Abstract
The thylakoid twin arginine protein translocation (Tat) system is thought to have a multivalent receptor complex with each cpTatC-Hcf106 pair constituting a signal peptide-binding unit. Conceptual models suggest that translocation of individual precursor proteins occurs upon assembly of a Tha4 oligomer with a precursor-occupied cpTatC-Hcf106. However, results reported here reveal that multiple precursor proteins bound to a single receptor complex can be transported together. Precursor proteins that contain one or two cysteine residues readily formed intermolecular disulphide bonds upon binding to the receptor complex, resulting in dimeric and tetrameric precursor proteins. Three lines of evidence indicate that all members of precursor oligomers were specifically bound to a receptor unit. Blue native–polyacrylamide gel electrophoresis analysis showed that oligomers were present on individual receptor complexes rather than bridging two or more receptor complexes. Upon energizing the membrane, the dimeric and tetrameric precursors were transported across the membrane with efficiencies comparable with that of monomeric precursors. These results imply a novel aspect of Tat systems, whereby multiple precursor-binding sites can act in concert to transport an interlinked oligo-precursor protein.
Keywords: chloroplasts, precursor protein oligomers, protein transport, receptor, twin arginine transport
Introduction
Twin arginine protein translocation systems, called Tat, are found in prokaryote cell membranes and in the thylakoid membranes of chloroplasts. Tat systems transport folded protein substrates across sealed membranes using only the proton electrochemical gradient as energy source (Berks et al, 2003; Muller and Klosgen, 2005; Lee et al, 2006; Sargent et al, 2006; Cline and Theg, 2007). This distinguishes Tat systems from most of the other protein transport systems, which transport proteins in unfolded conformation and are powered by NTP hydrolysis (Osborne et al, 2005; Neupert and Herrmann, 2007; Rounds et al, 2007). Substrates of Tat have hydrophobic signal peptides with a conserved and essential pair of adjacent arginine residues (RR) that are present at the beginning of the hydrophobic domain. Mutation of the arginines, even to two lysines, inactivates the signal peptide (Cline and Mori, 2001; Alami et al, 2003; Gerard and Cline, 2006). Most Tat systems consist of three membrane protein components of translocation machinery called Tha4, Hcf106 and cpTatC in chloroplasts, and TatA, TatB and TatC in bacteria, respectively. These components are organized into two complexes in the membrane. cpTatC and Hcf106 comprise a large (∼700 kDa) complex (Cline and Mori, 2001). Tha4 is found in a separate pool that may vary in size depending on the stage of protein transport (Dabney-Smith et al, 2006; Leake et al, 2008; Dabney-Smith and Cline, 2009).
cpTatC:Hcf106 is the primary receptor for the signal peptide of the precursor. Site-directed cross-linking in thylakoids and bacteria showed that the RR proximal region of the signal peptide is in close contact with cpTatC (TatC), whereas the hydrophobic domain and some parts of the adjacent mature domain are in close contact with Hcf106 (TatB) (Alami et al, 2003; Gerard and Cline, 2006). Gerard and Cline (2006) found that a precursor protein cross-linked between its RR proximal region and cpTatC was efficiently transported across the membrane. This result supports speculations that cpTatC (TatC) may provide the motive force for transmembrane protein movement (Bruser and Sanders, 2003; Dabney-Smith et al, 2006; Cline and McCaffery, 2007). On precursor binding in the presence of the proton gradient, Tha4 assembles with the receptor complex (Mori and Cline, 2002) and becomes organized as a homo-oligomer (Dabney-Smith and Cline, 2009). This has suggested that Tha4 has a function in transmembrane passage of precursor proteins (Berks et al, 2000; Mori and Cline, 2002; Bruser and Sanders, 2003; Gohlke et al, 2005; Dabney-Smith et al, 2006).
One curious aspect of the receptor complex is that it contains multiple copies of cpTatC and Hcf106 (Cline and Mori, unpublished) (McDevitt et al, 2005), suggesting that each receptor complex might be multivalent with respect to precursor binding and translocation sites. The stoichiometry of precursor proteins of a fully saturated receptor has not yet been determined. However, a recent structural analysis of precursor-bound receptor complex by single particle electron microscopy indicated that it contained one or two bound precursors and, in cases in which two precursors were bound, the pair occupied adjacent sites (Tarry et al, 2009). In imaged particles, the signal peptides seemed to be buried in the receptor complex with the mature domains projecting out from the circumference of the receptor complex at an angle of ∼50°, suggesting that there are potentially seven precursor-binding sites per complex.
We are attempting to determine the stoichiometry and organization of precursors on the receptor complex with biochemical approaches. Here, we show that bound precursor proteins that contain one or two cysteine residues in their mature domains spontaneously form disulphide bonds, resulting in dimeric and tetrameric precursor proteins. Surprisingly, efficient cross-linking was directed from a cysteine placed three residues from the signal peptide cleavage site. This same residue was earlier shown to cross-link to Hcf106, albeit weakly (Gerard and Cline, 2007). This indicates that the signal peptide-binding sites are very close in situ or are sufficiently flexible to closely approach one another. Blue native–polyacrylamide gel electrophoresis (BN–PAGE) analysis showed that precursor protein tetramers and dimers were present on individual receptor complexes rather than bridging two or more receptor complexes. Surprisingly, upon energizing the membrane with the proton gradient, the dimeric and tetrameric precursors were efficiently transported across the thylakoids and all signal peptides proteolytically removed. This suggests that multiple precursor-binding sites can act in concert for protein translocation.
Our results reveal a novel aspect of Tat system, which is already recognized as a unique protein transport system for its ability to transport folded protein domains across sealed membranes. Many protein transport systems in prokaryotes and eukaryotes have been characterized in considerable detail. One general conclusion is that a single ligand or an oligomeric ligand (in rare situations) interacts with a receptor and is transported one unit at a time. Here, we show evidence for the Tat system that multiple precursors bound to multiple-binding sites in a single translocase can be delivered across membrane together.
Results
Precursors with a Cys residue near the N-terminus of the mature domain dimerize through a disulphide linkage on binding to the thylakoid membrane
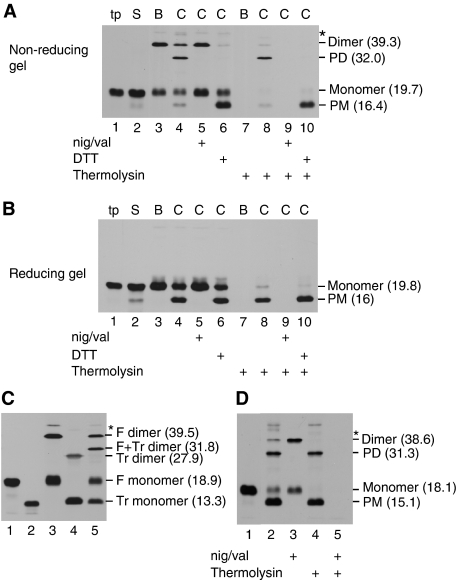
A library of single Cys-substituted tOE17 V-20F precursor proteins was constructed for use in site-directed cross-linking experiments. The tOE17 is a truncated version of the precursor for OE17 from maize that lacks several non-essential amino-proximal residues of the targeting peptide (Henry et al, 1997; Ma and Cline, 2000). The V-20F substitution places a phenylalanine at the twin arginine (RR)+2 position and results in a higher affinity binding to the Tat receptor complex (Gerard and Cline, 2007). The RR +2 F is found in most bacterial Tat substrates, in which it has an important function in transport efficiency (Stanley et al, 2000). During characterization experiments, we found that some of these Cys-labelled precursors formed higher molecular weight disulphide-linked products on binding to thylakoid membranes. This can be seen in Figure 1, in which a precursor containing a single Cys substitution at position 3, that is the third residue of the mature domain of OE17, when bound to thylakoids, produced an additional band on non-reducing SDS–PAGE at Mr 39.3 kDa, which is approximately twice that of the monomeric precursor (panel A, lane 3). The in vitro translated precursor (lane 1) and the supernatant from the binding reaction (lane 2) contained only monomeric tOE17, as did the membrane sample subjected to SDS–PAGE under reducing conditions (panel B, lane 3).
