Abstract
Substance P (SP) and its receptors are involved in anxiety-related behaviours and regulate the intake of drugs of abuse and alcohol. Within the midbrain ventral tegmental area (VTA), a region that is clearly involved in the control of these behaviours, SP is released by stress and has been shown to trigger relapse. SP activates neurokinin (NK) receptors, which excites midbrain dopamine (DA) neurons and leads to increased DA in target regions. In this study, we have investigated the mechanisms underlying SP actions in the VTA, specifically investigating interactions between SP and GABAB receptors. We show that in VTA neurons, NK receptor activation closes an inwardly rectifying potassium channel, and moreover inhibits GABAB receptor-mediated transmission through an interaction that depends upon phospholipase C (PLC), intracellular calcium and protein kinase C (PKC).
Introduction
The midbrain ventral tegmental area (VTA) is a key structure in the neural circuitry underlying the reinforcement of motivated behaviours, and changes in this circuit are considered responsible for the maladaptive behaviours associated with addiction (Hyman et al. 2006; Fields et al. 2007). Within the VTA, dopamine (DA)-, GABA- and glutamate-containing projection neurons receive excitatory glutamatergic inputs as well as inhibitory GABAergic inputs from a wide variety of brain regions (Fields et al. 2007). In addition, most of these afferent projections co-contain neuropeptides, which, when released, can presynaptically modulate neurotransmitter release and directly activate or inhibit neurons.
One key neuropeptide released in the VTA is the tachykinin-derived peptide substance P (SP), which is contained in a number of afferent projections to the VTA, most notably in inputs arising from the habenula and from the nucleus accumbens (Cuello et al. 1978; Lu et al. 1998). Microinjections of SP into the VTA increase DA in the nucleus accubmens (Deutch et al. 1985; Nikolaev et al. 2004), and electrophysiological studies have demonstrated that SP excites neurons in both the VTA as well as in the neighbouring substantia nigra pars compacta (Overton et al. 1992; Seabrook et al. 1995; Korotkova et al. 2006). Consistent with these studies, intra-VTA SP increases locomotion (Stinus et al. 1978), which can be blocked by DA receptor antagonists in the nucleus accumbens (Kelley et al. 1979). Moreover, intra-VTA administration of the SP analogue DiMe-C7 reinstates cocaine-seeking behaviour, which has been shown to depend upon DA (Placenza et al. 2004).
SP activates NK receptors and typically acts by closing G-protein-linked inwardly rectifying K+ (GIRK) channels and/or opening a non-selective cation current through a Gq, PLC-β, PKC-mediated pathway (Stanfield et al. 1985; Shen & North, 1992; Takano et al. 1995, 1996). In contrast, a recent study reported that SP can act in a G-protein-independent manner, via Src kinase, to activate a Na+ leak channel (NALCN) in VTA neurons (Lu et al. 2009). However, to isolate this current, the authors specifically blocked K+ currents. This approach made it impossible to determine the extent to which K+ channel effects contribute to typical SP-mediated depolarizations in VTA neurons.
In this study, we investigate the mechanisms underlying the actions of SP in the VTA under conditions where K+ channel effects can be measured. We find that the SP couples to GIRK channels in VTA neurons. Moreover, we show that SP can inhibit GIRK currents activated by other receptors, such as GABAB receptors.
Methods
Slice preparation and electrophysiology
All procedures were conducted in accordance to Ernest Gallo Clinic and Research Center animal care policy standards and comply with The Journal of Physiology and UK regulations on animal experimentation (Drummond, 2009).
Male, 4- to 6-week-old Sprague–Dawley rats (Harlan Laboratories, San Diego) were anaesthetized with isoflurane and decapitated. Horizontal brain slices (150–200 μm thick) including the VTA were prepared using a vibratome (Leica Instruments, Solm, Germany) in Ringer solution (119 mm NaCl, 2.5 mm KCl, 1.3 mm MgSO4, 1.0 mm NaH2PO4, 2.5 mm CaCl2, 26.2 mm NaHCO3 and 11 mm glucose saturated with 95% O2–5% CO2). Slices were visualized under an upright microscope with differential interference contrast optics and infrared illumination.
Whole-cell recordings were made at 33°C with 2.5–4 MΩ pipettes containing 123 mm potassium gluconate, 10 mm Hepes, 0.2 mm EGTA, 8 mm NaCl, 2 mm MgATP, 0.3 mm Na3GTP and 0.1% biocytin (pH 7.2, osmolarity adjusted to 275 mosmol l−1). In some experiments, 5 mm phosphocreatine was included in the whole-cell solution. The liquid junction potential (calculated at 15 mV) has been corrected for in the data shown in Fig. 3.
