Abstract
Adult obese Zucker rats (OZRs) have reduced sympathetic responses to evoked changes in arterial pressure (AP) compared to lean Zucker rats (LZRs). This study examined whether attenuated sympathetic baroreflexes in OZRs may be due to altered sensory or central mechanisms. The OZRs had elevated baseline splanchnic sympathetic nerve activity (SNA) and mean AP (MAP) compared to age-matched LZRs under urethane anaesthesia (P < 0.05). Aortic depressor nerve activity (ADNA) was measured while AP was altered by infusions of phenylephrine or nitroprusside (±60 mmHg over 60–90 s) in rats treated with atropine and propranolol to eliminate changes in heart rate. Although baseline ADNA was higher in the hypertensive OZRs, the relationship between MAP and ADNA was comparable in OZRs and LZRs. In contrast, electrical stimulation of the ADN afferent fibres (5 s train, 2 ms pulses, 4 V, 0.5–48 Hz) produced dramatically smaller reductions in SNA and MAP in OZRs compared to LZRs (P < 0.05). After blockade of α-adrenergic receptors to prevent sympathetically mediated depressor responses, OZRs still had reduced sympathetic responses to stimulation of the ADN. In addition, stimulation of vagal afferent nerves electrically or with phenylbiguanide (1, 2, 4 and 8 μg, i.v.) produced smaller inhibitions of SNA in OZRs compared with LZRs (P < 0.05). These data suggest that attenuated sympathetic baroreflexes are the result of altered central mechanisms in OZRs, and not deficits in the responsiveness of aortic baroreceptors to AP. Furthermore, central deficits in the regulation of SNA in OZRs extend to other sympathoinhibitory reflexes initiated by vagal afferent nerves.
Introduction
Obesity is associated with elevated vasomotor sympathetic nerve activity (SNA) and hypertension in humans and other animals (Tuck, 1992; Rocchini et al. 1999; Carlson et al. 2000). Short term autonomic regulation of heart rate (HR) and vascular resistance by arterial baroreflexes is impaired (Buñag et al. 1990; Grassi et al. 2000; Schreihofer et al. 2007), probably contributing to the altered variability in HR and mean arterial pressure (MAP) observed with obesity (Piccirillo et al. 1998; Carlson et al. 2000; Overton et al. 2001). Obese Zucker rats (OZRs), which have non-functional leptin receptors, become obese due to hyperphagia compared to lean Zucker rats (LZRs; Iida et al. 1996; Overton et al. 2001). The OZRs develop cardiovascular complications analogous to human obesity including higher resting SNA and MAP, reduced HR variability, and increased range of spontaneous MAP (Morgan et al. 1995; Carlson et al. 2000; Overton et al. 2001). These changes in basal function are linked to altered autonomic control of cardiovascular parameters. Adult OZRs have attenuated baroreflex-mediated changes in SNA to vascular targets, a deficit that emerges in adulthood after the onset of obesity and concomitant with rises in resting SNA and MAP (Schreihofer et al. 2007). In addition, reduced baroreflex-mediated changes in HR in OZRs are due to impairments in sympathetic and parasympathetic control of the heart (Buñag & Barringer, 1988; Barringer & Buñag, 1989). Mechanisms underlying these deficits associated with obesity are not known.
Many models of hypertension are associated with elevated SNA and impaired baroreflexes, but these deficits arise from multiple causes with potentially unique implications for cardiovascular regulation and treatment. In some cases, deficits at the sensory end of the reflex are observed, either due to altered stretch of the vessel or mechanotransduction at the receptor itself. For example, in baroreflex-impaired Dahl salt-sensitive rats, aortic depressor nerves have reduced activity and responsiveness to changes in MAP, but baroreflex responses to electrical activation of baroreceptor afferent fibres mediated through the central nervous system (CNS) are normal (Gordon & Mark, 1984; Miyajima & Buñag, 1986). In contrast, in spontaneously hypertensive rats (SHR) and renal wrap hypertensive rats, direct stimulation of arterial baroreceptor afferent fibres evokes blunted decreases in SNA and AP, suggesting that the processing of incoming baroreceptor-related information by the CNS is altered (Gonzalez et al. 1983; Zhang & Mifflin, 2000; Salgado et al. 2007). The aim of this study was to determine whether the OZRs display reduced sympathetic baroreflexes due to altered sensory or central mechanisms.
Methods
Ethical approval
Rats were housed in the animal care facility at the Medical College of Georgia, which is approved by the American Association for the Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee at the Medical College of Georgia approved the protocols. All experiments were conducted in agreement with the regulations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The authors have read and complied with guidelines for research in rodents outlined for The Journal of Physiology and UK regulations (Drummond, 2009).
Animal preparation and physiological measures
Age-matched adult (14–18 weeks old) male OZRs and LZRs (Harlan, Madison, WI, USA) were fed standard rat chow and tap water ad libitum. Rats were anaesthetized with isoflurane via a nose cone for surgery (induction with 5% and maintenance with 1.9–2.5% in 100% oxygen). Initial adequacy of anaesthesia was verified by the absence of withdrawal to a firm toe pinch. A catheter was implanted in a femoral vein to administer drugs and in a femoral artery to measure AP. The LZRs were artificially ventilated (55–65 strokes min−1 of 1 ml (100 g body weight)−1, Model 683, Harvard Apparatus). The OZRs were initially ventilated at the tidal volume of the age-matched LZRs, and volume was adjusted slightly upward to maintain end-tidal CO2 at 3.5–4.0% (Schreihofer et al. 2007). Rats were immobilized in a stereotaxic instrument (Kopf Instruments) in the prone position. The left greater splanchnic nerve was exposed retroperitoneally, placed on two Teflon-coated silver wires bared at the tips, and surrounded by dental impression material (Superdent, Darby Dental) as previously described (Mandel & Schreihofer, 2006; Schreihofer et al. 2007). The left ADN was identified as it joined the cervical vagus nerve near the superior laryngeal nerve and isolated from connective tissue (Huber et al. 2007). The ADN was placed on two Teflon-coated silver wires bared at the tips and surrounded by silicone elastomers (Kwik-Sil, World Precision Instruments). After surgical procedures were completed, isoflurane anaesthesia was replaced by urethane to facilitate observation of baroreflexes (1.5 g kg−1 LZR body weight in 1.5 g (5 ml)−1, 50 μl min−1, i.v.; Schreihofer et al. 2007). Once anaesthetized with urethane, rats were allowed to recover for 30–45 min. Rectal temperature was maintained at 37°C. Shortly before beginning experiments, adequacy of anaesthesia was confirmed (absence of corneal reflex, and <10 mmHg increase in AP to firm toe pinch), and then rats were neuromuscularly blocked (1 mg kg−1 pancuronium, i.v., Abbott Labs). At the end of each experiment the deeply anaesthetized rat was decapitated.
