Abstract
Previous studies have proposed a role for neuromedin B (NB), a bombesin-like peptide, in the control of body weight homeostasis. However, the nature of this role is unclear. The actions of NB are mediated preferentially by NB-preferring receptors (NBRs). Here we examined the consequences of targeted deletion of NBRs in female mice on body weight homeostasis in mice fed a normolipid diet (ND) or a high-fat diet (HFD) for 13 weeks. Body weight and food ingestion of neuromedin B receptor knockout (NBR-KO) mice fed a normolipid diet showed no difference in relation to wild-type (WT). However, the high-fat diet induced an 8.9- and 4.8-fold increase in body weight of WT and NBR-KO, respectively, compared to their controls maintained with a normolipid diet, even though the mice ingested the same amount of calories, regardless of genotype. Comparing mice fed the high-fat diet, NBR-KO mice accumulated approximately 45% less fat depot mass than WT, exhibited a lower percentage of fat in their carcasses (19.2 vs. 31.3%), and their adipocytes were less hypertrophied. Serum leptin and leptin mRNA in inguinal and perigonadal fat were lower in HFD NBR-KO than HFD WT, and serum adiponectin was similar among HFD groups and unaltered in comparison to ND-fed mice. HFD-fed WT mice developed glucose intolerance but not the HFD-fed NBR-KO mice, although they had similar glycaemia and insulinaemia. NBR-KO and WT mice on the normolipid diet showed no differences in any parameters, except for a trend to lower insulin levels. Therefore, disruption of the neuromedin B receptor pathway did not change body weight homeostasis in female mice fed a normolipid diet; however, it did result in partial resistance to diet-induced obesity.
Introduction
Neuromedin B (NB) and gastrin-releasing peptide (GRP), mammalian counterparts of the amphibian peptide bombesin, have a wide distribution in the central nervous system and peripheral tissues, especially in the gastrointestinal tract (Jensen et al. 2008). The administration of NB or GRP in mammals elicits several effects related to the control of endocrine and exocrine secretions, neurotransmission, cell growth and angiogenesis (Ohki-Hamazaki et al. 2005). Bombesin-related peptides act through G protein-coupled receptors and so far, three closely related receptors have been described in mammals, a NB-preferring receptor (NBR), a GRP-preferring receptor (GRPR), and an orphan receptor called bombesin-receptor subtype-3 (BRS-3), which has a very low affinity for both GRP and NB (Weber, 2009). Expression levels of NB-preferring receptor mRNA and binding studies have indicated the presence of high levels of receptors in the central nervous system, testis and gastro-intestinal smooth muscle cells (Ohki-Hamazaki et al. 1997a). In the brain, the NB receptor was detected in several regions including the arcuate nucleus and hypothalamus (Sano et al. 2004).
Previous studies have suggested the involvement of bombesin-like peptides and related receptors in the control of food intake and body weight. It has been reported that bombesin, GRP or NB acute administration into rodents have satiety effects (Gibbs & Smith, 1988; Ladenheim et al. 1994; Gonzalez et al. 2008), but whether these are pharmacological or physiological effects remains unclear, as well as the subtype of receptor involved. The lack of good specific antagonists had lead to the development of mice with targeted deletion of one of these receptors in order to better understand their physiological role.
BRS-3 knockout mice are hyperphagic and develop obesity and glucose intolerance at adulthood (Ohki-Hamazaki et al. 1997b). GRPR knockout mice also developed higher body weight, although much later in life, at the age of 45 weeks old (Ladenheim et al. 2002). No alterations in body weight have been reported for the neuromedin B receptor knockout mice (NBR-KO) (Oliveira et al. 2006, 2008), although, to the best of our knowledge, no systematic study has been performed to address the subject. In addition, the anorexic effect of acute administration of neuromedin B could not be demonstrated in NBR knockout mice or in their wild-type controls (Ohki-Hamazaki et al. 1999). Interestingly, both GRP and NB are potent stimulators of the electrical activity of proopiomelanocortic (POMC) neurons and neuropeptide Y neurons in isolated arcuate nucleus from mice (van del Pol et al. 2009), indicating that both anorexigenic and orexigenic pathways are activated by bombesin-like peptides, and the final effect in vivo remains to be elucidated, especially regarding neuromedin B. Neuromedin B is also involved in the central control of the hypothalamic-pituitary-thyroid hormones axis (Pazos-Moura et al. 1996; Ortiga-Carvalho et al. 1996, 1997, 2003; Oliveira et al. 2006, 2008), which is importantly involved in energy homeostasis (Moura & Pazos-Moura, 2004). NBR-KO mice presented alterations in defensive behaviour related to anxiety which was associated with increased levels of serotonin at the dorsal raphe nucleus (Yamada et al. 2002a,b,c, 2003; Yamano et al. 2002). Since serotonin has a known anorexigenic effect, it is feasible that neuromedin B may modulate anorexigenic pathways through regulation of serotonin neurons, although it has not been investigated. Recently, neuromedin B has been shown to be expressed in significant amounts in mouse and human white adipose tissue, where its expression is under the negative control of leptin (Hoggard et al. 2007). Since neuromedin B receptors are also present in the adipose tissue (Yang et al. 2003), a role for neuromedin B in energy homeostasis has been proposed, although it remains unknown. Additionally, in humans, polymorphisms of the neuromedin B gene, which were not functionally characterized, have been associated with increased susceptibility to obesity (Bouchard et al. 2004; Spálováet al. 2008).
Therefore, although much evidence suggests a role for neuromedin B in the control of body energy homeostasis, data are controversial, and the nature of this role is completely unclear. In this study, we sought to investigate whether the disruption of the neuromedin B-specific pathway in mice targeted deleted for the neuromedin B receptor would lead to an imbalance of energetic homeostasis in animals under a normolipid diet and when challenged with an excess calorie intake through the ingestion of a high-fat diet.
Methods
Animals and experimental design
The present experiments comply with the policies and regulations regarding ethical matters on animal experimentation described in Drummond (Drummond, 2009) and our protocol was approved by our Institutional Committee on Animal Care and Use from Instituto de Biofísica Carlos Chagas Filho.
