Abstract
The endothelium, a single layer of cells lining the entire circulatory system, plays a key role in maintaining vascular health. Endothelial dysfunction independently predicts cardiovascular events and improvement in endothelial function is associated with decreased vascular risk. Previous studies have suggested that exercise training improves endothelial function in macrovessels, a benefit mediated via repeated episodic increases in shear stress. However, less is known of the effects of shear stress modulation in microvessels. In the present study we examined the hypothesis that repeated skin heating improves cutaneous microvascular vasodilator function via a shear stress-dependent mechanism. We recruited 10 recreationally active males who underwent bilateral forearm immersion in warm water (42°C), 3 times per week for 30 min. During these immersion sessions, shear stress was manipulated in one arm by inflating a pneumatic cuff to 100 mmHg, whilst the other arm remained uncuffed. Vasodilatation to local heating, a NO-dependent response assessed using laser Doppler, improved across the 8 week intervention period in the uncuffed arm (cutaneous vascular conductance week 0 vs. week 4 at 41°C: 1.37 ± 0.45 vs. 2.0 ± 0.91 units, P= 0.04; 42°C: 2.06 ± 0.45 vs. 2.68 ± 0.83 units; P= 0.04), whereas no significant changes were evident in the cuffed arm. We conclude that increased blood flow, and the likely attendant increase in shear stress, is a key physiological stimulus for enhancing microvascular vasodilator function in humans.
Introduction
Located at the interface between the blood and vessel wall, the endothelium is essential for maintenance of vessel health and for the local regulation of vascular tone (Furchgott & Zawadzki, 1980). Endothelial dysfunction is associated with increased cardiovascular mortality and morbidity (Rossi et al. 2008; Shechter et al. 2009; Yeboah et al. 2009) and improvement in endothelial function decreases cardiovascular risk (Modena et al. 2002; Kitta et al. 2009). In conduit and resistance arteries, exercise training is associated with improvement in endothelial function (Green et al. 2004). One likely physiological mechanism involves the episodic increase in arterial shear stress associated with repeated bouts of exercise (Hambrecht et al. 2003; Tinken et al. 2010).
Less is known about the stimuli responsible for modulation of microvascular function in humans. We recently utilised a gradual local heating stimulus, which elicits cutaneous vasodilatation which is highly nitric oxide (NO) dependent, to assess the effects of fitness and exercise training on skin blood flow responses in humans (Black et al. 2008). Training was associated with enhanced vasodilator responses to local heating, with the magnitude of improvement largely attributable to enhanced NO-mediated vasodilatation (Black et al. 2008). However, it is not known whether this improvement in microvascular vasodilator function with exercise training is dependent upon increased microvascular shear stress, or some exercise-mediated effect independent of the increased blood flow and shear stress which is associated with exercise.
We therefore designed the present study to examine the effects of repeated increases in blood flow and shear stress, independent of exercise, on microvascular function. Skin blood flow responses to a NO-dependent gradual heating protocol were assessed before, during (week 4) and after an 8 week intervention period involving bilateral forearm immersion in warm water (42°C) for 30 min, 3 times per week. The placement of a pneumatic cuff around one forearm during each of these heating bouts minimised the increase in shear stress observed in the uncuffed limb. Using this within-subjects, simultaneous stimulus model, we examined the hypothesis that repetitive increases in blood flow and shear stress as a result of episodic heating would enhance microvascular function and that repeated heating, in the absence of increases in blood flow and shear stress, would not modify microvascular function.
Methods
Ethical approval
All study procedures were approved by the Human Research and Ethics Committee of the University of Western Australia. Written, informed consent was obtained from all subjects, and studies conformed to the Declaration of Helsinki.
Subject characteristics
Ten young recreationally active males (21.9 ± 1.7 years, Table 1) were recruited to undertake an 8 week experimental protocol. A pre-participation questionnaire was administered to exclude subjects with unsuitable lifestyle traits or medical issues such as cardiovascular disease, smoking, hypertension and hypercholesterolaemia. Due to possible anti-atherogenic effects of oestrogen, women were excluded from this study along with individuals taking medications or drugs of any kind.
Table 1.
