Abstract
Introduction
Three common haplotypes in the gene encoding catechol-O-methyltransferase (COMT) have been associated with pain modulation and the risk of developing chronic musculoskeletal pain, namely temporomandibular disorder (TMD). Haplotypes coding for higher enzymatic activity were correlated with lower pain perception. Rodent studies showed that COMT inhibition increases pain sensitivity via β2/3-adrenergic receptors. We hypothesized that the non-selective β-adrenergic antagonist propranolol will reduce clinical and experimental pain in TMD patients in a manner dependent on the subjects’ COMT diplotype.
Methods
40 female Caucasian participants meeting the Research Diagnostic Criteria for TMD were genotyped for COMT polymorphisms and completed a randomized, double–blind, placebo-controlled, two-period crossover pilot study. Each period consisted of a baseline assessment week followed by an intervention week (propranolol or placebo). Changes in clinical pain ratings, psychological status, and responses to heat and pressure stimuli between baseline and intervention weeks were compared across periods.
Results
The number of patients reporting a reduction in pain intensity rating was greater during propranolol treatment (p=0.014) compared with placebo. Propranolol significantly reduced a composite pain index (p=0.02) but did not decrease other clinical and experimental pain ratings. When stratified by the COMT high activity haplotype, a beneficial effect of propranolol on pain perception was noted in subjects not carrying this haplotype, a diminished benefit was observed in the heterozygotes, and no benefit was noted in the homozygotes.
Conclusion
COMT haplotypes may serve as genetic predictors of propranolol treatment outcome, identifying a subgroup of TMD patients who will benefit from propranolol therapy.
Keywords: propranolol, β-blockers, pharmacogenetics, chronic pain, temporomandibular joint disorder (TMD), catechol-O-methyltransferase (COMT), polymorphism
Introduction
Amongst the various chronic musculoskeletal pain conditions, temporomandibular disorder (TMD) is the most common. It is characterized by persistent pain in the masticatory and related muscles of the head and neck, pain in the temporomandibular joint(s) (TMJ), and limitation in jaw functions [1]. The prevalence of TMD ranges from 6% to 12 % in the general population [2-4]. Numerous epidemiological studies indicate a female preponderance [5], and women constitute up to 80% of the patients seeking treatment for TMD [6].
Consistent with a general state of pain amplification, TMD patients often complain of persistent pain in multiple body sites beyond the affected orofacial region [7, 8]. Compared to pain-free subjects, TMD patients reported greater sensitivity to noxious stimuli applied to remote body sites [9-12]. Between 20% to 80% of TMD patients reportedly suffer from a variety of other chronic pain disorders such as headache, low back pain, fibromyalgia, and irritable bowel syndrome [13-19]. Furthermore, elevated psychological distress, somatic body awareness, depression, and anxiety disorder are notable comorbidities [20-24]. Taken together, these findings suggest that TMD is a chronic pain disorder characterized by dysfunction of the nociceptive system resulting from a complex interplay of biological, psychological, and social factors [5].
Studies have provided substantial evidence that pain perception, and the susceptibility to chronic persistent pain conditions, are partly mediated by genetic factors [25]. Recent studies have identified several candidate genes associated with pain sensitivity, susceptibility to chronic pain disorders, and response to pain therapies [26]. The catechol-O-methyltransferase (COMT) gene which codes for an enzyme that metabolizes catecholamines (namely epinephrine, norepinephrine, and dopamine) is one such gene implicated in the regulation of pain perception. TMD patients exhibit lower COMT activity than pain-free controls [27]. The COMT gene contains multiple single-nucleotide polymorphisms (SNPs). However, a nonsynonymous amino acid exchange has been described only for SNP rs4680 (also called Val(108/158)Met), which codes for a valine-to-methionine substitution. This substitution reduces the activity of COMT enzyme by approximately fourfold [28]. The low activity Met allele is linked with increased sensory and affective ratings of experimentally evoked pain [29], fibromyalgia [30], migraine [31], and morphine efficacy in cancer pain treatment [32]. However, an association of Val(108/158)Met with various responses to experimental pain measures was not observed in acute postsurgical pain [33, 34].
Current research suggests that haplotype-based association methods are more powerful than analogous allele-based methods when multiple disease susceptibility variants occur in the same gene. We recently reported that COMT haplotypes, rather than individual SNPs, better accounted for the observed variability in pain sensitivity. Three major haplotypes of COMT, designated as low pain sensitive (LPS), average pain sensitive (APS), and high pain sensitive (HPS) according to the responses to experimental pain stimuli, accounted for 11% of variability in experimental pain sensitivity in women and were predictive of the risk of TMD onset [35, 36]. COMT haplotypes were also associated with pain severity in fibromyalgia patients and subjects with postsurgical shoulder pain [37, 38]. The LPS haplotype produces higher levels of COMT enzymatic activity than the APS or HPS haplotypes [39]. The pharmacological inhibition of COMT in rats resulted in mechanical and thermal hyperalgesia that was reversed by the non-selective β-adrenergic receptor antagonist propranolol, but not by dopaminergic, α-, or β1-adrenergic antagonists suggesting that the hyperalgesia is mediated by β2/3-adrenergic mechanism [40]. In fact, a recent study of a single-dose infusion of propranolol to TMD and fibromyalgia patients revealed a short-term improvement in clinical pain ratings [41].
To investigate the pharmacogenetic effect of COMT on treatment response to propranolol in chronic persistent pain conditions, we conducted a double-blind, placebo-controlled, two-period crossover pilot study of propranolol in TMD patients. We hypothesized that one week of treatment with propranolol (20 mg twice a day) would reduce clinical pain symptoms and experimental pain sensitivity in TMD patients significantly more than placebo. We further hypothesized that individuals with COMT haplotypes coding for reduced enzyme activity would experience relatively greater benefit from propranolol vs. placebo than participants with other COMT haplotypes.
Methods
Subjects
This study was approved by the Institutional Review Board at the University of North Carolina (UNC) at Chapel Hill. Informed consent was obtained from all subjects. Participants were recruited from patients who sought treatment at the Orofacial Pain Clinic, UNC at Chapel Hill School of Dentistry, and through advertisements.
