directed against proteolipid protein, epitope PLP139–151, which is first detected around 55 days post infection and then progresses to additional myelin antigens by epitope spreading around 90 days post infection (1). Most significantly, the autoreactive CD4+ T cell response plays a pathologic role in chronic disease progression as shown by reduction in disease progression following induction of peripheral tolerance to PLP antigens after disease onset (2). Therefore, TMEV infection of mice results in an autoimmune mediated demyelinating disease.
The innate immune response recognizes microbial infections and initiates the immune response to the infecting pathogen. The innate immune response recognizes pathogens by pathogen-associated molecular patterns (PAMPs) which enables the differentiation between self and non-self. Pattern recognition receptors, including the Toll like receptors (TLRs), recognize PAMPs and signal the cell to respond to the pathogen. Several agonists for TLRs have been determined including viral PAMPs such as double stranded RNA recognized by TLR3 and single stranded RNA recognized by TLR7/TLR8 (3,4). TLRs signal through a common adaptor protein MyD88 which leads to the downstream activation of NFkB family of transcription factors (5). However, some TLRs, including TLR3, signal through a MyD88 independent pathway that leads to downstream activation of interferon response factor, IRF-3, resulting in expression of IFN-inducible genes (6,7). Recent studies have shown that intracellular innate immune receptors RIG-I and MDA-5 can also recognize viral PAMPs (8). Overall, the engagement of TLRs or other innate immune receptors leads to the transcriptional activation of cytokines, chemokines, and effector molecules as well as costimulatory molecules and MHC class I and MHC class II. Therefore, the innate immune response directly influences the development of the adaptive immune response by influencing the activation of T cells and B cells. Most recently, the innate immune response has been associated with recognition of self tissue during tissue damage in the absence of pathogens. Damage associated molecular patterns (DAMPs) are released from dying cells during tissue damage and include high mobility group box 1 protein (HMGB-1), heat-shock proteins (HSPs), uric acid, altered matrix proteins, and S100 proteins. DAMPs are danger signals which can mediate an inflammatory response through TLRs and the receptor for advanced glycation end-products (RAGE) (9). A recent report has suggested a pivotal role for TLR2 and TLR4 in the immune response during spinal cord injury (10). Therefore, the innate immune response can recognize danger signals coming from pathogens during infection as well as from cell damage during injury.
Type I interferons, IFNα and IFNβ, are immediatelyinduced in response to virus infections and are identified as anti-viral proteins. However, several lines of evidence suggest that type I interferons have multiple functions in the immune response to virus infection. IFNα and IFNβ promote NK cell-mediated cytotoxicity as well as blastogenesis and proliferation (11). Type I interferons have also been shown to enhance production of IFNγ by T cells following virus infection (12,13). IFNα and IFNβ influence the adaptive immune response by driving the maturation of dendritic cells that promote CD4+ Th1 type response with high levels of IFNγ secretion and CD4+ T cell proliferation (14,15). Additional, studies have shown that type I interferons promote CD8+ T cell proliferation and survival (16) as well as enhance the B cell response (17). Type I interferon expression, specifically IFNα4 and IFNβ, is induced in virus-infected cells through activation (phosphorylation) of IRF-3 which is constitutively expressed at low levels (14). IFNα and IFNβ are then released from the infected cell and bind to the IFNα/β receptor on the cell surface which in turn induces expression of additional type I interferons through activation of IRF-7 via a positive feedback mechanism (18). Most interesting, IFNβ is currently one of the primary treatments for patients with MS, however, the mechanism by which IFNβ modulates disease has not been completely determined.
The innate immune response directly influences the development of the adaptive immune response through the expression of cytokines and chemokines as well activation of antigen presenting cells. The innate immune response may also be involved in activation of the autoimmune response through bystander activation of immune cells, especially autoreactive T cells, or through bystander damage of surrounding tissue. The current studies examined the effect of the innate immune response on development of demyelinating disease by altering the innate immune response during virus infection. Type I interferons are the predominant cytokines produced following virus infection and have been shown to influence the adaptive immune response, thus, type I interferons were altered during the innate immune response to TMEV to determine the effect on the development of demyelinating disease. Mice were administered type I interferons to increase the amount of type I interferons during the first four days of infection or mice were administered neutralizing antiserum to type I interferons to reduce the amount of type I interferons during the first four days of infection. In addition, mice were administered polyI:C which is a synthetic double stranded RNA that can activate the innate immune response to produce type I interferons. These studies demonstrate that altering the innate immune response by administration of agents following TMEV infection can directly affect the development of demyelinating disease suggesting the innate immune response can influence the activation of the autoimmune response and development of demyelinating disease.
Material and Methods
Infection of mice
Five- to six week old female SJL mice were purchased from Harlan Laboratories. The mice were housed at University of Wisconsin Research Animal Resource Center or housed at Northwestern University according to ACUC approved protocols at each institution. Mice were infected by intracerebral (i.c.) injection with 2 × 106 PFU of BeAn strain of TMEV. On the day of infection and 4 days post infection, mice were administered by intraperitonial (i.p.) injection: PBS (control), IFNβ (5000 U), αIFNβ (5000 NU), IFNα4 (5000 U), αIFNα (5000 NU) (R&D Systems), polyI:C (150μg) (Sigma), or rabbit serum (control). Mice were followed for clinical disease signs daily, and scores were assigned based on a scale of 0–5: score 1, mice show mild waddling gait; score 2, mice show more severe waddling gate; score 3, mice had a loss of righting ability associated with spastic hind limbs; score 4, mice had paralysis of hind limbs associated with dehydration; score 5- mice were moribund.
Viral plaque assay
The brain and spinal cord were removed from the infected mice at the indicated days post infection. The organs were homogenized and diluted in serum- free DMEM. BHK-21 cells were infected with the homogenized tissue dilutions. The cells were incubated at room temperature for one hour. A 2% agar solution was diluted in DMEM supplemented with 2% serum and 200μg/ml penicillin and 200μg/ml streptomycin and was added to the cells. The cells were incubated at 34°C for 5 days. The cells were fixed with methanol and stained with crystal violet solution (0.12% crystal violet). The plaques were counted on each plate and multiplied by the dilution and the amount of homogenate added to the plate to determine the PFU/ml. The weight of the tissue (mg) and homogenate volume was then used to calculate PFU/mg.
RNA isolation and real time PCR
Brain, spinal cord, spleen, and lymph nodes were removed from mice at the indicated days post infection. The RNA was isolated from the organs using Trizol (Invitrogen). The RNA was DNAse treated prior to converting the RNA into cDNA using oligo(dT)12–18 primers and Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science). Real time PCR reactions were conducted with FastStart SYBR Green Master Mix (Roche Applied Science). Briefly, 0.5μM primers, 1X SYBR Green Master Mix, and 2μl diluted cDNA were combined. The primers sequences were previously published (19). Real time PCR was conducted on a Rotorgene 6000 (Corbett Research) using a hot start with cycle conditions, 40 cycles; 95°C 15 seconds, 60°C 10 seconds, and 72°C 15 seconds; followed by a melt from 75°C to 95°C. Quantitation of the mRNA was based on standard curves derived from cDNA standards for each primer set that are run with the samples, and the samples are normalized using β-actin expression. Positive and negative cDNA controls were used for each primer set derived from known cell sources for each cytokine.
Cytokine secretion
Brain, spinal cord, and spleen were removed from mice at the indicated days post infection. The organs were homogenized, and the supernatant was collected and analyzed using a LiquiChip mouse cytokine kit (Qiagen) per the instructions. The beads were analyzed on Luminex100 machine (Qiagen) and the cytokines were quantitated based on a set of standards for the assay.
