Abstract
Increased Type I IFNs or IFN-I have been associated with human systemic lupus erythematosus. Interestingly augmenting or negating IFN-I activity in murine lupus not only modulates systemic autoimmunity, but also impacts lupus nephritis, suggesting that IFN-I may be acting at the level of the end-organ. We find resident renal cells to be a dominant source of IFN-I in an experimental model of autoantibody-induced nephritis. In this model, augmenting IFN-I amplified antibody-triggered nephritis, whereas ablating IFN-I activity ameliorated disease. One mechanism through which increased IFN-I drives immune-mediated nephritis might be operative through increased recruitment of inflammatory monocytes and neutrophils, though this hypothesis needs further validation. Collectively, these studies indicate that an important contribution of IFN-I toward the disease pathology seen in systemic autoimmunity may be exercised at the level of the end-organ.
The presence of self-reactive anti-nuclear Abs is a key characteristic in the development of the autoimmune disease, systemic lupus erythematosus (SLE).5 The deposition of anti-nuclear Abs, anti-glomerular Abs and related immune complexes within the end organs such as the kidney, play a significant role in the development of nephritis and death. Of the multiple cytokines implicated in disease progression, much research has focused on the key role of type I IFNs (IFN-I), particularly in human SLE. Early studies by Preble et al. (1, 2) demonstrated that α was detectable in the serum of SLE patients. In addition, it has been shown that sera from patients with SLE can stimulate normal PBMCs to produce IFNα (3, 4). More recently, several groups have demonstrated the presence of an IFN-I gene expression signature in the peripheral blood of patients with SLE, with some suggesting a correlation with disease severity and end organ disease (5–9). Whether the presence of this signature is a cause or a consequence of disease is yet to be determined. In this context, murine studies have been particularly informative.
First, negating IFN-I activity in the NZB/129 and B6.lpr strains ameliorated most of the component lupus phenotypes (10–12), though some exceptions have been noted (13). Second, deliberate administration of IFN-I, or the in vivo delivery of TLR ligands that trigger IFN-I production, augment various lupus phenotypes (11, 14–18). Interestingly, whereas antichromatin and other serum autoantibody levels were barely or modestly impacted in several of these studies, the severity of renal disease was significantly influenced in all of the above studies (15–20). Collectively, these findings suggest that one important mechanism through which IFN-I might be contributing to lupus pathogenesis is by directly impacting end organ disease. However, this hypothesis has not been directly tested.
The present work addresses the above hypothesis that IFN-I might directly modulate immune-mediated nephritis in lupus, based on studying a closely related experimental model, anti-glomerular basement membrane (GBM) nephritis. Though the initiating triggers differ in experimental anti-GBM disease and spontaneous lupus nephritis, both disease settings appear to share downstream effector mechanisms, as we have recently reviewed (21). Importantly, of 25 different molecules that have been examined for their functional relevance in two disease scenarios, all impacted both diseases concordantly, suggesting that the experimental anti-GBM model might be a reliable and useful tool for studying spontaneous lupus nephritis. We have previously reported that different inbred strains develop differing degrees of nephritis following challenge with anti-GBM sera (22, 23). In this communication, we examine how anti-GBM Ab-induced nephritis is affected by negating or augmenting IFN-I activity in an inbred strain that is modestly sensitive to nephritis, C57BL/6 (B6).
Materials and Methods
Reagents and mice
All mice were bred in the University of Texas Southwestern Medical Center. Breeding pairs for C57BL/6J (B6) mice were originally obtained from The Jackson Laboratory. B6.IFNAR−/− mice (lacking the common receptor for Type I IFNs) were obtained from Dr. Michel Aguet (ISREC Foundation, Epalinges, Switzerland) (24). The care and use of laboratory animals conformed to the National Institutes of Health guidelines and all experimental procedures conformed to an institutional animal care and use committee approved animal protocol.
Anti-GBM serum and disease induction
Anti-GBM serum was prepared as described previously (22). In brief, renal cortices of B6 mice were minced and then passed through sieves of decreasing pore size (150, 106, and 63 µm). Glomeruli were obtained from the finest sieve, washed in cold PBS and sonicated for 7 min. The glomerular sonicate was sent to the Lampire Laboratories for the generation of anti-GBM sera in rabbits. The specific binding of the immune rabbit sera to glomerular basement membrane was confirmed by direct immunofluoresence using frozen mouse kidney sections, and its efficacy in precipitating nephritis was tested in B6 and 129/sv mice, as described previously (23). Mice were presensitized with rabbit IgG (2.5 mg/ml with CFA, 100 µl/20g body weight) via the peritoneal cavity. Five days postinjection, mice were injected anti-GBM serum IV (170 µl/20g body weight). Blood and 24-h urine samples were collected before (day 0) and 14 days after induction of disease. In studies where this was combined with IFN-adenovirus (ADV), anti-GBM serum was administered IV at 130 µl/20g body weight to ensure the detection of any augmentation of disease. Prior trials using a lower dose of anti-GBM serum (100 µl/20g) did not have any effect with this combination.
IFN-ADV and IFN
IFN-I was deliberately administered to two cohorts of mice. In cohort 1, IFN-I (Universal type I IFN, no. 11200–2 PBL Laboratories) was injected i.p. at 10,000 U per mouse on days −4, 0, 4, 8, and 12 (where day 0 represents the day of rabbit Ig injection), as described previously (25). Mice in cohort II were administered IFN-I, using adenoviral delivery on D0 of the anti-GBM disease induction protocol. The IFN adenovirus (IFN-ADV) or control (ADV) was purchased from Q-Biogene. This was repeated twice and the data combined for analysis of renal disease. Ad5.CMV-mIFN-α expresses the murine IFNα5 gene under the control of the Cytomegalovirus-IE promoter/enhancer. Ad5.Null contains an empty expression vector and was used as a control (ADV). One × 109 IFN-ADV or ADV particles were injected, as previously described (14).
Assessment of renal disease
Mice were caged in metabolic cages and urine was collected over a 24 h period. Protein was measured using the Coommassie Plus Protein Assay kit (Pierce) as per the manufacturer’s instructions. BSA (Pierce) was used as a standard. Blood urea nitrogen (BUN) was assessed using the Quanti-Chrom Urea Assay Kit (BioAssay). Following euthanasia, kidneys were fixed in formalin and embedded in paraffin for blinded analysis by an independent pathologist (X.J.Z.), as previously described (22). The severity of GN was graded using the following guidelines set by the World Health Organization, based on light microscopy: 0, normal; 1, mild increase in mesangial cellularity and matrix; 2, moderate increase in mesangial cellularity and matrix, with thickening of the GBM; 3, focal endocapillary hypercellularity with obliteration of capillary lumina and a substantial increase in the thickness and irregularity of the GBM; and 4, diffuse endocapillary hypercellularity, with segmental necrosis, crescents, and hyalinized end-stage glomeruli. Tubular interstitial nephritis was graded on a 0–4 scale, as described (22).
Cell preparation and flow cytometry
Kidneys were prepared as described previously (18, 26). In brief, they were minced and resuspended into 0.75 ml PBS. Cells were spun down and the supernatant was kept at −20°C for cytokine analysis. Cells were resuspended in digestion buffer, consisting of collagenase (1 mg/ml) and DNase (1 µg/ml) in RPMI Complete Media and incubated at 37°C for 30 min.
