Abstract
Drosophila melanogaster, the fruit fly, is a commonly used model organism because of its homology to mammals and facile genetic manipulations. However, the size of the nervous system is very small. We report a method to evoke and detect rapid changes in extracellular dopamine in a single nerve cord isolated from a Drosophila larva. Flies were genetically modified to express Channelrhodopsin-2, a blue-light activated cation channel, in only dopaminergic neurons. Extracellular dopamine changes were measured with fast-scan cyclic voltammetry at an implanted carbon-fiber microelectrode. Seven-second stimulations with blue light result in an average peak dopamine concentration of 810 ± 60 nM, similar to electrically-stimulated release in mammals. Stimulations repeated at 15-minute intervals are stable for 65 minutes, allowing pharmacological experiments in the same sample. Peak duration is extended after cocaine or nisoxetine, inhibitors of the dopamine transporter (DAT). Release was reduced upon exposure to reserpine, which inhibits vesicular packaging. Chronic administration of NSD-1015, a dopamine synthesis inhibitor, decreased dopamine release and inhibited pupation, showing a link between neurotransmission and physiology. This is the first method to measure endogenous dopamine in an intact larval Drosophila nervous system and will allow studies of genetic and pharmacological manipulations of dopamine release and uptake.
Introduction
Drosophila melanogaster, the fruit fly, has been used for nearly 100 years as a model organism for understanding complex biological processes. For example, Drosophila has been used to study synaptic structure and neural circuitry 1–3 and as a model for human neurodegenerative diseases such as Amyotrophic lateral sclerosis, Parkinson’s disease and Alzheimer’s disease 4–6. About 75 % of human genes have a functional ortholog in the fruit fly 7;8. Drosophila has a short life span, large number of progeny, and can be easily genetically manipulated. These traits are amenable for the development of high throughput screenings because Drosophila genetic mutants can be created much quicker than in mammalian systems. For example, genetically-altered Drosophila can be produced in 1–2 months, while creating a knockout mouse can take up to 2 years. The limitation for using Drosophila to study neurotransmission has been lack of rapid measurement techniques.
Dopamine is an important neurotransmitter that is implicated in many human behaviors such as cognition, reward, addiction, motivation and motor function. Understanding dopamine regulation is essential for the treatment of many diseases including schizophrenia, attention-deficit disorder, and Parkinson’s disease. Dopamine regulatory functions such as synthesis and uptake are conserved between humans and Drosophila 9;10. Dopaminergic neurons have been characterized in Drosophila by immunohistochemistry11;12, which provides an understanding of neuronal morphology but not a direct measurement of neurotransmission. To quantitate dopamine tissue content in the fly central nervous system (CNS), tissue homogenates have been analyzed with HPLC or CE. These studies have estimated that total dopamine content ranges from 10 13 to 74 pg 14 in adult fly brains and 12–14 pg15 in the larval CNS. While these methods can measure multiple neurotransmitters, homogenization destroys the tissue so repeated measurements cannot be made in the same sample. In addition, they do not measure the functional pool of dopamine that is released by exocytosis and acts in the extracellular space as a neurotransmitter.
Direct measurement of dopamine in an intact Drosophila melanogaster CNS has been hindered both by the small size of the CNS and lack of a method to evoke endogenous release specifically from dopaminergic neurons. For Drosophila, small probes are needed because the CNS is only 100 μm wide and approximately 8 nL in volume. Therefore, the VNC is too small for both microdialysis probes and electrophysiology arrays, which are typically 100–200 μm. Detection of fast changes in dopamine concentrations has traditionally been performed in mammalian systems using fast-scan cyclic voltammetry (FSCV) at carbon-fiber microelectrodes 16. FSCV is advantageous because of its high sensitivity and subsecond temporal resolution 17. The 7 μm diameter carbon-fiber microelectrode is small enough to be implanted in the tiny fly CNS. The Ewing lab has developed a method using carbon-fiber microelectrodes to detect exogenously-applied dopamine in the adult Drosophila CNS 18. They evaluated dopamine clearance in normal flies and after pharmacological or genetic manipulation of the dopamine transporter (DAT), which is responsible for uptake. However, because this method does not measure endogenous dopamine, dopamine release cannot be studied.
Recently, our lab has developed a microelectrode method to detect endogenous serotonin release and uptake in the intact ventral nerve cord of a single Drosophila larva 19. While mammalian experiments have often evoked release using electrical stimulation, the bipolar electrical stimulating electrode used in rodents is larger than the length of the entire larval Drosophila VNC. Utilizing Drosophila’s genetic palpability, we have developed a method to evoke endogenous neurotransmitter release by genetically modifying flies to express Channelrhodopsin-2 (ChR2). ChR2 is a blue-light activated ion channel that can be inserted specifically into dopaminergic neurons using the commonly employed yeast GAL4/UAS system 20. Upon blue-light stimulation, ChR2 opens and the inward flow of calcium and sodium leads to exocytosis 21.
In this study, we develop and characterize a method to measure dopamine release in a single, isolated larval Drosophila CNS. Dopamine release is evoked by blue-light activation of ChR2 and detected using FSCV at carbon-fiber microelectrodes. The advantages of this method are that endogenous dopamine can be measured repeatedly in the same sample in an intact Drosophila CNS. Characterization using pharmacological agents that disrupt synthesis, uptake and vesicular packaging shows that the Drosophila dopaminergic system is similar to mammals. In addition, chronic administration of a synthesis inhibitor also disrupts pupation, a physiological process requiring dopamine. Therefore, our technique can be used to measure physiologically relevant dopamine changes and will allow Drosophila to be utilized as a model system to study dopamine regulation.
