Abstract
Reproduction is required for the survival of all animals, yet few reproductive genes have been shown to have a conserved requirement for fertility across the animal kingdom. Remarkably, the RNA binding protein BOULE, the oldest member of the DAZ (Deleted in AZoospermia) family of genes, appears to have maintained its conserved functional motif and spermatogenic expression from insects to humans. Boule mutations lead to a pachytene meiotic arrest before metaphase in Drosophila males and C. elegans females, and human BOULE can restore meiosis in the fly testis, suggesting a conserved meiotic function of human BOULE. However, the physiological function of BOULE in mammals is not yet known. We generated Boule knockout mice and found it to be required only for spermatogenesis, as in Drosophila. Interestingly, meiosis completed normally in the absence of Boule, and haploid round spermatids were readily detected. However, round spermatids did not progress beyond step 6, revealing a novel role for Boule in spermiogenesis, the differentiation of round spermatids into mature spermatozoa. Expression of key regulators of spermiogenesis was unaffected in Boule−/− mice, suggesting that Boule regulates germ-cell differentiation through a novel pathway.
INTRODUCTION
Animal models have contributed greatly to our understanding of the reproductive development processes underlying human infertility. However, genes associated with reproductive function diverge fast during evolution, and out of more than 400 mouse infertility mutations, only a small number have been implicated in human infertility (1–3). One of the most common human infertility factors in men is the DAZ (Deleted in AZoospemria) gene cluster on the Y chromosome (4). While DAZ is not absolutely required for spermatogenesis (5), DAZ deletions are present in 10–15% of azoospermic men (no sperm in semen), making it the most common known molecular cause of male infertility without pleiotropic effects (4). The DAZ cluster belongs to a reproductive-specific family of RNA-binding proteins comprised of DAZ, DAZL (DAZ-Like) and BOULE, whose homologs are involved in different stages of reproductive development in diverse species (4,6–10). Differentiation of human ES cells into primordial germ cells (PGCs) is enhanced by overexpression of DAZ family proteins, highlighting their important role in human gametogenesis (11), while evolutionary and comparative analysis of the DAZ family has provided the first molecular evidence for the conservation of the process of spermatogenesis between humans and insects (6,10). BOULE is the ancestral member of the DAZ family, with orthologs in nearly all metazoans, while DAZL homologs are present only in vertebrates (10,12) and the DAZ gene cluster is restricted to humans and higher primates (13–15).
DAZL homologs are expressed in both sexes and are important in primordial germ cells in zebrafish, Xenopus and mice (7,9,16–18). In mice, Dazl may be a key intrinsic factor necessary for PGCs to be competent to respond to extrinsic meiosis-inducing signals (19). In a mixed genetic background, however, some Dazl null germ cells exhibit defects in spermatogonial differentiation and meiotic progression, suggesting additional spermatogenic functions of Dazl (20,21). BOULE homologs are conserved from C. elegans through humans, and are primarily expressed in only one sex, predominantly males (8,10,22,23). Functional studies have shown BOULE homologs to be important for progression beyond pachynema of prophase I of meiosis in C. elegans oocytes and Drosophila testes (6,8,24). Human BOULE can function in the Drosophila testis to restore meiosis in a boule mutant background, suggesting that BOULE is functionally conserved among animals (25).
Though functions of DAZ and DAZL have been examined in mammals, the physiological role of mammalian BOULE has not yet been directly explored. Recently, Kee et al. (11) showed that ectopic expression of BOULE in human ES cells enhances their differentiation into PGCs, primarily in female cells. However, similar to studies in the fly (25), this study did not address the physiological role of BOULE in mammals. Here we report the characterization of Boule knockout mice, and describe a male-specific germ-cell requirement, while Boule null females are fertile. Surprisingly, we find that Boule is not required for meiosis in mammals, but its deletion produces a complete spermatogenic arrest during the round spermatid stage, prior to elongation. Expression of known regulators of spermiogenesis is not affected by the loss of Boule, indicating that Boule does not act directly through these pathways but instead acts through a novel spermiogenesis regulatory pathway.
RESULTS
Boule−/− mice are male sterile and azoospermic
In order to determine the role of Boule in mammalian spermatogenesis, we generated a Boule null allele in mice using targeted homologous recombination (Shah et al., in submission). Boule−/− mice are viable, and homozygous animals were produced in expected Mendelian ratios from heterozygous matings. Using sera we generated against the N-terminus of human BOULE, we detected two bands in wild-type and heterozygous testes, but not in homozygous mutants, and pre-incubation with the immunizing peptide failed to detect both bands (Fig. 1B). The lower band corresponds to the predicted Boule size of 33 kDa, while the upper band of ∼45 kDa is a newly identified Boule isoform in the mouse testis (Fig. 1B). Neither isoform was detected in null animals. Immunohistochemistry with this anti-sera recapitulated normal Boule expression in wild-type mice, with high levels in spermatocytes that peaked immediately before metaphase, dropped during meiotic division, increased in early round spermatids, tapering through step 5, after which signal was no longer detectable (Fig. 1A (10)). Boule expression in homozygous mutants was not detected by immunohistochemistry, confirming the generation of a null allele (Fig. 1A, bottom row). Additionally, the loss of Boule had no significant effect on spermatocyte and round spermatid expression of the closely related Dazl protein (Fig. 1A).
Figure 1.
Generation of Boule null mice. (A) Immunohistochemistry of wild-type and homozygous adult testis sections shows no detectable Boule protein in mutant animals. Preimmune sera was used as a control. Dazl expression in spermatocytes is unaffected by the loss of Boule, though may be reduced in spermatids. Scale bar: preimmune and α-Boule, 10 µm; α-Dazl, 5 µm. (B) Immunoblot of adult total testis lysates against Boule shows that no Boule protein is present in homozygous mutant animals. Two isoforms of ∼45 and 33 kDa (arrows) are detected only in wild-type and heterozygous animals by antisera generated against the N-terminal portion of Boule, but not by preimmune sera or antisera pre-incubated with the immunizing peptide (blocked). (C) Testes from homozygous mutant mice are 48% smaller by weight compared with those from wild-type and heterozygote mice.
Homozygous null females showed no obvious defects and were fertile, and heterozygote males were indistinguishable from wild-type males. Boule−/− males mated to wild-type females, however, failed to result in any pregnancies, despite the formation of copulation plugs. Testes of mutant animals were 48% smaller than wild-type (wild-type = 112.5 ± 16.1 mg, n = 12; mutant = 58.0 ± 12.3 mg, n = 7), though body weight was normal (Fig. 1C). Epididymal sperm counts of Boule null animals yielded no detectable sperm, indicating that the Boule−/− mice were azoospermic, similar to boule mutant flies and some men with DAZ deletions (4,6).
