Abstract
Background
The adeno-associated virus (AAV) has many safety features that favor its use in the treatment of arthritic conditions; however, the conventional, single-stranded vector is inefficient for gene delivery to fibroblastic cells that primarily populate articular tissues. This has been attributed to the inability of these cells to convert the vector to a double-stranded form. To overcome this, we evaluated double-stranded self-complementary (sc) AAV as a vehicle for intra-articular gene delivery.
Methods
Conventional and scAAV vectors were used to infect lapine articular fibroblasts in culture to determine transduction efficiency, transgene expression levels, and nuclear trafficking. scAAV containing the cDNA for interleukin (IL)-1 receptor antagonist (Ra) was delivered to the joints of naïve rabbits and those with IL-1β-induced arthritis. From lavage of the joint space, levels of transgenic expression and persistence were measured by enzyme-linked immunosorbent assay. Infiltrating leukocytes were quantified using a hemocytometer.
Results
Transgene expression from scAAV had an earlier onset and was approximately 25-fold greater than conventional AAV despite the presence of similar numbers of viral genomes in the nuclei of infected cells. Fibroblasts transduced with scAAV produced amounts of IL1-Ra comparable to those transduced with adenoviral and lentiviral vectors. IL1-Ra was present in lavage fluid of most animals for 2 weeks in sufficient quantities to inhibit inflammation of the IL-1β-driven model. Once lost, neither subsequent inflammatory events, nor re-administration of the virus could re-establish transgene expression.
Conclusions
scAAV-mediated intra-articular gene transfer is robust and similarly efficient in both normal and inflamed joints; the resulting transgenic expression is sufficient to achieve biological relevance in joints of human proportion.
Keywords: adeno-associated virus, arthritis, gene therapy, interleukin-1, interleukin-1 receptor antagonist
Introduction
Gene transfer has been proposed as a means to improve treatment of the arthritides [1]. By delivering cDNAs encoding anti-arthritic proteins to the cells in the capsular lining of joints, the gene products may be expressed and secreted locally into the joint space and neighboring tissues. Persistent expression of therapeutic gene products may provide a long-term benefit in the treatment of chronic joint diseases. Initial studies of the feasibility of this concept employed an ex vivo gene transfer approach [2,3]. Although effective, the expense and labor have led to the exploration of methods for delivering exogenous genes directly to joint lining cells in situ. Studies using adenovirus [4–8] and herpes simplex virus [8,9] as vectors for gene transfer have demonstrated that direct intra-articular gene delivery is feasible. Moreover, ensuing expression of certain therapeutic transgenes is sufficient to inhibit arthritic changes in certain animal models [7,9–13] Although both vector systems are highly efficient, gene expression from either is transient, generally persisting for no greater than 2–3 weeks. The loss of transgene expression frequently is accompanied by the onset of an inflammatory response resulting, at least in part, from the expression of viral proteins that remain encoded by these vector systems [14].
Recent studies by Gouze et al. [15,16] has demonstrated that direct intra-articular injection of VSV-G pseudotyped, HIV-based lentiviral vectors into the knees of rats results in expression of homologous transgene products at relevant levels for greater than 6 months. This demonstrates that certain populations of cells within the synovium and joint capsule are capable of maintaining an exogenous transgene for periods of time sufficient to treat chronic articular disease. Although lentiviral vectors encode no viral proteins and are powerful gene delivery tools, there may be considerable safety and psychological impediments with respect to the use of HIV-based vectors for in vivo gene delivery for nonfatal articular diseases.
Adeno-associated virus (AAV) has certain characteristics that may make it more suitable for gene delivery to joint tissues [17–19]. Wild-type AAV is nonpathogenic, and recombinant AAV vectors have been engineered that encode no viral proteins. Because the vector infects a variety of dividing and nondividing cells, in many applications, it can achieve significant levels of cellular transduction after delivery in vivo. Advancements in AAV technology, including the capacity to cross-package the vector in alternate capsid serotypes and methods for generating large-scale, high-titer, adenovirus-free preparations [20,21], have brought wider interest to the use of this vector system, including its potential for use in treating the arthritides.
In previous studies, we evaluated conventional, single-stranded AAV2 vectors and found them to be significantly less effective than HSV, adenovirus or lentivirus for intra-articular gene delivery. The onset of transgenic expression was significantly slower, requiring at least 1–2 weeks, and the resulting levels of expression were low in culture and borderline detectable after specific intra-articular injection in the knee joints of rats and rabbits (unpublished observations). Despite our poor results, there are reports available in the literature concerning the beneficial effects observed after local AAV-mediated delivery of anti-arthritic transgenes in the ankles and paws of rodents with experimental arthritis [22–29]. Unfortunately, the inflammatory pathology in models such as collagen-induced arthritis and streptococcal-wall induced arthritis occurs almost exclusively in the ankles and paws. In rodents, these arthrodial, or gliding joints, are extraordinarily small and architecturally complex, without a readily identifiable joint space, and cannot be reliably targeted for intra-articular injection. Interestingly, consistent with our findings, several studies from independent laboratories indicate that murine and human synovial fibroblasts are inherently resistant to transduction with conventional AAV based vectors [17,30,31]. Indeed, a study by Cottard et al. [23] indicates that the primary site of AAV2 transduction after injection in the ankle region is extra-articular muscle. Several groups have shown that certain stimuli, such as ultraviolet radiation, which increases the production of endogenous DNA repair and synthesis proteins, can significantly enhance intra-articular transgene expression from conventional AAV vectors [17,30,31]. This indicates that second-strand DNA synthesis is rate-limiting in AAV transduction of joint tissues.
