Abstract
Taxometric procedures were used to determine whether nicotine addiction is best conceptualized as a dimensional or a categorical (i.e., taxonic) phenomenon. Using data from the 2003 National Survey on Drug Use and Health (NSDUH; N = 12,467), results from MAMBAC, MAXEIG, and LMODE taxometric analyses provided strong evidence that nicotine addiction has a taxonic latent structure. Members of the addiction taxon, which constituted approximately 48% of those who reported smoking in the past 30 days, consumed a higher number of cigarettes per day, had stronger craving, higher levels of nicotine tolerance, more inflexible smoking patterns, and shorter latencies to smoking their first cigarette on waking compared with nontaxon members. These findings of a distinct addiction taxonic structure were replicated using a 2002 NSDUH sample (N = 12,224). Finally, the predictive validity of the taxon variable was compared with a continuous indicator sum. The taxon accounted for most of the predictive variance in the indicator sum, but the latter generally showed significant predictive power even after controlling for the former. Thus, these smoking variables may have both a categorical and a dimensional structure.
Keywords: nicotine addiction, taxometric analysis, cigarette smoking
More than 57 million (27%) American adults reported smoking cigarettes over the previous 30 days (Office of Applied Studies, 2004). The range of cigarette use among these people is exceptionally broad. Many are daily, high-rate smokers—approximately 35% smoke a pack (20 cigarettes) or more each day. Nearly 30% smoke less than a pack of cigarettes on a daily basis, and the remaining 35% do not smoke every day. Most nondaily smokers (72%) average 5 or fewer cigarettes on days on which they smoke. It is commonly assumed that the core difference between daily, high-rate smokers and those with lower levels of cigarette consumption arises from the development of nicotine addiction1 in the former group (Hughes, Helzer, & Lindberg, 2006; Piper, McCarthy, & Baker, 2006).
The nicotine addiction construct has been variously defined, but most conceptualizations have emphasized a particular constellation of primary features. These include high levels of chronic smoking, continued smoking despite knowledge of its harmful impact, impaired control over smoking, the emergence of nicotine withdrawal on cessation, and tolerance to nicotine’s effects (American Psychiatric Association, 2000; Piper et al., 2006; World Health Organization, 1992). Although there has been considerable research on the motivational processes presumably responsible for nicotine addiction (e.g., Brandon, Herzog, Irvin, & Gwaltney, 2004; Eissenberg, 2004; Glautier, 2004), little attention has been paid to the latent structure of this construct. In particular, there has been scant research on whether nicotine addiction varies quantitatively or qualitatively across the spectrum of smokers. For example, nicotine addiction may be a natural category such that daily, high-rate smokers differ qualitatively from people who smoke at lower levels. Alternatively, nicotine addiction may lie on a continuum of nicotine use, such that all smokers might be considered more or less addicted along one or more dimensions, with no natural threshold that distinguishes addicted from nonaddicted smokers.
As noted by Tiffany, Conklin, Shiffman, and Clayton (2004), most contemporary theories of nicotine addiction adopt a dimensional perspective with regard to the latent structure of the addiction construct. For example, models that presume that cigarette smoking is maintained by the positive reinforcing effects of nicotine generally posit that those processes are operative across all levels of cigarette consumption with no functional discontinuity between light and heavy smokers (see Glautier, 2004, for a review). Similarly, cognitively based and social learning models of addiction hypothesize that the key cognitive processes responsible for cigarette smoking are continuous across all levels of smoking (see Brandon et al., 2004, for review). Furthermore, approaches that emphasize affective– homeostatic mechanisms as central to addictive motivation typically describe those processes in fundamentally dimensional, not categorical, terms (Eissenberg, 2004). For instance, Koob and Le Moal (1997) hypothesized that repeated uses of nicotine modify the neurobiology of reward such that a person deprived of cigarettes is progressively more anhedonic. In this model, addiction reflects a continuous change in reward sensitivity over repeated nicotine exposures; the model posits no mechanistic threshold that would distinguish the addicted from the nonaddicted smoker.
In contrast, a categorical perspective on nicotine addiction is implicit in current diagnostic classification tools (e.g., the DSM–IV–TR; American Psychiatric Association, 2000), which define addiction as an assortment of behavioral attributes that collectively identify certain forms of smoking as qualitatively divergent from other kinds of smoking behavior. In the DSM–IV–TR, nicotine addiction is conceptualized as categorically different from nondependent smoking; that is, it is a taxon. According to J. Ruscio, Haslam, and Ruscio (2006), a taxon can be defined as a “grouping of cases that share an underlying commonality, a set of ‘deep’ properties that accounts for the group’s observable similarities” (p. 7). A taxon has boundaries and a distinct group of members, and the discontinuity between members and nonmembers occurs at the latent level. When DSM–IV–TR criteria are applied to current adult smokers, approximately 51% are diagnosed as addicted to nicotine (Grant, Hasin, Chou, Stinson, & Dawson, 2004).
The categorical approach adopted by the DSM–IV–TR is based on clinical consensus and diagnostic expediency and does not, sua sponte, provide sufficient justification for presuming the existence of any taxon (Lenzenweger, 2004). There is, however, ample empirical and theoretical justification for positing the existence of a nicotine addiction taxon. More important, there is a sizable literature on fundamental differences between daily, high-rate smokers and people who display sustained, low levels of cigarette consumption. Various terms have been used to describe the latter type of smoker, including chippers (Shiffman, 1989), occasional smokers (e.g., Hennrikus, Jeffery, & Lando, 1995), and intermittent smokers (McCarthy, Zhou, & Hser, 2001). Although inclusion criteria differ across studies, most researchers identify low-level smokers as people who smoke considerably fewer than 20 cigarettes per day and, in many studies, as those who do not smoke daily. Low-level smokers generally absorb significant amounts of nicotine when they smoke (Brauer, Hatsukami, Hanson, & Shiffman, 1996; Shiffman, Fischer, Zettler-Segal, & Benowitz, 1990), but do not maintain steady-state levels of nicotine (Shiffman, Paty, Kassel, Gnys, & Zettler-Segal, 1994) and do not experience withdrawal when they are abstinent from cigarettes (Shiffman, Paty, Gnys, Kassel, & Elash, 1995). Low-level smokers report experiencing craving in smoking situations (Shiffman & Paty, 2006) and display cue-specific craving when confronted with stimuli associated with smoking (Davies, Willner, & Morgan, 2000; Sayette, Martin, Wertz, Shiffman, & Perrott, 2001). But these smokers say that they do not have very much craving in periods between their cigarettes (Shiffman & Paty, 2006). In contrast, high-rate, daily smokers are more likely to report relatively elevated levels of craving even in the periods between smoking cigarettes (Shiffman & Patty, 2006; Tiffany, Warthen, & Goedeker, in press).
On one hand, low-level smokers do not appear to smoke to avoid nicotine withdrawal. On the other hand, as a consequence of their sustained high levels of nicotine exposure, high-rate, daily smokers exhibit a distinct withdrawal syndrome when they abstain from cigarettes (Hughes, Gust, Skoog, Keenan, & Fenwick, 1991). The idea that withdrawal relief or avoidance is at the heart of addictive drug use has long been formalized in several models of addictive motivation and has been forwarded by multiple researchers as the foundation of nicotine addiction (see Eissenberg, 2004, for review). Withdrawal-based models assume that nicotine withdrawal will emerge only after the smoker is exposed to a sufficient dose and frequency of nicotine. Thus, withdrawal relief or avoidance will not motivate use until the smoker escalates smoking to a point at which physical dependence emerges. Withdrawal, then, may provide a mechanism for distinguishing addicted from nonaddicted smokers. This hypothesis must be tempered in light of claims that with some drugs (most notably opiates), withdrawal signs and symptoms can emerge even with a single drug exposure (e.g., Stitzer, Wright, Bigelow, June, & Felch, 1991). This phenomenon of acute dependence, however, has not been demonstrated with nicotine in either human or animal studies (Eissenberg, 2004), and research has indicated that the development of nicotine withdrawal in rats requires uninterrupted exposure to nicotine sustained over several consecutive days (Vann, Balster, & Beardsley, 2005).