Figure 1.
tOE17-20F3C precursors form disulphide-linked dimers upon membrane binding that are subsequently transported to the thylakoid lumen. (A) In vitro translated [3H] tOE17-20F3C precursor containing a unique cysteine at residue 3 of the mature domain (tp) was incubated with washed thylakoids in a binding reaction. The assay mixture was separated by centrifugation into supernatant (lane S) and membranes, which were washed with IB (lane B). An aliquot of precursor-bound thylakoids was further incubated in a chase assay (lane C) in the absence (lanes 4 and 8) or presence (lanes 5 and 9) of nigericin and valinomycin (nig/val), or presence (lanes 6 and 10) of DTT as depicted below the panel. Aliquots of the thylakoid fraction were treated with the protease thermolysin (lanes 7–10). Samples were analysed by SDS–PAGE without reducing agent and fluorography. Putative precursor dimers and processed lumenal mature protein dimers (PD), monomer precursor and processed lumenal monomer (PM) are indicated to the right of the panel. (B) The same samples as in (A) were treated with β-mercaptoethanol before SDS–PAGE and fluorography. (C) Binding reactions were conducted with full length tOE17-20F3C and/or tOE17-20F3C truncated after residue 100 of the mature domain (labelled tOE17-20F3CTr). Lanes 1 and 2 contain the individual in vitro translation products of tOE17-20F3C and tOE17-20F3CTr. Lanes 3, 4 and 5 are recovered thylakoids membrane from binding assays with tOE17-20F3C, tOE17-20F3CTr and a mixture of the two precursors, respectively. tOE17-20F3C monomers and dimers (F monomer and F Dimer), tOE17-20F3CTr monomers and dimer (Tr monomer and Tr dimer) and the tOE17-20F3C:tOE17-20F3CTr heterodimer (F+Tr Dimer) are indicated to the right of the panel. Mrs in parentheses (in kilodaltons) of the different protein species were determined from their relative mobility fit to a curve of pre-stained molecular mass standards (Fisher Chemical Co.). (D) In vitro translated [3H] tOE17-20F3C precursor (lane 1) was incubated with washed thylakoids in a transport assay in the absence (lanes 2 and 4) or presence (lanes 3 and 5) of nigericin and valinomycin. Aliquots of recovered thylakoids were treated with thermolysin as designated below the panel. Samples were analysed by non-reducing SDS–PAGE and fluorography.
The Mr of the larger band in panel A (lane 3) suggested that it is a precursor homodimer. This conclusion was confirmed by a binding experiment with a mixture of tOE17-20F3C and a truncated version of the same precursor called tOE17-20F3CTr (panel C). When used for binding experiments separately, each produced a band twice the Mr of the monomeric precursor (lanes 3 and 4). When the precursors were combined and used for a binding experiment, an additional band appeared with a Mr equal to the sum of the two monomeric precursors (lane 5, F+Tr dimer). This strongly suggests that the major disulphide cross-linked product is a precursor dimer rather than a cross-linking product between precursor and an endogenous thylakoid protein.
In many of the experiments reported here, an additional ∼47 kDa tOE17-containing band was also present in the membrane samples (e.g. panel A, lanes 3–6, labelled with asterisk). This band is frequently difficult to reduce with our sample preparation method (see below Figure 7), but can be largely reduced by boiling samples in β-mercaptoethanol (data not shown). The identity of this cross-linking product is presently under investigation.
When the precursor-bound membranes were subjected to a chase reaction (i.e. transport from the bound state), two faster migrating species were produced (panel A, lane 4). The band at Mr 16.4 kDa is the monomeric mature form of OE17. The band at 32 kDa has the expected molecular weight of an mOE17 dimer, indicating that both signal peptides have been removed. mOE17 and mOE17 dimer were not produced in a chase reaction that contained ionophores that dissipate the proton gradient (lane 5). When DTT was included in the chase reaction, only the monomeric mOE17 was produced (lane 6). Protease post-treatment of the membranes confirmed that mOE17 monomer and dimer were transported to the lumen (lane 8). Trace quantities of tOE17 dimer and tOE17 monomer were protected from thermolysin, suggesting that some of the transported precursor was not processed by the signal peptidase (and see below, Figure 8). The complete thermolysin degradation of the dimeric and monomeric precursor present in the binding sample (lane 7) and chase with ionophores sample (lane 9) provide comparative evidence that the unprocessed species in the chase sample have been transported to the lumen.
To determine whether pre-binding of the precursor before transport is necessary for disulphide formation, a single stage transport reaction was conducted whereby the precursor protein was incubated with thylakoid membranes that were energized with a proton gradient (Figure 1D). Both the dimeric precursor (panel D, lane 2) and dimeric mOE17 (lanes 2 and 4) were produced during the reaction, indicating that disulphide linkage of precursors occurs during the normal time course of protein translocation.
Disulphide-linked precursors are primarily formed when Cys residues are located near the amino-terminus of the mature domain of the precursor
tOE17 V-20F with single Cys residues at varying locations in the signal peptide and mature domain (Figure 2A) were subjected to bind and chase assays to determine which regions of bound precursors are sufficiently close to form disulphides (Figure 2B). Precursors were bound to thylakoid membranes, which were then washed with import buffer (lane B). An aliquot of precursor-bound membranes was also treated with the oxidant copper (II)-1,10-phenanthroline (CuP) to actively promote disulphide formation (lane Cu). A second aliquot of precursor-bound membranes was subjected to a chase assay (lane C). As shown in Figure 2B, upper panel, Cys residues in the signal peptide, either amino-flanking or within the hydrophobic core did not form disulphides under any condition. Cys residues within the first 37 amino-proximal residues of the mature domain formed disulphides either with or without CuP (Figure 2B, middle panel). This region has a loosely packed structure, as determined from the crystal structure of the spinach protein, and is followed by a four-α-helix bundle (Balsera et al, 2005). The maize and spinach proteins share 70% identity and 90% similarity, suggesting that the maize OE17 used in this study has the same structural features as the spinach protein. Cys residues in different regions of the four-α-helix bundle region of OE17 also formed disulphides (Figure 2B, lower panel). Two of these variants (68C and 149C) only formed disulphides when CuP was used. The variant 137C formed very little dimer and only with CuP. This was expected because in correctly folded OE17, residue 137 is largely buried on the interior face of helix 4 (Balsera et al, 2005).
Figure 2.
Precursors oligomerize on the thylakoid membrane primarily through Cys residues near the amino-terminus of the mature domain. (A) Diagram of the maize tOE17 precursor protein sequence. Amino acids are numbered relative to the thylakoid processing signal peptidase (Tpp) cleavage site. The signal peptide and two structural domains of the mature protein (Balsera et al, 2005; Ristvejova et al, 2006) are depicted. Precursor proteins in (B) and (C) also contained a phenylalanine (F) in position −20 (Gerard and Cline, 2007). (B) Binding and chase assays with precursors substituted with unique Cys residues at positions designated in the figure. Membranes recovered from binding assays were washed with IB (lane B), treated with the oxidant CuP (lane Cu), or subjected to a chase assay (lane C). Lane S contains supernatant from the binding assay. Samples were analysed by non-reducing SDS–PAGE and fluorography. (C) Binding and chase assays with the double Cys precursor [3H] tOE17-25C-20F3C and precursor [3H] tOE17-20F3C17C. Designations of protein species are as in Figure 1 except that putative precursor tetramers and the processed tetramers (PT) are indicated. Their average Mrs (in kilodaltons) are indicated. (D) Binding and chase assays with [3H] tOE173C, which has the wild-type sequence in the hydrophobic core of the signal peptide.
