Abstract
Centrosomes are cellular organelles that have a major role in the spatial organisation of the microtubule network. The centrosome is comprised of two centrioles that duplicate only once during the cell cycle, generating a procentriole from each mature centriole. Despite the essential roles of centrosomes, the detailed structural mechanisms involved in centriole duplication remain largely unknown. Here, we describe human procentriole assembly using cryo-electron tomography. In centrosomes, isolated from human lymphoblasts, we observed that each one of the nine microtubule triplets grows independently around a periodic central structure. The proximal end of the A-microtubule is capped by a conical structure and the B- and C-microtubules elongate bidirectionally from its wall. These observations suggest that the gamma tubulin ring complex (γ-TuRC) has a fundamental role in procentriole formation by nucleating the A-microtubule that acts as a template for B-microtubule elongation that, in turn, supports C-microtubule growth. This study provides new insights into the initial structural events involved in procentriole assembly and establishes the basis for determining the molecular mechanisms of centriole duplication on the nanometric scale.
Keywords: centriolar barrel, centrosome, electron cryo tomography, microtubule triplet, procentiole
Introduction
The centrosome is a structure located near the geometric centre of interphase cells and duplicates only once during the cell cycle. The centrosome is comprised of two centrioles that are structurally similar to the basal body. Centrioles are surrounded by the pericentriolar material (PCM), which is responsible for the nucleation and organisation of the microtubule network (Paintrand et al, 1992). Centrioles exhibit a highly conserved nine-fold symmetry of stable microtubule blades, and are most often formed by microtubule triplets containing a complete A-microtubule and two incomplete B- and C-microtubules. During interphase, centrioles can give rise to cilia and flagella and are referred to basal bodies (Dutcher, 2003).
Numerous electron microscopy studies have established that centriole duplication begins at the G1/S transition when one procentriole appears next to the proximal end of each mature centrioles and elongates during late S/G2 phase, reaching their full length during the next cell cycle (Kuriyama and Borisy, 1981; Vorobjev and Chentsov Yu, 1982; Chrétien et al, 1997). Despite the recent discovery of proteins that have essential roles in centriole duplication (Strnad and Gonczy, 2008; Bettencourt-Dias and Glover, 2009), structural data are available only for Caenorhabditis elegans in which some details of the early procentriole formation steps have been revealed (Pelletier et al, 2006). First, a 60-nm-long central tube, oriented perpendicular to the wall of the mother centriole, is formed. Second, the diameter and the length of this tube increase while microtubules assemble around its circumference. The first step depends on SPD-2, ZYG-1, SAS-5, and SAS-6, whereas the second step involves SAS-4. However, C. elegans centrioles are singlet microtubules organised around a tube and differ from the triplets in mammalian centrioles, which are supposed to organise around a cartwheel (Cavalier-Smith, 1974; Azimzadeh and Bornens, 2007).
In this study, we report the structural morphogenesis of human procentriole. We show that procentriole organise around a cartwheel, composed by a periodical central hub. Our results describe the microtubule triplet formation. The A-microtubule is nucleated by a gamma tubulin ring complex (γ-TuRC)-like structure, whereas the B- and C-microtubules are formed from the wall of A- and B-microtubules, respectively, and grow bidirectionally. In addition, each of the nine microtubule triplets grows independently around this periodic central structure.
Results and discussion
To determine the first steps of human procentriole formation, we studied duplicating centrosomes isolated from human KE37 lymphoblasts (Bornens et al, 1987) using cryo-electron tomography. A total of 21 tomograms of centrosome showing a procentriole close to its parent were computed. The samples displayed good overall preservation, allowing the observation of centrioles surrounded by PCM (Figure 1; Supplementary movie 1). As expected, mature centrioles showed distal and subdistal appendages (Supplementary Figure S1A and B) and nine sets of angled microtubule triplets (Figure 1 and Supplementary Figure 1D). A careful observation of procentrioles revealed that they either show singlets, doublets, or microtubule triplets, suggesting that they are at different duplication stage. To classify our tomograms, we took advantage of basal-body assembly studies, which revealed that centriolar wall formation starts with singlet, then doublet, and finally triplet of microtubules (Dippell, 1968). Moreover, Kuriyama and Borisy (1981) and Chrétien et al (1997) have shown that the centrosome cycle can be defined by two criteria: the microtubule length and the number of microtubules in a triplet (initially one microtubule, followed by two and three microtubules). Accordingly, we classified our tomograms following these criteria, that is, the number of microtubules in the procentriolar wall and microtubule length.