Animals were maintained under controlled temperature (24 ± 1°C) and 12 h alternating darkness and artificial light cycles (light on at 7 am). Female wild-type (WT) and neuromedin B receptor knockout (NBR-KO) mice, 12 animals each, were used in this experiment. Heterozygous NBR+/− mice generated as described previously (Ohki-Hamazaki et al. 1999) were interbred to generate litters containing homozygous NBR−/− (NBR-KO) and NBR+/+ (WT) progeny. To confirm the genotype of the mice, genomic DNA was obtained from tail samples and analysed by polymerase chain reaction (PCR) using specific primers as described previously (Ohki-Hamazaki et al. 1999).
After weaning (day 21) animals were placed in cages containing three animals with the same genotype with water and standard chow (Bio-Tec, Brazil) ad libitum. Body weight was measured once a week from weaning until they were 13 weeks old. At this time, the standard chow was switched to one of two types of commercial diet: a normolipid diet (ND) or a high-fat diet (HFD) (Rhoster, Sao Paulo, Brazil). As indicated in Table 1, the composition of ND followed the recommendations of The American Institute of Nutrition for maintenance of rodents’ body weight (AIN93M, Reeves et al. 1993) providing the same percentage of nutrients and amount of calories, while HFD was modified to achieve 62% of the total calories from fat.
Table 1.
Nutritional composition of diets: normolipid diet (ND) and high-fat diet (HFD), used for wild-type (WT) and neuromedin B receptor knockout (NBR-KO) mice
| Ingredients (g) | AIN-93M | ND | HFD |
|---|---|---|---|
| Cornstarch | 465.692 | 432.87 | 204.11 |
| Casein | 140 | 172.82 | 236.58 |
| Dextrinized cornstarch | 155 | 155 | |
| Sucrose | 100 | 100 | 100 |
| Soybean oil | 40 | 40 | 40 |
| Lard | – | – | 320 |
| Cellulose | 50 | 50 | 50 |
| Mineral mixa | 35 | 35 | 35 |
| Vitamin mixb | 10 | 10 | 10 |
| l-Cystine | 1.8 | 1.8 | 1.8 |
| Choline bitartrate | 2.5 | 2.5 | 2.5 |
| Butylhydroquinone | 0.008 | 0.008 | 0.008 |
| Total (g) | 1000 | 1000 | 1000 |
| Carbohydrate (%) | 75.9 | 75 | 23 |
| Protein (%) | 14.1 | 15 | 15 |
| Lipid (%) | 10 | 10 | 62 |
| kcal g−1 | 3.6 | 3.6 | 5.2 |
Mineral mix (g kg−1): calcium, 357.0; phosphorus, 250.0; potassium, 74.6; sodium, 74.0; sulfur, 300; magnesium, 24.0; iron, 5.21; copper, 0.3; manganese, 0.63; zinc, 1.65; chromium, 0.27; iodine, 0.01; selenium, 0.01; boron, 0.08; molybdenum, 0.01; silicon, 1.45; nickel, 0.03; lithium, 0.02; vanadium, 0.007 (AIN-93 mineral mix; DYETS 210025; Dyets, Inc., Bethlehem, PA, USA).
Vitamin mix (g kg−1 diet): thiamine HCl, 0.6; riboflavin, 0.6; pyridoxine HCl, 0.7; niacin, 3.0; calcium pantothenate, 1.60; folic acid, 0.20; biotin, 0.02; vitamin B12, 2.5; vitamin A palmitate, 0.80 (500.00 IU g−1); vitamin E acetate, 15.0 (500 IU g−1); vitamin D3, 0.25 (400.00 IU g−1); vitamin K1, 0.75 (AIN-93 vitamin mix; DYETS 310025; Dyets, Inc.).
The body weight of the animals and food intake per cage were measured twice a week, and after 13 weeks on the diets animals were killed by immersing in an atmosphere of carbonic acid gas. After killing, trunk blood was collected and glycaemia was measured by an Optium Xceed meter (MediSense, UK), and serum was obtained after centrifugation and frozen at −20°C for measurements of serum hormones and lipids. Inguinal, perigonadal and retroperitoneal white adipose tissue and brown adipose tissue were excised and weighed. Inguinal and perigonadal white adipose tissue samples (70–80 mg) were collected and frozen at −70°C for RNA extraction, and other samples of the same tissues (50 mg) were maintained in buffered formalin (formaldehyde in phosphate-buffered saline) at 4°C for histological analysis. Residual adipose tissue was returned to carcasses which were eviscerated, weighed and frozen at −20°C for body composition analysis. Liver samples (50 mg) were collected, fixed in a 4% paraformaldehyde solution, kept in gradient sucrose solutions (10, 20 and 30%), washed in phosphate buffer, embedded using optimal cutting temperature compound (Tissue-Tek, CA, USA) and frozen at −70°C for histological evaluation.
Glucose tolerance test
A glucose tolerance test was performed after the mice had been on the diets for 2 months. After a 12 h fast, mice received an intraperitoneal injection of 2 mg (g body weight)−1d-(+)-glucose (Merck, Darmstadt, Germany) in phosphate-buffered saline. Blood glucose concentrations were measured via tail bleed before (0) and 20, 40, 60, 90 and 120 min after injection. Blood glucose was measured using an Optium Xceed meter.
Serum parameters
Serum leptin, adiponectin and insulin were determined by specific rodent radioimmunoassay kits (Linco Research, MA, USA), in accordance with the recommendations of the manufacturer. The sensitivity and intra-assay variation were 0.5 ng ml−1 and 5.3% for leptin, 0.78 ng ml−1 and 2.6% for adiponectin, 0.1 ng ml−1 and 8.8% for insulin, respectively. All samples were measured within the same assay.
Serum total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were measured by colorimetric assays using commercial kits (Applied BioSystems, CA, USA) and following the manufacturer's instructions.