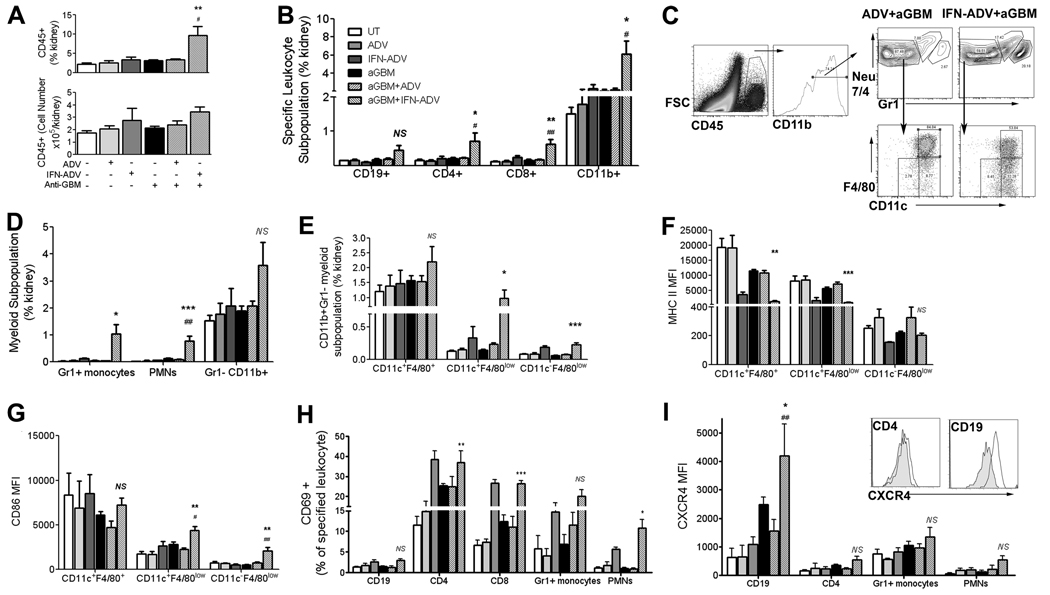
Cells were centrifuged and filtered through a 70-µM mesh and then mixed 1:1 with 40% Percoll solution. This was centrifuged at 3000 rpm for 20 min at room temperature with the brake off. The loose pellet was washed, counted, and resuspended in staining buffer. Analysis of myeloid subtypes was based upon analyses described by Geismann and colleagues, Taylor and colleagues and Soos et al. (27–29). Peripheral polymorphonucleocytes (neutrophils) were CD11b+, Neu 7/4+ Gr1+++, highly granular, and CD62L+, whereas the inflammatory monocyte population is Gr1+CD11b+Neu7/4+ (Fig. 4c) as described previously (18, 26). The Gr1−CD11b+ population was further characterized using the markers CD11c and F4/80 because an earlier report by Soos et al. (29) has described a complex network of interstitial dendritic cells throughout the kidney. The majority of these cells have been described as expressing CD11b, F4/80, and CD11c, and the use of a GFP transgenic demonstrated that they were also CX3CR1+. The strategy of our gating system is shown in Fig. 4c. Eluted leukocytes were enumerated using Sphero AccuCount particles (Spherotech), as per manufacturer’s instructions. Acquisition and analysis was completed using a BD LSR II with Diva software, and FlowJo 7.2 for Windows (Tree Star).
FIGURE 4.
Analysis of leukocyte infiltration into the kidney. Mice were injected on day 0 with IFN-ADV, ADV, and/or anti-GBM Abs, as indicated. Leukocyte infiltration into the kidney was assessed on day 14 (A) using flow cytometry. The frequency of various leukocyte subpopulations is shown in B. Myeloid subpopulations were also assessed (gating shown in C, statistics in D). These included inflammatory monocytes (Neu7/4+,Gr1+, CD11b+, SSClow), polymorphonucleocytes (Neu7/4+Gr12+CD11b+, SSChigh and the Gr1−CD11b+ population. This latter population was further analyzed for CD11c and F4/80 expression (gating in C, statistics in E), with the expression of MHC II (F) and CD86 (G) shown for each treatment group. Activation was detected using a variety of markers, including CD69 on multiple cell types (H). In addition, the expression level of CXCR4 was also assessed on the different cell types (I). The p value depicted pertains to ANOVA analysis with Bonferroni post hoc comparisons of the IFN-ADV plus aGBM group to ADV plus aGBM (*, p < 0.05; **p < 0.01; ***p < 0.001) and IFN-ADV to IFN-ADV plus aGBM (#, p < 0.05; ##, p < 0.01).
Cytokine multiplex analysis
The supernatants from kidney were analyzed using 22-plex murine cytokine assay with the Bio-Plex cytokine assay kit (Bio-Rad) as per the manufacturer’s protocol at BIIR Luminex Core, Dallas.
Assaying IFN-I protein and message
Kidneys were dissected out, chopped finely with a razor blade, and suspended in PBS. Kidney cells were spun to a pellet at 1100 rpm at 4°C for 6 min. The supernatant, enriched in interstitial fluid or “renal plasma”, was stored frozen at −20°C in aliquots until analysis. IFN-I protein was detected using an ELISA kit from PBL as per the manufacturer’s protocol. The kit measures the following subtypes: IFNaA, IFNα1, IFNα4, IFNα5, IFNα6, and IFNα9, with a detection range between 12.5–5000 pg/ml. RNA message was analyzed using Applied Biosystems Taqman Gene Expression Assays using primers for IFNα4 (Mm00833969_s1), IFNα5 (Mm00833976_s1), IFNβ1 (Mm00439546_s1), B2M (Mm00437762_m1), and CD45 (Mm01293570_m1, specific for a region outside the extra-cellular receptor splicing area). Absolute MAX QRT-PCR mix from Thermo Scientific was used for amplification, as per the manufacturer’s instructions. An ABI 7300 Real Time System, using Applied Biosystems Sequence Detection Software, Version 1.2.3 was used for amplification and analysis. The message levels of IFN-I genes were expressed after normalization to corresponding CD45 and B2M expression levels.
Statistical analyses
Results are expressed as the arithmetic mean ± SEM (SE). Study groups were analyzed for normal distribution using Kolmogorov-Smirnov test. Comparisons between two groups passing normality were assessed using the parametric Student t test, those that did not pass the normality test were assessed using the Mann-Whitney U test. Groups were compared using 1 or 2 way ANOVA with either Bonferroni or Dunn’s post hoc analyses for parametric and nonparametric data respectively. Correlations between two variables (Fig. 1, B and C) were calculated using Spearman’s rank non-parametric 2-way analyses, with rho, ρ and the probability, p, shown within the graph. Analyses were completed using InSTAT version 3.0 and Prism 5.0 for Windows (GraphPad Software) or Mcrosoft Excel.