Characteristic cellular associations are seen in mouse seminiferous tubules, allowing convenient staging of spermatogenesis (26). Surprisingly, spermatocytes at metaphase were clearly visible by hematoxylin and eosin (H&E) staining in stage XII tubules of Boule−/− animals (Fig. 2A and B), in contrast to the pachytene arrest seen in boule mutant flies (6). Round spermatids were present in stage I–VII tubules (Fig. 2C and D), and Periodic acid-Schiff (PAS) staining revealed acrosome-like structures in these cells (see Supplementary Material, Fig. S1). Multinucleate giant cells indicative of apoptosis (27) were present in stage VI–X tubules of Boule−/− animals, and occasionally in stage XI–XII tubules (Fig. 2F), and no elongating spermatids were seen as in wild-type (Fig. 2E). Unlike other spermatogenic arrest phenotypes, we did not see an increase in apoptosis throughout the mutant testes by TUNEL staining, but only detected apoptosis in the large cysts (Fig. 2G and H). Progressive germ-cell loss did not occur with age (up to 1 year), suggesting that Boule is specifically required for differentiation and not germ-cell maintenance.
Figure 2.
Histological defects in Boule null mice. (A–F) H&E stained adult testis sections. Representative wild-type and mutant sections showing stage XII (A and B), stage II–III (C and D) and stage IX (E and F) seminiferous tubules. Metaphase cells are clearly visible in stage XII homozygous null tubules, magnified in the inset. Round spermatids are present in mutant stage II–III, magnified in the inset. By stage IX, multinucleate giant cells populate the lumen of mutant tubules (asterisks) instead of elongating spermatids, as seen in wild-type tubules. (G and H) TUNEL staining of testis sections from wild-type and mutant adult animals. Multinucleate cysts are apoptotic, but general apoptosis is not increased in mutants. Me, metaphase spermatocyte; R spd, round spermatid; E spd, elongating spermatid; Lp, late pachytene. Scale bar: 50 µm; insets 5 µm.
Meiosis occurs normally in the absence of Boule
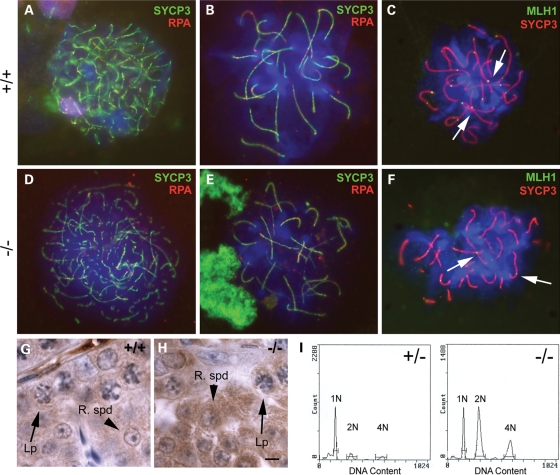
Although metaphase spermatocytes are present, it was possible that key events of meiotic prophase might be affected. To test this, we looked at immunofluorescent staining of meiotic chromosome spreads using specific markers (28). Synaptonemal complex protein 3 (SYCP3) marks the synaptonemal complex while replication protein A (RPA) and MutL homolog 1 (MLH1) mark early recombination nodules and later chiasmata, respectively (28). Chromosome pairing was normal and early recombination nodules formed properly in mutant animals when compared with wild-type (Fig. 3A and D). As prophase I progressed, mutant chromosomes continued to condense and were indistinguishable from wild-type spermatocytes (Fig. 3B and E). Normal late recombination nodules and condensed bivalents were present in mutant pachytene spermatocytes (Fig. 3C and F, arrows), indicating that prophase I of meiosis is normal in Boule−/− mice.
Figure 3.
Meiosis is normal in Boule−/− mice. (A–F) Immunofluorescent chromosome spread analysis of wild-type (A–C) and mutant (D–F) spermatocytes. SYCP3 (green) marks homologously paired synaptonemal complexes and RPA (red) marks early recombination nodules (A, B, D, E). Leptotene chromosomes begin to condense normally (D) and show successful homologous pairing by zygonema in mutant animals (E), as compared with wild-type animals (A and B). Normal pachytene late recombination nodules (arrows), eventual sites of chiasmata, are evident on wild-type and mutant pachytene chromosomes (C and F), marked by MLH1 (green). (G and H) Immunohistochemistry for Cdc25a shows that protein expression is unaffected in mutant animals (H) when compared with wild-type animals (G). Expression in both late pachytene spermatocytes (Lp, arrows) and round spermatids (R spd, arrowheads) is normal. (I) Cell cycle analysis from adult heterozygous (left) and homozygous mutant (right) testes shows haploid cells (1N) are present in mutant testes, with no aneuploid meiotic products. Scale bar (G, H): 5 µm.
In Drosophila, translation of the metaphase-promoting Cdc25 homolog twine is thought to be controlled by boule, and loss of twine protein in boule mutant flies leads to the observed pre-metaphase arrest (29). To determine whether Cdc25 translation was similarly disrupted in Boule−/− mice, we examined Cdc25 expression by immunohistochemistry. Mice have three Cdc25 homologs, only two of which, Cdc25a and Cdc25c, are expressed in testis germ cells (30,31). Expression of both Cdc25a and Cdc25c in pachytene spermatocytes was not affected in Boule−/− mice (Fig. 3G and H, data not shown), further confirming the normal progression of meiosis. Additionally, the proportion of seminiferous tubules containing metaphase spermatocytes in Boule−/− mice was similar to that seen in wild-type mice (3.9% in wild-type and 4.0% in mutant), indicating that progression to metaphase is not a rare event, and is unaffected by the loss of Boule.
To determine whether meiotic divisions occurred normally, we performed cell cycle analysis on adult testes. If chromosomes were mis-segregating in Boule−/− animals, we expected to see a smear of aneuploid cells centered on 1N. Instead, we saw a distinct haploid (1N) population (Fig. 3I, right). Notably, in null mice the amount of diploid spermatogonia/somatic cells (2N) and tetraploid spermatocytes (4N) was higher relative to the haploid cells (1N) when compared with heterozygotes (Fig. 3I, left). This is likely due to the absence of elongating spermatids in mutant animals, which caused a decrease in the total percentage of haploid cells and a corresponding increase in the percentage of non-haploid cells, and does not reflect a problem with meiosis. Together, these data show that Boule is not required for meiosis in mammals, in contrast to previously proposed models.
Global arrest of spermatogenesis at the round spermatid stage
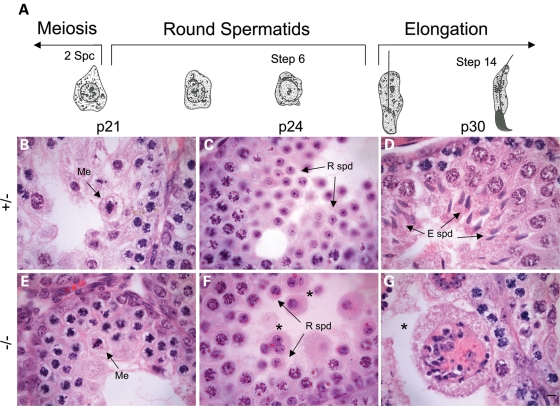
To determine when spermatogenic defects first arise, we examined prepubertal testis sections. The first wave of spermatogenesis is highly synchronized and begins after birth. Prophase spermatocytes first appear at postnatal day 10 (p10), metaphase is reached by p21, elongating spermatids are present at p30, with fully mature spermatozoa not present until p35 (32). As in heterozygotes, meiosis occurred normally at p21 and round spermatids were present by p24 in Boule−/− testes (Fig. 4), though occasionally multinucleate cells were also present (Fig. 4F). Notably, we noticed that some animals were developmentally delayed and by day 24 still did not have round spermatids. We observed this variability in both heterozygote and mutant mice, but this phenomenon occurs approximately twice as often in Boule−/− mice. However, when histologically similar samples are used for cell cycle analysis, cell-type ratios between heterozygote and Boule null testes are still comparable at both p20 and p24 (data not shown). By p30, elongating spermatids were abundant in heterozygotes but were completely absent in mutant mice, similar to the defects seen in adults (Fig. 4D and G). Thus, Boule is required specifically during the round spermatid stage both during the first wave of spermatogenesis and in later spermatogenesis in the adult. The greater tendency for developmental delay in Boule−/− mice may indicate an additional function for Boule in spermatocytes, though the primary requirement is in round spermatids.