Inclusion and Exclusion Criteria
Individuals were eligible for the study if they were between 18 and 60 years of age and met Research Diagnostic Criteria for TMD (RDC/TMD) [1], Only women were recruited as they constitute up to 80% of patients seeking treatment for TMD [6]. Only Caucasian patients were evaluated, due to the necessity of avoiding genetic population stratification bias [42] and the fact that Caucasians represent the majority of local referral patients.
Exclusion criteria were self-reported asthma, cardiac arrhythmia, coronary artery disease, renal failure or dialysis, diabetes mellitus, hyperthyroidism, major depression or other major psychiatric disorders, seizures, drug or alcohol abuse, current chemotherapy or radiation therapy, pregnancy or nursing, facial trauma or orofacial surgery within the last 6 weeks, and orthodontic treatment. To minimize the risk of an adverse hypotensive response to β-blockade, subjects with heart rate (HR) under 55 bpm and diastolic blood pressure below 50 mmHg were excluded from the study. Patients taking the following medications were also excluded: drugs with central nervous system activity (e.g., opioids, benzodiazepines, nonbenzodiazepine sedative hypnotics, tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and anticonvulsants) and β-blockers. The use of selective serotonin reuptake inhibitors (SSRIs) was permitted only if the subject had already been taking it for at least 1 month before study enrollment. Abortive medications for headaches (such as the triptans) and nonsteroidal anti-inflammatory drugs (NSAIDs) were not allowed during the last 2 days prior to the study visits.
Study design
This study was a randomized, double–blind, placebo-controlled, two-period crossover clinical trial. The Investigational Drug Service at the UNC Hospitals performed the randomization, and kept the master list of assignments. The treatment sequence code for each subject was not disclosed to the project investigators till completion of the entire research protocol. Following a 1-week baseline assessment period, patients were randomly allocated in blocks of 8 to commence in one of two study arms. The first arm started with 1week of active treatment, followed by a 1-week wash-out period, and then a 1-week placebo period. The second arm started with 1-week of placebo, followed by a 1-week wash-out period, and then a week of active treatment. During the active treatment period subjects received 20 mg of propranolol twice a day. Placebo pills were identical to the propranolol pills to ensure subject and investigator blinding. To monitor compliance, subjects returned all unused pills at the next study visit.
At initial screening, a RDC/TMD examination and electrocardiogram (ECG) was performed, medical history was recorded, and subjects completed a battery of psychological questionnaires (described below). At every subsequent study visit, the following were administered: 1) Pain self-report (PSR), 2) quantitative sensory testing (QST), 3) psychological questionnaires, and 4) cardiovascular system (CVS) measures.
Outcome measures
Pain self-report (PSR)
Pain intensity rating was obtained via the PSR based on a ‘0’ to ‘100’ numerical rating scale (NRS), whereby ‘0’ is ‘no pain at all’ and ‘100’ is ‘the most intense pain imaginable’. Subjects rated their lowest, average, and highest pain level for the past week. The average weekly pain intensity was the primary outcome variable for this study. Pain duration was determined by asking participants to denote the percentage of time during the waking day when they experienced pain. A pain index was calculated as a product of pain intensity by pain duration. Pain duration and pain index were considered as secondary outcome measures.
Quantitative sensory testing (QST)
Heat pain threshold and tolerance
Contact heat stimuli were delivered using a computer-controlled Medoc Thermal Sensory Analyzer (TSA-II, Ramat Yishai, Israel), a Peltier-element-based stimulator with a 15-15 mm surface area. Thermal threshold and tolerance were measured on the left ventral forearm by an ascending method of limits. Thermal pain threshold was defined as the temperature (°C) at which the subject first perceived heat pain, whereas thermal pain tolerance was defined as the temperature (°C) at which the subject could no longer tolerate the pain. The temperature increased from a baseline of 32°C with a 0.5°C/s rate of rise until the subject responded by pressing the button. The cutoff temperature for both measurements was 50°C. Average thermal threshold and tolerance values was calculated from 4 trials conducted with a 30-s inter-stimuli interval at different sites of the ventral forearm.
Pressure pain threshold (PPT)
The PPT was assessed over the right and left temporalis and masseter muscles, and the TMJs with a pressure algometer (Pain Diagnosis and Treatment, Great Neck, NY, USA). The PPT was defined as the amount of pressure (kg) at which the subject first perceived the stimulus to be painful. One pre-trial assessment was performed at each site followed by additional assessments until two consecutive measures differing by less than 0.2 kg were obtained. The maximum number of assessment at each site was 5. Pressure stimuli were delivered at an approximate rate of 1 kg/s. The cutoff pressure for all sites was 5 kg. The values from the right and left sides were averaged to obtain one PPT value per anatomical site. The heat pain threshold and tolerance and PPT values were secondary outcomes for this study.
Psychological questionnaires
The Beck Depression Inventory (BDI-II) is a 21-item self-report rating questionnaire that assesses depressive symptoms [43]. The State-Trait Anxiety Inventory (STAI) contains two 20-item instruments, one of which evaluates situational state anxiety (Form Y-1) and the other assesses trait anxiety (Form Y-2) [44]. The Perceived Stress Scale (PSS) assesses 10 sources of stress and produces an overall perceived stress rating [45]. Higher scores indicate greater level of psychological dysfunction in each of these questionnaires. All of them are validated and widely used in clinical pain studies.
Cardiovascular system (CVS) measures
The CVS measures included the systolic blood pressure (SBP), the diastolic blood pressure (DBP), and the heart rate (HR) measurements. They were assessed on the right arm using the Dinamap 1846 automatic vital signs monitor (Critikon, Inc., Tampa, FL, USA). Following a 15-minute seated rest period, 3 readings for each CVS measure recorded at 2-minute intervals were obtained. The averaged resting SBP, DBP, and HR were secondary outcomes for this study.