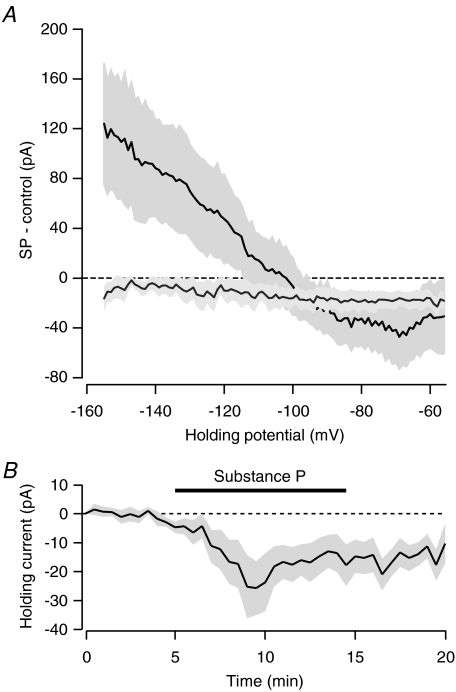
Figure 3. Current–voltage relationship for SP is inwardly rectifying.
A, difference between current–voltage curves recording in SP and during a control period (n= 4, dark line). This current is blocked by 500 μm barium (n= 5, grey line). Shaded area indicates s.e.m. Membrane potential has been corrected for the liquid junction potential. B, bath application of SP produces an inward current in voltage-clamped neurons recorded using a caesium based internal solution (n= 7).
Firing rate and membrane potential recordings were made in current-clamp (I= 0) mode using a Multiclamp 700 A amplifier (Molecular Devices), filtered at 2 kHz and collected at 5 kHz using acquisition procedures written for Igor Pro (Wavemetrics, Inc., Lake Oswego, OR, USA).
The presence of the hyperpolarization-activated non-specific cation current (Ih) is measured by voltage clamping cells at −60 mV and applying a series of steps from −40 to −120 mV. Current–voltage data were collected in voltage clamp by stepping from a holding potential of −60 mV to −40 mV and ramping down to −140 mV over a 2 s interval (−55 to −155 mV after correcting for the liquid junction potential). The current response to this ramp in SP was subtracted from the response recorded under control conditions and plotted against the holding potential. GABAB-mediated inhibitory postsynaptic currents (IPSCs) were evoked with a 100 Hz train of seven pulses given once every 30 s through a bipolar stimulating electrode placed ∼50 μm from the recorded neuron. GABAB IPSCs were pharmacologically isolated with picrotoxin (100 μm), DNQX (10 μm), sulpiride (10 μm), strychnine (1 μm) and d-AP5 (10 μm). IPSC amplitudes were calculated by comparing a 2 ms period at the peak of the response with a similar period just prior to the stimulus artifact. The significance of drug effects was determined comparing the last 4 min of baseline with the last 4 min of drug application. Baclofen currents were determined by measuring a 1 min average during the peak response. Unless otherwise noted, statistical analyses were performed using Student's t test, and significance was defined at P < 0.05. Results are presented as means ±s.e.m.
Drugs
Unless otherwise noted, drugs were applied by bath perfusion. Stock solutions of drugs were made and diluted (typically 1 in 1000) into Ringer solutions immediately before application. Drugs were obtained from Sigma or Tocris.
Immunocytochemistry
Slices used for electrophysiology were fixed immediately after recording in 4% formaldehyde for 2 h and then stored at 4°C in PBS. Slices were pre-blocked at room temperature for 2 h in PBS plus 0.3% (v/v) Tween 20, 0.2% BSA and 5% normal goat serum and then incubated at 4°C with a rabbit anti-tyrosine hydroxylase (TH) polyclonal antibody (1:100) for 48 h. The slices were then washed thoroughly in PBS with 0.3% Tween 20 and 0.2% BSA before being agitated overnight at 4°C with Cy5 anti-rabbit secondary antibody (1:100) and 3.25 μl ml−1 dichlorotriazinylaminofluorescein-conjugated streptavidin. Finally, sections were rinsed and mounted on slides with Fluoroguard Antifade reagent (Bio-Rad) mounting media and visualized under a Zeiss LSM 510 META confocal microscope. TH content or lack thereof was only accepted as valid if the biocytin-filled neuron was in proximity to and in the same focal plane as other clearly TH-labelled neurons in the same tissue section.