Figure 4.
Boule is required during the first wave of spermatogenesis. (A) Schematic representation of pre-pubertal spermatid development in the first wave of spermatogenesis. H&E stained sections from heterozygous (B–D) and mutant (E–G) postnatal mice indicate that Boule is not required until round spermatids are present. Images are representative of the most advanced tubules at each time point. Loss of Boule does not affect entry into meiotic divisions at p21, visualized by spermatocytes in metaphase (B and E, arrows). Round spermatids are abundant at day 24 (C and F, arrows), and small multinucleate cysts are seen in mutants (asterisks). By day 30, heterozygous animals have elongating spermatids (D, arrows), while mutant testes have numerous multinucleate giant cells (E, asterisk). Me, metaphase spermatocyte; R spd, round spermatid; E spd, elongating spermatid. Scale bar, 5 µm.
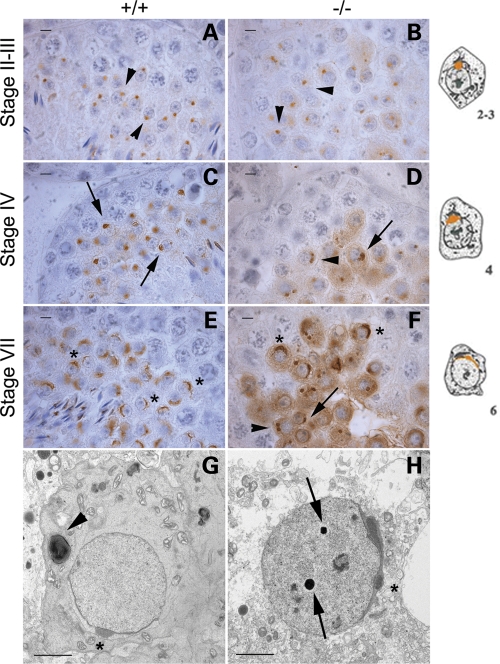
To pinpoint the exact arrest point in Boule−/− mice, we stained adult testis sections for the acrosome marker SP-10 (33,34). Spermiogenesis is divided into 16 steps, with the round spermatid steps distinguishable by the shape of the developing acrosome (26). Spermatid acrosomes in stage II–III mutant tubules (step 2–3 spermatids) showed the normal round, vesicular shape (Fig. 5A and B), but by stage IV, not all spermatids had the flattened acrosome morphology indicative of wild-type step 4, and many had two pre-acrosomal vesicles that failed to fuse (Fig. 5C and D). By stage VII, wild-type spermatids had a large arc-shaped acrosome (steps 6–7), but mutants had a range of morphologies. Most round spermatids had either step 2 acrosomes or two pre-acrosomal vesicles, some were suggestive of step 4, and a few had developed to step 6, but no further (Fig. 5E and F). These results indicate that most round spermatids do not mature past step 2 of spermiogenesis, and the final arrest occurs at step 6 in Boule−/− mice.
Figure 5.
Spermiogenesis does not proceed beyond step 6 in the absence of Boule. (A–F) Immunohistochemistry for the acrosome marker SP-10 in adult testis sections. Cartoon representations of SP-10 expression in spermatids at each step are shown at right. Step 2 acrosomes are small and rounded (A, arrowheads), then widen and begin to flatten during step 4 (C, arrows). By step 6, the acrosome is spread across the nucleus (E, asterisks). In mutant sections, step 2 is normal (B), but not all acrosomes progress to step 4 (D). Mutant stage VII tubules have spermatids with acrosomes indicative of steps 2, 4 and 6 (F, arrowhead, arrow and asterisks, respectively). (G and H) EM analysis shows that mutant spermatids are able to reach step 6 morphology (H, asterisk), similar to that seen in wild-type spermatids (G, asterisk). Electron-dense structures are often seen in mutant nuclei (H, arrows), but are infrequent in wild-type nuclei. A developing axoneme is seen adjacent to the wild-type nucleus (G, arrowhead), but these are rare in mutant spermatids. Scale bar (A–F): 5 µm; scale bar (G and H): 2 µm.
To further characterize the spermiogenesis defects, we looked at round spermatids by electron microscopy (EM). We observed similar acrosome defects in mutants and some normal step 6 acrosomes, but nothing more advanced (Fig. 5G and H). Additionally, the multinucleate cysts were clearly syncytia, with the multiple nuclei within each cyst showing a range of acrosome arrest morphologies, as detected by SP-10 staining. Some mutant spermatid nuclei had dark electron-dense structures not seen in wild-type animals (Fig. 5H, arrows), while axonemes and forming flagella were rare.
Boule is a key regulator of spermiogenesis, but does not act upstream of known regulators
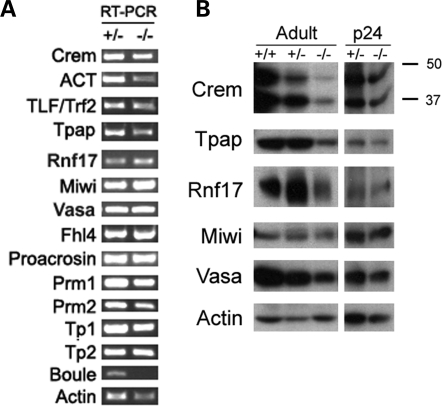
We next used RT–PCR to examine if Boule is required for expression of known spermiogenesis genes. While we could not quantify levels of expression with this assay, we qualitatively determined whether transcription was occurring and RNA was present. Though protein levels of the master spermiogenesis transcription factor Crem (Cyclic-AMP Response Element Modulator) (27,35) were reduced in Boule−/− mice (Fig. 6B), RNA of its target genes were present (Fig. 6A, Prm1, Prm2, TP1, TP2), indicating that transcription is active. In addition to the master transcription program, we detected RNA of other critical regulators of spermiogenesis (TLF/TRF2, Tpap, Rnf17 and Miwi) (36–40) indicating that Boule is not required for transcription of those genes.
Figure 6.
Boule does not act through known spermiogenesis regulators. (A) RT–PCR analysis of spermiogenesis genes indicates active transcription. Downstream genes of the transcription factors Crem and TLF/Trf2 (Tp1, Tp2, Prm1, Prm2) as well as other important spermiogenesis genes are present in mutants. Actin indicates equal loading, while Boule shows that lack of transcript in the null mutant can be verified by RT–PCR. (B) Immunoblots of adult and p24 whole testis lysates show reduced levels of Crem, Rnf17 and Tpap in adult mutants, but not at p24. Actin is a control for equal loading.