Assessments of ADNA in relation to MAP and SNA
Rats were treated with propranolol (5 mg kg−1, i.v.) and atropine (2 mg kg−1, i.v.) to prevent HR-related changes in ADNA. In the first protocol, changes from baseline ADNA were measured after evoked increases and decreases in AP by infusion of phenylephrine (50 μg ml−1) and sodium nitroprusside (50 μg ml−1), respectively. Infusions were regulated to alter MAP by 60 mmHg over 60–90 s (2.5 to 30 μg kg−1 min−1) using a syringe pump (Braintree Scientific, Braintree, MA, USA). To compare changes in multi-fibre ADNA and SNA from different rats, nerve activities were normalized as a percentage of baseline. In the second protocol, to eliminate potential differences in baseline ADNA, rats were treated with an autonomic ganglionic antagonist mecamylamine (10 mg kg−1, i.v.) or chlorisondamine (10 mg kg−1, i.v.) to eliminate SNA and reduce MAP below threshold for ADNA (Sapru & Wang, 1976). Here ADNA rectified voltage was measured as MAP was steadily increased from ∼60 to 200 mmHg over 3 min as a continuous ramp by increasing the infusion rate of phenylephrine (50 μg ml−1, 2.5 to 45 μg kg−1 min−1, i.v.; Gordon & Mark, 1984). The ADNA was quantified step-wise for each 10 mmHg and plotted with a sigmoidal curve fit. The threshold MAP for ADNA was estimated as the MAP that induced ADN bursts at three consecutives systolic pulses.
Assessment of stimulation ADN and other vagal afferents on SNA and MAP
In other rats, the ability of the CNS to inhibit SNA with direct electrical stimulation of baroreceptor afferent fibres of the ADN was measured. In addition, the ability of the CNS to inhibit SNA with electrical stimulation of isolated vagal afferent nerves independent of the ADN was measured.
In the first protocol, the ADN was placed on a bipolar electrode connected to a stimulator (model A300; World Precision Instruments) and isolated with Kwik-Sil for discrete and graded stimulations (5 s train, 4.0 V, 2.0 ms pulses at 0.5, 1, 2, 4, 8, 16, 24, 32, 40 and 48 Hz). The SNA, HR and MAP were continuously measured during alternating 3 min periods of control and ADN stimulation at each frequency. To eliminate potential differences in baroreceptor feedback due to concomitant changes in AP, the protocol was repeated after blockade of α-adrenergic receptors (phentolamine, 10 mg kg−1, i.v.) to eliminate sympathetically mediated changes in AP.
In the second protocol, while phentolamine continued to block sympathetically mediated changes in AP, the right vagus nerve was sectioned and the afferent end was placed on a bipolar electrode connected to a stimulator (model A300; World Precision Instruments). Following a 30 min recovery period, SNA, HR and MAP were continuously measured during alternating 3 min periods of control and stimulations of the vagal afferent nerve (5 s train, 4.0 V and 2.0 ms pulses at 0.5, 1, 2, 4 and 8 Hz).
With no antagonist pretreatment, other rats were injected with phenylbiguanide (1, 2, 4 and 8 μg, i.v.) to activate chemosensitive cardiopulmonary vagal afferents (Schreihofer & Guyenet, 2000) and determine reflex-mediated changes in SNA, HR and MAP. Values were allowed to recover to baseline for 5 min between doses. As the blood volume of age-matched adult OZRs and LZRs are comparable despite differences in body weight (Schreihofer et al. 2005), the bolus doses of phenylbiguanide were given by drug weight instead of body weight.
Data analysis and statistics
Analog physiological variables were converted to digital signals (Micro 1401, Cambridge Electronic Design) and viewed online (Spike2 software, Cambridge). Amplifiers and filters from the Neurolog system (Digitimer) were used to quantify AP and MAP. The HR was triggered from the rising phase of the AP pulse (Spike trigger, Neurolog). The ADNA and SNA were amplified and filtered (10 Hz to 3 kHz, 60 Hz notch filter, differential AC amplifier 1700, A-M Systems). Raw ADNA and SNA signals were full-wave rectified and averaged into 1 s bins. For estimation of baseline ADNA and SNA between OZRs and LZRs, the rectified voltage after subtraction of voltage due to noise was used. For estimation of per cent change in nerve activities, baseline (100%) was measured for 30 s immediately preceding each stimulus. Voltage due to noise was determined after ADNA or SNA were silenced by ganglionic blockade and set at 0%. Ganglionic blockade silenced SNA because recordings were from postganglionic nerves and silenced ADNA because AP was reduced below threshold for firing (Sapru & Wang, 1976). As previously described (Huber et al. 2007), the sensitivity of the ADN to changes in AP and baroreflex-mediated changes in SNA were assessed by generating a MAP–nerve activity curve fitted by a 4-parameter sigmoid regression (Sigma-Plot software) and the equation model: y= A1/1 + exp([A2(MAP − A3)]+ A4), where A1 is the range of the nerve activity, A2 is the gain coefficient, A3 is the MAP at the midpoint of the curve (MAP50) and A4 is the lower plateau.
All group data are expressed as mean ±s.e.m. Significant statistical difference was set at P < 0.05. Baseline values for body weight, MAP, HR, SNA, ADNA and parameters of sigmoid analyses were compared between OZRs and LZRs by unpaired t tests. For experiments utilizing a series of stimuli, the mean changes in SNA, HR and AP were each compared between OZRs and LZRs by two-way ANOVA with repeated measures followed by Tukey-Kramer post hoc tests (SigmaStat software).