Experimental Section
Chemicals
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used as received. All solutions were prepared using Milli-Q water (Millipore Billerica, MA). A modified Schneider’s buffer (15.2 mM MgSO4, 21 mM KCl, 3.3 mM KH2PO4, 36 mM NaCl, 5.8 mM NaH2PO4, 5.4 mM CaCl2, 11.1 mM glucose, 5.3 mM trehalose, pH = 6.2) was used in all calibration experiments and dissections. 50 μM cocaine and 20 μM nisoxetine solutions were made using the modified Schneider’s buffer. Similarly, a 100 μM reserpine solution in buffer was made by dilution of a 10 mM stock solution of reserpine dissolved in DMSO. A 20 μM NSD-1015 solution was made in water and mixed with the Drosophila food, Red Star yeast (Milwaukee, WI), making the total NSD-1015 concentration 6.6 μM. The drug-yeast mixture was fed to 3-day-old Drosophila larvae for 2 days prior to experimentation. Larvae were observed under a microscope and appeared healthy, but failed to show signs of pupation after 5 days.
Electrochemical Measurements
Carbon-fiber microelectrodes were fabricated by aspirating a single T-650 carbon fiber (Cytec Engineering Materials, West Patterson, NJ) into a 1.2 mm x 0.68 mm glass capillary (A-M systems, Carlsburg, WA). A vertical pipette puller (Narishige, model PE-21, East Meadow, NY) was used to form two electrodes. The carbon fiber was trimmed to a length of 40 – 60 μm with a scalpel. In order to seal the fiber in the glass, the electrodes were epoxied with Epon Resin 828 (Miller-Stephenson, Danbury, CT) mixed with 14% (w/w) m-phenylenediamine hardener (Fluka, Milwaukee, WI). The epoxy and hardener were heated to approximately 85°C and the electrodes were dipped into the mixture for 30 s and allowed to dry overnight. They were then cured in an oven at 100°C for two hours followed by 150°C overnight. The electrodes were soaked in isopropanol for 10 minutes and backfilled with 1 M KCl prior to use.
A Dagan Chem-Clamp potentiostat with modified gain settings was used to collect data (Dagan, Minneapolis, MN). Tar Heel CV software (gift of Mark Wightman, University of North Carolina) was used for data collection and analysis. A homemade breakout box and 2 computer interface boards (PCI 6052 and PCI 6711, National Instruments, Austin TX) were used to apply the cyclic voltammogram waveform and to collect data as previously described 22. The electrode was scanned at a scan rate of 400 V/s from −0.4 to 1.3 V and back at a repetition rate of 10 Hz. A Ag/AgCl wire was used as a reference electrode and was placed in the buffer bath outside the nerve cord. Electrodes were calibrated with a 1 μM dopamine solution before and after implantation into a VNC.
Statistics were performed using GraphPad Prism software. Data are reported as mean +/− standard error of the mean (SEM) for n number of different samples. Data were considered significantly different at the 95 % confidence level.
Preparation of ventral nerve cords
Flies containing UAS-ChR2 (a gift from Christian Schroll, Universitat Wurzburg) were crossed to flies expressing th-GAL4 (a gift from Jay Hirsh, University of Virginia) to generate homozygous lines with a th-GAL4;UAS-ChR2 genotype. Flies expressing th-GAL4 were crossed with flies containing UAS-GFP (Bloomington, IN) to generate homozygous lines with a th-GAL4;UAS-GFP genotype. 3-day-old larvae were fed yeast containing a 1 mM trans-retinal supplement for 2 days prior to dissection and were shielded from light. 5-day-old wandering 3rd instar larvae were dissected in the modified Schneider’s buffer unless otherwise noted. The entire CNS was removed and the optic lobes were removed by a horizontal cut across the anterior thorax region. The isolated ventral nerve cord (VNC) was adhered to the bottom of a plastic Petri dish (Becton Dickinson, Franklin Lakes, NJ) containing 4 mL of buffer. The dish was placed on a fluorescent microscope and a 40× water immersion lens was used to visualize the nerve cord while an electrode was inserted into the neuropil region using a micromanipulator. The electrode was inserted a distance of 4–5 segments away from the cut edge. For acute pharmacological experiments in different nerve cords, samples were dissected in the presence of drug and incubated for 20–30 minutes prior to electrode implantation to allow the drug to diffuse into the nerve cord. For experiments in the same nerve cord, a VNC was placed in a Petri dish with 3 mL modified Schneider’s buffer. After an initial stimulation, 1 mL of 80 μM nisoxetine was added to the Petri dish to provide a final concentration of 20 μM nisoxetine.
All experiments were performed in a dark room. The electrode was allowed to equilibrate after implantation for at least 5 minutes before the initiation of data collection. Unless otherwise indicated, 30 s of baseline electrochemistry data was collected before VNCs were exposed to 7 s of blue-light illumination from a 10 W halogen microscope bulb with a standard fluorescein emission filter (450 – 490 nm). Stimulations were repeated at 15 minute intervals to allow for recovery.
Immunohistochemistry Experiments
Flies expressing green fluorescent protein (GFP) in their dopaminergic neurons (th-GAL4;UAS-GFP) were mounted and stained using anti-chicken GFP polyclonal antibodies (Aves Labs, Tigard, Oregon) 23. Dopamine cell bodies were imaged with an Olympus 1×70 microscope with a Fluoview 3.3 laser scanning confocal system (Olympus America, Melville, NY). Image reconstitutions were performed with Volocity 2.0 (Improvision, Waltham, MA).
Results and Discussion
Dopaminergic signaling regulates a variety of complex behaviors in both flies and mammals including addiction, learning and sexuality 24;25. In flies, dopamine also regulates pupation. Because neurotransmitter signaling pathways have been previously shown to be conserved between species, Drosophila is a good model system to study dopamine regulation 10;26;27. We used 5-day old, 3rd instar larvae, which have been shown to have fully developed dopaminergic neurons 12;28. Drosophila larvae are also advantageous over adult flies because their CNS is easily removed and immobilized.
