Abstract
Introduction
Pulmonary hypoplasia is a condition of the newborn that is characterized by underdeveloped lungs and poor outcome. One strategy in the treatment of patients with hypoplasia is to augment underdeveloped lungs using biocompatible artificial lung tissue. However, one central challenge in current pulmonary tissue engineering efforts remains the development of a stable bio-mimetic alveolar-capillary membrane. Accordingly, we have built a series of bio-mimetic microfluidic devices that specifically model the alveolar-capillary membrane. Current designs include a single-layer microchip that exposes alveolar and endothelial cell types to controlled fluidic stimuli. A more advanced multi-layered device allows for alveolar cells to be cultured at an air-interface while allowing constant media nourishment and waste removal, thus better mimicking the physiologic milieu of the alveolar-capillary interface. Both devices possess the benefit of parallel testing.
Material and Methods
Microdevices were fabricated using soft lithography in a biocompatible transparent polymeric material, polydimethyl siloxane (PDMS), sealed covalently to glass. The multi-stage microdevice also integrated a suspended polyethylene terephthalate (PET) membrane connected via microfluidic channels to constant media and air access. Pulmonary endothelial (HMEC-1) and alveolar epithelial (A549) cell lines, along with fetal pulmonary cells harvested from Swiss Webster mice at day-18 gestational age, were studied under multiple hydrodynamic shear conditions and liquid-to-cell ratio regimes. Cultures were examined for cell viability, function and proliferation to confluent monolayers. A549 cells cultured at an air-interface in a microdevice was also tested for their ability to maintain cell phenotype and function.
Results
The single layer differential flow microdevice allowed for a systematic determination of the optimal growth conditions of various lung-specific cell types in a microfluidic environment. Our device showed a greater surfactant based decrease in surface tension of the alveolar hypophase in A549 cultures exposed to air as compared to submerged cultures.
Conclusions
We have successfully developed biomimetic microfluidic devices that specifically allow stable alveolar cell growth at the air-liquid interface. This work serves prerequisite towards an implantable artificial alveolar membrane.
Keywords: Microfluidic, alveolar cells, air-interface culture, endothelial cells, shear forces
Pulmonary hypoplasia is responsible for more than 2800 deaths annually in the United States [1] with preterm birth being a major contributor. It is a condition in neonates characterized by reduced lung mass and deficient surfactant production from inadequately differentiated alveolar epithelium, leading to an impeded alveolar gas exchange [2]. Current state-of-the-art treatment of pulmonary hypoplasia as well as neonatal respiratory failure has resulted in a significant improvement in outcomes of these infants but therapies are still associated with the known complications of persistent pulmonary hypertension, hyaline membrane disease, and acute respiratory distress syndrome [3;4]. With preterm birth (< 37weeks) constituting 13% of all deliveries in the USA in 2005 [5], there is a clear medical need for innovative research platforms to improve fundamental scientific understanding and regenerative medicine.
Gas exchange in humans occurs in the alveolar sacs of the lung which has a unique cellular environment due to the direct interaction of lung tissue with both air and blood. The sub-micron thickness of the alveolar surface, in combination with the layering of the lung surface between the air and blood allows gas exchange to occur in the lungs at a rate high enough to support metabolism. Although more than a dozen pulmonary cell types have been identified, three cell types predominate the alveolar-capillary interface: alveolar, mesenchymal and endothelial cells. Microfluidic cell culture devices developed utilizing MEMS (Micro Electro Mechanical Systems) technology provide a unique opportunity to engineer a model of the alveolar interface built to physiologically-relevant scale that provides both an artificial vasculature as well as a source of controlled airflow to the cultured cells, an ability pivotal to maintain stable differentiation of alveolar type II cells in culture [6]. Furthermore, these microfluidic cell culture devices allow simultaneous support of several cultures on a single microfluidic device.
This article addresses the initial efforts in fabricating a progressive series of microdevices that allow the study of pulmonary cells in a microenvironment, to pilot the development of a biomimetic model of the alveolar membrane. These results demonstrate successful design of a long term supportive growth environment at the alveolar-capillary level and will serve as the preliminary steps towards engineering a functional biocompatible lung tissue.