Baseline characteristics of study participants
| Age (years) | 21.9 ± 1.7 |
| Height (m) | 1.82 ± 0.07 |
| Weight (kg) | 79.1 ± 7.8 |
| BMI (kg m−2) | 23.8 ± 1.6 |
| SBP (mmHg) | 126 ± 8 |
| DBP (mmHg) | 58 ± 6 |
| MAP (mmHg) | 83 ± 6 |
| HR (beats min−1) | 61 ± 5 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Study design
Once enrolled in the study, all subjects underwent baseline assessments and were then required to attend the laboratory 3 times per week, for 8 weeks. Each visit involving bilateral forearm water bath exposure (42°C). Training began within 1 week of the baseline assessments. The experimental protocols were repeated during week 4 and again at the end of the training period (week 8). All studies were conducted at the same time of day to eliminate the possible effects of the circadian rhythm on vascular function.
Experimental procedures
Subjects arrived for testing having fasted for 8 h. In addition, they were advised to abstain from alcohol and/or caffeine and exercise for 24 h prior to testing. The laser Doppler probe sites on each forearm were shaved and cleaned 24 h prior to testing to avoid any inflammatory response that would otherwise affect skin blood flow. Similar placement sites were selected on each forearm and the location of these sites was recorded so that repeated measures at 4 and 8 weeks were undertaken at similar locations.
Upon arrival, subjects were seated and instrumented for ∼10 min and then began the ∼120 min heating protocol (see Black et al. 2008). Local heater discs (Perimed 455, Stockholm, Sweden) were attached to the forearms using double-sided adhesive rings. The laser Doppler probes, each with a 7-laser array (Model 413, Periflux 5001 System, Perimed AB), were then fitted into the middle of these localised heating discs to record change in red cell flux (in perfusion units, PU) (Cracowski et al. 2006).
Thermistors were placed and secured on five different sites (the calf, thigh, sternum and both forearms proximal to the heater discs) to continually record skin temperature throughout the protocol. This was to ascertain whether there were any increases in skin temperature at sites other than the forearms, which would imply a systemic thermoregulatory reflex in response to the forearm heating. Room temperature was also recorded throughout all assessments.
Once instrumented, recording commenced and the heater disc temperatures were increased to 33°C and maintained at this temperature for a 20 min ‘baseline’ period. Upon the completion of this baseline period, increments in heater disc temperature were gradual, so as to minimise any impact of axon reflexes, which are less NO dependent than the incremental heating response. Hence, after the 20 min at 33°C, the heaters increased in increments of 0.5°C every 5 min until 42°C was reached. Once the probes reached 42°C (∼90 min), they remained at this temperature for a period of 30 min. Blood pressure was recorded every 5 min at the ankle using a Dinamap automated monitor. Blood pressure measures were later corrected for the hydrostatic column (Groothuis et al. 2008) and used to calculate cutaneous vascular conductance (CVC), which accounts for skin blood flow changes which occur as a result of changes in blood pressure (Cracowski et al. 2006). This heating protocol is identical to that used in a recent study which established that skin blood flow responses to gradual incremental heating are highly dependent upon NO (Black et al. 2008).
All laser Doppler, skin thermistor, room and core temperature measurements were relayed and graphed in real time onto a laptop using the software program LabChart 6 (ADInstruments, Sydney, Australia).
Water bath ‘training’ protocol
Following the initial laser Doppler protocol described above, subjects were required to attend the laboratory 3 times per week for a period of 8 weeks. During each of the 30 min bilateral water bath exposures, a pneumatic cuff was placed on one forearm, below the elbow, and inflated to 100 mmHg using a rapid inflation/deflation pneumatic device (AG101 Hokanson, Bellevue, WA, USA). This cuff pressure was chosen following pilot trails to determine the optimal pressure required to maintain skin perfusion at near-baseline values during forearm heating. The contralateral arm remained uncuffed. The decision regarding left or right forearm cuff placement was randomised, but kept consistent for a given individual across the 24 water bath sessions.
Following cuff placement, both arms were immersed in warm water (42°C) up to or above the level of the elbow for 30 min. The water was maintained at a constant 42°C in a storage reservoir using a thermostatically controlled heating unit. This reservoir was connected via tubing to both forearm immersion tanks and a submersible pump ensured that water of identical temperature was continuously circulated to both forearm tanks.