Results
Adult OZRs weighed significantly more than LZRs (619 ± 8 g in 26 OZRs vs. 405 ± 6 g in 32 LZRs, P < 0.05). As previously reported for conscious adult Zucker rats (Carlson et al. 2000; Osmond et al. 2009), baseline MAP was higher in OZRs compared to LZRs when rats were initially anaesthetized with isoflurane (Table 1), and this difference was maintained when anaesthesia was switched to urethane for experimental protocols (Table 1). Rectified SNA voltage was also significantly higher in OZRs compared to LZRs under both anaesthetic conditions (Table 1), in agreement with a previous observation of increased renal SNA in OZRs (Morgan et al. 1995). In contrast, HR was not significantly different between LZRs and OZRs under either anaesthetic condition (Table 1).
Table 1.
Baseline values for splanchnic sympathetic nerve activity (SNA), mean arterial pressure (MAP) and heart rate (HR) for lean Zucker rats (LZRs) and obese Zucker rats (OZRs)
| Isoflurane anaesthesia |
Urethane anaesthesia |
||||||
|---|---|---|---|---|---|---|---|
| n | SNA (μV) | MAP (mmHg) | HR (beats min−1) | SNA (μV) | MAP (mmHg) | HR (beats min−1) | |
| LZR | 32 | 1.7 ± 0.2 | 116 ± 3 | 400 ± 7 | 1.2 ± 0.1 | 122 ± 3 | 424 ± 4 |
| OZR | 26 | 3.1 ± 0.2* | 137 ± 2* | 392 ± 5 | 2.9 ± 0.3* | 130 ± 3* | 413 ± 7 |
Values are mean ±s.e.m.
Significantly different from LZRs (P < 0.05).
Baroreflex-mediated responses to evoked changes in AP
Infusion of phenylephrine increased MAP and ADNA while evoking a reflexively mediated decrease in SNA (Fig. 1C and F). Conversely, infusion of nitroprusside decreased MAP and ADNA while evoking a reflexively mediated increase in SNA (Fig. 1A and D). Sigmoidal analysis revealed comparable changes in MAP and ADNA between OZRs and LZRs (Fig. 2A versus C, Table 2). In contrast, as previously shown (Schreihofer et al. 2007), baroreflex control of SNA was significantly blunted in OZR, as indicated by the reduced baroreflex sensitivity (slope) and range of the SNA in relation to AP (Fig. 2B versus D, Table 2). In particular, the evoked rise in AP effectively silenced SNA in LZRs but not in OZRs (Fig. 1C versus F, Table 2).
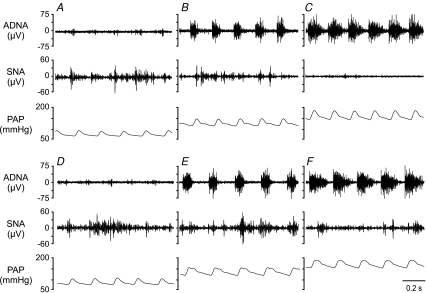
Figure 1. Typical responses of aortic depressor nerve and sympathetic nerve activity to evoked changes in arterial pressure.
Representative recordings of aortic depressor nerve activity (ADNA), splanchnic sympathetic nerve activity (SNA) and pulsatile arterial pressure (PAP) from one lean Zucker rat (LZR, top) and one obese Zucker rat (OZR, bottom). Minimal ADNA is observed when AP is reduced by nitroprusside (A and D). At baseline AP, ADNA shows bursts with each pulse of AP in both OZR and LZR (B and E). When AP is increased by infusion of phenylephrine, the bursts of ADNA are increased in both groups (C and F). Here, SNA is readily silenced in the LZR (C), but is still present in the OZR (F).
Figure 2. Typical baroreflex curves for ADNA and SNA in OZRs and LZRs.
Representative sigmoidal analyses for adult LZRs (A and B) and OZRs (C and D). Upper plots depict mean AP (MAP) versus ADNA from one LZR (A) and one OZR (B). Lower plots depict MAP versus SNA from one LZR (C) and one OZR (D).
Table 2.
Average parameters of sigmoidal baroreflex curves for LZRs and OZRs
| n | MAP50 (mmHg) | Slope (%/mmHg) | L Plateau (%) | Range (%) | |
|---|---|---|---|---|---|
| ADNA | |||||
| LZR | 11 | 144 ± 3 | 20 ± 2 | −7 ± 3 | 249 ± 21 |
| OZR | 9 | 139 ± 3 | 16 ± 2 | 1 ± 5 | 232 ± 43 |
| SNA | |||||
| LZR | 11 | 165 ± 2 | −13 ± 1 | −6 ± 5 | 129 ± 10 |
| OZR | 8 | 161 ± 7 | −8 ± 2* | 45 ± 4* | 56 ± 4* |
Values are mean ±s.e.m. ADNA, aortic depressor nerve activity; SNA, sympathetic nerve activity; MAP50, MAP at the midpoint of the curve; L Plateau, lower plateau; Range = upper plateau – lower plateau.
Significantly different from LZRs (P < 0.05).
As SNA baselines measured as voltages were significantly different between LZRs and OZRs (Table 1), baroreflex-mediated changes in SNA were also analysed as changes in full-wave rectified voltage. As observed with the analysis of SNA as a percentage change from baseline, the lower plateau SNA after infusion of phenylephrine was higher in OZRs (0.860 ± 0.247 μV, n= 12) compared to LZRs (0.083 ± 0.076 μV, n= 16, P < 0.05). However, the upper plateau SNA after unloading of baroreceptors with nitroprusside was comparable (3.161 ± 0.684 μV in OZRs and 2.619 ± 0.582 μV in LZRs). As observed with analysis of percentage change in SNA, the slope relating changes in the voltage of SNA versus AP was blunted in OZRs (−8.87 ± 1.22 μV mmHg−1) compared to LZRs (−13.04 ± 1.49 μV mmHg−1, P < 0.05).
After reducing AP below threshold for ADNA, the absolute rise in ADNA in response to a phenylephrine-induced ramp of increasing AP was indistinguishable between OZRs and LZRs (Fig. 3). The threshold MAP for the onset of ADNA was comparable between OZRs (83 ± 6 mmHg) and LZRs (86 ± 3 mmHg). In addition, raising MAP to 200 mmHg produced equivalent maximal ADNA in OZRs (3.2 ± 0.5 μV) and LZRs (3.0 ± 0.4 μV). These data suggest that despite the modest but significant hypertension observed in OZRs (Table 1), there is no resetting of baroreceptor afferent activity. Accordingly, baseline ADNA was significantly higher in hypertensive OZRs (2.3 ± 0.3 μV, n= 22) compared to LZRs (1.4 ± 0.2 μV, n= 21, P < 0.05).