DAZ family proteins are thought to activate the translation of target mRNAs in germ cells (41,42). If Boule is necessary for stimulation of translation of key spermiogenesis regulators, we would expect to find reduced protein levels in total testis extracts from Boule−/− mice, despite normal transcription. Interestingly, we detected a reduced amount of protein in adults of two genes necessary for spermiogenesis, testis-specific poly(A) polymerase (Tpap), and Rnf17 (37,39). Since Boule−/− mice arrest at the round spermatid stage, we asked if Tpap and Rnf17 proteins were absent in mutant round spermatids. Immunohistochemistry for both Tpap and Rnf17 showed normal expression patterns in Boule−/− mice, and protein was specifically detected in round spermatids (see Supplementary Material, Fig. S2). Expression of other key regulators for which antibodies were available was also normal (see Supplementary Material, Fig. S2). Since both Tpap and Rnf17 are highly expressed in haploid round spermatids, which were seen in lower relative numbers in adult mutant animals (Fig. 3I), the reduced protein levels detected by immunoblot could be due to the altered ratio of cell types in Boule−/− mice, and not a biological effect of the loss of Boule. To test this, we looked at protein expression in 24-day-old mice, when histological defects first arise in Boule−/− mice (Fig. 4), but relative cell type ratios are still comparable between mutants and littermate controls. Given the developmental variation we noted above, we used samples from mice that were matched histologically for this analysis. We saw no difference in protein levels or expression patterns between p24 heterozygotes and mutants for any gene examined (Fig. 6B, Supplementary Material, Fig. S3), indicating that the reduced protein levels seen in adults are a consequence of the reduced number of spermatids in Boule−/− mice, and not a direct effect of Boule on their translation. We therefore conclude that Boule does not act upstream of any known spermiogenesis regulators, but instead suggest that Boule acts through a novel pathway to regulate round spermatid differentiation.
DISCUSSION
Here we describe the function of Boule in mice, the last DAZ family member to be examined in mammals. Overexpression experiments have implicated human BOULE in both meiosis regulation and female PGC differentiation, but whether these functions were physiologically relevant was unknown (11,25). We found that Boule is required only for spermatogenesis, while null females exhibit normal fertility, suggesting that Boule is not essential for female germ-cell development in mice. Additionally, Boule is not necessary for meiosis or Cdc25 translation as in Drosophila (Fig. 3). Round spermatids in Boule null mice did not develop past step 6 of spermiogenesis, and often showed acrosome defects by step 2 (Fig. 5). These defects first occurred by p24 in the first wave of spermatogenesis, corresponding to the round spermatid stage (Fig. 4). Major abnormalities were not observed earlier, revealing a previously unexplored function of Boule in spermiogenesis.
Like DAZ and DAZL, BOULE is required for gametogenesis in mammals. Its specific requirement only in males is similar to DAZ in humans, providing a mammalian model to study DAZ-mediated human infertility. DAZ family genes show a remarkable ability to functionally replace each other in transgenic rescue studies in multiple species (7,25,43), suggesting that DAZ proteins function by a common mechanism. A better understanding of mouse Boule will help shed light on the role of DAZ in human infertility.
Mammalian Boule is necessary only for spermiogenesis, independent of known regulators
Only a handful of other genes have been identified as key regulators of spermiogenesis, with null phenotypes strikingly similar to that of Boule−/− mice. These genes are Crem, the master transcription factor for spermiogenesis; TLF/TRF2, a testis-specific TATA-binding protein-related factor; TPAP, a testis-specific cytoplasmic poly(A) polymerase; GRTH/Ddx25, a gonadotropin-regulated RNA helicase; Rnf17, a component of a newly identified spermatid cytoplasmic nuage; and Miwi, a mouse homolog of Piwi, a subclass of argonaute proteins that bind unique small RNAs found in germ cells (27,35–40,44). We found detectable RNA of these and other spermiogenesis genes, indicating that Boule is not necessary for transcription (Fig. 6A). Crem protein levels were reduced, however, which could explain the observed spermiogenesis arrest. While we cannot rule this out, more comparable levels of Crem in p24 mutants and the presence of its downstream targets in adults indicate that Crem-mediated transcription is active, and not the likely cause of the null phenotype. Protein expression of other regulators was also normal (Fig. 6B, Supplementary Material, Figs S2 and S3), suggesting that Boule is not required for their translation and does not act directly through these known regulatory pathways.
A notable feature of spermiogenesis is that many mRNAs are transcribed several days prior to their translation, necessitating a complex translational regulation network that allows mRNAs to be translated at precisely the right time (45–47). Much of this regulation is thought to occur in the chromatoid body, an enigmatic perinuclear structure to which many RNA binding proteins are localized (48). How these mRNAs are stored and properly translated is not known. Tpap is involved in posttranscriptional regulation, though which RNAs it polyadenylates is not yet known (37). GRTH/Ddx25 is involved in mRNA shuttling and translation, and its loss causes a moderate disruption of chromatoid bodies (44,49). Rnf17 is present in other germ-cell granules with unknown function (39). Miwi has been localized to the chromatoid body in round spermatids, suggesting a role in mRNA processing or translation (50,51). It has also been shown to bind a kinesin motor protein and transport mRNA for ACT (Activator of Crem in Testis) which is needed for proper Crem function (52). A class of small RNAs that interact with Miwi homologs, termed piRNAs (Piwi-interacting RNAs), have been found specifically in the germ line (53,54), some of which act to repress transposons in spermatogonia in conjunction with Miwi2, while others are present in spermatids and associate with polysomes (50), though their function remains unknown. These may somehow be involved in chromatoid body function and the regulation of spermiogenesis, possibly in the same pathway as Boule. Since Boule−/− mice show a similar phenotype to animals null for these genes and Boule is a known RNA-binding protein, it is likely that Boule interacts with these other RNA regulatory pathways in some way.
Conserved and novel roles of BOULE
The differing requirement of Boule in mammalian and fly spermatogenesis raises several questions: has mammalian Boule lost its meiotic regulatory role, or does it do so redundantly with another protein? Is the spermiogenesis function of Boule a novel development in mammals, or is it also present in Drosophila?
Though the specific arrest in Boule null flies and mice differs, our data do not contradict the model of a conserved meiosis role. Such a function is not required in mammals, and we propose that Boule and Dazl redundantly regulate meiosis in mammals. The similar pachytene requirement of Boule in both Drosophila and C. elegans, together with evidence that a human BOULE transgene can restore meiotic division in boule mutant flies, suggests a conservation of this function in animals (6,8,25). Additionally, mouse Dazl is known to be expressed in primary spermatocytes up through meiotic division (Fig. 1C (10,55)), and Xenopus xDazl can rescue progression through meiosis in boule mutant flies, showing that DAZL homologs can also promote metaphase (7). Boule and Dazl proteins are known to interact (10), and both Cdc25a and Cdc25c have been identified as potential targets of Dazl (56,57), providing evidence for a possible mechanism. In our model, both Boule and Dazl bind to Cdc25a and Cdc25c mRNA in wild-type mice and activate their translation, thus pushing spermatocytes into metaphase, while Dazl can still activate translation of Cdc25a and Cdc25c in the absence of Boule (Fig. 7A). Since Dazl−/− mice show a final arrest at leptonema (17,20), prior to the pachytene expression of Boule (10), Boule cannot reciprocally compensate for the loss of Dazl. Similarly, Dazl does not persist as long as Boule in round spermatids (10,23), preventing a compensatory role during spermiogenesis. It is not yet known whether mammalian Dazl can function to activate the transition into metaphase, and examination of Boule and Dazl double mutants will be needed to test this model.