Results
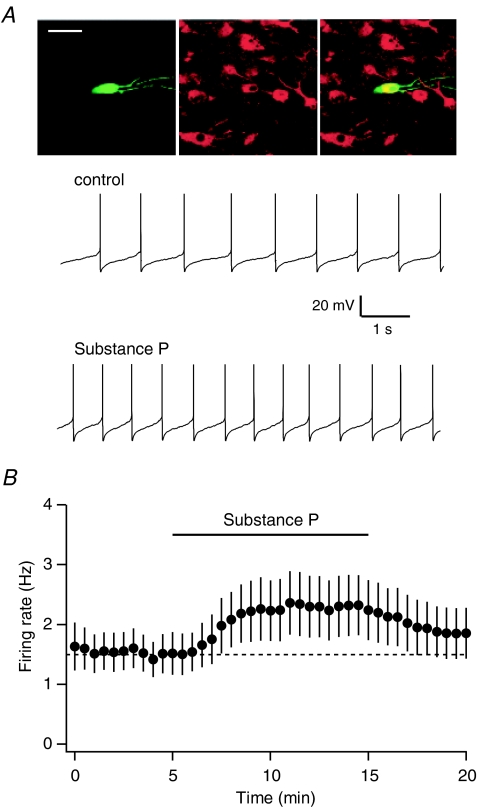
Whole-cell recordings were made from neurons throughout the VTA. In the VTA, DA neurons comprise a subset of the neurons that express Ih. Amongst Ih+ neurons, bath application of the neuropeptide SP (1 μm) produced a significant increase in the firing rate of spontaneously active neurons (Fig. 1A and B, firing rate increased from 1.5 ± 0.3 Hz to 2.2 ± 0.5 Hz, n= 12, paired t test, P < 0.05). In Ih+ neurons that were not firing spontaneously, application of SP depolarized the membrane potential (by 5.9 ± 1.4 mV, n= 14, paired t test, P < 0.01).
Figure 1. SP increases the firing rate of VTA neurons.
A, representative traces from an individual VTA neuron before and after bath application of 1 μm SP. This neuron was filled with biocytin during recording (green), and immunocytochemically confirmed as TH+ (red). Scale bar, 50 μm. B, average of 12 Ih-expressing neurons shows significant increase in firing following bath application of SP.
While all DA neurons express Ih, not all Ih+ neurons in the VTA are DAergic (Margolis et al. 2006). Therefore, we included biocytin in our whole-cell recording solution and immunocytochemically processed recorded neurons using antibodies for tyrosine hydroxylase (TH), a marker for DA neurons (example in Fig. 1A). In confirmed TH+ neurons, SP increased the firing rate and/or depolarized 6/7 neurons. On the other hand, amongst non-DA neurons (both Ih+ and Ih−, presumably including both GABA- and glutamate-containing neurons), only a minority were excited by SP (Table 1). Overall, there was a significant difference in the proportion of TH+ and TH− neurons that were sensitive to SP (Fisher exact test, P= 0.05).
Table 1.
Proportion of VTA neurons excited by SP depending on TH content
| Cell type | Excited | Not excited |
|---|---|---|
| Ih+ | 19 (73%) | 7 |
| TH+ | 6 (86%) | 1 |
| TH− | 0 (0%) | 2 |
| Ih− (all TH−) | 3 (38%) | 5 |
Includes data from both firing and non-firing neurons.
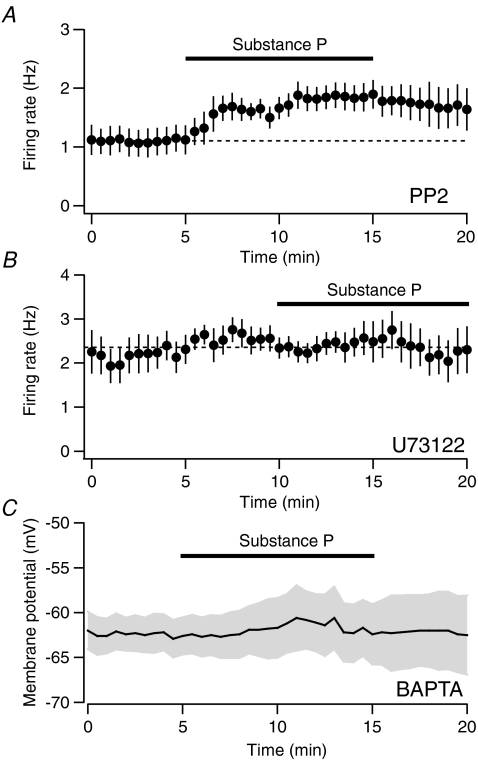
NK receptors are typically Gq coupled, the activation of which stimulates PLC-β (Hubbard & Hepler, 2006). However, a recent study using dissociated VTA neurons found that SP can act in a G-protein-independent manner, through the activation of a Src kinase (Lu et al. 2009). Therefore, we investigated whether inhibiting Src kinase alters the SP-mediated excitations in the VTA slice. Following bath application of the selective Src kinase inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine, 1 μm), SP still produced excitations in VTA Ih+ neurons (Fig. 2A (1.1 ± 0.2 Hz vs. 1.8 ± 0.2 Hz, n= 6, P < 0.01, paired t test), which were not different to the SP effects observed in control neurons (n.s., unpaired t test).
Figure 2. SP actions depend upon PLC and intracellular calcium.