Anatomy of Drosophila dopamine neurons
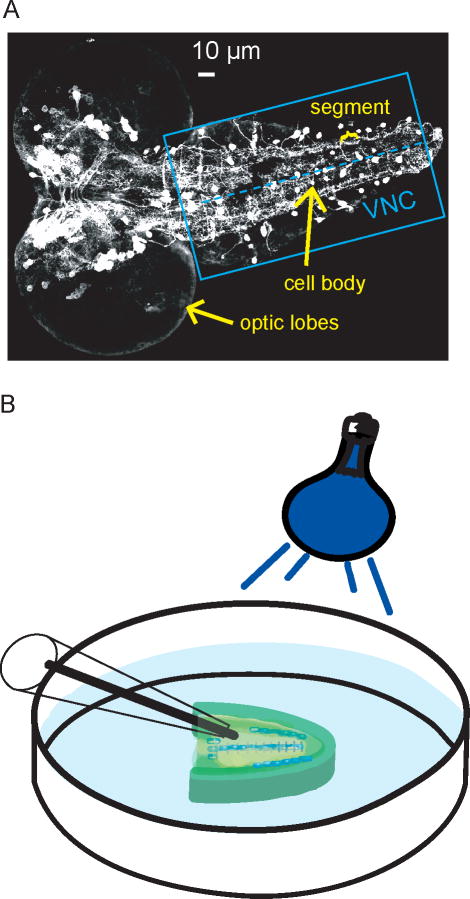
The larval Drosophila CNS is composed of optic lobes and a segmented ventral nerve cord (VNC). The CNS is small: approximately 100 μm wide and 250 μm in length. To visualize the location of dopamine neurons in a larva, a CNS was imaged from a fly expressing green-fluorescent protein in dopamine neurons. In Figure 1A, the optic lobes are the large round features on the left and the ventral nerve cord (VNC) extends out from them. Dopaminergic cell bodies are visualized as white circles and are evident throughout the CNS. In the ventral nerve cord (box), which is similar to the spinal cord in vertebrates, many cell bodies are located near the midline (dashed line). On either side of the midline, there is a region called the neuropil composed of a dense network of synapses and neurotransmitter release sites including dopaminergic terminals. For electrochemistry experiments, the whole CNS was dissected from the larva and the optic lobes of the CNS were removed to facilitate electrode insertion. Dissection and incubation of CNS tissue in a modified Schneider’s buffer has previously been shown to keep tissue viable for up to 6 hours after dissection19;28. The electrode was implanted in the neuropil to measure release from the dopamine terminals (Figure 1B).
Figure 1.
A) Fluorescence microscopy image of GFP-labeled dopaminergic neurons in a 5-day-old, 3rd instar larva CNS. The blue box indicates the ventral nerve cord, and the dashed line marks the midline, where many cell bodies (white circles) are located. On either side of the midline is the neuropil region, which is rich in dopamine terminals. B) Schematic of microelecrode placement into the neuropil region of the ventral nerve cord with blue-light stimulation. The optic lobes have been removed from the CNS.
Measurements of endogenous dopamine evoked by Channelrhodopsin2 stimulation
In order to elicit release from only one type of neuron, we expressed Channelrhodopsin-2 (ChR2) specifically in dopaminergic neurons using the yeast-based GAL4/UAS system. Flies containing ChR2, which are controlled by the upstream activating sequence (UAS) element, were crossed with flies containing GAL4. GAL4 was specifically targeted to cells containing tyrosine hydroxylase (th), a dopaminergic synthesis enzyme. Transcription of UAS requires GAL4; therefore, both UAS and GAL4 must be present in a cell for ChR2 to be expressed. The activation of ChR2 expressed in dopaminergic neurons causes depolarization and exocytosis 21.
This method of neuron-specific ChR2-mediated stimulation has numerous advantageous in comparison to electrical stimulation. First, it is specific and activates only one type of neuron. The dopaminergic projections are closely intertwined with other neurons, such as serotonergic projections, so electrical stimulation would be nonspecific. Second, larval dopaminergic neurons are small and the whole is nerve cord is smaller than most electrical stimulating electrodes, which are hundreds of microns in diameter. Finally, ChR2 stimulation is not action potential dependent, which might facilitate future studies that manipulate basal firing to determine the effects on stimulated release.
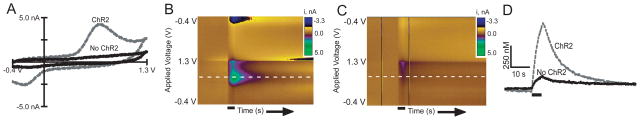
Evoked dopamine was measured at a carbon-fiber microelectrode that was inserted in the neuropil (Figure 1B). A triangular waveform (−0.4 V to 1.3 V and back at 400 V/s, 10 Hz) was constantly applied to the electrode. Figure 2A shows cyclic voltammograms collected after a 7 s stimulation with blue light. The cyclic voltammogram collected from a larva expressing ChR2 exhibits a peak shape and redox features consistent with dopamine release. However, a cyclic voltammogram from a parent fly lacking the ChR2 insertion (th-GAL4) contains no characteristic oxidation or reduction peaks for dopamine. Therefore, ChR2 expression is required to elicit dopamine release. Figures 2B and C show all the data collected in a three-dimensional false-color plot for the ChR2-expressing larva and the parent larva without ChR2, respectively. Consecutive cyclic voltammograms are plotted in time on the x-axis and the applied voltage is plotted on the y-axis. Change in current is represented by color. In Fig. 2B, the green area on the color plot corresponds to the oxidation of dopamine and the blue area to the reduction of dopamine. In the control sample without ChR2, a small change in current is observed at the switching potential (1.3 V), but there is an absence of dopamine oxidation or reduction peaks.
Figure 2.
