Abstract
Pathogenicity proteins (AL2/C2) of begomo- and curtoviruses suppress silencing through inhibition of the methyl cycle, as a consequence of inhibiting adenosine kinase (ADK). ADK phosphorylates cytokinin nucleosides, helping maintain a pool of bioactive cytokinins through inter-conversion of free-bases, nucleosides and nucleotides. We provide evidence that inhibiting ADK affects expression of primary cytokinin responsive genes. Specifically, we demonstrate increased activity of a primary cytokinin-responsive promoter in adk mutant Arabidopsis plants, and in response to silencing ADK expression or inhibiting ADK activity in transient assays. Similar changes in expression are observed in geminivirus infected tissue and when AL2/C2 are over-expressed. Increased cytokinin-responsive promoter activity may therefore be a consequence of an ADK/AL2/C2 interaction. Application of exogenous cytokinin increases susceptibility to geminivirus infection, characterized by a reduced mean latent period and enhanced viral replication. Thus, ADK appears to be a high value target of geminiviruses that includes increasing expression of primary cytokinin-responsive genes.
Keywords: Geminivirus, cytokinins, cell cycle, ADK, AL2, C2, silencing
Introduction
Geminiviruses are a diverse group of phytopathogenic viruses with a circular single-stranded (ss) DNA genome. These viruses replicate in the nuclei of infected plant cells through double-stranded (ds) DNA replicative form (RF) intermediates and rely extensively on the host for replication and transcription of the viral genome. Begomoviruses, including Cabbage leaf curl virus (CaLCuV) and Tomato golden mosaic virus (TGMV), and Curtoviruses, including Spinach curly top virus (SCTV), encode multifunctional proteins that function as pathogenicity factors (Baliji et al., 2007; Lacatus and Sunter, 2008; Sunter et al., 2001). The TGMV AL2 protein is a transcription factor, required for coat protein (CP) and nuclear shuttle protein (NSP) gene expression (Sunter and Bisaro, 1991; 1992), and localizes to both the cytoplasmic and nuclear compartments of the cell (Wang et al., 2003; Yang et al., 2007). By contrast, Beet curly top virus (BCTV) L2 and SCTV C2 share limited amino acid sequence homology with TGMV AL2, lack an obvious transcriptional activation domain, and are unable to complement a mutation in the TGMV AL2 gene (Baliji et al., 2004; 2007; Hormuzdi and Bisaro, 1995; Stanley et al., 1992; Sunter et al., 1994). Despite the lack of any sequence similarity, these proteins do share a common function in viral pathogenesis through interaction with two cellular kinases, Arabidopsis AKIN11 and adenosine kinase 2 (ADK2) (Baliji et al., 2007; Hao et al., 2003; Wang et al., 2003). AKIN11 is a serine/threonine kinase belonging to subgroup 1 of the SNF1-related protein kinases (SnRK1) that respond to nutritional and environmental stress. Closely related homologs include yeast SNF1 and mammalian AMP-activated (AMPK) protein kinases. When expressed as transgenes, a truncated TGMV AL2 and full-length BCTV L2 cause enhanced susceptibility to infection by TGMV, BCTV and an unrelated RNA virus, Tobacco mosaic virus (TMV) (Hao et al., 2003; Sunter et al., 2001). Observations that reducing expression of snRK1 also results in enhanced susceptibility, and over-expression of snRK1 leads to enhanced resistance to geminivirus infection, suggests that metabolic responses mediated by SnRK's are part of the plant innate defense response (Hao et al., 2003).
Evidence to date indicates that geminiviruses target ADK to suppress antiviral RNA silencing, most likely through inhibition of the methyl cycle (Buchmann et al., 2009; Raja et al., 2008; Wang et al., 2005). ADK is important for sustaining S-adenosylmethionine-dependent methyltransferase activity, and ADK deficiencies in yeast and Arabidopsis result in reduced methylation (Lecoq et al., 2001; Moffatt et al., 2002; Schoor and Moffatt, 2004; Weretilnyk et al., 2001). As histone and DNA methylation are known to be associated with RNA silencing this suggests a link between ADK inhibition by geminivirus AL2/C2 proteins and viral pathogenesis. A recent report suggests that AL2/C2 proteins suppress transcriptional gene silencing by inhibiting cellular transmethylation reactions thus counteracting methylation of the viral chromatin (Buchmann et al., 2009).
A model linking ADK and SnRK1 has been proposed whereby inhibition of ADK by geminivirus AL2/C2 would prevent inactivation of SnRK1 (Wang et al., 2003; 2005). ADK is a key component of the adenosine salvage pathway and catalyzes the conversion of adenosine to 5’AMP. This prevents accumulation of potentially inhibitory levels of purines and maintains intracellular AMP levels (Moffatt et al., 2000). Increasing intracellular AMP:ATP ratios generated during an infection would lead to a rapid metabolic response, mediated by activation of SnRK1 (Hao et al., 2003). The interaction between ADK and AL2/C2 would inhibit activation of SnRK1 by 5’ AMP. Although plant SnRK1 complexes are not known to be allosterically activated by AMP, inactivation of spinach SnRK1 is inhibited by low concentrations of 5'-AMP through binding to the kinase (Sugden et al., 1999).
A potential consequence of inhibiting ADK could relate to the role of ADK in cytokinin metabolism and cell cycle progression (Kwade et al., 2005). Cytokinins are phytohormones that act to promote cell proliferation and are found in almost all higher plants as well as mosses, fungi and bacteria. These compounds are N6-substituted adenine derivatives and ADK isolated from the moss Physcomitrella patens functions in the conversion of cytokinins to their nucleotide forms (von Schwartzenberg et al., 1998). ADK therefore appears to play an important role in regulating the relative levels of different cytokinin forms within the cell. Inhibition of ADK activity by AL2/C2 could therefore prevent phosphorylation of cytokinin nucleosides, blocking conversion to low activity nucleotide forms. This would increase the pool of bioactive cytokinins necessary for plant cell cycle progression, which geminiviruses rely on for replication of their DNA genome.
These observations prompted our current investigation into the role of geminivirus AL2/C2 in altering cytokinin responses through interaction with ADK. We provide evidence for a third, novel reason that geminiviruses target ADK: manipulating expression of cytokinin-responsive genes. We discuss the relevance of increased cytokinin levels for replication and maintenance of the viral genome.
Results
Activity of an Arabidopsis cytokinin responsive promoter increases in response to replicating geminiviruses in N.benthamiana leaves
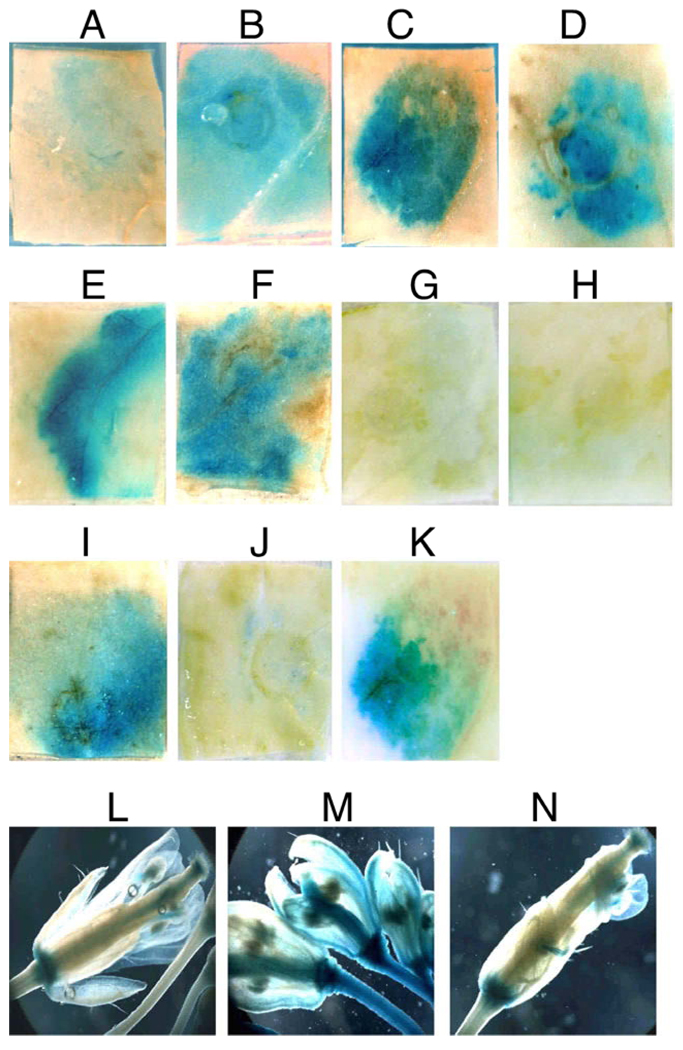
To determine whether geminivirus infection has an effect on cytokinin responses, we utilized a previously characterized cytokinin responsive gene. Arabidopsis response regulator 5 (ARR5) is a member of the Type A cytokinin primary response genes that provide the initial response to increases in active cytokinin signaling. When fused to the GUS reporter gene the ARR5 promoter (ARR5∷GUS) confers cytokinin-inducible gene expression ubiquitously (D'Agostino et al., 2000). To test whether the ARR5 promoter would be responsive to cytokinins in N.benthamiana, we first infused leaves with Agrobacterium cultures containing the ARR5∷GUS construct and analyzed GUS expression by histochemical staining six days post-infusion. Expression of the GUS reporter, visualized by a blue precipitate, is indicative of ARR5 promoter activity. A low level of GUS staining was detected in leaves co-infused with the ARR5∷GUS construct and an Agrobacterium culture containing empty vector (Fig. 1A), likely a response to the infusion process and/or Agrobacterium, which served as a negative control for our experiments. GUS staining increased when 5 µM benzylamino purine (BAP), a synthetic cytokinin, was included in the infusion (Fig. 1B). This confirms that the Arabidopsis ARR5 promoter is responsive to exogenous application of cytokinins in most cells of N.benthamiana leaves, as previously determined for Arabidopsis (D'Agostino et al., 2000; Hwang et al., 2002). We then tested the response to replicating geminiviruses by co-infusing N.benthamiana leaves with Agrobacterium cultures containing the ARR5∷GUS construct and cloned DNA containing tandemly-repeated copies of wild type genomes for either SCTV or TGMV DNA A. In the case of TGMV only the A component was used which removes any possible effects due to movement of the viral DNA (Sunter et al., 1987). Elevated levels of GUS staining were seen in leaves co-infused with the ARR5∷GUS construct and SCTV (Fig. 1C) or TGMV (Fig. 1D) when compared to the negative control (Fig. 1A). These results demonstrate that increased activity of the cytokinin responsive promoter correlates with the presence of geminivirus DNA. No increase in ARR5 promoter activity was detected when Agrobacterium containing a non-replicating TGMV A genome was used (data not shown). This suggests that the presence of replicating viral DNA is necessary for the observed increase in ARR5 promoter activity.