A, the increase in firing rate due to SP is not blocked by the SRK inhibitor PP2 (1 μm, n= 6). B, SP does not alter the firing rate of Ih-expressing neurons following incubation with the PLC inhibitor U73122 (10 μm, n= 6). C, SP does not depolarize neurons when BAPTA (10 mm) is included in the whole-cell solution (n= 8).
To test the role of PLC, we incubated slices in the PLC inhibitor U73122 (10 μm) for at least 15 min prior to making our electrophysiological recordings. In the presence of U73122, bath-applied SP had no effect on the firing rate of Ih-expressing neurons (Fig. 2B, 2.6 ± 0.3 Hz vs. 2.3 ± 0.5 Hz; n= 6, n.s, paired t test). This was significantly different to the firing rate increase observed in control cells following SP application (P < 0.05, unpaired t test).
We next tested whether the effects of SP depend on an increase in intracellular calcium by including the calcium buffer BAPTA (10 mm) in our internal solution. Buffering internal calcium prevented VTA neurons from firing spontaneously and significantly reduced the membrane potential depolarization in response to SP (Fig. 2C, −0.9 ± 1.7 mV; n= 8, P < 0.05, unpaired t test compared to SP effect in control experiments).
To determine the conductance responsible for the SP excitation, we applied voltage ramps before and during the application of SP. The current–voltage plot for this effect reversed at the potassium equilibrium potential (calculated at −103 mV for our internal solution, Fig. 3). Moreover, the curve showed inward rectification, consistent with the closing of a GIRK channel. To confirm this, we applied SP in the presence of barium (500 μm), which blocks inward-rectifying K+ channels. Barium clearly attenuated the SP current–voltage relationship (Fig. 3).
These data implicate GIRK function as the downstream effector of SP receptor activation as opposed to activating the NALCN as shown by Lu et al. (2009). However, we did observe an additional inward current in the presence of barium, making up roughly half of the response at −50 mV (Fig. 3A), consistent with SP coupling to both GIRK and NALCN. To confirm this, we bath-applied SP to neurons recorded using an internal solution that contained caesium, to block the GIRK. In voltage-clamped neurons, despite the caesium internal solution SP produced an inward current (Fig. 3B, 13.0 ± 5.2 pA, n= 7, P < 0.05, paired t test).
Thus, NK receptors couple to two channels in the VTA: they close a GIRK and simultaneously open the NALCN, with each contributing roughly half of the SP-induced inward current at the neuron's resting membrane potential. Consistent with this, there was no significant change in input resistance associated with the SP excitation (18.6 ± 11% increase, n= 13, n.s. paired t test).
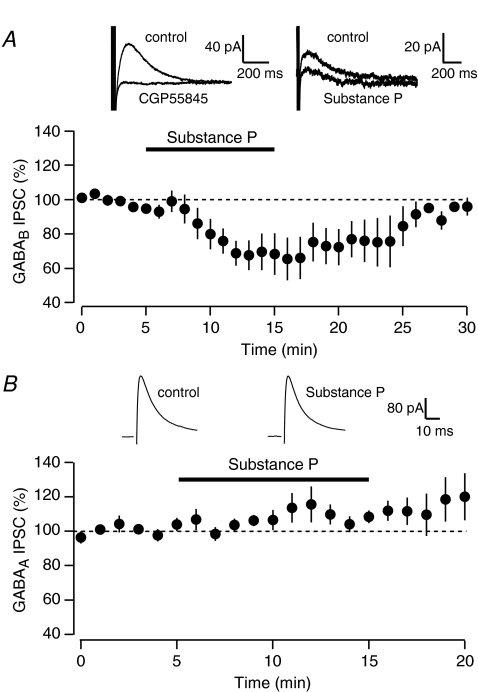
If GIRKs are a target for NK receptor signalling, it follows that SP may interact with the actions of other neurotransmitters whose receptors are coupled to GIRKs, such as the GABAB receptor (Dutar & Nicoll, 1988; Lüscher et al. 1997). Therefore, we tested whether SP alters signalling mediated by GABAB receptors in VTA neurons. To do so, we evoked pharmacologically isolated GABAB-mediated IPSCs once every 30 s with a bipolar stimulating electrode. These GABAB IPSCs were completely blocked by the selective antagonist CGP-55845 (1 μm, Fig. 4A). Following the bath application of SP, the amplitude of the evoked GABAB IPSCs was inhibited by 31.4 ± 9.2% (Fig. 4A, n= 7, P < 0.05, paired t test).
Figure 4. SP inhibits GABAB, but not GABAA-mediated IPSCs.
A, average of six neurons shows an SP-mediated inhibition of pharmacologically isolated GABAB IPSCs. Upper left inset: trace showing that the GABAB IPSC is completely blocked by the GABAB antagonist CGP55845 (1 μm). Upper right inset: trace showing example IPSC in control and following SP application. B, average of five neurons showing that GABAA IPSCs are not inhibited by SP. Insets: GABAA IPSCs during control (left) and following SP application (right).