Figure 7.
Proposed model of Boule spermatogenic function. In mice (A), Boule regulates spermiogenesis, likely through an unknown target. We propose that Dazl and Boule redundantly regulate progression to metaphase by activating translation of Cdc25. In flies (B), boule promotes translation of the Cdc25 homolog twine, and thus progression through meiosis. The spermiogenesis function seen in mice may also exist in Drosophila, though it has not yet been defined. Solid lines represent known pathways and dashed are proposed.
Additionally, we observed an increased frequency of developmental delay during the first wave of spermatogenesis in Boule−/− mice. Boule null mice showed a delay in the completion of meiosis approximately twice as often as heterozygous littermates, as indicated by a more frequent absence of round spermatids in 24-day-old knockout mice. This may indicate a role for Boule prior to spermatid differentiation, perhaps in meiosis. Though a minor phenotype, such delays in spermatogenesis in addition to a primary knockout phenotype have been reported in other mutants (58,59). Disruption of spermatid nuclear condensation is the primary defect in TSCL1 null mice, but a delay in round spermatid production was noted (59), while Parp2−/− mice showed a possible delay in meiosis in addition to more drastic defects in chromosome dynamics during pachynema (58). Further studies will be needed to determine how the loss of Boule contributes to delays in the first wave of spermatogenesis.
The primary defect of pachytene arrest in boule mutant flies suggests that the mammalian spermiogenesis function of Boule has recently evolved in mammals. However, close inspection of the Drosophila phenotype indicates that boule may also control spermiogenesis in flies (Fig. 7B). The arrested pre-meiotic cysts occasionally began spermiogenesis, but never progressed to the elongation phase (6), a feature not common to all meiotic arrest mutants. Flies with mutations in the Cdc25 homolog twine similarly fail to complete meiosis, but arrested cysts differentiate through spermatid elongation (60,61). Eberhart et al. (6) suggested that spermiogenic differentiation can be uncoupled from meiotic division, and that boule may play a role in both pathways. Transgenic, boule-independent expression of twine in boule mutant flies restores spermatocyte progression to metaphase, but spermatid differentiation remains arrested (30), providing further evidence for a dual role of boule in meiosis and spermiogenesis in Drosophila. In the case of C. elegans, the BOULE homolog daz-1 functions in oocytes and not spermatocytes, likely due to a divergence in that lineage. Caenorhabditis elegans are hermaphrodites, representing a unique evolution of their germline, and interestingly, C. elegans males do not undergo typical spermiogenesis or produce flagellated sperm. Whether or not the spermiogenesis role of BOULE is conserved remains to be seen, but our work raises the possibility that BOULE acts to bridge the meiotic division to ensure that sperm differentiation is properly timed and coordinated with meiosis. Such coordinated control of the meiotic cell cycle with spermatid differentiation has been observed in Drosophila spermatogenesis (62), and comparative analysis of Boule-mediated pathways in both Drosophila and mice could help determine to what extent any underlying molecular mechanism is conserved.
Our discovery of a novel requirement for BOULE in spermiogenesis has expanded the known roles of this conserved germ-cell regulator and defined its specific requirement in mammals. Though a short list of genes are already known to be key regulators of spermiogenesis, only Boule is specifically conserved in male gametogenesis in metazoans. This suggests that the unknown mechanism by which Boule regulates spermatogenesis is part of a fundamental process in male germ-cell development in animals, including humans.
MATERIALS AND METHODS
Animals
Mice were housed and bred in a barrier facility in the Northwestern University Center for Comparative Medicine. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Northwestern ACUC. Boule knockout mice in a mixed 129/B6 background were backcrossed into C57/B6 for three generations and no changes in spermatogenic defects were observed. All the tissues of wild-type, heterozygotes or homozygotes were from mixed background mice within the same litter.
Histology, immunohistochemistry and TUNEL staining
Dissected testes were fixed in Bouin's solution overnight at room temperature, and processed for H&E, PAS or immunohistochemistry according to standard protocols (10). TUNEL staining was performed according to the manufacturer's instructions (Chemicon ApopTag Peroxidase kit, S700). Meiotic chromosome spreads were prepared and immunostained as described previously, using immunofluorescence (63,64). Images were captured on a Leica DM5000B Microscope and processed using Adobe PhotoShop.
Cell cycle analysis
Dissected testes were collected and the tunica membrane removed, releasing the seminiferous tubules. Tubules were placed in 600 µl DM1 (1 mg/ml Collagenase IA (Sigma), 2 U/ml DNAse I (Sigma) in DMEM/F12 (Gibco)), cut into pieces and digested at 37°C for 30 min. The media was replaced with 700 µl DM2 (1 mg/ml Collagenase IA (Sigma), 2 mg/ml Hyaluronidase I-S (Sigma), 2 U/ml DNAse I (Sigma) in DMEM/F12 (Gibco)), and returned to 37°C for 30 min. Cells were pelleted at 400 g for 6 min at 4°C and washed twice with 1 ml of cold PBS. Cells were left in ∼50 µl PBS and resuspended, then fixed in 450 µl of cold methanol at 4°C for 2 h, or stored at −20°C. Following fixation, 1 ml PBS was added and cells were pelleted, then washed twice with 1 ml PBS. Cells were resuspended in 375 µl propidium iodide staining solution (50 µg/ml propidium iodide (Sigma), 0.2 mg/ml RNAse A (Sigma), 0.1% Triton-X in PBS) per 1 × 106 cells at 37°C for 20 min, while protected from light. Stained cells were filtered and counted on a Beckman Coulter Epics XL-MCL flow cytometer.
Immunoblots
Testes were dissected from euthanized mice, flash frozen in liquid nitrogen and either stored at −80°C or used immediately. Samples were ground in liquid nitrogen using a mortar and pestle and lysed in either 1x Laemmli buffer or polysome lysis buffer (10 mm Hepes, pH 7.0, 100 mm KCl, 5 mm MgCl2, 0.5% NP-40, 1 mm DTT, 0.2% ribonucleoside vanadyl complex (NEB), 100 U/ml RNAse Out (Invitrogen), protease inhibitor cocktail (Sigma)). Approximately 20 mg of protein was loaded for SDS–PAGE analysis, and transferred to PVDF membranes for subsequent immunoblotting.
RT–PCR
Testes were homogenized as above, lysed in Trizol (Invitrogen) and RNA was purified according to the manufacturer's instructions. Reverse transcription was performed on 8 µg RNA using Superscript III Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. cDNA was diluted appropriately to obtain equal starting amounts for PCR detection. Primer sequences are available upon request.
Antibodies
The first 498 bases of mouse Boule cDNA encoding residues 1–168 were PCR amplified and cloned into the GST fusion vector pGEX-4T-1 (GE Healthcare). Purified protein was injected into rabbits (Cocalico Biologicals), and resulting anti-serum 101 was used for western blot and immunohistochemistry. The specificity of the antibody was verified by pre-incubating antisera with immunizing peptide (1 µg/ml) for 30 min prior to immunoblotting.