Fig. 1.
Increased ARR5∷GUS promoter activity in response to geminiviruses. Panels A through K illustrate N.benthamiana leaf sections stained for the presence of GUS. Leaves were co-infused with Agrobacterium cultures containing the ARR5∷GUS promoter-reporter in the presence of: A) empty vector; B) 5 µM benzylamino purine; C) WT SCTV; D) WT TGMV DNA A; E) TGMV AL2; F) SCTV C2; G) TGMV AL1; H) TGMV AL3; I) dsADK; J) dsAKIN11; and K) ADK inhibitor A-134974. Tissue was analyzed six days post-infusion. Panels L, M and N illustrate GUS staining of inflorescences from transgenic Arabidopsis plants containing the ARR5∷GUS promoter-reporter, either mock inoculated (L), bolts exhibiting SCTV symptoms (M) or asymptomatic bolts from SCTV-infected plants (N).
Geminivirus pathogenicity factors AL2 and C2 increase the activity of a cytokinin responsive promoter
To determine which geminivirus gene/protein was responsible for the observed increases in cytokinin responsive promoter activity, we co-infused N.benthamiana leaves with the ARR5∷GUS construct in the presence of Agrobacterium cultures containing plasmid DNA capable of expressing individual geminivirus genes from the Cauliflower mosaic virus (CaMV) 35S promoter. As compared to the control (Fig. 1A) there is an increase in GUS staining in N.benthamiana leaves infused with Agrobacterium cultures containing either TGMV AL2 or SCTV C2 (Fig. 1E and F). In contrast, no increase above the control (Fig. 1A) was observed when N.benthamiana leaves were co-infused with Agrobacterium cultures containing constructs capable of expressing either TGMV AL1 or AL3 (Fig. 1G and H). This confirms that the TGMV AL2 and SCTV C2 gene products are sufficient to increase the activity of a primary cytokinin-responsive promoter in N.benthamiana leaves. The observation that SCTV C2, lacking a transcription function (Baliji et al., 2007), also results in increased ARR5 promoter activity would lead us to believe that this is not a direct effect of transcriptional activation by TGMV AL2. Similar increases in GUS staining were also observed when the ARR5∷GUS construct was co-infused with AL2 of Cabbage leaf curl virus (CaLCuV), another bipartite geminivirus (data not shown). This data suggests that response of a cytokinin-inducible promoter to geminivirus pathogenicity proteins is conserved across two genera within the Geminiviridae family.
Inhibition of ADK but not AKIN11 leads to increased activity of a cytokinin-responsive promoter
TGMV AL2 and SCTV C2 interact with and inhibit two cellular kinases, SnRK1 (AKIN11 in Arabidopsis) and ADK, both in vitro and in vivo (Baliji et al., 2007; Wang et al., 2003). We therefore tested whether increased ARR5 promoter activity is a consequence of one, or both, of these interactions. We first used an RNAi approach, using inverted repeat constructs designed to express dsRNA (dsADK and dsAKIN11) that is known to reduce their target mRNA levels in infused N.benthamiana leaves (Wang et al., 2005). Co-infusion of N.benthamiana leaves with Agrobacterium cultures containing ARR5∷GUS and dsADK constructs led to increased ARR5 promoter activity, as evidenced by increased accumulation of GUS staining (Fig. 1I) when compared with the control infusion (Fig. 1A). In contrast, no increase in GUS staining when compared to the control was observed when ARR5∷GUS was co-infused with Agrobacterium cultures containing the dsAKIN11 construct (Fig. 1J). These results indicate that silencing and/or suppression of endogenous ADK, but not SnRK1, mRNA leads to increased activity from a cytokinin responsive promoter.
To determine whether reduced ADK activity is required for the observed increases in cytokinin responsive promoter (Fig. 1I), we used the chemical inhibitor A-134974, an adenosine analogue, known to selectively inhibit rat ADK (McGaraughty et al., 2001) and to reduce ADK protein activity in plants (Wang et al., 2005). Increased GUS staining was observed when the ARR5∷GUS construct was co-infused with the ADK inhibitor indicating high ARR5 promoter activity (Fig. 1K). These results suggest that inhibition of ADK either by reducing ADK mRNA levels or inhibiting ADK activity, leads to increased ARR5 promoter activity. This data correlates with similar responses in ARR5 promoter activity observed in the presence of TGMV AL2 and SCTV C2 (Fig. 1C and 1D). Taken together, the results are consistent with increased expression of a cytokinin-responsive gene being a functional consequence of the interaction between ADK and AL2/C2. However the precise mechanism of interaction is yet to be understood.
Activity of a cytokinin responsive promoter increases in SCTV infected Arabidopsis plants
In transient leaf infusion assays we have shown that expression of either TGMV AL2 or SCTV C2, or inactivation of ADK, leads to increases in the activity of a cytokinin responsive promoter (Fig. 1. A–K). We therefore investigated whether similar increases could be detected in Arabidopsis plants systemically infected with SCTV. As TGMV does not infect Arabidopsis, we limited our study to SCTV infected plants. Rosette leaves on Arabidopsis plants transgenic for the ARR5∷GUS construct (D'Agostino et al., 2000) were agroinoculated with either empty vector (mock) or wild type SCTV and bolts, which are away from the site of inoculation, were stained for GUS expression seven to ten days post-inoculation. Symptoms typical of SCTV infection were observed in inoculated transgenic Arabidopsis plants and are comparable to symptoms observed in non-transgenic Arabidopsis plants (Baliji et al., 2007). Thus, presence of the transgene did not affect either symptom production or the overall infectivity of SCTV (data not shown). Intense GUS staining was observed at the base of the inflorescence from bolts of mock-inoculated plants (Fig. 1L), consistent with previously reported observations (D'Agostino et al., 2000). In contrast, intense GUS staining was detectable throughout the inflorescence and in the stems of bolts exhibiting symptoms typical of SCTV infection (Fig. 1M). It is important to note that GUS staining in asymptomatic bolts on SCTV-infected plants (Fig. 1N) was identical to bolts on mock-inoculated plants (Fig. 1L) indicating that the increase in cytokinin-responsive promoter activity is most likely specific to geminivirus infection. Not all new bolts that emerge exhibit symptoms when inoculated by the pin-prick method (Baliji et al., 2007), but asymptomatic bolts contain no detectable levels of viral DNA as observed by DNA gel blot hybridization (data not shown). These results demonstrate that increased activity of the ARR5 cytokinin-responsive promoter correlates with symptomatic tissues in SCTV-infected Arabidopsis, and strongly suggests that presence of the virus is needed to elicit the response.