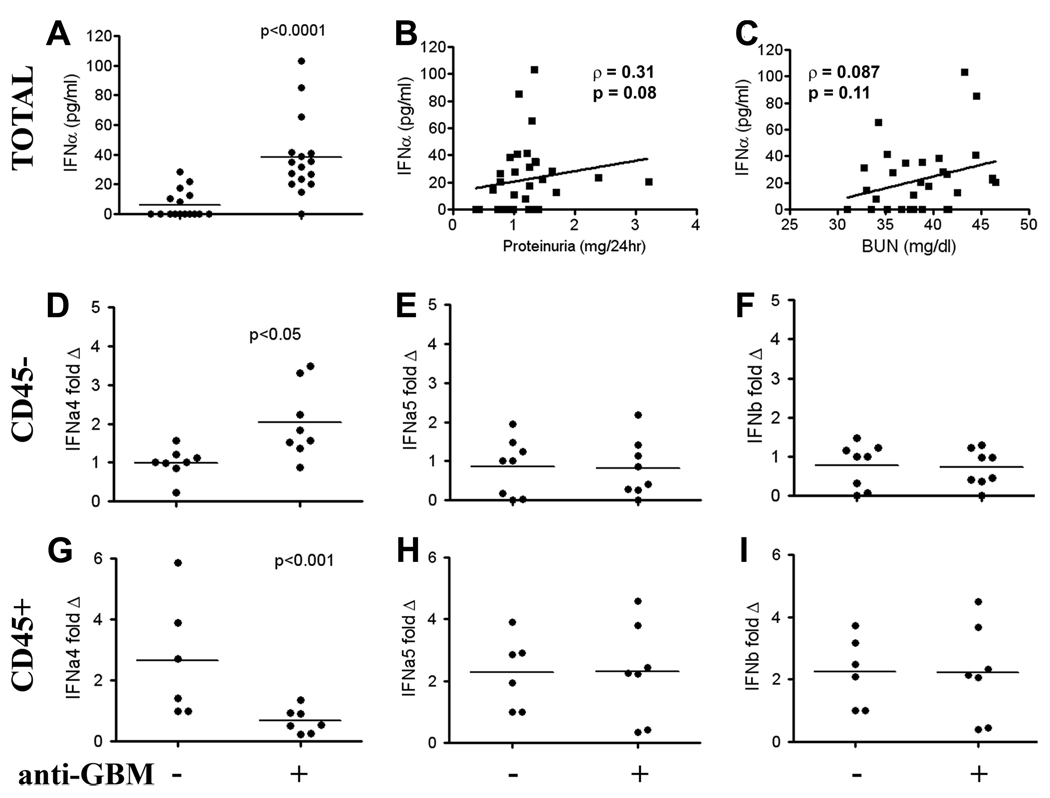
FIGURE 1.
Anti-GBM administration stimulates the production of IFN-I within the kidneys. B6 mice were injected with anti-GBM sera on day 5 (after presensitization with rabbit Ig on day 0). On day 14, total renal cells were extracted into a single cell suspension in PBS. The resulting supernatant from the untreated and anti-GBM challenged mice was assessed for IFNα protein, by ELISA (A). Shown in B and C are the correlation profiles of renal IFN-I levels with 24-h proteinuria or BUN levels in both groups of mice. The experiment was repeated, with the data combined in Fig. 1, A–C, with a final mouse number of 16 per group. In a separate study, CD45− resident renal cells were isolated using magnetic bead sorting and assessed for type I IFN mRNA (IFNa4, IFNa5, IFNb1) expression by real time PCR (D–F). CD45+ leukocytes were also assessed for IFN message expression (G–I; IFNa4, IFNa5, IFNb1). Units are fold change relative to the normalized expression level observed in control untreated mice. Each dot represents data obtained from an individual mouse.
Results
Resident renal cells may be the predominant source of IFN-I in immune nephritis
Nonautoimmune prone B6 mice were injected with rabbit anti-GBM Abs. As reported previously, B6 mice exhibit modest disease following anti-GBM Ab challenge (22). To determine whether disease was associated with increased IFN-I expression, kidneys from these mice were extracted and assessed for levels of soluble IFN-I in the renal interstitial fluid or “plasma” 14 days later. Although the secreted levels were low, the anti-GBM Ab assault was associated with a significant increase in IFN-I (Fig. 1A) and the levels of this cytokine trended with the levels of proteinuria and azotemia (Fig. 1, B and C), although this did not reach statistical significance, (p = 0.08 and p = 0.11, respectively). Because the elevated IFN-I may have been derived from infiltrating leukocytes or resident renal cells, and because the ELISA detects a number of IFN-I subtypes, we next analyzed IFN-I message levels in infiltrating vs resident kidney cells using real time PCR. Resident kidney cells were magnetic bead sorted away from infiltrating leukocytes with 99% purity, using CD45 beads. Message from the CD45+enriched and CD45−cell populations were standardized to both CD45 and B2M message levels for mRNA analysis. We specifically assayed the message levels of IFN-β1, IFNa4, and IFNa5, because highly specific PCR primers for these IFN-I genes could readily be designed. Interestingly, increased IFNα4 (p = 0.007) but not IFNα5 or IFNβ1 message was noted in CD45− resident renal cells (Fig. 1, D–F), following anti-GBM challenge. In contrast, analysis of mRNA in CD45+ leukocytes did not reveal any increase in IFN-I message (Fig. 1, G–I). Moreover, a significant decrease in the level of IFNa4 was observed in the CD45+ leukocyte population following anti-GBM challenge (Fig. 1G). This was repeated in a separate experiment using 129 mice. Interestingly, while IFNa4 mRNA was increased in CD45− renal cells following treatment with anti-GBM, consistent with the results from theB6 mice, there was no detectable change in expression in the infiltrating CD45+ leukocyte population following treatment (data not shown). Collectively, these findings indicate that intrarenal IFN-I is increased in anti-GBM disease, and arises predominantly from resident (CD45−) renal cells, rather than the infiltrating (CD45+) leukocytes.
Systemic IFNα administration amplifies experimental immune nephritis if administered chronically
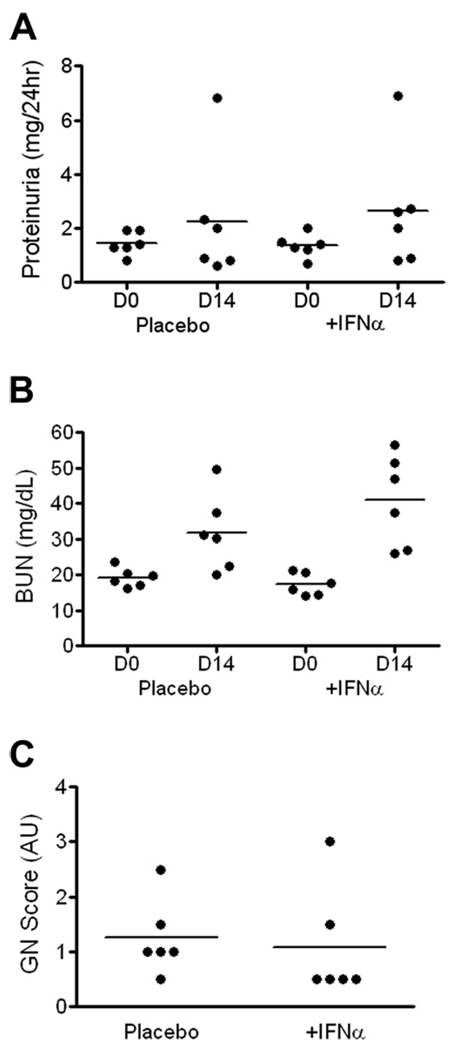
To examine the effect of exogenous IFNα on anti-GBM induced disease, a first cohort of mice were injected at 4 day intervals with recombinant IFNα, over the 14 day anti-GBM challenge/follow-up period. Analysis of proteinuria and BUN following anti-GBM assault revealed a modest increase in the IFN-I injected mice, although this was not significant (Fig. 2, A and B). Examination of renal pathology also demonstrated that recombinant IFN-I administered intermittently had no effect on anti-GBM induced disease (Fig. 2C). The grade of tubular and interstitial nephritis and the percentage of glomerular crescents were also unaffected by intermittent treatment with IFN-I (data not shown). Examination of serum at the end of the study (day 14) showed that IFN levels were rather low following intermittent administration (ranging from undetectable to 25 pg/ml).