Flow cytometry
Mice were perfused at the indicated days post infection, and the brain, spinal cord, and spleen were removed. The brain and spinal cord were minced and digested with collagenase type IV (Invitrogen) and DNAse (Invitrogen) for one hour at 37°C. The organs were then dissociated through nylon mesh before the mononuclear cells were separated on a 70/30 percoll gradient. The separated cells were washed with FACS buffer (saline with 5% normal goat serum) and blocked with antibody to CD16/32 (BD Bioscience) at 4°C for 30 minutes. The cells were then incubated for 45 minutes at 4°C with antibodies conjugated to fluoresceinated antibodies specific for CD4, CD8, CD11b, CD45, B220, and PanNK. The cells were washed and florescence was analyzed on a FACScalibur (BD Bioscience). The CD45 positive cells were analyzed to determine the percentage of CD4 and CD8 T cells, macrophage, B cells, and NK cells infiltrating the CNS.
T cell assays
Spleens and lymph nodes were removed from infected mice at the indicated days following infection and dissociated through a stainless steel screen to obtain a homogenous cell suspension. The red blood cells were lysed with Tris-ammonium chloride. The cells were then cultured at 1×106 cells per well (96-well plates) in HL-1 medium (BioWhittaker) supplemented with 2mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Peptides were added to the wells at increasing concentrations from 1–100 μM. PLP139–151 (HSLGKWLGHPDKF), PLP178–191 (NTWTTCQSIAFPSK), VP270–86 (WTTSQEAFSHIRIPLPH), and VP3159–166 (FNFTAPFI) were purchased from CPC Scientific. The amino acid composition was verified by mass spectrometry, and purity was assessed by HPLC. The plates were incubated at 37°C for 72 hours and then pulsed with 1μCi3[H]-TdR for 24 hours for proliferation assays. Proliferation was determined with triplicate wells for each peptide concentration and expressed as CPM ± SEM. For cytokine analysis, a duplicate set of proliferation wells were used to collect supernatants at 24, 48 and 72 hours, and supernatants were analyzed using a mouse cytokine bead detection kit and read on Luminex100 (Qiagen) machine as described above. ELISPOT assays were performed by coating 96-well plates with antibody for IFNγ, IL-2, IL-4, or TNFα (BD Bioscience) prior to addition of cells and peptides as described for proliferation. Following 24 hour incubation, the plates were washed and stained with the corresponding antibody pair and detected by colorimetric methods. The plates were analyzed on CTL ELISPOT reader.
Statistical analysis
A statistical comparison of the percentage of animals showing clinical disease between any two groups of mice will be performed byχ2test using Fisher’s exact probability. Comparisons of differences in immunological assays between any two groups was determined using students t test. Significance between any two groups was determined as ≥2 fold increase or decrease.
Results
The innate immune response influences the development of demyelinating disease following TMEV infection
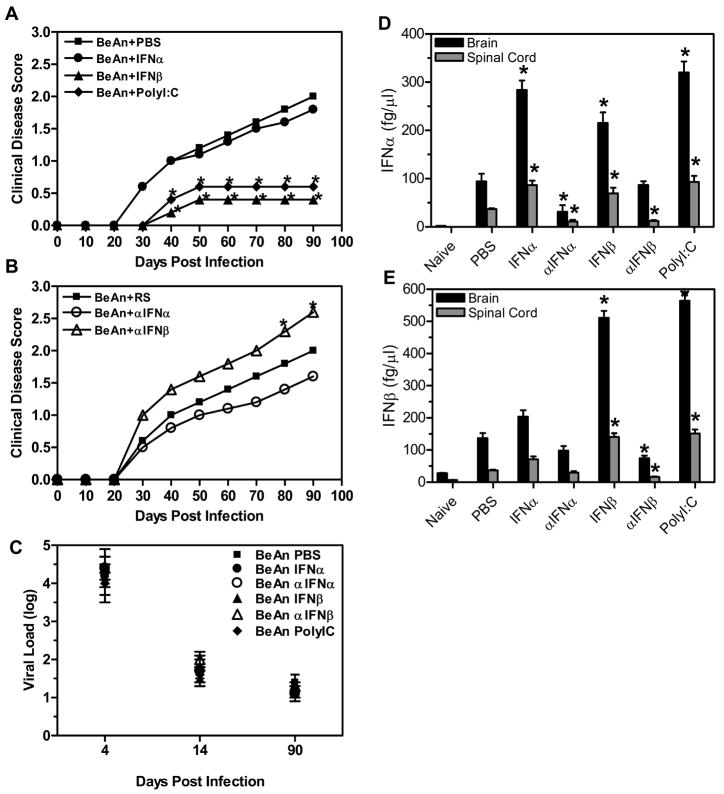
The innate immune response recognizes unique patterns on viruses such as double stranded RNA or single stranded RNA which activates a signaling cascade that results in expression of innate immune cytokines. The innate immune response to virus infections is predominantly mediated by expression of type I interferons, IFNα and IFNβ. Most importantly, the innate immune response to infections directly contributes to the development of the adaptive immune response and possibly the autoimmune response. Thus, to determine whether the innate immune response can influence the development of autoimmune demyelinating disease after virus infection, we altered the innate immune response during TMEV infection by administration of innate immune cytokines. Mice (10 mice per group) were administered IFNα4 or IFNβ on the day of TMEV infection and 4 days post infection. Another group of mice (n=10) was similarly administered polyI:C, a synthetic double stranded RNA, which is recognized by innate immune receptors and induces an innate immune response that includes the expression of type I interferons. The timing of the administration of type I interferons and polyI:C was chosen to clearly differentiate the role of these molecules on early virus infection versus the effect on ongoing demyelinating disease. The TMEV infected mice were monitored for the development and progression of demyelinating disease (Figure 1A). TMEV- infected mice administered IFNα developed clinical signs of demyelinating disease similar to control treated mice. Most interestingly, TMEV- infected mice administered IFNβ developed a less severe demyelinating disease which did not progress compared to control treated mice. Similarly, TMEV- infected mice administered polyI:C also developed a less severe demyelinating disease which did not progress. Co-administration of IFNα and IFNβ to TMEV- infected mice resulted in similar clinical disease as IFNβ alone, thus there was no apparent synergistic affect between the type I interferons (data not shown). Next, mice were administered neutralizing antiserum to type I interferons early during TMEV infection to determine if decreasing the innate immune cytokines altered development of subsequent demyelinating disease (Figure 1B). TMEV- infected mice administered antiserum to IFNα developed demyelinating disease which was slightly but not significantly reduced compared to control treated mice. However, TMEV- infected mice administered antiserum to IFNβ developed a more severe demyelinating disease than that in control treated mice. Therefore, administration of IFNβ to TMEV- infected mice during the innate immune response reduced the severity of demyelinating disease while administration of antiserum to IFNβ increased the severity of demyelinating disease. Most interestingly, administration of polyI:C which is an innate immune response stimulator resulted in significantly reduced demyelinating disease.
Figure 1. The innate immune response affects development of demyelinating disease.
SJL mice were infected with TMEV and administered IFNα, IFNβ, polyI:C, or PBS (A) (10 mice per group) on the day of infection and four days post infection. The mice were followed for clinical signs of demyelinating disease for 90 days following infection, PBS (square), IFNα (circle), IFNβ (triangle), and polyI:C (diamond). The stars above the line represent a statistically significant difference in the clinical score compared to TMEV- infected mice treated with PBS. TMEV- infected mice were administered antiserum to IFNα or IFNβ or rabbit serum (RS) (B) (10 mice per group) on the day of infection and four days post infection. Mice were followed for clinical signs of demyelinating disease for 90 days following infection, RS (square), αIFNα (open circle), andαIFNβ (open triangle). The stars above the line represent a significant difference in clinical disease score compared to TMEV- infected mice administered RS. (C) The brain and spinal cord were removed from TMEV- infected mice (3 per group) on 4, 14, and 90 days post infection and plaque assays were conducted to determine viral load. Viral load is the log of plaque forming units per milligram of tissue. The experiment was repeated five times with one representative experiment shown. (D, E) The brain and spinal cord were removed from mice (3 mice per group) at 7 days post infection, and RNA was isolated from the organs and converted to cDNA. Real time PCR was conducted on the cDNA using primers for IFNα (D) or IFNβ (E). The concentration for each primer was based on a standard curve with cDNA of known concentration. The experiment was repeated three times with a representative of one of the experiments shown in the graph. The stars above the bars indicate a significant difference (>3 fold increase or decrease) in expression based on the expression of control treated mice.