Geminivirus pathogenicity proteins increase expression of primary cytokinin response regulators in Arabidopsis plants
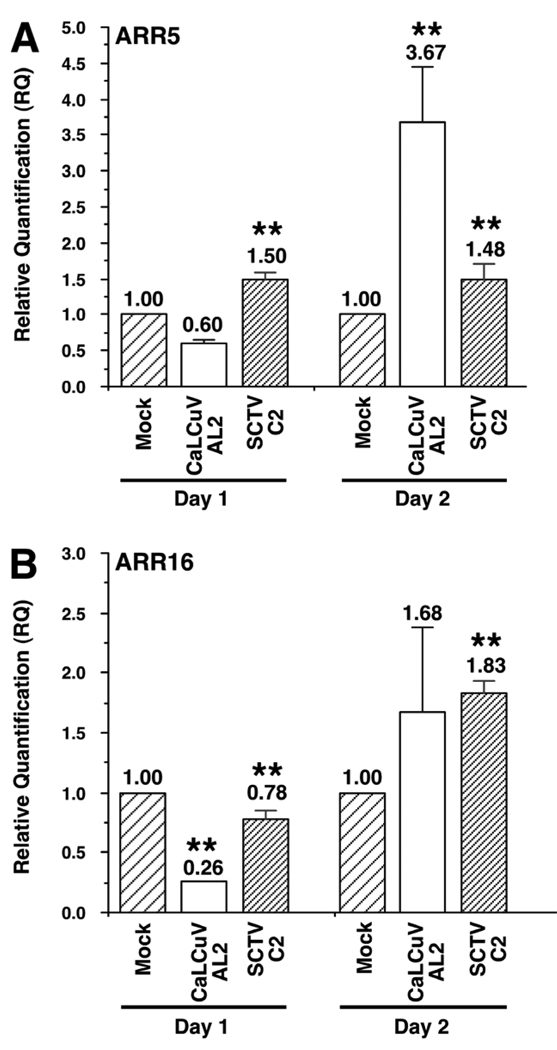
Our earlier results have shown that TGMV AL2 and SCTV C2 mediate an increase in the activity of the primary cytokinin responsive ARR5 promoter in N.benthamiana leaves (Fig. 1E and 1F). We then directly analyzed the response of endogenous ARR5 and a second primary cytokinin response regulator, ARR16, in Arabidopsis plants. It has been previously shown that ARR16 responds to cytokinins in a similar manner to ARR5 (D'Agostino et al., 2000). As TGMV does not infect Arabidopsis we used the AL2 gene from CaLCuV (a begomovirus) and the C2 gene from SCTV (a curtovirus) for these experiments. Whole Arabidopsis plants were vacuum infiltrated with Agrobacterium cultures containing constructs capable of constitutively expressing CaLCuV AL2, SCTV C2 or a construct containing empty vector as a mock treatment. Total RNA was isolated one and two days post-infusion (dpi) and quantitative real time RT-PCR (qRT-PCR) performed. Accumulation of RNA corresponding to CaLCuV AL2 and SCTV C2 was confirmed one and two days post-infiltration by RT-PCR using virus-specific primers followed by southern hybridization analysis of the amplified cDNA products (data not shown). After two days under these conditions it was observed that levels of RNA corresponding to CaLCuV AL2 and SCTV C2 decreased, and so the experiments were limited to two days post-infusion. A significant increase in expression of endogenous ARR5 of approximately four-fold (Student's t-test: P<0.05), was detected in response to CaLCuV AL2 at two dpi (Fig. 2A). We observed a similar trend with expression of ARR16 in response to CaLCuV AL2 where a smaller increase of approximately 1.5-fold was detected at 2 dpi (Fig. 2B), although there was greater variation in expression (Student's t-test: P<0.10). Interestingly, in CaLCuV AL2 infiltrated Arabidopsis plants, we detected an initial decrease in expression of both ARR5 and ARR16 at one dpi (Fig. 2A and B). To further extend these observations we tested the expression of endogenous ARR5 and ARR16 in Arabidopsis plants infiltrated with Agrobacterium capable of expressing SCTV C2. As can be seen (Fig. 2A), ARR5 expression increased approximately 1.5 fold in response to SCTV C2 at both one and two dpi. Although an initial decrease in ARR16 expression was observed at one dpi, ARR16 expression increased ∼two-fold at two dpi (Fig. 2B). Although there were some differences in the response of the two cytokinin responsive genes to AL2/C2, the data overall are consistent with the results observed in the N.benthamiana leaf infusion experiments. These results also suggest that the AL2/C2 gene from geminiviruses belonging to different genera is capable of inducing expression of primary cytokinin responsive genes, although the timing and extent may vary between different viruses and the type of response regulator.
Fig. 2.
Geminivirus pathogenicity proteins induce increased expression of endogenous primary cytokinin responsive genes in Arabidopsis. The graphs indicate relative levels of ARR5 (A) and ARR16 (B) mRNA. Values were determined by quantitative real time RT-PCR analysis of RNA isolated from Arabidopsis plants infused with Agrobacterium cultures containing DNA capable of expressing CaLCuV AL2, SCTV C2 or vector alone (Mock). Columns represent the fold change calculated from the mean ΔΔCt value from two (SCTV) or three (CaLCuV) independent experiments using RNA isolated one and two days post-infusion. Asterisks indicate significant differences in expression as determined using the Student's t-test (P<0.05). P values for all samples are given.
Mutation of endogenous ADK genes results in changes in gene expression similar to AL2/C2-infused Arabidopsis plants
Earlier results demonstrate that increased activity from a primary cytokinin responsive promoter is a consequence of geminivirus AL2/C2 interaction with ADK (Fig. 1) and suggests increased cytokinin accumulation and/or local signaling/transport. If the increase in activity of primary cytokinin responsive promoter activity is dependent on ADK inhibition, then we should see similar results in Arabidopsis plants that are defective for ADK function. To determine whether similar changes in expression of the endogenous ARR5 and ARR16 genes can occur through the direct inactivation of ADK, we used qRT-PCR to analyze RNA isolated from Arabidopsis plants deficient for either ADK1 (adk1-1) or ADK2 (adk2-1) (Young et al., 2006). Arabidopsis has two ADK genes, and each mutant was assayed separately as the loss of all ADK function is lethal (Moffatt et al., 2002). Expression of endogenous ARR5 is higher in both adk-1 and adk-2 mutant lines, as compared to wild type Arabidopsis plants (Table 1). In the case of ARR16, expression was higher in adk-1 but lower in adk-2 mutant Arabidopsis as compared to wild type plants (Table 1). The results do however generally confirm that elimination of one copy of ADK is sufficient to increase expression of some cytokinin responsive genes, similar to that observed in Arabidopsis plants expressing SCTV C2 or CaLCuV AL2 (Fig. 2).
Table 1.
Differences in expression of endogenous cytokinin-responsive genes in Arabidopsis adk knockout plants
| Genea | Sampleb | Fold-Changec | P Valued |
|---|---|---|---|
| ARR5 (At3g48100) | Wild type | 1.00 | NA |
| adk1-1 | 1.53 ± 0.14 | P < 0.001 | |
| adk2-1 | 1.55 ± 0.33 | P < 0.05 | |
| ARR16 (At2g40670) | Wild type | 1.00 | NA |
| adk1-1 | 1.61 ± 0.14 | P < 0.05 | |
| adk2-1 | 0.58 ± 0.33 | P < 0.05 |
Gene identification is according to The Arabidopsis Information Resource (TAIR) database.
Samples represent RNA isolated from wild type Arabidopsis plants or Arabidopsis plants containing T-DNA insertions in the ADK1 (adk1-1) or ADK2 (adk2-1) genes.
The fold-change represents an average calculated from ΔΔCt values using three technical replicates from each of two (ARR16) or four (ARR5) independent experiments using quantitative real time RT-PCR. ΔCt values were calculated relative to an endogenous control (β-tubulin; At2g29550). The standard error of the mean is given.
The P value was calculated using a Student's t-test on ΔCt values. Values were derived from three technical replicates from each of two (ARR16) or four (ARR5) independent experiments. NA = not applicable.