Characterization of dopamine signal evoked by ChR2 activation. Each larva was stimulated for 7 s. The electrode was scanned from −0.4 to 1.3 V and back at 400 V/s every 100 ms. A) Background subtracted cyclic voltammogram from a larva expressing ChR2 (dashed line) and one without ChR2 (solid line). The larva lacking the ChR2 insertion does not exhibit characteristic dopamine reduction and oxidization peaks. B) Color plot showing a 7 s blue light stimulation (denoted by line under figure) in a larva expressing ChR2. The green and blue areas show the oxidation and reduction peaks, respectively, for dopamine. C) Color plot from a larva without ChR2. The largest change in current corresponds to the switching potential. No dopamine peaks are present. The white dashed line marks the oxidation potential for dopamine. D) Current vs. time plots show that the dopamine signal in a ChR2 expressing larva increases upon blue-light stimulation and decreases after the blue light is turned off. The duration of the blue light stimulus is marked as a line under the trace. The current at the oxidation potential has been converted to concentration using a postcalibration value. The larva without ChR2 has a small change at the oxidation potential for dopamine, not from dopamine release but due to ionic changes in response to blue light.
A concentration vs time profile can be plotted by converting the current at the maximal oxidation potential for dopamine to concentration using an in vitro calibration. Figure 2D shows the evoked dopamine signal in the ChR2-expressing VNC increases quickly upon stimulation and decays rapidly after the blue-light is turned off, which is consistent with evoked dopamine release and fast clearance. In the larva without the ChR2 insertion, a small change in current is observed when the current at the oxidation potential for dopamine is plotted over time (Figure 2D). The change in current is not due to dopamine release, as confirmed by the cyclic voltammogram, but is detected at all potentials. This error is observed upon exposure to blue light, and not other wavelengths, and is likely due to changes in the background current caused by ionic changes during blue light activation. The current change in larva without ChR2 is only about 13 % of the average stimulated release and defines the lower limit of detection (~110 nM).
The average concentration of evoked dopamine measured in ChR2 expressing larvae is 810 ± 60 nM (n = 7), which is similar to concentrations observed in mammalian experiments. For example, stimulating a rat brain slice with a single electrical pulse results in approximately 2300 ± 400 nM dopamine in the caudate-putamen and from 540 ± 90 nM to 1500 ± 600 nM in the nucleus accumbens core 29;30. In vivo, peak dopamine concentrations between 250 and 600 nM have been observed after short, electrical stimulation pulse trains (0.4 to 2 s duration) 31–33. Longer stimulations were necessary to evoke ChR2-mediated neurotransmitter changes in Drosophila similar to electrically-stimulated experiments in mammals. This is most likely due to the low wattage of the halogen lamp and the difficulty of the light to penetrate the outermost glial cell layer of the nerve cord. Additionally, the extent to which ChR2 is expressed is unknown, so not all neurons may be depolarized as with electrical stimulation. Nevertheless, this similarity in magnitude of endogenous dopamine release between Drosophila and mammals validates using Drosophila as a model system.
Effects of stimulation parameters on evoked dopamine release
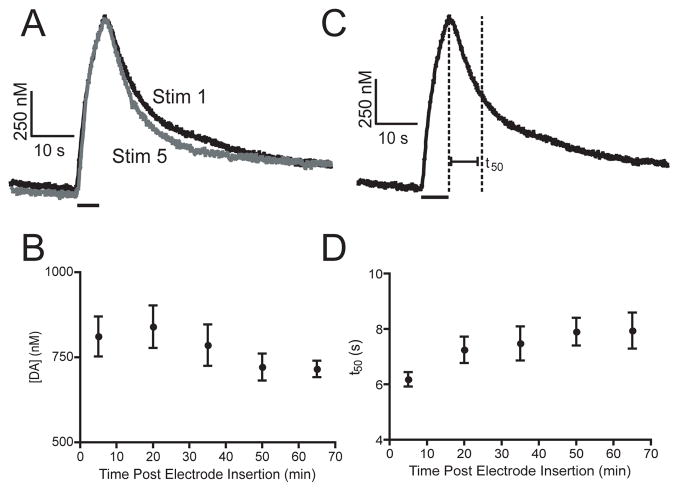
Stimulated release must be stable over a prolonged time interval in order to evaluate changes induced by pharmacological agents in the same sample. Stability parameters were established by varying stimulation length and recovery time. A stable, reproducible signal was achieved with repeated 7 s, blue-light stimulations every 15 minutes. For example, Figure 3A shows an initial 7 s stimulation and the fifth stimulation evoked 60 minutes later in the same VNC. The peak concentration evoked by each stimulation is nearly identical. Figure 3B demonstrates that repeating 7-s long stimulations at 15-minute intervals produces stable release for approximately 65 minutes. At longer times, evoked dopamine begins to decrease.
Figure 3.
Effect of repeated stimulations. Each VNC was stimulated for 7 s at 15-minute intervals. A) Concentration vs. time profiles from stimulations within the same VNC taken 60 min. B) Pooled data (mean +/− SEM, n = 7) show that the evoked dopamine concentration is stable for over an hour following nerve cord isolation. C) Diagram of the definition of t50. The half decay time is defined as the time from the end of the stimulation until the signal decays to 50 %. D) Pooled data (mean +/− SEM n = 7) show that t50 is stable for 65 minutes.
In order to evaluate the stability of the time course of signaling, the time to half decay (t50) was calculated from the individual current versus time profiles. t50 is defined as the time from the end of the stimulus until the half maximal concentration is reached (Figure 3C). This value can be used as an estimate of uptake by the dopamine transporter. Figure 3D shows t50 is constant for approximately 65 minutes, demonstrating that uptake is stable. Prolonged stability of both release and uptake during stimulated release experiments confirms that Drosophila is a viable model system for making repeated measurements of dopamine in the same sample.