Efficacy of independent variable manipulation
To determine whether differences in forearm and skin blood flow occurred between the cuffed and uncuffed arms during each water heating session, laser Dopplers were used to record changes in skin blood flow to each forearm during the submersion period in five subjects. Assessments of laser Doppler flux were made continuously in the cuffed and uncuffed arm immediately prior to warm water immersion and then throughout the 30 min immersion period. Finally, at the end of the 30 min immersion, we deflated the cuff whilst the arms remained immersed to determine whether any differences between the limbs were indeed due to cuff inflation. Skin temperatures (skin thermistors; calf, thigh, sternum and both forearms, MLT409, ADInstruments) and core temperature (RET-1, Physitemp Instruments, NJ, USA) were also measured throughout the 30 min warm water bath session in a subgroup of five subjects.
Data analysis
Laser Doppler protocol
Laser Doppler flux (LDF) from the cuffed and uncuffed arms was averaged over a stable 30 s period at the end of every 5 min interval to assess skin blood flow. Calibration of the probes was undertaken before and after the experiments using two generic points, 0 and 250 PU, in accordance with calibration guidelines using a zeroing disk and motility standard (Periflux System, Perimed AB). Measurements in perfusion units (PU) were converted to cutaneous vascular conductance (CVC) which was calculated as PU/Dinamap mean arterial pressure (MAP). All data from the gradual heating protocol were then normalised via expression as %CVCmax, using the maximum value recorded for each individual at the end of the prolonged heating phase of the protocol (Cracowski et al. 2006). All skin thermistor, core and room temperature readings were averaged at the end of each 5 min interval (in °C).
Statistics
Skin blood flow LDF and CVC outcome data were compared within subjects, across the three study time points (0, 4 and 8 weeks) using 2-factor ANOVA with planned comparisons performed on three temperature points: baseline (33°C), 41°C and peak (42°C). Post hoc Student's t tests were performed where significance was detected at the 0.05 level.
Results
Impact of cuff placement during bilateral forearm heating: efficacy of independent variable manipulation
Laser Doppler flux (LDF) and cutaneous vascular conductance (CVC)
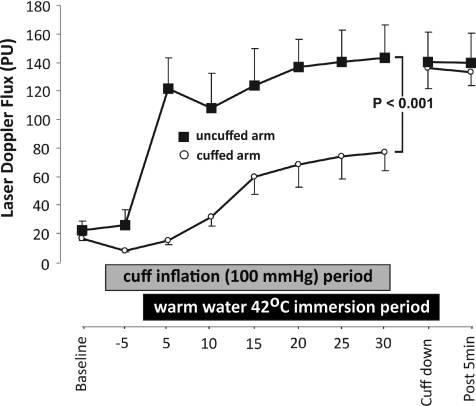
No significant differences were evident in LDF or CVC between the cuffed and uncuffed arms in the baseline period before the cuff was inflated (LDF 16.81 ± 2.02 vs. 22.26 ± 6.55, P= 0.36). In the absence of heating, inflation of the cuff around one arm caused a non-significant decrease in LDF and CVC in the cuffed arm (8.08 ± 0.55 cuffed vs. 26.14 ± 10.60 uncuffed, P= 0.16). However, once both arms where immersed in the warm water (42°C) there was a significant increase in the LDF (ANOVA, main effect for cuff placement, P= 0.001, Fig. 1) and CVC (ANOVA, main effect for cuff placement, P= 0.001) in the uncuffed arm, relative to the cuffed arm, across the entire immersion period. Cuff deflation at the end of the immersion period resulted in a rapid increase in cuffed arm LDF (76.7 ± 12.6 at 30 min vs. 136.3 ± 14.3 post-deflation, P= 0.004) and CVC (0.96 ± 0.15 at 30 min vs. 1.71 ± 0.19 post-deflation, P= 0.005) to values approximating those in the uncuffed arm (Fig. 1).
Figure 1. LDF differences between cuffed and uncuffed arms before and throughout water bath immersion.
Both limbs exhibited similar laser Doppler responses prior to cuff placement. Cuff placement in the absence of heating slightly decreased responses in the cuffed arm. Immersion in warm water exaggerated the difference between the limbs (P < 0.001). These differences resolved on cuff deflation.