Genotyping
This study was nested within a larger study designed to assess the biopsychosocial and genetic risk factors that contribute to the onset and persistence of TMD and related conditions. Genomic DNA was purified from whole blood samples by Cogenics Inc. (Morrisville, NC, USA) using the Gentra Puregene DNA Isolation Kit™ (Qiagen, Valencia, CA, USA) and genotyped on the Affymetrix CogPain GeneChip™ which contains 3,295 SNPs located within 358 loci of genes implicated in pain transmission and modulation. Samples and SNPs were subsequently subjected to a 95% call rate threshold and 99% filter for repeatability. SNPs which deviated from the Hardy-Weinberg equilibrium were identified and removed from the analysis. Genotyping data for the COMT gene alone was retrieved for this study.
Previous reports showed that 4 SNPs, namely rs6269, rs4633, rs4818, and rs4680 (SNPs number cited from NCBI databases) (Fig. 1), determined the 3 major COMT haplotypes that were associated with individual differences in a global pain measure as well as thermal pain sensitivity in healthy women [35, 36]. These 3 haplotypes were designated as LPS for GCGG, APS for ATCA, and HPS for ACCG allelic composition. To reconstruct these haplotypes, genotyping data was retrieved for the above mentioned SNPs. PLINK, version 1.06, software program was used for reconstructing the haplotypes from unphased genotype data [46] (http://pngu.mgh.harvard.edu/purcell/plink/).
Fig. 1.
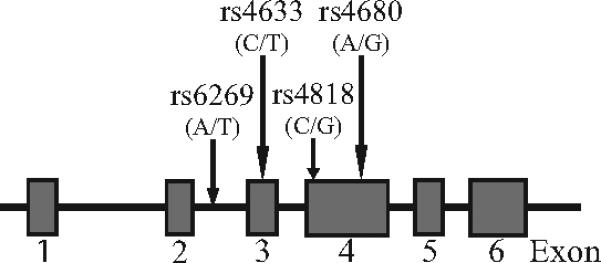
Schematic diagram of COMT gene. Boxes depict exons. Locations of the SNPs selected for the study are marked by arrows.
Statistical analysis
We estimated that with recruitment of 40 subjects, this pilot study would provide 80% power to detect a difference of 8 points between propranolol and placebo periods in the primary outcome variable, change in PSR pain intensity rating. A difference of 8 NRS points is equivalent to a 30% post-treatment reduction in PSR pain intensity rating for the propranolol period compared to a 5% reduction for the placebo period. The study also had 80% power to detect a difference among three COMT diplotypes of 25 NRS points in the net change in PSR pain intensity rating described below (i.e., within-subject difference between placebo and propranolol periods in change scores). The calculations were made using SAS PROC POWER for within each subject effects, specifying a two tailed, type I error of p=0.05 and assuming the observed standard deviation in net change of 16.8.
For each of the 14 outcome variables, within-subject change scores during each treatment period were computed by subtracting the value at the start of the treatment period from the value at the end of the treatment period. The within-subject, net outcome score was used to examine the relationship between propranolol efficacy and the number of LPS alleles. This measure of net change was calculated by subtracting the individual's change over the placebo period from the change over the propranolol period.
The PSR pain intensity rating was chosen a priori as the primary outcome variable, and thus the Type I error was controlled at the 2-sided 0.05 level for this variable. For the primary outcome variable, no adjustments were made for multiple comparisons because there was only one such variable. A multiplicity adjustment was not performed for the secondary measures because they were viewed as exploratory measures, and it was not the intent of the study to assess these secondary measures at the same experimental significance level as was established for the primary variable. Each outcome variable was tested for normality of its distribution using the Kolmogorov-Smirnov test and for homogeneity of variance by Levene's test. Psychological scores failed the test for normal distribution, so they were compared with Wilcoxon signed rank test or Kruskal-Wallis test. Other outcome variables, including the primary outcome, did not violate the diagnostic tests, and were evaluated with parametric tests.
A General Linear Model (GLM) procedure was used for repeated-measures analyses of the mean ratings from weekly PSR, experimental pain measurements, and CVS measures. In the GLM model, the treatment (propranolol or placebo) was the independent variable, and the only covariate was the study sequence, which was included to analyze paired data. A possible carryover effect was assessed by independent t-test, and no statistically significant carryover effect was observed for any study outcome (data not shown). Statistical evaluation of variation in the net outcome changes among the number of COMT LPS haplotype alleles was performed by one-way analysis of variance (ANOVA) with Tukey's post hoc test. Yates’ correction was implemented for Chi-square analysis of categorical data. Statistical analyses were done using SPSS software, version 16 (SPSS INC., Chicago, IL, USA).
The data derived from the study were analyzed using an intent-to-treat analysis. The intent-to-treat population included those subjects who, once randomized, had evidence of taking at least one dose of the study medication and attended at least one subsequent evaluation.
Results
Study population
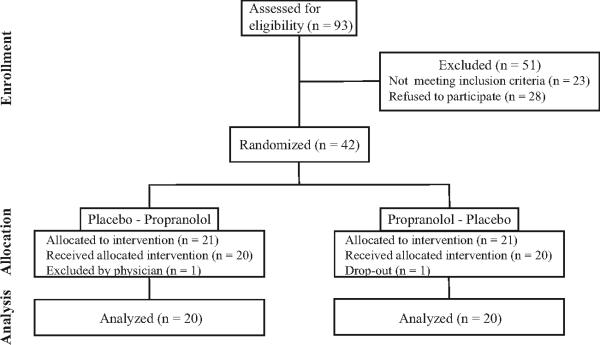
Of the 93 subjects screened, 42 subjects were eligible for this study (Fig. 2). One subject was excluded at the first visit because her DBP was lower than 50 mmHg, and another subject dropped-out of the study after the first baseline assessment citing time constrains. Therefore, 40 subjects successfully completed this study. Medication adherence estimate (propranolol and placebo) based on pill return was at 96.4 %. Subjects’ characteristics at baseline are presented in Table 1. When stratified by the number of COMT LPS alleles, no statistically significant difference between 3 subgroups was observed in any characteristic. Frequency of COMT diplotypes in the cohort is presented in Table 2.