1. Materials and methods
1. 1. Experimental Design
The first generation microdevice (Fig. 1) involves a single-layer microfluidic chip capable of providing continuous nutrient flow in direct contact with cultured endothelial and epithelial cell lines and shown to support cell growth for extended periods. This device was initially tested with cell lines representative of pulmonary cells, A549 (human alveolar epithelial cells) and HMEC-1 (human microvascular endothelial cells). Upon encouraging results with the cell lines [7], the device was applied to test the initial hypothesis that primary lung cells plated in the microfluidic device can receive adequate nutrition under flow to differentiate in culture. For this study, we employed fetal pulmonary cells harvested from Swiss Webster mice E18 day gestation. Fetal lung cells were cultured in the microculture chambers for a period of 10days under constant media perfusion. Cell viability, morphology, and proliferation were examined. Control groups were cells cultured in conventional flask culture.
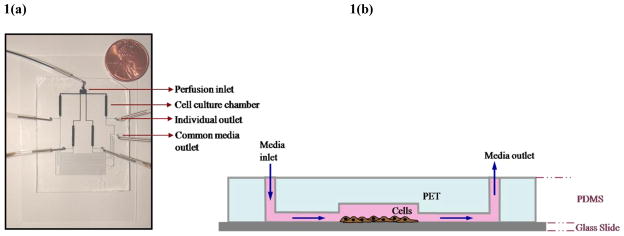
Figure 1. First generation microfluidic cell culture device.
Forming microfluidic channels in PDMS. (a) Digital image of the microdevice[7]. (b) Schematic of the cross-sectional view showing micro-channels and fluid access routes on-chip.
The second generation microdevice (Fig. 2) incorporates a multi-layer assembly of PDMS molds with a suspended PET membrane providing the culture surface. A549 monolayers cultured in these microdevices in two culture conditions were compared - exposed to ambient air and submerged in culture medium. Control groups were monolayers cultured in traditional transwells with similar cell culture surface. Cell functional and morphological analyses were performed to evaluate the micro-culture system in supporting air-exposed alveolar cultures.
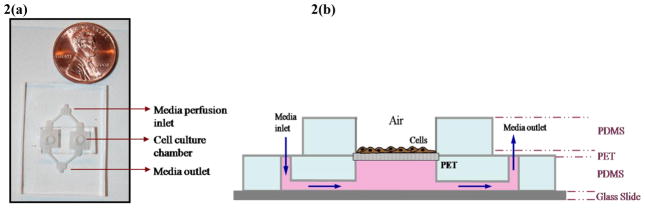
Figure 2. Second generation microfluidic cell culture device.
Forming microfluidic channels in PDMS. (a) Digital image of the microdevice[8]. (b) Schematic of the cross-sectional view showing micro-channels and fluid access routes on-chip
1. 2. Photolithography and Formation of Microfluidic Channels
Microfluidic network layouts created in L-Edit were used for lithographic masks and ~70μm thick templates made using SU-8 photoresist (SU-8 2050, Microchem Inc.) on silicon wafers. Briefly, the resist was spun at 3000rpm for 60s, soft-baked (5min at 65°C and 15min at 95°C) followed by UV exposure (400mJ/cm2, EVG 620 Maskaligner), post-baked (1min at 65°C followed by 10min at 95°C), and developed for 6min. The microfluidic pattern was molded in PDMS (Sylgard 184,Dow Corning) with precursors to curing agent ratio of 10:1 and allowed to crosslink for half hour at 80°C. The cured PDMS with the negative relief structure of the master template was used to form the microfluidic channels (Fig. 1 and 2).
First Generation Microdevices
Single-layer perfusion devices [17] (Fig. 1) were assembled by covalently bonding the PDMS molds to cell culture cover glass by exposure to oxygen plasma (2min at 200Torr and 25watt, PE II-A Plasma Asher, Technics West Inc.). All samples and cell suspensions were delivered to the microfluidic chips using 0.02inch tubing (Cole-Parmer, IL) fitted with steel needles. The sample injection rates were controlled by a syringe pump (NE 800, New Era Pump Systems Inc.).
Second Generation Microdevices
It is a multi-layered device in which the bottom layer of the microdevice, with embedded microfluidic channels, was covalently-sealed to a glass slide (Fig. 2), with a flat PDMS slab for the top layer [8]. Overlapping holes made in both layers served as the cell culture chambers. PET membranes (GE Osmonics) the size of the culture wells, with 0.4μm pores, were sandwiched between the two PDMS layers (Fig. 2) and sealed with liquid PDMS ‘mortar’ and slow-cured at 40°C for 4h.