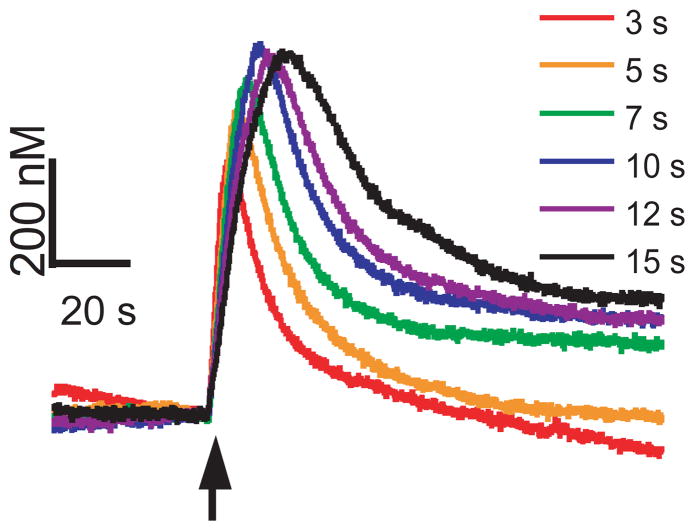
To test the effect of stimulation length on evoked dopamine, the duration of the blue-light stimulation was varied. VNCs were exposed to 3, 5, 7, 10, 12 or 15 s of blue-light stimulation, in that order, with 15 minutes rest between stimulations to allow the releasable pool of dopamine to recover (Figure 4). As the duration of activation increases, the amount of dopamine released also increases until a saturation point is reached around 10 s. For stimulations over 10 s, the peak height plateaus but the duration of signaling is longer. This suggests that either a maximal amount of release has been reached or that uptake is able to clear enough of the released dopamine during the longer stimulations that a steady-state curve is evident. Previously, ChR2 activation has been shown to occur on a millisecond time scale after which the channel reaches a steady state 34. Once open, ChR2 is responsive to continuous light for almost a full minute. Therefore, it is unlikely that the plateaus in peak height observed for longer stimulations are a result of desensitization of ChR2. A complete return by the signal to baseline is not observed for every stimulation, which is commonly observed in in vitro FSCV experiments 35. Therefore, the 7 s stimulation was chosen for pharmacology experiments, due to its robust, reproducible signal. However, in future experiments, 5 s stimulations may also be useful because evoked dopamine is well below maximal peak height.
Figure 4.
Effect of stimulation duration on dopamine signaling. Stimulations were performed for 3, 5, 7, 10, 12 or 15 seconds in one ventral nerve cord with 15 minutes of recovery time in between each stimulation. The stimulation start time is indicated by the black arrow.
Characterization of dopamine release using pharmacology
While fast-scan cyclic voltammetry provides a CV that allows chemical identification of the measured species, many neurotransmitters have CVs that are difficult to distinguish. For example, dopamine and norepinephrine have identical CVs, although the fly does not contain norepinephrine or epinephrine 24 so they are not possible interferents in Drosophila. Another method to identify the species being detected is to pharmacologically manipulate the specific neurotransmitter system 36. Therefore, in order to verify that dopamine was being detected, we inhibited dopamine synthesis pharmacologically with NSD-1015, which blocks the aromatic L-amino acid decarboxylase (AADC) enzyme. AADC catalyzes the decarboxylation of L-DOPA into dopamine, which is the second step in the dopamine synthesis pathway.
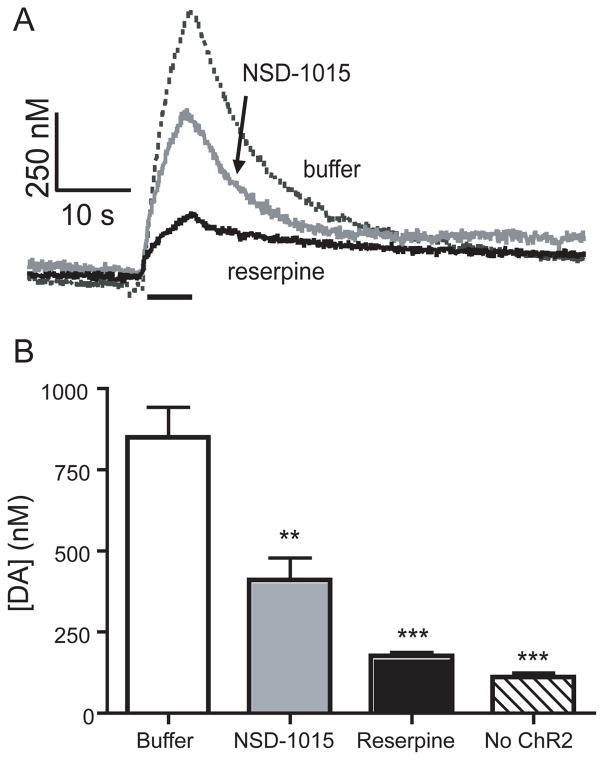
Twenty μM NSD-1015 was mixed with yeast and fed to larvae for 2 days prior to the experiment. When a larva is chronically exposed to NSD-1015, the amount of evoked dopamine decreases significantly (Figure 5A). The concentration of dopamine measured on the initial stimulation was 410 ± 80 nM (n = 6), significantly less than normal flies (unpaired t-test, p = 0.0038, Figure 5B). While stimulations repeated at 15-minute intervals evoke constant release in normal flies, subsequent stimulations from larvae fed NSD-1015 showed even lower release. For example, the second stimulation evokes on average only 43 ± 8 percent of the first, a significant decrease (n = 5, paired t-test, p = 0.004). The decrease in evoked release after NSD-1015 demonstrates that the measured neurotransmitter is dopamine and that synthesis is necessary to maintain the releasable pool of dopamine.
Figure 5.
Effect of pharmacological agents on evoked dopamine concentration. A) Concentration vs time profiles from individual VNCs show that peak dopamine concentration is reduced when larva are chronically fed NSD-1015 (6.6 μM), a synthesis inhibitor, or are incubated in reserpine (100 μM), a VMAT inhibitor that disrupts vesicular packaging. B) The concentration of dopamine elicited by the first stimulation is significantly less for NSD-1015 (** p < 0.01) and reserpine (*** p < 0.001) than in samples dissected in buffer, but more than control experiments where ChR2 was not expressed. * p < 0.05, ** p < 0.01, *** p < 0.001.
