Abstract
Endostatin, the C-terminal domain of collagen XVIII, binds to transglutaminase-2 (TG-2) in a cation-dependent manner. Recombinant human endostatin binds to TG-2 with an affinity in the nanomolar range (KD = 6.8 nM). Enzymatic assays indicated that, in contrast to other extracellular matrix proteins, endostatin is not a glutaminyl substrate of TG-2 and is not cross-linked to itself by the enzyme. Two arginine residues of endostatin, R27 and R139, are crucial for its binding to TG-2. They are also involved in the binding to heparin (Sasaki et al., EMBO J 18:6240–6248), and to α5β1 and αvβ3 integrins (Faye et al., J Biol Chem 284:22029–22040), suggesting that endostatin is not able to interact simultaneously with TG-2 and heparan sulfate, or with TG-2 and integrins. Inhibition experiments support the GTP binding site of TG-2 as a potential binding site for endostatin. Endostatin and TG-2 are colocalized in the extracellular matrix secreted by endothelial cells under hypoxia, that stimulates angiogenesis. This interaction occurring in a cellular context might participate in the concerted regulation of angiogenesis, and tumorigenesis by the two proteins.
Keywords: Endostatin, Protein-protein interaction, SPR binding assays, Transglutaminase-2
INTRODUCTION
Endostatin (ES) is a C-terminal fragment of the α1 chain of collagen XVIII, which inhibits angiogenesis, and tumor growth [1, 2]. It binds to α5β1 and αvβ3 integrins [3, 4] glypicans 1 and 4 [5], and to vascular endothelial growth factor receptor-2 (VEGFR-2, Flk1/KDR) [6] on the surface of endothelial cells. The binding of human endostatin to α5β1 integrin leads to the inhibition of focal adhesion kinase/c-Raf/MEK1/2/p38/ERK1 mitogen-activated protein kinase pathway [7]. Endostatin also inhibited the binding of VEGF165 to both endothelial cells and to the purified extracellular domain of KDR/Flk-1. Furthermore, endostatin blocks VEGF-induced tyrosine phosphorylation of KDR/Flk-1 and activation of ERK, p38 MAPK, and p125(FAK) in human umbilical vein endothelial cells [6].
Endothelial cells are a rich source of transglutaminase-2 (TG-2) [8], and this enzyme plays an important role at the surface of those cells. For example, evidence has been reported for its externalization and its co-localization with the β1 integrin [9]. Cell surface TG-2 interacts with integrins of the β1 and β3 subfamilies in focal adhesion sites [10], and the formation of a complex between TG-2 and VEGFR-2 has been proposed as a mechanism for modulation of endothelial cell response to VEGF [11]. Conflicting in vivo studies have proposed that the enzyme both stimulates and inhibits angiogenesis [12, 13]. However application of exogenous TG-2 blocks angiogenesis in a dose-dependent manner in an in vitro angiogenesis assay without causing cell death, by a mechanism that involves increased accumulation of extracellular matrix proteins [14]. This Ca2+-dependent enzyme catalyzes post-translational modification of proteins by the formation of γ-glutamyl-ε-lysine bonds between glutamine and lysine residues [15, 16, 17]. TG-2 is a multifunctional enzyme which undergoes a GTP-binding/GTPase cycle, with guanine nucleotide and calcium binding reciprocally regulating its transamidation activity [18].
Endostatin and TG-2 share several extracellular partners such as nidogen, SPARC, collagen VI, and the β-amyloid peptide [15, 19, 20, 21, 22]. Both proteins are also able to bind to heparin [19, 23, 24], α5β1 and αvβ3 integrins [3, 4, 10], and VEGF receptor-2 [6, 11]. They are involved in angiogenesis, and are increased in brain after trauma [25, 26]. Furthermore they may play a role in Alzheimer’s disease. Endostatin is released by neurons and accumulates in amyloid plaques [27] and TG-2 co-localizes with the pathological lesions in Alzheimer's disease brain [28, 29]. All these common properties prompted us to investigate the possible existence of an interaction between these two proteins, and we have recently shown that endostatin interacts with TG-2 [22]. We report here that endostatin binds to TG-2 with an affinity in the nanomolar range in a calcium-dependent manner. Endostatin is not a glutaminyl substrate of TG-2 in vitro, and it is not cross-linked to itself by the enzyme. Since endostatin and transglutaminase-2 are both involved in the regulation of angiogenesis, and have been implicated in Alzheimer’s disease, it is likely that their interaction is of major importance for the modulation of endothelial cell migration and/or proliferation, and for the formation and/or the stabilization of amyloid plaques in neurodegenerative diseases.
EXPERIMENTAL
Proteins and antibodies
Recombinant human TG-2 was purchased from two different suppliers (Immundiagnostik, Germany, and Covalab, France). Guinea pig TG-2 extracted from liver was from Sigma-Aldrich (St Quentin Fallavier, France). Recombinant endostatin, the C-terminal domain of collagen XVIII (NC1), and NC1 mutants (D104N and R27A/R139A) were produced in human embryonic kidney cells expressing Epstein-Barr virus nuclear antigen (HEK 293-EBNA cells, [4, 22, 23]. Transfected HEK 293 cells expressing wild type or mutant endostatin and the NC1(XVIII) domain were a generous gift of Naomi Fukai and Reidunn Jetne (Harvard Medical School, Boston, USA). To avoid the confusion due to two different numberings reported for endostatin in the literature, amino acids residues are numbered starting from the first amino acid residue of endostatin (i.e. His1, corresponds to His132 when numbering starts from the first amino acid of the entire C-terminal domain NC1 of collagen XVIII). Both proteins were tagged by the peptide Flag at the N- or the C-terminus for endostatin and at the C-terminus for the NC1 domain. The conditioned culture media were filtered through 0.22-µm filters and applied to a 5-ml heparin HiTrap (GE Healthcare, Uppsala, Sweden) for wild-type and mutant endostatin, and to an anti-Flag M2 affinity column (Sigma-Aldrich) for wild-type and mutant NC1. Endostatin was further purified by gel filtration on a Superdex S75 column (60 cm×2.6 cm, GE Healthcare), and the NC1 domain was further purified on a Sephacryl S200 column (60 cm×2.6 cm, GE Healthcare). The purity of proteins was assessed by SDS-PAGE and immunoblotting. The purified proteins were concentrated by ultrafiltration and stored at −80°C. Recombinant human endostatin expressed in Pichia pastoris was from Calbiochem (San Diego, CA). Anti-TG-2 monoclonal antibody (CUB7402) was from NeoMarker (Fremont, CA, USA). Bovine collagen XI was a generous gift from Dr. Marie-Claire Ronzière (UMR 5086, CNRS, University Lyon 1, France).
Preparation of a polyclonal antibody against human endostatin
Recombinant human endostatin with a Flag peptide at the C-terminus was used for the immunization of New Zealand white rabbits. Endostatin diluted in phosphate buffered saline (PBS, 660 µg/ml) emulsified with an equal volume of Freund's complete adjuvant was injected intra-dermally. Two booster injections with Freund's incomplete adjuvant were performed 14 and 28 days after the first injection. The IgG fraction was purified from the immunserum by affinity chromatography on Protein A Ceramic Hyper D®F (Pall Life Sciences, Saint-Germain en Laye, France). IgGs were eluted by 0.1 M citric acid pH 3, and neutralized with 1 M potassium phosphate buffer pH 9. The polyclonal antibody was assayed by immunoblotting, and solid-phase assays. The antibody reacted with wild type and mutant endostatin, and with the wild type and mutant NC1(XVIII) domains (Supplementary material, Fig S1).