Administration of IFNβ or polyI:C to TMEV- infected mice during the innate immune response to TMEV reduced demyelinating disease, however, these agents were administered by intraperitoneal injection while the infection is in the CNS. To determine whether the administration of IFNβ and polyI:C had an affect on the innate immune response in the CNS, the brain and spinal cord were analyzed for expression of type I interferons at 7 days post infection (Figure 1D and 1E). TMEV- infected mice administered IFNα had a significant increase in the expression of IFNα in the brain and spinal cord compared to control treated mice. Conversely, TMEV- infected mice administered antiserum to IFNα had a significant decrease in IFNα expression in the CNS compared to control treated mice. Administration of IFNβ led to a significant increase in expression of both IFNα and IFNβ in the CNS compared to control treated mice. Likewise, TMEV- infected mice administered polyI:C had a significant increase in both IFNα and IFNβ in the CNS. TMEV- infected mice administered antiserum to IFNβ had a significant decrease in expression of IFNβ in the CNS compared to control treated mice. Therefore, the peripheral administration of modulators of type I interferons had a direct affect on expression of type I interferons in the CNS of TMEV- infected mice. Further, these results suggests that type I interferons activate the innate immune response to TMEV through the IFNα/β receptor.
Type I interferons have been shown to have direct anti-viral activities and inhibit the replication of viruses. The development of demyelinating disease following TMEV infection has been associated with persistent virus infection of the CNS. Thus, to determine if the reduction in demyelinating disease was associated with a reduced virus load in the CNS, plaque assays were performed with the brain and spinal cord of the TMEV infected mice that were administered IFNα, IFNβ, or polyI:C or administered antiserum to IFNα or IFNβ (Figure 1C). Interestingly, the treatments did not result in significant differences in the CNS virus load in the mice at 4, 14, or 90 days post infection. Therefore, the reduced demyelinating disease observed in TMEV- infected mice administered IFNβ or polyI:C was not due to a reduction in infectious virus in the CNS of the mice.
Alteration of CNS cytokine expression following administration of IFNβ or polyI:C in TMEV-infected mice
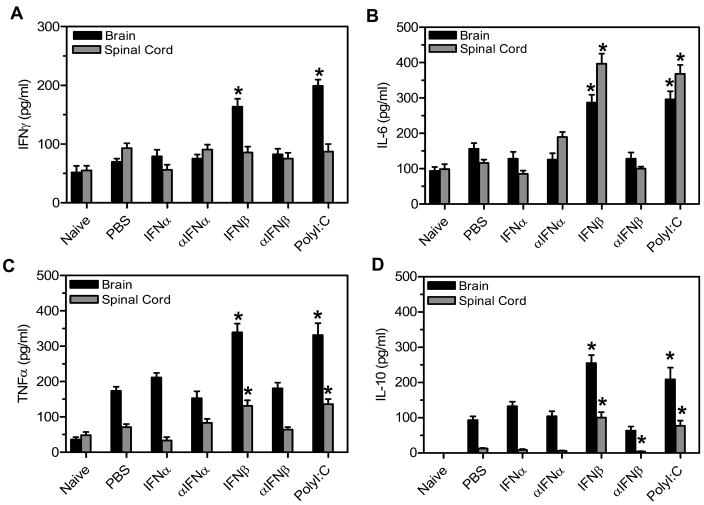
Next, we determined whether TMEV- infected mice administered IFNβ or polyI:C had altered expression of other cytokines in the CNS during the early anti-viral immune response. TMEV- infected mice were administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ and were examined at 7 days post infection for expression of cytokines in the CNS (Figure 2). TMEV-infected mice administered IFNβ or polyI:C had a significant increase in secretion of IFNγ in the brain compared to control treated mice (Figure 2A). TMEV- infected mice administered IFNβ or polyI:C had a significant increase in secretion of IL-6 and TNFα in the brain and spinal cord compared to control treated mice (Figure 2B and 2C). Most interestingly, TMEV- infected mice administered IFNβ or polyI:C had a significant increase in IL-10 expression in the brain and spinal cord compared to control treated mice (Figure 2D). TMEV-infected mice administered IFNα or antiserum to INFα had similar cytokine expression in the CNS as control treated mice. Administration of antiserum to IFNβ resulted in similar expression of IFNγ, IL-6, and TNFα in the CNS as controls, but these mice had decreased expression of IL-10. Thus, TMEV- infected mice administered IFNβ or polyI:C had increased expression of innate immune cytokines and adaptive immune cytokines such as IFNγ which is associated with a proinflammatory response and IL-10 which is associated with an anti-inflammatory response.
Figure 2. TMEV-infected mice administered IFNβ or polyI:C had increased expression of innate immune cytokines in the CNS.
TMEV- infected mice were administered IFNα, αIFNα, IFNβ, αIFNα, polyI:C, or PBS (control) on day 0 and day 4. The brain and spinal cord were removed from mice (3 mice per group) at 7 days post infection. The organs were dissociated and the supernatant was used in a cytokine bead array to determine the secretion of IFNγ (A), IL-6 (B), or TNFα (C). The RNA was isolated from the brain and spinal cord of mice (3 mice per group) at 7 days post infection and converted to cDNA. Real time PCR was conducted with primers for IL-10 (D). The experiment was repeated three times with one representative experiment shown. The stars above the bars indicate significant difference in expression levels compared to control (PBS) treated mice.
Infiltration of immune cells into the CNS following TMEV infection was altered following administration of IFNβ or polyI:C
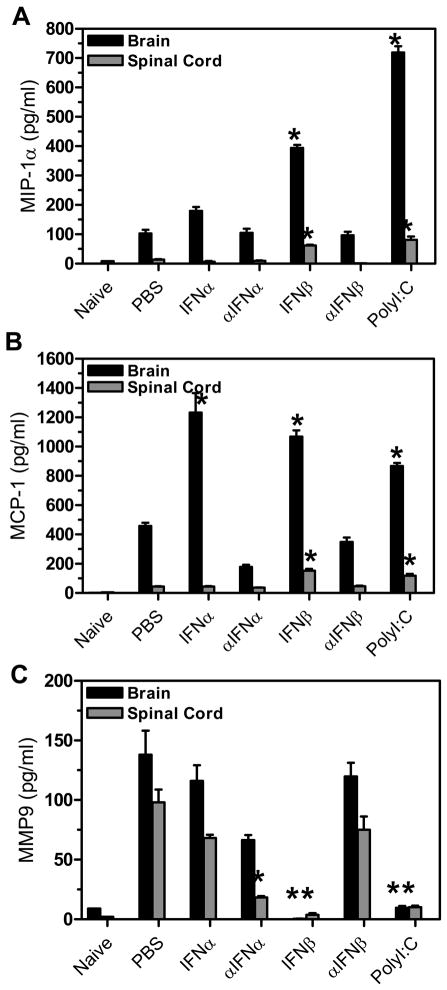
We next examined TMEV-infected mice to determine whether the chemokine expression may be altered in mice administered IFNβ or polyI:C which develop a reduced demyelinating disease. Chemokines play an important role in trafficking immune cells to the site of virus infection. TMEV-infected mice were administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ at day 0 and day 4 post infection, and CNS chemokine expression was analyzed at 7 days post infection (Figure 3). TMEV- infected mice administered IFNβ or polyI:C had increased expression of MIP-1α and MCP-1 in the brain and spinal cord while mice administered IFNα had increased expression of MCP-1 in the brain (Figure 3A and 3B) suggesting that increased expression of type I interferons leads to increased expression of chemokines in the CNS. Another group of molecules which may also be important for trafficking cells to the CNS are matrix metalloproteases (MMP) which modify matrix components. MMP9 can promote extravasation of T cells and macrophage into the CNS and has been associated with promoting breakdown of the blood brain barrier in MS patients (20). Therefore, MMP9 expression was analyzed in TMEV-infected mice administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ (Figure 3C). TMEV-infected mice administered IFNβ or polyI:C had decreased expression of MMP9 in the brain and spinal cord compared to control treated mice. In addition, TMEV-infected mice administered antiserum to IFNα also had decreased expression of MMP9 in the spinal cord. Therefore, TMEV-infected mice administered IFNβ or polyI:C develop a less severe demyelinating disease associated with decreased expression of MMP9.