Core and skin temperatures during forearm immersion
Repeated measures ANOVA revealed that core body temperature increased modestly, but significantly, with heating (37.7 ± 1.3°C to 37.9 ± 0.1°C). Post hoc t tests revealed that there were differences between baseline core temperature and core temperatures at 20, 25 and 30 min of heating. Mean skin temperature, calculated from the thigh, leg and sternum placement sites, decreased significantly from baseline (32.2 ± 0.6°C) during immersion of the arms in warm water (30 min value: 30.6 ± 0.6°C).
Impact of repetitive heating on skin blood flow responses
Comparison of perfusion unit data at baseline, and weeks 4 and 8
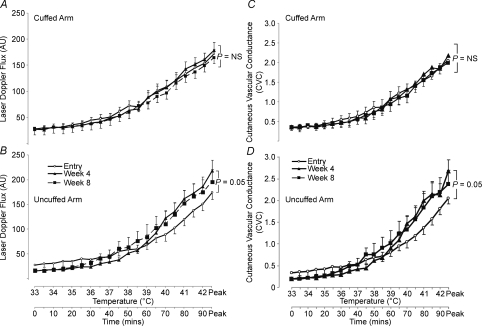
Mean PU values at 33°C were similar in the cuffed limb between weeks 0, 4 and 8 (27.6 ± 14.8 versus 27.0 ± 18.8 versus 28.1 ± 18.9; ANOVA P= 0.99, Fig. 2A). Similarly, 2-way ANOVA on temperature (33°C, 41°C and peak (42°C)) revealed no significant difference in the cuffed arm between weeks 0, 4 and 8 (P= 0.44) and no differences between any of the time-points at any given temperature by paired t test (Fig. 2A). No differences were evident between peak PU values at 0, 4 and 8 weeks (172.0 ± 26.1 versus 178.9 ± 35.9 versus 164.5 ± 37, ANOVA P= 0.57, Fig. 2A).
Figure 2. Averaged LDF and CVC data.
Averaged laser Doppler flux (LDF) data between weeks 0, 4 and 8 at all temperature points in the cuffed (A) and uncuffed (B) limbs. AU, arbitrary units; NS, no significance. Averaged cutaneous vascular conductance (CVC) data between weeks 0, 4 and 8 at all temperature points is shown in the cuffed (C) and uncuffed (D) limbs.
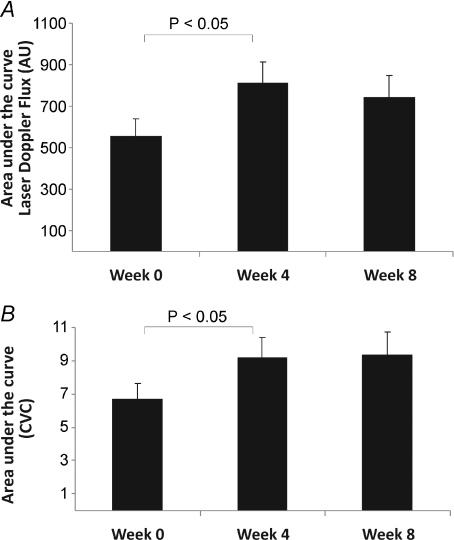
ANOVA on temperature points at 33°C, 41°C and peak (42°C) revealed a significance value of P= 0.05 between uncuffed arm PU data at weeks 0, 4 and 8 (Fig. 2B). A t test performed on the area under the curve between weeks 0 and 4, for temperature data from the onset of the heating response (36°C) to peak heating (42°C) was significant (P= 0.02; Fig. 3A). Individual heating point comparisons between weeks 0 and 4 at 41°C (119.5 ± 42.8 versus 161.4 ± 59.9; P= 0.06) and peak heating approached significance (42°C; 174.4 ± 50.9 versus 218.1 ± 61.7 PU; P= 0.06) (Fig. 2B).
Figure 3. Skin vascular responses in cuffed and uncuffed arms.
Averaged (±s.e.m.) area under the curve for: A, uncuffed forearm laser Doppler flux (perfusion units) at entry, 4 and 8 weeks and B, uncuffed forearm cutaneous vascular conductance (CVC) at entry, 4 and 8 weeks. *P < 0.05.