1. 3. Cell Culture Experiments
A549, human alveolar basal epithelial cells, (passage 2–8, ATCC#CCL-185) cultured in F-12K Nutrient Mixture (Cellgro) with 2mM L-Glutamine, 1% pen-strep (P4458, Sigma-Aldrich) and 10% FBS (A-111-L, Hyclone Labs) was used. HMEC-1, human microvascular endothelial cells, (passage 2–8, CDC, Atlanta) were maintained in MCDB 131 medium (10372, Gibco) supplemented with 10% FBS, 10ng/ml EGF (354001, BD), 1% pen-strep and 1μg/ml hydrocortisone (H0888, Sigma-Aldrich).
Primary fetal cell isolation and culture
All animal procedures were carried out in accordance with a protocol approved by the University Animal Care and Use Committee (Protocol# MO06M368). Fetal pulmonary cells were isolated by a previously described method [9]. Briefly, surgically harvested fetal lungs were rinsed in HBSS and digested with 0.05% trypsin in PBS for 30min. DMEM containing 10% FBS was used to neutralize the trypsin and the resulting cell suspension filtered through a 70μm filter followed by centrifugation at 800rpm for 5min. The cell pellet was resuspended in complete medium (DMEM with 10% FBS and Pen-Strep) and plated for culture.
Experimental set up
PDMS microdevices and all microfluidic components (syringes, needles, and tubes) were UV-sterilized prior to use in experiments. The microchannels were filled with sterile PBS and trapped air bubbles were purged by applying back-pressure on the fluid network, forcing the trapped air through PDMS with high permeability to gases.
A549 and HMEC-1 cells
Microfluidic culture chambers were coated with 0.1% gelatin (3hr at 37°C). Cells suspended from conventional flask culture using 0.05% trypsin-EDTA (Gibco 25300–054) were seeded into microfluidic chips to yield on-chip cell seeding densities of 1.5×105 cells/ml.
Fetal Pulmonary Cells
For primary cells, the channels were coated with 0.5% collagen (Type IV) and allowed to incubate for 1hr at 37°C. Channels were post-treated as mentioned above, and fetal pulmonary cells (freshly harvested and Passage-2) were seeded into the micro-chambers to obtain uniform on-chip cell densities. For high cell density experiments, seeding was repeated every half hour for a total of 4hrs until concentrated seeding was achieved.
The culture chips were maintained in a controlled 37°C and 5% CO2 environment and a syringe pump was used to initiate and maintain flow of fresh media at a constant flow rate of 0.05μl/min (unless otherwise stated).
Transwell Culture
Cell culture media was added to the top and bottom wells of Corning transwells (0.4μm pores, CLS3460, Sigma Aldrich), and allowed to equilibrate for at least an hour in the cell culture incubator. Trypsinized A549 cells were then seeded onto the membrane surface at 0.3×104 cells/cm2. Cells were sustained on F-12K medium with 10% FBS and allowed to proliferate to confluence over a period of 3 to 4 days. For experiments with cells exposed to air, cell culture media in the upper well of the transwell system was removed while the media in the lower basal compartment was retained for cell sustenance. Thereafter, spent medium was replaced with a fresh supply once every 3days for culture periods up to 3weeks.
For air-exposed cultures of A549 cells on chip, the protocol followed was identical to transwell culture, except for the continuous replenishment of media infused by a syringe pump at 0.3μl/min.
1. 4. Cell Viability and Morphology
Phase contrast images of the culture wells were acquired daily (Zeiss Axiovert 200) and analyzed using Metamorph Imaging software. Average cell count data was used to determine the mean cell growth rate [7]. Statistical significance was assessed using the student t-test for which results were considered significant when p<0.05. All data is presented as the average +/− standard error of the mean.
1. 5. Characterization of Cell Monolayer Function – Surfactant Droplet Test
An established method [10] of using a mixture of hydrophobic aliphatic and aromatic compounds to measure surface tension was employed to determine the change in surface tension values from production of surfactant proteins by alveolar cells. Briefly, 20μl test droplets of DMP/O (4:1 v/v Dimethylphthalate/n-Octanol) stained with Crystal Violet is deposited on the cell monolayer surface and surface tension of the hypophase is estimated from the ratio of the diameter of the droplet deposited (d) to that prior deposition (d0). High resolution digital images were used for the measurements and surface tension values determined from a calibration curve [10], for thin liquid substrates, with surface tension values for a range of (d/d0) values.