Fig. 2.
Flow of the subjects through the study.
Table 1.
Baseline subject characteristics (Mean(SE)) categorized by the number of LPS alleles of COMT gene
| Characteristic | Total (n=40) | 0 LPS (n=13) | 1 LPS (n=19) | 2 LPS (n=8) | P value |
|---|---|---|---|---|---|
| Age (years) | 35.4 (1.7) | 38.4(3.7) | 34.0(2.3) | 34.1(3.2) | 0.510 |
| BMI (kg/m2) | 24.4 (0.7) | 26.2(1.7) | 23.1(0.6) | 24.7(1.4) | 0.140 |
| Clinical pain: | |||||
| Pain intensity (NRS 0-100) | 32.8 (3.0) | 38.8(5.3) | 31.1(4.5) | 26.9(6.4) | 0.343 |
| Pain duration (% of day) | 54.6(5.1) | 67.7(7.5) | 53.7(6.9) | 35.6(13.1) | 0.079 |
| Pain index | 2013(283) | 2577(451) | 1833(375) | 1522(827) | 0.361 |
| Experimental heat pain: | |||||
| Thermal Threshold (°C) | 43.1 (0.3) | 43.4(0.5) | 43.0(0.4) | 42.9(0.6) | 0.735 |
| Thermal Tolerance (°C) | 47.2 (0.2) | 47.4(0.3) | 47.3(0.3) | 46.5(0.5) | 0.238 |
| Experimental pressure pain: | |||||
| M. temporalis PPT (kg) | 2.30 (0.12) | 2.27(0.22) | 2.43(0.19) | 2.04(0.14) | 0.466 |
| M. masseter PPT (kg) | 1.92 (0.08) | 1.84(0.12) | 1.97(0.15) | 1.91(0.13) | 0.806 |
| TMJ PPT (kg) | 2.17 (0.11) | 2.18(0.21) | 2.23(0.18) | 2.05(0.12) | 0.840 |
| Psychological measures: | |||||
| BDI (range 0-63) | 7.5 (0.9) | 7.0(1.6) | 8.7(1.5) | 5.3(1.4) | 0.446 |
| STAI Y-1 (range 20-80) | 30.8 (1.5) | 30.7(3.2) | 32.7(2.0) | 26.4(2.1) | 0.212 |
| STAI Y-2 (range 20-80) | 39.1 (1.4) | 37.3(2.0) | 40.6(2.1) | 38.2(3.6) | 0.594 |
| PSS (range 0-40) | 14.8 (1.0) | 13.5(1.5) | 15.7(1.7) | 14.8(1.9) | 0.617 |
| CVS measures: | |||||
| SBP (mmHg) | 112.9 (1.9) | 115.1(3.7) | 111.1(2.4) | 113.1(4.4) | 0.666 |
| DBP (mmHg) | 64.9 (1.5) | 64.0(2.6) | 65.8(1.7) | 64.6(4.4) | 0.857 |
| HR (bpm) | 68.1 (1.5) | 68.7(2.4) | 66.9(2.5) | 70.0(1.5) | 0.739 |
BMI, body mass index; PPT, pressure pain threshold; TMJ, temporomandibular joint; BDI, Beck depression inventory; STAI, state-trait anxiety inventory; PSS, perceived stress scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
Table 2.
Frequency of COMT diplotypes (n=40)
| Diplotype | SNPs allele | Number (%) |
|---|---|---|
| APS/HPS | ATCA/ACCG | 1 (2.5%) |
| APS/APS | ATCA/ATCA | 12 (30%) |
| LPS/APS | GCGG/ATCA | 19 (47.5%) |
| LPS/LPS | GCGG/GCGG | 8 (20%) |
SNP, single nucleotide polymorphism
Efficacy of propranolol treatment
Clinical pain perception
A significantly greater percentage of patients (67.5% vs. 32.5%) experienced reduction in PSR pain intensity rating during the propranolol treatment period than the placebo treatment period (chi square = 8.450, p = 0.014). However, reduction in PSR pain intensity rating comparing propranolol treatment to placebo treatment did not reach the statistical significance (Wilks’ lambda = 0.932, p = 0.104) (Table 3). Reduction in pain duration was also nonsignificant, while the change in pain index was statistically significant (Wilks’ lambda = 0.866, p = 0.02) (Table 3).
Table 3.
Comparison of outcome changes in placebo and propranolol arm (n=40)
| Outcome | Placebo Mean (SD) | Propranolol Mean (SD) | P value |
|---|---|---|---|
| Clinical pain: | |||
| Pain intensity (NRS 0-100) | -0.1 (10.8) | -4.6 (13.3) | 0.104 |
| Pain duration (% of day) | -3.5(18.1) | -4.5(16.7) | 0.802 |
| Pain index | 34(862) | -453(1167) | 0.020 |
| Experimental heat pain: | |||
| Thermal Threshold (°C) | 0.22 (1.26) | 0.43 (1.16) | 0.449 |
| Thermal Tolerance (°C) | -0.02 (0.82) | 0.26 (0.61) | 0.081 |
| Experimental pressure pain: | |||
| M. temporalis PPT (kg) | 0.10 (0.41) | 0.11 (0.50) | 0.944 |
| M. masseter PPT (kg) | 0.16 (0.38) | 0.04 (0.34) | 0.214 |
| TMJ PPT (kg) | 0.04 (0.41) | 0.11 (0.52) | 0.576 |
| Psychological measures: | |||
| BDI (range 0-63) | -1.8 (3.5) | -0.5 (2.9) | 0.206 |
| STAI Y-1 (range 20-80) | -1.1 (5.6) | -0.8 (6.9) | 0.689 |
| PSS (range 0-40) | 0.1 (3.7) | -1.4 (3.1) | 0.087 |
| CVS measures: | |||
| SBP (mmHg) | -1.8 (6.3) | -4.3 (6.7) | 0.131 |
| DBP (mmHg) | -1.2 (6.2) | -3.3 (5.2) | 0.117 |
| HR (bpm) | -0.1 (6.0) | -7.1 (9.4) | 0.001 |
PPT, pressure pain threshold; TMJ, temporomandibular joint; BDI, Beck depression inventory; STAI, state-trait anxiety inventory; PSS, perceived stress scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
Experimental pain perception
No significant treatment effect of propranolol on experimental pain perception (thermal or pressure) was observed. However, the change in thermal pain tolerance measure compared between treatment periods was close to the threshold for statistical significance (Wilks'lambda = 0.922, p = 0.081) (Table 3).