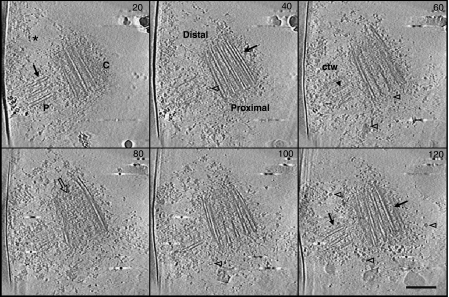
Figure 1.
Cryo-electron tomography of purified human centrosomes. Z slices (20 nm spacing) from a three-dimensional reconstruction (140 slices) of a centrosome, showing longitudinal sections of a centriole and its procentriole surrounded by the pericentriolar material (*: PCM). The procentriole (P) is perpendicular to the proximal part of the mature centriole (C). Arrows point towards microtubule blades. Ring-shaped objects (open arrowheads) are visible in the PCM. The central structure of the cartwheel (ctw) is distinguished in the procentriole (arrowhead in slice 60). The distal extremity of the mature centriole is filled with electron dense material (open arrow in slice 80). Scale bar, 250 nm.
The central hub of the cartwheel is a periodic structure
A 100-nm central structure having a 20-nm diameter at the proximal end of the procentriole is observed from the earliest stage of duplication (procentriole with one microtubule) until late duplication stage (procentriole with nine long microtubule triplets; Figure 2A and B; Supplementary Table I). This central structure is reminiscent of the central hub of the basal body cartwheel (Dippell, 1968; Cavalier-Smith, 1974), but the radial spokes cannot be distinguished in our data, probably due to the resolution of 4 nm. Moreover, a 110-nm stalk seemed to connect the central hub to the parent centriole (Figure 2A and B; Supplementary movie 2). However, the cartwheel and the stalk are visible only in few tomograms probably due to the high protein density of PCM.
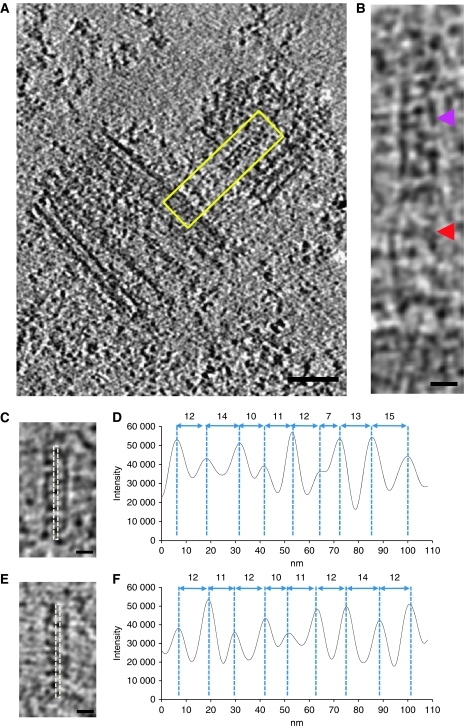
Figure 2.
Visualisation of the initial procentriole structures. (A) Z-section of a tomogram showing the central structure of the cartwheel and the connecting stalk (boxed in yellow). (B) Magnified view of the boxed region in (A), showing the central tube of the cartwheel (purple arrowhead) and the connecting stalk (red arrowhead). (C, E) Projection images of 23 Z-sections from two cryo-tomograms containing the central cartwheel structure. (D, F) Profile plots obtained from corresponding images (dotted lines) in (C, E). Maxima appear each 11.75 nm (with a s.d. of 2.5 and 1.16 nm, respectively). Scale bars: 100 nm (A) and 20 nm (B, C, E).