2. Results
2. 1. Flow Dependant Growth of Pulmonary Cell Cultures
The first generation differential flow microdevice allowed for systematic characterization of cell behavior in response to various fluid flow regimes. Optimal culture conditions with respect to seeding density, surface coating and media flow rates were determined for pulmonary cell types, A549 and HMEC-1; and fetal pulmonary cells in their 1st and 2nd passages.
Average cell count data was used to determine the mean cell growth rate [7] using the exponential growth model: dN/dt = rN, where ‘N’ is the cell number at time ‘t’, and ‘r’ is the growth rate (per unit time). The growth rates were determined from the semi-logarithmic plot of N versus time (in hours). Cell growth plateaued near 80% confluence, hence data from the initial culture days, while cells were in their exponential growth phase, were used in determining the growth rates.
Cell types representative of pulmonary cells that have been well-studied in several in vitro models of the alveolo-pulmonary barrier [11;12], A549 and HMEC-1 cells were chosen to provide an initial assessment of cell growth characteristics in microculture environment (growth curves under supplementary data). Figures 4a and 4b depict near confluent cultures of A549 and HMEC-1 cells respectively. These cultures were immunostained with cell-specific markers SP-A (Surfactant protein A) and CD31, indicating maintenance of cell phenotype. In case of A549s characterized as alveolar Type II cells that produce surfactant proteins to reduce alveolar surface tension, positive staining for SP-A indicated preservation of Type II differentiated state. Although cell growth rates were determined from the first week of culture in the microchambers, the cultures were sustained with fresh media for 3weeks to test their viability over long periods.
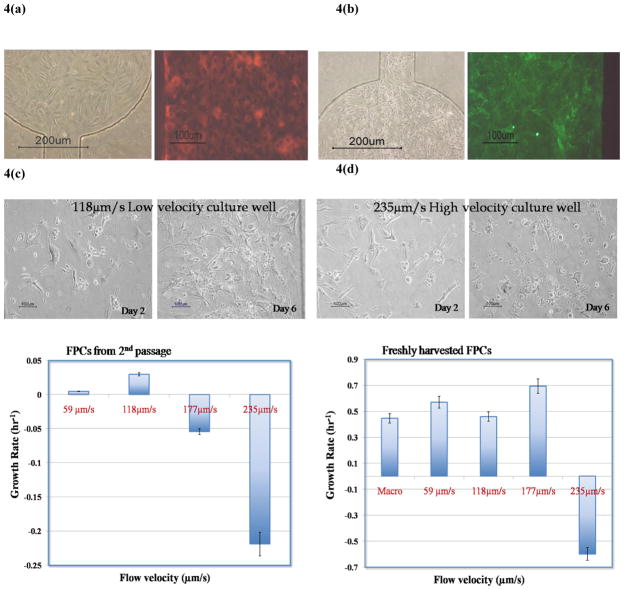
Figure 4.
Phase contrast and fluorescent images of (a) A549 and (b) HMEC-1 cells immunostained for SPA and CD31 respectively. (c) Phase contrast micrographs of FPCs in culture chambers with the highest and lowest flow velocities (within the range presented) (d) Average growth rate of FPCs in the microfluidic wells. The data point marked ‘macro’ corresponds to cells maintained in conventional flask culture.
A mixed-population of cells, from fetal lungs, was used to ascertain cell viability, proliferation and selective survival of different cell types exposed to a dynamic flow environment. Experimental protocol similar to that detailed above was used to assess growth of fetal pulmonary cells using the first generation microdevice (Fig. 4c and 4d). Growth kinetics (from averaged cell populations over entire culture wells) of the mixed population of cells (Fig. 4d) was recorded for each perfusion condition over a 7-day period, following a 24hr static-culture period during which cell growth lagged.