FIGURE 2.
The impact of intermittent recombinant IFNα in anti-GBM disease. Recombinant IFNα was administered to a cohort of anti-GBM injected B6 mice on days −4, 0, 4, 8, and 12, as detailed in Materials and Methods. Twenty-four hour proteinuria was assessed (A), together with BUN (B). Glomerulonephritis was assessed blinded (C), using the WHO classification scheme. The placebo group received PBS.
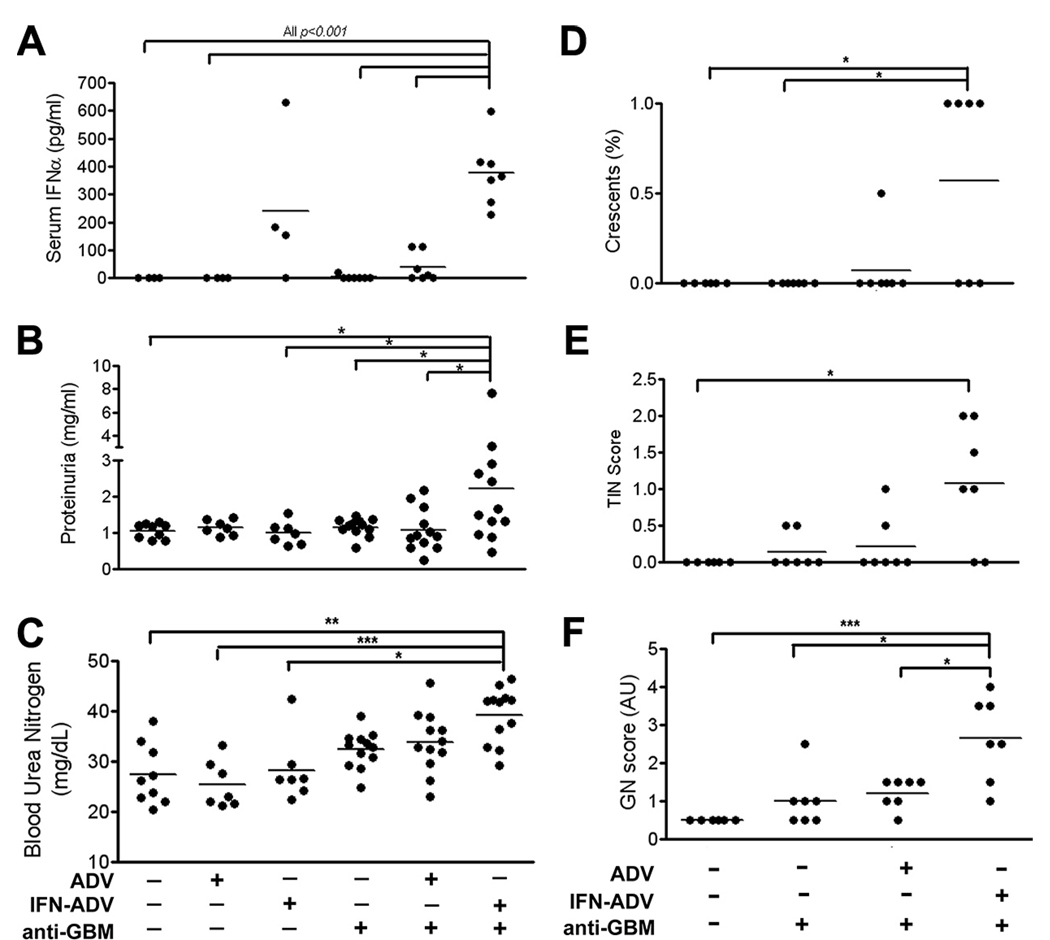
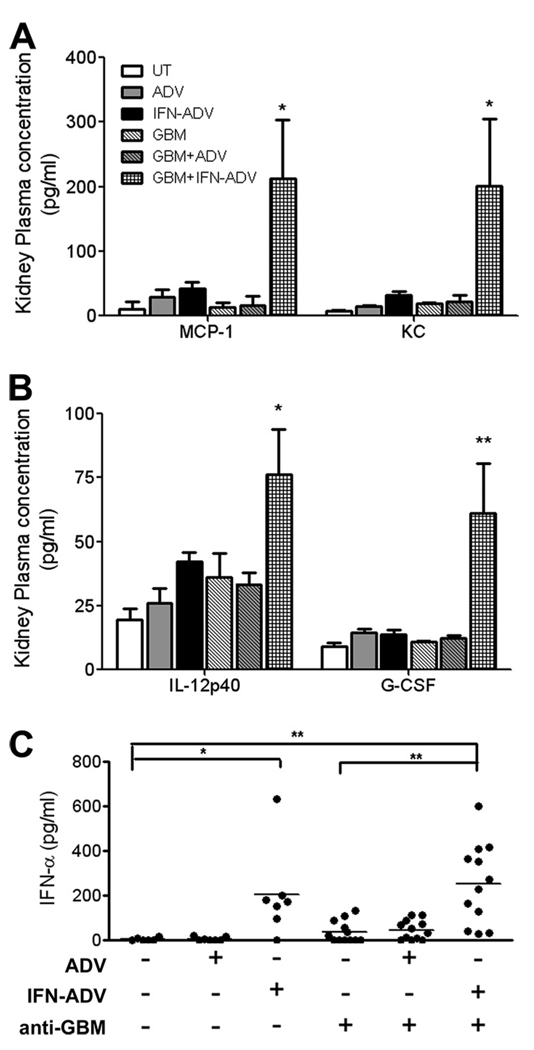
Because the levels of serum IFNα following intermittent administration of recombinant IFN-I were low, we next used an adenoviral vector system for sustained delivery of IFNα. Serum levels at the end of the study using this system averaged 240 pg/ml for IFN-ADV alone, and 380pg/ml for IFN-ADV plus anti-GBM (Fig. 3A). Interestingly, a combination of the control ADV vector and anti-GBM Ab resulted in low, but detectable levels of serum IFNα. Analysis of protein in the urine demonstrated a significant increase following combined exposure to IFN-ADV and anti-GBM Ab (Fig. 3B), compared with mice receiving either alone. This was also accompanied by a visible increase in BUN (Fig. 3C), though not significant.
FIGURE 3.
IFN-ADV delivery significantly amplifies anti-GBM induced nephritis. Mice were injected on day 0 with IFN-ADV, ADV, and/or anti-GBM Abs, as indicated. Serum IFNα (A; all comparisons shown, p < 0.001), Proteinuria (B) and BUN (C) were assessed on day 14 following challenge. Kidneys were assessed for histological nephritis as described in methods (D–F). Shown p values pertain to 1-way ANOVA with post hoc analyses comparing to the IFN-ADV plus anti-GBM injected group of mice. The experiment was repeated three times, with data combined for B and C; serum IFNα and renal pathology was acquired from two independent experiments (*, p < 0.05; **p < 0.01; ***p < 0.001).
Blinded analysis of renal histology by an independent pathologist demonstrated a significant increase in glomerulonephritis, tubulo-interstitial nephritis, and glomerular crescent formation after anti-GBM/IFN-ADV, compared with the controls (Fig. 3, D–F). No other single or combination treatment group demonstrated a significant increase in disease.