In order to pupate, Drosophila metabolizes dopamine into melanin 24. Interestingly, larvae fed NSD-1015 exhibited delayed pupation by 3–4 days or failed to pupate at all. Our electrochemistry data show a loss of releasable dopamine and the lack of pupation demonstrates that lower dopamine has a physiological effect. Because NSD-1015 was chronically administered to larvae, this technique is suitable for future studies of the effects of chronic drug exposure on dopamine levels.
To test whether the dopamine release is vesicular, we dissected and incubated VNCs in reserpine. Reserpine inhibits the vesicular monoamine transporter (VMAT) and prevents dopamine from being packaged into vesicles. In mammals, reserpine has been shown to diminish stimulated dopamine release 37. Because Drosophila possess a functional ortholog of VMAT, the isoform dVMAT-A, the response to reserpine should be conserved between species 38. Figure 5A shows that 30 min incubation in reserpine significantly reduces dopamine signaling. Evoked release was 180 ± 10 nM, significantly less than buffer-incubated animals (n = 7, unpaired t-test, p < 0.0001), but higher than in control subjects without ChR2 (n = 7, unpaired t-test, p < 0.0001), indicating that a small amount of dopamine is still detected (Figure 5B). Because reserpine effectively reduced the evoked dopamine signal, dopamine release is indeed vesicular. Therefore, Drosophila is useful in studying mechanisms of vesicular packaging and exocytosis.
Characterization of dopamine uptake using pharmacology
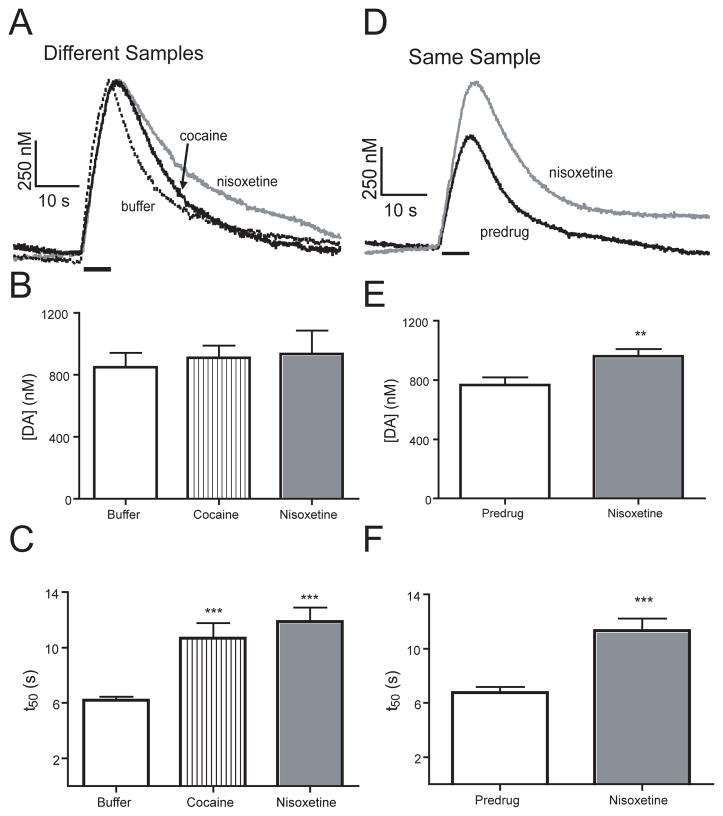
Dopamine is taken up from the extracellular space back into the cell by the dopamine transporter (DAT). Therefore, if the transporter is blocked, the dopamine clearance rate decreases and dopamine signaling is prolonged, which has been observed in mammalian systems 39. To test the effect of blocking uptake in Drosophila, VNCs were dissected and incubated in 50 μM cocaine, a drug of abuse, and known DAT inhibitor 26;39. Figure 6A shows example traces of dopamine after uptake inhibition. The evoked peak height is similar for the cocaine and buffer traces, but the signal takes longer to return to baseline after cocaine. The peak concentration elicited by the initial stimulation was 910 ± 80 nM, which is not significantly different from buffer controls (n = 7, unpaired t test, p = 0.6107) (Figure 6B). The half decay time (t50) for cocaine-exposed nerve cords increased by nearly 50 % and was significantly larger than buffer-incubated controls (n = 6, unpaired t-test, p = 0.0001, Figure 6C), which indicates uptake plays a role in dopamine clearance.
Figure 6.
Effect of DAT inhibitors on dopamine signaling. A) Concentration versus time profiles from individual VNCs dissected in buffer or incubated in 50 μM cocaine or 20 μM nisoxetine DAT inhibition increases the clearance time for dopamine. B) The initial concentration of dopamine measured in VNCs in the presence of 50 μM cocaine (n=6) or 20 μM nisoxetine (n=6) was not significantly different than that measured in buffer (n=7). C) The half decay time (t50) for VNCs dissected in buffer (n = 7) is significantly less (*** p < 0.001) than for cocaine (n=6) or nisoxetine (n=6). D) Concentration versus time profiles from a same-sample experiment. An initial 7 s blue-light stimulation was performed, then the nerve cord incubated in 20 μM nisoxetine and another stimulation performed 30 min later. DAT inhibition increases the clearance time and concentration of dopamine. E) Evoked release significantly increased (** p < 0.01) in the same-sample experiment after nisoxetine was added (n = 6). F) The half decay time (t50) significantly increased (*** p < 0.001) after nisoxetine.