Figure 3. Chemokine and MMP expression was altered in TMEV-infected mice administered IFNβ or polyI:C.
TMEV-infected mice were administered IFNα, αIFNα, IFNβ, αIFNβ, polyI:C or PBS (control) on day 0 and day 4 post infection. The brain and spinal cord was removed from mice (3 mice per group) at 7 days post infection. The RNA was isolated from the organs, converted to cDNA, and real time PCR was conducted with primers specific for MIP-1α (A), MCP-1 (B), or MMP9 (C). The experiment was repeated three times with one representative experiment shown. The stars above the bars represent a significant difference (increase or decrease) in expression compared to control (PBS) treated mice.
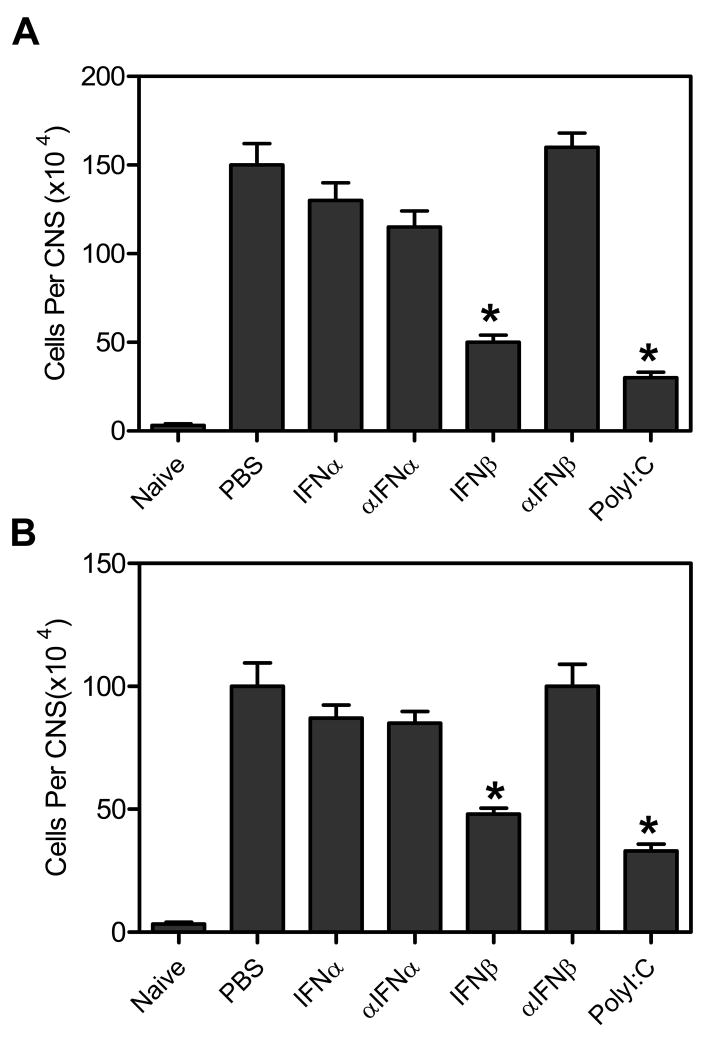
As discussed above, TMEV-infected mice administered IFNβ or polyI:C have altered chemokine and MMP9 expression compared to control treated mice suggesting that immune cell infiltration into the CNS following administration of IFNβ or polyI:C may be altered. To determine whether the immune cells entering the CNS after TMEV infection were altered, TMEV- infected mice were administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ and examined for infiltrating immune cells in the CNS at 7 days post infection (Figure 4). TMEV- infected mice administered IFNβ or polyI:C had reduced numbers of CD4+ and CD8+ T cells in the CNS compared to control treated mice. Likewise, TMEV-infected mice administered IFNβ or polyI:C had a slight reduction in macrophage and neutrophils infiltrating into the CNS however the reduction was not significant compared to control treated mice (data not shown). Thus, administration of IFNβ or polyI:C reduced immune cell infiltration, specifically T cells, into the CNS compared to control treated mice.
Figure 4. TMEV- infected mice administered IFNβ or polyI:C had reduced immune cell infiltration into the CNS early during infection.
TMEV-infected mice were administered IFNα, αIFNα, IFNβ, αIFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The brain and spinal cord was removed from the mice (3 mice per group) at 7 days post infection, and the mononuclear cells were isolated. The mononuclear cells were stained with fluoresceinated antibodies specific for CD4 (A) or CD8 (B). The number of cells staining positive for each antibody was determined based on the total number of CD45 positive cells in the CNS of each mouse. The experiment was repeated four times with one representative experiment shown. The stars above the bars represent a significant difference compared to control (PBS) treated mice.
T cell response in TMEV-infected mice administered IFNβ or polyI:C
The CD4+ and CD8+ T cell response to TMEV is activated by 7 days post infection which correlates with peak virus loads in the CNS. We next examined whether TMEV-infected mice administered IFNβ or polyI:C have an altered virus specific CD4+ and CD8+ T cell responses. TMEV-infected mice were administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ at day 0 and day 4 post infection, and the T cell responses in the spleen and lymph nodes were determined at 7 days post infection to TMEV-specific CD4+ T cell epitope VP270–86 or TMEV-specific CD8+ T cell epitope VP3159–166 (Figure 5). Both the VP270–86 specific CD4+ T cell response and the VP3159–166 specific CD8+ T cell response in the TMEV- infected mice administered IFNβ or polyI:C were similar to the response in the control treated mice. Therefore, administration of IFNβ or polyI:C to TMEV- infected mice does not alter the peripheral T cell response to virus. Thus, TMEV-infected mice administered IFNβ or polyI:C have similar virus loads in the CNS and similar virus- specific T cell responses as control treated mice suggesting that IFNβ and polyI:C do not affect virus clearance by altering the virus-specific T cell response.
Figure 5. The CD4+ and CD8+ T cell responses against TMEV were not affected by altering the innate immune response.
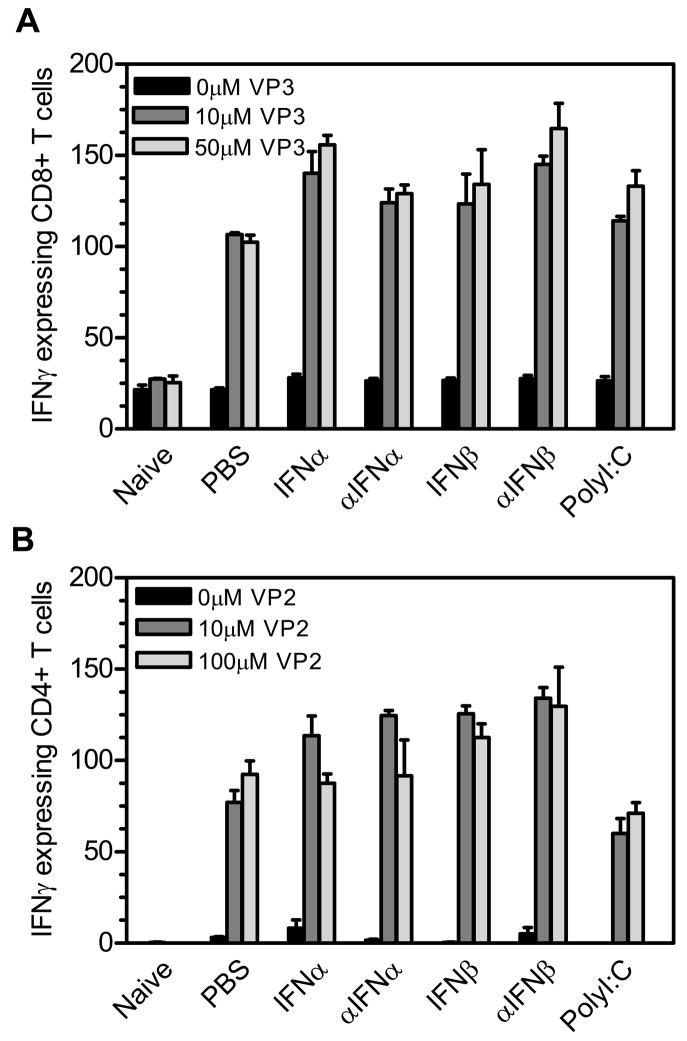
TMEV- infected mice were administered IFNα, αIFNα, IFNβ, αIFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The spleens were removed from the mice (3 mice per group) at 7 days post infection, and a single cell suspension was obtained followed by lysis of the red blood cells. IFNγ-specific ELISPOT assays were conducted by adding the splenocytes and CD8+ T cell specific peptide VP3159–166 (A) or CD4+ T cell specific peptide VP270–86 (B). The number of IFNγ expressing cells per 5 × 105 splenocytes was determined. The experiment was repeated five times with one representative experiment shown.