A recent model of addiction motivation forwarded by Baker, Piper, McCarthy, Majeskie, and Fiore (2004) suggested an additional mechanism whereby high-rate smokers might be categorically distinct from low-level smokers. These authors hypothesized that addictive behavior is typified by signaled avoidance learning—in essence, addicts learn to avoid the negative affective consequences of abstinence by using drugs when confronted with interoceptive and exteroceptive cues that signal the onset of withdrawal. Behavior supported by avoidance learning is highly resistant to extinction and prone to reinstatement (Bouton, 2000). Furthermore, to the extent that avoidance effectively forestalls the aversive unconditioned stimulus and terminates signals of impending withdrawal, there should be little overt indication in the behavior of addicted smokers that their smoking is driven by attempts to evade withdrawal. As escape learning generally precedes avoidance learning, a major milestone in the emergence of addictive smoking might be a relatively abrupt transition from escape learning to avoidance learning (Tiffany et al., 2004). A categorically distinct form of addiction may emerge when smokers learn to manage the aversive effects of nicotine withdrawal by maintaining a level of drug use that largely avoids withdrawal experiences.
Given the purported limitations of a purely categorical taxonomy for diagnosing substance use disorders, some authors have recommended that DSM–V dependence criteria include dimensionality such that symptoms are rated in terms of severity (Helzer, van den Brink, & Guth, 2006). However, these recommendations are not based on empirical evidence of the latent structure of addiction. The extent to which nicotine addiction is purely dimensional or might have categorical elements has not, with the exception of one study, been directly addressed. Muthén and Asparouhov (2006) applied several latent variable models to data on seven DSM–IV–TR nicotine dependence features assessed in current smokers participating in the 2001–2002 National Epidemiological Survey on Alcohol and Related Conditions (Grant, Moore, & Kaplan, 2003). Their comparison of several analyses favored a hybrid model (factor mixture analysis) of nicotine addiction with three classes of smokers: a zero class (i.e., those who endorsed no DSM–IV–TR features), a low nonzero class, and a high nonzero class. People in the nonzero classes reported at least one feature of nicotine dependence. The primary characteristic that distinguished membership in those latter two classes was the profile of responses across the seven DSM–IV–TR dependence criteria. Those in the high nonzero class were more likely to endorse difficulty quitting or cutting down and continued smoking despite emotional or physical problems from smoking than those in the low nonzero class. This model also included a single dimensional factor across the two nonzero classes, which Muthén and Asparouhov interpreted as a severity dimension. Because this research was intended primarily to be illustrative of the potential of hybrid latent variable models, several substantive and descriptive features of the study were omitted. Nonetheless, the research supports the possibility that nicotine addiction may have a categorical or taxonic structure.
Another group of statistical procedures, collectively known as taxometric analyses, can be deployed to discover whether there are distinct groups, or taxa, contained within a construct (Meehl, 1973; Meehl & Golden, 1982; Meehl & Yonce, 1994; Waller & Meehl, 1998). These methods have been used to evaluate the psychopathological structures that underlie depression (e.g., Haslam & Beck, 1994), dissociative identity disorder (e.g., Waller, Putnam, & Carlson, 1996), eating disorders (e.g., Williamson et al., 2002), posttraumatic stress disorder (e.g., A. M. Ruscio, Ruscio, & Keane, 2002), and schizotypy (e.g., Korfine & Lenzenweger, 1995). To date, only one study has applied taxometric procedures to investigate addictive disorders. Denson and Earleywine (2006) used taxometric analysis on data from the 2001–2002 National Epidemiological Survey on Alcohol and Related Conditions and concluded that cannabis addiction has a dimensional, not a taxonic, latent structure.
To determine the underlying structure of nicotine addiction and to meet the necessary taxometric requirements, the ideal data set would be collected from a large-scale, population-based sample and contain multiple items related to smoking behavior and addiction. The National Survey of Drug Use and Health (NSDUH) is the only survey that produces ongoing estimates of drug use among noninstitutionalized individuals in the United States (for specific information about sample stratification and weighting, see Office of Applied Studies, 2004). The NSDUH contains several possible indicators of smoking addiction, including characteristics of smoking behavior (e.g., the number of cigarettes smoked each day and latency to smoke first cigarette upon waking) and items from the Nicotine Dependence Syndrome Scale (NDSS, Shiffman, Waters, & Hickcox, 2004). The NDSS is a multidimensional instrument used to assess nicotine addiction. Five subscales (i.e., Drive, Priority, Tolerance, Continuity, and Stereotypy) are theorized to tap multiple dimensions that characterize nicotine addiction. These five subscales of the NDSS retained this factor structure when applied to the 2001 NSDUH sample in adults (Flaherty & Shiffman, 2004b). Additionally, the Drive, Tolerance, and Continuity scales appear to be related to measures of smoking history, latency to first cigarette, and current smoking taken from the 2001 NSDUH, suggesting that the instrument has an adequate level of construct validity (Flaherty & Shiffman, 2004a).
We conducted taxometric analyses on selected indicators from the NSDUH to determine whether the resulting data conformed more clearly to a taxonic (categorical) or a dimensional latent structure. A categorical model of nicotine addiction would be supported if results from several taxometric procedures consistently provided evidence of taxa via graphical plots, converging estimates of base rates, and marked resemblance to plots from simulated taxonic data sets. The proposition that nicotine addiction has a primarily dimensional latent structure would be supported by consistent data plots that did not uncover taxa, high variability of base rate estimates within and across various analyses, and greater similarity to plots created from simulated dimensional data sets.
Method
Participants
These analyses used data from individuals who completed the NSDUH in 2003 and 2002 (Office of Applied Studies, 2003, 2004). Detailed descriptions of the sampling procedures for the NSDUH are described elsewhere (Office of Applied Studies, 2004). A total of 67,784 individuals completed the NSDUH in 2003. The sample size in 2002 was 68,126 individuals. Individuals included in the final taxometric analyses were those who reported smoking at least one cigarette in the past 30 days and who were age 18 or older with no missing data (2003 NSDUH: N = 12,467; 2002 NSDUH: N = 12,224; see Table 1 for demographic characteristics). The decision to exclude smokers under the age of 18 was based on research suggesting that most of these individuals are uptake smokers with unstable smoking patterns (Kandel & Logan, 1984; U.S. Department of Health & Human Services, 1989). Furthermore, Flaherty and Shiffman (2004a) found that the NDSS five-factor model did not fit data collected from adolescents.
Table 1.
Demographic Characteristics of 2003 and 2002 National Survey on Drug Use and Health Samples
| Demographic characteristic | 2003 sample (N = 12,467; n[%]) |
2002 sample (N = 12,224; n[%]) |
|---|---|---|
| Age | ||
| 18–25 | 7,256 (58.2) | 7,106 (58.1) |
| 26–34 | 1,844 (14.8) | 1,746 (14.3) |
| 35 or older | 3,367 (27.0) | 3,372 (27.6) |
| Gender | ||
| Male | 6,430 (51.6) | 6,196 (50.7) |
| Female | 6,037 (48.4) | 6,028 (49.3) |
| Ethnicity | ||
| Non-Hispanic White | 8,976 (72.0) | 9,195 (75.2) |
| Non-Hispanic Black/African American | 1,229 (9.9) | 1,158 (9.5) |
| Non-Hispanic Native American/Alaskan Native | 234 (1.9) | 180 (1.5) |
| Non-Hispanic Native Hawaiian/Other Pacific Islander | 66 (0.5) | 55 (0.4) |
| Non-Hispanic Asian | 241 (1.9) | 227 (1.9) |
| Non-Hispanic more than one race | 296 (2.4) | 228 (1.9) |
| Hispanic | 1,425 (11.4) | 1,181 (9.6) |
Candidate Indicators
Items that measured various aspects of smoking behavior and nicotine addiction on the NSDUH were chosen as candidate indictors. Potential indicators were restricted to measures that used continuous or ordinal scales, as taxometric procedures are not particularly informative when dichotomous variables are used as indicators (J. Ruscio et al., 2006). Smoking behavior variables available for analyses included the number of days the participant smoked in the past 30 days, latency to smoking the first cigarette after waking, the age when an individual began to smoke, and the average number of cigarettes smoked per day during the days an individual smoked.