The following antibodies were used for IHC: Preimmune serum, 1:200; Boule anti-serum 101, 1:200; anti-Dazl anti-serum-149, 1:200 (55); anti-Cdc25a, 1:40 (Neomarkers ab3); anti-SP-10, 1:400 (a gift of Dr P. Reddi, University of Virginia); anti-Rnf17, 1:150 (a gift of Dr P.J. Wang, University of Pennsylvania (39)); anti-Tpap, 1:100 (a gift of Dr T. Baba, University of Tsukuba, Japan (65)); anti-Vasa, 1:200 (Abcam ab13840); anti-Crem, 1:100 (a gift of Dr P. Sassone-Corsi, University of California, Irvine (66)); and anti-Miwi, 1:100 (Abcam ab12337). For immunofluorescence we used: anti-SYCP3, (Abcam); anti-RPA, (Cell Signaling 2208); and anti-MLH, (BD Pharmigen 550838). For western blotting, the following antibodies were used: anti-β-Actin, 1:1000 (Santa Cruz Biotechnology SC-1615 HRP); Preimmune serum, 1:1000; Boule anti-serum 101; anti-Crem, 1:500; anti-Rnf17, 1:400; anti-Tpap, 1:500; anti-Vasa, 1:2000; and anti-Miwi, 1:1000. HRP-conjugated secondary antibodies were used (anti-mouse, Biorad; anti-rabbit and anti-goat, Dako).
SUPPLEMENTARY MATERIAL
FUNDING
This project was supported by NIH U01 HD045871 and Northwestern Memorial Hospital EAM grants (E.Y.X.); M.J.W.V. was supported by the Cell and Molecular Basis of Disease training grant, funded by NIH T32 GM08061.
Supplementary Material
ACKNOWLEDGEMENTS
We thank L. Reynolds for EM; T. Kurita, H. Ishikawa for help with IHC; M. Laronda and J. Marvin for flow cytometry assistance; C. Shah and J. Wei for technical assistance; P. Reddi (University of Virginia) for SP-10 antibody; P. Sassone-Corsi (University of California, Irvine) for Crem antibody; T. Baba (University of Tsukuba, Japan) for Tpap antibody; P.J. Wang (University of Pennsylvania) for Rnf17 antibody; and T. Kurita, E. Goldberg, H.J. Cooke, J. Weiss, D. Chakravarti, T. Woodruff and S. Huang for comments on the manuscript.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Matzuk M.M., Lamb D.J. Genetic dissection of mammalian fertility pathways. Nat. Cell Biol. 2002;4(Suppl.):s41–s49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 2.Swanson W.J., Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002;3:137–144. doi: 10.1038/nrg733. doi:10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- 3.Wyckoff G.J., Wang W., Wu C.I. Rapid evolution of male reproductive genes in the descent of man. Nature. 2000;403:304–309. doi: 10.1038/35002070. [DOI] [PubMed] [Google Scholar]
- 4.Reijo R., Lee T.Y., Salo P., Alagappan R., Brown L.G., Rosenberg M., Rozen S., Jaffe T., Straus D., Hovatta O., et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat. Genet. 1995;10:383–393. doi: 10.1038/ng0895-383. doi:10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 5.Reijo R., Alagappan R.K., Patrizio P., Page D.C. Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome. Lancet. 1996;347:1290–1293. doi: 10.1016/s0140-6736(96)90938-1. doi:10.1016/S0140-6736(96)90938-1. [DOI] [PubMed] [Google Scholar]
- 6.Eberhart C.G., Maines J.Z., Wasserman S.A. Meiotic cell cycle requirement for a fly homologue of human Deleted in Azoospermia. Nature. 1996;381:783–785. doi: 10.1038/381783a0. doi:10.1038/381783a0. [DOI] [PubMed] [Google Scholar]
- 7.Houston D.W., Zhang J., Maines J.Z., Wasserman S.A., King M.L. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development. 1998;125:171–180. doi: 10.1242/dev.125.2.171. [DOI] [PubMed] [Google Scholar]
- 8.Karashima T., Sugimoto A., Yamamoto M. Caenorhabditis elegans homologue of the human azoospermia factor DAZ is required for oogenesis but not for spermatogenesis. Development. 2000;127:1069–1079. doi: 10.1242/dev.127.5.1069. [DOI] [PubMed] [Google Scholar]
- 9.Ruggiu M., Speed R., Taggart M., McKay S.J., Kilanowski F., Saunders P., Dorin J., Cooke H.J. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 10.Xu E.Y., Moore F.L., Pera R.A. A gene family required for human germ cell development evolved from an ancient meiotic gene conserved in metazoans. Proc. Natl Acad. Sci. USA. 2001;98:7414–7419. doi: 10.1073/pnas.131090498. doi:10.1073/pnas.131090498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kee K., Angeles V.T., Flores M., Nguyen H.N., Reijo Pera R.A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. doi:10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H., Li Z., Li M., Wang L., Hong Y. Boule is present in fish and bisexually expressed in adult and embryonic germ cells of medaka. PLoS One. 2009;4:e6097. doi: 10.1371/journal.pone.0006097. doi:10.1371/journal.pone.0006097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agulnik A.I., Zharkikh A., Boettger-Tong H., Bourgeron T., McElreavey K., Bishop C.E. Evolution of the DAZ gene family suggests that Y-linked DAZ plays little, or a limited, role in spermatogenesis but underlines a recent African origin for human populations. Hum. Mol. Genet. 1998;7:1371–1377. doi: 10.1093/hmg/7.9.1371. doi:10.1093/hmg/7.9.1371. [DOI] [PubMed] [Google Scholar]
- 14.Gromoll J., Weinbauer G.F., Skaletsky H., Schlatt S., Rocchietti-March M., Page D.C., Nieschlag E. The Old World monkey DAZ (Deleted in AZoospermia) gene yields insights into the evolution of the DAZ gene cluster on the human Y chromosome. Hum. Mol. Genet. 1999;8:2017–2024. doi: 10.1093/hmg/8.11.2017. doi:10.1093/hmg/8.11.2017. [DOI] [PubMed] [Google Scholar]
- 15.Saxena R., Brown L.G., Hawkins T., Alagappan R.K., Skaletsky H., Reeve M.P., Reijo R., Rozen S., Dinulos M.B., Disteche C.M., et al. The DAZ gene cluster on the human Y chromosome arose from an autosomal gene that was transposed, repeatedly amplified and pruned. Nat. Genet. 1996;14:292–299. doi: 10.1038/ng1196-292. doi:10.1038/ng1196-292. [DOI] [PubMed] [Google Scholar]
- 16.Houston D.W., King M.L. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development. 2000;127:447–456. doi: 10.1242/dev.127.3.447. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y., Page D.C. Dazl deficiency leads to embryonic arrest of germ cell development in XY C57BL/6 mice. Dev. Biol. 2005;288:309–316. doi: 10.1016/j.ydbio.2005.06.032. doi:10.1016/j.ydbio.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Maegawa S., Yasuda K., Inoue K. Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev. 1999;81:223–226. doi: 10.1016/s0925-4773(98)00242-1. doi:10.1016/S0925-4773(98)00242-1. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y., Gill M.E., Koubova J., Page D.C. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. doi:10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 20.Saunders P.T., Turner J.M., Ruggiu M., Taggart M., Burgoyne P.S., Elliott D., Cooke H.J. Absence of mDazl produces a final block on germ cell development at meiosis. Reproduction. 2003;126:589–597. doi: 10.1530/rep.0.1260589. doi:10.1530/rep.0.1260589. [DOI] [PubMed] [Google Scholar]