Solid-phase binding assays
Transglutaminase was diluted in 25 mM Tris·HCl, 150 mM NaCl, pH 7.4 (TBS) for coating. Endostatin and the NC1(XVIII) domain were diluted in 10 mM PBS pH 7.4 containing 138 mM NaCl, 27 mM KCl, or in 25 mM Tris·HCl, 150 mM NaCl, pH 7.4 (TBS). Aliquots (100 µl) were added to the wells of a 96-well microplate (MaxiSorp, Nunc, Thermo Fisher Scientific, Roskilde, Denmark). Plates were incubated overnight at 4°C, and wells were blocked for 2 h with 5% (w/v) BSA in TBS. The plates were incubated for 2 h at room temperature with TG-2 diluted in TBS containing 1 mM EDTA with or without cations (8 mM CaCl2, MgCl2, or MnCl2). The wells were washed three times with TBS containing 0.1% (v/v) Tween 20. Bound transglutaminase was detected by a monoclonal anti-TG-2 antibody diluted 1:1000 in TBS + 0.1% (v/v) Tween 20 for 1 h at room temperature. Bound endostatin was detected with the polyclonal anti-endostatin antibody diluted 1:1000. The wells were washed 3 times with TBS, 0.1% (v/v) Tween 20, and were then incubated with either alkaline phosphatase-conjugated or peroxidase-conjugated secondary antibodies. The immunological reaction was detected by adding p-nitrophenyl phosphate (absorbance measured at 405 nm), or 3,3',5,5'-tetramethylbenzidine (absorbance was measured at 450 nm). Nonspecific binding was measured in BSA-coated wells, and was subtracted from the values measured in endostatin-, or transglutaminase-coated wells. For inhibition experiments by GTP, endostatin was diluted in 25 mM Tris·HCl, 150 M NaCl pH 7.4 (0.1 µg/well), and incubated overnight at 4°C in a 96-well microplate (MaxiSorp, Nunc). Wells were blocked for 2 h with 5% (w/v) BSA in TBS. TG-2 was preincubated in TBS containing 8 mM CaCl2 with several concentrations of GTP (0–100 µM) for 1 h at room temperature, before incubation for 2 h at room temperature. The binding of TG-2 to endostatin was performed as described above. All assays were performed in triplicate.
Surface plasmon resonance arrays
SPR arrays were performed using a Biacore Flexchip system (GE Healthcare, Sweden) as described previously [22]. This available high-density array platform is able to follow the binding of a single analyte to 400 target spots at a time. Proteins (50 and 200 µg/ml) were spotted in triplicate at two concentrations onto the surface of a Gold Affinity chip (GE Healthcare) using a non-contact PiezoArray spotter (Perkin Elmer, Courtaboeuf, France). Six drops of 330 pl each were delivered to the surface of the chip (spot diameter: 250–300 µm, spotted amount: 100–400 pg/spot). The chips were then dried at room temperature and stored under vacuum at 4°C until their insertion into the Biacore Flexchip instrument. The chip was blocked with Superblock buffer (Pierce, Thermo Scientific, Brebières, France) for 5×5 min. The blocked chip was then equilibrated with PBS, 0.05% (v/v) Tween 20 at 500 µl/min for 90 min. TG-2 (500 nM) was flowed at 25°C over the chip surface for 25 min at the same flow rate. The dissociation of the endostatin-TG-2 complex was monitored in buffer flow - PBS, 0.05% (v/v) Tween 20 - for 40 min. Data collected from reference spots (gold surface), and from buffer spots were subtracted from those collected on spotted proteins to obtain specific binding curves.
Surface plasmon resonance binding assays
The SPR binding assays were performed in a Biacore T100 instruments (GE Healthcare). Recombinant human endostatin (50 µg/ml in 10 mM maleate buffer, pH 6.2) was covalently immobilized to the dextran matrix of a CM5 sensor chip via its primary amine groups according to the manufacturer's instruction (amine coupling kit, GE Healthcare) at a flow rate of 5 µl/min with 10 mM Hepes buffer pH 7.4 containing 0.15 M NaCl and 0.05% (v/v) P20 (HBS-P+ buffer, GE Healthcare) as running buffer. An immobilization level ranging between 1800 and 2000 resonance units (RU) was obtained; a control flow cell was prepared with 10 mM maleate buffer pH 6.2. ZnCl2(1 mM) was added to the running buffer for binding experiments. Sensorgrams collected on the control flow cell were automatically subtracted from the sensorgrams obtained on immobilized endostatin to yield specific binding responses. Binding assays were performed at 25 °C, but the sample compartment of the Biacore T100 was kept at 4°C to maintain transglutaminase-2 in its native state. Kinetic and affinity constants were calculated by injecting several concentrations of transglutaminase-2 (8–130 nM) in the presence of 2 mM CaCl2, over immobilized endostatin at 60 µl/min. The complexes were dissociated by a pulse (60 s) of 1.5 M NaCl + 2 M guanidinium chloride. The kinetic rates, ka and kd(association and dissociation rate constants, respectively), were calculated using the Biacore T100 evaluation software 2.0.1. Apparent equilibrium dissociation constants (KD) were calculated as the ratio of kd/ka for a 1:1 (Langmuir) interaction model. Inhibition experiments were carried out by preincubating TG-2 (130 nM) with 1 mM GTP for 15 min at room temperature before injecting the mixture over endostatin immobilized on the sensor chip.
Cross-linking experiments
Guinea pig TG-2 (5 mU/ml, Sigma-Aldrich) was incubated with recombinant human endostatin expressed in mammalian cells (10 µg/ml), the biotinylated peptide TVQQEL used as an acyl donor (30) (Assay mix A5731, Sigma-Aldrich) and 1 mM dithiothreitol. The reaction was carried out for 2, 5, 10, 20, 30 and 60 min at room temperature and was stopped by addition of 20 mM EDTA. Control experiments were performed with TG-2 and endostatin alone. The reaction products were analyzed by SDS-PAGE and by immunoblotting with either streptavidin-peroxidase, or with the polyclonal anti-endostatin antibody, followed by chemiluminescence detection (ECL, GE Healthcare). In another series of experiments, TG-2 (10 mU/ml) was incubated with endostatin (10 µg/ml), biotin-X-cadaverin (2 mM, Sigma-Aldrich), 8 mM CaCl2 and 1 mM dithiothreitol. The reaction was carried out for 120 min at room temperature, and was stopped by addition of 20 mM EDTA. Control experiments were performed with either TG-2 or endostatin alone. The reaction products were analyzed as described above.
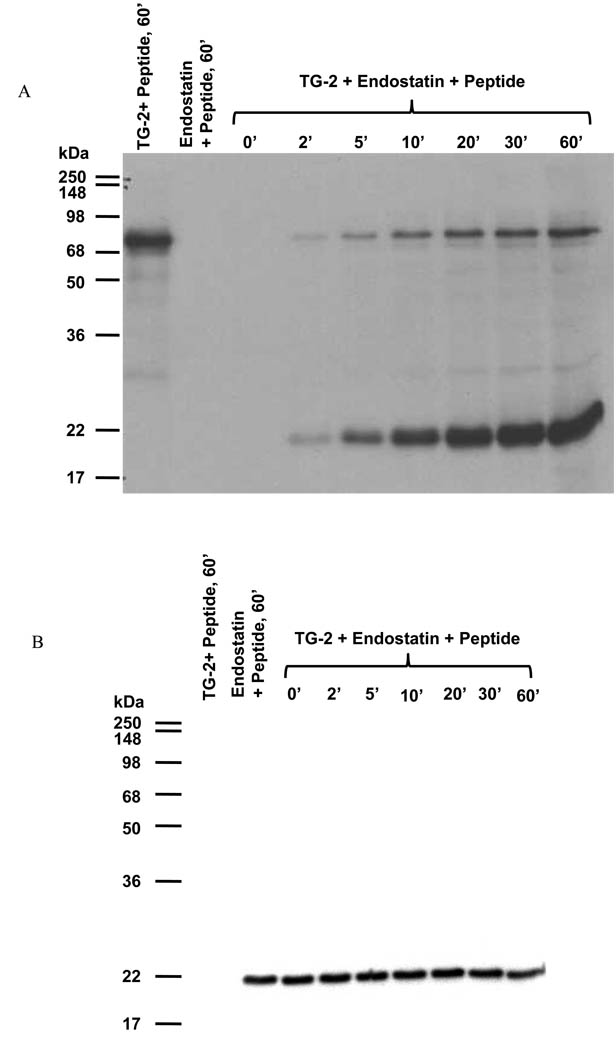
Cross-linking experiments: Reaction between endostatin (476 nM) and biotinylated peptide TVQQEL-OH mediated by guinea pig TG-2 (5 mU/ml). Reactions were carried out for 60 min at room temperature with or without GTP (µM). Results were analyzed by immunoblotting using A) extravidin-peroxidase, and B) polyclonal antibody against endostatin.