Average scores of items that loaded on the five factor scales of the NDSS (Drive, Priority, Tolerance, Continuity, and Stereotypy) were also selected as possible indicators. NDSS items were rated on a scale ranging from 1 to 5, representing various levels of intensity or severity. Some items from the NDSS were reverse scored so that higher scores reflected higher levels of addiction. The reliabilities (alpha) of the Drive, Priority, Tolerance, Continuity, and Stereotypy scales of the NDSS as estimated from the 2003 NSDUH were .83, .65, .84, .83, and .40, respectively.
Analytic Procedures
The initial analysis examined whether the proposed indicator variables uncovered evidence of taxonicity by using mean above minus below a cut (MAMBAC; Meehl & Yonce, 1994). We conducted further analyses, maximum eigenvalue (MAXEIG; Waller & Meehl, 1998) and latent modes of factor score density plot (LMODE; Waller & Meehl, 1998), to provide converging lines of evidence regarding the latent structure of nicotine addiction.
To clarify interpretation of the taxometric results, we used J. Ruscio et al.’s (2006) method of creating simulated data sets based on the parameters of the research data. This bootstrap method generates simulated samples of taxonic and dimensional data sets with the same sample size, indicator distributions, and indicator correlations as the research data. These simulated data are then subjected to the same taxometric analyses applied to the research data. Ten sets of simulated dimensional data and 10 sets of simulated taxometric data were created for each analytic procedure. The graphical output from this procedure depicts the average analysis curve across the 10 simulated data sets as well as curves representing the outcome of the analysis on each data set. The comparison curve fit index (CCFI) quantifies the extent to which graphical plots from the research data match simulated plots (see J. Ruscio et al., 2006, for further discussion). The CCFI2 can range from 0 to 1, with values greater than 0.5 suggesting taxonic latent structures and values less than 0.5 providing support for dimensional structures.
Finally, taxometric procedures provided an estimation of base rates. Base rate variability across indicator curves and across the various taxometric procedures was assessed. In general, widely disparate estimates of base rates indicate a dimensional structure, and narrower ranges of consistent values are representative of a taxonic structure. Base rate estimates were only interpreted if the taxometric analyses provided evidence of a taxon within the dataset.
The taxometric procedures were applied in a replication analysis with data from the 2002 NSDUH (using the same inclusion criteria described for the selection of the 2003 sample). Given that the results suggested a taxonic latent structure, we also conducted analyses of associations between theoretically relevant variables (i.e., smoking history, demographic variables, and other drug addiction variables) and taxon group membership using logistic regression procedures.
Results
Indicator Validity
We first assessed the nine candidate indicator variables for their potential utility for taxometric analyses (J. Ruscio et al., 2006; Waller & Meehl, 1998). These analyses were conducted with provisional taxon and nontaxon samples generated using an a priori base rate. Approximately 42% of the 2003 NSDUH sample met the recommended NDSS-derived addiction criterion (Office of Applied Studies, 2004). Therefore, we used an a priori base rate estimate of .40 for the initial validity analyses. (This same a priori base rate was used to generate the simulated taxonic data sets.) Those who were in the top 40% of a total score aggregated across the candidate indicators were assigned to a provisional taxon group, and the remaining cases were assigned to its complement. We calculated average interindicator correlations between the variables selected as potential indicators to assess their suitability for taxometric analysis. In addition, we calculated the average of each indicator score for the provisional taxon and nontaxon samples, and the difference of these scores, expressed as Cohen’s d, was used as an index of the potential taxometric utility of the candidate indicator. Guidelines in the taxometric literature suggest using indicators that have an average interitem correlation of less than .30 within the provisional taxon and nontaxon groups, have a Cohen’s d of at least 1.25, and are not overly skewed (Meehl, 1995; J. Ruscio et al., 2006).
Results of the indicator validity analysis (Table 2) revealed that five of the nine candidate variables met the standards for taxometric analyses: average number of cigarettes smoked per day, latency to first cigarette after waking, and the composite scores for the Tolerance, Drive, and Continuity scales of the NDSS. These variables were used as indicators for all taxometric analyses. The age of an individual’s first cigarette and the NDSS Stereotypy and Priority scales were excluded for not meeting the Cohen’s d criterion; the number of days smoked in the past 30 days was excluded because it was excessively skewed.
Table 2.
Indicator Distribution and Correlation Statistics for 2003 National Survey on Drug Use and Health Using an a Priori Base Rate of .40 to Form Provisional Samples (N = 11,441)
| Average correlation |
||||||
|---|---|---|---|---|---|---|
| Candidate indicators | Cohen’s d | Skew | Kurtosis | Full sample | Nontaxon | Taxon |
| Number of days smoked in the past 30 days | 1.28 | −1.11 | −0.49 | .381 | .236 | −.013 |
| Age of first cigarette | 0.89 | −1.38 | 11.00 | .121 | −.090 | −.111 |
| Latency to first cigarettea | 1.37 | 0.23 | −1.41 | .386 | .224 | .100 |
| Average no. cigarettesa | 1.43 | −0.11 | −0.29 | .422 | .286 | .090 |
| Nicotine Dependence Symptom Scale | ||||||
| Drivea | 1.66 | 0.11 | −0.98 | .453 | .298 | .132 |
| Priority | 0.59 | 1.75 | 3.15 | .215 | .096 | .093 |
| Continuitya | 1.55 | 0.09 | −1.12 | .425 | .277 | .093 |
| Stereotypy | 0.40 | 0.29 | −0.43 | .158 | .120 | −.025 |
| Tolerancea | 1.31 | 0.63 | −0.70 | .391 | .249 | .102 |
Note. Because these analyses required complete data sets, participants with any missing data were deleted, and the sample size was attenuated here relative to the sample used in the taxometric analyses. Average correlation = mean interindicator correlation for the full sample and the provisional nontaxon and taxon samples.
Indicators used in taxometric analyses.
MAMBAC
In MAMBAC analysis, one indicator served as an input variable, and another served as the output variable. The mean of the output variable below a sliding cut on the input variable was subtracted from the mean above the cut for each pair, resulting in a mean difference score. In our analysis, 500 evenly spaced sliding cuts were made, beginning 250 cases from either extreme. Each indicator served as both an input and an output variable in every possible pairwise combination. The MAMBAC function was then graphed for each of the 20 pairwise combinations. Data supporting a taxonic latent structure would produce a plot that appeared peaked or humped. If the data were better represented by a dimensional structure, the plot would appear disk shaped or concave.
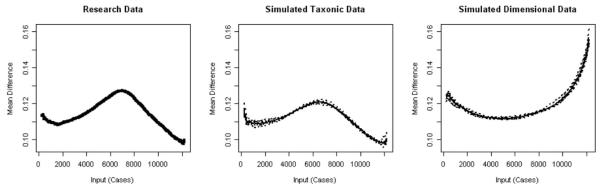
Across the 20 MAMBAC plots, each indicator produced an inverted-U–shaped peak consistent with a taxonic latent structure. None of the individual MAMBAC graphs appeared to be predominantly concave when inspected visually. The MAMBAC curves were averaged, and the average MAMBAC curve was compared with the curves from simulated dimensional and simulated taxonic data (Figure 1). The averaged MAMBAC curve appeared to be more similar to the simulated peaked taxonic plot than the simulated dimensional plot, which had a concave shape. The CCFI was .827, indicating that the research data were strongly supportive of a taxonic structure. The estimated taxon base rates for the individual MAMBAC curves ranged from .481 to .591 (M = .529, SD = .045). The relatively narrow range of the estimated base rate estimates provided further support for a taxonic latent structure for nicotine addiction. (See Table 3 for base rate and CCFI estimates for each taxometric procedure.)
Figure 1.
2003 National Survey on Drug Use and Health averaged mean above minus below a cut plot for research data (left panel) and simulated data (middle and right panels).
Table 3.
Estimated Base Rates and Comparison Curve Fit Index of Taxometric Analyses on the National Survey on Drug Use and Health
| Estimate | 2003 sample (N = 12,467) |
2002 sample (N = 12,224) |
|---|---|---|
| Base rate | ||
| MAMBAC | 0.529 | 0.548 |
| MAXEIG | 0.477 | 0.483 |
| LMODE | 0.467 | 0.461 |
| Comparison curve fit index | ||
| MAMBAC | 0.827 | 0.820 |
| MAXEIG | 0.948 | 0.921 |
Note. MAMBAC = mean above minus below a cut; MAXEIG = maximum eigenvalue; LMODE = latent mode.
