EMBO J 29 10, 1637–1651 (2010); published online April012010
Balancing the flow of organelles and molecular complexes to and from the cell periphery is very important, and this is especially true for neurons, which are an extreme example of polarised cells. Neurons need to precisely regulate anterograde and retrograde transport of many cargoes such as mRNA, mitochondria and signalling molecules in time and space. Hence, there is a great deal of interest in identifying adaptor proteins controlling the association of molecular motors with different cargoes. One such class of adaptors is the Bicaudal-D (BICD) protein family. This issue of the EMBO Journal presents a study by Schlager et al, which describes two new members of the BICD family, Bicaudal-D-related protein-1 and 2 (BICDR-1 and BICDR-2), and identifies an essential role for BICDR-1 in neurons.
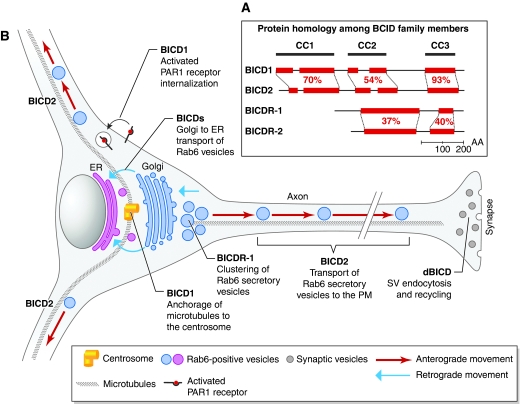
Bicaudal-D (BICD) (meaning ‘two tails') was first identified in Drosophila and named after the striking phenotype of its mutant, where embryos have an anterior-to-posterior transformation. BICD is an adaptor for dynein-dependent transport along microtubules. Only one BICD gene is present in invertebrates, whereas in mammals there are two, BICD1 and BICD2. In Drosophila, BICD is in a complex with Egalitarian (Egl) and has an essential role in dynein-mediated transport of mRNAs during oogenesis and early embryonic development (Bullock and Ish-Horowicz, 2001; Bullock, 2007). Both mammalian BICDs interact with the dynein–dynactin motor complex and the small GTPase Rab6 (Hoogenraad et al, 2001), and have been implicated in the dynein-dependent Golgi-to-ER transport of Rab6-positive vesicles (Matanis et al, 2002). BICD2 also has a role in the kinesin-1-mediated transport of Rab6-positive secretory granules containing exogenous neuropeptide-Y (Grigoriev et al, 2007) (see Figure 1 for a summary of some BICD functions).
Figure 1.
(A) The domain structure of the mammalian BICD proteins. The percentage of similarity between the different coil-coiled regions (CC; in red) is indicated. The similarity between CCs in BICDs and BICDRs is below 30% (Probir Chakravarty, Cancer Research UK London Research Institute). (B) Summary of some of the possible roles of BICD family members in neurons.
This recent study by Schlager et al (2010) identifies two novel Bicaudal-D-related genes, BICDR-1 and BICDR-2. BICDR-1 is conserved in vertebrates and its expression is restricted to the developing brain, eye, dorsal root ganglia and kidney. BICDR-1 binds to cytoplasmic dynein and is associated with the centrosome; it also directly interacts with Rab6 determining a pericentrosomal localisation of Rab6-positive vesicles. Importantly, BICDR-1 regulates the recruitment and/or activity of the anterograde kinesin motor Kif1C. As a consequence, its overexpression perturbs neurite extension in hippocampal neurons, phenocopying Rab6 silencing and p50 overexpression. Taken together, these observations suggest that BICDR-1 has non-redundant roles in neurons by regulating the trafficking of Rab6-positive secretory vesicles and localising them at the cell centre. The resulting perinuclear trapping of this secretory compartment might prevent the secretion of important factors required for neurite extension. By contrast, BICD2- and Rab6-positive vesicles are transported towards the cell periphery (Grigoriev et al, 2007), suggesting that BICDR-1 and BICD2 might compete with one another in maintaining the balance between anterograde and retrograde transport of specific cargoes.
These findings elucidate an exciting role for a new member of the BICD family in the regulation of intracellular trafficking in polarised cells, and show a reciprocal relationship between its levels of expression and neurite extension. This is the first function ascribed to any of the known BICD related proteins, suggesting that BICDR-1 and classical BICDs may function together to maintain essential aspects of nervous system development. Importantly, BICD1 is involved in microtubule anchorage at the centrosome and recruits ninein (Fumoto et al, 2006), which is crucial for centrosome homeostasis. It is intriguing that both BICD1 and BICDR-1 are both closely associated with centrosomes, which are fundamental for asymmetric cell division and radial glia migration during neurogenesis (Wang et al, 2009).
In addition, recent evidence suggests that BICD proteins control not only secretion but also endocytosis. Indeed, BICD1 has been implicated in clathrin- and dynamin-dependent uptake of activated PAR1 receptor (Swift et al, 2010; Figure 1). A recent paper by Li et al (2010) has also suggested a new role for BicD in synaptic vesicle recycling through transport of free clathrin back to the plasma membrane in Drosophila neurons. It remains to be determined whether BICDRs have a similar function in the context of endocytosis.
On the basis of these findings, BICD family members seem to have pleiotrophic roles in intracellular trafficking in distinct organisms. It is still unclear whether the function of BICD in mRNA localisation is conserved in mammalian cells, and how different BICDs are orchestrated to ensure correct development and cell homeostasis. In this regard, assessing the expression pattern of BICDs during development and their relative expression levels would greatly enhance our understanding of the interplay between the members of the BICD family, and help us to build up better models of the spatio-temporal control of molecular motors in neurons and other cells.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bullock SL (2007) Translocation of mRNAs by molecular motors: think complex? Semin Cell Dev Biol 18: 194–201 [DOI] [PubMed] [Google Scholar]
- Bullock SL, Ish-Horowicz D (2001) Conserved signals and machinery for RNA transport in Drosophila oogenesis and embryogenesis. Nature 414: 611–616 [DOI] [PubMed] [Google Scholar]
- Fumoto K, Hoogenraad CC, Kikuchi A (2006) GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J 25: 5670–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A (2007) Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 13: 305–314 [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A, Howell SA, Dortland BR, De Zeeuw CI, Willemsen R, Visser P, Grosveld F, Galjart N (2001) Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J 20: 4041–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kuromi H, Briggs L, Green DB, Rocha JJ, Sweeney ST, Bullock SL (2010) Bicaudal-D binds clathrin heavy chain to promote its transport and augments synaptic vesicle recycling. EMBO J 29: 992–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, Barnekow A, Hoogenraad CC (2002) Bicaudal-D regulates COPI-independent Golgi–ER transport by recruiting the dynein–dynactin motor complex. Nat Cell Biol 4: 986–992 [DOI] [PubMed] [Google Scholar]
- Schlager MA, Kapitein LC, Grigoriev I, Burzynski GM, Wulf PS, Keijzer N, de Graaff E, Fukuda M, Sheperd IT, Akhmanova A, Hoogenraad CC (2010) Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D related protein-1 (BICDR-1) regulates neuritogenesis. EMBO J 29: 1637–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Xu J, Trivedi V, Austin KM, Tressel SL, Zhang L, Covic L, Kuliopulos A (2010) A novel par1-interactor, bicaudal-D1, regulates G protein signaling and internalization. J Biol Chem 285: 11402–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH (2009) Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]