While cocaine has been traditionally used to inhibit DAT in mammals, in Drosophila, cocaine has a higher affinity for the serotonin transporter (SERT) than DAT 21. Therefore, to prove that DAT is specifically responsible for dopamine clearance, we performed measurements in a different experimental group that was treated with nisoxetine. Nisoxetine has a Ki value three orders of magnitude higher for DAT than SERT in Drosophila (5.6 and 5000 μM, respectively). An example trace after 20 μM nisoxetine shows an increase in clearance time compared to samples incubated in buffer (Figure 6A). The concentration of evoked dopamine after nisoxetine incubation is 930 ± 150 nM, which is not significantly different from the buffer group (n = 7 unpaired t test, p = 0.6351) (Figure 6B). Nisoxetine also significantly increased t50 (Figure 6C, n = 7, unpaired t test, p = 0.0001) in a similar manner as cocaine. However the dose is lower for nisoxetine, indicating nisoxetine has a higher affinity for DAT, as has been reported for in vitro studies 10. These studies indicate that the dopamine transporter is specifically responsible for dopamine uptake.
Slowed clearance time after uptake inhibition is consistent with results found in mammalian studies. Measurements in the rat striatum show that cocaine increases the time course of signal decay by 50% 40. Additionally, uptake inhibition is expected to increase the peak concentration, since no dopamine will be cleared during the stimulation. For example, brain slice measurements in the nucleus accumbens and striatum of rats have shown significant increases in maximal dopamine concentration after cocaine administration29;41. Peak height increases after cocaine have been also been attributed to diffusion from distant sites or release effects 42;43 We observed a trend towards higher release after uptake inhibition, but observing a significant increase in Drosophila might require a large number of samples because of the biological variability between samples. Within subject studies might better elucidate these differences.
Thus, we repeated the experiments with nisoxetine in the same sample for comparison. For these experiments, a VNC was dissected in buffer and an initial 7 s, blue-light stimulation performed. Then, nisoxetine was added to the Petri dish to provide a final concentration of 20 μM and the same VNC stimulated at 15 min intervals. The example traces from the same-sample experiment show both an increase in peak height and decay time 30 min after nisoxetine application (Figure 6D). Evoked dopamine increased on average from 810 ± 60 nM to 960 ± 50 nM, which is statistically significant (n = 6, paired t test, p = 0.0046, Figure 6E). This 20 % increase is similar to increases in peak height after uptake inhibition observed in mammalian experiments 43. In same-sample measurements, addition of nisoxetine significantly increased clearance time as well (n = 6, paired t-test, p = 0.0005). The t50 values from the separate group and same sample experiments are not significantly different (p=6, unpaired t-test p = 0.6943, compare Figures 6C and F). Therefore, the prior stimulation in the same sample experiment has no effect on the clearance time. These experiments demonstrate that our technique makes Drosophila amenable for studies of dopamine uptake inhibitors and drugs of abuse.
Comparison to other techniques
We demonstrate the first direct, real-time measurement of endogenous dopamine release in a single Drosophila larva. Previously, HPLC and CE studies measured total dopamine tissue content in Drosophila, but provided no time course information about extracellular dopamine signaling13;14. Dopamine uptake has been measured in adult fruit flies by the Ewing group 18. They implanted a microelectrode in the protocerebral anterior medial brain area of an immobilized, intact adult fly. The time course of clearance of exogenously applied dopamine was measured using FSCV or amperometry. This experimental design provides information about uptake and they found that dopamine signaling was prolonged in mutant flies lacking DAT function or after administration of cocaine. Our data using cocaine in the larval CNS is consistent with their findings that dopamine uptake in Drosophila is slowed in the presence of a DAT inhibitor. For measuring uptake, our method and the Ewing method are complementary and both indicate Drosophila is a valid model system.
The primary advantage of our method is that we are able to evoke and detect endogenous dopamine release. The ChR2 stimulation is specific to one neuronal type and permits optical control of dopamine release. Our method will allow a range of experiments that test the effects of different pharmacological and genetic manipulations on dopamine release. For example, we could study exocytosis or the function of dopamine autoreceptors on the regulation of release. While it is not possible to make measurements in the intact larva because of body-wall contractions, our method takes advantage of whole CNS culture techniques that have been standardized in Drosophila. Therefore, neuronal connections are maintained, an advantage of using Drosophila larvae over mammalian brain slice experiments.
Conclusions
We have shown that fast-scan cyclic voltammetry at carbon-fiber microelectrodes can be used to measure endogenous dopamine release evoked in an isolated Drosophila melanogaster larval ventral nerve cord. The release of dopamine measured in the presence of pharmacological agents in Drosophila is consistent with data collected from mammalian studies; therefore, dopaminergic signaling in Drosophila is homologous to signaling in mammals. Drosophila is a valuable model system to study dopamine regulation and the results could be useful for designing better mammalian experiments to reduce the use of higher order animals. For example, our method could be used to examine how chronic cocaine administration or cocaine withdrawal influences dopamine clearance in Drosophila. Additionally, we could monitor dopamine release in flies that have been genetically modified to explore exocytosis or genetic elements affecting DAT. Once dopamine regulation is better characterized in Drosophila, this system could be used to study flies with genetic mutations mimicking human diseases.
Acknowledgments
This work was funded by the National Science Foundation (CHE 0645587522) to BJV and NIH to BC (RO1 DA020942) and BJV (R01 MH085159).