Expression of endogenous cytokinin-responsive genes increases during geminivirus infection
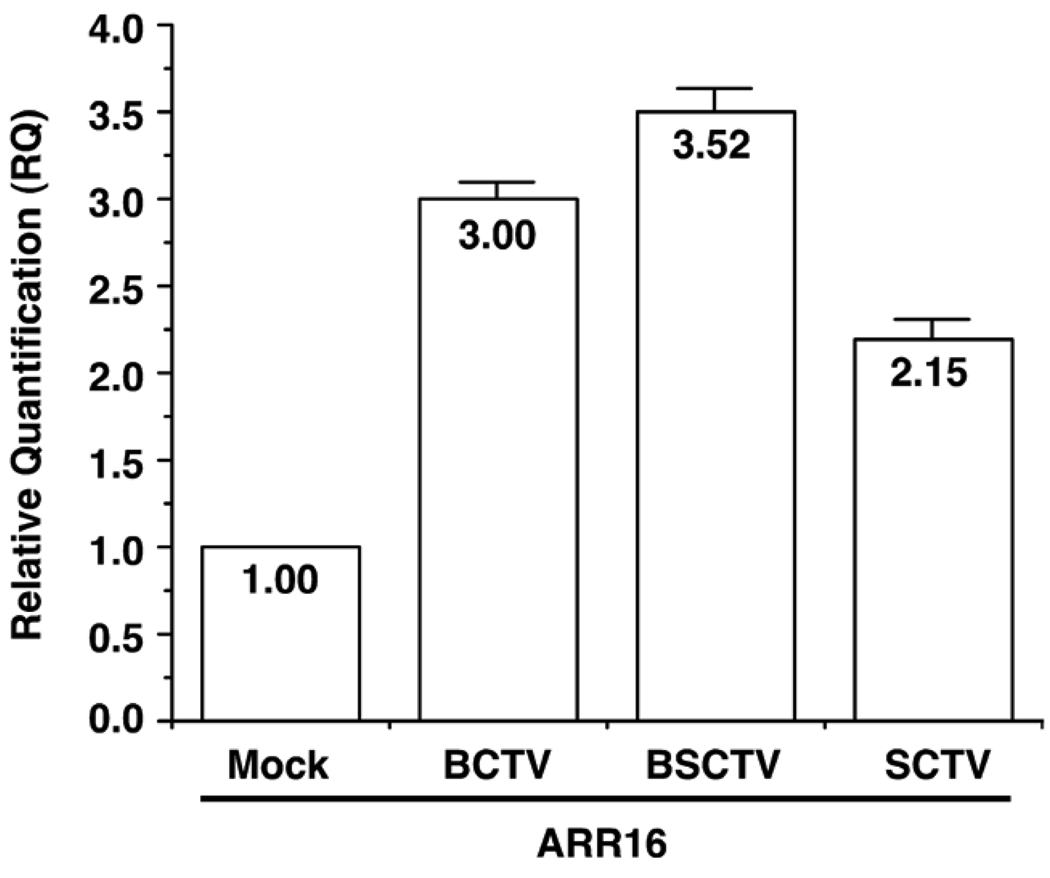
Earlier experiments demonstrated that geminivirus AL2/C2, when over-expressed in Arabidopsis plants, induce increase expression of endogenous cytokinin-responsive genes (Fig. 2). To determine whether similar increases in the expression of endogenous cytokinin-responsive genes also occur in geminivirus infected plants, we examined the expression of ARR16 in Arabidopsis rosette leaves after agroinoculation with three different monopartite curly top viruses (BCTV; Beet severe curly top virus, BSCTV; and SCTV). We chose ARR16 over ARR5, as previous studies show increased accumulation of ARR16 transcripts in cytokinin-treated rosette leaves of wild-type Arabidopsis Col. plants (Kiba et al., 1999). To better synchronize the infection, Arabidopsis plants were grown under short day conditions (8 h light, 16 h dark, 21°C) to induce the growth of rosette leaves (Samach and Coupland, 2000) and to prevent plants from flowering (bolting). It should be noted that under conditions where Arabidopsis plants inoculated with SCTV are allowed to bolt, no viral DNA can be detected in rosette leaves (data not shown). Total RNA was isolated 10 dpi at which time rosette leaves displayed symptoms typical of a curly top infection. As shown (Fig. 3), increases in expression of endogenous ARR16 mRNA of 2.2 to 3.5-fold were detected in rosette leaves from Arabidopsis plants infected with SCTV, BCTV and BSCTV, as compared to a vector control (mock). These results are again consistent with geminivirus infection causing increased expression of primary cytokinin responsive genes.
Fig. 3.
Geminivirus infection induces expression of endogenous primary cytokinin responsive genes in Arabidopsis. The graph represents the relative quantification of ARR16 mRNA levels observed in different treatments. Values were determined by quantitative real time RT-PCR analysis of RNA isolated from Arabidopsis rosette leaves systemically infected with Beet curly top virus (BCTV), Beet severe curly top virus (BSCTV) or Spinach curly top virus (SCTV). The columns represent relative differences in ARR16 mRNA levels compared to that present in mock-inoculated plants.
Application of exogenous BAP increases replication of wild type and c2-4 mutant SCTV in N.benthamiana leaves
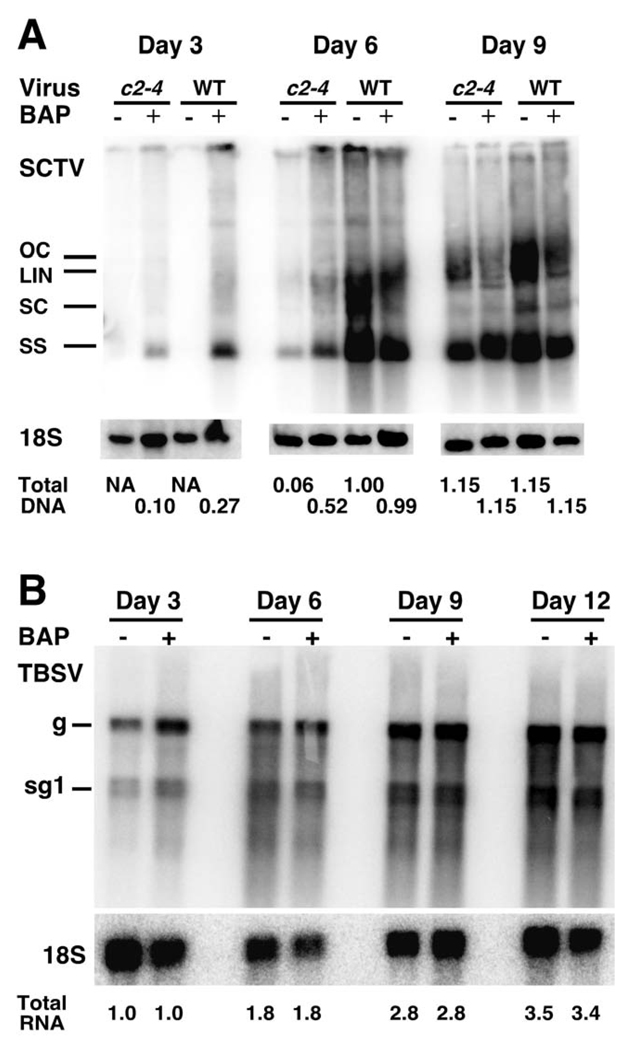
The above results indicate that expression of AL2/C2 leads to an increase in cytokinin-responsive gene expression, although this could be part of an overall global effect of virus infection. If the interaction of AL2/C2 with ADK influences the expression of cytokinin-responsive genes, we expect this to be important for pathogenesis. As cytokinins are important for cell cycle progression this would be expected to be critical for DNA virus replication. We therefore extended our analysis by testing the effect of exogenous cytokinin on geminivirus replication using a transient leaf infusion assay (Johansen and Carrington 2001). SCTV was chosen for this experiment due to the availability of SCTV DNA containing a mutation in the C2 gene exhibits a two to three-fold reduction in viral DNA accumulation relative to wild type virus (Baliji et al., 2007). Viral DNA levels eventually become comparable in extracts from plants infected with either mutant or wild type SCTV (Baliji et al., 2007). N.benthamiana leaves were infused with Agrobacterium containing DNA capable of releasing wild type or c2-4 mutant SCTV genomes in the absence or presence of 5 µM BAP, which elicits a significant cytokinin response (D'Agostino et al., 2000). Total DNA was isolated from infused leaves three to nine days post infusion and viral DNA forms identified by DNA gel blot hybridization. The total amount of viral DNA was normalized to 18S rDNA by phosphorimager analysis. Viral DNA can be detected in leaves infused with both wild type and c2-4 mutant virus (Fig. 4A). However, differences in the kinetics of viral DNA accumulation were observed. In the absence of BAP, replicating viral DNA derived from wild type or c2-4 mutant SCTV is detectable after six days. However, the amount of c2-4 mutant DNA is approximately 16-fold lower than wild type SCTV DNA at this time point (Fig. 4A). By nine days post-infusion little difference is observed in wild type and mutant viral DNA accumulation. This is consistent with previous data in protoplasts where the kinetics of c2-4 mutant virus accumulation is delayed (Baliji et al., 2007). When exogenous BAP is added with the virus inoculum, both wild type and mutant viral DNA are now detectable after three days, although the level of viral DNA derived from c2-4 mutant SCTV is approximately three-fold lower than wild type SCTV DNA (Fig. 4A). The total amount of mutant viral DNA detected at three days post-infusion in the presence of BAP, is approximately two-fold higher than that detected at six days post-infusion in the absence of BAP. Together this data suggests that increasing cytokinin levels, through the application of exogenous BAP, partially complements a c2 mutation, resulting in an increase in viral DNA replication.
Fig. 4.