To improve the central hub analysis, volumes presenting the best visualisation (nominal defocus of ∼ 4 μm) were cropped from two tomograms. The density profiles of their corresponding 2D projections demonstrated the presence of structures periodically repeating every 12 nm (Figure 2C and F). On the basis of the 100-nm length and the 12-nm repeat, we propose that the central hub is composed by eight or nine rod-like structures. This periodic structure, which was not previously observed in human centrioles, may contribute to the nine-fold symmetry of the centrosome. In other organisms, the cartwheel has been suggested to participate in the establishment of the nine-fold centriolar symmetry on the basis of different arguments: (1) bld12 (Sas-6 homologue) null-mutants in Chlamydomonas lack the central hub of the cartwheel in which bld12 localises, and a variable number of triplets are observed (Nakazawa et al, 2007); (2) human Sas-6 has been localised to the proximal end of the procentriole and disappears in the mature centriole when the cartwheel is absent (Strnad et al, 2007; Strnad and Gonczy, 2008); (3) Overexpression of SAS6 reveals its involvement in organising a tube-like centriole precursor in Drosophila (Rodrigues-Martins et al, 2007); (4) Bld10, another cartwheel protein, stabilises the nine-fold symmetry of centrioles (Hiraki et al, 2007).
A γ-TuRC-like stucture is required for the nucleation of the A-microtubule
In the nascent procentriole, singlets corresponding to the A-microtubule were observed (Figures 3A and B and 4A and B). Interestingly, all A-microtubules appeared to be closed at their proximal end, whatever procentrioles showed singlet, doublet or triplet microtubules. (Figure 3A and E; Supplementary Table I). However, in fully developed mature centrioles this cap was no longer present (Figure 3G). This cap displays a conical shape and presents an asymmetry reminiscent of the γ-TuRC structure (Moritz et al, 2000; Zhang et al, 2000). This structure is strikingly similar to that found adjacent to the spindle pole body in budding yeast (O'Toole et al, 1999), and to the minus end of microtubules nucleated from Drosophila melagonaster centrosomes or from isolated γ-TuRC (Moritz et al, 1995, 2000). Therefore, it seems likely that this cap-like structure, observed here at the proximal end of the A-microtubule, corresponds to the γ-TuRC. To the best of our knowledge, this is the first time that a γ-TuRC-like structure has been described at the proximal end of the centriolar microtubule, suggesting its crucial involvement in nucleating and stabilising the A-microtubule. In fact, γ-tubulin and nedd1 recruitment of γ-TuRC have been shown to be essential for centrosome duplication (Ruiz et al, 1999; Garreau de Loubresse et al, 2001; Shang et al, 2002; Dammermann et al, 2004, 2008; Haren et al, 2006). Moreover, immuno EM labelling of γ-tubulin has been performed previously on isolated centrosomes after post-embedding (Moudjou et al, 1996) or on freeze-substituted samples (Fuller et al, 1995). Both studies reported gold particles close to the proximal end of the mature centriole, suggesting that the procentriole formation was requiring γ-tubulin.
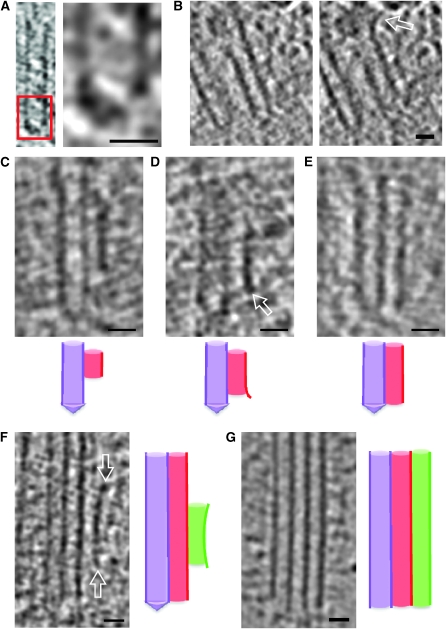
Figure 3.