Comparisons of cutaneous vascular conductance (CVC) at baseline, week 4 and week 8 of repeated bilateral heating
Raw PU skin blood flow data can be affected by changes in arterial blood pressure, so it is conventionally converted to cutaneous vascular conductance (PU/MAP) (Cracowski et al. 2006). Values for CVC at baseline (33°C) in the cuffed limb were similar between weeks 0, 4 and 8 (0.35 ± 0.22 versus 0.34 ± 0.25 versus 0.35 ± 0.26; ANOVA P= 0.99, Fig. 2C). Furthermore, a 2-way ANOVA on temperature data points at 33°C, 41°C and peak (42°C) revealed no significant difference in the cuffed arm between weeks 0, 4 and 8 (P= 0.48) and, similar to cuffed PU data, there were no differences between any of the weeks at any given temperature by paired t test (Fig. 2C). No difference was evident between peak CVC values at weeks 0, 4 or 8 (2.06 ± 0.27 versus 2.18 ± 0.4 versus 2.0 ± 0.48; ANOVA P= 0.60).
Repeated water bath exposure significantly enhanced the uncuffed arm CVC between temperature data points at 33°C, 41°C and peak (42°C) between weeks 0, 4 and 8 (2-way ANOVA; P= 0.03, Fig. 2D). Specifically, significant differences between weeks 0 and 4 at temperature points 41°C (1.37 ± 0.45 versus 2.0 ± 0.91; P= 0.04), 41.5°C (1.58 ± 0.47 versus 2.15 ± 0.81; P= 0.05) and peak 42°C (2.06 ± 0.45 versus 2.68 ± 0.83; P= 0.04) were evident. In addition, a t test performed on the area under the curve for weeks 0 and 4 data from the onset of the heating response (36°C) to peak heating (42°C) proved significant (P= 0.04, Fig. 2D).
Discussion
We recently demonstrated that exercise training enhances the largely NO-dependent skin blood flow response to incremental heating in humans (Black et al. 2008). The aim of the present study was to determine whether repeated episodic skin vasodilatation, independent of exercise, improves NO-mediated microvascular function. We also assessed the contribution of shear stress to this adaptation using cuff inflation on one arm during each heating bout to bilaterally manipulate skin blood flow and shear stress. Our results indicate that warm water immersion was associated with a significant increase in skin blood flow, that cuff inflation significantly attenuated this increase in skin blood flow during forearm heating, that repeated exposure to forearm heating significantly enhanced skin blood flow responses to a NO-dependent incremental local heating protocol and that no such improvement was evident when the blood flow and therefore the shear stress response to heating were significantly attenuated by cuff placement. We submit that increased blood flow, and the likely attendant increase in shear stress, is a key physiological stimulus for enhancing cutaneous microvascular vasodilator function in humans in response to heating.
The results of this study are consistent with the few previous studies that have assessed exercise training effects on endothelial function in microvessels (Boegli et al. 2003; Lenasi & Strucl, 2004; Black et al. 2008). However, our results suggest that increases in blood flow which are independent of an exercise stimulus also induce improvements in microvascular function in the skin. Given that the gradual heating protocol we employed to measure skin blood flow elicits a largely NO-dependent vasodilator response (Black et al. 2008), our findings therefore suggest that localised improvements in microvascular function in the uncuffed arm result from enhanced NO-dependent vasodilatation.
Studies investigating the effects of exercise in larger arteries in humans have concluded that exercise induces improvement in NO-mediated endothelial function which is shear stress mediated (Green et al. 2004; Tinken et al. 2010). For example, Hambrecht demonstrated an increase in internal mammary artery endothelial NO synthase mRNA and protein content, phosphorlyation of shear-sensitive endothelial nitric oxide synthase (eNOS) moieties and enhanced in vitro and in vivo NO vasodilator function as a result of exercise training (Hambrecht et al. 2003). More recently, Tinken et al. (2010) demonstrated that increased shear stress is a necessary precondition for enhanced skeletal muscle conduit and resistance vessel adaptation in healthy young subjects. In keeping with these larger artery studies, we previously observed increases in NO-mediated vasodilator responses in skin microvessels as a consequence of exercise training (Black et al. 2008). However, links between shear stress and change in microvascular function have not previously been made, possibly due to perceived differences in the magnitude of exposure of smaller vessels to shear stress. In addition, microvessels may possess less eNOS content than larger arteries (Laughlin et al. 2003). Our results are therefore the first, to our knowledge, to indicate that manipulation of blood flow and shear stress induces differential adaptation in cutaneous microvascular function in humans.
