Abstract
During 1994–2005, we isolated Mycobacterium microti from 5 animals and 4 humans. Only 1 person was immunocompromised. Spoligotyping showed 3 patterns: vole type, llama type, and a new variant llama type.
Keywords: Tuberculosis, zoonoses, domestic animals, wild animals, zoo, immunocompromised patients, molecular epidemiology, Scotland, dispatch
Naturally occurring mycobacteria that are part of the Mycobacterium tuberculosis complex include M. tuberculosis, M. bovis, M. caprae, M. africanum, M. microti, and M. pinnipedii. Although these species show remarkable genetic homology, there are notable phenotypic differences, particularly in their relative pathogenicity for different mammalian species.
Tuberculosis in wild rodents was first studied in 1937 as part of an investigation of cyclical changes in the population density of voles (1). Field voles, bank voles, wood mice, and shrews are particularly susceptible to infection with M. microti (2). However, other small mammals such as guinea pigs, rabbits, mice, and rats are resistant to M.microti infection, even at high doses of infection. More recently, sporadic cases have been described in larger mammals (3–6).
There have been only 6 published reports of human infections, comprising 13 patients in total (7–11). Salient information from these reports is summarized in Table 1.
Table 1. Summary of all reported cases of human infections with Mycobacterium microti*.
| Case-patient no. | Ref | Age, sex, country | Immune status | Infection site | Animal contact | Laboratory findings | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | (8) | 48 y, M, Germany | HIV positive | Lung | None | Llama type. Good growth in liquid medium. Poor on pyruvate. Drug susceptible. Curved bacilli. | Cured |
| 2 | (7,10) | 39 y, M, the Netherlands | HIV positive | Lung; lymph nodes | House mice | Cultures negative; curved bacilli in sputum. Vole type on direct spoligotyping. | Cured after prolonged therapy† |
| 3 | (7) | 12 y, M, the Netherlands | Renal transplant | Lung meninges | None | Vole type. Other details unavailable. | Cured |
| 4 | (7) | 41 y, M, the Netherlands | Renal transplant | Peritoneal | Wild small rodents | Vole type. Other details unavailable. | Died despite therapy |
| 5 | (7) | 34 y, M, the Netherlands | Normal | Lung | Lived in mobile home | Vole type. Other details unavailable. | Cured |
| 6 | (9) | 53 y, M, Germany | Normal | Lung | None | Llama type. AFB film negative. Liquid culture better than pyruvate agar. No growth on normal egg media. Fully drug susceptible. Noncurved bacilli. | Cured |
| 7 | (9) | 58 y, M, Germany | Diabetic | Lung | None | Vole type. Growth on liquid culture only (poor). Susceptibility not done. Noncurved bacilli. | Cured |
| 8 | (5) | Not known; England or Wales | Not known | Not known | Not known | Llama type. | Not known |
| 9 to 12 | (5) | Not known; England or Wales | Not known | Not known | Not known | Vole type. | Not known |
| 13 | (11) | 69 y, sex not known, Germany | Normal | Abdominal/ miliary | Not known | Vole type. Primary culture in liquid. Subculture in solid agar. | Died, despite appropriate therapy |
*Ref, reference; M, male; AFB, acid-fast bacilli. †Three household contacts were found to be tuberculin positive.
M. microti has been used in extensive trials to assess its efficacy and safety as a vaccine. Percutaneously administered M. microti vaccine was found to be safe but no more effective than M. bovis BCG (12). The low virulence and poor immunogenicity are due to several key genetic deletions, resulting in the inability to produce the strongly immunogenic T-cell antigens ESAT-6 and CFP-10 (13).
Several genotypes of M. microti have been recognized by spacer oligotyping (spoligotyping). The llama-type (presence of spacers 4–7, 23, 24, 26, 37, 38) and the vole-type (only 2 spacers, 37 and 38) have been well described; both types are involved in human infections (5,7). The international spoligotyping database (SpolDB4) (14) includes 40 M. microti strains, 37 of which are from the United Kingdom and Western Europe. Although there are no published reports of M. microti infections from the United States, 3 of the strains in SpolDB4 are from this country. M. microti strains yield broadly similar, high–copy number fingerprints by the insertion sequence 6110–based restriction fragment length polymorphism method (IS6110 RFLP) (7).