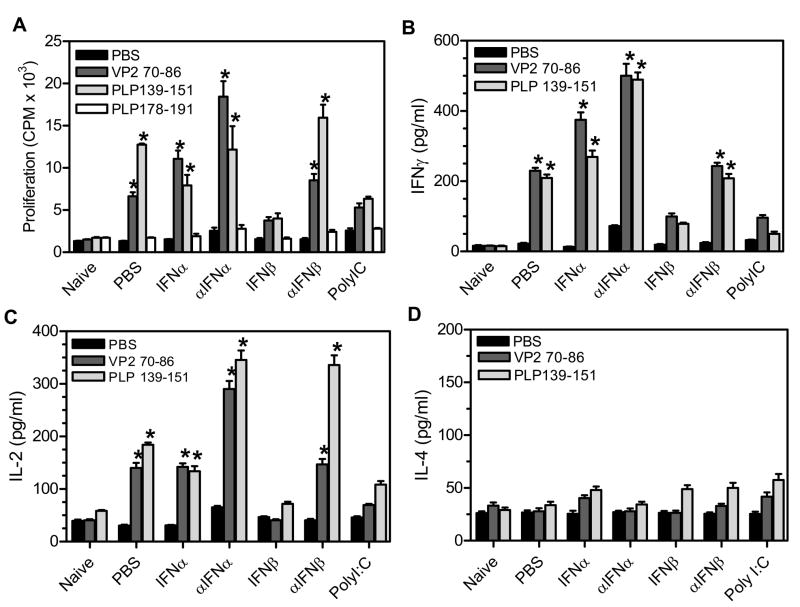
TMEV- infected mice develop an autoimmune CD4+ T cell response to myelin antigens which can first be detected around 55 days post infection and is directed to immunodomintant proteolipid protein epitope, PLP139–151 (1). As disease progresses, epitope spreading of the CD4+ T cell response results in reactivity to additional myelin antigens, such as PLP178–191, at around 90 days post infection. Previous studies have shown that tolerizing TMEV- infected mice to myelin-specific CD4+ T cells reduced disease progression which directly demonstrated that the myelin-specific CD4+ T cell response is involved in chronic TMEV-induced demyelinating disease (2). Since TMEV-infected mice treated with IFNβ or polyI:C develop a reduced demyelinating disease, these mice were examined for development of myelin-specific CD4+ T cell responses. TMEV-infected mice were administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ at days 0 and 4 post infection. At 63 days post infection, mice were analyzed for the CD4+ T cell response to virus and myelin antigens in the spleen and lymph nodes by performing a proliferation assay (Figure 6A). TMEV- infected mice have a viral- specific VP270–86 CD4+ T cell response throughout TMEV- induced demyelinating disease. At 63 days post infection, TMEV-infected mice have a myelin, PLP139–151 -specific CD4+ T cell response but have not yet developed a PLP178–191 -specific CD4+ T cell response. TMEV- infected mice administered IFNβ or polyI:C did not develop a significant virus, VP270–86 specific CD4+ T cell response. Most interestingly, TMEV- infected mice administered IFNβ or polyI:C did not develop myelin- specific CD4+ T cell responses to immunodominant myelin epitope, PLP139–151, or to spread epitope, PLP178–191. Next, the supernatants from the proliferation assays were examined to examine the activation of CD4+ T cell and to determine whether the CD4+ T cell responses were Th1 or Th2 type responses (Figure 6B, 6C, and 6D). TMEV- infected mice had virus- and myelin- specific CD4+ T cells which secrete IL-2 and IFNγ but not IL-4 demonstrating activation of the CD4+ T cells to a Th1 type phenotype. TMEV-infected mice administered IFNβ or polyI:C did not develop virus or myelin specific CD4+ T cell responses that secrete IL-2, IFNγ, or IL-4 during demyelinating disease. Therefore, TMEV- infected mice administered IFNβ or polyI:C during the innate immune response do not develop myelin-specific CD4+ T cell responses corresponding with the reduced demyelinating disease in these mice.
Figure 6. TMEV-infected mice administered IFNβ or polyI:C had reduced autoimmune CD4+ T cell responses.
TMEV-infected mice were administered IFNα, αIFNα, IFNβ, αIFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The spleen was removed from mice (3 mice per group) at 60 days post infection and then dissociated into a single cell suspension. The splenocytes were used in a proliferation assay with CD4+ T cell peptides specific for virus antigen, VP270–86, or myelin antigens, PLP139–151 and PLP178–191 (A). Proliferation was determined by pulsing the cells with Ci3[H]-TdR for the last 24 hours of culture. The counts per minutes (CPM) were determined for each peptide group. The supernatants from the proliferation cultures were removed and analyzed for IFNγ (B), IL-2 (C), or IL-4 (D) specific responses using ELISA kits. The stars above the bars indicate a significant increase compared to naïve mice. The experiment was repeated five times with one representative experiment shown.
Alteration of immune cell infiltration into the CNS during demyelinating disease in mice administered IFNβ or polyI:C
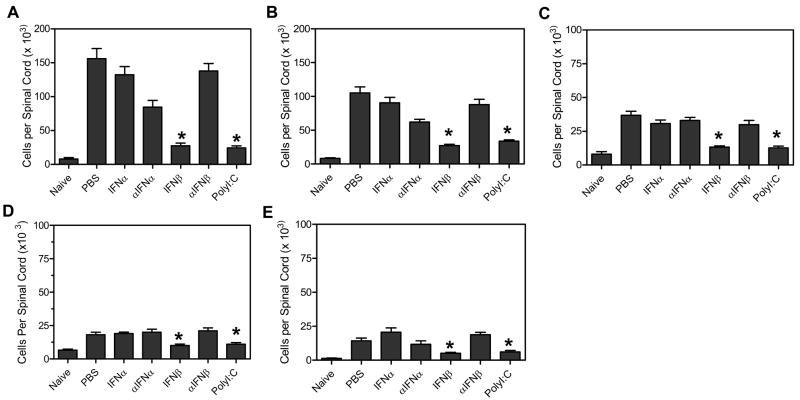
As shown above, TMEV- infected mice administered IFNβ or polyI:C develop a reduced demyelinating disease which is associated with reduced autoreactive CD4+ T cell responses. TMEV- induced demyelinating disease is associated with infiltration of activated T cells and macrophage into the spinal cord which mediate myelin destruction. Therefore, TMEV- infected mice administered type I interferons or polyI:C were next examined for infiltration of immune cells into the CNS during demyelinating disease. TMEV- infected mice were administered IFNα, IFNβ, polyI:C or antiserum to IFNα or IFNβ at day 0 and day 4 post infection. At 63 days post infection, the mice were examined for infiltration of immune cells into the CNS by flow cytometric analysis (Figure 7). TMEV- infected mice had significant numbers of CD4+ T cells, CD8+ T cells, and macrophage (CD11b+ CD11c − CD45hi) in the spinal cord with fewer numbers of B cells and dendritic cells (CD11c+) (Figure 7). Administration of IFNβ or polyI:C significantly reduced the numbers of CD4+ T cells, CD8+ T cells, macrophage, B cells, and dendritic cells in the CNS compared to control treated mice during demyelinating disease which correlates with reduced demyelination (data not shown) and reduced clinical disease in these mice.