This inhibition of a GIRK-mediated signal raises the possibility of an intracellular interaction between SP and GABAB signalling. However, in other brain regions SP can presynaptically modulate GABA release (Kombian et al. 2003; Bailey et al. 2004). Therefore, to determine whether the SP-mediated inhibition of GABAB signalling we observed is due to a postsynaptic interaction or a presynaptic change in GABA release, we measured the effect of SP on pharmacologically isolated evoked GABAA-mediated IPSCs. Bath application of SP did not cause a significant change on the amplitude of GABAA IPSCs (Fig. 4B, 110.5 ± 6.7% of baseline, n= 5, n.s. paired t test).
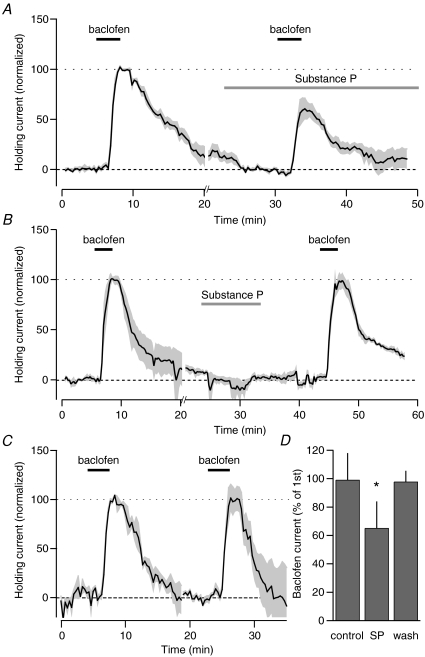
This differential effect of SP on GABAA and GABAB receptor-mediated IPSCs argues against a presynaptic mechanism for SP. To confirm that SP is acting directly on the postsynaptic neuron to alter GABAB signalling, we investigated the effects of SP on the direct effects of the GABAB agonist baclofen. A brief, 3 min application of a saturating dose (20 μm) of baclofen produced a large outward current in neurons voltage clamped at −60 mV. A second application of baclofen, given in the presence of SP exhibited a reduced response (Fig. 5A, 61.3 ± 11.2% of the peak of the first baclofen application, n= 5, P < 0.05 paired t test). This SP-mediated inhibition reversed following the washout of SP: when the second baclofen application was given 10 min after the washout of SP, the peak current was similar to the peak of the initial application (Fig. 5B, 97.7 ± 7.2%, n= 5, n.s. paired t test). The effect of SP on the baclofen current is not due to receptor desensitization, since under control conditions baclofen can be bath-applied multiple times without attenuation (Fig. 5C, 99.7 ± 13.0% of first application, n= 4, n.s. paired t test). These data indicate that GABAB receptor-mediated synaptic transmission is attenuated by SP through a postsynaptic mechanism.
Figure 5. SP inhibits baclofen-mediated currents onto VTA neurons.
A, average holding current change in response to 20 μm baclofen before and during bath application of SP (1 μm). Individual experiments are normalized so that the peak of the initial baclofen application is 100%. Responses are re-zeroed prior to 2nd baclofen application for comparison (hash marks on x-axis indicate the point of renormalization). B, application of baclofen 10 min after washout of SP shows that the inhibition of the baclofen current by SP reverses. C, control experiments showing that multiple applications of baclofen do not display desensitization when applications are separated by 20 min. D, summary data for control (n= 4), SP experiments (n= 5), and following washout of SP (n= 5). *P < 0.05 compared to control, one-way ANOVA with a Holm–Sidak post-hoc test.
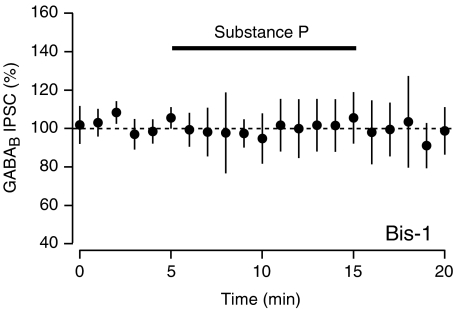
We next tested whether the SP-mediated inhibition of the GABAB IPSC is dependent upon PKC. Incubating slices in the PKC inhibitor bisindolylmaleimide-1 (BIS-1, 10 μm) for at least 30 min prior to SP application completely blocked the SP-mediated inhibition of the evoked GABAB IPSC (Fig. 6, 101.8 ± 12.5% of baseline, n= 7, n.s.), indicating that activation of PKC is required for this effect.
Figure 6. Inhibition of GABAB IPSCs requires PKC.
Average of five neurons shows that GABAB IPSCs are not inhibited by SP in slices that have been incubated in the PKC inhibitor Bis-1 (10 μm).