Procentriole microtubule triplet growth. (A) Microtubule singlet of a procentriole. The proximal part of the microtubule is capped by a conical structure. The right panel is a 3 × magnification of the boxed region. (B) Two Z sections of an A-microtubule (singlet) spaced by 5 nm. The distal part of the microtubule forms an outwardly curved extension (open arrow). (C–E) Microtubule doublets from three different tomograms. Under each panel, a schematic representation of microtubule organisation is shown: A-microtubule is in purple, B-microtubule is in red (C). The B-microtubule is 35 nm from the tip of the A-microtubule cap. (D) The B-microtubule starts at 18 nm from the tip of the A-microtubule cap and shows an outwardly curved extension at its proximal extremity (open arrow). (E) The B-microtubule is at the level of the tip of the A-microtubule cap. (F) Microtubule triplet of a procentriole. The C-microtubule is attached to the side of the B-microtubule at a distance of 46 nm from the distal tip of the cap. The distal and proximal extremities of the C-microtubule display curved extensions (open arrows). (G) Microtubule triplet of a mature centriole. The microtubules are opened at their distal and proximal extremities. (F, G) Next to the panel, a schematic representation of microtubule organisation is shown: A-microtubule is in purple, B-microtubule is in red, and C-microtubule is in green. Scale bar: 20 nm.
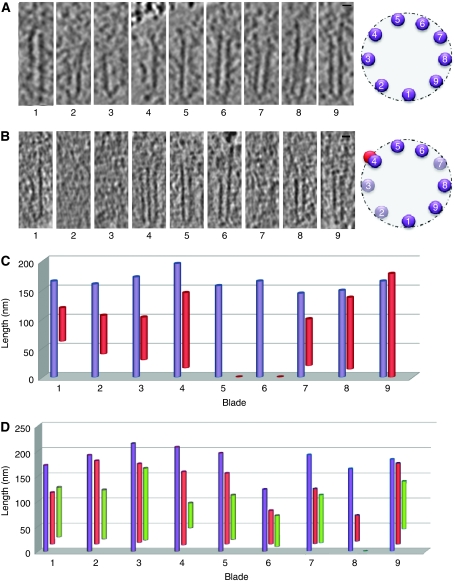
Figure 4.
The nine microtubule blades of the centriolar barrel. (A) Microtubule singlets present in a procentriole. Note that the length of each microtubule differs and is not arranged in order of size around the centriolar wall. Next to the panel, a schematic representation of the microtubule organisation in the centriolar wall is shown. A-microtubules are represented by a purple circle and numbered. (B) Procentriole displaying singlet or doublet microtubules. Note that three microtubule blades are not yet formed (2, 3, 7), whereas the blade 4 already shows a doublet microtubule. Next to the panel, a schematic representation of the microtubule organisation in the centriolar wall is shown. Present A-microtubules are represented by a purple circle and numbered, absent A-microtubules are in light purple, and B-microtubule is in red. Scale bar: 20 nm. (C, D) The length of the A-microtubule is measured from the proximal tip to its distal end. (C) Procentriole with nine blades of singlet (blade 5 and 6) or doublet microtubules. Note that the length of the A-microtubule is variable (in purple). The B-microtubule (in red) already reaches a length of 180 nm in blade 9, whereas the others are only 60 nm long (blade 1 or 2) or have not yet assembled (blades 5 and 6). (D) Procentriole with nine blades of doublet or triplet microtubules. Note that the C-microtubule (in green) in blade 8 is absent.