Body composition analysis
Body composition (fat and protein masses) analysis was determined by the carcass method, previously described in detail (Rodrigues et al. 2009; Souza et al. 2009). Briefly, frozen eviscerated and weighed carcasses were autoclaved and homogenized in distilled water (1:1 w/v). Proteins were extracted from 1 g of homogenates using potassium hydroxide and total protein was quantified by the Bradford method (Bradford, 1976). Three grams of homogenate was used to determine fat mass gravimetrically. Samples were hydrolysed with potassium hydroxide and ethanol, and after the addition of sulfuric acid, total lipids were extracted by three successive washes with petroleum ether. The samples were dried at room temperature until constant weight was obtained. Both measurements were expressed as grams of protein or fat per 100 g carcass.
White adipose tissue histology and morphometry
Fixed samples of inguinal and perigonadal white adipose tissue (WAT) were dehydrated in ethanol and diaphanized in xylol and then embedded in paraffin. Fat pads were cut into 5 μm sections at 250 μm intervals, mounted on slides and stained with haematoxylin and eosin. Images were captured at ×20 magnification and adipose cell area was calculated with Image/J software (Image Processing and Analysis in Java, NIH) by manually tracing at least 48 adipocytes in three sections for each tissue per mouse, and a total of four animals per group were evaluated. The total adipocyte number in WAT was determined by dividing the total WAT mass excised after the animals were killed by the estimated mean adipocyte mass. The latter parameter was calculated by multiplying adipocyte density (0.948 mg ml−1, triolein) by mean adipocyte volume, which was determined from the mean value of adipocyte diameter (from area) (Johmura et al. 2009).
Liver Oil Red O staining
Embedded tissues were cryosectioned at −22°C into 5 μm slices, adhered onto glass slides and kept at −20°C overnight. Sections were washed in phosphate-buffered saline followed by propylene glycol and were stained with Oil Red O. Stained sections were rinsed with propylene glycol and distilled water followed by haematoxylin staining and glycerol/phosphate buffer 1:1 covering. Images were captured at ×40 magnification and lipid deposition was quantified by Image/J software using a modified protocol (Goodpaster et al. 2000). Briefly, 10 arbitrary fields with the same area and approximately 20 nuclei were analysed in 2 sections per animal. The threshold for the intensity of staining was adjusted in order to pick up only the droplets of lipid, and the full range of greyscale imaging was from 0 (complete staining) to 255 (no staining) arbitrary units. Data were expressed as the percentage of the sum of Oil Red O stained area in relation to the total area of the image field.
White adipose tissue leptin mRNA analysis
Real-time PCR
Total inguinal and perigonadal white adipose tissue RNA was isolated from samples using the commercially available RNeasy lipid tissue mini kit (Qiagen, TX, USA). Total RNA was reverse transcribed using 1 μg RNA and the Superscript III kit (Invitrogen, CA, USA). Leptin primer was designed as follows: forward: 5′-CATCTGCTGGCCTTCTCCAA-3′ reverse: 5′-ATCCAGGCTCTCTGGCTTCTG-3′. 36B4 primer was used as control as previously described (Machado et al. 2009). Products were amplified on Applied Biosystems 7500 Real-Time PCR System (Life Technologies Corp., MD, USA) using SYBR Green PCR Master Mix (Applied BioSystems, MD, USA). Cycle parameters were: 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, 60°C for 30 s, and 70°C for 45 s. Product purity was confirmed by agarose gel analysis. Changes in mRNA expression were calculated from the cycle threshold, after correcting for 36B4. Data are expressed as fold induction over control group, which was set to 1.
Statistical analyses
Data are expressed as means ±s.e.m. and were evaluated using GraphPad Prism 5 (GraphPad Prism Software, Inc., CA, USA). Data were analysed by two-way ANOVA followed by a Bonferroni post test. Differences were considered to be significant at P≤ 0.05.
Results
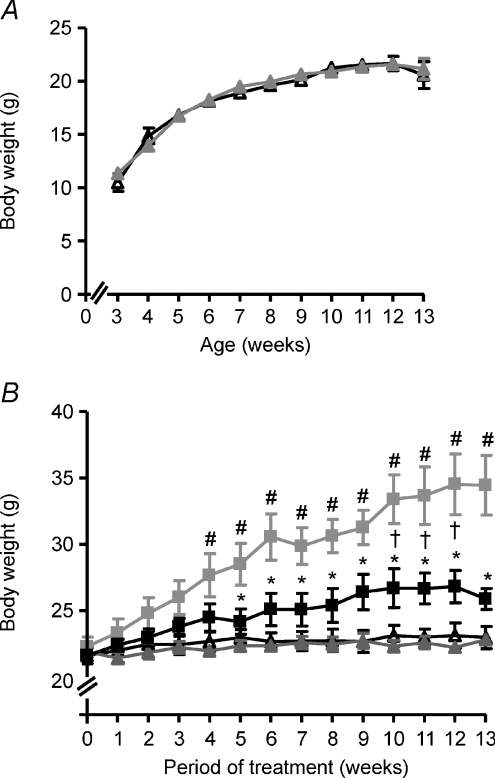
As depicted in Fig. 1A, NBR-KO mice and their wild-type controls fed standard chow exhibited similar body weights from weaning at 3 weeks old until 13 weeks old. Also, they ingested a similar amount of chow (data not shown).
Figure 1.
A, body weight of wild-type (WT, black triangles) and neuromedin B receptor knockout (NBR-KO, grey triangles) mice fed standard chow from weaning at 3 weeks old to 13 weeks old. Values are expressed as means ±s.e.m. WT, n= 12; NBR-KO, n= 12. B, body weight of WT and NBR-KO mice fed a normolipid diet (WT ND, black triangles; NBR-KO ND, grey triangles) or high-fat diet (WT HFD, grey squares; NBR-KO HFD, black squares) over 13 weeks, starting when animals were 13 weeks old. Values are expressed as means ±s.e.m.n= 6 animals per group. # WT ND vs. WT HFD, † NBR-KO ND vs. NBR-KO HFD, * WT HFD vs. NBR-KO HFD (P < 0.05).