To our knowledge, no study has previously assessed the impact of repeated localised skin heating, with manipulation of the attendant hyperaemia, on cutaneous microvascular function in humans. No improvements were observed across the 8 weeks of repeated heating exposure in the cuffed arm in this experiment, indicating that heating alone, in the absence of changes in blood flow and shear stress, has negligible impact on cutaneous vascular function. This finding suggests that hyperaemia and increased shear stress, rather than heat per se, are obligatory for microvascular adaptation in response to repeated heating of the skin in humans. This finding requires further investigation and verification.
We could not find any previous studies investigating the effect of repeated localised skin heating on microvascular adaptation in humans. However, Fox et al. (1963b) reported increased forearm skin blood flow after a period of 10–12 daily sessions of heat acclimation using a more systemic stimulus of warm water immersion to the xiphoid process (with arms not submerged). A more recent study using sauna exposure in chronic heart failure patients also revealed that a systemic heating stimulus improved macrovascular endothelial function, although microvascular function was not directly measured (Kihara et al. 2002). These studies suggest that the classical signs of heat acclimation (e.g. increased skin blood flow) can be achieved by passive increases in core temperature (Fox et al. 1963a) that stimulate the central thermoregulatory reflexes to modify cutaneous microvascular vasomotor control. In the present study, although core temperature increased modestly, the usual acclimation response of increased skin blood flow was not seen in the cuffed limb. This suggests that cutaneous adaptive responses cannot be achieved without an obligatory increase in flow and shear stress. In order to examine this hypothesis, further studies should be undertaken using models which manipulate and uncouple the relationship between central thermal drive and peripheral adaptations.
Although we have previously demonstrated that the protocol used in this study induces a largely NO-mediated vasodilator response (Black et al. 2008), we did not employ microdialysis or iontophoresis methodologies to block NO-dependent vasodilatation in the present study. Indeed, we measured a general vasodilator response of microvessels which probably incorporates numerous vasodilator systems and we cannot exclude the possible contribution of other vasodilator pathways to the enhanced heating-induced microvascular function we observed. Future studies, involving specific blockers, will be necessary to tease out the precise mechanisms responsible for our findings. Nonetheless, this is the first study to describe a solely hyperaemia- and shear stress-dependent increase in microvascular vasodilator function in humans and our previous work suggests a large role for NO in the adaptations we observed (Black et al. 2008).
Another limitation of our experiment was that we recruited males only. This was intentional, as we wished to avoid the cyclical effects of the menstrual cycle on skin blood flow responses. To date, the effects of shear modulation on microvascular endothelial function in women across the menstrual cycle remains unknown and it is feasible that shear stress may have supplementary effects in combination with those associated with oestrogen. In addition, studies in populations known to possess endothelial dysfunction, such as those with heart disease and diabetes, may also reveal important findings and the possibility of using repeated heating, in the absence of exercise, to enhance microvascular function has not been comprehensively addressed to date.
Finally, we cannot rule out a contribution of the veno-arteriolar reflex to adaptations we observed in the present study (Johnson, 2002). However, the response to our local heating protocol did not decrease in the cuffed arm between weeks 0, 4 and 8, as may have been expected if repeated elicitation of the veno-arteriolar reflex vasoconstriction induced chronic microvascular adaptation. There is also the recent suggestion that heating diminishes the vasoconstriction evoked by the veno-arteriolar reflex (Brothers et al. 2009), suggesting that repeated heating may have a smaller impact on reflex veno-arteriolar vasoconstriction than expected. Future studies might investigate the role of this reflex in our bilateral heating model using brachial artery compression in preference to cuff occlusion, as the former affects arterial inflow without modifying venous congestion. Similarly, other approaches to the manipulation of shear stress, such as acute and chronic changes in viscosity, may extend our understanding of the role of shear in microvascular adaptation.