In contrast to the closed proximal ends of the A-microtubules, distal ends were open and showed a frequent long and slightly outwardly curved extension (Figure 3B). Such a microtubule structure was previously described for growing microtubules in vitro (Chrétien et al, 1995) and in vivo (Koning et al, 2008). Taken together, our ex vivo results suggest that the procentriolar A-microtubule grows unidirectionally from the proximal (minus) to the distal (plus) end, and are nucleated by a γ-TuRC like structure. This observation is in contrast with that of Pelletier et al (2006) in C. elegans, in which microtubules did not seem to grow preferentially at the distal or proximal extremities, though a slight positional bias for the distal region of the central tube was observed. This difference may reflect divergent mechanisms of assembly between the human and C. elegans centrioles, which involve the formation of a cartwheel structure in the former case and a tube in the later.
The A- and B-microtubules act as templates for the bidirectional growth of the B- and C-microtubules, respectively
In contrast to the A-microtubule, which was always capped at its proximal end, B- and C-microtubules were never closed at their extremities (Figure 3C and G; Supplementary Table I). This suggests that γ-TuRC may not be required for B- and C-microtubule nucleation. In addition, the proximal extremity of the B-microtubule was found at different heights with respect to the A-microtubule tip (Figure 3C and E), which was as far as 60 nm from the cap tip in several blades, at the same height of the tip, or even below in others (Supplementary Figure S2A and C). In addition, both the proximal and distal extremities of the B-microtubule showed outwardly curved extensions in growing procentriole (Figure 3D and F), suggesting that elongation took place at both extremities. Interestingly, the proximal end of the B-microtubule appeared blunt when it reached the proximal extremity of the A-microtubule (Figure 3E and F), whereas the distal end remained curved. This observation suggests that the B-microtubule no longer grows towards the proximal end but continues growing at the distal end. Similar observations were made for the C-microtubule (Figure 3F). These observations suggest that B- and C-microtubules are nucleated without γ-TuRC and that the A- and B-microtubules act as templates for the bidirectional growth of the B- and C-microtubules, respectively. Moreover, all microtubule triplets in the mature centriole were blunt and open at their proximal extremity (Figure 3G), suggesting that the γ-TuRC is no longer necessary and is removed from the A-microtubule ends when microtubule blades are fully developed and stabilised. This stabilisation has been attributed to tubulin modifications (Bobinnec et al, 1998) or to the presence of rare tubulins, such as δ- and ɛ-tubulins (Goodenough and StClair, 1975; Dupuis-Williams et al, 2002; Dutcher, 2003). In addition, the distal extremity of the microtubule triplet does not present outwardly curved extensions in agreement with the fact that mature centriole does not grow anymore (Kochanski and Borisy, 1990).
Asynchronous growth of the centriolar wall
To get deeper insight into centriolar wall formation, we examined the order of appearance of the nine A-microtubules in the nascent procentriole (Figure 4A–D). The formation of the first A-microtubules was not directly related to a specific position. Moreover, the position of each A-microtubule at the centriole wall did not correlate with its size, arguing against a sequential growth process. These findings suggest that each A-microtubule, and thus each blade in a procentriole, assembles in an independent manner. To confirm this hypothesis, we also analysed procentrioles with doublets or triplets. The B-microtubule was already present in some blades, whereas some A-microtubules were absent in others. Similar observations were made for C-microtubules with regards to B-microtubules (Figure 4D). These observations support an asynchronous growth model in which all blades assemble without any specific order. A sequential growth model has been proposed for Paramecium basal bodies (Dippell, 1968), which involves three rounds of microtubule formation: the second starts before completion of the first, and the third before completion of the second. Here, in contrast, triplets appeared independently, which might also reflect divergent mechanisms of assembly between Paramecium basal bodies and human centrioles.