At that age, NBR-KO and WT were started on a normolipid diet (ND) or a high-fat diet (HFD) and were fed with these diets for 13 weeks. The body weights of NBR-KO and WT mice fed the normolipid diet did not differ during the experiment which ended when they were 26 weeks old (Fig. 1B). Therefore, deletion of the neuromedin B receptor did not interfere with growth and body weight gain of mice fed a normolipid diet. However, the genotype interfered with the response to a high-fat diet (Fig. 1B). WT mice fed a high-fat diet exhibited significantly higher body weights than the WT animals fed a normolipid diet (P < 0.05), starting at 4 weeks on the diets until the end of the experiment (54.6%). NBR-KO mice on the high-fat diet also gained more weight than NBR-KO on the normolipid diet (19.7%); however, the difference in body weight was lower than that of the WT groups, and became statistically significant only after 10 weeks on the diets (P < 0.05). Therefore, for animals fed a high-fat diet, the gain in body weight was significantly smaller for NBR-KO than WT mice from 5 to 13 weeks on the diets, when the cumulative body weight gain of HFD-fed WT mice was 8.9-fold while that of the NBR-KO was 4.8-fold compared to animals of the same genotype on the normolipid diet (P < 0.05).
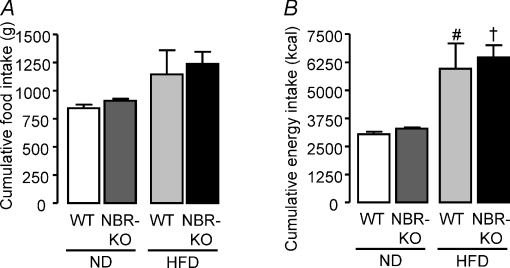
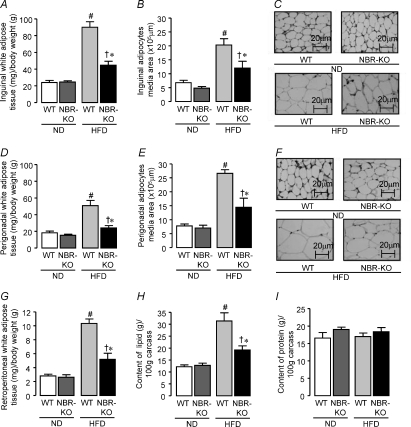
The total amounts of food intake (grams) and energy intake (calories) during the 13 weeks were not modified by the genotype, and NBR-KO and WT mice had similar energy intakes, when fed either the normolipid diet or the high-fat diet (Fig. 2A and B). The mass of white adipose tissue depots as well as the size of their adipocytes did not differ between NBR-KO and WT mice fed the normolipid diet. However, NBR-KO mice accumulated less fat than wild-type in response to the high-fat diet (Fig. 3, P < 0.05). The relative mass (corrected by body weight) of inguinal, perigonadal and retroperitoneal fat depots of HFD-fed NBR-KO mice was 44, 40 and 50%, respectively, lower than that of HFD-fed WT mice (Fig. 3A, D and G, P < 0.05). Also, the relative percentage of fat in the eviscerated carcasses of HFD-fed NBR-KO mice was lower than in HFD-fed WT mice (19.2% and 31.3%, respectively, Fig. 3H, P < 0.05), and no differences were observed in terms of protein content in their carcasses (Fig. 3I). The histological and morphometric analysis of adipocytes of inguinal and perigonadal fat depots revealed that the degree of hypertrophy caused by the high-fat diet was lower in NBR-KO mice than in WT (Fig. 3C and F). Compared to animals fed the normolipid diet, the mean areas of inguinal and perigonadal adipocytes were increased by 3- and 3.5-fold, respectively, in HFD-fed WT mice, while the increases were 2.5- and 2-fold, respectively, in the HFD-fed NBR-KO mice (Fig. 3B and E, P < 0.05). Therefore, for HFD-fed mice, the mean areas of inguinal and perigonadal adipocytes were 41% and 46%, respectively, lower in NBR-KO than in WT (P < 0.05). No influence of genotype or diet was observed in terms of the estimated number of adipocytes (WT ND: 51 ± 10, 40 ± 7; NBR-KO ND: 79 ± 13, 45 ± 6; WT HFD: 50 ± 5, 32 ± 4; NBR-KO HFD: 46 ± 11, 33 ± 4 (×104); inguinal and perigonadal, respectively), and presumably, hyperplasia was not contributing to the increased adiposity of mice fed the high-fat diet.
Figure 2.
Total amount of food intake (A) and energy intake (B) of WT and NBR-KO mice fed a normolipid diet (ND) or a high-fat diet (HFD) for 13 weeks. Values are expressed as means ±s.e.m.n= 2 cages with 3 mice per group. # WT ND vs. WT HFD, † NBR-KO ND vs. NBR-KO HFD (P < 0.05).
Figure 3. White adipose tissue morphology in WT and NBR-KO mice fed a normolipid or high-fat diet for 13 weeks.
Mass of inguinal (A), perigonadal (D) and retroperitoneal (G) white adipose tissue. Area of inguinal (B) and perigonadal (E) white adipocytes. Haematoxylin–eosin-stained histological sections of inguinal (C) and perigonadal (F) white adipose tissue. Percentage lipid (H) and protein (I) contents in eviscerated carcasses. Values are expressed as means ±s.e.m.n= 6 animals per group. # WT ND vs. WT HFD, † NBR-KO ND vs. NBR-KO HFD, * WT HFD vs. NBR-KO HFD (P < 0.05).
Brown adipose tissue mass relative to body weight was not modified by diet or genotype (in mg (g body weight)−1: WT ND, 0.104 ± 0.008; NBR-KO ND, 0.108 ± 0.019; WT HFD, 0.139 ± 0.010; NBR-KO HFD, 0.109 ± 0.007).