Figure 3. Relationship between AP and ADNA after ganglionic blockade.
Groups of LZRs (n= 7) and OZRs (n= 7) were treated with chlorisondamine to decrease AP and minimize ADNA. Then, AP was steadily raised by infusion of phenylephrine. The plot shows MAP versus ADNA (expressed in μV) fitted by four-parameters logistic sigmoidal regression.
Changes in SNA, HR and MAP due to stimulation of the ADN
Electrical stimulation of ADN afferent fibres produced significantly smaller inhibitions of SNA and MAP in OZRs compared to LZRs at 4–48 Hz (Fig. 4A and B). Although there was a trend for blunted HR responses in OZRs, they were not significantly different from LZRs (e.g. −12 ± 4 versus−19 ± 3 beats min−1 at 48 Hz). After antagonism of α1-adrenergic receptors with phentolamine to prevent sympathetically mediated depressor responses, OZRs still had reduced inhibition of SNA to stimulation of the ADN compared with LZRs at 8–48 Hz (Fig. 4C). Under these conditions, ADN-evoked changes in MAP were absent (Fig. 4D), indicating effective adrenergic receptor blockade. The decreases in HR in response to ADN stimulation were greatly reduced by phentolamine, but were not significantly different between OZRs and LZRs (−8 ± 3 versus−7 ± 1 beats min−1 at 48 Hz).
Figure 4. Effects of ADN stimulation on SNA and AP in LZRs and OZRs.
Frequency-dependent changes in SNA (A) and MAP (B) in response to electrical stimulation of the left ADN in LZRs (filled bars; n= 9) and in OZRs (open bars; n= 9). After sympathetically mediated changes in AP were clamped by injection of phentolamine, stimulation of the ADN still evoked reduced inhibitions of SNA (C) in OZRs compared to LZRs. There were no significant changes in MAP under these conditions (D). *Significant difference from LZRs at that stimulus intensity (P < 0.05).
Changes in SNA and MAP due to vagal stimulation
Electrical stimulation
This protocol was performed after treatment with phentolamine and stimulation of the ADN. Electrical stimulation of the vagal afferent nerve produced two phases of responses that were dependent upon the frequency of the stimulation due to the activation of different sets of afferent nerves and reflex pathways (Sun & Guyenet, 1987). Therefore, the sympathoinhibitory responses evoked by low-frequency stimulation (0.5–8 Hz; Fig. 5A) were analysed separately from the sympathoexcitatory responses evoked by high-frequency stimulation (16–48 Hz; Fig. 5B). Low-frequency stimulation of vagal afferent nerves produced a significantly smaller inhibition of SNA in OZRs compared to LZRs at 8 Hz (Fig. 5A). In contrast, high-frequency stimulation evoked increases in SNA that were not significantly different between OZRs and LZRs (Fig. 5B).
Figure 5. Effects of vagal afferent nerve stimulation on SNA in LZRs and OZRs.
Electrical stimulation of right vagal afferent nerves in LZRs (n= 11, filled bars) and OZRs (n= 10, open bars) after injection of phentolamine to prevent sympathetically mediated changes in AP. In the low frequency range (A) inhibition of SNA was attenuated in OZRs at the highest frequency of stimulus. In the high frequency range (B) increases in SNA were comparable in LZRs and OZRs. Data are means ±s.e.m. *Significant difference from LZRs at that stimulus intensity (P < 0.05).
Chemical stimulation
Chemosensitive cardiopulmonary vagal afferent nerves were activated by the intravenous injection of the serotonin 5HT3 agonist phenylbiguanide (Verberne & Guyenet, 1992; Schreihofer & Guyenet, 2000). This protocol was performed before the antagonism of α-adrenergic receptors with phentolamine, so changes in AP were intact. The sympathoinhibition evoked by phenylbiguanide was significantly smaller in OZRs than LZRs for all four doses examined (Fig. 6). However, the decreases in HR (−100 ± 16 versus−86 ± 16 beats−1 with 8 μg) and AP (−48 ± 3 versus−47 ± 3 mmHg at 8 μg) were not significantly different between OZRs and LZRs at the four doses examined.
Figure 6. Effects of bolus injection of phenylbiguanide (PBG) on SNA.
Filled bars: LZRs (n= 10); open bars: OZRs (n= 11). *Significant difference from LZRs at that dose of PBG (P < 0.05).
Discussion
Many forms of hypertension are associated with impaired arterial baroreflex control of SNA and HR (Gonzalez et al. 1983; Miyajima & Buñag, 1986; Buñag & Barringer, 1988; Grassi et al. 2000; Zhang & Mifflin, 2000), although the mechanisms underlying these deficits vary with the hypertensive model. The aim of this study was to determine whether impaired sympathetic baroreflexes in hypertensive OZRs could be explained by a reduced function of baroreceptor afferent nerves. Our results showed that the sensitivity of baroreceptor afferent nerves, as gauged by the activity of the ADN, were normal in OZRs. In contrast, bypassing the sensory endings with direct stimulation of ADN afferent fibres yielded attenuated inhibition of SNA, as observed with pharmacologically evoked changes in AP. In addition, electrical or chemical activation of other vagal afferent nerves also produced blunted inhibition of SNA in OZRs compared to LZRs. Together these data suggest that changes at sites beyond the sensory endings, such as the CNS, are responsible for attenuated sympathoinhibitory reflexes in OZRs.
In the present study the ADNA and SNA in OZRs and LZRs were analysed in two ways. To facilitate comparisons of changes in activity between groups, we calculated percentage changes from a baseline of 100% with the voltage due to noise set at 0%. Using this measure, we observed blunted ADN-mediated decreases in SNA that were in agreement with the reduced depressor responses. Moreover, reduction in gain of baroreflex-mediated changes in SNA in OZRs was comparable whether measured by per cent or absolute change in SNA. In contrast, we observed a normal percentage change in ADNA that was corroborated by examination of voltage changes in ADNA. These data suggest that the percentage change of ongoing activity is a relevant measure even with differences in baseline activity. This type of analysis normalizes for potential differences in the recordings from rat to rat due to contact of the wires to fibres, physical properties of the nerves, or differences in baseline activity, allowing for significant differences to be detected in smaller groups of rats. However, analysis of percentage change does not allow for comparison of baseline activity or maximal changes in absolute activity. For this purpose, we examined raw rectified voltages of SNA and ADNA. In these studies, recordings were performed by one person using one set of recording wires and amplifiers at uniform amplification settings. Consistent with a previous report that the firing rate and integrated voltage of renal SNA is elevated in OZRs compared to LZRs (Morgan et al. 1995), we observed that the splanchnic SNA measured in voltage was higher in OZRs. Furthermore, the voltage for baseline ADNA was higher in OZRs, in agreement with the observation of a higher baseline AP with no change in threshold or sensitivity of ADNA in OZRs. These data suggest that both types of analysis are valuable for estimating properties of nerve activity.