MAXEIG
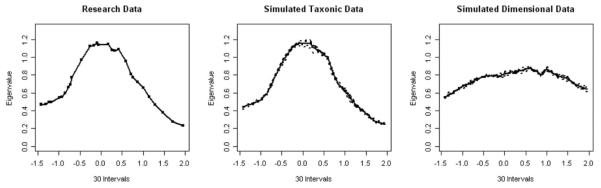
MAXEIG is a multivariate taxometric procedure in which one of the indicator variables is sorted in ascending order, and the remaining variables are used as output variables. The first eigenvalue of the covariance matrix of output variables is plotted within successive intervals. In this analysis, 30 equal-sized intervals (416 cases per interval) were used. A separate graph was produced for each input variable; plots consisted of the smoothed eigenvalues on the y-axis and the midpoints of the intervals of each input variable on the x-axis. Similar to MAMBAC, evidence of taxonicity in the plot of eigenvalues produces a peak, whereas a relatively flat plot suggests dimensionality.
Visual inspection of each of the five MAXEIG graphs revealed peaks rather than flat plots, which was consistent with a taxonic interpretation. Furthermore, the averaged MAXEIG appeared very similar to the graph of the simulated taxonic data, as these graphs were peaked, whereas the graph for the simulated dimensional data appeared relatively flat (Figure 2). The CCFI was .948, supplying more support for taxonicity in the data. The estimated base rates for the individual MAXEIG curves ranged from .453 to .495 (M = .477, SD = .016); the relatively low variation in base rate estimates provided additional support for a taxonic interpretation of the indicator data.
Figure 2.
2003 National Survey on Drug Use and Health averaged maximum eigenvalue plot for research data (left panel) and simulated data (middle and right panels).
LMODE
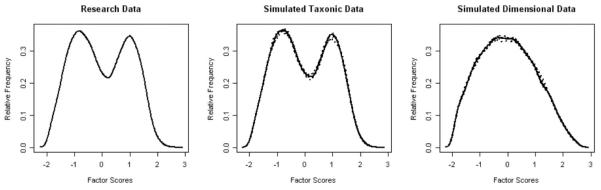
In LMODE, the indicators were factor analyzed, and a distribution of estimated true factor scores on the first principal factor was plotted. Factor score density plots from taxonic data would be bimodal, that is, would have two humps, whereas factor score density plots from dimensional data would be unimodal, with one hump. Although LMODE procedures provide visual evidence and an estimate of base rates, they do not allow for a calculation of CCFI.
The graph of the LMODE analyses revealed a distinct bimodal distribution, suggesting taxonicity (Figure 3). Furthermore, the LMODE graph of the actual NSDUH data closely resembled the graph of the simulated taxonic data. The estimated base rate from the LMODE analysis was .467, which was similar to the estimates derived from the MAMBAC and MAXEIG analyses.
Figure 3.
2003 National Survey on Drug Use and Health latent mode plot for research data (panel1) and simulated data (panels 2 and 3).
Taxometric Analyses Using 2002 NSDUH Data
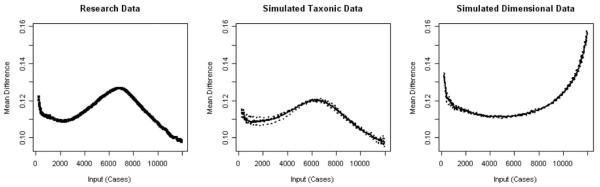
The taxometric procedures were applied to data from the 2002 NSDUH in an attempt to replicate the profile of effects found in the 2003 NSDUH sample. The taxometric procedures, which were conducted using the same indicators as in the examination of the 2003 NSDUH data,3 provided clear evidence of a taxonic structure underlying nicotine addiction (Figures 4, 5 and 6; Table 3). Overall, the individual MAMBAC plots were peaked. The averaged MAMBAC curve appeared more similar to the graph of the simulated taxonic data than to the graph of the simulated dimensional data. The MAMBAC CCFI of .82 was similar to the results from the 2003 NSDUH sample. Base rate estimates did not vary widely (M = .548, SD =.075, range = .476 –.673). Furthermore, the individual MAXEIG curves were consistently peaked in the 2002 NSDUH sample, and the average MAXEIG graph resulted in a peaked shape that was highly similar to the graph generated from the simulated taxonic data (CCFI = .921). Estimated base rates from the MAXEIG sample continued to vary little, ranging from .451 to .499 (M = .483, SD =.019). Results from LMODE analyses resulted in a bimodal distribution, which matched the bimodal distribution generated from the simulated taxonic data. The estimated base rate was .455; this estimate was similar to those from the other taxonic procedures using the 2002 and 2003 NSDUH samples.4
Figure 4.
2002 National Survey on Drug Use and Health averaged mean above minus below a cut plot for research data (left panel) and simulated data (middle and right panels).
Figure 5.
2002 National Survey on Drug Use and Health averaged maximum eigenvalue plot for research data (left panel) and simulated data (middle and right panels).
Figure 6.
2002 National Survey on Drug Use and Health latent mode plot for research data (left panel) and simulated data (middle and right panels).
Characteristics of Group Membership on Taxometric Indicators
The base rate generated from the taxometric analysis was applied to the 2003 NSDUH data to describe characteristics of the typical taxon and nontaxon member for the taxometric indicators. J. Ruscio et al. (2006) outlined a technique to estimate the optimum cutting score for MAXEIG in which the indicator values are summed for each case and then sorted in descending order by the total indicator sum.5 The estimated base rate (in this case, .477) was used as a cut point. Cases above this cut were assigned to the taxon group, and cases below this cut were assigned to the nontaxon group. Using this method, the cut point in our analysis was 14.33. The estimated base rate of .477 from the MAXEIG procedures was selected because it was the intermediary between the higher estimate produced by MAMBAC (0.529) and the lower estimate produced by LMODE (0.467). Table 4 shows the average indicator scores for the members of the taxon and nontaxon groups.
Table 4.
Mean Indicator Values for Taxon and Nontaxon Members Using the 2003 National Survey on Drug Use and Health
| M (SD) |
||
|---|---|---|
| Indicator | Taxon (N = 5,947) | Nontaxon (N = 6,520) |
| Latency to first cigarette: On the days that you smoke, how soon after you wake up do you have your first cigarette? (with point values) |
3.08 (0.83) | 1.51 (0.83) |
| Within the first 5 minutes (4) | ||
| Between 6 and 30 minutes (3) | ||
| Between 31 and 60 minutes (2) | ||
| More than 60 minutes (1) | ||
| Average no. cigarettes: On the days you smoked cigarettes during the past 30 days, how many cigarettes did you smoke per day, on average? (with point values) |
4.72 (0.96) | 2.88 (1.07 |
| 1 cigarette per day (2) | ||
| 2 to 5 cigarettes per day (3) | ||
| 6 to 15 cigarettes per day (about one-half pack) (4) | ||
| 16 to 25 cigarettes per day (about one pack) (5) | ||
| 26 to 35 cigarettes per day (about one-and-a-half packs) (6) | ||
| More than 35 cigarettes per day (about two packs or more) (7) | ||
| NDSS drivea | 3.61 (0.81) | 2.03 (0.79) |
|
||
| NDSS continuitya | 3.68 (0.87) | 1.94 (0.9) |
|
||
| NDSS tolerancea | 3.06 (1.11) | 1.60 (0.75) |
|
||
Note. NDSS = Nicotine Dependence Symptom Scale.
NDSS item response options and point values: not at all true (1), somewhat true (2), moderately true (3), very true (4), and extremely true (5).
Correlates of Taxon Membership
The association between taxon membership and several variables including smoking history, drug addiction, health, and selected demographic characteristics was examined (see Table 5). We used logistic regression to evaluate the prediction of taxon group membership with the listed variables as predictors. The overall model was highly significant: Wald χ2(11) = 2,447.10, Cox and Snell R2 = .305, p < 0001. Examination of the odds ratios from the multivariate logistic regression showed that 7 of the 11 variables made significant independent contributions to the prediction of membership in the taxon group. Relative to the nontaxon group, members of the nicotine addiction taxon smoked their first cigarette at a younger age, reported smoking more days out of the past 30 days, and were more likely to purchase their cigarettes by the carton than by the pack. The taxon and nontaxon members did not differ significantly in terms of rates of alcohol abuse or dependence or of rates of marijuana abuse or dependence. Those in the taxon group, however, were more likely to abuse or be dependent on illicit drugs (excluding marijuana). Moreover, taxon members reported significantly more symptoms of psychological distress than nontaxon members in the previous year and rated their overall health as poorer. Finally, the education level of taxon members was lower than that of nontaxon members.