Immunocytochemistry
Primary cultures of human umbilical vein endothelial cells (HUVEC) were grown in endothelial cell growth medium 2 (PromoCell, Heidelberg, Germany). The cells were used for immunofluorescence experiments between passages 2 and 5. HUVEC were seeded on glass coverslips and cultivated in complete medium in normoxia or hypoxia (1% O2). The cells were then fixed with cold methanol for 5 minutes before blocking with 10% (v/v) normal goat serum in PBS. They were then incubated either with the anti-endostatin antibody, or with the antibody directed against TG-2 diluted in PBS containing 1% (v/v) normal goat serum. Secondary goat antibodies directed against rabbit or mouse IgG coupled to AlexaFluor-488 or -555 were from Invitrogen (Cergy Pontoise, France). Nuclei were stained with TO-PRO-3 (Invitrogen). Coverslips were mounted with Mowiol and observed with a TCS SP2 confocal microscope (Leica Microsystems, Nanterre, France).
RESULTS
Transglutaminase binds to monomeric endostatin and to the C-terminal domain of collagen XVIII
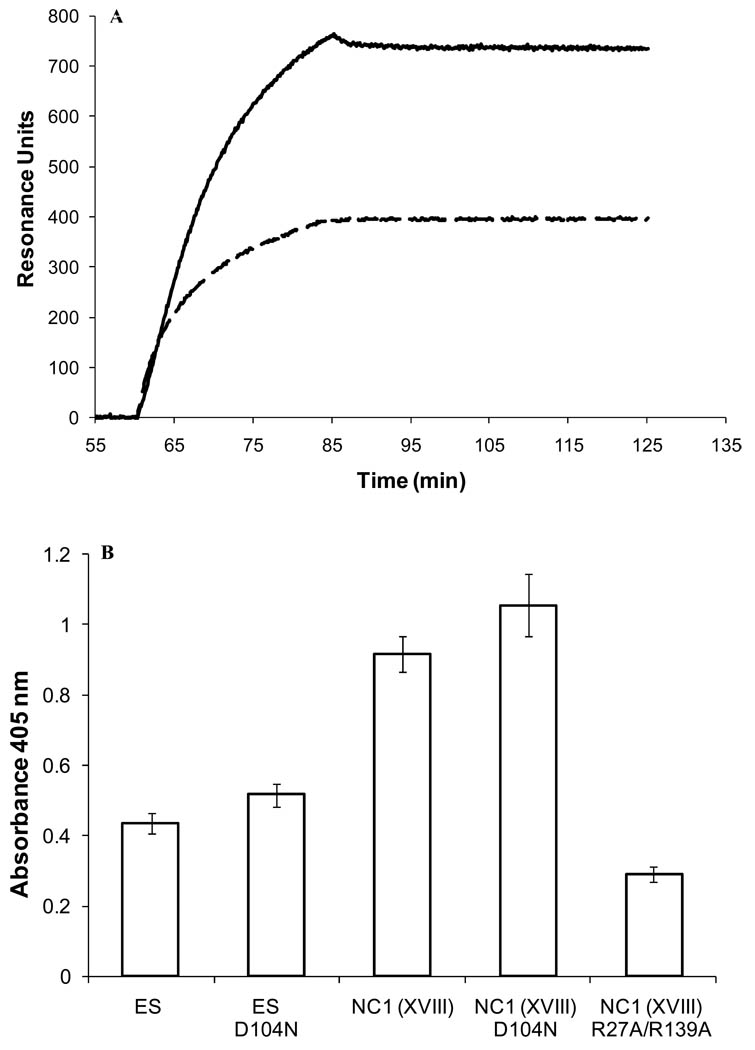
The binding of human TG-2 to endostatin and to the trimeric NC1 domain of collagen XVIII was demonstrated by SPR arrays (Figure 1A). Under the same experimental conditions, TG-2 was found previously to bind to known ligands, heparin and heparan sulfate [31), collagen XI [32] (Supplementary material, Fig. S2), α5β1 and αvβ3 integrins [10], and amyloid Aβ peptide [26]. The interaction was confirmed by solid-phase assays (Figure 1B), and was also observed with guinea pig TG-2 (Figure 2B). Several sources of TG-2 and endostatin were used to support the existence of the interaction between these two molecules.
Figure 1. TG-2 binds to endostatin and to the trimeric C-terminal NC1 domain of collagen XVIII.
A) Overlay of sensorgrams resulting from the injection of human TG-2 (500 nM) over immobilized wild-type NC1 (—, 154 pg) and wild-type endostatin (–––, 300 pg) (flow rate 500 µl/min). B) Solid-phase binding assay. Binding of human TG-2 (350 nM) to immobilized wild type and mutant endostatin and NC1(XVIII) domain (0.5 µg/well).
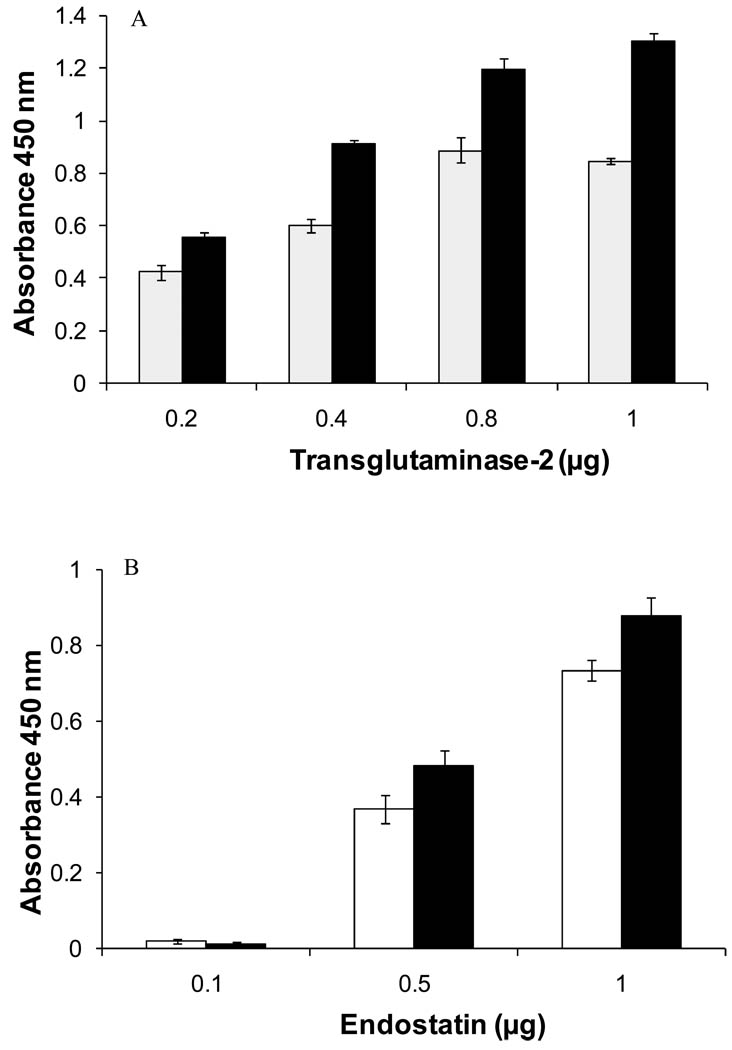
Figure 2. Soluble and immobilized endostatin bind to TG-2.
Solid-phase binding assays. A) Binding of recombinant human endostatin (500 nM) produced in mammalian (■) or yeast (□) expression systems to immobilized TG-2 (mean of 3 experiments ±SD). B) Binding of human (□, 350 nM) and guinea pig TG-2 (■, 350 nM) to immobilized mammalian human endostatin. The values are expressed as mean ± SD (n=3).