Next, leukocyte infiltration into the kidney was assessed using flow cytometry. Analyses demonstrated that a combination of sustained IFNα administration and anti-GBM Ab injection resulted in a significant increase in the percentage of CD45+ leukocytes within the kidney (9.65 ± 2.34, IFN-ADV plus anti-GBM compared with 3.39 ± 0.21, ADV plus anti-GBM; Fig. 4A, top). The overall numbers of leukocytes within the kidney showed a moderate, but nonsignificant increase, which suggests a loss of renal cells possibly due to the disease (Fig. 4A, bottom). An alternative reason for the disparity between the percentage infiltrate and total cell numbers is that the processing procedures of the kidney (collagenase and percoll, see Materials and Methods) may have created variability between samples. However, leukocyte infiltration does appear to be real, because additional assays on IFN-ADV treated B6 mice published previously have shown minimal infiltration following ADV or IFN-ADV treatment alone (18).
Further analyses on the CD45+ population suggested a moderate increase in B and T lymphocyte infiltration and a significant increase in the myeloid population in the IFN-ADV plus anti-GBM group (Fig. 4B). Gating strategies for analyzing the myeloid lineage are shown in Fig. 4C. Cells were gated on CD11b (CD19−CD3−) and initially through Neu7/4 and Gr1 which showed that both Gr1+ inflammatory monocytes and neutrophils increased in response to the combination of IFN-ADV and anti-GBM (11- and 3-fold greater numbers than anti-GBM alone for Gr1 plus monocytes and neutrophils respectively, data not shown and Fig. 4D), The Gr1− CD11b+ population was further subgated using CD11c and F4/80 as described in the Materials and Methods (Fig. 4C, bottom). The CD11c− F4/80low population, together with the CD11c+ F4/80low population showed a significant expansion in response to anti-GBM plus IFN-ADV (Fig. 4E). Analyses of surface receptor expression showed a surprising decrease in MHC II expression in response to IFN-ADV. This was an IFN-specific response because the IFN-ADV alone had a similar effect (Fig. 4F). However, surface CD86 expression was increased following a combination of IFN-ADV and anti-GBM in both the CD11c+ and CD11c− F4/80low populations which was not due to IFN-ADV (Fig. 4G).
Analysis of CD69 positivity in multiple cell types suggested trends in activation following IFNα administration independent of an anti-GBM insult (Fig. 4H). This may not be a surprise because CD69 is expressed very early on lymphocytes following activation from multiple challenges, including IFNα (30–32). Further analysis of CXCR4 expression, a molecule we have found to be important in the pathogenesis of lupus nephritis (33), also demonstrated an increase in activation in multiple cell types following the administration of IFN-ADV and anti-GBM Abs (Fig. 4I). Overall, this data demonstrates that while IFNα can mediate activation across multiple cell types, these effects are not necessarily associated with the development of disease. In particular, increased expression of CXCR4 and CD86 occur preferentially in mice exposed to anti-GBM and IFN-ADV, which develop nephritis.
Analyses of the renal interstitial fluid or plasma using a multiplex cytokine luminex assay demonstrated small nonsignificant increases in the myeloid attractant chemokines MCP-1 and keratinocyte chemoattractant (KC, Groα, CXCL1) (Fig. 5A) and the proinflammatory cytokines IL-12p40 and G–CSF (Fig. 5B) following IFN-ADV treatment alone. The elevation in these specific mediators was significantly higher in mice treated with a combination of anti-GBM and IFN-ADV (Fig. 5, A and B). Other cytokines and chemokines that were detected in the kidney, but exhibited no change following treatment were IL-1α, IL-1β, IL-6, IL-9, IL-13, and eotaxin (data not shown). In addition, MIP-1α, MIP-1β, IL-10, IL-2, IL-3, IL-4, IL-5, IFN-γ, GM-CSF, IL-17, and IL-12p70 were expressed at low or undetectable levels and did not increase following any treatment (data not shown). Examination of IFNα in the renal interstitial fluid demonstrated that although there is detectable IFNα following ADV plus anti-GBM, this is no different from the levels in the anti-GBM alone mice, in contrast to the observations in the sera (Fig. 5C). In addition, there does not seem to be any effect of ADV alone in any other measure of renal disease.
FIGURE 5.
Analysis of cytokines and chemokines within the kidney. Mice were injected as described in Fig. 4. Kidneys were minced and re-suspended in PBS. Cytokine and chemokine analysis of the samples was completed using the Luminex bead array system. Levels of IFNα in the kidney supernatant were examined using an ELISA, as described in the Materials and Methods. The p value depicted pertains to ANOVA analysis with Bonferroni post hoc test comparisons of the IFN-ADV plus aGBM group to ADV plus aGBM (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
IFNAR elimination ameliorates anti-GBM disease
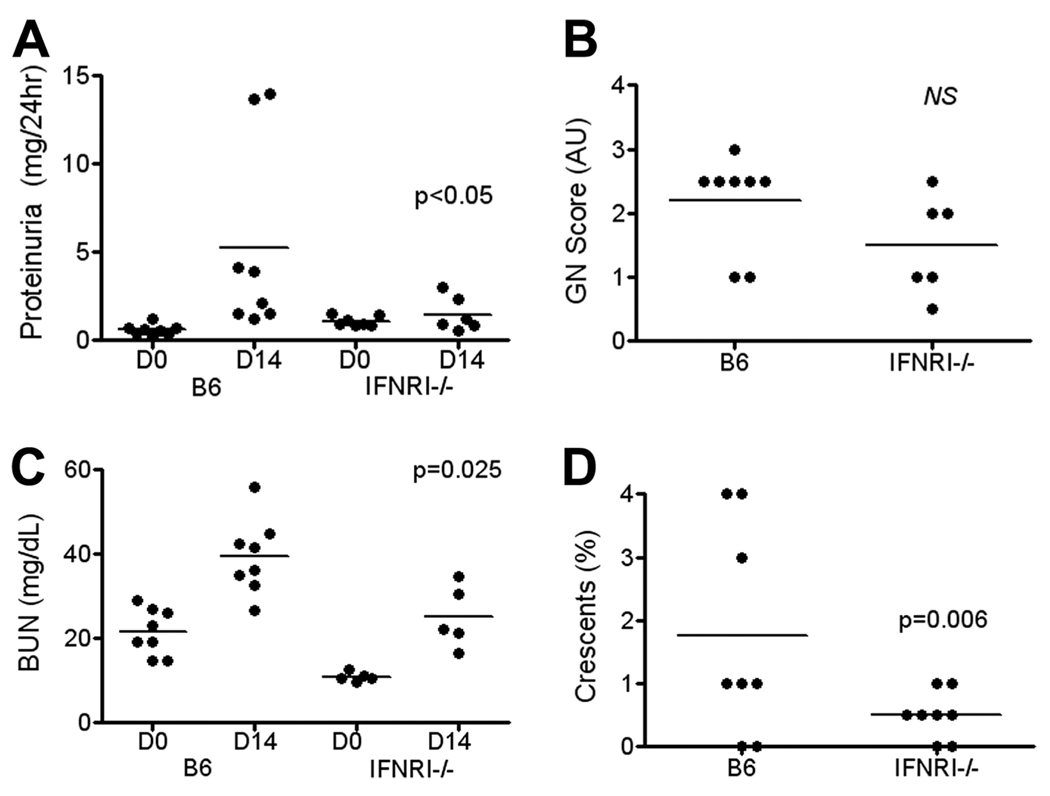
Because intrarenal IFN-I was up-regulated in anti-GBM disease (Fig. 1), we next asked whether IFN-I was playing an essential role in mediating anti-GBM disease. To test this, we used an IFNAR-deficient strain lacking the common receptor for Type IIFN, on the B6 background (B6.IFNAR−/−). Following challenge with the anti-GBM Abs, the significant increase in proteinuria observed in the B6 control strain was absent in the knockout mice (Fig. 6A). Anti-GBM challenge increased BUN in both B6 and B6.IFNAR−/−mice (Fig. 6B). However, the induced level of BUN was significantly less in the knockout animals compared with the levels in the B6 controls. Likewise, the degree of GN and glomerular crescent formation in IFNAR−/− mice following anti-GBM challenge was lower in the knockouts compared with the B6 controls (Fig. 6, C and D). These initial findings were reproduced in an additional study, as detailed in the legend. Overall, the deficiency of IFNAR ameliorated all aspects of the renal disease following the immune challenge, indicating that the up-regulated IFN-I during the course of anti-GBM induced nephritis was playing an essential role in the pathogenesis of this disease.