Introduction of two Cys residues in amino-proximal mature domain of the precursor, that is at positions +3 and +17, resulted in a precursor dimer band and a band that migrated at the expected Mr for a precursor tetramer (Figure 2C, lane 5). When the membranes were incubated under transport promoting conditions, the putative tetramer was processed to a smaller size (lane 6) and transported to the lumen as determined by protection against thermolysin treatment (see below in Figure 8). The processed form of the tetramer migrated with a Mr consistent with the removal of four signal peptides. This suggests an organization of receptor-bound precursors in which the mature domain amino-proximal regions of at least four precursors are spatially close enough to form disulphide cross-links. The tetramer could not be detected with precursor tOE17-25C-20F3C (lanes 2–3), although it also contains two Cys residues. This is likely due to inability of the −25C in the signal peptide region to form disulphides efficiently. Also shown in Figure 2D is that the tight binding afforded to precursors by phenylalanine in the RR +2 position is not essential for disulphide formation. tOE173C, with the wild-type sequence in the hydrophobic core, formed disulphide-linked dimer very efficiently.
Disulphide formation occurs on the membrane
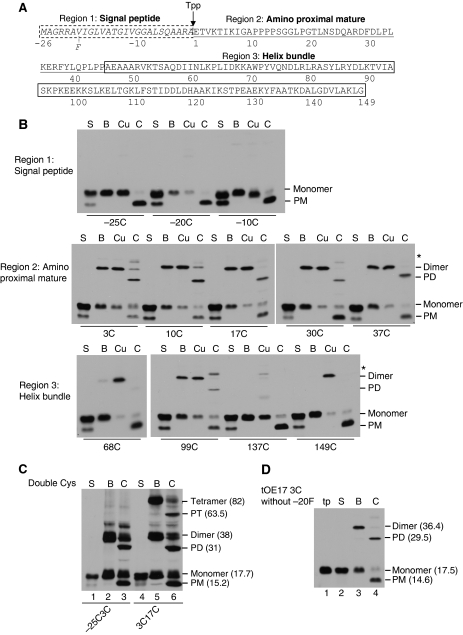
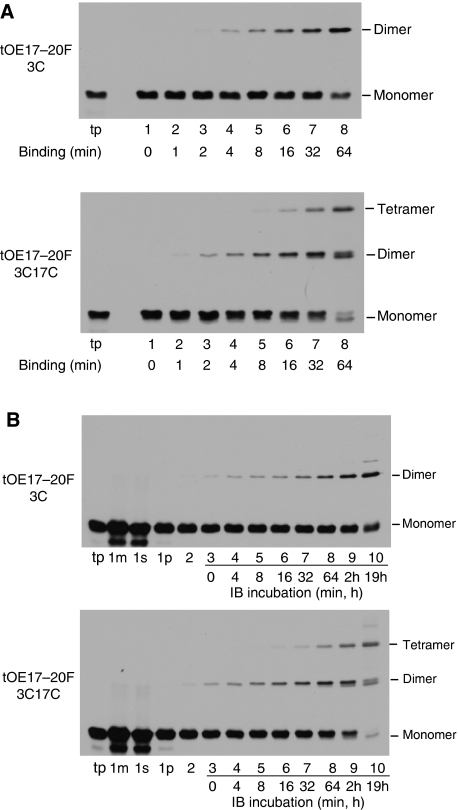
Experiments in Figures 1 and 2 suggest that dimerization through disulphide formation occurs on or after precursor binding. This question was examined more fully in the experiments in Figure 3. A time course experiment of binding and disulphide formation was conducted with tOE17-20F3C or tOE17-20F3C17C that were desalted to remove the reducing agent DTT present in the translation reaction (Figure 3A). At each time point, N-ethyl maleimide (NEM) was added to inactivate free sulfhydryls and thereby quench disulphide formation. Control experiments showed that NEM stopped disulphide formation within 10 s of its addition (data not shown). Virtually identical results were obtained when disulphide formation was quenched with trichloroacetic acid, which rapidly adjusts the sample to a pH non-permissive for disulphide formation (Supplementary Figure S1). On fractionation of samples, precursor dimer and tetramer were found in the thylakoid membrane fraction (Figure 3A), but not the supernatant (data not shown). Precursor binding to the membrane occurred as rapidly as could be measured with this method, whereas disulphide formation occurred more slowly. Nevertheless, dimers could be detected as soon as 1 min after the start of the assay and tetramers as soon as 8 min. Importantly, this occurred in the absence of any oxidant to accelerate the reaction.
Figure 3.
Oligomerization occurs after precursor binding to the thylakoid membrane. (A) Time course analysis of oligomerization during binding of desalted in vitro translated [3H] tOE17-20F3C (upper panel) or [3H] tOE17-20F3C17C (lower panel) at 0°C. At the times indicated, disulphide formation was quenched with 4 mM NEM and membranes recovered from assay mixtures were washed with buffer containing 5 mM NEM. Samples were analysed by non-reducing SDS–PAGE. (B) Time course analysis of oligomerization after precursor binding. tOE17-20F3C (upper panel) or tOE17-20F3C17C (lower panel) were incubated with thylakoids in the presence of 2 mM DTT. The assay mixture (lane 1m), supernatant fraction (lane 1s) and thylakoid fraction (lane 1p) of binding assays were analysed directly with SDS–PAGE. Thylakoids were then washed with IB containing 2 mM DTT (lane 2), and then quickly washed twice with import buffer lacking DTT (total time, ∼7 min), and were incubated in import buffer on ice for the times indicated. Disulphide formation was quenched by non-reducing urea-SDS sample buffer containing 5 mM NEM.
To specifically determine whether precursors already bound to thylakoids could subsequently form disulphide bonds, the timing of disulphide formation was further investigated by uncoupling it temporally from binding. Precursors were bound to thylakoids in the presence of 2 mM DTT, and the membranes recovered and subsequently washed in the presence of 2 mM DTT to prevent disulphide formation (Figure 3B, lanes 1m, 1s, 1p and 2). The thylakoids were then quickly washed with IB lacking DTT and incubated on ice. At each time point, an aliquot was removed and quenched with SDS sample buffer containing NEM (lanes 3–10). The results indicate that precursor oligomerization occurs after binding as rapidly as could be measured with this assay (lane 3), and gradually increased over time (lanes 4–10). One interesting aspect of these time course experiments is that precursor trimers were not detected with tOE17-20F3C17C at any time point, even though disulphide formation was rapidly quenched with NEM to prevent post-assay disulphide formation.
During the later time points of tOE17-20F3C17C oligomerization, a dimer band with higher mobility on the SDS gel appeared (Figure 3A, lane 8; Figure 3B, lane 10). This raised the possibility that the faster band represented an inefficiently formed C3–C17 disulphide. However, this seems not to be the case. A time course of binding and oligomerization was conducted with a mixture of tOE17-20F17C and a C-terminally truncated tOE17-20F3C (Supplementary Figure S2). The mixed dimer, which migrated at an intermediate location in the gel, formed as rapidly as the homodimer.