The binding properties of the D104N mutant were investigated because individuals with the homozygous D104N polymorphism in the COL18A1 gene have a high risk of occurrence of cancer [33, 34]. The D104N mutation did not alter the folding of endostatin and of the NC1(XVIII) domain as assessed by intrinsic fluorescence (Supplementary material Fig. S3). This mutation did not affect significantly the ability of endostatin, or of the NC1 domain of collagen XVIII, to bind to transglutaminase-2 (Figure 1B, and Figure 3B). In contrast, the double R27A/R139A NC1 mutant that has lost its capacity to bind heparin [19] but is properly folded as assessed by fluorescence spectroscopy (Supplementary material Fig. S3), bound to TG-2 to a lesser extent (31% compared to the wild type NC1 domain) (Figure 1B).
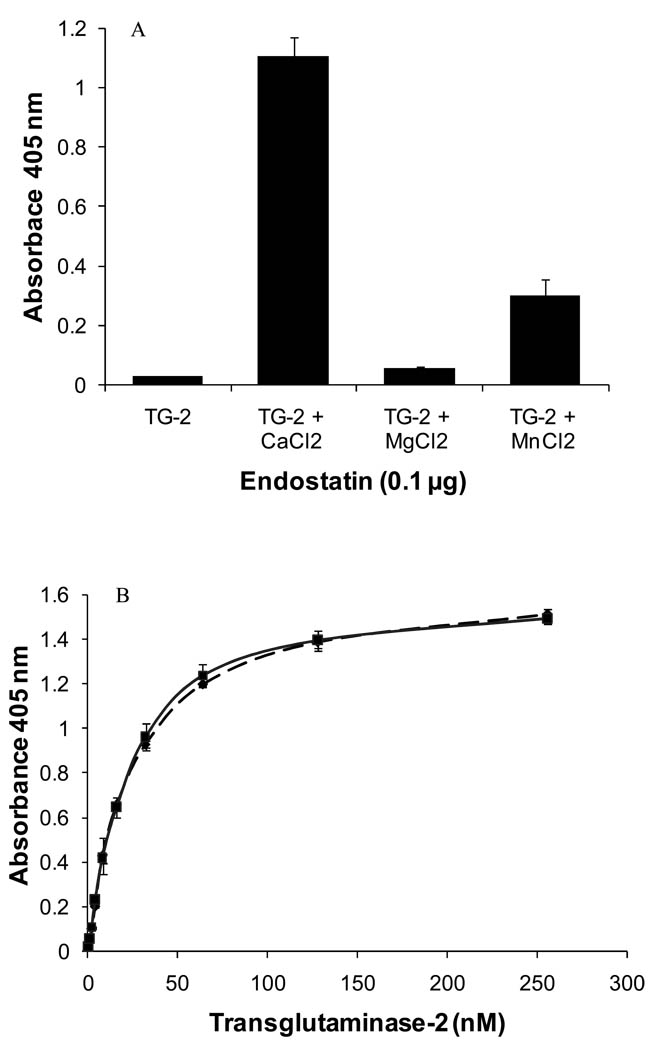
Figure 3. The binding of endostatin to TG-2 is cation-dependent.
A) Solid-phase assays: effect of cations. Binding of human TG-2 (64 nM) to coated endostatin produced in HEK 293 cells (0.1 µg/well) in the presence of cations (8 mM). (mean of 3 experiments ±SD) B) Binding of human TG-2 (0, 1, 2, 4, 8, 16, 32, 64, 128, 256 nM to immobilized human endostatin (––– wild-type endostatin, — D104N mutant endostatin; 0.1 µg/well) in the presence of 8 mM CaCl2. The values are expressed as mean ± SD (n=3).
The binding of recombinant endostatin (i.e. expressed in yeast or in mammalian cells) to immobilized TG-2 was concentration-dependent (Figure 2A). Similar results were obtained in the reverse orientation when TG-2 was incubated with endostatin-coated wells (Figure 2B).
The binding of endostatin to transglutaminase-2 is dependent upon cations
Binding assays were performed with human recombinant TG-2 in the presence of 1 mM EDTA to remove residual cations from the sample and to analyze the effects of different cations added at 8 mM. In absence of any cation, TG-2 did not bind significantly to endostatin (Figure 3A). The addition of cations increased the binding by ~ 2-fold for MgCl2, ~ 10-fold for MnCl2, and ~ 38-fold for CaCl2 (Figure 3A). Similar increase in binding was obtained with guinea pig TG-2 in the presence of cations (Supplementary material Fig. S4). Calcium was used for further experiments because it was the most efficient in promoting endostatin binding. In presence of 8 mM CaCl2, the binding of TG-2 to wild-type endostatin and to the D104 N endostatin mutant was saturable (Figure 3B).
Endostatin binds to transglutaminase with high affinity
Kinetic analysis was performed by injecting guinea pig TG-2 at several concentrations over immobilized endostatin produced either in mammalian cells (Supplementary material Fig. S5A) or in yeast (Supplementary material Fig. S5B). The sensorgrams were fitted to a 1:1 Langmuir model using the Biacore T100 evaluation software (2.0.1), and kinetics and affinity constants were calculated (Table 1). The affinity constants were 6.8 nM, and 5.6 nM for recombinant endostatin produced from mammalian cells and yeast, respectively (Table 1).
Table 1. Kinetic and equilibrium dissociation constants of the binding of transglutaminase-2 to endostatin.
Rate and affinity constants of the binding of soluble transglutaminase-2 to immobilized endostatin calculated from SPR binding assays using the Biacore T100 evaluation software 2.0.1.
| Endostatin (Immobilized on the chip) |
HEK 293 EBNA cells |
P. pastoris |
|---|---|---|
| Guinea pig TG-2 Injected in soluble form |
+ 2 mM CaCl2 | + 2 mM CaCl2 |
| ka (M−1s−1) | 1.12×105 M−1 s−1 | 6.10 ×104 M−1 s−1 |
| kd (s−1) | 7.65×10−4 s−1 | 3.41×10−4 s−1 |
| KD (M) | 6.8 nM | 5.59 nM |
| Chi2 | 4.1 | 8.81 |
| U-value | 1 | 1 |
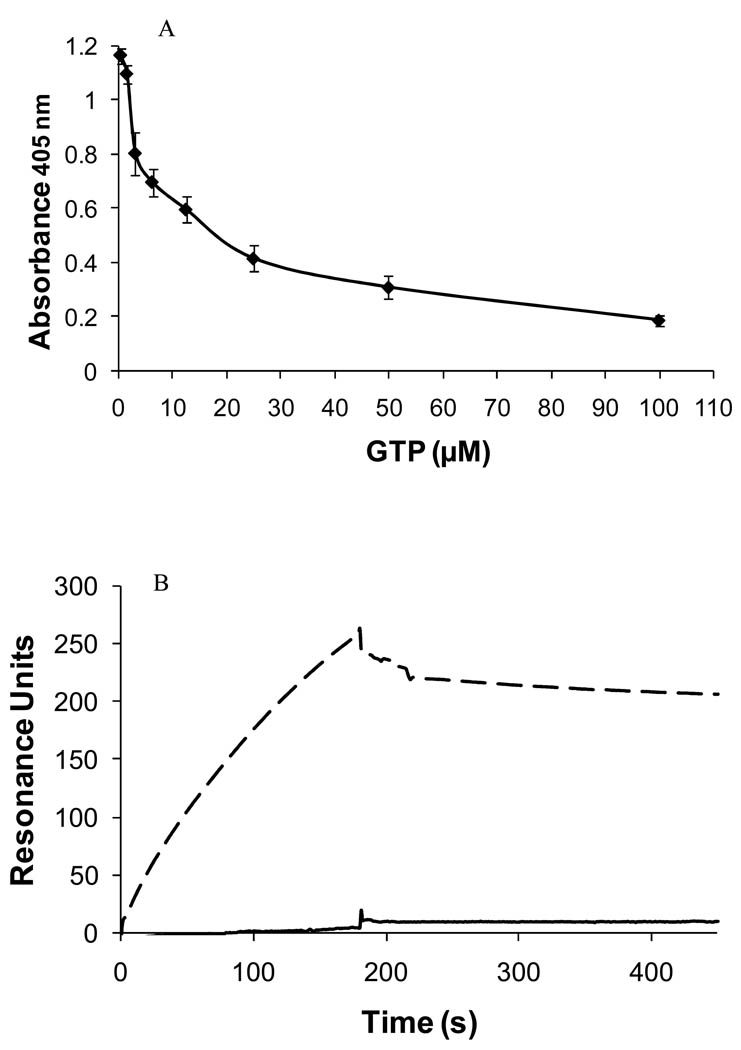
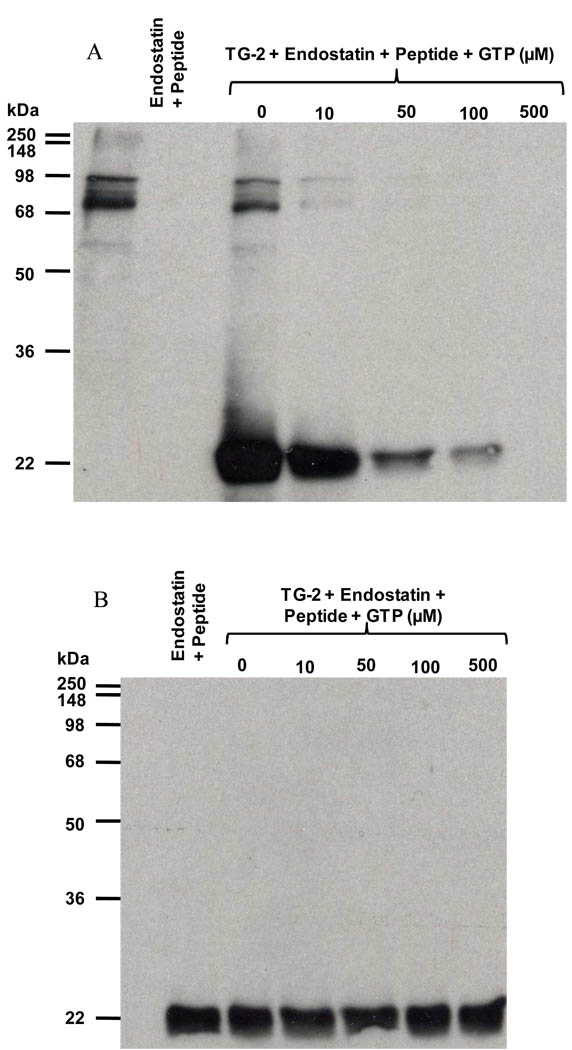