The effect of exogenous cytokinin on accumulation of viral nucleic acid in N.benthamiana leaves. N.benthamiana leaves were infused with Agrobacterium cultures containing wild type or c2-4 mutant SCTV DNA (A), or mechanically inoculated with TBSV (B), in the absence or presence of 5µM Benzylaminopurine (−/+ BAP). Total DNA or RNA was isolated from leaves three to 12 days post-infusion and hybridized to probes specific for SCTV (A) or TBSV (B). To the left of each panel double-stranded supercoiled (SC), open circular (OC) and linear (LIN), and single-stranded (SS) viral DNA forms of SCTV, and genomic (g) and subgenomic (sg1) forms of TBSV are shown. Hybridization was normalized to either 18S rDNA (A) or 18S rRNA (B). The relative levels of total viral DNA or RNA are indicated below each respective panel. For SCTV a value of 1 was arbitrarily assigned to the total amount of viral DNA present in samples from leaves infused with wild type SCTV in the absence of BAP at six days post-infusion (A). For TBSV a value of 1 was arbitrarily assigned to the total amount of viral RNA present in samples from leaves inoculated in the absence of BAP at three days post-infusion (B).
We expect this effect to be limited to DNA viruses that require re-initiation of the plant cell cycle. To test if application of exogenous BAP had a specific effect we performed similar experiments using Tomato bushy stunt virus (TBSV) a (+) sense single-stranded RNA virus (Kim et al., 2007). For these experiments TBSV carrying a mutation in the P19 silencing suppressor was used, as wild type TBSV causes lethal necrosis upon systemic infection of N.benthamiana (Scholthof et al., 1995). As can be seen (Fig. 4B), no difference in TBSV replication was detected in the absence or presence of BAP, even 12 days post-TBSV infection. This result demonstrates that the effect of increased SCTV DNA replication in the presence of exogenous cytokinins appears to be specific to geminiviruses. However, whether this effect is general for DNA viruses still needs to be determined. These results provide further evidence that increased expression of cytokinin-responsive genes is important for geminiviral pathogenesis.
Application of exogenous BAP reduces the mean latent period for geminivirus infection
To further examine the biological significance of increased cytokinins we analyzed the effect of exogenous application of BAP on geminivirus infectivity. N.benthamiana plants were inoculated with differing inoculum doses of Agrobacterium containing DNA capable of releasing wild type TGMV A and B genomes in the absence or presence of 5 µM BAP. Inoculum doses of 1.0 and 0.2 (OD600nm) were chosen based on previous observations that infectivity drops below 50% at lower doses (Sunter et al., 2001). Plants were scored for the time to first appearance of symptoms (mean latent period) typical of a TGMV infection (Table 2). Plants inoculated in the presence of 5 µM BAP exhibited systemic symptoms typical of TGMV infection one to two days earlier than plants inoculated in the absence of BAP, depending on the inoculum dose. ANOVA confirmed that mean latent period reductions observed were statistically different (P < 0.05).
Table 2.
Mean latent period following TGMV or SCTV inoculation of Nicotiana benthamiana plants in the absence or presence of exogenous cytokinin treatment
| Virus Inoculum | Dose | − BAP | + BAP |
|---|---|---|---|
| TGMV A + B | 1.0 | 13.9 ± 1.3 (15/18) | 12.7 ± 0.7 (18/18) a |
| 0.2 | 13.4 ± 1.8 (11/15) | 11.4 ± 0.9 (17/18) a | |
| Wild type SCTV | 0.04 | 8.6 ± 0.7 (18/18) | 7.9 ± 0.4 (18/18) a |
| 0.008 | 9.1 ± 0.7 (18/18) | 7.8 ± 0.4 (18/18) a | |
| SCTV c2-4 mutant | 0.04 | 9.4 ± 0.8 (17/17) | 9.1 ± 0.6 (17/17) b |
| 0.008 | 11.0 ± 1.2 (17/17) | 9.0 ± 0.5 (18/18) a |
Note: Plants were agro-inoculated with TGMV or SCTV as described in the Materials and Methods. Plants were scored for the appearance of systemic symptoms typical of TGMV or SCTV infection. Lower case superscripts indicate significant differences in the mean latent period (days post inoculation ± SE) observed in the absence or presence of BAP as confirmed by ANOVA
P<0.05;
P<0.10
Numbers in parentheses indicate infectivity (number of plants infected/number plants inoculated).
In similar experiments, N.benthamiana plants were inoculated with differing inoculum doses of Agrobacterium containing DNA capable of releasing wild type or c2-4 mutant SCTV genomes in the absence or presence of 5 µM BAP. In this case, inoculum doses of 0.04 and 0.008 (OD600nm), were chosen based on observations that ∼100% of plants become infected even at these lower inoculum doses (Baliji et al., 2007). Plants inoculated in the presence of BAP exhibited systemic symptoms typical of SCTV infection approximately one day earlier than plants inoculated in the absence of BAP (Table 2) at inoculum doses of 0.04 and 0.008 (P<0.05). As the application of exogenous BAP results in increased replication of c2-4 mutant SCTV in leaves (Fig. 4A), we analyzed the infectivity of mutant virus in the presence of BAP (Table 2). A small reduction in mean latent period was observed at an inoculum dose of 0.04 (P<0.10) whereas a reduction of two days was observed at an inoculum dose of 0.008 (P<0.05). Overall, the results demonstrate a trend towards a reduction in the mean latent period for TGMV and SCTV infection in the presence of exogenously applied cytokinin. This is consistent with the presence of increased levels of cytokinins responses being important for geminivirus pathogenesis.
Discussion
Geminiviruses typically infect terminally differentiated plant cells and reprogram the cellular environment for successful viral amplification and pathogenesis. Several geminiviral proteins have been shown to interact with host factors to facilitate infection. One such example is the interaction between geminivirus AL2/C2/L2 proteins and two cellular kinases, SnRK1 (AKIN11) and ADK, which is thought to represent a dual strategy for countering host defense mechanisms directed against geminiviruses (Hao et al., 2003; Wang et al., 2003; Baliji et al., 2007). Plants transgenic for either TGMV AL2 or BCTV L2 exhibit an enhanced susceptibility phenotype that is likely due to direct inactivation of SnRK1 (Sunter et al., 2001; Hao et al., 2003). A second interaction with ADK is predicted to lead to loss of AMP by preventing the conversion of adenosine to 5’-AMP (Hao et al., 2003; Wang et al., 2003; Baliji et al., 2007). It has been reported that 5'-AMP inhibits inactivation of spinach SnRK1 through binding of AMP suggesting plant SnRK1 kinases are regulated by AMP in a manner similar to their mammalian counterparts (Sugden et al., 1999). Thus, geminiviruses might target an ADK/SnRK1 complex to facilitate infection (Wang et al., 2003). TGMV AL2 and BCTV L2 proteins also suppress antiviral gene silencing through interfering with the methyl cycle (Wang et al., 2005; Raja et al., 2008). As histone and DNA methylation are known to be associated with RNA silencing this suggests a link between ADK inhibition by geminivirus AL2/C2 proteins and silencing suppression. Thus geminivirus AL2/C2/L2 gene products employ multiple strategies for countering antiviral defenses.
The evidence presented here provides a third possible reason why geminiviruses target ADK during infection. ADK appears to play a role in regulating the relative levels of different cytokinin forms within the cell (von Schwartzenberg et al., 1998) and recent evidence indicates a possible link to cell cycle progression (Kwade et al., 2005). Naturally occurring cytokinins are N6-substituted adenine derivatives that exist in multiple forms (Hutchison and Kieber, 2002), with the cytokinin free-base being the biologically active phytohormone (Astot et al., 2000). The interconversion of free-bases to nucleosides and nucleotides, also plays an important role in cytokinin metabolism (Zrenner et al., 2006). ADK converts cytokinin nucleosides to low activity nucleotide forms through phosphorylation to help reduce the bioactive pool of cytokinin and maintain interconversion (von Schwartzenberg et al., 1998; Moffatt et al., 2000). Inactivation of ADK would therefore most likely lead to an increase in the pool of bioactive cytokinin. In support of this we have shown that activity of a primary cytokinin responsive promoter (ARR5) increases in geminivirus infected Arabidopsis plants (Fig. 1). This correlates with an increase in endogenous mRNA accumulation (Fig. 1 and Table 1) when ADK is inactivated chemically or by RNAi. A similar increase in ARR5 promoter activity is observed when the TGMV AL2 or SCTV C2 gene is over expressed (Fig. 1 and Table 1). As these proteins are known to inactivate ADK (Wang et al., 2003) it might be logical to assume that the ADK/C2 interaction leads to increased ARR5 promoter activity. However, mutations that specifically disrupt this interaction have not been mapped and interpretation of results from such a mutant protein might be challenging given that the interaction domain overlaps with domains necessary for interaction with SnRK1 and self-interaction. The observable increase does not however appear to be consequence of the transcriptional activity of TGMV AL2 as induction of the ARR5 promoter was detected in tissue expressing SCTV C2 that lacks any transcription factor activity (Baliji et al., 2007). Similar increases in endogenous ARR5 expression were observed in Arabidopsis plants infected with SCTV. Increases in ARR5 expression were not observed in uninfected tissue indicating that the presence of virus appears to be necessary for the observed induction in expression. This result would also argue against the possibility of a signaling event that leads to systemic increases in cytokinin responsive promoter activity that is initiated at the site of inoculation. An interesting question is whether increased cytokinin promoter activity correlates with tissue tropism of the virus. However, our leaf infusion data might argue against this as both SCTV, a phloem limited virus, and TGMV, non-phloem limited, appear to give the same staining pattern in N.benthamiana (Fig. 1). While this does not rule out a correlation between promoter activity and tissue tropism of the virus in Arabidopsis, the observable increases in promoter activity could be a result of increased cytokinin accumulation, signaling and/or changes in transport, which could not be distinguished in this study.