Psychological and CVS measures
There were no statistically significant differences between propranolol and placebo periods in any measure of psychological distress (Table 3). Among CVS measures, propranolol significantly reduced HR only (Wilks’ lambda = 0.732, p = 0.001) (Table 3), indicating good patient compliance with the protocol.
Pharmacogenetic evaluation of propranolol response based on COMT haplotype
Clinical pain perception
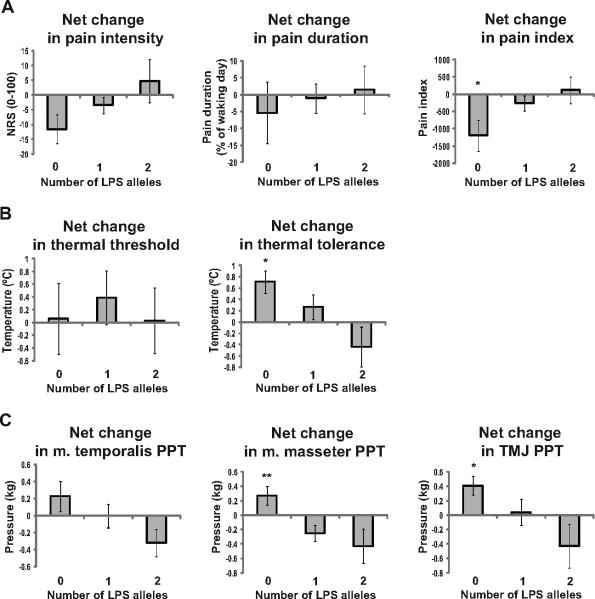
A net outcome change was used to evaluate the contribution of the LPS haplotype number in the effects of propranolol on an individual pain variable. The number of LPS alleles was associated with statistically significant variation in the net change in pain index (F(2,37) = 3.836, p =0.031). Association between the number of LPS alleles and the net change in PSR pain intensity rating approached statistical significance (F(2,37) = 2.626, p = 0.086) (Fig. 3). Subjects with no LPS alleles (~ 33% of the examined population sample, Table 2) showed the greatest response. An intermediate effect was observed in the heterozygotes and no effect was noted in LPS homozygotes (Fig. 3).
Fig. 3.
Net changes in measures of the reported pain and experimental pain sensitivity categorized by the number of LPS alleles of COMT. Subjects not carrying LPS allele show the best response to propranolol therapy in all pain measures except thermal threshold. Data are expressed as Means±SE. *p<0.05 different from LPS homozygotes, **p<0.01 different from LPS hetero- and homozygotes with Tukey's post hoc test.
Experimental pain perception
The number of LPS alleles was also significantly associated with the net change in thermal tolerance (F (2,37) = 4.160, p = 0.023), masseter muscle PPT (F (2,37) = 5.555, p = 0.008), and TMJ PPT (F(2,37)=3.318, p = 0.047) (Fig. 3). Association between the number of LPS alleles and the net change in temporalis muscle PPT approached statistical significance (F(2,37) = 2.097, p = 0.137). However, the net change in thermal threshold demonstrated no association with COMT haplotypes (F(2,37) = 0.182, p = 0.835) (this likely resulted from the higher variability associated with this measure).
Psychological and CVS measures
In contrast to the measures of pain perception, no association with the number of COMT LPS alleles was found for the net changes in psychological measures (Table 4), providing evidence that the nature of the effect of propranolol on pain perception is not neuropsychiatric. A significant association with the number of COMT LPS alleles was observed for the net HR change (F(2,35) = 4.551, p = 0.017) but not for the measures of resting arterial blood pressure (Table 4).
Table 4.
Net changes in psychological and CVS measures (Mean (SE)) categorized by the number of LPS alleles of COMT gene
| Outcome | 0 LPS (n=13) | 1 LPS (n=19) | 2 LPS (n=8) | P value |
|---|---|---|---|---|
| Psychological measures: | ||||
| BDI | 0.5 (1.4) | 1.9 (1.3) | 0.8 (1.2) | 0.714 |
| STAI Y-1 | -2.2 (2.5) | 1.3 (2.4) | 1.8 (1.5) | 0.630 |
| PSS | 1.2 (1.2) | 2.7 (1.1) | -0.9 (1.8) | 0.342 |
| CVS measures: | ||||
| SBP (mmHg) | -4.4 (3.7) | -2.6 (1.9) | 0.8 (2.1) | 0.499 |
| DBP (mmHg) | -1.8 (3.3) | -3.4 (1.6) | 0.4 (4.7) | 0.583 |
| HR (bpm) | -9.9 (3.5) | -9.7 (2.0) | 3.3 (4.4) | 0.017 |
BDI, Beck depression inventory; STAI, state-trait anxiety inventory; PSS, perceived stress scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
Safety
At least one treatment-emergent adverse event was reported by 5 subjects (12.5%) during propranolol period (namely nausea, diarrhea, insomnia, dizziness, and fatigue) and 4 subjects (10%) during the placebo period (namely diarrhea, gastrointestinal bloating, fatigue, and headache). All adverse events were mild and did not result in discontinuation of the study.
Discussion
In this study, we determined that common COMT haplotypes are associated with different response to β-blocker treatment in a common chronic musculoskeletal pain disorder. This is the first study that has identified a genetic marker that is associated with the efficacy of propranolol treatment in patients with TMD. The present findings are consistent with a recent study that reported that TMD and fibromyalgia patients showed a dysregulation of β-adrenergic activity which contributed to altered cardiovascular, catecholamine, and clinical pain responses to psychological and physical stressors. Concordant with our data, acute treatment with intravenous low-dose propranolol also produced improvement in clinical pain report [41].