TG-2 is regulated by calcium and GTP. The transamidating activity of the enzyme is inhibited by GTP in an allosteric fashion, whereas CaCl2 partially reverses GTP inhibition [35]. We performed inhibition experiments with GTP, a reversible inhibitor of the cross-linking activity of TG-2, to determine if it was able to interfere with the binding of TG-2 to endostatin. Recombinant TG-2 in TBS containing 8 mM CaCl2 was preincubated with increased concentrations of GTP (0–100 µM) for 1 h at room temperature. It was then added to a 96-well microplate coated with recombinant human endostatin expressed in mammalian cells and incubated for 2 h at room temperature. We observed a decrease in the binding level of TG-2 to endostatin upon the addition of increasing concentrations of GTP (Figure 4A). This inhibition was confirmed by SPR experiments where the binding of guinea pig TG-2 to recombinant human endostatin expressed in mammalian cells was nearly abolished (96.7% inhibition) by 1 mM GTP (Figure 4B). A similar effect was observed with human endostatin produced in yeast (100% inhibition, data not shown). The above results suggest that either endostatin binds to TG-2 at the same site as GTP, or that the interaction with endostatin requires TG-2 to be in an open conformation, fully activated by calcium.
Figure 4. GTP inhibits the binding of TG-2 to endostatin.
A) Solid-phase binding assays: Effect of GTP (0–100 µM) on the binding of human TG-2 (64 nM) to endostatin (expressed in HEK cells; 0.1 µg/well) in presence of 8 mM CaCl2. The values are expressed as mean ± SD (n=3); B) SPR binding assays: injection of guinea pig TG-2 (130 nM) with (—) or without (–––) 1 mM GTP over immobilized human endostatin.
Endostatin is not a glutaminyl substrate of transglutaminase-2
We performed enzymatic assays to determine if endostatin was a substrate of TG-2. Endostatin was incubated with the enzyme in the presence of an acyl donor, i.e. the biotinylated peptide TVQQEL. Analysis of the reaction products by SDS-PAGE and immunoblotting showed that the biotinylated peptide was cross-linked to endostatin, and to a lesser extent to the enzyme (Figure 5A). The amount of incorporated peptide increased as a function of time. In absence of TG-2, or in presence of a Ca2+ ion chelator (20 mM EDTA), the biotinylated peptide was not cross-linked with either endostatin or TG-2. Immunodetection with a polyclonal anti-endostatin antibody showed that endostatin was not cross-linked to itself or to TG-2 under those experimental conditions (Figure 5B). When endostatin was reacted with the biotinylated peptide TVQQEL in the presence of TG-2 and GTP, the amount of peptide incorporated into endostatin decreased when GTP concentration increased (Figure 6A), whereas the amount of endostatin did not change in these conditions (Figure 6B). The incorporation of the peptide was totally inhibited by 500 µM GTP as shown in Figure 6 A. Since GTP is an inhibitor of the transamidating activity of TG-2, these results confirm that the cross-linking of endostatin to TVQQEL is mediated by the transamidating activity of TG-2 and that endostatin behaves as an acyl acceptor in the transamidation reaction.
Figure 5. Endostatin is as an acyl acceptor in the transamidation reaction catalyzed by TG-2.
Cross-linking experiments: Reaction between endostatin (476 nM) and the biotinylated peptide TVQQEL-OH mediated by guinea pig TG-2 (5 mU/ml). The reaction was carried out for 0, 2, 5, 10, 20, 30 and 60 min at room temperature. Results were analyzed by immunoblotting using A) extravidin-peroxidase, and B) polyclonal antibody against endostatin.
Figure 6. GTP inhibits the cross-linking between endostatin and an acyl donor mediated by guinea pig TG-2.
Reaction between endostatin (476 nM) and the biotinylated peptide TVQQEL-OH mediated by guinea pig TG-2 (5 mU/ml). Reactions were carried out for 60 min at room temperature in absence of GTP or in presence of increased GTP concentrations (10 – 500 µM). Results were analyzed by immunoblotting using A) extravidin-peroxidase, and B) polyclonal antibody against endostatin.
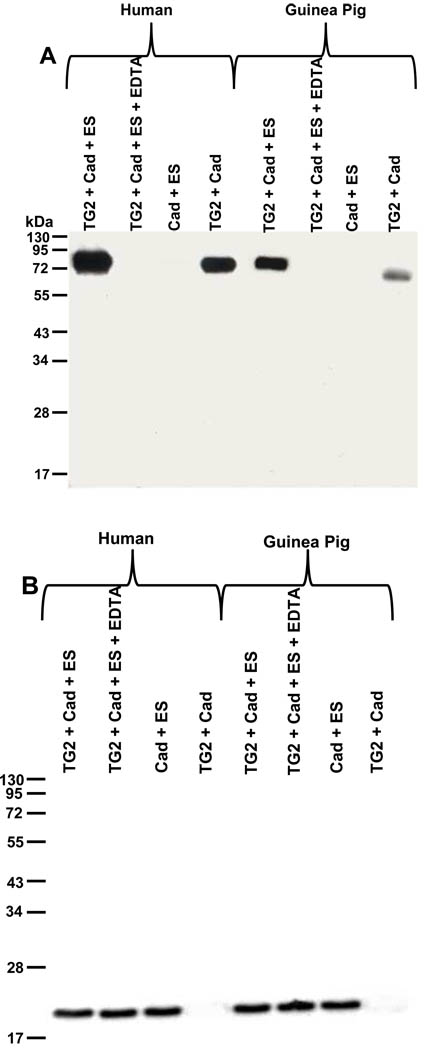
When endostatin was incubated with TG-2 and cadaverin, an acyl acceptor, no crosslinking reaction was detected, even when the reaction was performed with an increased amount of enzyme (10 U/ml instead of 5 mU/ml) and for a longer period time (120 min versus 60 min, Figure 7A and B). These results show that endostatin is an acyl acceptor, but not an acyl donor in the transamidation reaction catalyzed by TG-2. Preliminary results showed that endostatin did not inhibit the cross-linking activity of transglutaminase-2 in vitro (Supplementary material Fig. S6), suggesting that endostatin does not bind to the transamidating active site of TG-2.