Disulphide formation occurs between precursors that are each specifically bound to a receptor unit
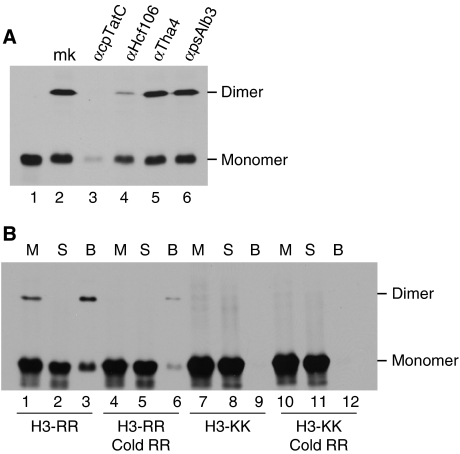
Experiments were conducted to assess whether disulphide formation results from specific binding to the receptor complex, that is as opposed to non-specific interaction of precursor with thylakoids or association of an unbound precursor with a receptor-bound precursor. First, thylakoids were pre-treated with IgGs directed against cpTatC or Hcf106 and, as controls, with IgGs against Tha4, as Tha4 is not involved in precursor binding (Cline and Mori, 2001), or psAlb3, an integrase protein of the chloroplast SRP pathway (Figure 4A). As expected, dimer formation was unaffected by treatment with either αTha4 or αpsAlb3 IgGs (lanes 5 and 6). Dimer formation was eliminated by αcpTatC IgGs and substantially reduced by αHcf106 IgGs. This experiment indicates that precursor binding to the receptor complex is required for disulphide formation between precursors.
Figure 4.
Dimer formation results from specific binding of both cross-linked precursors. (A) Precursor dimerization requires a functional cpTatC-Hcf106 complex. Thylakoids were treated with IgGs to individual Tat pathway transport machinery components as indicated and to anti-psAlb3 as a negative control. The resulting thylakoids along with mock-treated thylakoids (lane mk) were then incubated with tOE17-20F3C in binding reactions. Lane 1 is an aliquot of translation product used for assays. (B) Both of the cross-linked precursors must specifically bind to the thylakoid membrane to form a dimer. [3H] tOE17-20F3C (H3-RR) or the non-functional [3H] KKtOE17-20F3C (H3-KK), either alone or mixed with unlabelled tOE17-20F3C (Cold RR), were incubated in binding reactions. Aliquots of binding mixture (lane M), the supernatant fraction (lane S) and the membrane fraction (lane B) were analysed by non-reducing SDS–PAGE and fluorography.
A binding experiment was conducted with a mutant twin lysine (KK) precursor containing a Cys residue at residue 3 to examine whether both members of the precursor dimer must be specifically bound (Figure 4B). In earlier experiments, twin lysine precursors bound to thylakoids in trace amounts could not be cross-linked to the receptor and were not recovered with the receptor complex on BN–PAGE (Cline and Mori, 2001; Gerard and Cline, 2006). As shown in Figure 4B, lanes 7–9, KK precursor dimer was not detected in the binding mixture (M, lane 7), the supernatant (S, lane 8) or the membranes (B, lane 9), and the KK precursor was virtually absent from the membrane fraction (B, lane 9). To test the possibility that the KK precursor might cross-link to a specifically bound precursor, the binding reaction was conducted with a mixture of the KK precursor and an unlabelled RR precursor (lanes 10–12). Again, virtually no KK precursor was associated with the membranes (lane 12), and dimer was not detected. As a control to verify the effect of the unlabelled RR precursor, it was included in a binding experiment with radiolabelled RR precursor (lanes 4–6). As expected, it competed for binding and reduced the amount of radiolabelled dimeric and monomeric precursor (lanes 4–6; compare with lanes 1–3).
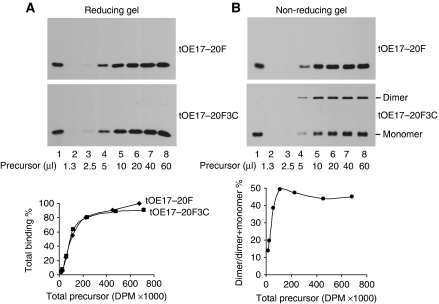
Finally, to determine whether both precursors of a dimer must be bound to the receptor complex, precursor-binding saturation experiments were conducted. If only one member of the dimer is specifically bound, then at saturation, substantially more precursors should be associated with the membranes than the saturation quantity of precursor lacking Cys. As seen from the reducing SDS–PAGE analysis of samples (Figure 5A), binding with and without dimer formation was nearly identical. Analysis by non-reducing SDS–PAGE (Figure 5B) shows that in this experiment, dimer accounted for ∼50% of the total-bound precursor at higher amounts of precursor. These results support the interpretation that at the time of cross-linking to dimer, both precursors are specifically bound to a receptor unit. They also indicate that free precursors did not exchange with bound precursors for receptor sites after cross-linking, as this would also increase the total amount of precursor at saturation. Figure 5B also shows that dimeric precursor is relatively less abundant than monomeric precursor at low precursor concentrations. This is the expected result if multiple occupancy of the same receptor complex is requisite for disulphide cross-linking.
Figure 5.
Dimerization does not increase total precursor binding to thylakoids. Thylakoids (20 μg chlorophyll) were incubated in a binding assay for 30 min with 1.3–60 μl of [3H] tOE17-20F or [3H] tOE17-20F3C precursor as indicated in lanes 2 through 8 and were subsequently washed twice before analysis. Lane 1 is an aliquot of translation product. (A) Samples were analysed by reducing SDS–PAGE to determine the total amount of precursor bound, which was quantified by scintillation counting of extracted gel bands (Cline, 1986) and plotted below the fluorogram. Total binding from the 60 μl tOE17-20F reaction was arbitrarily set at 100%. (B) Samples in (A) were analysed by non-reducing SDS–PAGE to determine the relative amounts of precursor dimer and monomer in each sample. The plot below the fluorogram depicts the dimer yield as a percentage of the total amount of precursor bound (i.e. dimer plus monomer).
Multiple precursors are bound to the same receptor complex
BN–PAGE of digitonin-solubilized thylakoids readily detects the cpTat receptor at ∼700 kDa and precursor-bound receptor complexes at ∼750 kDa (Cline and Mori, 2001). Thylakoid membranes from binding assays with tOE17-20F, tOE17-20F3C or tOE17-20F3C17C were dissolved with digitonin and subjected to BN–PAGE on a 5–13.5% gel (Figure 6). One half of the gel was processed for fluorography to locate the bound radiolabelled precursor (Figure 6A). Identical lanes in the other half of the BN gel were subjected to a second dimension SDS–PAGE (Figure 6C–E). As shown in panel A (lane 1), receptor-bound tOE17-20F migrated predominantly as a single band on the blue native gel at ∼750 kDa, but with some smearing up to 800 kDa. tOE17-20F3C and tOE17-20F3C17C migrated at ∼750 kDa, but with pronounced band(s) at ∼800 kDa. On the second dimension SDS gel, it can be seen that the dimer (panels D and E) and tetramer (panel E) bands are shifted slightly towards the ∼800 kDa region with respect to the monomer. Importantly, the dimer and tetramer bands did not migrate any larger than the 880 kDa ferritin standard. This indicates that dimeric and tetrameric precursors are on individual receptor complexes rather than bridging two receptor complexes, which would have migrated at ∼1400 kDa. One interesting aspect of the 2D gel of tOE17-20F3C17C (panel E) is that the tetramer band is more heavily represented than tetramer in the SDS-standard in lane 1 (leftmost pattern). The possibility that additional tetramer formed subsequent to digitonin solubilization was tested by SDS–PAGE analysis of samples of the digitonin-solubilized membranes that were stored at 4°C until after the 2D gel was completed (Figure 6B, lanes 4–6). In those samples, the relative abundance of tetramer, dimer and monomer was similar to that in the 2D gel (compare lane 6 of panels B and E). This indicates that the precursors can form additional disulphide linkages after detergent solubilization.