Table 5.
Results of Logistic Regression of Taxon and Nontaxon Members in the 2003 National Survey on Drug Use and Health
| Variable | Taxon M (SD) | Nontaxon M (SD) | Odds ratio | 95% confidence interval |
|---|---|---|---|---|
| Age at first cigarette | 14.12 (3.49) | 15.26 (3.76) | 0.945** | 0.934–0.957 |
| No. days smoked cigarettes in the past 30 days | 28.47 (5.53) | 17.69 (11.66) | 1.132** | 1.125–1.139 |
| Buy cigarettes by pack (1) or carton (2) | 1.31 (0.46) | 1.08 (0.26) | 3.725** | 3.302–4.202 |
| Perceived risk of smoking one or more packs of cigarettes per day on a scale ranging from 1 (no risk) to 4 (great risk) |
3.35 (0.74) | 3.47 (0.72) | 0.960 | 0.905–1.081 |
| Alcohol abuse or dependence (past year; 1 = yes) | 0.209 (0.41) | 0.219 (0.42) | 1.009 | 0.904–1.125 |
| Marijuana abuse or dependence (past year; 1 = yes) | 0.081 (0.27) | 0.066 (0.25) | 1.165 | 0.981–1.384 |
| Illicit drug abuse or dependence (excluding marijuana, past year; 1 = yes) |
0.061 (0.24) | 0.042 (0.18) | 1.438* | 1.154–1.793 |
| Level of selected symptoms of emotional distress (past year) on a scale of 0 to 24 |
7.01 (6.23) | 5.70 (5.57) | 1.040** | 1.032–1.048 |
| Overall health on a scale ranging from 1 (excellent) to 5 (poor) |
2.48 (0.95) | 2.18 (0.91) | 1.177** | 1.122–1.235 |
| Education level on a scale ranging from 1 (fifth grade or less) to 11 (college senior/16th year or higher) |
7.94 (1.69) | 8.43 (1.88) | 0.887** | 0.864–0.910 |
| Income level on a scale from 1 (less than $20,000) to 4 ($75,000 or more) |
2.07 (0.95) | 2.12 (1.03) | 0.974 | 0.931–1.019 |
Note. Alcohol, marijuana, and illicit drug abuse or dependence assessed as meeting Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; American Psychological Association, 2000) abuse or dependence criteria for the respective drug category. Level of selected symptoms of emotional distress was calculated by assigning a value of 0 to 4 to each reported response regarding the frequency of six symptoms of distress: Nervous, feeling hopeless, restless or fidgety, so sad or depressed that nothing could cheer you up, everything was an effort, and feeling no good or worthless.
p < .01.
p < .0001.
We also conducted a series of bivariate regressions to compare the validity of taxon membership relative to the summed indicator variable as predictors for each of the 11 variables listed in Table 6 as dependent variables. In the case of dichotomous dependent variables, we used logistic regression and calculated generalized —all other analyses used ordinary least squares regression with conventional . With the exception of one set of analyses (alcohol abuse and dependence), both the indicator sum and the taxon variables were significant predictors of the dependent variables (all ps < .01; see Table 6). Of note, additional models evaluating the predictive validity of the residual of the indicator sum variable (with taxon membership covaried out of the continuous variable; analyses not shown) revealed that the residual was also significantly associated with each of the dependent variables (with the exception of marijuana abuse or dependence). We also calculated a relative R2 for each dependent variable as the ratio of the R2 for the taxon variable to the R2 for the indicator sum variable. In all analyses but one (income), the variance attributed to taxon membership accounted for a majority of the predictive variance in the indicator sum variable, with an average of 69% across the 10 analyses showing significant regression effects.
Table 6.
Regression Analyses Comparing Taxon With Sum of Indicators as Predictor Variables
| Predictor R2 |
|||
|---|---|---|---|
| Dependent variable | Group | Indicator sum | Relative R2 |
| Age at first cigarette | .0241 | .0312 | .77 |
| Past 30 days’ smoking | .2483 | .4141 | .60 |
| Pack or cartona | .0909 | .1204 | .75 |
| Risk of smoking | .0068 | .0100 | .68 |
| Alcohol abuse/dependencea | .0001 | .0000 | — |
| Marijuana abuse/dependencea | .0007 | .0007 | 1.0 |
| Illicit drug abuse/dependencea | .0031 | .0048 | .65 |
| Emotional distress symptoms | .0128 | .0185 | .69 |
| Overall health | .0237 | .0363 | .65 |
| Education level | .0186 | .0278 | .67 |
| Income level | .0007 | .0017 | .41 |
Note. Group = taxon or nontaxon; relative R2 = ratio of group R2 to indicator sum R2.
Logistic regression (generalized R2).
Finally, examination of the means for days smoked out of the past 30 suggested that taxon membership might be strongly associated with daily smoking. To explore this relationship further, we converted the past 30 days smoking variable into a dichotomous measure on the basis of whether participants reported smoking on each of the past 30 days. An analysis of the two-way contingency table between taxon membership and daily smoking for the 2003 NSDUH data (Table 7) revealed that daily smoking showed considerable sensitivity for assignment to the taxon group (sensitivity = .891) but substantially less specificity (.638), with a large number of daily smokers in the nontaxon group. These results indicated that daily smoking might be a necessary but not a sufficient condition for taxon membership.
Table 7.
Relationship Between Taxon Membership and Daily Smoking in the 2003 National Survey on Drug Use and Health
| Past 30 days smoking | Taxon | Nontaxon |
|---|---|---|
| Daily | 5,300 | 2,360 |
| Nondaily | 647 | 4,160 |
Note. Daily = people who reported smoking on each of the past 30 days
Discussion
This study is the first to investigate the latent structure of nicotine addiction using taxometric analyses. Multiple lines of evidence from three statistically distinct procedures (MAMBAC, MAXEIG, and LMODE) applied to large, nationally representative samples of adult smokers showed that the selected indicators had a marked taxonic structure. Moreover, evidence for this addiction taxon was found across datasets from different years. None of the analyses provided support for a purely dimensional structure. Overall, the results were consistent with the proposition that there is a qualitative difference between addicted and nonaddicted smokers. The analyses showed that relative to nonaddicted smokers, members of the addiction taxon could be distinguished as people who consumed a high number of cigarettes per day and had strong craving to smoke, high tolerance to nicotine, relatively unvarying smoking patterns, and shorter latencies to smoke their first cigarette on waking.
Characterization of the Nicotine Addiction Taxon
The five indicators providing evidence of an addiction taxon included items related to NDSS Drive, Continuity, and Tolerance and latency to first cigarette on waking and average number of cigarettes smoked per day. According to Shiffman et al. (2004), items on the Drive scale relate to craving to smoke, a loss of control over smoking, and the experience of negative affect when abstinent from cigarettes. Although craving is not listed in DSM–IV–TR as a critical component of drug dependence, findings from several lines of research have consistently suggested that craving is a central component of nicotine withdrawal (Colby et al., 2004). Our results support the proposal that craving may be a core feature of nicotine addiction (Tiffany et al., in press).
The items contributing to the Continuity factor represent smoking patterns that are uniform within and across days. Like craving, an uninterrupted, stable pattern of smoking is not included in DSM–IV–TR nicotine dependence criteria. The item related to latency to smoke was originally derived from the Fagerström Tolerance Questionnaire (Fagerström, 1978). Subsequent research has found that two of the Fagerström Tolerance Questionnaire items, latency to first cigarette and number of cigarettes per day, are the variables most consistently related to other measures of smoking behavior (Lichtenstein & Mermelstein, 1986). Recently, Baker et al. (2007) reported that latency to the first cigarette of the day was the single best predictor of relapse among people attempting to quit smoking. These findings complement the present results, which indicate that latency to smoke and the number of cigarettes consumed per day are important behavioral attributes of a nicotine addiction taxon.