Figure 7. TMEV- infected mice administered IFNβ or polyI:C had reduced immune cell infiltration into the CNS during demyelinating disease.
TMEV-infected mice were administered IFNα, αIFNα, IFNβ, αIFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The spinal cord was removed from mice (3 mice per group) at 60 days post infection. The mononuclear cells were isolated from the organs and stained with fluoresceinated antibodies specific for CD45, CD4, CD8, CD11b, B220, and CD11c. Flow cytometric analysis was conducted to determine the number of CD45hi cells in the CNS per mouse that were (A) CD4+ T cells, (B) CD8+ T cells, (C) macrophages (CD45hiCD11b+CD11c−), (D) B cells (B220+), and (E) dendritic cells (CD11c+ and CD11b+CD11c+). The stars above the bars represent a significant difference in the number of cells in the CNS compared to control (PBS) treated mice. The experiment was repeated five times with one representative experiment shown.
Alteration of cytokine expression during demyelinating disease in mice administered IFNβ or polyI:C
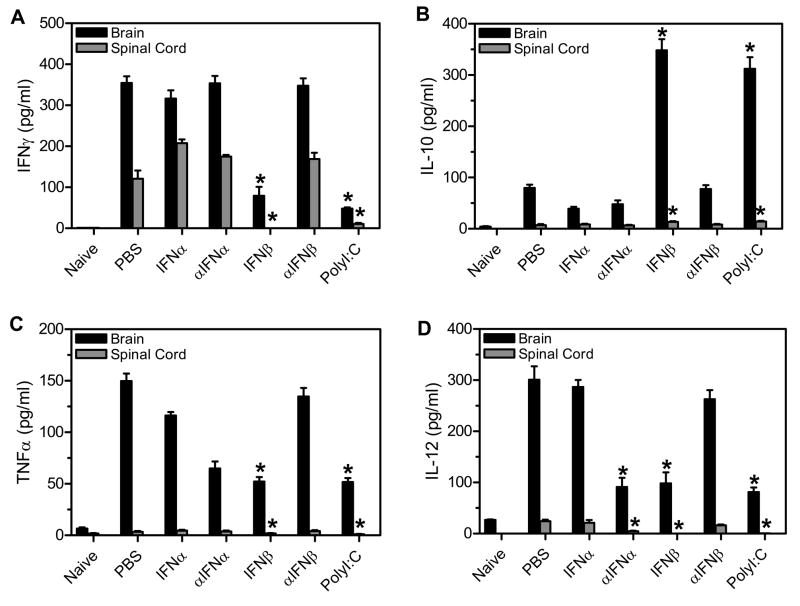
Demyelinating disease is associated with a Th1 type, pro-inflammatory immune response in the CNS, and reduction in demyelinating disease has been associated with a Th2 type response. Therefore, to determine whether the TMEV-infected mice administered IFNβ or polyI:C have altered cytokine expression in the CNS, mice were analyzed for expression of cytokines which promote either a Th1 or Th2 type immune response. TMEV- infected mice were administered IFNα, IFNβ, polyI:C, or antiserum to IFNα or IFNβ during the innate immune response. At 63 days post infection, the brain and spinal cord were removed from the mice and analyzed for expression of IFNγ, TNFα, IL-12, or IL-10 (Figure 8). TMEV- infected mice expressed significant amounts of pro-inflammatory cytokines, IFNγ, TNFα, and IL-12 in the CNS during demyelinating disease. Administration of IFNβ or polyI:C significantly reduced expression of IFNγ, TNFα, and IL-12 in the CNS compared to control treated mice. Most interestingly, TMEV- infected mice administered IFNβ or polyI:C had significantly increased expression of IL-10 in the CNS compared to control treated mice. The increased expression of IL-10 was mediated by both resident microglia cells as well as infiltrating cells which included macrophage, and CD4+ and CD8+ T cells (Supplemental Figure 1). TMEV-infected mice administered antiserum to IFNα had significantly reduced expression of IL-12 in the CNS as well as slightly reduced expression of TNFα. Therefore, administration of IFNβ or polyI:C during the innate immune response resulted in long- lasting alteration of cytokine expression in the CNS during demyelinating with decreased expression of pro-inflammatory cytokines and increased expression of anti- inflammatory cytokine, IL-10.
Figure 8. TMEV-infected mice administered IFNβ or polyI:C had reduced expression of proinflammatory cytokines in the CNS during demyelinating disease.
TMEV- infected mice were administered IFNα, αIFNα, IFNβ, αIFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The brain and spinal cord were removed from mice (3 mice per group) at 60 days post infection. The RNA was isolated from the organs, converted to cDNA, and real time PCR was conducted with primers specific for IFNγ (A), IL-10 (B), TNFα (C), or IL-12 (D). The concentration for each primer was determined by a standard curve with cDNA of a known concentration. The stars above the graph represent a significant difference in expression compared to control (PBS) treated mice. The experiment was repeated three times with one representative experiment shown.
Discussion
TMEV infection of SJL mice results in a persistent virus infection of the CNS which leads to the development of a chronic progressive demyelinating disease that has immunological and pathological similarities to MS. MS has been associated with an inflammatory immune response in the CNS, and myelin- specific CD4+ T cells have been isolated from MS patients. The causative agent of MS is unknown, however, epidemiological evidence suggests that an infectious agent may be involved in initiation of disease. Viruses have been proposed to trigger the development of autoimmune diseases through bystander activation of immune cells or bystander damage of local tissue during infection. The innate immune response is the immediate immune response to a virus infection, and it distinguishes self from non-self. The innate immune response induces the secretion of cytokines which initiate the anti-viral immune response and influence the development of the adaptive immune response. The innate immune response also induces the secretion of chemokines which attract immune cells to the site of infection. Finally, the innate immune response activates NK cells and neutrophils which control the spread of the virus and activates antigen presenting cells which mediate the antigen- specific adaptive immune response. Thus, the innate immune response controls the spread of infection and damage to local tissue as well as activates the adaptive immune response which will ultimately clear the virus from the host. Therefore, the innate immune response may influence the development of an autoimmune response following virus infection through bystander activation of autoreactive T cells at the site of infection or through bystander damage to tissue at the site of infection. We examined the role of the innate immune response during early TMEV infection on development and progression of autoimmune demyelinating disease. Since the innate immune response to virus infection is predominantly mediated by type I interferons, IFNα and IFNβ, our studies focused on determining the effect of altering the amount of type I interferon present during the innate immune response to TMEV on development of the anti-myelin specific immune responses and on development of demyelinating disease.
Administration of either type I interferons, IFNα and IFNβ, or polyI:C to TMEV- infected mice during the innate immune response resulted in increased expression of the respective cytokines in the CNS. The most abundant source of type I interferon expression in the CNS following TMEV infection are microglial cells and to a lesser extent astrocytes (21,22). Conversely, administration of antiserum to IFNα or IFNβ during the innate immune response in TMEV- infected mice led to decreased CNS expression of IFNα or IFNβ. Manipulations of the levels of IFNβ in TMEV- infected mice led to significant changes in subsequent disease in that administration of IFNβ resulted in less severe demyelinating disease while administration of antiserum to IFNβ resulted in a more severe demyelinating disease. These results indicate that IFNβ expressed during the innate immune response mediates protection from development of demyelinating disease. In contrast, manipulations of the levels of IFNα in TMEV-infected mice had no significant effects on disease development. IFNα and IFNβ share a common receptor complex composed of two major subunits, IFNAR-1 and IFNAR-2. Although IFNα and IFNβ bind to the same receptor complex and can elicit similar responses, IFNα and IFNβ can also have different effects on the immune response. Previous studies have suggested that IFNβ may form a more stable interaction with the receptor complex than IFNα leading to conformational differences that influence the response elicited by receptor engagement (23). Thus, even though IFNα and IFNβ bind to the same receptor, our results suggest that they have different effects on the innate immune response and development of demyelinating disease. Most interestingly, administration of polyI:C in TMEV- infected mice during the innate immune response resulted in less severe demyelinating disease. These results show that altering the innate immune response during early virus infection directly affects the development of demyelinating disease indicating that the innate immune response to virus infection directly affects development of autoimmune disease.