Figure 6.
Cross-linked precursor dimers and tetramers are formed within individual receptor complexes. (A) [3H] tOE17-20F (lane 1), [3H] tOE17-20F3C (lane 2) or [3H] tOE17-20F3C17C (lane 3) precursors were incubated with thylakoids in binding reactions. Recovered thylakoids were solubilized in 1% digitonin and analysed by BN–PAGE and fluorography. Locations of molecular weight markers ferritin monomer and dimer are indicated on the left of the panel. (B) tOE17-20F (lanes 1 and 4), tOE17-20F3C (lanes 2 and 5) or tOE17-20F3C17C (lanes 3 and 6) precursor-bound thylakoids from (A) were either dissolved in urea-SDS sample buffer (lanes 1–3) or first solubilized in buffer containing 1% digitonin and then >24 h later mixed with urea-SDS sample buffer (lanes 4–6). Samples were analysed by non-reducing SDS–PAGE. (C–E) BN–PAGE lanes [3H] tOE17-20F (C), [3H] tOE17-20F3C (D) or [3H] tOE17-20F3C17C (E) were subjected to second dimension SDS–PAGE. An aliquot of respective binding sample in urea-SDS, mixed with molecular weight markers, was run on the left side of the SDS–PAGE gels (lane 1).
Cross-linked precursor is transported without breaking the linkage
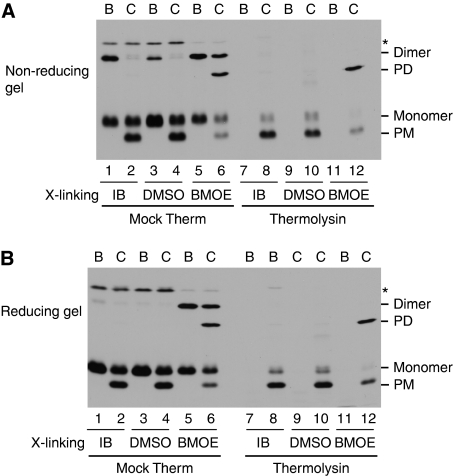
The results in Figures 1 and 2 suggest that precursors are transported across the membrane while still cross-linked. However, the formal possibility remained that the disulphide linkage was broken during the chase reaction and reformed in the lumen. To address this possibility, irreversible cross-links were made between bound tOE17-20F3C precursors by incubation with the bis-maleimide cross-linker bis-maleimidoethane (BMOE) (Figure 7, lanes 5, 6, 11 and 12). The cross-linked precursor-bound membranes were then subjected to a chase reaction in the presence of DTT to reduce any disulphide present. Controls included incubation of bound tOE17-20F3C with IB (lanes 1, 2, 7 and 8) or with IB containing DMSO (lanes 3, 4, 9 and 10), the solvent for BMOE. Samples were analysed by non-reducing SDS–PAGE (panel A) and reducing SDS–PAGE (panel B). As shown in Figure 7A, dimeric precursor was formed either naturally by disulphide formation (lanes 1 and 3) or mediated by BMOE (lane 5). When analysed on reducing SDS–PAGE (Figure 7B), the disulphide-linked dimer was dissociated to monomer (lanes 1 and 3), whereas the BMOE-linked dimer was unaffected (lane 5). As shown, a decrease of BMOE-linked dimer was accompanied by a corresponding appearance of BMOE-linked dimeric mature protein (lane 6), which was present in the lumen as evident by protection against thermolysin post-treatment (lane 12). As expected, on the reducing SDS gel, only monomeric species were present without BMOE (panel B, lanes 1–4, 7–10). Therefore, dimeric precursors, each of which is bound to a receptor site, are transported across the membrane while irreversibly linked. A similar experiment, conducted with the tOE17-20F3C17C precursor, showed that the tetrameric precursor was transported without breaking the linkage (Supplementary Figure S3).
Figure 7.
Cross-linked precursor dimer can be transported without breaking the linkage. tOE17-20F3C precursor was incubated with thylakoids in binding reactions without any additions (lanes 1, 2, 7 and 8) or with 0.5 mM TCEP to prevent disulphide formation (lanes 3–6, 9–12). The thylakoids were then subjected to cross-linking with BMOE from a DMSO stock solution (lanes 5, 6, 11 and 12), mock treated with DMSO (lanes 3, 4, 9 and 10) or mock treated with import buffer (IB). The thylakoids were buffer washed without (lanes 1, 2, 7 and 8) or with (lanes 3–6, 9–12) 2 mM DTT. Recovered thylakoids were either analysed directly as binding samples (lane B) or used for further chase assays as described in Materials and methods (lane C). Aliquots of the recovered thylakoids were treated with the thermolysin (lanes 7–12). Samples were analysed by non-reducing SDS–PAGE and fluorography (A) or reducing SDS–PAGE and fluorography (B).
Oligomeric precursors are transported with efficiencies similar to the monomeric precursor
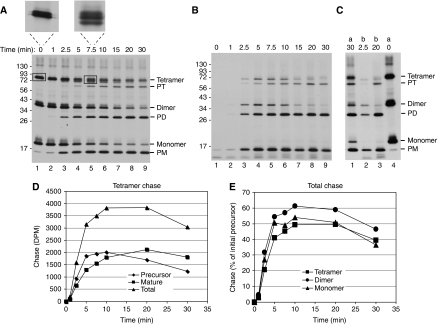
A time course of chase of the oligomeric precursors of tOE17-20F3C17C was conducted to determine the relative efficiencies with which oligomers are transported. The chase reaction was conducted without stromal extract or DTT to prevent reduction of disulphides during the chase. Aliquots were removed at varying times; the chase stopped with ionophores and recovered membranes analysed without or with post-treatment with thermolysin to degrade non-transported species. The appearance of the processed mature forms in the untreated membrane samples suggests that transport of dimeric precursor lags behind that of monomer and that transport of tetrameric precursor lags behind dimer (Figure 8A). However, this is misleading because transported tetrameric and dimeric precursors are processed slowly. This can be seen in panel B, in which significant amounts of tetrameric and dimeric precursors are resistant to protease, showing their presence in the lumen (e.g. compare the 5 and 7.5 min time points to the 0 time point). Closer inspection of the tetrameric precursor band after 5–10 min chase shows the appearance of two smaller bands, indicating partial processing of signal peptides (panel A insets). In support of this interpretation is a side-by-side comparison showing that the protease-resistant tetrameric precursor is smaller than the initial tetrameric precursor band (panel C). It is likely that the disulphide formation through Cys +3, which is very close to the processing site, impairs the action of the thylakoid signal peptidase. Quantification of protease-resistant tetramer precursor and fully mature form (panel D) shows the early appearance and slow decline of protease-resistant precursor, and the corresponding accumulation of mature form. When protease-resistant precursor and mature forms are summed and plotted (panel E), it can be seen that the chase of tetramer, dimer and monomer are similar, with only a small lag of tetramer chase with respect to monomer and dimer chase. It can also be seen that there are no protease-protected bands between the positions of processed tetramer and precursor dimer (panel B). This might have been expected if the individual mature domains of the tetramer were transported separately.
Figure 8.