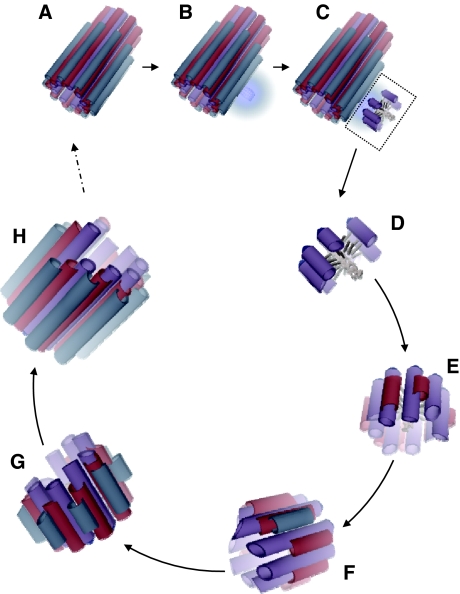
Model of human procentriole assembly
On the basis of our results, we present a scenario for procentriole morphogenesis (Figure 5). First, the central hub linked to parent centriole by a stalk is formed and directs the nine-fold symmetry. Second, the γ-TuRC is recruited around the parent centriole (Dammermann et al, 2004), allowing A-microtubule growth. The B-microtubules assemble using the outer surface of the growing A-microtubules as a template and polymerise bidirectionally, that is, from their plus and minus ends (Bergen and Borisy, 1980). Similarly, the C-microtubules assemble using the outer surface of the previously formed B-microtubules as a template. The distal end of the triplet continues growing while the proximal end stops, leading to blunt extremities just above the γ-TuRC. In the mature centriole, the A-microtubules are no longer capped, suggesting that the γ-TuRC remains present during B- and C-microtubule growth until the centriolar microtubule wall is stabilised (Goodenough and StClair, 1975; Bobinnec et al, 1998; Dupuis-Williams et al, 2002; Dutcher, 2003). Finally, according to the mechanism of centriole barrel construction presented in this study, the microtubule triplets assemble independently from each other.
Figure 5.
Model of human procentriole assembly. (A) Mature centriole. (B, C) The first step of procentriole assembly involves the formation of a stalk and the central hub of the cartwheel at the proximal end of the mature centriole (A). (C) The cartwheel assembles and organises the procentriolar wall, in which some A-microtubules start growing from the γ-TuRCs. (D) Enlargment of the procentriole shown in (C). (E) Before completion of the nine A-microtubule round, some B-microtubules start growing from the wall of A-microtubules. (F) B-microtubules grow bidirectionally, and C-microtubules start growing from the wall of the B-microtubules. (G) C-microtubules grow bidirectionally until the B- and C-microtubules reach the proximal end of the A-microtubule. (H) Growth continues at the distal end until completion of the microtubule triplet blades.
Therefore, these results define a structural pathway for the assembly of a daughter centriole around a transient cartwheel and led us to reconstruct a scenario for microtubule triplet formation and the centriolar barrel, which has never been described before. It will be of interest to understand the structural assembly differences observed in centrioles between C. elegans and mammals (Strnad and Gonczy, 2008; Loncarek and Khodjakov, 2009), whereas the molecular pathway involved in centriole duplication is conserved throughout the evolution.
Materials and methods
Centrosome purification
Centrosomes were purified from KE-37 cells as described previously (Bornens et al, 1987). Immunofluorescence study, using ctr453 and anti-γ-tubulin antibodies, was performed on each sucrose fraction to estimate the centrosome concentration.
Sample preparation and freezing
Purified centrosomes were centrifuged at 10 000 g in an Eppendorf tube after dilution in 10 mM K-PIPES (pH 7.2). The centrosomes were resuspended in 10 μl of 10 mM K-PIPES and mixed with 1 μl of 15-nm gold particles. Approximately 5 μl of this sample was deposited onto a Lacey carbon film grid (300 microMesh) and blotted for 3 s. The grid was plunged into liquid ethane using a Leica CFC.