In the 12-year period from 1994 through 2005, we isolated M. microti from 4 humans and from 5 animals (2 cats, a llama, a badger, and a ferret). No clinical details were available for the animal cases. The animal and human cases were from different locations in Scotland. No epidemiologic links were apparent.
The Patients
Patient 1 was a 41-year-old woman in whom sputum smear–positive tuberculosis was diagnosed in 2001. She was treated with isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months and for 4 months more with rifampin and isoniazid. She made good clinical progress, but sputum samples remained positive for acid-fast bacilli (AFB), although cultures were negative. She was re-treated with isoniazid, rifampin, ethambutol, and pyrazinamide for 6 months. She became sputum negative and remained clinically well at her 6-month follow-up visit. She was not immunocompromised. No other patients with tuberculosis were identified in contacts, and no relevant animal contact had occurred.
Patient 2 was a 39-year-old man for whom HIV was diagnosed in 2003, who had bilateral pulmonary consolidation. The patient lived on a farm. He was initially treated with co-trimoxazole for suspected Pneumocystis carinii infection, and rifampin, isoniazid, and pyrazinamide were added when AFB were seen in the sputum sample. The patient’s condition deteriorated, and he died despite this drug treatment and intensive therapy unit support. No other patients with tuberculosis were identified in connection with this case.
Patient 3 was a 76-year-old woman who had received a diagnosis of pulmonary tuberculosis in 2005. She made an uneventful recovery following standard therapy with isoniazid, rifampin, and ethambutol for 2 months, followed by rifampin and isoniazid for a further 4 months. She was not immunocompromised, and she reported no major animal contact. No cases of tuberculosis were identified in connection with this patient.
Patient 4 was a 45-year-old woman who was seen in 2005 for hemoptysis; a diagnosis of cavitating pulmonary tuberculosis was made. She received treatment with isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months and rifampin and isoniazid for 4 months more. She remained unwell, with further hemoptysis, and a residual cavity was shown on chest x-ray. Chemotherapy was reintroduced. She was not known to be immunocompromised. She had a pet cat and a dog, both in good health. No cases of tuberculosis were identified in contacts.
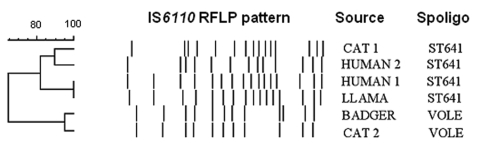
The laboratory characteristics of the isolates are shown in Table 2. Biochemical tests were not possible because of sparse growth. Isolates were identified as M. tuberculosis complex by using the Accuprobe culture confirmation assay (GenProbe, San Diego, CA, USA), and species identification as M. microti was confirmed by spoligotyping. Since we do not perform drug susceptibility testing using solid media, only the 3 strains that grew well in liquid subculture were tested. Genotyping data on our isolates are summarized in Table 2 and the Figure.
Table 2. Laboratory features of Mycobacterium microti isolates from Scotland*†.