- 21.Schrans-Stassen B.H., Saunders P.T., Cooke H.J., de Rooij D.G. Nature of the spermatogenic arrest in Dazl −/− mice. Biol. Reprod. 2001;65:771–776. doi: 10.1095/biolreprod65.3.771. doi:10.1095/biolreprod65.3.771. [DOI] [PubMed] [Google Scholar]
- 22.Cheng M.H., Maines J.Z., Wasserman S.A. Biphasic subcellular localization of the DAZL-related protein boule in Drosophila spermatogenesis. Dev. Biol. 1998;204:567–576. doi: 10.1006/dbio.1998.9098. doi:10.1006/dbio.1998.9098. [DOI] [PubMed] [Google Scholar]
- 23.Tung J.Y., Luetjens C.M., Wistuba J., Xu E.Y., Reijo Pera R.A., Gromoll J. Evolutionary comparison of the reproductive genes, DAZL and BOULE, in primates with and without DAZ. Dev. Genes Evol. 2006;216:158–168. doi: 10.1007/s00427-005-0039-2. doi:10.1007/s00427-005-0039-2. [DOI] [PubMed] [Google Scholar]
- 24.Otori M., Karashima T., Yamamoto M. The Caenorhabditis elegans homologue of deleted in azoospermia is involved in the sperm/oocyte switch. Mol. Biol. Cell. 2006;17:3147–3155. doi: 10.1091/mbc.E05-11-1067. doi:10.1091/mbc.E05-11-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu E.Y., Lee D.F., Klebes A., Turek P.J., Kornberg T.B., Reijo Pera R.A. Human BOULE gene rescues meiotic defects in infertile flies. Hum. Mol. Genet. 2003;12:169–175. doi: 10.1093/hmg/ddg017. doi:10.1093/hmg/ddg017. [DOI] [PubMed] [Google Scholar]
- 26.Russell L.D., Ettlin R.A., Sinha Hikim A.P., Clegg E.D. Histological and Histopathological Evaluation of the Testis. 1 edn. St. Louis, MO: Cache River Press; 1990. [Google Scholar]
- 27.Nantel F., Monaco L., Foulkes N.S., Masquilier D., LeMeur M., Henriksen K., Dierich A., Parvinen M., Sassone-Corsi P. Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature. 1996;380:159–162. doi: 10.1038/380159a0. doi:10.1038/380159a0. [DOI] [PubMed] [Google Scholar]
- 28.Ashley T. The mouse ‘tool box’ for meiotic studies. Cytogenet. Genome Res. 2004;105:166–171. doi: 10.1159/000078186. doi:10.1159/000078186. [DOI] [PubMed] [Google Scholar]
- 29.Maines J.Z., Wasserman S.A. Post-transcriptional regulation of the meiotic Cdc25 protein Twine by the Dazl orthologue Boule. Nat. Cell Biol. 1999;1:171–174. doi: 10.1038/11091. [DOI] [PubMed] [Google Scholar]
- 30.Wickramasinghe D., Becker S., Ernst M.K., Resnick J.L., Centanni J.M., Tessarollo L., Grabel L.B., Donovan P.J. Two CDC25 homologues are differentially expressed during mouse development. Development. 1995;121:2047–2056. doi: 10.1242/dev.121.7.2047. [DOI] [PubMed] [Google Scholar]
- 31.Wu S., Wolgemuth D.J. The distinct and developmentally regulated patterns of expression of members of the mouse Cdc25 gene family suggest differential functions during gametogenesis. Dev. Biol. 1995;170:195–206. doi: 10.1006/dbio.1995.1207. doi:10.1006/dbio.1995.1207. [DOI] [PubMed] [Google Scholar]
- 32.Bellve A.R., Cavicchia J.C., Millette C.F., O'Brien D.A., Bhatnagar Y.M., Dym M. Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J. Cell Biol. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. doi:10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herr J.C., Flickinger C.J., Homyk M., Klotz K., John E. Biochemical and morphological characterization of the intra-acrosomal antigen SP-10 from human sperm. Biol. Reprod. 1990;42:181–193. doi: 10.1095/biolreprod42.1.181. doi:10.1095/biolreprod42.1.181. [DOI] [PubMed] [Google Scholar]
- 34.Reddi P.P., Flickinger C.J., Herr J.C. Round spermatid-specific transcription of the mouse SP-10 gene is mediated by a 294-base pair proximal promoter. Biol. Reprod. 1999;61:1256–1266. doi: 10.1095/biolreprod61.5.1256. doi:10.1095/biolreprod61.5.1256. [DOI] [PubMed] [Google Scholar]
- 35.Blendy J.A., Kaestner K.H., Weinbauer G.F., Nieschlag E., Schutz G. Severe impairment of spermatogenesis in mice lacking the CREM gene. Nature. 1996;380:162–165. doi: 10.1038/380162a0. doi:10.1038/380162a0. [DOI] [PubMed] [Google Scholar]
- 36.Deng W., Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. doi:10.1016/S1534-5807(02)00165-X. [DOI] [PubMed] [Google Scholar]
- 37.Kashiwabara S., Noguchi J., Zhuang T., Ohmura K., Honda A., Sugiura S., Miyamoto K., Takahashi S., Inoue K., Ogura A., et al. Regulation of spermatogenesis by testis-specific, cytoplasmic poly(A) polymerase TPAP. Science. 2002;298:1999–2002. doi: 10.1126/science.1074632. doi:10.1126/science.1074632. [DOI] [PubMed] [Google Scholar]
- 38.Martianov I., Fimia G.M., Dierich A., Parvinen M., Sassone-Corsi P., Davidson I. Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol. Cell. 2001;7:509–515. doi: 10.1016/s1097-2765(01)00198-8. doi:10.1016/S1097-2765(01)00198-8. [DOI] [PubMed] [Google Scholar]
- 39.Pan J., Goodheart M., Chuma S., Nakatsuji N., Page D.C., Wang P.J. RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis. Development. 2005;132:4029–4039. doi: 10.1242/dev.02003. doi:10.1242/dev.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D., Penttila T.L., Morris P.L., Teichmann M., Roeder R.G. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science. 2001;292:1153–1155. doi: 10.1126/science.1059188. doi:10.1126/science.1059188. [DOI] [PubMed] [Google Scholar]
- 41.Collier B., Gorgoni B., Loveridge C., Cooke H.J., Gray N.K. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 2005;24:2656–2666. doi: 10.1038/sj.emboj.7600738. doi:10.1038/sj.emboj.7600738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds N., Collier B., Bingham V., Gray N.K., Cooke H.J. Translation of the synaptonemal complex component Sycp3 is enhanced in vivo by the germ cell specific regulator Dazl. RNA. 2007;13:974–981. doi: 10.1261/rna.465507. doi:10.1261/rna.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel T., Speed R.M., Ross A., Cooke H.J. Partial rescue of the Dazl knockout mouse by the human DAZL gene. Mol. Hum. Reprod. 2002;8:797–804. doi: 10.1093/molehr/8.9.797. doi:10.1093/molehr/8.9.797. [DOI] [PubMed] [Google Scholar]