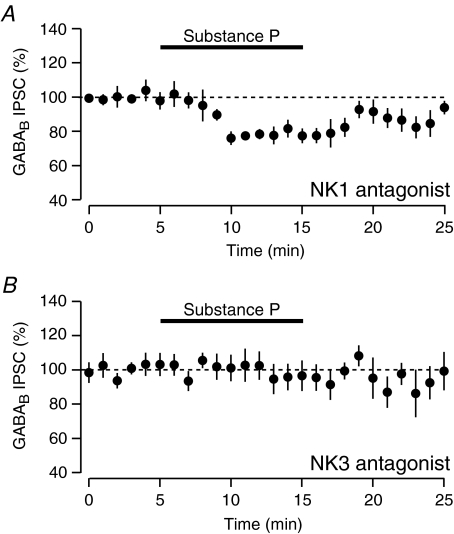
Since SP can activate both NK1 and NK3 receptors, we investigated which receptor subtype mediates the inhibition of the GABAB IPSCs in the VTA. The NK1 antagonist L-732,138 (10 μm) had no effect on the magnitude of SP-mediated inhibition of the GABAB IPSC (Fig. 7A, inhibited by 21.4%± 3.5, n= 7, not significantly different to SP inhibition in control conditions, unpaired t test). On the other hand, following application of the NK3 antagonist SB222200 (2 μm), SP no longer inhibited the GABAB IPSC (Fig. 7B, inhibited by 4.1 ± 7.5%, n= 7, significantly different to SP inhibition in control conditions, P < 0.05 unpaired t test).
Figure 7. Inhibition of GABAB is mediated through NK3 receptors.
A, SP-mediated inhibition of evoked GABAB IPSCs is unaffected by the NK1 antagonist L-732,138 (10 μm, n= 7). B, inhibition of GABAB IPSCs by SP is blocked by the NK3 antagonist SB222200 (2 μm, n= 7).
Discussion
We have shown that SP depolarizes VTA neurons by closing an inwardly rectifying K+ channel. This coupling depends upon PLC, requires an increase in internal calcium and is PKC dependent. Moreover, SP inhibits GABAB receptor-mediated IPSCs. These data appear to contrast with a recent study using dissociated VTA neurons, where SP acted in a G-protein-independent manner to open NALCN (Lu et al. 2009). This activation was absent in a NALCN knockout mouse. Importantly, Lu et al. (2009) blocked all K+-mediated effects of SP by using a caesium-based internal solution, therefore only permitting the observation of NK receptor coupling to NALCN. As NK receptors can couple to multiple effector pathways, it is reasonable to conclude that SP inhibits a K+ channel while simultaneously activating NALCN. The physiological impact of coupling to both an inhibitory and an excitatory conductance is unclear. Importantly, the balance between K+ channel inhibition versus NALCN activation will be determined by the level of tonic GIRK activation, which depends in part on the activity of GABAergic afferents acting at GABAB receptors. It should also be noted that independent of their regulation by SP, the two channels will interact in a complex manner: the increased membrane resistance associated with GIRK inactivation should increase NALCN depolarizations while the GIRK-mediated depolarization will decrease the driving force for the NALCN. The extent of such an interaction will ultimately depend upon the cellular distribution of the two channels.
Lu et al. (2009) found that SP coupled to NALCN through Src kinase. In our hands, an Src kinase inhibitor had no effect on SP-mediated excitations in our VTA slice preparation, while it was entirely blocked by inhibiting PLC. The reason for this discrepancy is not clear, but at the least indicates that the NK receptor coupling to GIRK is independent of Src kinase.
GABAB receptors activate GIRKs through direct binding of the Gβγ subunit to the channel. Previous studies have demonstrated that Gq-coupled receptors can inhibit GIRK channels either through activation of PKC or through the depletion of PIP2 (Takano et al. 1995; Keselman et al. 2007; Sohn et al. 2007). In VTA neurons, SP-mediated inhibition of GABAB signalling clearly depends upon PKC activation. Mutation of key PKC phosphorylation sites on GIRK1 and GIRK4 subunits abolishes the inhibition by SP when the mutated GIRK subunits were co-expressed with NK1 receptors in Xenopus oocytes (Mao et al. 2004), pointing to a direct interaction between PKC and the GIRK channel. However, it is also possible that PKC alters function by phosphorylating the GABAB receptor itself or an intermediary protein, such as a regulator of G-protein signaling RGS protein.
While the NK1 receptor has the highest affinity to SP, our data indicate that the SP-mediated inhibition of GABAB IPSCs is mediated through the NK3 receptor. This is consistent with results from a previous slice electrophysiology study using selective NK agonists, which found that NK3 but not NK1 agonists increased the firing rate of putative DA neurons in the VTA (Seabrook et al. 1995). EM studies also support this finding: while both NK1 and NK3 receptors are observed in the VTA (Lessard & Pickel, 2005; Lessard et al. 2007), the distribution of NK1 receptors is predominantly cytoplasmic (Lessard & Pickel, 2005).