The ADN was chosen as the representative baroreceptor sensory afferent nerve because it contains exclusively baroreceptor afferent fibres (Sapru & Krieger, 1977), whereas the carotid sinus nerve, which also contributes to the generation of arterial baroreflexes (Ohta & Talman, 1995), also contains chemosensitive fibres that mediate peripheral chemoreflexes (Sapru & Krieger, 1977). Although, it is not likely that carotid sinus baroreceptor afferent nerves would be affected in the OZRs in the absence of a change in ADNA, this study cannot rule out potential alterations in this additional set of baroreceptor afferent fibres. In addition, whole nerve recordings of ADNA did not distinguish between myelinated and unmyelinated fibres. Conservatively, the data in the present study suggest that the integrated discharge from baroreceptor afferents arising from the aortic arch provide comparable inputs to the CNS over a wide range of AP levels in LZRs and OZRs. However, because the elevated baseline MAP was associated with higher ADNA in OZRs compared to LZRs, the OZRs appear to receive chronically elevated inputs from the ADN under resting conditions.
The blunted baroreflexes in OZRs do not appear to be due to a diminished ability of aortic baroreceptors to sense AP. However, at this age the adult OZRs display significant changes in the structure and function of the aorta, the source of baroreceptors that give rise to the ADN, including increased aortic vascular stiffness and loss of anistropy compared to age-matched LZRs (Sista et al. 2005). This development of arteriosclerosis is observed in patients with insulin resistance, a trait observed in OZRs at this age (Liu et al. 2002; Sista et al. 2005). Increased aortic vascular stiffness in other hypertensive models is associated with diminished suprathreshold baroreceptor sensitivity without a change in the baroreceptor threshold pressure (Andresen, 1984). Nevertheless, in the present study the relationship between MAP and ADNA was indistinguishable between OZRs and LZRs regardless of how ADNA was measured. Specifically, the pressure threshold for onset of ADNA, rise in ADNA with evoked increases in AP, and maximal ADNA were comparable. Thus, impairments in aortic vascular function in OZRs do not appear to translate into a diminished ability of baroreceptors to sense AP or respond to changes in AP.
The observation that baroreceptor afferent nerves do not reset in the face of a sustained significantly higher AP was surprising in light of the abundance of existing data demonstrating elevations in threshold and suprathreshold sensitivity in ADNA with elevated AP (Munch et al. 1983; Krieger, 1988). A number of these studies examined afferent activity with large changes in AP in a short time span, although changes are also seen in chronically hypertensive rat models (Krieger, 1970; Munch et al. 1983). The adult OZRs have significantly higher AP (∼15 mmHg) demonstrated by telemetric recordings in the conscious state (Osmond et al. 2009), but perhaps the gradual onset of this moderate hypertension is insufficient to measurably alter whole ADNA activity. Indeed, baroreceptor resetting is not complete under most conditions, as the upward shift in threshold is only a fraction of the magnitude of the elevated AP (Munch et al. 1983). The onset of reduced baroreceptor afferent function with modest hypertension may also require sufficient time for significant alterations in baroreceptor afferent activity to occur. In 16-week-old SHRs, hypertension is clearly established but the thresholds for c-fibre baroreceptor afferent activity are not reset until 36 weeks of age (Thoren et al. 1983). In addition, whether circulating factors in the obese state contribute to the sensitivity of baroreceptor afferents warrants future investigation. Indeed, the threshold for baroreceptor afferent activity does not reset in rabbits made hypertensive by a high-cholesterol diet, in contrast to an increased threshold observed by the same measures in a renal model of hypertension (Angell-James, 1973, 1974). Regardless of the underlying mechanisms for normal baroreceptor afferent activity in hypertensive OZRs, the central processing of baroreceptor inputs and other vagal inputs in the OZRs are clearly altered and yield a reduced ability to restrain SNA. This autonomic deficit occurs in the absence of or prior to changes in baroreceptor afferent nerve sensitivity.
Electrical stimulation of ADN evoked smaller inhibitions of SNA in an intensity-dependent manner that coincided with reduced depressor responses and persisted in the absence of changes in AP. These results mimic the diminished sympathoinhibition evoked by increasing AP with the α1-adrenergic vasoconstrictor phenylephrine in OZRs (Fig. 2D, Table 2; Schreihofer et al. 2007). Altered central processing of incoming baroreceptor signals is present in several other models of hypertension, such as the SHRs (Gonzalez et al. 1983; Salgado et al. 2007) and one-kidney, renal wrap hypertensive rats (Zhang & Mifflin, 2000) and dogs (Seeber et al. 1972). Like the OZRs, SHRs also show diminished inhibition of SNA and AP with chemical activation of vagal afferents with phenylbiguanide (Catelli & Sved, 1988), suggesting changes at sites within the central pathway common to these two reflexes, namely the nucleus of the solitary tract (NTS), the caudal ventrolateral medulla (CVLM) and the rostral ventrolateral medulla (RVLM; Sun & Guyenet, 1987). In renal wrap hypertensive rats, blunted baroreflexes are associated with altered processing of baroreceptor inputs at the NTS (Zhang & Mifflin, 2000). In addition, in SHRs the regulation of SNA and AP by the ventrolateral medulla appears to promote elevated SNA. Specifically, tonic inhibition of SNA by the CVLM appears to be reduced in SHRs compared to normotensive Wistar–Kyoto rats (Smith & Barron, 1990; Arnolda et al. 1992), whereas the glutamatergic drive to the RVLM appears to be enhanced (Ito et al. 2000). In OZRs the critical regions of the CNS that promote elevated SNA or blunted baroreflexes are not known. Clearly, the OZRs have SNA that is resistant to inhibition by stimulation of vagal afferent nerves that is not apparent in LZRs. Whether the baroreceptor-independent SNA is due to elevated excitatory drive to presympathetic neurons or a reduction in the activation or influence of inhibitory pathways remains to be determined. The patterns of autonomic deficits in OZRs may provide clues for sources of disrupted autonomic control. Although sympathoinihibitory reflexes were blunted in OZRs, rises in SNA evoked by high-frequency stimulation of vagal afferent fibres were not attenuated. Similarly, stimulation of sciatic nerve in OZRs yields exaggerated sympathetically mediated increases in AP (Ruggeri et al. 2006). Central mechanisms underlying these opposing sympathetic reflex responses are distinct, producing changes in the activity of presympathetic neurons in the RVLM by different neurotransmitters. Sympathoinhibitory vagal and arterial baroreceptor reflexes are initiated by activation of the NTS and lead to GABAergic inhibition of the RVLM to reduce SNA (Sun & Guyenet, 1987; Verberne & Guyenet, 1992). In contrast, although sympathoactivation evoked by high-frequency stimulation of vagal afferent nerves is also initiated in the NTS, the rise in SNA is produced by glutamatergic activation of the RVLM (Sun & Guyenet, 1987). Similarly, stimulation of the sciatic nerve increases SNA via a glutamatergic activation of the RVLM (Kiely & Gordon, 1994). Thus, perhaps the disparate states of these reflexes in OZRs may be due to alterations in GABAergic or glutamatergic inputs to this critical region for sympathetic regulation. The RVLM appears to contribute to the elevation in SNA in diet-induced obese rats (Stocker et al. 2007), suggesting this medullary region could be a key central site for altered autonomic regulation in obesity.