Figure 7. Endostatin is not a glutaminyl substrate of TG-2.
Cross-linking experiments: Reaction between endostatin (ES, 476 nM) and biotin-X-cadaverin (Cad) in presence of human or guinea pig TG-2 (10 mU/ml). Analysis of the reaction products by immunoblotting using A) extravidin-peroxidase, and B) anti endostatin antibody.
Endostatin and transglutaminase-2 are co-localized in the extracellular matrix secreted by endothelial cells and in vessel walls
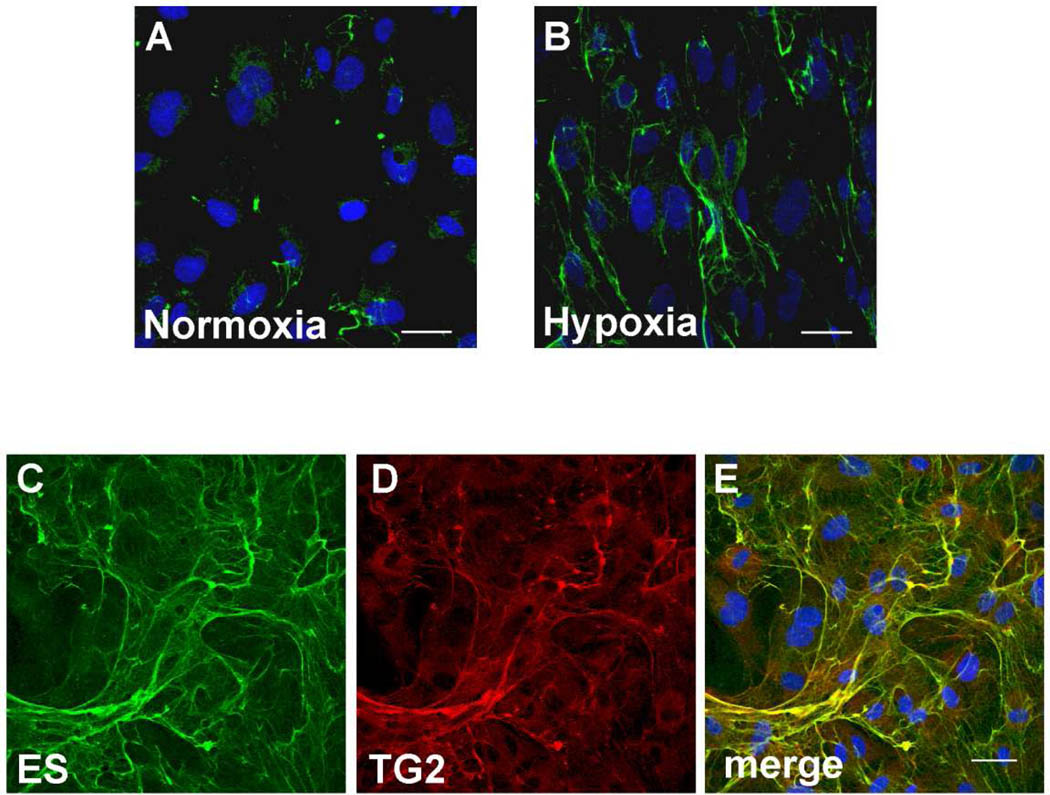
To determine if the binding of endostatin with TG-2 occurs within the extracellular matrix secreted by endothelial cells and/or at the cell surface, immunostaining experiments were performed with anti-endostatin and anti-transglutaminase antibodies. Since hypoxia induces COL18A1 gene in a HIF-1α dependent manner in endothelial cells [36], human umbilical cord endothelial cells (HUVEC) were incubated in a hypoxic environment for six days to enhance detection of endostatin (i.e. by immunofluorescence). Indeed an increased level of endostatin was detected in the extracellular matrix synthesized by cultured endothelial cells under hypoxia, that stimulates angiogenesis, compared to normoxia (Figure 8A and B). As can be seen from Figure 8, transglutaminase-2 co-localized with endostatin/collagen XVIII in the extracellular matrix secreted by HUVEC cultured under hypoxic conditions (Figure 8C–E). We have confirmed the co-localization of transglutaminase-2 and endostatin in tissue by performing double immunostaining of foreskin sections with antibodies directed against TG-2 and endostatin. TG-2 and endostatin were co-localized in vessel walls (Supplementary material Fig. S7).
Figure 8. Endostatin and TG-2 are co-localized in the extracellular matrix synthesized by HUVECs under hypoxia.
Confocal microscopy: immunostaining of endostatin/ collagen XVIII (A–C), and TG-2 (D), merge (E) in the extracellular matrix secreted by cultured HUVECs. Cells were grown to confluence in normoxia (A) or hypoxia (B–E). Nuclei (blue) were stained using TO-PRO-3. Bar = 30 µm.
DISCUSSION
The interaction between endostatin and TG-2 was demonstrated by SPR and by solid-phase assays with two different sources of transglutaminase (human recombinant TG-2 and guinea pig TG-2 extracted from liver), and two different sources of recombinant human endostatin (produced by HEK 293 EBNA cells or in yeast). These experiments revealed that endostatin binds to transglutaminase with affinity in the nanomolar range and in a cation-dependent manner, calcium ions being the most potent among those assayed.
TG-2 catalyzes post-translational modification of proteins by the formation of γ-glutamyl-ε-lysine bonds between glutamine and lysine residues. Endostatin contains eight glutamine residues that could potentially act as glutaminyl substrates (acyl donors) in the transamidation reaction. However, endostatin is not a glutaminyl substrate of TG-2 in vitro, but rather an acyl acceptor providing lysine residues in the transamidation reaction. This is in agreement with the fact that none of the glutamine residues of endostatin is found within a QXP sequence, a preferred sequence for glutamine-donor substrates where X represents a variable amino acid [37] and with the fact that the N-terminus of endostatin contains a QP (Q7P8) sequence reported to abolish transamidation [37]. The LGQS sequence found in the N-propeptide of the α1 chain of procollagen III, a physiological substrate of TG-2 [38], is present in endostatin (residues 158–161) but it is located on the side of endostatin that is not involved in the binding to TG-2. Therefore the glutamine residue 160 does not appear to be a glutaminyl substrate of TG-2.
GTP inhibits the binding of endostatin to tranglutaminase-2, suggesting that endostatin could bind to the GTP binding site on TG-2. However, the binding of endostatin to TG-2 is calcium-dependent and calcium is known to activate TG-2 by switching on its transamidating (i.e. cross-linking) activity [15]. Since the activation of TG-2 by Ca2+ can be counteracted by the allosteric inhibitor GTP [35], the inhibition of endostatin binding by GTP might alternatively suggest that endostatin binds to the transamidating active form of the enzyme. Thus, we used TG-2 in an extended, active, conformation [39] to further investigate the location of the endostatin binding site on TG-2. We have shown here that R27 and/or R139 residues participate in the binding of endostatin to TG-2. These residues both form part of a large basic patch on endostatin, comprising the heparin-binding site [19], that is likely to interact with negatively charged amino acids on TG-2 surface. In addition to our experimental data, we have used the Connolly molecular surface of TG-2 to have clues on possible binding site(s) of endostatin on TG-2. There are three negatively charged areas on the enzyme: one in the N-terminal β-sandwich domain, one close to transamidating catalytic core, and a third one close to the GTP-binding site. We have shown that endostatin does not interfere with TG-2 cross-linking activity in vitro and that GTP blocks the binding of endostatin to TG-2. These results suggest that endostatin may bind to, or close to, the GTP binding site but not to the transamidating active site of TG-2.