At a circuit level, the interaction between NK and GABAB receptors is intriguing. It has been demonstrated that there are differences in GABAB receptor function on VTA DA and GABA neurons (Cruz et al. 2004; Labouebe et al. 2007). Specifically, the GABAB receptors on DA neurons have a lower affinity for agonists compared to GABA neurons. Thus, at low concentrations, GABAB agonists can lead to the disinhibition of DA neurons. As SP preferentially acts on DA neurons, release of SP should amplify this disinhibitory effect.
SP is co-localized in GABA-containing terminals arising from the nucleus accumbens (Lu et al. 1998). It has been proposed that GABAA and GABAB receptors segregate to separate subpopulations of VTA synapses and this particular projection activates GABAB receptors (Sugita et al. 1992; Cameron & Williams, 1993). Moreover, the patterns of stimulation required to release dense core vesicles are plausibly similar to the stimulation used to activate GABAB receptors. In this scenario, the actions of these two neurotransmitters will counteract each other, at least in SP-sensitive neurons.
NK receptors have recently been suggested as a target for the treatment of alcoholism and addiction (Foroud et al. 2008; George et al. 2008; Kuehn, 2009) In rats, microinjection of SP and/or SP analogues into the VTA elicit locomotor activation (Kelley et al. 1979), and induce reinstatement to cocaine-seeking in rats (Placenza et al. 2004). Further, stress, which is clearly implicated in relapse to drug seeking in both animal models of addiction and in humans, causes release of SP into the VTA (Lisoprawski et al. 1981; Bannon et al. 1983). Conversely, GABAB agonists reduce ethanol intake (Colombo et al. 2003) and cocaine self-administration (reviewed in Roberts, 2005). Our data indicate that the release of endogenous SP into the VTA, such as during periods of stress, would act to decrease the efficacy of GABAB agonists in the treatment of addiction. This suggests that NK receptor antagonists may synergize with GABAB agonists to more effectively prevent relapse.
Acknowledgments
The authors thank J. Driscoll for technical help. This work was funded by ABMRF/The Foundation for Alcohol Research, The Wheeler Center for the Neurobiology of Addiction and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco.
Glossary
Abbreviations
- DA
dopamine
- GIRK
G-protein linked inwardly rectifying K+
- Ih
hyperpolarization-activated non-specific cation current
- NALCN
Na+ leak channel
- NK
neurokinin
- VTA
ventral tegmental area
Author contributions
Y.-F.X., E.B.M. and G.O.H. designed the project, wrote the manuscript and approved the final version to be published. Y.-F.X. conducted experiments and analysed data.
References
- Bailey CP, Maubach KA, Jones RS. Neurokinin-1 receptors in the rat nucleus tractus solitarius: pre- and postsynaptic modulation of glutamate and GABA release. Neuroscience. 2004;127:467–479. doi: 10.1016/j.neuroscience.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Elliott PJ, Alpert JE, Goedert M, Iversen SD, Iversen LL. Role of endogenous substance P in stress-induced activation of mesocortical dopamine neurones. Nature. 1983;306:791–792. doi: 10.1038/306791a0. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmacology (Berl) 2003;167:221–224. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Luscher C. Bi-directional effects of GABAB receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Emson PC, Paxinos G, Jessell T. Substance P containing and cholinergic projections from the habenula. Brain Res. 1978;149:413–429. doi: 10.1016/0006-8993(78)90484-5. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Maggio JE, Bannon MJ, Kalivas PW, Tam SY, Goldstein M, Roth RH. Substance K and substance P differentially modulate mesolimbic and mesocortical systems. Peptides. 1985;6:113–122. doi: 10.1016/0196-9781(85)90143-3. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behaviour and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Foroud T, Wetherill LF, Kramer J, Tischfield JA, Nurnberger JI, Jr, Schuckit MA, Xuei X, Edenberg HJ. The tachykinin receptor 3 is associated with alcohol and cocaine dependence. Alcohol Clin Exp Res. 2008;32:1023–1030. doi: 10.1111/j.1530-0277.2008.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Hubbard KB, Hepler JR. Cell signalling diversity of the Gqα family of heterotrimeric G proteins. Cell Signal. 2006;18:135–150. doi: 10.1016/j.cellsig.