The basis for the onset of reduced sympathetic baroreflexes with obesity is not known. The lack of functional leptin receptors cannot be the sole cause for altered baroreflex function in OZRs because juvenile OZRs have fully functional sympathetic baroreflexes (Schreihofer et al. 2007). Instead, attenuation of baroreflexes emerges in adulthood long after the onset of obesity with the establishment of metabolic disorder. As seen with obese humans, adult OZRs present with type 2 diabetic traits such as insulin resistance and increased basal plasma insulin and glucose levels in addition to elevated circulating levels of free fatty acids and triglycerides, and cholesterol (Alemzadeh & Tushaus, 2004; Frisbee, 2005; Osmond et al. 2009). Type 1 diabetic rats, who lack insulin but have elevated blood glucose levels, also have impaired baroreflexes associated with normal baroreceptor afferent nerve function (do Carmo et al. 2007, 2008), suggesting blood glucose may be an important regulator of baroreflex function by the brain. Indeed, infusion of leptin into the brain of streptozotocin-treated rats restores impaired baroreflex control of HR, and this effect appears to depend on the leptin-induced reduction of blood glucose levels (do Carmo et al. 2008). Paradoxically, antecedent periods of insulin-induced hypoglycaemia also impair baroreflexes in normotensive patients with a body mass index of <25 but not in insulin-infused patients maintained euglycaemic (Adler et al. 2009), highlighting the importance of strict glycaemic control in both directions. Additionally, the hyperinsulinaemia or insulin resistance observed in OZRs may also affect baroreflex function. Although infusion of insulin into Sprague–Dawley rats either peripherally or centrally appears to increase baroreflex sensitivity independent of blood glucose levels (Hong & Hsieh, 2007; Pritcher et al. 2008), insulin resistance can be associated with reduced transport of insulin to the brain. For example, baroreflexes are reduced in pregnancy in association with insulin resistance and decreased concentrations of insulin in the cerebrospinal fluid, and baroreflex function is restored by administration of the insulin sensitizer rosiglitazone (Daubert et al. 2007). Future studies will be necessary to determine contributions of diabetic complications of obesity for the onset and maintenance of attenuated sympathetic baroreflexes in OZRs.
Whether the impairment of arterial baroreflexes in OZRs precedes development of hypertension or emerges after AP is elevated has not been determined. At 7–8 weeks of age male OZRs are normotensive (Schreihofer et al. 2007; Osmond et al. 2009) and have a normal range of changes in SNA in response to evoked changes in MAP (Schreihofer et al. 2007). In contrast, by 13 weeks of age OZRs have increased MAP and frank impairment of baroreflexes (Schreihofer et al. 2007; Osmond et al. 2009). In one report utilizing conscious female Zucker rats, blunted baroreflexes were present in OZRs without observed differences in baseline MAP from LZRs (Buñag & Barringer, 1988). In this study, the rats were 3 months old, and MAP was measured with indwelling vascular catheters. As male rats are hypertensive by this age when MAP is measured by telemetry (Osmond et al. 2009), it is not clear whether the difference is due to gender or difficulty in detecting modest differences in MAP in OZRs and LZRs at the onset of hypertension with small group sizes. In renal models of hypertension, the rise in AP clearly precedes the onset of impaired baroreceptor processing by the CNS and may be a consequence of the hypertension (Seeber et al. 1972; Zhang & Mifflin, 2000). However, in other models of modest hypertension, such as exposure to chronic intermittent hypoxia, rats can have elevated basal SNA and AP with normal phenylephrine-induced inhibition of SNA (Greenberg et al. 1999). These data suggest that this magnitude of sympathetically mediated hypertension, which is similar to OZRs, is not sufficient to attenuate the baroreflex control of SNA, and that the two sympathetic regulatory deficits may have distinct underlying mechanisms.
Perspectives
Obesity is a major independent risk factor for the development of cardiovascular disease, and the incidence of obesity in adults and children has reached epidemic proportions. Despite continued efforts to combat obesity, many continue to suffer the consequences of their condition, necessitating a better understanding of obesity-associated diseases. Despite altered aortic vascular function in OZRs, this study suggests that aortic baroreceptors are able to sense and detect changes in AP normally. However, in this hyperphagic model of obesity the brain's ability to combat changes in AP are compromised, probably contributing to the elevated SNA and increased variability of MAP observed in OZRs. Additional studies will be required to determine key brain regions for obesity-induced impairment of baroreflex function, and whether diabetic complications of obesity play a causative or exacerbating role in the impairment of neural control of the circulation observed with obesity.
Acknowledgments
This work was supported by a National Institutes of Health grant to A.M.S. (R01HL086759).