Chase of oligomeric precursor proteins. Washed thylakoids were incubated with [3H] tOE17-20F3C17C and unlabelled mTha4 at 15°C in darkness for 20 min. Thylakoids were recovered, washed twice with IB, resuspended in IB containing 10 mM MgCl2 and stored on ice for 50 min. Precursor-bound thylakoids were then mixed with an equal volume of 10 mM ATP and transferred to a 25°C light bath. Aliquots were removed at the times indicated at the top of the panel and diluted six-fold in ice-cold IB containing 0.75 μM nigericin and 1.5 μM valinomycin. Each aliquot was divided into two portions: one was analysed directly by non-reducing SDS–PAGE/fluorography and the other was thermolysin treated before analysis. Radioactive proteins were quantified by scintillation counting of bands extracted from gel slices. (A) Fluorogram of samples analysed directly. Insets above show magnifications of the tetramer precursor region from the designated lanes (boxed bands). (B) Fluorogram thermolysin-treated samples. (C) Side by side comparison of selected samples from A and B, showing that the protease-protected tetramer precursor bands are partially processed. Lanes 1 and 4 are from (A); lanes 2 and 3 are from (B). (D) Quantification of the protease-protected tetramer precursor and mature forms showing the slow rate of signal peptide processing. (E) Chase of all species. The percentage was calculated based on the amount of precursor form present at the beginning of the chase and included a correction for the additional two leucines in the signal peptide. The precursors for tetramer, dimer and monomer were initially present at a ratio of 1 : 1.25 : 1.04, respectively.
Discussion
This study provides several new insights into the function of the Tat transport apparatus. First, we found that each receptor complex can bind more than one precursor protein. Second, precursors bound to the same receptor complex are sufficiently close that they form disulphides without an oxidant. Third, oligomers as large as tetramers are collectively transported to the lumen with efficiency nearly as great as that of monomers.
Earlier structural results of the bacterial Tat receptor complex found at most two precursors per complex (Tarry et al, 2009). In that study, the precursor-bound complexes were obtained from substrate-saturated bacterial membranes by detergent solubilization and subsequent chromatography procedures that may have released or dislodged a significant amount of precursor. The results reported here (Figures 2, 3, 4, 5, 6, 7 and 8) show that on the membrane, substrate-saturated receptor complexes bind at least four precursors. Site-directed cross-linking studies indicate that cpTatC (TatC) is the main recognition component for the signal peptide (Alami et al, 2003; Gerard and Cline, 2006) and the number of cpTatC (TatC) per receptor complex has been estimated at 7–13 based on the apparent size and composition of detergent-solubilized complexes (Mori and Cline, unpublished) (Gohlke et al, 2005). Structural studies support the implication; the geometry of precursor protrusions from the Tat receptor complex observed by Tarry et al (2009) suggest seven potential precursor-binding sites per receptor complex. This raises the possibility that in situ even more than four precursors might be bound to a single receptor complex. Our current efforts are focused on determining the stoichiometry of a fully saturated receptor complex.
The observation that a precursor with two Cys residues in the amino-proximal mature domain produced dimers and tetramers, but not trimers, is interesting. Trimers were absent even when reactions were quenched with NEM to rapidly inactivate all remaining sulfhydryls (Figure 3). In principle, trimers are possible with double Cys-substituted proteins, as exemplified by double Cys variants of the Tat component Tha4 (Dabney-Smith and Cline, 2009), wherein trimers were readily detected as major disulphide cross-linking products. One possibility is that structural constraints of the OE17 moiety hinder trimer formation. A second possibility is that dimers, not trimers, are an intermediate stage in the formation of tetramers. In support of this interpretation, Tarry et al (2009) observed that when two precursors were bound to receptor complexes, they invariably occupied adjacent sites. In addition, the time course studies of oligomerization (Figure 3) show that dimers appeared well in advance of tetramers.
Our results with different Cys variant precursors (Figure 2) provide clues to the topography of bound precursors in situ. Cys residues placed within the signal peptide did not result in precursor dimers, suggesting that each signal peptide is bound to an isolated binding site. This is consistent with earlier analyses of signal peptide interactions with components of the receptor complex. Photocross-linking with (Tmd)-Phe translationally inserted within signal peptide regions of tOE17 resulted in cross-linking to either cpTatC or Hcf106 (Gerard and Cline, 2006). Very weak, if any, cross-linking was observed between amino-proximal mature domain residues and Tat components. Similar results were obtained for preSufI and the bacterial Tat system (Alami et al, 2003). Structural analysis of the preSufI precursor bound to isolated bacterial receptor complexes also suggests that the signal peptide, but not the mature domain, is buried in the receptor (Tarry et al, 2009).
The efficient disulphide cross-linking directed by Cys three residues from the signal peptidase cleavage site (Figure 1) was surprising when considered in the context of the structural model, wherein the pre-SufI mature domains project with an angle of 50° from an ∼10 nm diameter receptor complex (Tarry et al, 2009). This would place the emergence of the first few mature domain residues at a far greater distance than the required ∼4 Å between cysteine β-carbons of adjacently bound precursors needed to form a disulphide. Disulphide formation between the more distal residues, that is 10C through 37C (Figure 2), is more understandable, considering the apparent structural flexibility of the OE17 amino-proximal mature domain (Balsera et al, 2005) and the projected structure of preSufI mature domains from the bound complex, wherein certain loops of adjacent-bound preSufI mature domains appear to closely approach each other (Tarry et al, 2009).
On the other hand, the notion that the in situ conformation of precursors bound to receptors is different from that of isolated complexes also deserves consideration. Examination of membrane-bound precursors by indirect methods suggests that the amino-proximal mature domain of some precursors is not accessible to exogenous agents. For example, thylakoid bound tOE17-20F with engineered cleavage sites in carboxyl proximal signal peptide flanking domains up to residue +39 was not readily accessible to cleavage by factor Xa, whereas the unbound precursor was readily cleaved (Gerard and Cline, 2007). Other experiments suggest that precursors insert into membranes and/or receptor complexes through a hairpin loop consisting of the signal peptide and some of the amino-proximal mature domain (Fincher et al, 1998; Hou et al, 2006). In such a configuration, amino-proximal mature domain residues of adjacent precursors might be better spaced to form disulphides. In addition, the implied immediate environment of such a configuration might be more conducive to spontaneous formation of disulphides. Solubilization of receptor complexes with detergents may remove the constraints of membrane that stabilize such a configuration. Certainly, these considerations highlight the need for a more detailed description of the conformation of precursors bound to receptor units, as this is the antecedent to the conformation during translocation.
Regardless of the actual organization of precursors in the receptor complex that allows them to form disulphides, it seems that the cross-linked structure is very compatible with the translocation step. As shown in Figures 7 and 8 and Supplementary Figure S3, cross-linked dimer and tetramer are readily transported into the lumen without breaking the linkage. The implication of these results is that multiple-occupied receptor units operate coordinately to transport the oligomer. Although our data do not rule out the possibility that only one signal peptide of a cross-linked tetramer directs transport, characteristics of tOE17-20F binding to the receptor suggest that members of a cross-linked oligomer would not dissociate from their binding sites before translocation. The tOE17-20F precursor used to construct the Cys-labelled precursors has a very high affinity for the receptor and a very low rate of dissociation. Incubation in buffer solutions for 30 min releases <5% of the bound precursor protein, and substantial dissociation of the precursor from its binding site requires extraction with salt plus urea (Gerard and Cline, 2007). Rapid exchange of precursors between the receptor bound and non-specifically bound precursor protein has been documented for some precursors in thylakoids and bacteria (Musser and Theg, 2000; Bageshwar et al, 2009). However, such exchange is also unlikely because of the very low level of non-specifically bound precursor that results from our assay conditions as determined by anti-cpTatC pre-treatment of membranes (Figure 4A), protease pre-treatment of membranes (Gerard and Cline, 2007) and binding of the non-functional twin lysine precursor (Figure 4B).