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L, Iversen SD. Behavioural activation induced in the rat by substance P infusion into ventral tegmental area: implication of dopaminergic A10 neurones. Neurosci Lett. 1979;11:335–339. doi: 10.1016/0304-3940(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Keselman I, Fribourg M, Felsenfeld DP, Logothetis DE. Mechanism of PLC-mediated Kir3 current inhibition. Channels (Austin) 2007;1:113–123. doi: 10.4161/chan.4321. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Ananthalakshmi KV, Parvathy SS, Matowe WC. Dopamine and adenosine mediate substance P-induced depression of evoked IPSCs in the rat nucleus accumbens in vitro. Eur J Neurosci. 2003;18:303–311. doi: 10.1046/j.1460-9568.2003.02753.x. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Findings on alcohol dependence point to promising avenues for targeted therapies. JAMA. 2009;301:1643–1645. doi: 10.1001/jama.2009.535. [DOI] [PubMed] [Google Scholar]
- Labouebe G, Lomazzi M, Cruz HG, Creton C, Lujan R, Li M, Yanagawa Y, Obata K, Watanabe M, Wickman K, Boyer SB, Slesinger PA, Luscher C. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- Lessard A, Grady EF, Bunnett NW, Pickel VM. Predominant surface distribution of neurokinin-3 receptors in non-dopaminergic dendrites in the rat substantia nigra and ventral tegmental area. Neuroscience. 2007;144:1393–1408. doi: 10.1016/j.neuroscience.2006.10.058. [DOI] [PubMed] [Google Scholar]
- Lessard A, Pickel VM. Subcellular distribution and plasticity of neurokinin-1 receptors in the rat substantia nigra and ventral tegmental area. Neuroscience. 2005;135:1309–1323. doi: 10.1016/j.neuroscience.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Lisoprawski A, Blanc G, Glowinski J. Activation by stress of the habenulo-interpeduncular substance P neurons in the rat. Neurosci Lett. 1981;25:47–51. doi: 10.1016/0304-3940(81)90099-9. [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang X, Chen F, Wang R, Rojas A, Shi Y, Piao H, Jiang C. Molecular basis for the inhibition of G protein-coupled inward rectifier K+ channels by protein kinase C. Proc Natl Acad Sci U S A. 2004;101:1087–1092. doi: 10.1073/pnas.0304827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev SV, Lebedev AA, Bychkov ER, Oblyapin AV, Dambinova SA, Shabanov PD. The effects of substance P after central administration on the activity of the mesolimbic system of the rat brain as studied by microdialysis. Neurosci Behav Physiol. 2004;34:743–746. doi: 10.1023/b:neab.0000036016.65208.27. [DOI] [PubMed] [Google Scholar]
- Overton P, Elliott PJ, Hagan RM, Clark D. Neurokinin agonists differentially affect A9 and A10 dopamine cells in the rat. Eur J Pharmacol. 1992;213:165–166. doi: 10.1016/0014-2999(92)90251-x. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Fletcher PJ, Rotzinger S, Vaccarino FJ. Infusion of the substance P analogue, DiMe-C7, into the ventral tegmental area induces reinstatement of cocaine-seeking behaviour in rats. Psychopharmacology (Berl) 2004;177:111–120. doi: 10.1007/s00213-004-1912-9. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Bowery BJ, Hill RG. Pharmacology of tachykinin receptors on neurones in the ventral tegmental area of rat brain slices. Eur J Pharmacol. 1995;273:113–119. doi: 10.1016/0014-2999(94)00681-v. [DOI] [PubMed] [Google Scholar]
- Shen KZ, North RA. Substance P opens cation channels and closes potassium channels in rat locus coeruleus neurons. Neuroscience. 1992;50:345–353. doi: 10.1016/0306-4522(92)90428-5. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Lim A, Lee SH, Ho WK. Decrease in PIP2 channel interactions is the final common mechanism involved in PKC- and arachidonic acid-mediated inhibitions of GABAB-activated K+ current. J Physiol. 2007;582:1037–1046. doi: 10.1113/jphysiol.2007.137265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima Y, Yamaguchi K. Substance P raises neuronal membrane excitability by reducing inward rectification. Nature. 1985;315:498–501. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- Stinus L, Kelley AE, Iversen SD. Increased spontaneous activity following substance P infusion into A10 dopaminergic area. Nature. 1978;276:616–618. doi: 10.1038/276616a0. [DOI] [PubMed] [Google Scholar]
- Sugita S, Johnson SW, North RA. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- Takano K, Stanfield PR, Nakajima S, Nakajima Y. Protein kinase C-mediated inhibition of an inward rectifier potassium channel by substance P in nucleus basalis neurons. Neuron. 1995;14:999–1008. doi: 10.1016/0896-6273(95)90338-0. [DOI] [PubMed] [Google Scholar]
- Takano K, Yasufuku-Takano J, Kozasa T, Singer WD, Nakajima S, Nakajima Y. Gq/11 and PLC-β1 mediate the substance P-induced inhibition of an inward rectifier K+ channel in brain neurons. J Neurophysiol. 1996;76:2131–2136. doi: 10.1152/jn.1996.76.3.2131. [DOI] [PubMed] [Google Scholar]