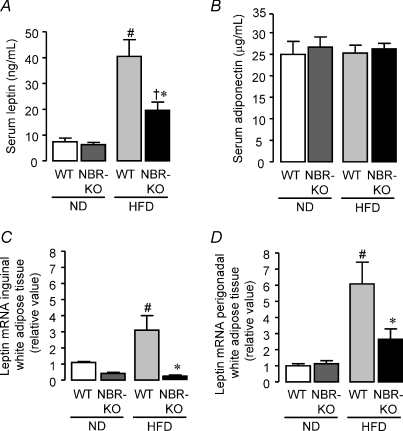
As expected, mice fed the high-fat diet exhibited increased serum leptin compared to mice fed the normolipid diet; however, the increase was lower in NBR-KO (3.1-fold) mice than in WT (5.4-fold) (Fig. 4A, P < 0.05). Serum leptin was 2.1-fold higher in HFD-fed WT mice than in HFD-fed NBR-KO. No significant difference related to genotype was observed in mice fed the normolipid diet. The same pattern of response to normolipid and high-fat diet occurred in terms of leptin mRNA expression in inguinal and perigonadal white adipose tissues. Interestingly, the high-fat diet induced a higher increment in leptin mRNA expression in perigonadal WAT than in inguinal WAT, regardless of genotype. Compared to animals of the same genotype fed normolipid diet, HFD-fed WT mice exhibited a 6- and 3-fold increase in leptin mRNA expression in perigonadal and inguinal WAT, respectively (Fig. 4C and D), while NBR-KO mice presented no increase in leptin mRNA expression in inguinal and a small, not statistically significant increase in leptin mRNA of perigonadal WAT (Fig. 4C and D).
Figure 4. Adipocyte hormones in WT and NBR-KO mice fed a normolipid or high-fat diet for 13 weeks.
A, serum leptin, n= 6 per group, except for NBR-KO ND (n= 5). B, serum adiponectin, n= 6 per group, except for NBR-KO ND (n= 5). C, relative expression of inguinal white adipose tissue leptin mRNA (normalized by 36B4) to values of WT animals on normolipid diet (set as 1). Data were obtained by quantitative PCR. Values are means ±s.e.m.n= 5 per group, except for WT ND (n= 4). D, relative expression of perigonadal white adipose tissue leptin mRNA (normalized by 36B4) to values of WT animals on a normolipid diet (set as 1). Values are means ±s.e.m.n= 6 per group, except for WT HFD (n= 5). # WT ND vs. WT HFD, † NBR-KO ND vs. NBR-KO HFD, * WT HFD vs. NBR-KO HFD (P < 0.05).
Serum adiponectin was similar between groups (Fig. 4B), but correcting serum values per total white adipose tissue mass excised, animals fed the high-fat diet showed lower values than those fed the normolipid diet; however, HFD-fed NBR-KO mice presented a 2.8-fold higher relative adiponectin than HFD-fed WT mice (WT ND, 0.044 ± 0.007; NBR-KO ND, 0.055 ± 0.007; WT HFD, 0.010 ± 0.001; NBR-KO HFD, 0.028 ± 0.003 μg ml−1 (g body weight)−1, P < 0.05).
Regarding serum lipids, total cholesterol, triglycerides and HDL cholesterol exhibited no significant differences among all experimental groups, either comparing diets or genotype (Table 2).
Table 2.
Serum lipids of wild-type mice (WT) and neuromedin B receptor knockout mice (NBR-KO) fed a normolipid diet (ND) or high-fat diet (HFD) for 13 weeks
| Serum lipids | WT ND | NBR-KO ND | WT HFD | NBR-KO HFD |
|---|---|---|---|---|
| Total cholesterol (mg dl−1) | 129 ± 8 | 124 ± 4 | 147 ± 15 | 143 ± 8 |
| HDL cholesterol (mg dl−1) | 74 ± 5 | 74 ± 4 | 81 ± 5 | 90 ± 7 |
| Triglycerides (mg dl−1) | 89 ± 12 | 84 ± 8 | 69 ± 3 | 103 ± 18 |
n= 6 per group.
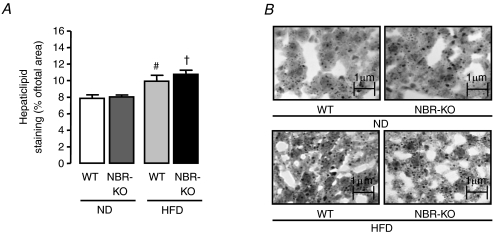
Oil Red O staining in liver samples (Fig. 5A and B) revealed that the percentage of area occupied by lipid staining in liver from mice fed a high-fat diet was higher than in mice fed the normolipid diet (P < 0.05) and no significant difference was observed between genotypes in either diet.
Figure 5. Hepatic lipid deposition of WT and NBR-KO mice fed a normolipid or high-fat diet for 13 weeks.
A, percentage of the sum of Oil Red O-stained area in relation to the total analysed area. B, Oil Red O-stained sections of liver. Values are means ±s.e.m.n= 5 animals per group. # WT ND vs. WT HFD, † NBR-KO ND vs. NBR-KO HFD (P < 0.05).
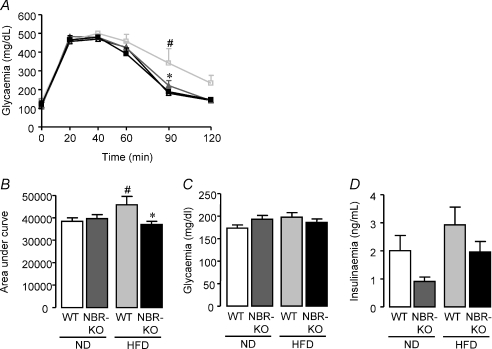
As depicted in Fig. 6A, NBR-KO and WT fed the normolipid diet showed similar glucose tolerance curves. Fasting glycaemia (time zero) and all other time-points after intraperitoneal injection of glucose were similar. The high-fat diet caused an imbalance in glucose homeostasis in WT mice, but not in NBR-KO mice, as revealed by the glucose tolerance curves. HFD-fed WT mice exhibited an 89% higher blood glucose than ND-fed WT mice at 90 min after the intraperitoneal injection of glucose, and the area under the curve of HFD-fed mice was increased by 19% in comparison to ND-fed WT mice (Fig. 6B, P < 0.05). However, NBR-KO on a high-fat diet showed a similar glucose tolerance curve to mice on the normolipid diet. Glycaemia of animals, who were not fasted overnight, was similar among groups regardless of diet or genotype (Fig. 6C). Serum insulin of the same animals showed important trends, which did not reach statistically significant differences (Fig. 6D). Comparing mice fed the normolipid diet, NBR-KO mice exhibited a 55% lower serum insulin than WT. HFD-fed WT and NBR-KO animals showed a 1.45-fold and 2.15-fold higher serum insulin, respectively, than mice of the same genotype on a normolipid diet, and comparing mice fed the high-fat diet, NBR-KO mice presented a 23% lower serum insulin than WT.