Glossary
Abbreviations
- ADN
aortic depressor nerve
- ADNA
aortic depressor nerve activity
- AP
arterial pressure
- CNS
central nervous system
- CVLM
caudal ventrolateral medulla
- GABA
γ-aminobutyric acid
- LZR
lean Zucker rat
- HR
heart rate
- MAP
mean arterial pressure
- NTS
nucleus of the solitary tract
- OZR
obese Zucker rat
- RVLM
rostral ventrolateral medulla
- SHR
spontaneously hypertensive rats
- SNA
sympathetic nerve activity
Author contributions
D.A.H. and A.M.S. contributed to the conception and the design of the study. The experiments were performed by D.A.H. with the guidance of A.M.S. The manuscript was prepared and edited by both authors who approved the submitted version of the manuscript. The experiments were conducted at the Medical College of Georgia in Augusta, GA, USA.
References
- Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes. 2009;58:360–366. doi: 10.2337/db08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemzadeh R, Tushaus KM. Modulation of adipoinsular axis in prediabetic Zucker diabetic fatty rats by diazoxide. Endocrinol. 2004;145:5476–5484. doi: 10.1210/en.2003-1523. [DOI] [PubMed] [Google Scholar]
- Andresen MC. Short- and long-term determinants of baroreceptor function in aged normotensive and spontaneously hypertensive rats. Circ Res. 1984;54:750–759. doi: 10.1161/01.res.54.6.750. [DOI] [PubMed] [Google Scholar]
- Angell-James Characteristics of single aortic and right subclavian baroreceptor fibre activity in rabbits with chronic renal hypertension. Circ Res. 1973;32:149–161. doi: 10.1161/01.res.32.2.149. [DOI] [PubMed] [Google Scholar]
- Angell-James Arterial baroreceptor activity in rabbits with experimental atherosclerosis. Circ Res. 1974;34:27–39. doi: 10.1161/01.res.40.4.27. [DOI] [PubMed] [Google Scholar]
- Arnolda L, Minson J, Kapoor V, Pilowsky P, Llewellyn-Smith I, Chalmers J. Amino acid neurotransmitters in hypertension. Kidney Int Suppl. 1992;37:S2–S7. [PubMed] [Google Scholar]
- Barringer DL, Buñag RD. Uneven blunting of chronotropic baroreflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 1989;256:H417–H421. doi: 10.1152/ajpheart.1989.256.2.H417. [DOI] [PubMed] [Google Scholar]
- Buñag RD, Barringer DL. Obese Zucker rats, though still normotensive, already have impaired chronotropic baroreflexes. Clin Exp Hypertens. 1988;10:257–262. doi: 10.3109/10641968809075977. [DOI] [PubMed] [Google Scholar]
- Buñag RD, Eriksson, Krizsan D. Baroreceptor reflex impairment and mild hypertension in rats with dietary-induced obesity. Hypertension. 1990;15:397–406. doi: 10.1161/01.hyp.15.4.397. [DOI] [PubMed] [Google Scholar]
- Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension. 2000;35:403–408. doi: 10.1161/01.hyp.35.1.403. [DOI] [PubMed] [Google Scholar]
- Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur J Pharmacol. 1988;151:243–248. doi: 10.1016/0014-2999(88)90804-7. [DOI] [PubMed] [Google Scholar]
- Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2188–R2195. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- do Carmo JM, Hall JE, da Silva AA. Chronic central leptin infusion restores cardiac sympathetic-vagal balance and baroreflex sensitivity in diabetic rats. Am J Physiol Heart Circ Physiol. 2008;295:H1974–H1981. doi: 10.1152/ajpheart.00265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Carmo JM, Huber DA, Castania JA, Fazan VP, Fazan R, Jr, Salgado HC. Aortic depressor nerve function examined in diabetic rats by means of two different approaches. J Neurosci Methods. 2007;161:17–22. doi: 10.1016/j.jneumeth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC. Hypertension-independent microvascular rarefaction in the obese Zucker rat model of the metabolic syndrome. Microcirculation. 2005;12:383–392. doi: 10.1080/10739680590960241. [DOI] [PubMed] [Google Scholar]
- Gonzalez ER, Krieger AJ, Sapru HN. Central resetting of baroreflex in the spontaneously hypertensive rat. Hypertension. 1983;5:346–352. doi: 10.1161/01.hyp.5.3.346. [DOI] [PubMed] [Google Scholar]
- Gordon FJ, Mark AL. Mechanism of impaired baroreflex control in prehypertensive Dahl salt-sensitive rats. Circ Res. 1984;54:378–387. doi: 10.1161/01.res.54.4.378. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol. 1999;86:298–305. doi: 10.1152/jappl.1999.86.1.298. [DOI] [PubMed] [Google Scholar]
- Hong LZ, Hsieh PS. Hyperinsulinemia instead of insulin resistance induces baroreflex dysfunction in chronic insulin-infused rats. Am J Hypertens. 2007;20:451–458. doi: 10.1016/j.amjhyper.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Huber DA, do Carmo JM, Castania JA, Fazan R, Jr, Salgado HC. Does acute hyperglycemia alter rat aortic depressor nerve function? Braz J Med Biol Res. 2007;40:1567–1576. doi: 10.1590/s0100-879x2007001100017. [DOI] [PubMed] [Google Scholar]
- Iida M, Murakami T, Ishida K, Mizumo A, Kuwajima M, Sharma K. Substitution at codon 269 (glutamine → proline) of the leptin receptor (OB-R) cDNA is the only mutation found in the Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;224:597–604. doi: 10.1006/bbrc.1996.1070. [DOI] [PubMed] [Google Scholar]
- Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- Kiely JM, Gordon FJ. Role of rostral ventrolateral medulla in centrally mediated pressor responses. Am J Physiol Heart Circ Physiol. 1994;267:H1549–H1556. doi: 10.1152/ajpheart.1994.267.4.H1549. [DOI] [PubMed] [Google Scholar]
- Krieger EM. Time course of baroreceptor resetting in acute hypertension. Am J Physiol. 1970;218:486–490. doi: 10.1152/ajplegacy.1970.218.2.486. [DOI] [PubMed] [Google Scholar]
- Krieger EM. Mechanisms of complete baroreceptor resetting in hypertension. Drugs. 1988;35:98–103. doi: 10.2165/00003495-198800356-00014. [DOI] [PubMed] [Google Scholar]