The question of whether or not all signal peptides of the oligomer remain bound during the translocation step is more difficult to answer. Tat systems in bacteria transport very large precursor proteins (Berks et al, 2003) and even heterodimers directed by a single signal peptide (Rodrigue et al, 1999). Thus, in principle, it should be possible to transport an 80 kDa tetrameric precursor through a single signal peptide. However, the thylakoid in vitro Tat system seems not to be as robust as the bacterial Tat system. Substrates smaller than the ∼80 kDa tetramer reported here stalled during transport, and polyphenol oxidase (∼60 kDa), the largest known substrate of the thylakoid Tat system, is not transported significantly in our in vitro assay (Cline and McCaffery, 2007). Thus, the comparable transport efficiencies of tetramer, dimer and monomer (Figure 8) support the interpretation that multiple signal peptides of the cross-linked oligomer remain bound to receptor sites and active for translocation.
If our interpretations are correct, then this leads to the exciting possibility that multiple precursor-bound receptor units can act in concert to transport a large precursor protein. Current models for the operation of the Tat system suggest that precursor proteins bind to the receptor, which triggers assembly of a Tha4 oligomer and that the Tha4 oligomer facilitates movement across the bilayer (Bruser and Sanders, 2003; Gohlke et al, 2005). By inference, our results suggest that at least four adjacent sites can simultaneously recruit a sufficient amount of Tha4 to allow transport of all members of the cross-linked precursor. The situation described in this work does not allow conclusions regarding the normal mode of transport in situ because the transport of a tetramer was enforced by disulphide cross-linking. However, it is interesting to note that the glucose-fructose oxidoreductase of Zymomonas mobilis is synthesized as an enzymatically active tetrameric ∼180 kDa precursor, in which each subunit possesses a twin arginine signal peptide (Nurizzo et al, 2001). The fact that this enzyme is transported in fully folded form suggests that collaboration of signal peptide-bound units for translocation of large oligomers may be a natural strategy of Tat systems. Perhaps the translocation kinetics of precursors exhibiting different levels of receptor complex occupancy can resolve this interesting question.
Materials and methods
Plasmid construction and mutagenesis
Transcription plasmids for the various precursors were constructed by PCR mutagenesis with the QuikChange mutagenesis kit from Stratagene. The clones for tOE17 (Henry et al, 1997) and tOE17V-20F (Gerard and Cline, 2007) were used as template DNA. Cysteine substitutions were obtained by replacing the relevant codons with TGC, and lysine substitutions of the twin arginine motif with AAG.
Preparation of precursor proteins
Capped mRNAs were transcribed in vitro with SP6 polymerase (Promega) and were translated in vitro in the presence of [3H]-leucine or unlabelled leucine with a homemade wheat germ translation system (Cline, 1986). The radiolabelled in vitro translation products are designated as [3H] precursors. When needed, the in vitro translation products were buffer exchanged with desalting spin columns equilibrated with import buffer (IB: 50 mM HEPES/KOH, pH 8.0, 0.33 M sorbitol) containing 2% BSA protein as described by the manufacture (Pierce).
Preparation of chloroplasts, thylakoids and stromal extract
Intact chloroplasts were isolated from 9 to 10-day-old pea seedlings (Cline et al, 1993). Chloroplast pellets were lysed at 2 mg of chlorophyll per ml in 10 mM HEPES/KOH, pH 8.0, containing 10 mM MgCl2 at 0°C for 6 min and adjusted to import buffer, 5 mM MgCl2. Thylakoids pellets were obtained from lysates by centrifugation at 3300 g for 8 min, were washed with IB and then resuspended in IB containing 10 mM MgCl2 at 1 mg of chlorophyll per ml before use. Stromal extract was obtained from the first supernatant by additional centrifugation at 12 000 g for 10 min. Chlorophyll concentrations were determined according to Arnon (1949).
Precursor-binding, chase and transport assays
Binding reactions were conducted by mixing apyrase-treated in vitro translated precursors with thylakoids as follows. After translation, precursors were mixed 1 : 1 with 60 mM leucine in 2 × IB and incubated with apyrase (10 units per 100 μl translation) for 10 min at 0°C. Unless stated otherwise, binding assays contained apyrase-treated precursor, thylakoids and IB in a 1 : 1 : 1 ratio and were incubated for 30 min in darkness at 0°C. Thylakoid membranes were then recovered by centrifugation, washed twice with IB and analysed as binding samples. As noted in the figure legends, some binding assays were treated with 1 mM CuP oxidant to accelerate disulphide formation (Dabney-Smith et al, 2006). After 2 min, oxidation was terminated with 1 mM EDTA and 4 mM NEM. Chase assays generally contained 20 μg chlorophyll of precursor-bound thylakoids, a stoichiometric amount of stromal extract, and 5 mM Mg-ATP in IB containing 3 mM MgCl2. Assays were incubated for 15 min at 25°C in ∼100 μmol/m2/s white light. Thylakoid membranes were recovered by centrifugation and analysed directly or first treated with thermolysin (Cline et al, 1993). Assays for protein transport into isolated thylakoids were conducted essentially as described earlier (Cline et al, 1993). Certain assays also contained 0.5 μM nigericin plus 1.0 μM valinomycin (Nig/Val) to dissipate the protonmotive force across thylakoid membrane. Binding assays with IgG- pre-treated thylakoids were conducted as described (Mori et al, 1999) with 25 μg chlorophyll of thylakoids treated with 37.5 μg of respective IgG.
SDS–PAGE
For non-reducing SDS–PAGE, the thylakoids samples were resuspended in 20 mM EDTA, pH 8.0 and then mixed with an equivalent volume of 2 × SDS sample buffer (100 mM Tris–HCl (pH 6.8), 8 M urea, 5 mM EDTA, 5% SDS and 30% glycerol) at 0.25 or 0.5 mg chlorophyll per ml. The samples were left at 25°C for at least 1 h and then analysed by 11.5% SDS–PAGE and fluorography. Typical sample loading was 2.5–5 μg chlorophyll. For reducing SDS–PAGE, 5% β-mercaptoethanol was added to the sample buffer and the samples were incubated for 15 min at 37°C and 45 min at 25°C.
BN–PAGE
Thylakoid membranes were solubilized in 1% digitonin, 0.5 × IB, 20% glycerol and 0.5 M amino-caproic acid at 1 mg chlorophyll per ml. BN–PAGE on 5–13.5% gradient 0.75-mm-thick gels and fluorography were as described (Cline and Mori, 2001; Gerard and Cline, 2007). For 2D analysis, excised BN–PAGE lanes were soaked in 1 × urea-SDS sample buffer for 10 min and were layered onto 1-mm-thick 11.5% SDS–PAGE.
BMOE cross-linking of bound precursors
Precursor-bound thylakoids were washed twice with IB in the presence of 0.5 mM of the reducing agent TCEP (tris-(2-carboxyethyl) phosphine) from Invitrogen. The thylakoids were then incubated with 1 mM BMOE (Pierce Chemical Co.), dispensed from a 40 mM stock in DMSO, in IB, pH 6.8, 2 mM EDTA and 0.5 mM TCEP. Cross-linking was for 8 min at 25°C. The thylakoids were then recovered by centrifugation and washed with IB containing 2 mM DTT. Recovered thylakoids were analysed directly or subjected to a chase assay. For chase, the thylakoids, at 1 mg chlorophyll per ml in IB, 10 mM MgCl2, were incubated with one volume of IB and one volume of unlabelled Tha4 translation products for 15 min at room temperature, were recovered by centrifugation and subjected to chase assays as described above.