Figure 6. Glucose homeostasis in WT and NBR-KO mice fed a normolipid or high-fat diet for 13 weeks.
A, glucose tolerance test: blood glucose was measured at time zero (following an overnight fast), and after intraperitoneal injection of glucose. n= 6 per group (WT ND, black squares; NBR-KO ND, black triangles; WT HFD, grey squares; NBR-KO HFD, grey triangles). B, area under the curve during the glucose tolerance test of WT and NBR-KO fed ND or HFD. C, fed blood glucose concentrations of WT and NBR-KO on ND or HFD. Values are expressed as means ±s.e.m.n= 6 per group. D, fed serum insulin concentrations of WT and NBR-KO on ND or HFD. Values are expressed as means ±s.e.m.n= 6 per group, except for NBR-KO HFD (n= 5). # WT ND vs. WT HFD, * WT HFD vs. NBR-KO HFD (P < 0.05).
Discussion
The main finding of the present study was that female neuromedin B receptor knockout mice developed a partial resistance to diet-induced obesity, suggesting the involvement of the NB–NBR pathway in the control of body energetic homeostasis.
Previous studies have demonstrated, in short-term feeding tests, that neuromedin B has an acute anorexic effect when administered centrally or systemically, as a single dose, in rats (Gibbs & Smith, 1988), although it could not be reproduced in mice, either wild-type or NBR-KO (Ohki-Hamazaki et al. 1999). Our results do not support an important influence of NB and NBR on the control of long-term appetite in female mice fed a normolipid diet or a high-fat diet, since they showed similar food intake. A previous study demonstrated that NB stimulated the electrical activity of both proopiomelanocortic (POMC) and neuropeptide Y neurons of the arcuate nucleus (van del Pol et al. 2009). Our study suggests that, in vivo, at least in female mice, the final result of the lack of neuromedin B action via its specific receptor does not favour an orexigenic or anorexigenic pathway, which may reflect the fact previously reported that NB acted with similar potency on those antagonist neurons (van del Pol et al. 2009).
The fact that serum lipids are not altered in animals fed HFD, independent of genotype, suggests that those animals still had the ability to metabolize and deposit this excess of ingested lipids in the adipose tissue, and also in the liver. In other studies, serum lipids of mice on high-fat diets were reported to be normal (Sheng et al. 2008) or diminished (Guo et al. 2009), probably depending on mice strain and diet composition and duration of administration. The observation that adipose tissue of HFD-fed NBR-KO is less hypertrophied is consistent with the lower adiposity of these mice. This is probability the result of a higher capacity to burn calories, decreasing the availability of lipids to be deposited in adipose tissue. However, since neuromedin B is abundant in adipose tissue and its role in the tissue physiology is unknown, we cannot rule out the existence of some abnormality in the ability of adipocytes of NBR-KO to accumulate triglycerides, which is not the case for the liver where lipid deposition was similar between genotypes. In addition, the reduced leptin levels in HFD-fed NBR-KO mice may be directly related to their decreased amount of adipose fat; however, we also cannot exclude the possibility of impaired leptin production, since although it is known that leptin modulates the expression of neuromedin B in epididymal fat and pituitary gland (Hoggard et al. 2007; Ortiga-Carvalho et al. 2002) it is currently unknown whether neuromedin B may affect leptin production.
Exogenous neuromedin B, as well as GRP, has shown the ability to stimulate insulin secretion in vivo and in isolated pancreatic preparations (Namba et al. 1984; Otsuki et al. 1987). However, our study suggests that disruption of NB receptor signalling does not result in a major imbalance of glucose homeostasis, although this remains to be further investigated with proper measurements of insulin secretion. Since there was a non-significant trend to reduced insulin levels in the fed state of NBR-KO mice on a normolipid diet, there is the possibility that NBR-KO mice might exhibit abnormalities of insulin secretion and action, as demonstrated for the GRPR knockout mice. Although GRP also has a direct stimulatory effect on insulin secretion, GRPR knockout mice have adaptive responses leading to increased cholinergic-induced insulin secretion (Persson et al. 2000, 2002). Furthermore, the responses of GRPR knockout mice and female NBR-KO mice to diet-induced obesity are different, since high-fat-diet-fed GRPR knockout mice behave similarly to wild-type, developing obesity and glucose intolerance (Persson et al. 2002).
A role for NB in energy expenditure could not be demonstrated in a previous study that showed no effect of the acute administration of NB on oxygen consumption or locomotor activity (Hoggard et al. 2007). Indeed, in our study when fed normolipid diets, there was no difference in adiposity between NBR-KO and wild-type mice, suggesting that long-term disruption of the neuromedin B signalling pathway does not cause an imbalance in energy expenditure. However, the lower accumulation of fat in adipose tissue of NBR-KO mice in response to a high-fat diet, maintaining the same caloric intake as wild-type, and the absence of an alteration in stools consistency, suggest that NBR-KO mice have a higher energy expenditure in response to a high-fat diet. Since the biological role of neuromedin B in integrative physiology is not at all clear, an intensive investigation will be needed to clarify the mechanisms leading to partial resistance to diet-induced obesity in female NBR-KO mice.
The extent to which resistance to diet-induced obesity is primarily due to disruption of the neuromedin B receptor signalling pathway or may reflect an adaptative mechanism to that disruption is unknown. The possibility that an up-regulation of GRP receptors may participate in this response is unlikely since it has been previously demonstrated that NBR-KO mice have no increase in GRPR expression in whole brain and pituitary (Ohki-Hamazaki et al. 1999; Oliveira et al. 2006), and more importantly, GRP receptor knockout mice did not exhibit abnormalities in food ingestion or body weight at ages comparable to the NBR-KO mice in our study, even when fed a high-fat diet (Persson et al. 2002).