- 44.Tsai-Morris C.H., Sheng Y., Lee E., Lei K.J., Dufau M.L. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc. Natl Acad. Sci. USA. 2004;101:6373–6378. doi: 10.1073/pnas.0401855101. doi:10.1073/pnas.0401855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braun R.E. Post-transcriptional control of gene expression during spermatogenesis. Semin. Cell Dev. Biol. 1998;9:483–489. doi: 10.1006/scdb.1998.0226. doi:10.1006/scdb.1998.0226. [DOI] [PubMed] [Google Scholar]
- 46.Hecht N.B. Molecular mechanisms of male germ cell differentiation. Bioessays. 1998;20:555–561. doi: 10.1002/(SICI)1521-1878(199807)20:7<555::AID-BIES6>3.0.CO;2-J. doi:10.1002/(SICI)1521-1878(199807)20:7<555::AID-BIES6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 47.Kleene K.C. Patterns of translational regulation in the mammalian testis. Mol. Reprod. Dev. 1996;43:268–281. doi: 10.1002/(SICI)1098-2795(199602)43:2<268::AID-MRD17>3.0.CO;2-#. doi:10.1002/(SICI)1098-2795(199602)43:2<268::AID-MRD17>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Kotaja N., Sassone-Corsi P. The chromatoid body: a germ-cell-specific RNA-processing centre. Nat. Rev. Mol. Cell Biol. 2007;8:85–90. doi: 10.1038/nrm2081. doi:10.1038/nrm2081. [DOI] [PubMed] [Google Scholar]
- 49.Sheng Y., Tsai-Morris C.H., Gutti R., Maeda Y., Dufau M.L. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is a transport protein involved in gene-specific mRNA export and protein translation during spermatogenesis. J. Biol. Chem. 2006;281:35048–35056. doi: 10.1074/jbc.M605086200. doi:10.1074/jbc.M605086200. [DOI] [PubMed] [Google Scholar]
- 50.Grivna S.T., Pyhtila B., Lin H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc. Natl Acad. Sci. USA. 2006;103:13415–13420. doi: 10.1073/pnas.0605506103. doi:10.1073/pnas.0605506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotaja N., Bhattacharyya S.N., Jaskiewicz L., Kimmins S., Parvinen M., Filipowicz W., Sassone-Corsi P. The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc. Natl Acad. Sci. USA. 2006;103:2647–2652. doi: 10.1073/pnas.0509333103. doi:10.1073/pnas.0509333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotaja N., Lin H., Parvinen M., Sassone-Corsi P. Interplay of PIWI/Argonaute protein MIWI and kinesin KIF17b in chromatoid bodies of male germ cells. J. Cell Sci. 2006;119:2819–282553. doi: 10.1242/jcs.03022. [DOI] [PubMed] [Google Scholar]
- 53.Aravin A., Gaidatzis D., Pfeffer S., Lagos-Quintana M., Landgraf P., Iovino N., Morris P., Brownstein M.J., Kuramochi-Miyagawa S., Nakano T., et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 54.Grivna S.T., Beyret E., Wang Z., Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–1714. doi: 10.1101/gad.1434406. doi:10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reijo R.A., Dorfman D.M., Slee R., Renshaw A.A., Loughlin K.R., Cooke H., Page D.C. DAZ family proteins exist throughout male germ cell development and transit from nucleus to cytoplasm at meiosis in humans and mice. Biol. Reprod. 2000;63:1490–1496. doi: 10.1095/biolreprod63.5.1490. doi:10.1095/biolreprod63.5.1490. [DOI] [PubMed] [Google Scholar]
- 56.Jiao X., Trifillis P., Kiledjian M. Identification of target messenger RNA substrates for the murine deleted in azoospermia-like RNA-binding protein. Biol. Reprod. 2002;66:475–485. doi: 10.1095/biolreprod66.2.475. doi:10.1095/biolreprod66.2.475. [DOI] [PubMed] [Google Scholar]
- 57.Venables J.P., Ruggiu M., Cooke H.J. The RNA-binding specificity of the mouse Dazl protein. Nucleic Acids Res. 2001;29:2479–2483. doi: 10.1093/nar/29.12.2479. doi:10.1093/nar/29.12.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dantzer F., Mark M., Quenet D., Scherthan H., Huber A., Liebe B., Monaco L., Chicheportiche A., Sassone-Corsi P., de Murcia G., et al. Poly(ADP-ribose) polymerase-2 contributes to the fidelity of male meiosis I and spermiogenesis. Proc. Natl Acad. Sci. USA. 2006;103:14854–14859. doi: 10.1073/pnas.0604252103. doi:10.1073/pnas.0604252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Weyden L., Arends M.J., Chausiaux O.E., Ellis P.J., Lange U.C., Surani M.A., Affara N., Murakami Y., Adams D.J., Bradley A. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Mol. Cell. Biol. 2006;26:3595–3609. doi: 10.1128/MCB.26.9.3595-3609.2006. doi:10.1128/MCB.26.9.3595-3609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courtot C., Fankhauser C., Simanis V., Lehner C.F. The Drosophila cdc25 homolog twine is required for meiosis. Development. 1992;116:405–416. doi: 10.1242/dev.116.2.405. [DOI] [PubMed] [Google Scholar]
- 61.Alphey L., Jimenez J., White-Cooper H., Dawson I., Nurse P., Glover D.M. twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell. 1992;69:977–988. doi: 10.1016/0092-8674(92)90616-k. doi:10.1016/0092-8674(92)90616-K. [DOI] [PubMed] [Google Scholar]
- 62.Lin T.Y., Viswanathan S., Wood C., Wilson P.G., Wolf N., Fuller M.T. Coordinate developmental control of the meiotic cell cycle and spermatid differentiation in Drosophila males. Development. 1996;122:1331–1341. doi: 10.1242/dev.122.4.1331. [DOI] [PubMed] [Google Scholar]
- 63.Moens P.B., Heyting C., Dietrich A.J., van Raamsdonk W., Chen Q. Synaptonemal complex antigen location and conservation. J. Cell Biol. 1987;105:93–103. doi: 10.1083/jcb.105.1.93. doi:10.1083/jcb.105.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peters A.H., Plug A.W., van Vugt M.J., de Boer P. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 1997;5:66–68. doi: 10.1023/a:1018445520117. doi:10.1023/A:1018445520117. [DOI] [PubMed] [Google Scholar]
- 65.Kashiwabara S., Zhuang T., Yamagata K., Noguchi J., Fukamizu A., Baba T. Identification of a novel isoform of poly(A) polymerase, TPAP, specifically present in the cytoplasm of spermatogenic cells. Dev. Biol. 2000;228:106–115. doi: 10.1006/dbio.2000.9894. doi:10.1006/dbio.2000.9894. [DOI] [PubMed] [Google Scholar]
- 66.Delmas V., van der Hoorn F., Mellstrom B., Jegou B., Sassone-Corsi P. Induction of CREM activator proteins in spermatids: down-stream targets and implications for haploid germ cell differentiation. Mol. Endocrinol. 1993;7:1502–1514. doi: 10.1210/mend.7.11.8114765. doi:10.1210/me.7.11.1502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.