Variation in ARR5 promoter activity as measured either by histochemical GUS staining (Fig. 1) or analysis of endogenous transcript levels (Fig. 2 and Fig. 3) could reflect differences in the stability of GUS and ARR5 mRNA, the long half-life of GUS protein, and/or a missing ARR5 regulatory sequence as previously outlined (D'Agostino et al., 2000). Some differences were apparent depending on the response regulator tested and the virus gene tested. One possible explanation is that differences could relate to the tissue specific expression of the two response regulators. ARR5 is primarily expressed in root and shoot meristems in the absence of exogenous cytokinin (D'Agostino et al., 2000), but expression expands to tissues around the shoot meristematic region and in all root tissues (To et al., 2004). In contrast, cytokinin appears to induce expression of ARR16 primarily within root endodermis of young seedlings (Kiba et al., 2002), but also shows increased accumulation in cytokinin-treated leaves of wild-type Arabidopsis Col. plants (Kiba et al., 1999). Initial decreases in expression could be a result of a stress-response that inhibits ARR5 and ARR16 expression, or this could reflect the amount of time required for AL2/C2 protein to accumulate and inactivate ADK, therefore inducing ARR5 and ARR16 promoter activity. Despite these alternatives, our data does suggest that induction of primary cytokinin response genes is consistent with the ability of geminivirus pathogenicity (AL2/C2) proteins of viruses from two genera to interact with ADK (Wang et al., 2003).
The observable increases in ARR5 promoter activity could be a consequence of the interaction of AL2/C2 with ADK, as induction can be detected when ADK, but not SnRK1, is inhibited by targeting either RNA, protein or by mutation (Fig. 1 and Table 1). Inactivation of ADK would prevent conversion of cytokinin nucleosides to the low activity nucleotide forms thus maintaining higher levels of bioactive cytokinins. This conclusion is supported by data that demonstrates reduced ADK activity in plants infected with either TGMV or BCTV, but not with BCTV l2 mutant virus (Wang et al., 2003) that can only produce the first 73 amino acids of L2 (Hormuzdi and Bisaro 1995). Arabidopsis plants containing mutations in the adk-1 or adk-2 alleles are extremely sensitive to infection with geminiviruses (Raja et al., 2008), although symptoms appear more enhanced in adk-1 versus adk-2 mutant plants. This could reflect the multiple pathways affected by the ADK/AL2/C2 interaction. Another possible explanation for increases in cytokinin responsive gene expression upon inactivation of ADK is a general reduction in methylation. This is also consistent with recent observations that inhibition of ADK by geminivirus AL2/C2 proteins can interfere with methylation (Buchmann et al., 2009). However, in our analysis we also observed decreases in the expression of ARR5 and ARR16 one day after infusion of Arabidopsis with CaLCuV AL2 (Fig. 2), which would suggest that increases in expression of cytokinin responsive genes is not a consequence of general demethylation of host chromatin.
Support for a role of cytokinins in geminiviral pathogenesis is provided by observations that exogenous application of cytokinin results in an increase in the kinetics of viral DNA accumulation (Fig. 4) and reduces the mean latent period for symptom appearance of both TGMV and SCTV (Table 2). In addition, a mutation in the c2 gene in SCTV, which results in delayed replication kinetics and milder symptoms as compared to wild type virus (Baliji et al., 2007), can be partially complemented by the addition of exogenous cytokinin (Fig. 4A). Although the reduction in mean latent period is only one to two days, this could be considered an increase in virulence. Although we did not observe any obvious enhancement of disease symptoms, we did detect increases in replication (Fig. 4A) upon treatment with exogenous cytokinin. This result is in contrast to the enhanced susceptibility (ES) phenotype associated with the interaction of geminivirus AL2/C2 with SnRK1, where no increase in viral nucleic acids is observed (Sunter et al., 2001).
One benefit to geminiviruses of increasing cytokinin levels would be to promote cell proliferation. Previous data has elegantly shown that the TGMV AL1 and AL3 proteins relieve E2F-mediated repression of genes required for replication through interaction with pRBR (Egelkrout et al., 2001). However, in our experiments we did not see an increase in activity of the ARR5 cytokinin-responsive promoter when AL1 or AL3 was present (Fig. 1). This might imply that although the proteins involved in geminivirus replication can promote transition of cells from G1 to S, this is not sufficient to induce increases in expression of cytokinin response regulators that may be needed to maintain an active state of replication. Thus, it is possible that this is achieved through the interaction of AL2 with ADK, and that viral infection requires AL1, AL2 and AL3 to efficiently promote replication. A simplistic model would be that upon infection geminiviruses express early viral genes required for replication (AL1 and AL3) and suppression of defense responses (AL2). The AL1 and AL3 proteins interact with pRBR to release E2F transcription factors responsible for expression of genes required for cell cycle progression (Egelkrout et al., 2001). In this model inactivation of ADK by AL2/C2 would cause an increase in cytokinin levels by preventing conversion of active cytokinins to lower activity forms. Observations that ADK activity increases during S-phase, reaching a peak at G2 (Kwade et al., 2005), are consistent with this idea. In addition, application of exogenous cytokinins or transgenic plants over-expressing cytokinins has been shown to delay senescence (Ori et al., 1999). Senescence eventually leads to death of the leaf as a consequence of mobilizing nutrients from senescing leaves to other parts of the plant (Li et al., 1992). This could be potentially detrimental to the virus and preventing this would therefore be advantageous to virus survival. Regardless, it is clear that geminiviruses have evolved multiple strategies to provide a cellular environment conducive to viral amplification and our results suggest that geminiviruses target ADK for several important reasons: suppression of silencing through inhibition of the methyl cycle (Wang et al., 2005), blocking activation of SnRK1 (Hao et al., 2003) and increasing cytokinin levels (this report). It therefore seems that the AL2/ADK/SnRK1 interaction is an extremely important interaction for viral pathogenesis to enable efficient amplification and successful infection. We are currently investigating several aspects of this model, including analysis of additional cytokinin-responsive and senescence-related genes.
Materials and Methods
DNA constructs and plant material
Cloned DNA (pIB-1.6TC) containing the 1.6 kbp fragment of the promoter region of the Arabidopsis ARR5 gene fused upstream of the β-glucuronidase (GUS) reporter gene in the Agrobacterium Ti plasmid vector, pBI101.1, and transgenic Arabidopsis ecotype Wassilewskija containing pIB-1.6TC (D'Agostino et al. 2000) were kindly provided by Dr. J. Kieber (Dept of Biology, University of North Carolina, Chapel Hill, NC). Plasmid pIB-1.6TC was introduced into Agrobacterium strain C58 (provided by Dr. Bisaro, The Ohio State University, Columbus, OH) by triparental mating and the resulting construct (ARR5∷GUS) used for infusion assays.
Plasmid DNAs containing tandemly repeated copies of wild type (pSCTV-WT) or c2-4 mutant (pSCTV C2-4) SCTV DNA in a Ti plasmid vector have been described (Baliji et al., 2004; Baliji et al., 2007). Cloned DNAs capable of expressing SCTV C2 or CaLCuV AL2 from the CaMV 35S promoter were generated by PCR. The SCTV C2 ORF (444 bp) was amplified using C2 specific primers (forward: 5’-gcgagatctccatggaagcgcttaagtcctggacgc-3’ and reverse: 5’-gcgctgcagctcgagttaatagagatcgtttccctc-3’) from pSCTV-WT (Baliji et al., 2004). The PCR product was cleaved with XhoI (underlined, reverse primer), klenow treated and digested with BglII (underlined, forward primer). The resulting product was inserted into BglII and SmaI cleaved pMON530 (Rogers et al., 1987) to generate p35s-SCTV C2. The CaLCuV AL2 ORF (390 bp) was amplified using AL2 specific primers (forward: 5'-gcgagatctccatggatgcaaaattcatcactcttg-3' and reverse 5'- gcgaagcttggatccctacttaaatatgtcggccc-3') from CaLCuV DNA A (Abouzid et al., 1992). The PCR product was cleaved with HindIII (underlined, reverse primer), klenow treated and digested with BglII (underlined, forward primer). The resulting PCR product was inserted into BglII and SmaI cleaved pMON530 (Rogers et al., 1987) to generate p35s-CaLCuV AL2. The presence of each ORF in the sense orientation was confirmed by DNA sequencing. The resulting Ti plasmid constructs were mobilized into Agrobacterium strain GV3111SE by triparental mating (Rogers et al., 1987). Plasmid constructs containing dsADK and dsSNF1, and Arabidopsis plants deficient for either ADK1 (adk1-1: SALK_040957/ 11 At3g09820.1) or ADK2 (adk2-1: SALK_000565/ At5g03300) were obtained from Dr. D.M. Bisaro (The Ohio State University, Columbus, OH).