Cryo-electron tomography
Grids were transferred into a JEOL JEM 2200FS cryo-electron microscope equipped with an Ω filter. Single-axis Z-loss tomographic tilt series at 4–13 μm underfocus (First 0 of the CTF, between 3.3 and 5.5 nm, respectively) were acquired applying the Saxton acquisition scheme, in which the higher tilt angles are sampled more than those close to 0°, improving the quality of single-axis reconstructions. Tilt angles vary between −60 and +60°. The microscope was operated at 200 kV, and images were acquired using a 2-k Ultrascan Gatan Camera used in binning 2 (Gatan, Pleasanton, CA, USA). The nominal magnification and energy window used were × 20 000 and 10 eV, respectively. Under these conditions, the pixel size corresponds to 1 nm. The maximum estimated total dose for a single tilt series was 75 e−/Å2.
Image processing and 3D rendering
Tilt series were aligned using the etomo software (http://bio3d.colorado.edu/imod/doc/etomoTutorial.html). The WBP and SART reconstructions were generated using etomo and TomoJ (http://u759.curie.u-psud.fr/softwaresu759.html), respectively. The tomogram visualisation and analysis were carried out using ImageJ software after applying a band-pass filter at the estimated resolution of 4 nm. The 3D rendering of the central hub and modelling were performed using Chimera (http://www.cgl.ucsf.edu/chimera).
Supplementary Material
Acknowledgments
We thank Dr C Kervran and S Blestel for their help in image analysis of the tomograms; Dr R Basto and Dr M Bornens for their critical reading of the paper; and G Keryer and L Mouawad for useful discussions. This study was supported by funds from the Agence National de la Recherche (ANR-PCV06-142771; to DC and SM); the 3DEM European network (LSHG-CT-2004-502828; to SM); the FRM and Canceropole idf (to SM); the Institut Fédératif de Recherche IFR 140 (to DC); and by a grant from the French Research Ministry (to PG).
Footnotes
The authors declare that they have no conflict of interest.
References
- Azimzadeh J, Bornens M (2007) Structure and duplication of the centrosome. J Cell Sci 120: 2139–2142 [DOI] [PubMed] [Google Scholar]
- Bergen LG, Borisy GG (1980) Head-to-tail polymerization of microtubules in vitro. Electron microscope analysis of seeded assembly. J Cell Biol 84: 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt-Dias M, Glover DM (2009) SnapShot: centriole biogenesis. Cell 136: 188–188.e1 [DOI] [PubMed] [Google Scholar]
- Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Edde B, Bornens M (1998) Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol 143: 1575–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornens M, Paintrand M, Berges J, Marty MC, Karsenti E (1987) Structural and chemical characterization of isolated centrosomes. Cell Motil Cytoskeleton 8: 238–249 [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T (1974) Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci 16: 529–556 [DOI] [PubMed] [Google Scholar]
- Chrétien D, Buendia B, Fuller SD, Karsenti E (1997) Reconstruction of the centrosome cycle from cryoelectron micrographs. J Struct Biol 120: 117–133 [DOI] [PubMed] [Google Scholar]
- Chrétien D, Fuller SD, Karsenti E (1995) Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J Cell Biol 129: 1311–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A, Maddox PS, Desai A, Oegema K (2008) SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the gamma-tubulin-mediated addition of centriolar microtubules. J Cell Biol 180: 771–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammermann A, Muller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K (2004) Centriole assembly requires both centriolar and pericentriolar material proteins. Dev Cell 7: 815–829 [DOI] [PubMed] [Google Scholar]
- Dippell RV (1968) The development of basal bodies in paramecium. Proc Natl Acad Sci USA 61: 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis-Williams P, Fleury-Aubusson A, de Loubresse NG, Geoffroy H, Vayssie L, Galvani A, Espigat A, Rossier J (2002) Functional role of epsilon-tubulin in the assembly of the centriolar microtubule scaffold. J Cell Biol 158: 1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK (2003) Elucidation of basal body and centriole functions in Chlamydomonas reinhardtii. Traffic 4: 443–451 [DOI] [PubMed] [Google Scholar]