The regression procedures identified additional features that significantly differentiated the addicted from the nonaddicted smoker. Smokers in the addiction taxon reported smoking nearly every day of the previous month, whereas those in the nonaddicted group smoked cigarettes on a little more than half of the preceding 30 days. Smokers in the taxon group were more likely to purchase cigarettes by the carton and more likely to begin smoking at a younger age—on average, smokers in the taxon group had their first cigarette more than a year earlier than smokers in the nonaddicted group. Although the two groups did not differ in their rates of alcohol or marijuana abuse or dependence, those in the addiction taxon had higher rates of illicit drug abuse or dependence, reported more symptoms of psychological distress, described themselves as being in poorer health, and had a lower level of education than those in the nonaddicted group. Many of these characteristics reflect features previously reported in the literature as distinguishing heavy smokers from low-level smokers (e.g., deBry & Tiffany, 2008; Gilpin, Cavin, & Pierce, 1997; Presson, Chassin, & Sherman, 2002; Pomerleau, Collins, Shiffman, and Pomerleau, 1993; Shiffman & Paty, 2006).
Theoretical and Heuristic Implications
These taxometric results do not support the assumption implicit in several addiction theories that smokers, regardless of their level of cigarette use, only differ in terms of their severity of nicotine addiction. The results are consistent with models of drug addiction that presume that high-rate, daily smokers display a profile of cigarette smoking and related behavior that is categorically distinct from the smoking behaviors of low-level smokers. As noted in the introduction, withdrawal-based models that assume that the withdrawal syndrome emerges only after sustained, continuous exposure to an abused drug would be most compatible with the proposal that nicotine addiction represents a qualitatively discrete form of smoking behavior. Certain aspects of the findings were clearly congruent with this conceptualization of addictive smoking. First, the prototypical smoker in the addiction taxon tended to smoke a relatively large number of cigarettes on a daily basis. This pattern of uninterrupted smoking would be most likely to support the emergence of a pronounced withdrawal syndrome on cessation of smoking. Second, although none of the indicators uniquely and directly indexed the withdrawal syndrome, the NDSS Drive subscale contains one item that would qualify as a principal feature of nicotine withdrawal, namely, “After not smoking for a while, you need to smoke in order to feel less restless and irritable.” Indeed, Baker et al. (2004) have proposed that negative affectivity following suspension of drug use is the defining feature of drug withdrawal. Third, the NDSS Tolerance subscale also contributed to the identification of the taxon. Tolerance, a reduction in drug effect after repeated exposure to the drug, has been portrayed by some researchers as a manifestation of the same adaptive process that gives rise to the withdrawal syndrome in the abstinent state (e.g., Siegel, Baptista, Kim, McDonald, & Weise-Kelly, 2000).
The apparent qualitative distinction between addicted and non-addicted smokers raises questions about the development of nicotine addiction. Most contemporary models propose that the emergence of nicotine addiction is a dynamic process that unfolds continuously over time and situations (Tiffany et al., 2004). Moreover, some researchers (e.g., DiFranza & Wellman, 2005) have hypothesized that individual symptoms of nicotine addiction readily develop over initial, intermittent exposures to tobacco and that addictive smoking, characterized by loss of autonomy over smoking, emerges very early during the course of smoking history. That proposal stands in contrast to the present findings, which suggest that the threshold for nicotine addiction is not reached until a person is smoking at relatively high rates on a daily basis. Issues regarding transitions from nonaddicted to addicted smoking may be best addressed by focusing on the trajectories of taxonic indicators in longitudinal studies that begin tracking people relatively early in the course of their smoking history.
It may also be instructive to study smokers whose data fall near the boundary between the addicted and nonaddicted groups—for example, individuals who smoke at high rates but are not in the taxon because of their low scores on other indicators. These investigations would allow us to determine the “sharpness” of the demarcation line between addicted and nonaddicted smokers (Lenzenweger, 2004) and point to fundamental psychological and biological processes responsible for the development of nicotine addiction. Studies of the genetics of smoking may also be informed by the findings of the present research. The heritability of nicotine addiction, variously defined, is at least 50% (see Batra, Patkar, Berrettini, Weinstein, & Leone, 2003, for a review). Most studies on the genetics of smoking have operationalized addiction phenotypes through arbitrary cutoffs on diagnostic criteria or on scales putatively indexing addiction. The taxon identified in this research represents an excellent starting point for a more refined, empirically validated phenotyping of the nicotine addiction construct.
Although the present results appear most consistent with withdrawal-based models of addiction, it is important to note that the indicators available for analyses were not distinctively representative of key facets of withdrawal-based models. Moreover, even if withdrawal is a defining feature of the proposed nicotine addiction taxon, there is nothing in this research that allows us to discern whether withdrawal-based processes are causes or consequences of addictive smoking. Moreover, critical variables suggested by other models of addiction, such as essential manifestations of positive reinforcement processes, were not available as indicators. Thus, this study is not dispositive with regard to any specific model of addiction. Nonetheless, these results, if replicated and validated, indicate that any comprehensive theory of nicotine addiction might have to account for the apparent categorical aspects of the latent structure of smoking-related behavior.
Considerations for the Diagnosis of Nicotine Addiction
The taxonic conceptualization is superficially consistent with DSM–IV–TR and International Statistical Classification of Diseases and Related Health Problems (World Health Organization, 1992) categorical models of substance dependence. But the DSM–IV–TR approach to nicotine dependence does not map fully on to the findings generated by the present study. Although the taxometric indicators and DSM–IV–TR dependence criteria do share some features (i.e., high levels of smoking and tolerance), they do not completely overlap. Furthermore, although the base rate of the nicotine addiction taxon identified in this study (nearly .50) is remarkably close to the incidence of nicotine dependence among current adult smokers reported in some studies (e.g., 51%; Grant et al., 2004), these estimates are derived from somewhat dissimilar approaches. The taxometric analyses use empirically generated cut points along the indicator distributions of symptom severity to distinguish between addicted and nonaddicted smokers. DSM–IV–TR uses a clinically defined cut-off on a total symptom count to establish the boundaries of these two groups.
This investigation focused on the latent structure of nicotine addiction and excluded other types of substance use disorders, whereas DSM–IV–TR uses the same criteria for all drug-dependence disorders. It is uncertain whether our findings reflect a taxon specific to nicotine, or whether nicotine addiction shares a common latent structure with all addictive disorders or represents a more general vulnerability for externalizing disorders (Hicks et al., 2007; Krueger, Markon, Patrick, & Iacono, 2005). Given that rates of alcohol and marijuana use disorders did not significantly discriminate between smokers in and out of the nicotine addiction taxon, the taxon identified here may be somewhat independent of these drug abuse disorders. This possibility must be qualified in light of the fact that the drug use predictor variables lumped abuse and dependence into a single measure and the finding that a variable representing illicit drug abuse or dependence did discriminate the two smoking groups. Currently, only one published study has used taxometric methods to investigate drugs other than nicotine, and results supported a dimensional structure underlying cannabis addiction (Denson & Earleywine, 2006). More studies are needed to evaluate this finding, as that investigation had several limitations. More important, the study only used dichotomous indicators (i.e., either endorsing or not endorsing each symptom of DSM–IV–TR cannabis dependence), which are not well suited for taxometric analyses. Future investigations should reexamine marijuana addiction using continuous indicators. Additional taxometric studies should also be conducted to explore the latent structures underlying other major drug addictions including alcohol, opioid, and cocaine addiction.
Clinical Implications
Ideally, the manifest system adopted for the description and diagnosis of nicotine addiction would match the latent structure of the addiction construct. The present results support the feasibility of diagnosing nicotine addiction via an empirically derived boundary distinguishing addicted from nonaddicted smokers. Beyond its diagnostic function, we would expect that this taxometrically derived classification would have prognostic and treatment utility. With regard to prognosis, smokers meeting criteria for the addiction taxon would most likely have considerably greater difficulty quitting smoking and have a substantially greater probability of relapse than smokers not in the taxon group. Moreover, smokers’ sensitivity to selected treatments may vary systematically as a function of whether they are in the addiction taxon. For example, treatments specifically targeting nicotine withdrawal, such as nicotine replacement therapies, may be effective only for smokers in the nicotine addiction taxon.