The development of demyelinating disease following TMEV infection is dependent on the establishment of persistent virus infection of microglia and macrophage in the CNS (22). Type I interferons are antiviral proteins which can directly inhibit virus replication. Therefore, one possible explanation for the less severe demyelinating disease may be reduced levels of infectious virus in the CNS of the mice. However, the TMEV-infected mice administered type I interferons during the innate immune response had similar amounts of infectious virus in the CNS compared to controls suggesting that the reduction in demyelinating disease may be due to an affect on the immune response during virus infection.
The innate immune response is mediated by the expression of innate immune cytokines which coordinate the innate immune response as well as influence the development of the adaptive immune response. TMEV is associated with the development of a Th1- type, pro- inflammatory immune response in the CNS. TMEV- infected mice administered IFNβ or polyI:C had increased expression of pro-inflammatory cytokines (IFNγ, IL-6, and TNFα) during the innate immune response compared to control treated mice. Most interestingly, TMEV-infected mice administered IFNβ or polyI:C also had increased the expression of IL-10 which is normally associated with an anti-inflammatory immune response. These results suggest that the innate immune response was enhanced in the mice administered IFNβ or polyI:C and that the increased expression of IL-10 may play a role in reducing the development of the pro-inflammatory immune response associated with demyelinating disease. Further, the innate immune response induces the expression of chemokines and trafficking molecules that aid in the attraction of immune cells to the site of infection. TMEV- infected mice administered IFNβ or polyI:C had increased expression of chemokines early post- infection, however, these mice had decreased infiltration of T cells and macrophage into the CNS. Interestingly, TMEV- infected mice administered IFNβ or polyI:C had decreased expression of MMP9 which is a molecule that facilitates cell migration across the blood brain barrier (24). Therefore, IFNβ and polyI:C may be mediating the trafficking of cells within the CNS but may be reducing the number of immune cells infiltrating from the periphery by maintaining the integrity of the blood brain barrier.
TMEV infection is associated with a virus- specific CD4+ and CD8+ T cell response which can be detected at 7 post infection which correlates with peak virus load in the periphery and CNS, and the virus- specific CD4+ T cell response can be detected throughout the persistent infection of the CNS. The demyelinating phase of the disease is associated with development of a myelin- specific CD4+ Th1 type T cell response directed against immunodominant myelin epitope, PLP139–151, which arises via epitope spreading. Interestingly, TMEV- infected mice administered type I interferons or polyI:C have similar virus- specific CD4+ and CD8+ T cell responses as the controls suggesting that the virus-specific immune response was not significantly altered by the innate immune response. In contrast, TMEV- infected mice administered IFNβ or polyI:C did not develop autoreactive CD4+ T cell responses to either the immunodominant myelin antigen, PLP139–151, or to other identified myelin antigens. Further, administration of IFNβ or polyI:C reduced the expression of Th1- type cytokines in the CNS during chronic demyelinating disease and increased the expression of IL-10 which has been associated with a Th2- type response. Therefore, altering the innate immune response to TMEV significantly affected the development of the autoreactive CD4+ T cell response.
IFNβ was approved for MS treatment over 10 years ago and remains a predominate treatment for MS. IFNβ administration to patients with relapsing remitting disease reduces the relapse rate by 30–50% and reduces progression to disability by 30% (25). Patients also show a reduction in active lesions in the CNS by magnetic resonance imaging (26). The mechanism by which IFNβ reduces MS progression is not completely understood, however studies suggest that the main action of IFNβ is through modulation of the inflammatory response. IFNβ has been shown to reduce the proliferation of T cells and restore suppressor functions to T cells (27). Treatment with IFNβ has also been associated with a reduction in the pro-inflammatory Th1 type cytokines and an increase in Th2 type cytokines such as IL-10 resulting in a decrease in the inflammatory response. Further, treatment with IFNβ in MS patients has been shown to decrease the expression of adhesion molecules and MMP9 which prevent autoreactive T cells from entering the CNS (28). The results from our studies demonstrate that administration of IFNβ to TMEV- infected mice in only two doses during the innate immune response resulted in the alteration of cytokine expression from a Th1- to a Th2- type response, the reduction of T cell infiltration associated with decreased in MMP9 expression, and the decreased activation/proliferation of autoreactive myelin- specific T cells. Thus, IFNβ expression may be affecting several mechanisms of the immune response which alter the progression of demyelinating disease. In addition, our studies show that when these affects are induced during the innate immune response, IFNβ can inhibit the development of autoimmune disease. Thus, if MS is exacerbated by virus infections or sustained by a persistent/latent infection, IFNβ may be reducing the effects of the virus infection on CNS disease progression.
PolyI:C is a synthetic double stranded RNA that mimics viral RNA and has been shown to be a potent activator of the innate immune response. Our previous studies have shown that microglia isolated from the CNS of mice are activated following stimulation with polyI:C to express innate and adaptive immune cytokines and to become effective antigen presenting cells (19). The current studies have shown that administration of polyI:C to mice during the innate immune response to TMEV alters the development of autoimmune demyelinating disease. The reduction in demeylinating disease following polyI:C administration was associated with altered cytokine expression from a Th1 to a Th2 type response, reduced infiltration of T cells and macrophage into the CNS, and reduced activation of autoreactive T cells. Thus, polyI:C had a similar affect on the immune response as IFNβ and is likely exerting its affect by inducing the expression of IFNβ. PolyI:C also reduced EAE disease when administered prior to disease onset, and the action of polyI:C was shown to be mediated through IFNβ production and the induction of MCP-1 (29). However, polyI:C is an agonist for TLR3 as well as Mda5 both of which are innate immune receptors that upon recognition of polyI:C can activate several components of the adaptive immune response. These include activation of antigen presenting cells, specifically dendritic cells; induction of CD8+ T cell expansion; and the production of antibodies (30). PolyI:C does not induce T cell proliferation and does not induce disease when used as an adjuvant for EAE induction (31). Thus, polyI:C may also be exerting its affects on development of autoimmune disease through mechanisms other than expression of type I interferons. Further studies will be required to differentiate the mechanism of action by which polyI:C alters the immune response during TMEV- induced demyelinating disease.
The current studies demonstrate that altering the innate immune response during TMEV infection of the CNS resulted in an alteration in the development and progression of chronic autoimmune mediated demyelinating disease. The results suggest that the innate immune response to CNS virus infections may be involved in development of autoimmune demyelinating disease through bystander activation of immune cells, including autoreactive T cells. Administration of IFNβ or polyI:C reduced the infiltration of immune cells into the CNS, specifically T cells, which could be activated to become autoreactive cells. This is consistent with our recent finding that naïve PLP139–151- specific CD4+ T cells get activated in the inflamed CNS in TMEV-infected mice. Administration of IFNβ or polyI:C also altered the cytokine expression to reduce the inflammatory response and to switch the response towards a Th2 response which does not promote activation of autoreactive T cells. Thus, these results suggest that a more robust innate immune response may inhibit bystander activation of immune cells in the CNS. Likewise, the innate immune response to virus infection may also affect the development of autoimmune demyelinating disease through bystander damage. Virus- infected cells are killed directly by the virus as well as by components of the innate and adaptive response in an effort to reduce the spread of infection. The damaged tissue may form DAMPs that are recognized by innate immune receptors which may promote a pro-inflammatory immune response. The increased expression of IFNβ may protect the CNS cells from the cytolytic actions of the virus and reduce cell death of neurons during the innate immune response (32,33). Therefore, the innate immune response to virus infection of the CNS may be involved in the development of autoimmune demyelinating disease by bystander activation or bystander damage. However, depending on the particular innate immune response initiated, the virus can either promote or inhibit the development of autoimmune disease. Interestingly, several viruses, including encephalitis viruses that infect the CNS, encode a protein that inhibits the expression of IFNβ which may promote initiation of autoimmune disease (34). Further, MS patients have been shown to express lower levels of IFNβ than control patients, thus, the innate immune response to a particular virus may vary between individuals which could result in differential development of autoimmune disease in individuals infected with the same virus (35).