- Fuller SD, Gowen BE, Reinsch S, Sawyer A, Buendia B, Wepf R, Karsenti E (1995) The core of the mammalian centriole contains gamma-tubulin. Curr Biol 5: 1384–1393 [DOI] [PubMed] [Google Scholar]
- Garreau de Loubresse N, Ruiz F, Beisson J, Klotz C (2001) Role of delta-tubulin and the C-tubule in assembly of Paramecium basal bodies. BMC Cell Biol 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, StClair HS (1975) BALD-2: a mutation affecting the formation of doublet and triplet sets of microtubules in Chlamydomonas reinhardtii. J Cell Biol 66: 480–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren L, Remy MH, Bazin I, Callebaut I, Wright M, Merdes A (2006) NEDD1-dependent recruitment of the gamma-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J Cell Biol 172: 505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki M, Nakazawa Y, Kamiya R, Hirono M (2007) Bld10p constitutes the cartwheel-spoke tip and stabilizes the nine-fold symmetry of the centriole. Curr Biol 17: 1778–1783 [DOI] [PubMed] [Google Scholar]
- Kochanski RS, Borisy GG (1990) Mode of centriole duplication and distribution. J Cell Biol 110: 1599–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning RI, Zovko S, Barcena M, Oostergetel GT, Koerten HK, Galjart N, Koster AJ, Mieke Mommaas A (2008) Cryo electron tomography of vitrified fibroblasts: microtubule plus ends in situ. J Struct Biol 161: 459–468 [DOI] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG (1981) Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol 91: 814–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J, Khodjakov A (2009) Ab ovo or de novo? Mechanisms of centriole duplication. Mol Cells 27: 135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Guenebaut V, Heuser J, Agard DA (2000) Structure of the gamma-tubulin ring complex: a template for microtubule nucleation. Nat Cell Biol 2: 365–370 [DOI] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA (1995) Microtubule nucleation by gamma-tubulin-containing rings in the centrosome. Nature 378: 638–640 [DOI] [PubMed] [Google Scholar]
- Moudjou M, Bordes N, Paintrand M, Bornens M (1996) gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci 109(Pt 4): 875–887 [DOI] [PubMed] [Google Scholar]
- Nakazawa Y, Hiraki M, Kamiya R, Hirono M (2007) SAS-6 is a cartwheel protein that establishes the nine-fold symmetry of the centriole. Curr Biol 17: 2169–2174 [DOI] [PubMed] [Google Scholar]
- O'Toole ET, Winey M, McIntosh JR (1999) High-voltage electron tomography of spindle pole bodies and early mitotic spindles in the yeast Saccharomyces cerevisiae. Mol Biol Cell 10: 2017–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintrand M, Moudjou M, Delacroix H, Bornens M (1992) Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struct Biol 108: 107–128 [DOI] [PubMed] [Google Scholar]
- Pelletier L, O'Toole E, Schwager A, Hyman AA, Muller-Reichert T (2006) Centriole assembly in Caenorhabditis elegans. Nature 444: 619–623 [DOI] [PubMed] [Google Scholar]
- Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, Glover DM (2007) DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr Biol 17: 1465–1472 [DOI] [PubMed] [Google Scholar]
- Ruiz F, Beisson J, Rossier J, Dupuis-Williams P (1999) Basal body duplication in Paramecium requires gamma-tubulin. Curr Biol 9: 43–46 [DOI] [PubMed] [Google Scholar]
- Shang Y, Li B, Gorovsky MA (2002) Tetrahymena thermophila contains a conventional gamma-tubulin that is differentially required for the maintenance of different microtubule-organizing centers. J Cell Biol 158: 1195–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad P, Gonczy P (2008) Mechanisms of procentriole formation. Trends Cell Biol 18: 389–396 [DOI] [PubMed] [Google Scholar]
- Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P (2007) Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell 13: 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev IA, Chentsov Yu S (1982) Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol 93: 938–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Keating TJ, Wilde A, Borisy GG, Zheng Y (2000) The role of Xgrip210 in gamma-tubulin ring complex assembly and centrosome recruitment. J Cell Biol 151: 1525–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.