The finding of resistance to diet-induced obesity in female mice raises the question of whether oestrogen and neuromedin B might have some association in terms of the control of energy metabolism, which is presently unknown. However, we have shown before that oestrogen up-regulates NB expression at the pituitary, and the oestrogen status of female rats modulates the response of thyrotrophs and lactotrophs to the action of neuromedin B on thyrotropin and prolactin secretion (Moreira et al. 2003).
In conclusion, this study shows that disruption of the neuromedin B receptor pathway did not change body weight or food intake in female mice fed a normolipid diet; however, it did result in partial resistance to diet-induced obesity, not accounted for alterations in food intake.
Acknowledgments
This research was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We also thank Uilian Calixto for technical assistance.
Glossary
Abbreviations
- GRP
gastrin-releasing peptide
- GRPR
GRP receptor
- HDL
high-density lipoprotein
- HFD
high-fat diet
- NB
neuromedin B
- NBR-KO
NB receptor knockout
- ND
normolipid diet
- WAT
white adipose tissue
- WT
wild-type
Author contributions
The conception and design of this study was conducted by G.S.M.P., K.J.O. and C.C.P.-M. with contributions from V.M.-C. and T.M.O.-C. E.W. was responsible for the generation of neuromedin B receptor knockout mice. Experiments were performed by G.S.M.P., K.J.O., L.L.S., A.C. and F.F.B. All authors contributed to the analysis and interpretation of data as well as writing, revising and final approval of the version to be published. The study was conducted at the Instituto de Biofisica Carlos Chagas Filho, Federal University of Rio de Janeiro, Brazil.
References
- Bouchard L, Drapeau V, Provencher V, Lemieux S, Chagnon Y, Rice T, Rao DC, Vohl MC, Tremblay A, Bouchard C, Pérusse L. Neuromedin β: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. 2004;80:1478–1486. doi: 10.1093/ajcn/80.6.1478. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Smith GP. The actions of bombesin-like peptides on food intake. Ann N Y Acad Sci. 1988;547:210–216. doi: 10.1111/j.1749-6632.1988.tb23889.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diab Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- Guo J, Jou W, Gavrilova O, Hall KD. Persistent diet-induced obesity in male C57BL/6 mice resulting from temporary obesigenic diets. PloS One. 2009;4:e5370. doi: 10.1371/journal.pone.0005370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard N, Bashir S, Cruickshank M, Miller JD, Speakman JR. Expression of neuromedin B in adipose tissue and its regulation by changes in energy balance. J Mol Endocrinol. 2007;39:199–210. doi: 10.1677/JME-07-0071. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signalling, and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johmura Y, Watanabe K, Kishimoto K, Ueda T, Shimada S, Osada S, Nishizuka M, Imagawa M. Fad24 causes hyperplasia in adipose tissue and improves glucose metabolism. Biol Pharm Bull. 2009;32:1656–1664. doi: 10.1248/bpb.32.1656. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Hampton LL, Whitney AC, White WO, Battey JF, Moran TH. Disruptions in feeding and body weight control in gastrin-releasing peptide receptor deficient mice. J Endocrinol. 2002;174:273–281. doi: 10.1677/joe.0.1740273. [DOI] [PubMed] [Google Scholar]
- Ladenheim EE, Taylor JE, Coy DH, Moran TH. Blockade of feeding inhibition by neuromedin B using a selective receptor antagonist. Eur J Pharmacol. 1994;271:7–9. doi: 10.1016/0014-2999(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Machado DS, Sabet A, Santiago LA, Sidhaye AR, Chiamolera MI, Ortiga-Carvalho TM, Wondisford FE. A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expressionin vivo. Proc Natl Acad Sci U S A. 2009;106:9441–9446. doi: 10.1073/pnas.0903227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira RM, Curty FH, Lisboa PC, D Amaral, Ortiga-Carvalho TM, Pazos-Moura CC. Estrogen modulates neuromedin B effects on thyrotropin and prolactin release in vitro. Life Sci. 2003;72:917–923. doi: 10.1016/s0024-3205(02)02351-2. [DOI] [PubMed] [Google Scholar]
- Moura EG, Pazos-Moura CC. Regulation of thyrotropin synthesis and secretion. Arquivos Brasileiros de Endocrinologia e Metabologia. 2004;48:40–52. doi: 10.1590/s0004-27302004000100006. [DOI] [PubMed] [Google Scholar]
- Namba M, Gathei MA, Adrian TE, Bacarese-Hamilton AJ, Mulderry PK, Bloom SR. Effect of neuromedin B on gut hormone secretion in the rat. Biomed Res. 1984;5:229–234. [Google Scholar]
- Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and function of bombesin-like peptides, and their receptors. Int J Dev Biol. 2005;49:293–300. doi: 10.1387/ijdb.041954ho. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Sakai Y, Kamata K, Ogura H, Okuyama S, Watase K, Yamada K, Wada K. Functional properties of two bombesin-like peptide receptors revealed by the analysis of mice lacking neuromedin B receptor. J Neurosci. 1999;19:948–954. doi: 10.1523/JNEUROSCI.19-03-00948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Wada E, Matsui K, Wada K. Cloning and expression of the neuromedin B receptor and the third subtype of bombesin receptor genes in the mouse. Brain Res. 1997a;762:165–172a. doi: 10.1016/s0006-8993(97)00380-6. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K, Maeno H, Imaki J, Kikuyama S, Wada E, Wada K. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997b;390:165–169. doi: 10.1038/36568. [DOI] [PubMed] [Google Scholar]