Supplementary Material
TMEV-infected mice were administered IFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The brain and spinal cord were removed from mice (3 mice per group) at 60 days post infection. The mononuclear cells were isolated from the organs and stained with fluoresceinated antibodies specific for IL-10 and CD45, and CD4, CD8, CD11b, or CD11c. Flow cyotmetric analysis was conducted by examining the resident microglia, CD45intermediateCD11b+ cells (A) or infiltrating cells CD45high (B) and CD11b+ macrophage (C), CD4+ T cells (D), CD8+ T cells (E), or CD11c+ dendritic cells (F).
Acknowledgments
We thank Jenna Bowen and Erin Johnson at University of Wisconsin for technical assistance on the cytokine expression assays.
This work was supported by grants from the National Multiple Sclerosis Society RG3625 and RG3629 and NIH NS023349.
Abbreviations
- MS
multiple sclerosis
- PLP
proteolipid protein
- TMEV
Theiler’s murine encephalomyelitis virus
References
- 1.Katz-Levy Y, Neville KL, Padilla J, Rahbe SM, Begolka WS, Girvin AM, Olson JK, Vanderlugt CL, Miller SD. Temporal development of autoreactive Th1 responses and endogenous antigen presentation of self myelin epitopes by CNS-resident APCs in Theiler’s virus-infected mice. J Immunol. 2000;165:5304–5314. doi: 10.4049/jimmunol.165.9.5304. [DOI] [PubMed] [Google Scholar]
- 2.Neville KL, Padilla J, Miller SD. Myelin-specific tolerance attenuates the progression of a virus-induced demyelinating disease: Implications for the treatment of MS. J Neuroimmunol. 2002;123:18–29. doi: 10.1016/s0165-5728(01)00479-9. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll- like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 4.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol. 2003;5:143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, Brint E, Dunne A, Gray P, Harte MT, McMurray D, Smith DE, Sims JE, Bird TA, O’Neill LA. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 7.Malakhova O, Malakhov M, Hetherington C, Zhang DE. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J Biol Chem. 2002;277:14703–14711. doi: 10.1074/jbc.M111527200. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Taniguchi T. Toll-like receptor signaling and IRF transcription factors. IUBMB Life. 2006;58:290–295. doi: 10.1080/15216540600702206. [DOI] [PubMed] [Google Scholar]
- 9.Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3:382–390. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 10.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- 11.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 12.Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-alpha/beta and IL-18 synergistically enhance IFN-gamma gene expression in human T cells. J Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- 13.Cousens LP, Orange JS, Biron CA. Endogenous IL-2 contributes to T cell expansion and IFN-gamma production during lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–5699. [PubMed] [Google Scholar]
- 14.Biron CA. Interferons alpha and beta as immune regulators--a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 15.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 16.Tough DF, Sun S, Sprent J. T cell stimulation in vivo by lipopolysaccharide (LPS) J Exp Med. 1997;185:2089–2094. doi: 10.1084/jem.185.12.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Bon A, Schiavoni G, D’Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 19.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg GA, Dencoff JE, Correa N, Jr, Reiners M, Ford CC. Effect of steroids on CSF matrix metalloproteinases in multiple sclerosis: relation to blood-brain barrier injury. Neurology. 1996;46:1626–1632. doi: 10.1212/wnl.46.6.1626. [DOI] [PubMed] [Google Scholar]
- 21.Olson JK, Girvin AM, Miller SD. Direct activation of innate and antigen presenting functions of microglia following infection with Theiler’s virus. J Virol. 2001;75:9780–9789. doi: 10.1128/JVI.75.20.9780-9789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 23.Brierley MM, Fish EN. IFNα/β receptor interactions to biological outcomes: understanding the circuitry. J Interferon Cytokine Res. 2002;22:835–845. doi: 10.1089/107999002760274845. [DOI] [PubMed] [Google Scholar]
- 24.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 25.Clerico M, Contessa G, Durelli L. Interferon-beta1a for the treatment of multiple sclerosis. Expert Opin Biol Ther. 2007;7:535–542. doi: 10.1517/14712598.7.4.535. [DOI] [PubMed] [Google Scholar]
- 26.Bermel RA, Rudick RA. Interferon-beta treatment for multiple sclerosis. Neurotherapeutics. 2007;4:633–646. doi: 10.1016/j.nurt.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markowitz CE. Interferon-beta: mechanism of action and dosing issues. Neurology. 2007;68:S8–11. doi: 10.1212/01.wnl.0000277703.74115.d2. [DOI] [PubMed] [Google Scholar]
- 28.Avolio C, Filippi M, Tortorella C, Rocca MA, Ruggieri M, Agosta F, Tomassini V, Pozzilli C, Stecchi S, Giaquinto P, Livrea P, Trojano M. Serum MMP-9/TIMP-1 and MMP-2/TIMP-2 ratios in multiple sclerosis: relationships with different magnetic resonance imaging measures of disease activity during IFN-beta-1a treatment. Mult Scler. 2005;11:441–446. doi: 10.1191/1352458505ms1193oa. [DOI] [PubMed] [Google Scholar]
- 29.Touil T, Fitzgerald D, Zhang G, Rostami A, Gran B. TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-β. J Immunol. 2006;177:7505–7509. doi: 10.4049/jimmunol.177.11.7505. [DOI] [PubMed] [Google Scholar]
- 30.Kumar H, Koyama S, Ishii KJ, Kawai T, Akira S. Cutting Edge: Cooperation of IPS-1- and TRIF-Dependent Pathways in Poly IC-Enhanced Antibody Production and Cytotoxic T Cell Responses. J Immunol. 2008;180:683–687. doi: 10.4049/jimmunol.180.2.683. [DOI] [PubMed] [Google Scholar]
- 31.Hansen BS, Hussain RZ, Lovett-Racke AE, Thomas JA, Racke MK. Multiple toll-like receptor agonists act as potent adjuvants in the induction of autoimmunity. J Neuroimmunol. 2006;172:94–103. doi: 10.1016/j.jneuroim.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89:770–778. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Kieseier BC, Hartung HP. Interferon-beta and neuroprotection in multiple sclerosis--facts, hopes and phantasies. Exp Neurol. 2007;203:1–4. doi: 10.1016/j.expneurol.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Blakqori G, Delhaye S, Habjan M, Blair CD, Sanchez-Vargas I, Olson K, Attarzadeh-Yazdi G, Fragkoudis R, Kohl A, Kalinke U, Weiss S, Michiels T, Staeheli P, Weber F. La Crosse Bunyavirus Nonstructural Protein NSs Serves to Suppress the Type I Interferon System of Mammalian Hosts. J Virol. 2007;81(10):4991–4999. doi: 10.1128/JVI.01933-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wandinger K, Wessel K, Neustock P, Siekhaus A, Kirchner H. Diminished production of type-I interferons and interleukin-2 in patients with multiple sclerosis. J Neurol Sci. 1997;149:87–93. doi: 10.1016/S0022-510X(97)05383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TMEV-infected mice were administered IFNβ, polyI:C, or PBS (control) on day 0 and day 4 post infection. The brain and spinal cord were removed from mice (3 mice per group) at 60 days post infection. The mononuclear cells were isolated from the organs and stained with fluoresceinated antibodies specific for IL-10 and CD45, and CD4, CD8, CD11b, or CD11c. Flow cyotmetric analysis was conducted by examining the resident microglia, CD45intermediateCD11b+ cells (A) or infiltrating cells CD45high (B) and CD11b+ macrophage (C), CD4+ T cells (D), CD8+ T cells (E), or CD11c+ dendritic cells (F).