The outcome of this proof-of-concept study supports the feasibility and merit of conducting a larger trial. The trend for improvement in the PSR pain intensity rating and significant improvement in the pain index supports a likely therapeutic effect of propranolol in a substantial percentage of TMD patients. It is interesting to note that the pain index , which is a product of two measures that each yielded smaller estimates of effect and larger p-values in comparing treatment periods, reached the threshold of statistical significance. This apparent paradox can be explained by the fact that the individual measures were only moderately correlated (Pearson's r = 0.5), and it is a tenet of psychometric theory that orthogonal measures that each are moderately associated with a third variable will yield a stronger association when the two are combined. While it might be tempting to conclude that the pain index is a statistically superior measure of propranolol efficacy, we caution that the result may be idiosyncratic, and recommend that the index be evaluated in studies with larger samples conducted in other populations.
While propranolol treatment did not affect measures of sensitivity to experimental pain, a “dose-response” effect by the number of COMT LPS alleles was observed in the majority of measures of experimental and clinical pain, with a greater improvement in subjects not carrying a LPS haplotype, an intermediate effect in the heterozygotes, and no beneficial effect in LPS homozygotes. The relative benefit for TMD patients without the LPS haplotype identified a large subgroup (~33% of our sample, Table 2) where β-blocker treatment was advantageous. In comparison, 20% of the patients showed either no effect or a worsening of their pain condition in response to propranolol therapy.
The neural mechanisms by which propranolol produces an analgesic effect under conditions of low COMT activity and high catecholamine bioavailability are poorly understood. However, both central and peripheral mechanisms have been proposed. In support of a peripheral mechanism, the intraplantar injection of epinephrine (EPI) produced a peripherally mediated hyperalgesia in rats, which was blocked by propranolol [47]. Similarly, the intramuscular injection of low-dose propranolol in rats with carageenan-induced inflammation of the gastrocnemius muscle reduced inflammatory pain locally [41]. Both propranolol and a selective β2-adrenoreceptor antagonist reportedly impaired pain behavior in a rodent model of TMD [48]. Finally, propranolol may produce analgesia by blocking tetrodotoxin-resistant sodium channels in sensory neurons; however, this is unlikely to contribute to our findings since relatively high doses are required to observe such effects [49].
The analgesic effect of propranolol may also be mediated via a central mode of action. Propranolol impairs memory storage and reconsolidation of negative emotional events in rodents and humans [50-54]. Propranolol is also known to have central nervous system modulating activities and anxiolytic effects [55, 56]. Intraplantar formaline injection in rats increased extracellular norepinephrine (NE) levels within the ventral part of the bed nucleus of the stria terminalis (vBNST), brain area which had been implicated in stress responses and negative affective states, and the injection of the β-adrenergic antagonist timolol into this brain region dose-dependently attenuated the negative affective component of pain [57].
Catecholamine-induced influence on the autonomic control of the BP may indirectly regulate pain since increase in BP has been associated with reduced pain sensitivity [58]. In this study, the chosen dosage of propranolol (20 mg twice a day) was intentionally low to minimize any reduction in BP which may alter endogenous pain modulation via the baroreceptor reflex, thereby increasing pain [59, 60].
In contrast to the effects of propranolol on pain perception, its effect on anxiety, depression, and perceived stress were not associated with COMT haplotypes. These results are consistent with the observation that psychological scores do not vary among various COMT haplotype groups in healthy female volunteers, and that psychological factors influenced the risk of TMD onset independent of the COMT haplotypes [61]. A recent open trial of β-adrenergic receptor/5-HT1A presynaptic autoreceptor antagonist, pindolol, in the fibromyalgia patients reported no change in the anxiety and depression scores although a significant effect on pain sensitivity was observed [62]. The lack of change in psychological variables in our study may be partly explained by the relatively low levels of psychological distress reported by this cohort, the low dose of propranolol administered, and the lack of effect of propranolol on the psychological status of TMD patients.
In line with the variable effect of propranolol on pain perception, it is generally recognized that the pharmacodynamic responses to β-adrenergic receptor blockade are highly inconsistent. For example, 30-60% of patients with hypertension who were treated with β-blocker monotherapy failed to achieve adequate BP control [63, 64]. In migraineurs, response rates to propranolol therapy ranged from 33 to 44% [65]. Variable effects in response to propranolol treatment have also been reported for anxiety, psychocardiac disorders, aggressive behavior, and substance withdrawal [56, 66-68]. This variability may be accounted for, in part, by COMT genetic polymorphism.
In this study, a haplotype-based analysis was used instead of a single SNP analysis because the linkage disequilibrium between several markers showed stronger association with a disease than individual SNPs, as was observed by Shifman et al. [69]. Consistent with this view, we previously showed a functional effect of COMT haplotypes on enzyme activity and protein levels [39]. Nevertheless, in this cohort with only one subject carrying the HPS haplotype, an association analysis based on Val(108/158)Met polymorphism would lead to a very similar result compared to a haplotype-based approach.
This study has a few limitations. First, this study was designed as a proof-of-concept trial recruiting a relatively small number of subjects. Therefore, our findings need to be replicated in a larger sample set. Second, our results are based on a 1-week low-dose propranolol treatment. Future studies with a longer duration of treatment and higher dosage levels are desirable. Third, this study included only Caucasian females because the majority of TMD patients are women [6]. Hence, the results may not be generalized to men or non-Caucasian females. Future studies with diverse ethnicity and involving both sexes are needed.
In conclusion, COMT gene polymorphism contributes to the variable pharmacodynamic responses to propranolol in patients with chronic musculoskeletal pain. COMT haplotypes may serve as genetic predictors of treatment outcome and permit the identification of the subgroup of patients who will benefit from propranolol therapy. The results of this study may also explain the variability of treatment responses to β-blockers for a broad spectrum of diseases.