FIGURE 6.
Deletion of the IFNAR receptor significantly reduces anti-GBM nephritis. B6 and B6.IFNAR−/− mice were injected with anti-GBM sera. Twenty-four hour proteinuria (A), BUN (B), and renal pathology (C and D) were assessed at the end of the study, on day 14. Shown p values pertain to comparisons of the day 14 data from the B6.IFNAR−/− mice against corresponding data from the B6 controls, using Student’s t test or the Mann-Whitney U test (1). The fraction of mice with severe GN (score >2) in the different study groups were compared using the Fisher’s exact test (2). The findings were reproduced in an additional study in which B6.IFNAR−/− mice had reduced proteinuria (2.3 mg/24 h vs 5.2 mg/24 h, p < 0.01, n = 4–5 per group) and reduced BUN (32 mg/dL vs 166 mg/dL, p < 0.03), compared with anti-GBM challenged B6 controls.
Ablating IFN-I activity does not appear to dampen systemic immune responses
We reasoned that the reduced anti-GBM-induced renal disease observed in B6.IFNAR−/− mice may relate either to reduced systemic xenogenic immune response to the administered rabbit Ig or to reduced local inflammatory events within the kidneys. To distinguish between these possibilities, we next examined the serum levels of anti-rabbit xenogenic immune response in the anti-GBM challenged B6.IFNAR−/− mice and B6 controls. B6.IFNAR−/− mice did not exhibit reduced anti-rabbit alloimmune response, indicating that the reduced disease in these mice is not simply the consequence of reduced systemic adaptive immune responsiveness to the administered anti-GBM Abs (data not shown).
Discussion
Several studies in human SLE have pointed to the strong association of elevated IFN-I with disease. In addition, the published studies reveal that elevated IFN-I is often associated or correlated with various end-organ disease manifestations in SLE, including nephritis and cutaneous symptoms (34–36). Reports have also demonstrated a pivotal role for IFNα in pure cutaneous lupus where the disease is limited to the skin (37). Other studies have shown that IFNα is detectable in the CSF of SLE patients with neuropsychiatric syndromes (38). Moreover, hepatitis and malignant melanoma patients treated with IFN-I have a tendency to develop neurological disorders (38). In the above human SLE studies, the degree to which various end organ disease manifestations are dictated by local expression of IFN-I within the end-organs has not been ascertained.
Similarly, in murine lupus, we and others have reported that increased IFN-I is associated with more severe disease (11, 14–18), though exceptions have been noted (13). Whereas ablating the IFNAR ameliorates systemic lupus and lupus nephritis in polygenic models of lupus (10–12), deliberate administration of IFN-I or TLR ligands that elicit IFN-I production, invariably augment disease (11, 14–18). Again, in all of these studies, it is not clear whether the observed change in renal disease is due to the direct impact of IFN-I on the end-organs, or they simply follow as a consequence of changes in autoantibody titers and/or leukocyte function. The present study contributes a novel perspective to this important question. This is the first demonstration that the bulk of IFN-I expressed in a targeted end-organ (in any autoimmune disease) is generated by resident cells in the organ, as opposed to infiltrating leukocytes. Moreover, infiltrating leukocytes appear to down-regulate their IFNa4 mRNA expression in this strain, perhaps in a compensatory role to the inflammatory environment.
Primary candidate receptors responsible for the production of type I IFN include antiviral immune receptors, RIG-I and Mda-5, and the intracellular TLRs. Although TLR7 and TLR9 are not expressed by renal cells, Anders and colleagues (39–41) have shown that TLR3 is expressed by multiple cells within the kidney, and it is increased in response to an inflammatory challenge. Furthermore, this group has also demonstrated that viral triphosphate RNA (3-P RNA), a non-TLR agonist, aggravates lupus nephritis in MRLlpr mice, increasing systemic IFNα (42). Furthermore, 3-P RNA binds directly to glomerular and tubular epithelial cells, possibly activating the cells through RIG-I. Moreover, we have shown previously that costimulation of TLR3, facilitates anti-glomerular Ab-elicited nephritis (43). Therefore, it is possible that TLR3 is up-regulated and activated by cellular debris from the initial inflammation that ensues following the anti-GBM challenge. Further work is necessary to test this hypothesis.
Given that the experimental anti-GBM nephritis model closely parallels spontaneous lupus nephritis in terms of their downstream effector mechanisms (21), it is tempting to speculate that IFN-I might be playing a similar role in engendering end-organ disease in spontaneous lupus nephritis. Studies are in progress to directly ascertain whether this is also true in spontaneous lupus nephritis. Determining whether resident renal cells are the major source of IFN-I in lupus nephritis could have important diagnostic and therapeutic implications.
A second novel contribution of this study is the demonstration that altering IFN-I levels can directly alter the degree of renal disease following an anti-glomerular Ab-mediated assault. Whereas ablating IFN-I activity altogether ameliorates immune nephritis, the deliberate administration of IFN-I amplifies end-organ disease. Moreover, following a combination of systemic IFN-I and anti-GBM serum (Fig. 4), we observed a leukocyte infiltration pattern in the assaulted kidney akin to our previous observations in two different spontaneous models of SLE (18, 26). Specifically, an increase in the numbers of inflammatory monocytes, neutrophils and activated CD4+ T cells within the kidney appears to be a key feature of severe nephritis associated with elevated IFN-I. Moreover, following treatment with IFNα and anti-GBM, we detected a significant increase in the chemokine MCP-1, which is known to be associated with disease pathogenesis in multiple murine models and in human disease (44–49). Increased levels of MCP-1 mRNA and protein have been detected within the NZB/W strains and inhibition of MCP-1 ameliorates the disease progression in both the NZB/W and MRLlpr SLE strains, decreasing the myeloid recruitment into the kidney (44–46, 48, 50). In addition MCP-1 is increased in sera and kidney of patients with SLE, correlating with disease activity manifestations (47, 49). In addition, both keratinocyte chemoattractant and G–CSF were significantly higher within the kidney following combined treatment with anti-GBM and IFNα. These molecules are primarily responsible for the recruitment of neutrophils into the site of inflammation. In addition, G–CSF has been found to increase nephritis upon low dose administration in the MRLlpr strain, and it may drive the differentiation of monocytes into dendritic cells; both of these findings thus place it as an important mediator in disease progression (51, 52). IL-12p40 was also increased in diseased mice treated with the combination of IFNα and anti-GBM. We and others have previously demonstrated an elevated production of IL-12, which is important for T cell activation and proliferation, across multiple murine lupus models (53–57).