Freshly isolated fetal pulmonary cells and those in their second passage [initially seeded in cell culture flasks] were used to determine their ability to acclimate to the microenvironment. Although newly isolated cells were able to survive and proliferate in the micro culture chambers, cells that were initially seeded in culture flasks and harvested three days later were unable to survive and proliferate to the same degree (Fig. 4d). In addition, cells harvested from culture flasks in their second passage seemed to have a higher percentage of cells with distended cytoplasm as exhibited by fibroblasts or Type I cells. Also, within the initial category (recently isolated), cells failed to sustain growth past 3days in the culture well corresponding to the highest flow velocity (Fig. 4c). Cells proliferated with growth rates comparable to conventional culture (Macro data in Fig. 4d) in the other three wells. Although the wells with the higher velocity had a shorter media turnover rate [turnover rate related to the time required to replace spent media in the culture chamber with fresh media], the high fluid shear forces seemed to be detrimental to the cells (well with 235μm/s velocity flow, Fig. 4c–d). Cells grown in wells that exhibited proliferation showed well-adhered, healthy morphology compared to static culture with comparable growth rates as in conventional flask cultures.
2. 2. Maintenance of Cell Phenotype and Function in Pulmonary Cell Monolayers Cultured at the Air-Liquid Interface
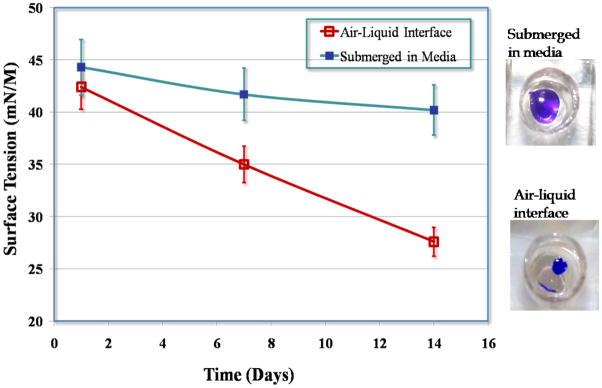
The second generation device was tested using the A549 epithelial cell line [8]. A549 cells were successfully supported at an air-liquid interface (Fig. 2) in a microfluidic environment for up to 2 weeks without loss in cell function. Cells cultured at the air-liquid interface better mimic their physiological environment, and also demonstrated a decrease in surface tension of the hypophase compared to cells cultured in media (Fig. 5). This was visible in the phase contrast images of the monolayers, with the air-interface cultures yielding images with greater contrast than the submerged cultures. This could be explained by the fact that the air-exposed cultures with a greater concentration of soluble surfactant proteins lead to decreased surface tension that allowed the formation of a protective thin-film around the cells, whereas in submerged cultures the higher surface tension hindered the formation of the thin film. This variation in hypophase composition in air-exposed cultures, allowed culture medium to enter the crevices of cell-cell junctions and highlight the ridges optically leading to images with higher contrast. The monolayer integrity was monitored using TEER (Trans Epithelial Electrical Resistance) measurements (data not shown) over the course of the culture period [8].
Figure 5.
Variation in surface tension of the A549 cell hypophase with time, dependant on culture conditions: submerged in media and at an air-liquid interface.
3. Discussion
This study involves the analysis of cell growth and behavior of primary cells, harvested from murine fetal lungs, in a microfluidic cell culture device exposed to various fluidic shear conditions. For a given inlet media flowrate, the microfluidic device was capable of routing the media into four culture wells at different flow velocities dictated by the variable resistances offered to each well. The resistance to fluid flow was manipulated by altering the dimensions [length and width] of the outlet fluidic channels leading to the common outlet (as shown in Fig. 1a).
Using microfluidic technology provides several advantages over traditional culture platforms. Firstly, it provides a unique microenvironment with physiologically comparable length scales and cell volume to extracellular fluid ratio. Secondly, it allows for high throughput parallel processing with near-identical experimental conditions thereby reducing errors. Thirdly, it accommodates complex architectures allowing the fabrication of 3-dimensional structures to mimic the complex in vivo structure.
The critical task is the determination of optimized culture conditions within the microenvironment, balancing various factors affecting cell adhesion and proliferation such as media turnover rates, surface coating, seeding density, and maximum sustainable shear forces. The pulmonary cell harvest from entire fetal lung yielded a mixed population of cells with each cell type carrying specific requirements in terms of media replenishment (dependant on metabolic activity), cell-to-cell proximity/interaction, surface coating, and resistance to shear forces. Our first generation microculture device was optimized with respect to the above mentioned critical growth parameters to yield cell growth rates comparable to conventional cultures in addition to exposing cells to a complex milieu of shear forces and large cell-to-extra cellular fluid ratios (CV/EV), unlike the static-cultures in petri-dishes/flasks with very small EC-to-EV ratios.