Supplementary Material
Acknowledgments
We thank Cassie Patricia Aldridge, Jonathan Martin, Jose Celedon, Ricardo Rodrigues and Michael McCaffery for critical review of the paper. We also thank Michael McCaffery for excellent technical assistance. This work was supported in part by National Institutes of Health grant R01 GM46951 to KC.
Footnotes
The authors declare that they have no conflict of interest.
References
- Alami M, Luke I, Deitermann S, Eisner G, Koch HG, Brunner J, Muller M (2003) Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell 12: 937–946 [DOI] [PubMed] [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bageshwar UK, Whitaker N, Liang FC, Musser SM (2009) Interconvertibility of lipid- and translocon-bound forms of the bacterial Tat precursor pre-SufI. Mol Microbiol 74: 209–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsera M, Arellano JB, Revuelta JL, de las Rivas J, Hermoso JA (2005) The 1.49 A resolution crystal structure of PsbQ from photosystem II of Spinacia oleracea reveals a PPII structure in the N-terminal region. J Mol Biol 350: 1051–1060 [DOI] [PubMed] [Google Scholar]
- Berks BC, Palmer T, Sargent F (2003) The Tat protein translocation pathway and its role in microbial physiology. Adv Microb Physiol 47: 187–254 [DOI] [PubMed] [Google Scholar]
- Berks BC, Sargent F, Palmer T (2000) The Tat protein export pathway. Mol Microbiol 35: 260–274 [DOI] [PubMed] [Google Scholar]
- Bruser T, Sanders C (2003) An alternative model of the twin arginine translocation system. Microbiol Res 158: 7–17 [DOI] [PubMed] [Google Scholar]
- Cline K (1986) Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem 261: 14804–14810 [PubMed] [Google Scholar]
- Cline K, Henry R, Li C, Yuan J (1993) Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J 12: 4105–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, McCaffery M (2007) Evidence for a dynamic and transient pathway through the TAT protein transport machinery. EMBO J 26: 3039–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Mori H (2001) Thylakoid DeltapH-dependent precursor proteins bind to a cpTatC-Hcf106 complex before Tha4-dependent transport. J Cell Biol 154: 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Theg SM (2007) The Sec and Tat protein translocation pathways in chloroplasts. In Molecular Machines Involved in Protein Transport across Cellular Membranes, RE Dalbey CM Koehler, F Tamanoi (eds) Vol. XXV, pp 463–492. London: Elsevier [Google Scholar]
- Dabney-Smith C, Cline K (2009) Clustering of C-terminal stromal domains of Tha4 homo-oligomers during translocation by the Tat protein transport system. Mol Biol Cell 20: 2060–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabney-Smith C, Mori H, Cline K (2006) Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J Biol Chem 281: 5476–5483 [DOI] [PubMed] [Google Scholar]
- Fincher V, McCaffery M, Cline K (1998) Evidence for a loop mechanism of protein transport by the thylakoid Delta pH pathway. FEBS Lett 423: 66–70 [DOI] [PubMed] [Google Scholar]
- Gerard F, Cline K (2006) Efficient twin arginine translocation (Tat) pathway transport of a precursor protein covalently anchored to its initial cpTatC binding site. J Biol Chem 281: 6130–6135 [DOI] [PubMed] [Google Scholar]
- Gerard F, Cline K (2007) The thylakoid proton gradient promotes an advanced stage of signal peptide binding deep within the Tat pathway receptor complex. J Biol Chem 282: 5263–5272 [DOI] [PubMed] [Google Scholar]
- Gohlke U, Pullan L, McDevitt CA, Porcelli I, de Leeuw E, Palmer T, Saibil HR, Berks BC (2005) The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc Natl Acad Sci USA 102: 10482–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R, Carrigan M, McCaffrey M, Ma X, Cline K (1997) Targeting determinants and proposed evolutionary basis for the Sec and the Delta pH protein transport systems in chloroplast thylakoid membranes. J Cell Biol 136: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Frielingsdorf S, Klosgen RB (2006) Unassisted membrane insertion as the initial step in DeltapH/Tat-dependent protein transport. J Mol Biol 355: 957–967 [DOI] [PubMed] [Google Scholar]
- Leake MC, Greene NP, Godun RM, Granjon T, Buchanan G, Chen S, Berry RM, Palmer T, Berks BC (2008) Variable stoichiometry of the TatA component of the twin-arginine protein transport system observed by in vivo single-molecule imaging. Proc Natl Acad Sci USA 105: 15376–15381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, Tullman-Ercek D, Georgiou G (2006) The bacterial twin-arginine translocation pathway. Annu Rev Microbiol 60: 373–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cline K (2000) Precursors bind to specific sites on thylakoid membranes prior to transport on the delta pH protein translocation system. J Biol Chem 275: 10016–10022 [DOI] [PubMed] [Google Scholar]
- McDevitt CA, Hicks MG, Palmer T, Berks BC (2005) Characterisation of Tat protein transport complexes carrying inactivating mutations. Biochem Biophys Res Commun 329: 693–698 [DOI] [PubMed] [Google Scholar]
- Mori H, Cline K (2002) A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid [Delta]pH/Tat translocase. J Cell Biol 157: 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Summer EJ, Ma X, Cline K (1999) Component specificity for the thylakoidal Sec and Delta pH-dependent protein transport pathways. J Cell Biol 146: 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Klosgen RB (2005) The Tat pathway in bacteria and chloroplasts (review). Mol Membr Biol 22: 113–121 [DOI] [PubMed] [Google Scholar]
- Musser SM, Theg SM (2000) Characterization of the early steps of OE17 precursor transport by the thylakoid DeltapH/Tat machinery. Eur J Biochem 267: 2588–2598 [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann JM (2007) Translocation of proteins into mitochondria. Annu Rev Biochem 76: 723–749 [DOI] [PubMed] [Google Scholar]
- Nurizzo D, Halbig D, Sprenger GA, Baker EN (2001) Crystal structures of the precursor form of glucose-fructose oxidoreductase from Zymomonas mobilis and its complexes with bound ligands. Biochemistry 40: 13857–13867 [DOI] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B (2005) Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol 21: 529–550 [DOI] [PubMed] [Google Scholar]
- Ristvejova J, Kopecky V Jr, Sovova Z, Balsera M, Arellano JB, Green M, Ettrich R (2006) Structure and dynamics of the N-terminal loop of PsbQ from photosystem II of Spinacia oleracea. Biochem Biophys Res Commun 345: 287–291 [DOI] [PubMed] [Google Scholar]
- Rodrigue A, Chanal A, Beck K, Muller M, Wu LF (1999) Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial tat pathway. J Biol Chem 274: 13223–13228 [DOI] [PubMed] [Google Scholar]
- Rounds C, Wang F, Schnell DJ (2007) The Toc machinery of the protein import apparatus of chloroplasts. In Molecular Machines Involved in Protein Transport across Cellular Membranes, RE Dalbey CM Koehler, F Tamanoi (eds) Vol. XXV, pp 415–438. London: Elsevier [Google Scholar]
- Sargent F, Berks BC, Palmer T (2006) Pathfinders and trailblazers: a prokaryotic targeting system for transport of folded proteins. FEMS Microbiol Lett 254: 198–207 [DOI] [PubMed] [Google Scholar]
- Stanley NR, Palmer T, Berks BC (2000) The twin arginine consensus motif of Tat signal peptides is involved in Sec-independent protein targeting in Escherichia coli. J Biol Chem 275: 11591–11596 [DOI] [PubMed] [Google Scholar]
- Tarry MJ, Schafer E, Chen S, Buchanan G, Greene NP, Lea SM, Palmer T, Saibil HR, Berks BC (2009) Structural analysis of substrate binding by the TatBC component of the twin-arginine protein transport system. Proc Natl Acad Sci USA 106: 13284–13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.