Importantly, all of these changes appear to be occurring at the level of the end-organ, because parallel changes were not observed in the degree of systemic immune responsiveness to the administered rabbit Ig (data not shown). Given the local expression of IFN-I within the end-organs (rather than infiltrating leukocytes), and its apparent association with disease severity in immune nephritis, it is important to carefully evaluate the role of renal-expressed IFN-I in spontaneous lupus nephritis.
We propose that the anti-GBM model of experimentally induced nephritis offers an accessible snapshot into the pathogenesis of spontaneous lupus nephritis, offering us the means to assess the initiation and progression of disease within a defined time frame. Specifically, in the 2-wk experimental model of nephritis, the anti-GBM challenge is targeting the kidney directly via exogenous kidney-specific autoantibodies. The immune response, which leads to nephritis, appears to parallel events that take place in spontaneous models, including a requirement for T cells and myeloid infiltration. However, eventually the inflammation is resolved in nonautoimmune prone strains, which is likely due to the clearance of the immune complexes within the kidney, in the absence of an additional challenge. This is unlike the nephritis in spontaneous models, where there is a continuous cycle of inflammatory challenge, with the endogenous Abs and immune complexes persisting far beyond a 2 wk period.
We have previously described a multistep model in which at least three genetically determined pathways lead to the development of SLE (58–60). The first stage of disease development results in a loss of tolerance to nuclear Ags and the production of autoantibodies, as exemplified by Sle1. This is not sufficient to drive full disease pathology. The second pathway or checkpoint involves the peripheral dysregulation in innate and/or adaptive immunity, including myeloid cell hyperactivity or up-regulated TLR expression, as exemplified by Sle3 and Yaa. The third pathway or checkpoint dictates the severity of end organ damage, once pathogenic autoantibodies are formed.
The reported findings indicate that at least the third pathway or checkpoint that is operative within the end organs is susceptible to IFN-I driven amplification. Whether IFN-I has the capacity to drive any of the other checkpoints in lupus development warrants further investigation. The suggestion that locally produced IFN-I could be critical in driving end-organ pathology in autoimmunity could have profound implications toward our understanding of how IFN-I contributes to SLE, and how we manage it clinically.
Acknowledgments
We thank Jose Casco for breeding mice, Elizabeth Curry and Angela Mobley from the UT Southwestern Flow Cytometry Core, and Sun-Hee Hwang, Ferdicia Carr-Johnson (UTSW) and Jill Plants (BIIR) for technical assistance.
Footnotes
This work was supported by funding from the National Institutes of Health, Medimmune and the Alliance for Lupus Research.
Abbreviations used in this paper: SLE, systemic lupus erythematosus; IFN-I, type I IFN; BUN, blood urea nitrogen; GBM, glomerular basement membrane; ADV, adenovirus.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science. 1982;216:429–431. doi: 10.1126/science.6176024. [DOI] [PubMed] [Google Scholar]
- 2.Preble OT, Rothko K, Klippel JH, Friedman RM, Johnston MI. Interferon-induced 2′-5′ adenylate synthetase in vivo and interferon production in vitro by lymphocytes from systemic lupus erythematosus patients with and without circulating interferon. J. Exp. Med. 1983;157:2140–2146. doi: 10.1084/jem.157.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vallin H, Perers A, Alm GV, Ronnblom L. Anti-dsDNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-α inducer in systemic lupus erythematosus. J. Immunol. 1999;163:6306–6313. [PubMed] [Google Scholar]
- 4.Vallin H, Blomberg S, Alm GV, Cederblad B, Ronnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-α (IFN-α) production acting on leucocytes resembling immature dendritic cells. Clin. Exp. Immunol. 1999;115:196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han GM, Chen SL, Shen N, Ye S, Bao CD, Gu YY. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 2003;4:177–186. doi: 10.1038/sj.gene.6363966. [DOI] [PubMed] [Google Scholar]
- 6.Crow MK, Wohlgemuth J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 2003;5:279–287. doi: 10.1186/ar1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crow MK, Kirou KA, Wohlgemuth J. Microarray analysis of interferon-regulated genes in SLE. Autoimmunity. 2003;36:481–490. doi: 10.1080/08916930310001625952. [DOI] [PubMed] [Google Scholar]
- 8.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun D, Geraldes P, Demengeot J. Type I interferon controls the onset and severity of autoimmune manifestations in lpr mice. J. Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 12.Kono DH, Baccala R, Theofilopoulos AN. Inhibition of lupus by genetic alteration of the interferon-α/β receptor. Autoimmunity. 2003;36:503–510. doi: 10.1080/08916930310001624665. [DOI] [PubMed] [Google Scholar]
- 13.Hron JD, Peng SL. Type I IFN protects against murine lupus. J. Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 14.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-α induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J. Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 15.Pawar RD, Patole PS, Zecher D, Segerer S, Kretzler M, Schlondorff D, Anders HJ. Toll-like receptor-7 modulates immune complex glomerulonephritis. J. Am. Soc. Nephrol. 2006;17:141–149. doi: 10.1681/ASN.2005070714. [DOI] [PubMed] [Google Scholar]
- 16.Patole PS, Grone HJ, Segerer S, Ciubar R, Belemezova E, Henger A, Kretzler M, Schlondorff D, Anders HJ. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J. Am. Soc. Nephrol. 2005;16:1326–1338. doi: 10.1681/ASN.2004100820. [DOI] [PubMed] [Google Scholar]
- 17.Anders HJ, Vielhauer V, Eis V, Linde Y, Kretzler M, Perez de Lema G, Strutz F, Bauer S, Rutz M, Wagner H, et al. Activation of toll-like receptor-9 induces progression of renal disease in MRL-Fas(lpr) mice. FASEB J. 2004;18:534–536. doi: 10.1096/fj.03-0646fje. [DOI] [PubMed] [Google Scholar]
- 18.Fairhurst AM, Mathian A, Connolly JE, Wang A, Gray HF, George TA, Boudreaux CD, Zhou XJ, Li QZ, Koutouzov S, Banchereau J, Wakeland EK. Systemic IFN-α drives kidney nephritis in B6.Sle123 mice. Eur. J. Immunol. 2008;38:1948–1960. doi: 10.1002/eji.200837925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anders HJ, Banas B, Linde Y, Weller L, Cohen CD, Kretzler M, Martin S, Vielhauer V, Schlondorff D, Grone HJ. Bacterial CpG-DNA aggravates immune complex glomerulonephritis: role of TLR9-mediated expression of chemokines and chemokine receptors. J. Am. Soc. Nephrol. 2003;14:317–326. doi: 10.1097/01.asn.0000042169.23931.73. [DOI] [PubMed] [Google Scholar]
- 20.Anders HJ. A Toll for lupus. Lupus. 2005;14:417–422. doi: 10.1191/0961203305lu2102rr. [DOI] [PubMed] [Google Scholar]
- 21.Fu Y, Du Y, Mohan C. Experimental anti-GBM disease as a tool for studying spontaneous lupus nephritis. Clin. Immunol. 2007;124:109–118. doi: 10.1016/j.clim.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Xie C, Zhou XJ, Liu X, Mohan C. Enhanced susceptibility to end-organ disease in the lupus-facilitating NZW mouse strain. Arthritis Rheum. 2003;48:1080–1092. doi: 10.1002/art.10887. [DOI] [PubMed] [Google Scholar]