| Source | Specimen | Direct AFB | Growth on primary isolation |
Drug susceptibility | Genotype | |||
|---|---|---|---|---|---|---|---|---|
| Solid culture |
Liquid culture |
|||||||
| IUT | PYR | MB/MGIT | ||||||
| Human 1 | Sputum | Positive | – | + | – | Failed to grow in liquid cultures | Llama type SIT641 (spacers 4–7, 23,24, 37, and 38) | |
| Human 2 | Sputum | Positive (many) | – | + | – | Failed to grow in liquid cultures | Llama type SIT641 | |
| Human 3 | Sputum | Positive (many) | – | – | + | Susceptible to R, I, E; resistant to P | Llama type SIT641 | |
| Human 4 | Sputum | Positive (few) | + | + | – | Failed to grow in liquid culture | Llama type (spacers 4–7 and 23,24 only) | |
| Cat 1 | Tissue/lymph node | Negative | – | + | – | Failed to grow in liquid cultures | Llama type SIT641 | |
| Badger | Tissue/lung | Strongly positive | + | + | ± | Inadequate growth | Vole type SIT 539 (spacers 37 and 38) | |
| Cat 2 | Tissue/lymph node | Negative | – | + | – | Susceptible to R,I,E,P (grew on liquid subculture) | Vole type SIT539 | |
| Llama | Tissue/lung | Positive | + | + | – | Failed to grow in liquid culture | Llama type SIT641 | |
| Ferret | Tissue | Positive | ± | ± | ± | Susceptible to R,I,E,P | Not tested | |
*AFB, acid-fast bacilli; IUT, International Union Against Tuberculosis formulation of solid egg medium; PYR, IUT medium with pyruvate supplementation; MB, MBBact, Biomerieux, Basingstoke, United Kingdom; MGIT, Mycobacteria Growth Indicator Tube, Becton Dickinson; R,I,E,P, rifampin, isoniazid, ethambutol, pyrazinamide; SIT, Spoligo-International-Type. †SIT numbers: designations in International spoligotyping database (Spol DB4) (14).
Figure.
Comparison of the restriction fragment length polymorphism patterns of Mycobacterium microti strains from Scotland. Spoligo, spoligotyping.
Conclusions
M. microti infection is widespread in wild small rodent populations in the United Kingdom (2). There are sporadic reports, all from the United Kingdom and Western Europe, of M. microti infection in other mammals. Certain animals, such as cats (4,5) and New World camelids domesticated in Europe (6), seem to be particularly susceptible. The reported animal cases have all been detected in clinical veterinary practice and are unlikely to reflect the true field incidence. Difficulties with laboratory diagnosis probably further contribute to the underestimation of the incidence. M. microti grows poorly on traditional solid egg media, and modern automated liquid culture techniques do not seem to yield better results. Moreover, even when a mycobacterial infection is diagnosed, routine veterinary diagnostic procedures often do not identify the mycobacterium to species level. It is likely also that known animal cases are not all formally reported in the literature.
The transmission of M. microti to pets, particularly cats, is of particular concern. Cats are assumed to acquire the M. microti infection from infected wild rodents, but this assumption is not supported by the genotyping evidence. Most of the strains isolated from cats are genotypically very distinct from wild rodent strains, as shown in our cases and in the literature (5). Very little is known about the incidence and ecology of M. microti infection in farm and domestic animals.
Many of the human patients with M. microti infection appear to have no immunologic deficits (3 of our 4 patients and 3 of the 8 published cases for which relevant clinical details were available). However, inherited defects of interleukin receptor function are known to specifically predispose to intracellular infections, particularly mycobacterial infection (15). Therefore, some persons with apparently normal immunity infected with M. microti may in fact have undetected specific immune defects.
Human-to-human transmission of M. microti infection seems rare. In the single instance in which this possibility is moot, the secondary cases all occurred in the same mice-infested household (10).
Extensive trials of M. microti as a vaccine suggest that it lacks virulence for humans with normal immunity. However, it remains a potential threat to the substantial pool of persons with compromised immunity, including the unknown number who may have genetic defects specifically predisposing to mycobacterial infections.
Acknowledgments
We thank the microbiologists and physicians from hospitals in Scotland for discussions and clinical information relating to the cases reported in this article. We particularly acknowledge David Hamilton, Michael Lockhart, Ken Dagg, David Thetford, and Tim Brown.
This research is part of the remit of the Scottish Mycobacteria Reference Laboratory and was funded entirely through its contract with Health Protection Scotland.
Biography
Dr Emmanuel retired recently from his post as consultant medical microbiologist at the Department of Laboratory Medicine at the Royal Infirmary of Edinburgh. The department incorporates the Scottish Mycobacteria Reference Laboratory, which he directed during the period that this research was carried out. His research interests include the molecular epidemiology of Mycobacterium tuberculosis complex.