Limitations
Several variables available for analyses did not meet our selection criteria for inclusion as indicators (i.e., number of days smoked in the past 30 days, age at first cigarette, NDSS Priority, and NDSS Stereotypy). The extent to which these constructs are meaningful with regard to understanding the latent structure of smoking addiction remains an open question. Some of the variables used to represent these constructs on the NSDUH were psychometrically inadequate. For example, both the NDSS Priority and the NDSS Stereotypy scales had very low levels of reliability. Even for indicators found to be taxometrically useful, their sensitivity could be enhanced. For instance, given that average number of cigarettes consumed per day appeared to be one of the defining attributes of an addiction taxon, this measure could be improved with a more precise estimate of daily nicotine exposure as opposed to the relatively coarse assessment used in the NSDUH.
A number of potentially valid indicators of nicotine addiction, such as nicotine withdrawal, are not captured well by the NDSS scales, and it will be useful in future studies to collect data from self-report instruments beyond those analyzed in the present research. Preferably, candidate indicators would be collected across a wide range of behavioral and biological domains so as to diminish the reliance on self-report, which may be biased or incomplete. This may allow the discovery of other features that distinguish the taxon group from the nontaxon group beyond the data gathered through the NDSS and other self-report items on the NSDUH.
The taxometric analyses were designed to determine whether the data were best represented by a one-group (dimensional) or a two-group (taxonic) latent structure (J. Ruscio et al., 2006). The identification of a single taxonic boundary does not mean that the latent structure of nicotine addiction could not be differently parsed into subgroups and/or multiple dimensions on the basis of additional analyses on these and other indicators. For instance, Muthén and Asparouhov (2006) described a hybrid multiclass model of nicotine addiction derived from DSM–IV–TR criteria with two classes of smokers distinguished primarily on the basis of two diagnostic features: attempts to cut down or quit smoking and continued smoking despite emotional and physical problems from cigarette use. It would be useful to apply those modeling procedures to the data used in the present research to determine the extent to which the latent structure identified by a hybrid multiclass model maps onto the results generated through taxometric procedures.
The taxonic solution supported by these analyses also does not preclude the possibility that there are levels of severity of nicotine addiction within the taxon group or meaningful dimensional variation within the nontaxon group (see Waller & Meehl, 1998, and J. Ruscio et al., 2006, for discussions of the potential importance of dimensional variation within taxon and/or nontaxon groups). For example, Shiffman and Sayette (2005) found that factor scales from the NDSS clearly discriminated heavy smokers from low-level smokers and that even among low-level smokers, selected NDSS scales were significantly predictive of other smoking-related variables. Our finding that variance attributable to taxon membership did not account completely for the predictive validity of the continuous indicator sum variable is entirely consistent with the view that the latent structure of the indicator variables may have both categorical and dimensional features. Furthermore, the death, disease, and disability caused by cigarette smoking are determined by the net exposure to cigarette smoke over time (Burns, Major, Shanks, Thun, & Samet, 2001). Consequently, for any smoker a dimensional variable, the level of sustained cigarette exposure, has critical health implications irrespective of that person’s standing with regard to nicotine addiction. Nonetheless, the strong evidence of a taxon in this research suggests that there is a natural, latent category in smoking-related behaviors. A fuller understanding of the processes responsible for the formation of that category should lead to more clinically useful assessments of nicotine addiction and more effective treatments for cigarette smokers.
Acknowledgments
This research was supported, in part, by National Institutes of Health Grant R01CA120412 to Stephen T. Tiffany.
Footnotes
Following the recommendation of Baker et al. (2004) and O’Brien, Volkow, and Li (2006), we use the term addiction rather than dependence in this article. We reserve the latter term to refer to the condition whereby, following chronic exposure to a drug, a withdrawal syndrome emerges when drug levels in the organism decline. We also use dependence when talking specifically about Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev., or DSM–IV–TR; American Psychiatric Association, 2000) dependence criteria and diagnoses.
The CCFI is derived by first calculating the root-mean-square residual of the y values on the values of the averaged plots from the research data and the simulated dimensional and taxonic plots: where yres.data is a data point on the averaged plot of the research data, ysim.data is the corresponding data point on the averaged taxonic or dimensional data plot, and N is the number of data points on each curve. The two fit values, FitRMSR-dim and FitRSMR-tax are integrated into one index (CCFI) by the following equation: CCFI = FitRSMR-dim/(FitRSMR-dim + FitRMSR-tax).
Preliminary validity analyses conducted on the nine candidate indicators with the 2002 NSDUH data produced a profile of effects identical to that identified with the 2003 NSDUH data.
Taxometric analyses conducted separately by gender for the 2003 and 2002 NSDUH data produced patterns of results and taxometric indices in women and men nearly identical to those observed in the full samples.
The indicator sum was calculated by adding the point values of the 12 items making up the five taxonic indicators. See Table 4 for point values for each item.
Contributor Information
Katherine C. Goedeker, Department of Psychological Sciences, Purdue University
Stephen T. Tiffany, Department of Psychology, University at Buffalo, State University of New York.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 2000. text rev. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim S-Y, et al. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine & Tobacco Research. 2007;9(Suppl. 4):S555–S570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Batra VB, Patkar AA, Berrettini WH, Weinstein SP, Leone FT. The genetic determinants of smoking. Chest. 2003;123:1730–1739. doi: 10.1378/chest.123.5.1730. [DOI] [PubMed] [Google Scholar]
- Bouton M. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2000;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Brandon T, Herzog T, Irvin J, Gwaltney C. Cognitive and social learning models of drug dependence: Implications for the assessment of tobacco dependence in adolescents. Addiction. 2004;99(Suppl. 1):51–77. doi: 10.1111/j.1360-0443.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Hatsukami D, Hanson K, Shiffman S. Smoking topography in tobacco chippers and dependent smokers. Addictive Behaviors. 1996;21:233–238. doi: 10.1016/0306-4603(95)00054-2. [DOI] [PubMed] [Google Scholar]
- Burns DM, Major JM, Shanks TG, Thun MJ, Samet JM. Shopland D, Burns D, Benowitz N, Amacher R, editors. Smoking lower yield cigarettes and disease risks. U.S. Department of Health & Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. 2001:65–158. National Cancer Institute Smoking and Tobacco Control Monograph No. 13, NIH Pub. No. 02–5074.