Acknowledgements
We thank Dr. Muhammad Siddiqi, Vanessa Miller, Parisa Kasravi, Dmitry Chivilev, and Dustin Gibson for clinical and technical assistance, and Olivier Monbureau for the development of the QST software. The authors would also like to thank Dr. Alan L. Hinderliter, Division of Cardiology, UNC at Chapel Hill, for ECG consulting. This work was supported by NIH grants R01 DE016558 to L.D. and P01 NS045685 to W.M
This work was supported by NIH grants R01 DE016558 to L.D. and P01 NS045685 to W.M.
Footnotes
Conflicts of Interest: Drs. G.D.S, L.D., S.A.M., and W.M. are officers and share holders in Algynomics Inc. Drs. G.D.S., L.D., and W.M. are co-inventors on a pending patent which covers functional genetic variants of COMT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 1992;6:301–55. [PubMed] [Google Scholar]
- 2.De Leeuw R. Orofacial pain: guidelines for assessment, diagnosis, and management. 4th edition Quintessence Publishing; Chicago: 2008. [Google Scholar]
- 3.Dworkin SF, Huggins KH, LeResche L, Von Korff M, Howard J, Truelove E, et al. Epidemiology of signs and symptoms in temporomandibular disorders: clinical signs in cases and controls. J Am Dent Assoc. 1990;120:273–81. doi: 10.14219/jada.archive.1990.0043. [DOI] [PubMed] [Google Scholar]
- 4.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Oro-facial pain in the community: prevalence and associated impact. Community Dent Oral Epidemiol. 2002;30:52–60. doi: 10.1034/j.1600-0528.2002.300108.x. [DOI] [PubMed] [Google Scholar]
- 5.Suvinen TI, Reade PC, Kemppainen P, Kononen M, Dworkin SF. Review of aetiological concepts of temporomandibular pain disorders: towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factors. Eur J Pain. 2005;9:613–33. doi: 10.1016/j.ejpain.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Warren MP, Fried JL. Temporomandibular disorders and hormones in women. Cells Tissues Organs. 2001;169:187–92. doi: 10.1159/000047881. [DOI] [PubMed] [Google Scholar]
- 7.Hagberg C, Hagberg M, Kopp S. Musculoskeletal symptoms and psychosocial factors among patients with craniomandibular disorders. Acta Odontol Scand. 1994;52:170–7. doi: 10.3109/00016359409027592. [DOI] [PubMed] [Google Scholar]
- 8.Turp JC, Kowalski CJ, O'Leary N, Stohler CS. Pain maps from facial pain patients indicate a broad pain geography. J Dent Res. 1998;77:1465–72. doi: 10.1177/00220345980770061101. [DOI] [PubMed] [Google Scholar]
- 9.Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MB. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain. 1996;12:260–9. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–51. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 11.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 12.Sarlani E, Greenspan JD. Evidence for generalized hyperalgesia in temporomandibular disorders patients. Pain. 2003;102:221–6. doi: 10.1016/S0304-3959(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 13.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221–7. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 14.Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia. 2008;28:832–41. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 15.Cooper BC, Kleinberg I. Relationship of temporomandibular disorders to muscle tension-type headaches and a neuromuscular orthosis approach to treatment. Cranio. 2009;27:101–8. doi: 10.1179/crn.2009.016. [DOI] [PubMed] [Google Scholar]
- 16.Graff-Radford SB. Temporomandibular disorders and headache. Dent Clin North Am. 2007;51:129–44. vi–vii. doi: 10.1016/j.cden.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Plesh O, Wolfe F, Lane N. The relationship between fibromyalgia and temporomandibular disorders: prevalence and symptom severity. J Rheumatol. 1996;23:1948–52. [PubMed] [Google Scholar]
- 18.Raphael KG, Marbach JJ, Klausner J. Myofascial face pain. Clinical characteristics of those with regional vs. widespread pain. J Am Dent Assoc. 2000;131:161–71. doi: 10.14219/jada.archive.2000.0143. [DOI] [PubMed] [Google Scholar]
- 19.Wiesinger B, Malker H, Englund E, Wanman A. Does a dose-response relation exist between spinal pain and temporomandibular disorders? BMC Musculoskelet Disord. 2009;10:28. doi: 10.1186/1471-2474-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonjardim LR, Gaviao MB, Pereira LJ, Castelo PM. Anxiety and depression in adolescents and their relationship with signs and symptoms of temporomandibular disorders. Int J Prosthodont. 2005;18:347–52. [PubMed] [Google Scholar]
- 21.Fricton JR. Masticatory myofascial pain: an explanatory model integrating clinical, epidemiological and basic science research. Bull Group Int Rech Sci Stomatol Odontol. 1999;41:14–25. doi: 10.3201/eid0801.010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gameiro GH, da Silva Andrade A, Nouer DF, Ferraz de Arruda Veiga MC. How may stressful experiences contribute to the development of temporomandibular disorders? Clin Oral Investig. 2006;10:261–8. doi: 10.1007/s00784-006-0064-1. [DOI] [PubMed] [Google Scholar]
- 23.Korszun A, Hinderstein B, Wong M. Comorbidity of depression with chronic facial pain and temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:496–500. doi: 10.1016/s1079-2104(96)80192-2. [DOI] [PubMed] [Google Scholar]
- 24.Yap AU, Dworkin SF, Chua EK, List T, Tan KB, Tan HH. Prevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patients. J Orofac Pain. 2003;17:21–8. [PubMed] [Google Scholar]
- 25.Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–30. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Diatchenko L, Nackley AG, Tchivileva IE, Shabalina SA, Maixner W. Genetic architecture of human pain perception. Trends Genet. 2007;23:605–13. doi: 10.1016/j.tig.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Marbach JJ, Levitt M. Erythrocyte catechol-O-methyltransferase activity in facial pain patients. J Dent Res. 1976;55:711. doi: 10.1177/00220345760550043801. [DOI] [PubMed] [Google Scholar]