- Liu RH, Mizuta M, Kurose T, Matsukura S. Early events involved in the development of insulin resistance in Zucker fatty rat. Int J Obes Relat Metab Disord. 2002;26:318–326. doi: 10.1038/sj.ijo.0801924. [DOI] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima E, Buñag RD. Impaired sympathetic baroreflexes in prehypertensive Dahl hypertension-sensitive rats. Clin Exp Hypertens. 1986;8:1049–1061. doi: 10.3109/10641968609044085. [DOI] [PubMed] [Google Scholar]
- Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension. 1995;25:834–838. doi: 10.1161/01.hyp.25.4.834. [DOI] [PubMed] [Google Scholar]
- Munch PA, Andresen MC, Brown AM. Rapid resetting of aortic baroreceptors in vitro. Am J Physiol Heart Circ Physiol. 1983;244:H672–H680. doi: 10.1152/ajpheart.1983.244.5.H672. [DOI] [PubMed] [Google Scholar]
- Ohta H, Talman WT. Baroreceptors in the carotid sinus contribute to arterial baroreceptor reflexes in normotensive rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:S62–S63. doi: 10.1111/j.1440-1681.1995.tb02971.x. [DOI] [PubMed] [Google Scholar]
- Osmond JM, Mintz JD, Dalton B, Stepp DW. Obesity increases blood pressure, cerebral vascular remodelling, and severity of stroke in the Zucker rat. Hypertension. 2009;53:381–386. doi: 10.1161/HYPERTENSIONAHA.108.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton JM, Williams TD, Chambers JB, Rashotte ME. Cardiovascular and metabolic responses to fasting and thermoneutrality are conserved in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1007–R1015. doi: 10.1152/ajpregu.2001.280.4.R1007. [DOI] [PubMed] [Google Scholar]
- Piccirillo G, Vetta F, Viola E, Santagada E, Ronzoni S, Cacciafesta M, Marigliano V. Heart rate and blood pressure variability in obese normotensive subjects. Int J Obes Relat Metab Disord. 1998;22:741–750. doi: 10.1038/sj.ijo.0800650. [DOI] [PubMed] [Google Scholar]
- Pritcher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension. 2008;51:514–520. doi: 10.1161/HYPERTENSIONAHA.107.102608. [DOI] [PubMed] [Google Scholar]
- Rocchini AP, Mao HZ, Babu K, Marker P, Rocchini AJ. Clonidine prevents insulin resistance and hypertension in obese dogs. Hypertension. 1999;33:548–553. doi: 10.1161/01.hyp.33.1.548. [DOI] [PubMed] [Google Scholar]
- Ruggeri P, Brunori A, Cogo CE, Storace D, Di Nardo F, Burattini R. Enhanced sympathetic reactivity associates with insulin resistance in the young Zucker rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R376–R382. doi: 10.1152/ajpregu.00644.2005. [DOI] [PubMed] [Google Scholar]
- Salgado HC, Barale AR, Castania JA, Machado BH, Chapleau MW, Fazan R., Jr Baroreflex responses to electrical stimulation of aortic depressor nerve in conscious SHR. Am J Physiol Heart Circ Physiol. 2007;292:H593–H600. doi: 10.1152/ajpheart.00181.2006. [DOI] [PubMed] [Google Scholar]
- Sapru HN, Krieger AJ. Carotid and aortic chemoreceptor function in the rat. J Appl Physiol. 1977;42:344–348. doi: 10.1152/jappl.1977.42.3.344. [DOI] [PubMed] [Google Scholar]
- Sapru HN, Wang SC. Modification of aortic baroreceptor resetting in the spontaneously hypertensive rat. Am J Physiol. 1976;230:664–674. doi: 10.1152/ajplegacy.1976.230.3.664. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol. 2000;279:R729–R742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Hair CD, Stepp DW. Reduced plasma volume and mesenteric vascular reactivity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R253–R261. doi: 10.1152/ajpregu.00498.2004. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;293:H2543–H2549. doi: 10.1152/ajpheart.01201.2006. [DOI] [PubMed] [Google Scholar]
- Seeber AM, Kgrdenat RK, Spickler JW. Stimulation of the aortic nerve in normal and hypertensive dogs. Medicina. 1972;32:640–645. [PubMed] [Google Scholar]
- Sista AK, O’Connell MK, Hinohara T, Oommen SS, Fenster BE, Glassford AJ, Schwartz EA, Taylor CA, Reaven GM, Tsao PS. Increased aortic stiffness in the insulin-resistant Zucker fa/fa rat. Am J Physiol Heart Circ Physiol. 2005;289:H845–H851. doi: 10.1152/ajpheart.00134.2005. [DOI] [PubMed] [Google Scholar]
- Smith JK, Barron KW. Cardiovascular effects of L-glutamate and tetrodotoxin microinjected into the rostral and caudal ventrolateral medulla in normotensive and spontaneously hypertensive rats. Brain Res. 1990;506:1–8. [PubMed] [Google Scholar]
- Stocker SD, Meador R, Adams JM. Neurons of the rostral ventrolateral medulla contribute to obesity-induced hypertension in rats. Hypertension. 2007;49:640–646. doi: 10.1161/01.HYP.0000254828.71253.dc. [DOI] [PubMed] [Google Scholar]
- Sun M-K, Guyenet PG. Arterial baroreceptor and vagal inputs to sympathoexcitatory neurons in rat medulla. Am J Physiol Regul Integr Comp Physiol. 1987;252:R699–R709. doi: 10.1152/ajpregu.1987.252.4.R699. [DOI] [PubMed] [Google Scholar]
- Thoren P, Andresen MC, Brown AM. Resetting of aortic baroreceptors with non-myelinated afferent fibres in spontaneously hypertensive rats. Acta Physiol Scand. 1983;117:91–97. doi: 10.1111/j.1748-1716.1983.tb07182.x. [DOI] [PubMed] [Google Scholar]
- Tuck ML. Obesity, the sympathetic nervous system, and essential hypertension. Hypertension. 1992;19:I67–I77. doi: 10.1161/01.hyp.19.1_suppl.i67. [DOI] [PubMed] [Google Scholar]
- Verberne AJ, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am J Physiol Regul Integr Comp Physiol. 1992;263:R1195–R1202. doi: 10.1152/ajpregu.1992.263.6.R1195. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Integration of aortic nerve inputs in hypertensive rats. Hypertension. 2000;35:430–436. doi: 10.1161/01.hyp.35.1.430. [DOI] [PubMed] [Google Scholar]