- 23.Xie C, Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J. Immunol. 2004;172:5047–5055. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 24.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Liu Y, Xie C, Zhu J, Kreska D, Morel L, Mohan C. Deficiency of type I interferon contributes to Sle2-associated component lupus phenotypes. Arthritis Rheum. 2005;52:3063–3072. doi: 10.1002/art.21307. [DOI] [PubMed] [Google Scholar]
- 26.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, Casco J, Li QZ, Connolly JE, Wakeland EK. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PR, Brown GD, Geldhof AB, Martinez-Pomares L, Gordon S. Pattern recognition receptors and differentiation antigens define murine myeloid cell heterogeneity ex vivo. Eur. J. Immunol. 2003;33:2090–2097. doi: 10.1002/eji.200324003. [DOI] [PubMed] [Google Scholar]
- 29.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–596. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 30.Deblandre GA, Leo O, Huez GA, Wathelet MG. CD69 is expressed on Daudi cells in response to interferon-α. Cytokine. 1992;4:36–43. doi: 10.1016/1043-4666(92)90034-o. [DOI] [PubMed] [Google Scholar]
- 31.Lanier LL, Buck DW, Rhodes L, Ding A, Evans E, Barney C, Phillips JH. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J. Exp. Med. 1988;167:1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Testi R, Phillips JH, Lanier LL. Leu 23 induction as an early marker of functional CD3/T cell antigen receptor triggering: requirement for receptor cross-linking, prolonged elevation of intracellular [Ca2+] and stimulation of protein kinase C. J. Immunol. 1989;142:1854–1860. [PubMed] [Google Scholar]
- 33.Wang A, Fairhurst AM, Tus K, Subramanian S, Liu Y, Lin F, Igarashi P, Zhou XJ, Batteux F, Wong D, Wakeland EK, Mohan C. CXCR4/ CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J. Immunol. 2009;182:4448–4458. doi: 10.4049/jimmunol.0801920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng X, Wu H, Grossman JM, Hanvivadhanakul P, FitzGerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 35.Dall’era MC, Cardarelli PM, Preston BT, Witte A, Davis JC., Jr Type I interferon correlates with serological and clinical manifestations of SLE. Ann. Rheum. Dis. 2005;64:1692–1697. doi: 10.1136/ard.2004.033753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 37.Wenzel J, Tuting T. Identification of type I interferon-associated inflammation in the pathogenesis of cutaneous lupus erythematosus opens up options for novel therapeutic approaches. Exp. Dermatol. 2007;16:454–463. doi: 10.1111/j.1600-0625.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 38.Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, Grunze H, Lieb K. Interferon α (IFNα) and psychiatric syndromes: a review. Prog. Neuropsychopharm. Biol. Psy. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 39.Patole PS, Pawar RD, Lech M, Zecher D, Schmidt H, Segerer S, Ellwart A, Henger A, Kretzler M, Anders HJ. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol. Dial. Transplant. 2006;21:3062–3073. doi: 10.1093/ndt/gfl336. [DOI] [PubMed] [Google Scholar]
- 40.Anders HJ. Innate pathogen recognition in the kidney: toll-like receptors, NOD-like receptors, and RIG-like helicases. Kidney Int. 2007;72:1051–1056. doi: 10.1038/sj.ki.5002436. [DOI] [PubMed] [Google Scholar]
- 41.Anders HJ, Schlondorff D. Toll-like receptors: emerging concepts in kidney disease. Curr. Opin. Nephrol. Hypertens. 2007;16:177–183. doi: 10.1097/MNH.0b013e32803fb767. [DOI] [PubMed] [Google Scholar]
- 42.Allam R, Pawar RD, Kulkarni OP, Hornung V, Hartmann G, Segerer S, Akira S, Endres S, Anders HJ. Viral 5′-triphosphate RNA and non-CpG DNA aggravate autoimmunity and lupus nephritis via distinct TLR-independent immune responses. Eur. J. Immunol. 2008;38:3487–3498. doi: 10.1002/eji.200838604. [DOI] [PubMed] [Google Scholar]
- 43.Fu Y, Xie C, Chen J, Zhu J, Zhou H, Thomas J, Zhou XJ, Mohan C. Innate stimuli accentuate end-organ damage by nephrotoxic antibodies via Fc receptor and TLR stimulation and IL-1/TNF-α production. J. Immunol. 2006;176:632–639. doi: 10.4049/jimmunol.176.1.632. [DOI] [PubMed] [Google Scholar]
- 44.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J. Exp. Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sironi M, Guglielmotti A, Polentarutti N, Fioretti F, Milanese C, Romano M, Vigini C, Coletta I, Sozzani S, Bernasconi S, et al. A small synthetic molecule capable of preferentially inhibiting the production of the CC chemokine monocyte chemotactic protein-1. Eur. Cytokine Network. 1999;10:437–442. [PubMed] [Google Scholar]
- 46.Perez de Lema G, Maier H, Franz TJ, Escribese M, Chilla S, Segerer S, Camarasa N, Schmid H, Banas B, Kalaydjiev S, et al. Chemokine receptor Ccr2 deficiency reduces renal disease and prolongs survival in MRL/lpr lupus-prone mice. J. Am. Soc. Nephrol. 2005;16:3592–3601. doi: 10.1681/ASN.2005040426. [DOI] [PubMed] [Google Scholar]
- 47.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2006;65:209–215. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulkarni O, Pawar RD, Purschke W, Eulberg D, Selve N, Buchner K, Ninichuk V, Segerer S, Vielhauer V, Klussmann S, Anders HJ. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 49.Chan RW, Lai FM, Li EK, Tam LS, Chow KM, Lai KB, Li PK, Szeto CC. Intrarenal cytokine gene expression in lupus nephritis. Ann. Rheum. Dis. 2007;66:886–892. doi: 10.1136/ard.2006.063123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zoja C, Liu XH, Donadelli R, Abbate M, Testa D, Corna D, Taraboletti G, Vecchi A, Dong QG, Rollins BJ, et al. Renal expression of monocyte chemoattractant protein-1 in lupus autoimmune mice. J. Am. Soc. Nephrol. 1997;8:720–729. doi: 10.1681/ASN.V85720. [DOI] [PubMed] [Google Scholar]
- 51.Zavala F, Masson A, Hadaya K, Ezine S, Schneider E, Babin O, Bach JF. Granulocyte-colony stimulating factor treatment of lupus autoimmune disease in MRL-lpr/lpr mice. J. Immunol. 1999;163:5125–5132. [PubMed] [Google Scholar]
- 52.Rutella S. Granulocyte colony-stimulating factor for the induction of T-cell tolerance. Transplantation. 2007;84:S26–S30. doi: 10.1097/01.tp.0000269611.66517.bf. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, Wakeland EK, Mohan C. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J. Clin. Invest. 2005;115:1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sela U, Sharabi A, Dayan M, Hershkoviz R, Mozes E. The role of dendritic cells in the mechanism of action of a peptide that ameliorates lupus in murine models. Immunology. 2009;128:e395–e405. doi: 10.1111/j.1365-2567.2008.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J. Immunol. 2006;177:5878–5889. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 57.Decker P, Kotter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology. 2006;45:1087–1095. doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 58.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv. Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 59.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 60.Kanta H, Mohan C. Three checkpoints in lupus development: central tolerance in adaptive immunity, peripheral amplification by innate immunity and end-organ inflammation. Genes Immun. 2009;10:390–396. doi: 10.1038/gene.2009.6. [DOI] [PubMed] [Google Scholar]