- 28.Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–10. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- 29.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 30.Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–7. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 31.Erdal ME, Herken H, Yilmaz M, Bayazit YA. Significance of the catechol-O-methyltransferase gene polymorphism in migraine. Brain Res Mol Brain Res. 2001;94:193–6. doi: 10.1016/s0169-328x(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 32.Rakvag TT, Klepstad P, Baar C, Kvam TM, Dale O, Kaasa S, et al. The Val158Met polymorphism of the human catechol-O-methyltransferase (COMT) gene may influence morphine requirements in cancer pain patients. Pain. 2005;116:73–8. doi: 10.1016/j.pain.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Lee H, Rowan J, Brahim J, Dionne RA. Genetic polymorphisms in monoamine neurotransmitter systems show only weak association with acute post-surgical pain in humans. Mol Pain. 2006;2:24. doi: 10.1186/1744-8069-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, et al. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–96. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 2006;125:216–24. doi: 10.1016/j.pain.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 37.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, 3rd, Sack BK, et al. Evidence for a biopsychosocial influence on shoulder pain: pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, Vargas A, Lao-Villadoniga JI, Garcia-Fructuoso F, et al. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Res Ther. 2007;9:R110. doi: 10.1186/ar2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- 40.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2-and beta3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Light KC, Bragdon EE, Grewen KM, Brownley KA, Girdler SS, Maixner W. Adrenergic dysregulation and pain with and without acute beta-blockade in women with fibromyalgia and temporomandibular disorder. J Pain. 2009;10:542–52. doi: 10.1016/j.jpain.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Healy DG. Case-control studies in the genomic era: a clinician's guide. Lancet Neurol. 2006;5:701–7. doi: 10.1016/S1474-4422(06)70524-5. [DOI] [PubMed] [Google Scholar]
- 43.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger CDGR, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y1) Comsulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 45.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 46.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–12. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues LL, Oliveira MC, Pelegrini-da-Silva A, de Arruda Veiga MC, Parada CA, Tambeli CH. Peripheral sympathetic component of the temporomandibular joint inflammatory pain in rats. J Pain. 2006;7:929–36. doi: 10.1016/j.jpain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Chidlow G, Melena J, Osborne NN. Betaxolol, a beta(1)-adrenoceptor antagonist, reduces Na(+) influx into cortical synaptosomes by direct interaction with Na(+) channels: comparison with other beta-adrenoceptor antagonists. Br J Pharmacol. 2000;130:759–66. doi: 10.1038/sj.bjp.0703369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta-adrenergic blockade. Neurobiol Learn Mem. 2000;74:259–66. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- 51.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–4. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 52.Chamberlain SR, Muller U, Blackwell AD, Robbins TW, Sahakian BJ. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology (Berl) 2006;188:397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- 53.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–8. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 54.Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Ann N Y Acad Sci. 1997;821:247–58. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- 55.Laverdure B, Boulenger JP. [Beta-blocking drugs and anxiety. A proven therapeutic value]. Encephale. 1991;17:481–92. [PubMed] [Google Scholar]
- 56.Peet M, Ali S. Propranolol and atenolol in the treatment of anxiety. Int Clin Psychopharmacol. 1986;1:314–9. doi: 10.1097/00004850-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Deyama S, Katayama T, Ohno A, Nakagawa T, Kaneko S, Yamaguchi T, et al. Activation of the beta-adrenoceptor-protein kinase A signaling pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain in rats. J Neurosci. 2008;28:7728–36. doi: 10.1523/JNEUROSCI.1480-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Randich A, Maixner W. The role of sinoaortic and cardiopulmonary baroreceptor reflex arcs in nociception and stress-induced analgesia. Ann N Y Acad Sci. 1986;467:385–401. doi: 10.1111/j.1749-6632.1986.tb14642.x. [DOI] [PubMed] [Google Scholar]
- 59.de Abreu TC, Nilner M, Thulin T, Vallon D. Office and ambulatory blood pressure in patients with craniomandibular disorders. Acta Odontol Scand. 1993;51:161–70. doi: 10.3109/00016359309041162. [DOI] [PubMed] [Google Scholar]
- 60.Mohn C, Vassend O, Knardahl S. Experimental pain sensitivity in women with temporomandibular disorders and pain-free controls: the relationship to orofacial muscular contraction and cardiovascular responses. Clin J Pain. 2008;24:343–52. doi: 10.1097/AJP.0b013e318162eaf4. [DOI] [PubMed] [Google Scholar]
- 61.Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, et al. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 2007;86:1120–5. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- 62.Wood PB, Kablinger AS, Caldito GS. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother. 2005;39:1812–6. doi: 10.1345/aph.1G014. [DOI] [PubMed] [Google Scholar]
- 63.Veterans Administration Cooperative Study Group on Antihypertensive Agents Comparison of propranolol and hydrochlorothiazide for the initial treatment of hypertension. II. Results of long-term therapy. JAMA. 1982;248:2004–11. [PubMed] [Google Scholar]
- 64.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, et al. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. N Engl J Med. 1993;328:914–21. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 65.Tfelt-Hansen P, Rolan P. Beta-adrenoreceptor blocking drugs in migraine prophylaxis. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The headaches. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 519–28. [Google Scholar]
- 66.Ipser JC, Kariuki CM, Stein DJ. Pharmacotherapy for social anxiety disorder: a systematic review. Expert Rev Neurother. 2008;8:235–57. doi: 10.1586/14737175.8.2.235. [DOI] [PubMed] [Google Scholar]
- 67.Lader M. Beta-adrenoceptor antagonists in neuropsychiatry: an update. J Clin Psychiatry. 1988;49:213–23. [PubMed] [Google Scholar]
- 68.Meibach RC, Dunner D, Wilson LG, Ishiki D, Dager SR. Comparative efficacy of propranolol, chlordiazepoxide, and placebo in the treatment of anxiety: a double-blind trial. J Clin Psychiatry. 1987;48:355–8. [PubMed] [Google Scholar]
- 69.Shifman S, Bronstein M, Sternfeld M, Pisante-Shalom A, Lev-Lehman E, Weizman A, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]