Taken together, this study demonstrated optimal growth of pulmonary cell types and their ability to maintain monolayer integrity for over 2 weeks within continuously perfused microfluidic devices and serves as a first step towards the development of a functional alveolar gas-exchange membrane mimicking in vivo pulmonary tissue. Future work will relate to making the microchannel constructs more physiologic and biocompatible, with increased scalability and ability to incorporate a co-culture of alveolar and endothelial cells on either side of the membrane.
Supplementary Material
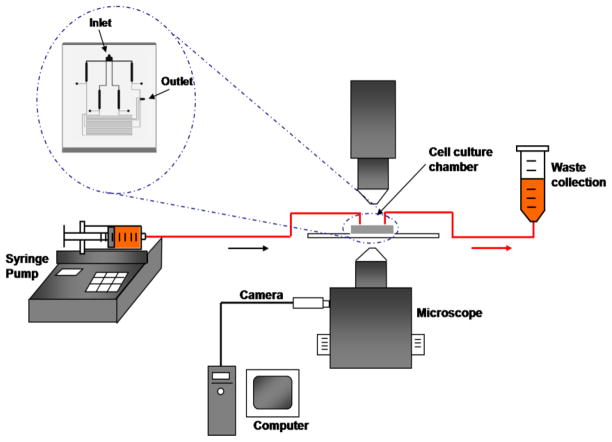
Figure 3.
Schematic of the experimental set-up.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mondrinos MJ, Koutzaki S, Jiwanmall E, et al. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 2.Sherer DM, Davis JM, Woods JR., Jr Pulmonary hypoplasia: a review. Obstet Gynecol Surv. 1990;45:792–803. doi: 10.1097/00006254-199011000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Sakurai Y, Azarow K, Cutz E, et al. Pulmonary barotrauma in congenital diaphragmatic hernia: a clinicopathological correlation. J Pediatr Surg. 1999;34:1813–1817. doi: 10.1016/s0022-3468(99)90319-6. [DOI] [PubMed] [Google Scholar]
- 4.Migliazza L, Bellan C, Alberti D, et al. Retrospective study of 111 cases of congenital diaphragmatic hernia treated with early high-frequency oscillatory ventilation and presurgical stabilization. J Pediatr Surg. 2007;42:1526–1532. doi: 10.1016/j.jpedsurg.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Center for Disease Control. 2005 http://www.cdc.gov/nchs/fastats/birthwt.htm.
- 6.Dobbs LG, Pian MS, Maglio M, et al. Maintenance of the differentiated type II cell phenotype by culture with an apical air surface. Am J Physiol. 1997;273:L347–L354. doi: 10.1152/ajplung.1997.273.2.L347. [DOI] [PubMed] [Google Scholar]
- 7.Nalayanda DD, Puleo CM, Fulton WB, et al. Characterization of pulmonary cell growth parameters in a continuous perfusion microfluidic environment. Exp Lung Res. 2007;33:321–335. doi: 10.1080/01902140701557754. [DOI] [PubMed] [Google Scholar]
- 8.Nalayanda DD, Puleo C, Fulton WB, et al. An open-access microfluidic model for lung-specific functional studies at an air-liquid interface. Biomed Microdevices. 2009 doi: 10.1007/s10544-009-9325-5. (in press) [DOI] [PubMed] [Google Scholar]
- 9.Mondrinos MJ, Koutzaki S, Jiwanmall E, et al. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 10.Schurch S, Goerke J, Clements JA. Direct determination of surface tension in the lung. Proc Natl Acad Sci USA. 1976;73:4698–4702. doi: 10.1073/pnas.73.12.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carterson AJ, Honer zu BK, Ott CM, et al. A549 lung epithelial cells grown as three-dimensional aggregates: alternative tissue culture model for Pseudomonas aeruginosa pathogenesis. Infect Immun. 2005;73:1129–1140. doi: 10.1128/IAI.73.2.1129-1140.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly JJ, Moore TM, Babal P, et al. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol. 1998;274:L810–L819. doi: 10.1152/ajplung.1998.274.5.L810. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.