- Colby SM, Rohsenow DJ, Monti PM, Gwaltney CJ, Gulliver SB, Abrams DB, et al. Effects of tobacco deprivation on alcohol cue reactivity and drinking among young adults. Addictive Behaviors. 2004;29:879–892. doi: 10.1016/j.addbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Davies GM, Willner P, Morgan MJ. Smoking-related cues elicit craving in tobacco “chippers”: A replication and validation of the two-factor structure of the Questionnaire of Smoking Urges. Psychop-harmacology. 2000;152:334–342. doi: 10.1007/s002130000526. [DOI] [PubMed] [Google Scholar]
- deBry SC, Tiffany ST. Tobacco induced neurotoxicity of adolescent cognitive development (TINACD): A proposed model for the development of impulsivity in nicotine dependence. Nicotine & Tobacco Research. 2008;10:11–25. doi: 10.1080/14622200701767811. [DOI] [PubMed] [Google Scholar]
- Denson T, Earleywine M. Pothead or pot smoker? A taxometric investigation of cannabis dependence. Substance Abuse Treatment, Prevention, and Policy. 2006;1:22–32. doi: 10.1186/1747-597X-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: Integrating the clinical and basic science literature. Nicotine & Tobacco Research. 2005;7:9–26. doi: 10.1080/14622200412331328538. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: The contribution of negative reinforcement models. Addiction. 2004;99(Suppl. 1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Measuring the degree of physical dependency to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Flaherty BP, Shiffman S. Correlates of the Nicotine Dependence Syndrome Scale in a representative U.S. sample. Presented at the annual meeting of the Society for Research on Nicotine and Tobacco in Scottsdale, AZ.Feb, 2004a. [Google Scholar]
- Flaherty BP, Shiffman S. Factor structure of the Nicotine Dependence Syndrome Scale in the 2001 National Household Survey of Drug Abuse. Presented at the annual meeting of the Society for Research on Nicotine and Tobacco in Scottsdale, AZ.Feb, 2004b. [Google Scholar]
- Gilpin E, Cavin SW, Pierce JP. Adult smokers who do not smoke daily. Addiction. 1997;92:473–480. [PubMed] [Google Scholar]
- Glautier S. Measures and models of nicotine dependence: Positive reinforcement. Addiction. 2004;99(Suppl. 1):30–50. doi: 10.1111/j.1360-0443.2004.00736.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Grant BF, Moore TC, Kaplan K. Source and Accuracy Statement: Wave 1 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) National Institute on Alcohol Abuse & Alcoholism; Bethesda, MD: 2003. [Google Scholar]
- Haslam N, Beck A. Subtyping major depression: A taxometric analysis. Journal of Abnormal Psychology. 1994;103:686–692. doi: 10.1037//0021-843x.103.4.686. [DOI] [PubMed] [Google Scholar]
- Helzer JE, van den Brink W, Guth SE. Should there be both categorical and dimensional criteria for the substance use disorders in DSM-V? Addiction. 2006;101(Suppl. 1):17–22. doi: 10.1111/j.1360-0443.2006.01587.x. [DOI] [PubMed] [Google Scholar]
- Hennrikus DJ, Jeffery RW, Lando HA. The smoking cessation process: Longitudinal observations in a working population. Preventive Medicine. 1995;24:235–244. doi: 10.1006/pmed.1995.1039. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Bernat D, Malon SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal: A replication and extension. Archives of General Psychiatry. 1991;48:52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Helzer JE, Lindberg SA. Prevalence of DSM-ICD-defined nicotine dependence. Drug and Alcohol Dependence. 2006;85:91–102. doi: 10.1016/j.drugalcdep.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Logan JA. Patterns of drug use from adolescence to young adulthood: I. Periods of risk for initiation, continued use, and discontinuation. American Journal of Public Health. 1984;74:660–666. doi: 10.2105/ajph.74.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997 October 3;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Korfine L, Lenzenweger MF. The taxonicity of schizotypy: A replication. Journal of Abnormal Psychology. 1995;104:26–31. doi: 10.1037//0021-843x.104.1.26. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM–V. Journal of Abnormal Psychology. 2005;114:537–550. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzenweger M. Consideration of the challenges, complications, and pitfalls of taxometric analysis. Journal of Abnormal Psychology. 2004;113:10–23. doi: 10.1037/0021-843X.113.1.10. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Mermelstein RJ. Some methodological cautions in the use of the tolerance questionnaire. Addictive Behaviors. 1986;11:439–442. doi: 10.1016/0306-4603(86)90024-9. [DOI] [PubMed] [Google Scholar]
- McCarthy WJ, Zhou Y, Hser Y. Individual change amid stable smoking patterns in polydrug users over three years. Addictive Behaviors. 2001;26:143–149. doi: 10.1016/s0306-4603(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Meehl P. MAXCOV-HITMAX: A taxometric search method for loose genetic syndromes. In: Meehl P, editor. Psychodiagnosis: Selected papers. University of Minnesota Press; Minneapolis: 1973. pp. 200–224. [Google Scholar]
- Meehl P. Bootstrap taxometrics: Solving the classification problem in psychopathology. American Psychologist. 1995;50:266–275. doi: 10.1037//0003-066x.50.4.266. [DOI] [PubMed] [Google Scholar]
- Meehl P, Golden R. Taxometric methods. In: Kendall P, Butcher J, editors. Handbook of research methods in clinical psychology. Wiley; New York: 1982. pp. 127–181. [Google Scholar]
- Meehl PE, Yonce LJ. Taxometric analysis. I: Detecting taxonicity with two quantitative indicators using means above and below a sliding cut (MAMBAC procedure) Psychological Reports. 1994;74:1059–1274. [Google Scholar]
- Muthén B, Asparouhov T. Item response mixture modeling: Application to tobacco dependence criteria. Addictive Behaviors. 2006;31:1050–1066. doi: 10.1016/j.addbeh.2006.03.026. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Volkow N, Li TK. What’s in a word? Addiction versus dependence in DSM-V. American Journal of Psychiatry. 2006;163:764–765. doi: 10.1176/ajp.2006.163.5.764. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies, Substance Abuse & Mental Health Services Administration . State estimates of substance use from the 2001 National Household Survey on Drug Abuse. Volume II. Individual state tables and technical appendices. Author; Rockville, MD: 2003. (NHSDA Series H-20). DHHS Publication No. SMA 03-2826. [Google Scholar]
- Office of Applied Studies, Substance Abuse and Mental Health Services Administration . Results from the 2003 National Survey on Drug Use and Health: National Findings. Author; Rockville, MD: 2004. (NSDUH Series H-25). DHHS Publication No. SMA 04-3964. [Google Scholar]
- Piper J, McCarthy D, Baker T. Assessing tobacco dependence: A guide to measure evaluation and selection. Nicotine & Tobacco Research. 2006;8:339–351. doi: 10.1080/14622200600672765. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Collins AC, Shiffman S, Pomerleau C. Why some people smoke and others do not: New perspectives. Journal of Consulting and Clinical Psychology. 1993;61:723–731. doi: 10.1037//0022-006x.61.5.723. [DOI] [PubMed] [Google Scholar]
- Presson CC, Chassin L, Sherman SJ. Psychosocial antecedents of tobacco chipping. Health Psychology. 2002;21:384–392. doi: 10.1037//0278-6133.21.4.384. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Ruscio J, Keane TM. The latent structure of posttraumatic stress disorder: A taxometric investigation of reactions to extreme stress. Journal of Abnormal Psychology. 2002;111:290–301. [PubMed] [Google Scholar]
- Ruscio J, Haslam N, Ruscio AM. Introduction to the taxometric method: A practical guide. Erlbaum; London: 2006. [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman S, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2001;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Tobacco “chippers”: Individual differences in tobacco dependence. Psychopharmacology. 1989;97:539–547. doi: 10.1007/BF00439561. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Fischer LB, Zettler-Segal M, Benowitz NL. Nicotine exposure among nondependent smokers. Archives of General Psychiatry. 1990;47:333–336. doi: 10.1001/archpsyc.1990.01810160033006. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: Contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115:509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychology. 1995;14:301–309. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. [Google Scholar]
- Shiffman S, Sayette MA. Validation of the Nicotine Dependence Syndrome Scale (NDSS): A criterion-group design contrasting chippers and regular smokers. Drug and Alcohol Dependence. 2005;79:45–52. doi: 10.1016/j.drugalcdep.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ, Hickcox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: The associative basis of tolerance. Experimental and Clinical Psychopharmacology. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Stitzer ML, Wright C, Bigelow GE, June HL, Felch LJ. Time course of naloxone-precipitated withdrawal after acute methadone exposure in humans. Drug and Alcohol Dependence. 1991;29:39–46. doi: 10.1016/0376-8716(91)90020-y. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA, Shiffman S, Clayton RR. What can dependence theories tell us about assessing the emergence of tobacco dependence? Addiction. 2004;99(Supp. 1):78–86. doi: 10.1111/j.1360-0443.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Warthen MW, Goedeker KC. The functional significance of craving in nicotine dependence. In: Bevins R, Caggiula A, editors. Nebraska Symposium on Motivation. Vol. 51: The motivational impact of nicotine and its role in tobacco use. University of Nebraska Press; Lincoln: (in press) [Google Scholar]
- U.S. Department of Health & Human Services Centers for Disease Control, Office on Smoking & Health; Atlanta, GA: Reducing the health consequences of smoking: 25 years of progress. A report of the Surgeon General, 1989. 1989 (DHHS Pub. No [CDC] 89-8411)
- Vann RE, Balster RL, Beardsley PM. Dose, duration, and pattern of nicotine administration as determinants of behavioral dependence in rats. Psychopharmacology. 2005;184:482–493. doi: 10.1007/s00213-005-0037-0. [DOI] [PubMed] [Google Scholar]
- Waller NG, Meehl PE. Multivariate taxometric procedures: Distinguishing types from continua. Sage Publications; Newbury Park, CA: 1998. [Google Scholar]
- Waller NG, Putnam FW, Carlson EB. Types of dissociation and dissociative types: A taxometric analysis of dissociative experiences. Psychological Methods. 1996;1:300–321. [Google Scholar]
- Williamson DA, Womble LG, Smeets MAM, Netemeyer RG, Thaw JM, Kutlesic V, Gleaves DH. Latent structure of eating disorder symptoms: A factor analytic and taxometric investigation. American Journal of Psychiatry. 2002;159:412–415. doi: 10.1176/appi.ajp.159.3.412. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. Author; Geneva: 1992. [Google Scholar]