Plant inoculation
Arabidopsis and N.benthamiana plants were inoculated with Agrobacterium cultures containing tandemly repeated copies of wild type SCTV, c2-4 mutant SCTV or wild type TGMV DNA A and B using different dilutions of a standard dose (OD600=1.0) as described previously (Sunter et al., 2001; Baliji et al., 2004). Negative controls consisted of plants mock inoculated with Agrobacterium cultures containing the Ti plasmid vector alone. Inoculated plants were scored for appearance of symptoms typical of TGMV or SCTV infection. Mean latent periods were recorded and statistical analysis performed by ANOVA as described (Sunter et al., 2001). Symptomatic, asymptomatic, and mock-inoculated plant samples were stained for GUS activity six days post inoculation. Photographs were taken with Canon PowerShot A530 digital camera.
Agrobacterium infusion assays
Agrobacterium cultures containing individual plasmid constructs were incubated overnight in infiltration medium at room temperature as described (Johansen and Carrington, 2001). Where necessary cultures were mixed prior to infusion by combining equal volumes of a standard dose (OD600=1.0). Healthy N. benthamiana plants at the four to six leaf stage were infused with the cultures on the underside of the leaves using a 3-cc syringe. In some infusion experiments, the ADK inhibitor A-134974 (Sigma) was mixed with Agrobacterium cultures to a final concentration of 100 µM just prior to leaf infusions as described (Wang et al., 2005). Infused N.benthamiana leaf tissue was analyzed for GUS expression using a histochemical assay as previously described (Sunter and Bisaro, 1991; 1997). Infusion experiments were repeated a minimum of twice. Photographs of the infusion sites were taken with either a Canon PowerShot A530 digital camera for macroscopic observation or infused leaves were mounted on microscopic slides, visualized and photographed using a light microscope equipped with a digital camera (Axioskop, Carl Zeiss).
For vacuum infiltration of whole Arabidopsis plants Agrobacterium cultures were poured into a beaker of an appropriate size and placed into a vacuum jar and the solution degassed by drawing vacuum until bubbles formed. Arabidopsis Col-0 plants (4–6 weeks old) were sprinkled with water prior to infiltration and then whole plants submerged in the Agrobacterium culture ensuring all rosette leaves were submerged in the solution. Vacuum was drawn for 20–30 min at a pressure of approximately 0.05 Bar. Plants were removed from the beaker, replanted into moist soil, covered and placed in a growth chamber under long day conditions (16 h light and 8 h dark, 21°C) unless otherwise specified.
RNA isolation and RT-PCR
Total RNA was isolated from infiltrated leaves of Arabidopsis using Plant RNA Reagent as described by the manufacturer (Invitrogen, Carlsbad, CA), treated with DNaseI (Ambion, Austin, TX) and purified through RNeasy MiniElute clean up kit (Qiagen, Valencia, CA). Total RNA, free from contaminating DNA, was stored at −80°C. The presence of mRNA specific to the SCTV C2 or CaLCuV AL2 coding regions was confirmed by RT-PCR analysis using C2 or AL2 ORF specific primers. Specific cDNA products were detected by hybridization to 32P-labeled probes specific for either, SCTV C2 or CaLCuV AL2, generated by random priming (DECA Prime II labeling kit, Ambion, Austin, TX). DNA was identified by phosphorimager (Molecular Imager FX, Bio-Rad, Hercules, CA).
Quantitative real-time PCR analysis
The identification of differentially expressed genes depends on the ability to distinguish steady state mRNA levels. Thus, real time quantitative RT-PCR (qRT-PCR) was used to assess differences in expression of genes in response to the proteins of interest by comparison to a vector treated control. Total RNA (1 µg) isolated from Arabidopsis tissue as described above, and reverse transcribed using a high-capacity cDNA archive kit (Applied Biosystems, Foster city, CA). Real time PCR analysis was performed using a 7500 Real-time PCR system (Applied Biosystems, Foster city, CA) with TaqMan Gene Expression Assays (FAM reporter dye at the 5’ end of the TaqMan MGB probe and a non-fluorescent quencher at the 3’ end of the probe) at a concentration of 18µM for each primer and 5µM for the probe. Assays for Arabidopsis genes ARR5 (At3g48100) and ARR16 (At2g40670) were used. For each experiment, target samples were also analyzed using TaqMan Gene Expression Assays for Arabidopsis β-tubulin (At2g29550) as an endogenous control. Independent biological samples from two to four experiments were used for the analysis. For each biological sample we used three to four replicates. Ct values for each well position were examined prior to data analysis. Differences in gene expression between the target gene and endogenous control (ΔCt) for each replicate were calculated and used to measure differences between treatments (ΔΔCt) using 7500 System SDS software (Applied Biosystems, Foster city, CA). ΔCt values for all replicates within the control or treatment groups were tested for statistical variation (Yuan et al., 2006) using the parametric Student's t-test if the data indicated a normal distribution with equal variance. If this was not the case, then the non-parametric Wilcoxon Signed Rank test was used (Zar, 1974). Standard errors were calculated using the mean ΔCt values for control and treatment samples.
Southern and Northern Blot Hybridization
DNA or RNA was isolated from virus-infected N.benthamiana leaves and analyzed by DNA or RNA gel blot hybridization as described previously (Baliji et al., 2007). The presence of SCTV DNA or TBSV RNA was assessed by DNA or RNA gel blot hybridization to 32P-labeled probes specific for SCTV or TBSV. Probes were prepared by random priming (DECA Prime II labeling kit, Ambion, Austin, TX), using PCR products generated with primers specific for the SCTV C2 coding region (Baliji et al., 2007) or TBSV (Primers TBSV2F and TBSV 2R: Kim et al., 2007). Hybridization signals were quantified by phosphorimager analysis (Molecular Imager FX, Bio-Rad, Hercules, CA) and normalized to hybridization signals for 18S rDNA or 18S rRNA as appropriate (Sunter et al., 2001).