The requirement of calcium ions for endostatin binding to TG-2 suggests as discussed above that endostatin binds to the open form of the enzyme. Extracellular TG-2 is predominantly maintained in the catalytically inactive closed conformation under ordinary physiological conditions, and is able to promote cell adhesion, spreading, migration, or differentiation independently of its catalytic activity [15]. Extracellular TG-2 is transiently activated in response to innate immune signals such as exposure to polyinosinic-polycytidylic acid, a potent ligand of the toll-like receptor TLR-3 [40]. The stimulation of innate immune pattern recognition receptors such as TLR3 triggers the release of extracellular TG-2 from cell surface integrins and the concomitant enzyme activation [40]. It is thus possible that in the course of angiogenesis up-regulated innate immune receptors trigger the activation of extracellular TG-2 leading in turn to endostatin binding. This hypothesis is supported by the fact that multiple human endothelial cell types express surface TLR3, which play a key role in disrupting the haemostasis balance on endothelial cells [41], and by the suppression of sequence- and target-independent angiogenesis by siRNA via TLR3 [42].
Endostatin might cooperate with TG-2 in several physio-pathological processes either through direct interactions or through more complex mechanisms involving multimolecular complexes. Direct interactions between endostatin and TG-2 might play a role in organizing the extracellular matrix in skin basement membrane, endostatin being present in this location [43], and TG-2 stabilizing basement membranes [44]. At the endothelial cell surface, endostatin inhibits both the extracellular activation of proMMP-2 by inhibition of MT1-MMP as well as the catalytic activity of MMP-2, thus blocking the invasiveness of tumor cells [45]. MMP-2, functioning in concert with MT1-MMP, hydrolyzes cell-surface-associated TG-2, and the cleavage eliminates both the adhesion and the enzymatic activity of TG-2 [46]. It should be noted that endostatin and TG-2 bind to the catalytic domain of MMP-2, and both proteins inhibit MMP-2 maturation [46, 47].
Besides MMP-2, endostatin and TG-2 share heparan sulfate, VEGF-R2 and integrins as binding partners at the cell surface, and both regulate endothelial cell adhesion [3, 6, 10, 11, 48]. As reported here, two arginine residues implicated in the binding of endostatin (R27 and/or R139) to TG-2 are also involved in the binding to α5β1 and αvβ3 integrins [4], and to heparin [19]. This suggests that endostatin is not able to bind simultaneously to TG-2 and α5β1/αvβ3 integrins or to TG-2 and heparan sulfate. The co-localization of endostatin/collagen XVIII and TG-2 in the extracellular matrix secreted by cultured endothelial cells indicates that this interaction may occur in a cellular context and supports its physiological relevance. These proteins are co-localized in the extracellular matrix synthesized under hypoxia that stimulates angiogenesis. This raises the possibility that endostatin and TG-2 regulate endothelial cell behaviour and control angiogenesis in a concerted fashion. However, their molecular interplay in the extracellular matrix secreted by endothelial cells and/or at the surface of these cells remains to be deciphered.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. C. Marquette and Prof. L. Blum (UMR 5246 CNRS- University Lyon 1) for their help in spotting on Gold affinity chips, Sylviane Guerret (Novotec, Lyon, France) for her skillful assistance in the production of the polyclonal anti-endostatin antibody, and Naomi Fukai and Reidunn Jetne for their generous gift of transfected HEK 293 cells expressing wild type or mutant endostatin and the NC1(XVIII) domain. The support of GE Healthcare (Dr. C. Quétard, P. Linden, T. Salomon, X. Rousselot and A. Sylvan) is gratefully acknowledged. SPR experiments were performed in the facility of IFR 128 Gerland Lyon Sud (France).
FUNDING
This work was supported by the Association pour la Recherche contre le Cancer [grant 3652 to SRB], GIS Maladies Rares [INSERM A04115SP to SRB], CPER Rhône-Alpes to CF and SRB), the Emergence Research Program (Région Rhône-Alpes to SRB), a BQR grant from University Lyon-1 [to SRB], and NIH [36820 to BO].
Abbreviations
- BSA
Bovine serum albumin
- ES
endostatin
- HBS
Hepes-Buffered-Saline
- GTP
Guanosine 5'-triphosphate
- HEK
Human embryonic kidney
- HIF
Hypoxia-inducible factor
- HUVEC
Human umbilical vein endothelial cells
- MMP-2
Matrix metalloproteinase-2
- MT-MMP1
Membrane type matrix metalloproteinase-1
- PBS
Phosphate-buffered-saline
- PNPP
p-nitrophenyl phosphate
- RU
Resonance unit
- SPR
Surface plasmon resonance
- TBS
Tris-buffered-saline
- TG-2
Transglutaminase-2
- TLR3
Toll-like receptor-3
- TMB
3,3',5,5'-Tetramethylbenzidine
- VEGF
Vascular endothelial growth factor
REFERENCES
- 1.Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp. Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y. Molecular mechanisms and therapeutic development of angiogenesis inhibitors. Adv. Cancer Res. 2008;100:113–131. doi: 10.1016/S0065-230X(08)00004-3. [DOI] [PubMed] [Google Scholar]
- 3.Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K. Interaction of endostatin with integrins implicated in angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1024–1029. doi: 10.1073/pnas.031564998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faye C, Moreau C, Chautard E, Jetne R, Fukai N, Ruggiero F, Humphries MJ, Olsen BR, Ricard-Blum S. Molecular interplay between endostatin, integrins, and heparan sulfate. J. Biol. Chem. 2009;284:22029–22040. doi: 10.1074/jbc.M109.002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karumanchi SA, Jha V, Ramchandran R, Karihaloo A, Tsiokas L, Chan B, Dhanabal M, Hanai JI, Venkataraman G, Shriver Z, Keiser N, Kalluri R, Zeng H, Mukhopadhyay D, Chen RL, Lander AD, Hagihara K, Yamaguchi Y, Sasisekharan R, Cantley L, Sukhatme VP. Cell surface glypicans are low-affinity endostatin receptors. Mol. Cell. 2001;7:811–822. doi: 10.1016/s1097-2765(01)00225-8. [DOI] [PubMed] [Google Scholar]
- 6.Kim YM, Hwang S, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. Endostatin blocks vascular endothelial growth factor-mediated signaling via direct interaction with KDR/Flk-1. J. Biol. Chem. 2002;277:27872–27879. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- 7.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4766–4771. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 8.Korner G, Schneider DE, Purdon MA, Bjornsson TD. Bovine aortic endothelial cell transglutaminase. Enzyme characterization and regulation of activity. Biochem. J. 1989;262:633–641. doi: 10.1042/bj2620633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaudry CA, Verderio E, Jones RA, Smith C, Griffin M. Tissue transglutaminase is an important player at the surface of human endothelial cells: evidence for its externalization and its colocalization with the beta(1) integrin. Exp. Cell Res. 1999;252:104–113. doi: 10.1006/excr.1999.4633. [DOI] [PubMed] [Google Scholar]
- 10.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dardik R, Inbal A. Complex formation between tissue transglutaminase II (tTG) and vascular endothelial growth factor receptor 2 (VEGFR-2): proposed mechanism for modulation of endothelial cell response to VEGF. Exp. Cell Res. 2006;312:2973–2982. doi: 10.1016/j.yexcr.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J. 1999;13:1787–1795. doi: 10.1096/fasebj.13.13.1787. [DOI] [PubMed] [Google Scholar]
- 13.Haroon ZA, Lai TS, Hettasch JM, Lindberg RA, Dewhirst MW, Greenberg CS. Tissue transglutaminase is expressed as a host response to tumor invasion and inhibits tumor growth. Lab. Invest. 1999;79:1679–1686. [PubMed] [Google Scholar]