Footnotes
Suggested citation for this article: Emmanuel FX, Seagar A-L, Doig C, Rayner A, Claxton P, Laurenson I. Human and animal infections with Mycobacterium microti, Scotland. Emerg Infect Dis [serial on the Internet]. 2007 Dec [date cited]. Available from http://www.cdc.gov/EID/content/13/12/1924.htm
References
- 1.Wells AQ. Tuberculosis in wild voles. Lancet. 1937;i:1221. 10.1016/S0140-6736(00)83505-9 [DOI] [Google Scholar]
- 2.Cavanagh R, Begon M, Bennett M, Ergon T, Graham IM, De Haas PE, et al. Mycobacterium microti infection (vole tuberculosis) in wild rodent populations. J Clin Microbiol. 2002;40:3281–5. 10.1128/JCM.40.9.3281-3285.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahans K, Palmer S, Inwald J, Brown J, Abayakoon S. Isolation of Mycobacterium microti from a male Charolais-Hereford cross. Vet Rec. 2004;155:373–4. [PubMed] [Google Scholar]
- 4.Gunn-Moore DA, Jenkins PA, Lucke VM. Feline tuberculosis: a literature review and discussion of 19 cases caused by an unusual mycobacterial variant. Vet Rec. 1996;138:53–8. [DOI] [PubMed] [Google Scholar]
- 5.Kremer K, Van Soolingen D, Van Embden J, Hughes S, Inwald J, Hewinson G. Mycobacterium microti: more widespread than previously thought. J Clin Microbiol. 1998;36:2793–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oevermann A, Pfyffer GE, Zanolari P, Meylan M, Robert N. Generalized tuberculosis in llamas (Lama glama) due to Mycobacterium microti. J Clin Microbiol. 2004;42:1818–21. 10.1128/JCM.42.4.1818-1821.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Soolingen D, Van der Zanden AG, De Haas PE, Noordhoek GT, Kiers A, Foudraine NA, et al. Diagnosis of Mycobacterium microti infections among humans by using novel genetic markers. J Clin Microbiol. 1998;36:1840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horstkotte MA, Sobottka I, Schewe CK, Schäfer P, Laufs R, Rüsch-Gerdes S, et al. Mycobacterium microti llama-type infection presenting as pulmonary tuberculosis in a human immunodeficiency virus-positive patient. J Clin Microbiol. 2001;39:406–7. 10.1128/JCM.39.1.406-407.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemann S, Richter E, Dalügge-Tamm H, Schlesinger H, Graupner D, Königstein B, et al. Two cases of Mycobacterium microti–derived tuberculosis in HIV-negative immunocompetent patients. Emerg Infect Dis. 2000;6:539–42. 10.3201/eid0605.000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foudraine NA, Van Soolingen D, Noordhoek GT, Reiss P. Pulmonary tuberculosis due to Mycobacterium microti in a human immunodeficiency virus–infected patient. Clin Infect Dis. 1998;27:1543–4. 10.1086/517747 [DOI] [PubMed] [Google Scholar]
- 11.Geiss HK, Feldhues R, Neimann O, Nolte R, Reiker R. Landousy septicaemia (sepsis tuberculosa acutissima) due to Mycobacterium microti in an immunocompetent man. Infection. 2005;33:393–6. 10.1007/s15010-005-5075-3 [DOI] [PubMed] [Google Scholar]
- 12.Hart PDA, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. BMJ. 1977;2:293–5. 10.1136/bmj.2.6082.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Pelayo MC, Caimi KC, Inwald JK, Hinds J, Bigi F, Romano MI, et al. Microarray analysis of Mycobacterium microti reveals deletion of genes encoding PE-PPE proteins and ESAT-6 family antigens. Tuberculosis (Edinb). 2004;84:159–66. 10.1016/j.tube.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 14.Brudey K, Driscoll JR, Rigouts L, Prodinger WM, Gori A, Al-Hajoj SA, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6:23. 10.1186/1471-2180-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, et al. Low penetrance, broad resistance, and favourable outcome of interleukin 12 receptor beta 1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–35. 10.1084/jem.20021769 [DOI] [PMC free article] [PubMed] [Google Scholar]