Reference List
- 1.Collins CA, DiAntonio A. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Prokop A, Meinertzhagen IA. Semin Cell Dev Biol. 2006;17:20–30. doi: 10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Olsen SR, Wilson RI. Trends Neurosci. 2008;31:512–20. doi: 10.1016/j.tins.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson MR, Lagow RD, Xu K, Zhang B, Bonini NM. J Biol Chem. 2008;283:24972–81. doi: 10.1074/jbc.M804817200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M. Mol Cell Neurosci. 2004;26:365–75. doi: 10.1016/j.mcn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Feany MB, Bender WW. Nature. 2000;404:394–98. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 7.Rubin GM, Yandell MD, Wortman JR, Gabor Miklos GL, Nelson CR, Hariharan IK, Fortini ME, Li PW, Apweiler R, Fleischmann W, Cherry JM, Henikoff S, Skupski MP, Misra S, Ashburner M, Birney E, Boguski MS, Brody T, Brokstein P, Celniker SE, Chervitz SA, Coates D, Cravchik A, Gabrielian A, Galle RF, Gelbart WM, George RA, Goldstein LS, Gong F, Guan P, Harris NL, Hay BA, Hoskins RA, Li J, Li Z, Hynes RO, Jones SJ, Kuehl PM, Lemaitre B, Littleton JT, Morrison DK, Mungall C, O’Farrell PH, Pickeral OK, Shue C, Vosshall LB, Zhang J, Zhao Q, Zheng XH, Lewis S. Science. 2000;287:2204–15. doi: 10.1126/science.287.5461.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bier E. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 9.Neckameyer WS, Holt B, Paradowski TJ. Biochem Genet. 2005;43:425–43. doi: 10.1007/s10528-005-6781-3. [DOI] [PubMed] [Google Scholar]
- 10.Porzgen P, Park SK, Hirsh J, Sonders MS, Amara SG. Mol Pharmacol. 2001;59:83–95. doi: 10.1124/mol.59.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Hsouna A, Lawal HO, Izevbaye I, Hsu T, O’Donnell JM. Dev Biol. 2007;308:30–43. doi: 10.1016/j.ydbio.2007.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budnik V, White K. J Comp Neurol. 1988;268:400–13. doi: 10.1002/cne.902680309. [DOI] [PubMed] [Google Scholar]
- 13.Paxon TL, Powell PR, Lee HG, Han KA, Ewing AG. Anal Chem. 2005;77:5349–55. doi: 10.1021/ac050474m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie SL, Hirsh J. J Neurosci Methods. 2006;153:243–49. doi: 10.1016/j.jneumeth.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Dasari S, Viele K, Turner AC, Cooper RL. Eur J Neurosci. 2007;26:424–38. doi: 10.1111/j.1460-9568.2007.05655.x. [DOI] [PubMed] [Google Scholar]
- 16.Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Neuroscience. 1988;25:513–23. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- 17.Robinson DL, Venton BJ, Heien ML, Wightman RM. Clin Chem. 2003;49:1763–73. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 18.Makos MA, Kim YC, Han KA, Heien ML, Ewing AG. Anal Chem. 2009:1848–54. doi: 10.1021/ac802297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borue X, Cooper S, Hirsh J, Condron B, Venton BJ. J Neurosci Meth. 2009;179:300–08. doi: 10.1016/j.jneumeth.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuire SE, Roman G, Davis RL. Trends Genet. 2004;20:384–91. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Schroll C, Riemensperger T, Bucher D, Ehmer J, Voller T, Erbguth K, Gerber B, Hendel T, Nagel G, Buchner E, Fiala A. Curr Biol. 2006;16:1741–47. doi: 10.1016/j.cub.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. Analyst. 2003;128:1413–19. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 23.Daubert EA, Condron BG. Cold Spring Harbor Protocols. Vol. 2007. 2007. db. [Google Scholar]
- 24.Wright TR. Adv Genet. 1987;24:127–222. [PubMed] [Google Scholar]
- 25.Neckameyer WS. J Neurogenet. 1998;12:101–14. doi: 10.3109/01677069809167259. [DOI] [PubMed] [Google Scholar]
- 26.McClung C, Hirsh J. Curr Biol. 1998;8:109–12. doi: 10.1016/s0960-9822(98)70041-7. [DOI] [PubMed] [Google Scholar]
- 27.Torres G, Horowitz JM. Synapse. 1998;29:148–61. doi: 10.1002/(SICI)1098-2396(199806)29:2<148::AID-SYN6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Sykes PA, Condron BG. Dev Biol. 2005;286:207–16. doi: 10.1016/j.ydbio.2005.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones SR, Garris PA, Kilts CD, Wightman RM. J Neurochem. 1995;64:2581–89. doi: 10.1046/j.1471-4159.1995.64062581.x. [DOI] [PubMed] [Google Scholar]
- 30.Budygin EA, John CE, Mateo Y, Jones SR. J Neurosci. 2002;22:RC222. doi: 10.1523/JNEUROSCI.22-10-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. J Neurosci. 1994;14:6084–93. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Nature. 2003;422:614–18. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 33.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. J Neurosci. 2004;24:4393–400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Proc Natl Acad Sci U S A. 2005;102:17816–21. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal Chem. 2000;72:5994–6002. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 36.Phillips PE, Wightman RM. Trend Anal Chem. 2003;22:509–14. [Google Scholar]
- 37.Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA. Exp Brain Res. 1994;100:395–406. doi: 10.1007/BF02738400. [DOI] [PubMed] [Google Scholar]
- 38.Chang HY, Grygoruk A, Brooks ES, Ackerson LC, Maidment NT, Bainton RJ, Krantz DE. Mol Psychiatry. 2006;11:99–113. doi: 10.1038/sj.mp.4001742. [DOI] [PubMed] [Google Scholar]
- 39.Greco PG, Garris PA. Eur J Pharmacol. 2003;479:117–25. doi: 10.1016/j.ejphar.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 40.Zahniser NR, Larson GA, Gerhardt GA. J Pharmacol Exp Ther. 1999;289:266–77. [PubMed] [Google Scholar]
- 41.Jones SR, Garris PA, Wightman RM. J Pharmacol Exp Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- 42.Hafizi S, Palij P, Stamford JA. Neuropharmacology. 1992;31:817–24. doi: 10.1016/0028-3908(92)90046-r. [DOI] [PubMed] [Google Scholar]
- 43.Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, Wightman RM. J Neurosci. 2006;26:3206–09. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]