- 14.Jones RA, Kotsakis P, Johnson TS, Chau DY, Ali S, Melino G, Griffin M. Matrix changes induced by transglutaminase 2 lead to inhibition of angiogenesis and tumor growth. Cell Death Differ. 2006;13:1442–1453. doi: 10.1038/sj.cdd.4401816. [DOI] [PubMed] [Google Scholar]
- 15.Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem. J. 2002;368:377–396. doi: 10.1042/BJ20021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol. Cell. Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 17.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2009;36:659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 18.Ruan Q, Tucholski J, Gundemir S, Johnson Voll GV. The Differential Effects of R580A Mutation on Transamidation and GTP Binding Activity of Rat and Human Type 2 Transglutaminase. Int. J. Clin. Exp. Med. 2008;1:248–259. [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki T, Larsson H, Kreuger J, Salmivirta M, Claesson-Welsh L, Lindahl U, Hohenester E, Timpl R. Structural basis and potential role of heparin/heparan sulfate binding to the angiogenesis inhibitor endostatin. EMBO J. 1999;18:6240–6248. doi: 10.1093/emboj/18.22.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dieterich W, Esslinger B, Trapp D, Hahn E, Huff T, Seilmeier W, Wieser H, Schuppan D. Cross linking to tissue transglutaminase and collagen favours gliadin toxicity in coeliac disease. Gut. 2006;55:478–484. doi: 10.1136/gut.2005.069385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley DM, Zhao C, Speier AC, Woodard GA, Li S, Li Z, Walz T. Transglutaminase induces protofibril-like amyloid beta-protein assemblies that are protease-resistant and inhibit long-term potentiation. J. Biol. Chem. 2008;283:16790–16800. doi: 10.1074/jbc.M802215200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faye C, Chautard E, Olsen BR, Ricard-Blum S. The first draft of the endostatin interaction network. J. Biol. Chem. 2009;284:22041–22047. doi: 10.1074/jbc.M109.002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricard-Blum S, Feraud O, Lortat-Jacob H, Rencurosi A, Fukai N, Dkhissi F, Vittet D, Imberty A, Olsen BR, van der Rest M. Characterization of endostatin binding to heparin and heparan sulfate by surface plasmon resonance and molecular modeling: role of divalent cations. J. Biol. Chem. 2004;279:2927–2936. doi: 10.1074/jbc.M309868200. [DOI] [PubMed] [Google Scholar]
- 24.Gambetti S, Dondi A, Cervellati C, Squerzanti M, Pansini FS, Bergamini CM. Interaction with heparin protects tissue transglutaminase against inactivation by heating and by proteolysis. Biochimie. 2005;87:551–555. doi: 10.1016/j.biochi.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Deininger MH, Meyermann R, Schluesener HJ. Endostatin/collagen XVIII accumulates in patients with traumatic brain injury. J. Neurotrauma. 2006;23:1103–1110. doi: 10.1089/neu.2006.23.1103. [DOI] [PubMed] [Google Scholar]
- 26.Wang DS, Dickson DW, Malter JS. Tissue Transglutaminase, Protein Cross-linking and Alzheimer's Disease: Review and Views. Int. J. Clin. Exp. Pathol. 2008;1:5–18. [PMC free article] [PubMed] [Google Scholar]
- 27.Deininger MH, Fimmen BA, Thal DR, Schluesener HJ, Meyermann R. Aberrant neuronal and paracellular deposition of endostatin in brains of patients with Alzheimer's disease. J. Neurosci. 2002;22:10621–10626. doi: 10.1523/JNEUROSCI.22-24-10621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Johnson BR, Suri DE, Martinez J, Bjornsson TD. Immunohistochemical demonstration of tissue transglutaminase in amyloid plaques. Acta Neuropathol. 1998;96:395–400. doi: 10.1007/s004010050910. [DOI] [PubMed] [Google Scholar]
- 29.Wilhelmus MM, Grunberg SC, Bol JG, van Dam AM, Hoozemans JJ, Rozemuller AJ, Drukarch B. Transglutaminases and transglutaminase-catalyzed cross-links colocalize with the pathological lesions in Alzheimer's disease brain. Brain Pathol. 2009;19:612–622. doi: 10.1111/j.1750-3639.2008.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruoppolo M, Orru S, D'Amato A, Francese S, Rovero P, Marino G, Esposito C. Analysis of transglutaminase protein substrates by functional proteomics. Protein Sci. 2003;12:1290–1297. doi: 10.1110/ps.0239103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EA. Heparan sulfate proteoglycans are receptors for the cellsurface trafficking and biological activity of transglutaminase-2. J. Biol. Chem. 2009;284:18411–18423. doi: 10.1074/jbc.M109.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kleman JP, Aeschlimann D, Paulsson M, van der Rest M. Transglutaminase-catalyzed cross-linking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry. 1995;34:13768–13775. doi: 10.1021/bi00042a007. [DOI] [PubMed] [Google Scholar]
- 33.Lourenco GJ, Cardoso-Filho C, Goncales NS, Shinzato JY, Zeferino LC, Nascimento H, Costa FF, Gurgel MS, Lima CS. A high risk of occurrence of sporadic breast cancer in individuals with the 104NN polymorphism of the COL18A1 gene. Breast Cancer Res. Treat. 2006;100:335–338. doi: 10.1007/s10549-006-9259-z. [DOI] [PubMed] [Google Scholar]
- 34.Nascimento H, Lourenco GJ, Ortega MM, Yamaguti GG, Costa FF, Lima CS. D104N polymorphism in endostatin, an angiogenesis inhibitor, in acute and chronic myeloid leukaemia. Leuk. Res. 2007;31:1158–1159. doi: 10.1016/j.leukres.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Achyuthan KE, Greenberg CS. Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J. Biol. Chem. 1987;262:1901–1906. [PubMed] [Google Scholar]
- 36.Paddenberg R, Faulhammer P, Goldenberg A, Kummer W. Hypoxia-induced increase of endostatin in murine aorta and lung. Histochem. Histochem. Cell Biol. 2006;125:497–508. doi: 10.1007/s00418-006-0158-5. [DOI] [PubMed] [Google Scholar]
- 37.Fleckenstein B, Molberg O, Qiao SW, Schmid DG, von der Mulbe F, Elgstoen K, Jung G, Sollid LM. Gliadin T cell epitope selection by tissue transglutaminase in celiac disease. Role of enzyme specificity and pH influence on the transamidation versus deamidation process. J. Biol. Chem. 2002;277:34109–34116. doi: 10.1074/jbc.M204521200. [DOI] [PubMed] [Google Scholar]
- 38.Bowness JM, Folk JE, Timpl R. Identification of a substrate site for liver transglutaminase on the aminopropeptide of type III collagen. J. Biol. Chem. 1987;262:1022–1024. [PubMed] [Google Scholar]
- 39.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibamiya A, Hersemeyer K, Schmidt Woll T, Sedding D, Daniel JM, Bauer S, Koyama T, Preissner KT, Kanse SM. A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood. 2009;113:714–722. doi: 10.1182/blood-2008-02-137901. [DOI] [PubMed] [Google Scholar]
- 42.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elamaa H, Sormunen R, Rehn M, Soininen R, Pihlajaniemi T. Endostatin overexpression specifically in the lens and skin leads to cataract and ultrastructural alterations in basement membranes. Am. J. Pathol. 2005;166:221–229. doi: 10.1016/S0002-9440(10)62246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aeschlimann D, Paulsson M. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. J. Biol. Chem. 1991;266:15308–15317. [PubMed] [Google Scholar]
- 45.Kim YM, Jang JW, Lee OH, Yeon J, Choi EY, Kim KW, Lee ST, Kwon YG. Endostatin inhibits endothelial and tumor cellular invasion by blocking the activation and catalytic activity of matrix metalloproteinase. Cancer Res. 2000;60:5410–5413. [PubMed] [Google Scholar]
- 46.Belkin AM, Zemskov EA, Hang J, Akimov SS, Sikora S, Strongin AY. Cell-surface-associated tissue transglutaminase is a target of MMP-2 proteolysis. Biochemistry. 2004;43:11760–11769. doi: 10.1021/bi049266z. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Jang JW, Kim YM, Lee HI, Jeon JY, Kwon YG, Lee ST. Endostatin binds to the catalytic domain of matrix metalloproteinase-2. FEBS Lett. 2002;519:147–152. doi: 10.1016/s0014-5793(02)02742-4. [DOI] [PubMed] [Google Scholar]
- 48.Dixelius J, Cross M, Matsumoto T, Sasaki T, Timpl R, Claesson-Welsh L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002;62:1944–1947. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.