Acknowledgements
We thank Janet Sunter for maintenance and generation of N.benthamiana cell cultures and plants and for assistance with protoplast transfection experiments. We also thank Dr. Jun Tu for critical reading of the manuscript. Research supported by a National Institutes of Health grant (SO6-GM08194: MBRS/SCORE) awarded to GS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abouzid AM, Hiebert E, Strandberg JO. Cloning, identification and partial sequencing of the genomic components of a geminivirus infecting Brassicaceae. Phytopathology. 1992;82:1070–1074. [Google Scholar]
- Astot C, Dolezal K, Nordstrom A, Wang Q, Kunkel T, Moritz T, Chua N-H, Sandberg G. An alternative cytokinin biosynthesis pathway. Proc. Natl. Acad. Sci. USA. 2000;26:14778–14783. doi: 10.1073/pnas.260504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliji S, Sunter J, Sunter G. Transcriptional analysis of complementary sense genes in Spinach curly top virus and the functional role of C2 in pathogenesis. Mol. Plant-Microbe Interact. 2007;20:194–206. doi: 10.1094/MPMI-20-2-0194. [DOI] [PubMed] [Google Scholar]
- Baliji S, Black MC, French R, Stenger DC, Sunter G. Spinach curly top virus: A new curtovirus species from southwest Texas displaying incongruent gene phylogenies that suggest a history of recombination among curtoviruses. Phytopathology. 2004;94:772–779. doi: 10.1094/PHYTO.2004.94.7.772. [DOI] [PubMed] [Google Scholar]
- Brandstatter I, Kieber JJ. Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell. 1998;10:1009–1019. doi: 10.1105/tpc.10.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann RC, Asad S, Wolf JN, Mohannath G, Bisaro DM. Geminivirus AL2 and L2 proteins suppress transcriptional gene silencing and cause genome-wide reductions in cytosine methylation. J. Virol. 2009;83:5005–5013. doi: 10.1128/JVI.01771-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino IB, Deruere J, Kieber JJ. Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol. 2000;124:1706–1717. doi: 10.1104/pp.124.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelkrout EM, Robertson D, Hanley-Bowdoin L. Proliferating cell nuclear antigen transcription is repressed through an E2F consensus element and activated by geminivirus infection in mature leaves. Plant Cell. 2001;13:1437–1452. doi: 10.1105/tpc.13.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Wang H, Sunter G, Bisaro DM. Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. Plant Cell. 2003;15:1034–1048. doi: 10.1105/tpc.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi SG, Bisaro DM. Genetic analysis of Beet curly top virus: Examination of the roles of L2 and L3 genes in viral pathogenesis. Virology. 1995;206:1044–1054. doi: 10.1006/viro.1995.1027. [DOI] [PubMed] [Google Scholar]
- Hutchison CE, Kieber JJ. Cytokinin signaling in Arabidopsis. Plant Cell. 2002;14 Supplement:s47–s59. doi: 10.1105/tpc.010444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Chen H-C, Sheen J. Two-component signal transduction pathways in Arabidopsis. Plant Physiol. 2002;129:500–515. doi: 10.1104/pp.005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen LK, Carrington JC. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 2001;126:930–938. doi: 10.1104/pp.126.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Yamada H, Mizuno T. Characterization of the ARR15 and ARR16 response regulators with special reference to the cytokinin signaling pathway mediated by the AHK4 histidine kinase in roots of Arabidopsis thaliana. Plant Cell Physiol. 2002;43:1059–1066. doi: 10.1093/pcp/pcf121. [DOI] [PubMed] [Google Scholar]
- Kiba T, Taniguchi M, Imamura A, Ueguchi C, Mizuno T, Sugiyama T. Differential expression of genes for response regulators in response to cytokinins and nitrate in Arabidopsis thaliana. Plant Cell Physiol. 1999;40:767–771. doi: 10.1093/oxfordjournals.pcp.a029604. [DOI] [PubMed] [Google Scholar]
- Kim M, Kwak H-R, Jeong S-G, Ko S-J, Lee S-H, Park J-W, Kim K-H, Choi H-S, Cha B-J. First report on Tomato bushy stunt virus infecting tomato in Korea. Plant Pathol J. 2007;23:143–150. [Google Scholar]
- Kwade Z, Swiatek A, Azmi A, Goossens A, Inze D, Van Onckelen H, Roef L. Identification of four adenosine kinase isoforms in Tobacco BY-2 cells and their putative role in the cell cycle-regulated cytokinin metabolism. J. Biol. Chem. 2005;280:17512–17519. doi: 10.1074/jbc.M411428200. [DOI] [PubMed] [Google Scholar]
- Lecoq K, Belloc I, Desgranges C, Daignan-Fornier B. Role of adenosine kinase in Saccharomyces cerevisiae. identification of the ADO1 gene and study of the mutant phenotypes. Yeast. 2001;18:335–342. doi: 10.1002/1097-0061(20010315)18:4<335::AID-YEA674>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Lacatus G, Sunter G. Functional analysis of bipartite begomovirus coat protein promoter sequences. Virology. 2008;376:79–89. doi: 10.1016/j.virol.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Develop Biol. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Wismer CT, Mikusa J, Zhu CZ, Cowart M, Kowaluk EA, Jarvis MF. Effects of A-134974, a novel adenosine kinase inhibitor, on carrageenan-induced inflammatory hyperalgesia and locomotor activity in rats: evaluation of the sites of action. J. Pharmacol. Exp. Ther. 2001;296:501–509. [PubMed] [Google Scholar]
- Moffatt BA, Wang L, Allen MS, Stevens YY, Qin W, Snider JD, von Schwartzenberg K. Adenosine kinase of Arabidopsis. Kinetic properties and gene expression. Plant Physiol. 2000;124:1775–1785. doi: 10.1104/pp.124.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt BA, Stevens YY, Allen MS, Snider JD, Periera LA, Todorova MI, Summers PS, Weretilnyk EA, Martin-Caffrey L, Wagner C. Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol. 2002;128:812–821. doi: 10.1104/pp.010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell. 1999;11:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Raja P, Sanville BC, Buchmann RC, Bisaro DM. Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 2008;82:8997–9007. doi: 10.1128/JVI.00719-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SG, Klee HJ, Horsch RB, Fraley RT. Improved vectors for plant transformation: Expression cassette vectors and new selectable markers. Methods in Enzymol. 1987;153:253–277. [Google Scholar]
- Samach A, Coupland G. Time measurement and the control of flowering in plants. Bioessays. 2000;22:38–47. doi: 10.1002/(SICI)1521-1878(200001)22:1<38::AID-BIES8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Scholthof HB, Scholthof KBG, Jackson AO. Identification of Tomato Bushy Stunt Virus host-specific symptom determinants by expression of individual genes from a Potato virus X vector. Plant Cell. 1995;7:1157–1172. doi: 10.1105/tpc.7.8.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoor S, Moffatt BA. Applying high throughput techniques in the study of adenosine kinase in plant metabolism and development. Front. Biosci. 2004;9:1771–1781. doi: 10.2741/1346. [DOI] [PubMed] [Google Scholar]
- Stanley J, Latham J, Pinner MS, Bedford I, Markham PG. Mutational analysis of the monopartite geminivirus Beet curly top virus. Virology. 1992;191:396–405. doi: 10.1016/0042-6822(92)90201-y. [DOI] [PubMed] [Google Scholar]
- Sugden C, Crawford RM, Halford NG, Hardie DG. Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5'-AMP. Plant J. 1999;19:433–439. doi: 10.1046/j.1365-313x.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- Sunter G, Bisaro DM. Transactivation in a geminivirus: AL2 gene product is needed for coat protein expression. Virology. 1991;180:416–419. doi: 10.1016/0042-6822(91)90049-h. [DOI] [PubMed] [Google Scholar]
- Sunter G, Bisaro DM. Transactivation of geminivirus AR1 and BR1 gene expression by the viral AL2 gene product occurs at the level of transcription. Plant Cell. 1992;4:1321–1331. doi: 10.1105/tpc.4.10.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter G, Bisaro DM. Regulation of a geminivirus coat protein promoter by AL2 protein (TrAP): Evidence for activation and derepression mechanisms. Virology. 1997;232:269–280. doi: 10.1006/viro.1997.8549. [DOI] [PubMed] [Google Scholar]
- Sunter G, Gardiner WE, Rushing AE, Rogers SG, Bisaro DM. Independent encapsidation of Tomato golden mosaic virus A component DNA in transgenic plants. Plant Mol. Biol. 1987;8:477–484. doi: 10.1007/BF00017993. [DOI] [PubMed] [Google Scholar]
- Sunter G, Stenger DC, Bisaro DM. Heterologous complementation by geminivirus AL2 and AL3 genes. Virology. 1994;203:203–210. doi: 10.1006/viro.1994.1477. [DOI] [PubMed] [Google Scholar]
- Sunter G, Sunter JL, Bisaro DM. Plants expressing Tomato golden mosaic virus AL2 or Beet curly top virus L2 transgenes show enhanced susceptibility to infection by DNA and RNA viruses. Virology. 2001;285:59–70. doi: 10.1006/viro.2001.0950. [DOI] [PubMed] [Google Scholar]
- To JPC, Haberer G, Ferreira FJ, Deruère J, Mason MG, Schaller GE, Alonso JM, Ecker JR, Kiebera JJ. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schwartzenberg K, Kruse S, Reski R, Moffatt BA, Laloue M. Cloning and characterization of an adenosine kinase from Physcomitrella involved in cytokinin metabolism. Plant J. 1998;13:249–257. doi: 10.1046/j.1365-313x.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Hao L, Shung C-Y, Sunter G, Bisaro DM. Adenosine kinase is inactivated by geminivirus AL2 and L2 proteins. Plant Cell. 2003;15:3020–3032. doi: 10.1105/tpc.015180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Buckley KJ, Yang X, Buchmann RC, Bisaro DM. Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J. Virol. 2005;79:7410–7418. doi: 10.1128/JVI.79.12.7410-7418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weretilnyk EA, Alexander KJ, Drebenstedt M, Snider JD, Summers PS, Moffatt BA. Maintaining methylation activities during salt stress: the involvement of adenosine kinase. Plant Physiol. 2001;125:856–865. doi: 10.1104/pp.125.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Baliji S, Woody S, Buchmann RC, Wang H, Lindbo J, Sunter G, Bisaro DM. Functional modulation of the geminivirus AL2 transcription factor and silencing suppressor by self-interaction. J. Virol. 2007;81:11972–11981. doi: 10.1128/JVI.00617-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L-S, Harrison BR, Murthy N, Moffatt BA, Gilroy S, Masson PH. Adenosine kinase modulates root gravitropism and cap morphogenesis in Arabidopsis. Plant Physiol. 2006;142:564–573. doi: 10.1104/pp.106.084798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85–96. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. In: McElroy WD, Swanson CP, editors. Englewood Cliffs: Prentice-Hall; 1974. [Google Scholar]
- Zrenner R, Stitt M, Sonnewald U, Boldt R. Pyrimidine and purine biosynthesis and degradation in plants. Ann. Rev. Plant Biol. 2006;57:805–836. doi: 10.1146/annurev.arplant.57.032905.105421. [DOI] [PubMed] [Google Scholar]