In summary, we found that repeated heating of the skin can induce improvement in microvascular function if it is associated with hyperaemia and increased shear stress. We have also demonstrated that hyperaemia or shear-mediated improvement in microvascular function can occur in the absence of exercise as a stimulus. Finally, when the skin was exposed to heating in the absence of hyperaemia and increased shear, then no adaptation in NO-mediated function was apparent. Given that NO is an important molecule in preventing the development of atherosclerosis, this study has important implications in terms of strategies aimed at preventing microvascular disease in humans.
Acknowledgments
Professor Green's research is supported by a grant from the Australian Research Council. None of the authors have conflict to disclosures
Glossary
Abbreviations
- CVC
cutaneous vascular conductance
- LDF
laser Doppler flux
- PU
perfusion units
Author contributions
The study was performed in the laboratory of D.J.G. in the School of Sports Science, Exercise and Health of The University of Western Australia. D.J.G. conceived and designed the experiment. L.H.N., D.J.G., M.G.F. and H.C. contributed to the collection and analysis of the data whilst D.H.J.T. and N.T.C. contributed critically to the interpretation of the data and writing of the manuscript. All authors helped draft and/or revise the manuscript critically. All authors have approved the final version of the submitted manuscript.
References
- Black MA, Green DJ, Cable NT. Exercise training prevents age-related decline in nitric oxide (NO)-mediated vasodilator function in human microvessels. J Physiol. 2008;586:3511–3524. doi: 10.1113/jphysiol.2008.153742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boegli Y, Gremion G, Golay S, Kubli S, Liaudet L, Leyvraz P-F, Waeber B, Feihl F. Endurance training enhances vasodilation induced by nitric oxide in human skin. J Invest Dermatol. 2003;121:1187–1204. doi: 10.1046/j.1523-1747.2003.12518.x. [DOI] [PubMed] [Google Scholar]
- Brothers RM, Wingo JE, Hubing KA, Del Coso J, Crandall CG. Effect of whole body heat stress on peripheral vasoconstriction during leg dependency. J Appl Physiol. 2009;107:1704–1709. doi: 10.1152/japplphysiol.00711.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracowski J-L, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Acclimatization to heat in man by controlled elevation of body temperature. J Physiol. 1963a;166:530–547. doi: 10.1113/jphysiol.1963.sp007121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RH, Goldsmith R, Kidd DJ, Lewis HE. Blood flow and other thermoregulatory changes with acclimatization to heat. J Physiol. 1963b;166:548–562. doi: 10.1113/jphysiol.1963.sp007122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana AJ, O’Driscoll G, Taylor R. Effects of exercise training on vascular endothelial nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis JT, Poelkens F, Wouters CW, Kooijman M, Hopman MTE. Leg intravenous pressure during head-up tilt. J Appl Physiol. 2008;105:811–815. doi: 10.1152/japplphysiol.90304.2008. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Geilen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Johnson JM. How do veins talk to arteries. J Physiol. 2002;538:341. doi: 10.1113/jphysiol.2001.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T, Biro S, Imamura M, Yoshifuku S, Takasaki K, Ikeda Y, Otuji Y, Minagoe S, Toyama Y, Tei C. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol. 2002;39:754–759. doi: 10.1016/s0735-1097(01)01824-1. [DOI] [PubMed] [Google Scholar]
- Kitta Y, Obata J-e, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K-i, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Turk JR, Schrage WG, Woodman CR, Price EM. Influence of coronary artery diameter on eNOS protein content. Am J Physiol Heart Circ Physiol. 2003;284:H1307–H1312. doi: 10.1152/ajpheart.00792.2002. [DOI] [PubMed] [Google Scholar]
- Lenasi H, Strucl M. Effect of regular physical training on cutaneous microvascular reactivity. Med Sci Sports Exerc. 2004;36:606–612. doi: 10.1249/01.mss.0000121948.86377.51. [DOI] [PubMed] [Google Scholar]
- Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002;40:505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Schecter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol. 2009;134:52–58. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Hopkins ND, Dawson EA, Cable NT, Green DJ. Shear stress mediates vascular adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]