- Oliveira KJ, Cabanelas A, Veiga MA, Paula GS, Ortiga-Carvalho TM, Wada E, Wada K, Pazos-Moura CC. Impaired serum thyrotropin response to hypothyroidism in mice with disruption of neuromedin B receptor. Regul Pept. 2008;146:213–217. doi: 10.1016/j.regpep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Oliveira KJ, Ortiga-Carvalho TM, Cabanelas A, Veiga MA, Aoki K, Ohki-Hamazaki H, Wada K, Wada E, Pazos-Moura CC. Disruption of neuromedin B receptor gene results in dysregulation of the pituitary–thyroid axis. J Mol Endocrinol. 2006;36:73–80. doi: 10.1677/jme.1.01892. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Curty FH, Nascimento-Saba CC, Moura EG, Polak J, Pazos-Moura CC. Pituitary neuromedin B content in experimental fasting and diabetes mellitus and correlation with thyrotropin secretion. Metab Clin Exp. 1997;46:149–153. doi: 10.1016/s0026-0495(97)90293-6. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Oliveira KJ, Morales MM, Martins VP, Pazos-Moura CC. Thyrotropin secretagogues reduce rat pituitary neuromedin B, a local thyrotropin release inhibitor. Exp Biol Med. 2003;228:1083–1088. doi: 10.1177/153537020322800916. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Oliveira KJ, Soares BA, Pazos-Moura CC. The role of leptin in the regulation of TSH secretion in the fed state: in vivo and in vitro studies. J Endocrinol. 2002;174:121–125. doi: 10.1677/joe.0.1740121. [DOI] [PubMed] [Google Scholar]
- Ortiga-Carvalho TM, Polak J, McCann S, Pazos-Moura CC. Effect of thyroid hormones on pituitary neuromedin B and possible interaction between thyroid hormones and neuromedin B on thyrotropin secretion. Regul Pept. 1996;67:47–53. doi: 10.1016/s0167-0115(96)00106-1. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Fujii M, Nakamura T, Tani S, Oka T, Yajima H, Baba S. Effects of neuromedin B and neuromedin C on exocrine and endocrine rat pancreas. Am J Physiol Gastrointest Liver Physiol. 1987;252:G491–G498. doi: 10.1152/ajpgi.1987.252.4.G491. [DOI] [PubMed] [Google Scholar]
- Pazos-Moura CC, Moura EG, Rettori V, Polak J, McCann S. Role of neuromedin B in the in vitro thyrotropin release in response to thyrotropin-releasing hormone (TRH) from anterior pituitaries of eu-, hypo- and hyperthyroid rats. Proc Soc Exp Biol Med. 1996;211:353–358. doi: 10.3181/00379727-211-43980. [DOI] [PubMed] [Google Scholar]
- Persson K, Gingerich RL, Nayak S, Wada K, Wada E, Ahrén B. Reduced GLP-1 and insulin responses and glucose intolerance after gastric glucose in GRP receptor deleted mice. Am J Physiol Endocrinol Metab. 2000;279:E956–E962. doi: 10.1152/ajpendo.2000.279.5.E956. [DOI] [PubMed] [Google Scholar]
- Persson K, Pacini G, Sundler F, Ahrén B. Islet function phenotype in gastrin-releasing peptide receptor gene-deficient mice. Endocrinology. 2002;143:3717–3726. doi: 10.1210/en.2002-220371. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;1223:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rodrigues AL, Moura EG, Passos MCF, Dutra SCP, Lisboa PC. Postnatal early overnutrition changes the leptin signalling pathway in the hypothalamic-pituitary-thyroid axis of young and adult rats. J Physiol. 2009;587:2647–2661. doi: 10.1113/jphysiol.2009.169045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H, Feighner SD, Hreniuk DL, Iwaasa H, Sailer AW, Pan J, Reitman ML, Kanatani A, Howard AD, Tan CP. Characterization of the bombesin-like peptide receptor family in primates. Genomics. 2004;84:139–146. doi: 10.1016/j.ygeno.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Sheng X, Zhang Y, Gong Z, Huang C, Zang YQ. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008;2008:581348. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza LL, Nunes MO, Paula GSM, Cordeiro A, Penha-Pinto V, Neto JFN, Oliveira KJ, Tavares do Carmo MG, Pazos-Moura CC. Effects of dietary fish oil on thyroid hormone signaling in the liver. J Nutr Biochem. 2009 doi: 10.1016/j.jnutbio.2009.07.008. DOI 10.1016/j.jnutbio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Spálová J, Zamrazilová H, Vcelák J, Vanková M, Lukásová P, Hill M, Hlavatá K, Srámková P, Fried M, Aldhoon B, Kunesová M, Bendlová B, Hainer V. Neuromedin beta: P73T polymorphism in overweight and obese subjects. Physiol Res. 2008;57(Suppl. 1):S39–S48. doi: 10.33549/physiolres.931488. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber HC. Regulation and signalling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diab Obes. 2009;16:66–71. doi: 10.1097/med.0b013e32831cf5aa. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Restraint stress impaired maternal behaviour in female mice lacking the neuromedin B receptor (NMB-R) gene. Neurosci Lett. 2002c;330:163–166. doi: 10.1016/s0304-3940(02)00771-1. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada E, Wada K. Role of bombesin (BN)-like peptides/receptors in emotional behaviour by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry. 2002a;7:113–117. doi: 10.1038/sj.mp.4000974. [DOI] [PubMed] [Google Scholar]
- Yamada K, Santo-Yamada Y, Wada K. Stress-induced impairment of inhibitory avoidance learning in female neuromedin B receptor-deficient mice. Physiol Behav. 2003;78:303–309. doi: 10.1016/s0031-9384(02)00979-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, Wada E, Yamano M, Sun YJ, Ohara-Imaizumi M, Nagamatsu S, Wada K. Decreased marble burying behaviour in female mice lacking neuromedin-B receptor (NMB-R) implies the involvement of NMB/NMB-R in 5-HT neuron function. Brain Res. 2002b;942:71–78. doi: 10.1016/s0006-8993(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Yamano M, Ogura H, Okuyama S, Ohki-Hamazaki H. Modulation of 5-HT system in mice with a targeted disruption of neuromedin B receptor. J Neurosci Res. 2002;68:59–64. doi: 10.1002/jnr.10194. [DOI] [PubMed] [Google Scholar]
- Yang YS, Song HD, Li RY, Zhou LB, Zhu ZD, Hu RM, Han ZG, Chen JL. The gene expression profiling of human visceral adipose tissue and its secretory functions. Biochem Biophys Res Commun. 2003;300:839–846. doi: 10.1016/s0006-291x(02)02843-7. [DOI] [PubMed] [Google Scholar]