The recent development of double-stranded, self-complementary (sc) AAV vectors bypasses the need for single-strand to double-strand genome conversion and has shown dramatically increased transduction efficiency in many tissues compared to conventional AAV vectors [32,33]. scAAV vectors can be produced either by generation of vector plasmids that are approximately half-genome sized combined with selective purification of the infectious double-stranded form [32], or through the use of half-genome sized vector plasmids containing a mutation in one of the terminal resolution sequences of the AAV inverted terminal repeats [33]. Both strategies generate + and − strand viral genomes that are covalently linked at one terminal repeat. Because the genomes of scAAV are half wild-type size (approximately 2.5 kb), the resulting 2 × viral construct (approximately 5 kb) can be packaged into the normal AAV capsid.
In the present study, we tested the hypothesis that scAAV vectors provide improved transduction of articular fibroblasts over conventional AAV vectors to enable rapid expression of functional levels of transgene expression in joint tissues.
Materials and methods
Construction and generation of AAV vectors
The cDNA encoding green fluorescent protein (GFP) was cloned into the conventional AAV packaging vector pTRUF2 as a NotI-SalI fragment. For generation of scAAV vector plasmids, the cDNAs for GFP and human interleukin (IL)-1 receptor antagonist (Ra) were directionally inserted into the SacII, NotI sites of pHpa-trs-SK plasmid [33]. For all AAV vector constructs, transcription was driven by the cytomegalovirus (CMV) promoter/enhancer.
AAV vectors were propagated using an adenovirus-free, two plasmid transfection system. Using ten-layer, cell factories (Nunc, Rochester, NY, USA), the respective AAV vector plasmids were co-transfected into 293 cells by CaPO4 precipitation with the pDG packaging/helper plasmid [21]. The pDG plasmid contains the rep and cap genes from AAV2, and complementing adenoviral functions required for amplification and packaging of the AAV genome. Sixty hours post-transfection, cells were harvested with phosphate-buffered saline containing 10 mM ethylenediaminetetraacetic acid, pelleted, resuspended in low salt buffer and lysed by three rounds of freeze-thaw. Cellular nucleic acids were digested by incubation with Benzonase (Sigma, St Louis, MO, USA). Purification of AAV from the crude lysate was performed using iodixanol gradients followed by fast protein liquid chromatography affinity chromatography over a mono-Q column. The eluate was desalted and concentrated with a Millipore Biomax 100K filter (Millipore, Billerica, MA, USA), aliquotted and stored at −80°C. Viral titers were determined by quantitative competitive polymerase chain reaction (PCR) assay relative to well-characterized AAV viral reference standards. Each viral preparation was examined for purity by resolution of the viral proteins by sodium dodecyl sulfate-polyarcylamide gel electrophoresis and silver stain.
Isolation and infection of primary articular fibroblasts
Over the course of our in vitro experiments, four New Zealand white rabbits were euthanized and the capsular tissues from both knee joints were harvested. To isolate fibroblastic cells for experimentation, the noncollagenous soft tissues, including the synovial lining and subsynovium, were scraped from the dense supporting fibrous tendon and ligamentous tissue of the capsule using a scalpel. Under aseptic conditions, the fresh isolates of rabbit synovial/capsular (articular) tissue were minced with a razor blade and digested in approximately 30 ml of saline solution with 0.2% collagenase for 2 h at 37°C with constant stirring. Afterward, the suspension was passed through a nylon mesh to remove undigested tissue. The cells in the filtrate were then pelleted, washed in saline and plated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) with 1% penicillin/streptomycin. Twenty-four hours later, the cultures were washed to remove non-adherent cells and debris; the medium was replaced, and the cultures returned to the incubator.
For viral infection, unless otherwise indicated, cells were plated in 12-well plates and grown to approximately 70% confluence. Prior to infection, cells were washed two times with serum free media (DMEM). AAV vector from stock solutions was mixed with serum free media to produce working solutions containing appropriate DNAse resistant viral genomes/per cell and placed on cell cultures. For experiments involving the GFP transgene and intracellular trafficking, viral doses of 104 viral genomes per cell were used. For those involving IL-1Ra, viral doses ranged from 103 to 105 viral genomes per cell. After incubation with virus for 2 h, complete media was added to each well and the cells returned to the incubator. For quantification of IL-1Ra, at 24-h intervals after infection, the media from each well was harvested, and replaced with fresh media. Harvested media from selected days was stored frozen at −80°C. Each viral dose was added to four individual wells and supernatant from each well was tested individually by an enzyle-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA).
Quantification of viral genomes in cytoplasmic and nuclear cell fractions
Viral DNA from fractionated cells was isolated from a procedure adapted from Zhao et al. [34]. Briefly, lapine articular fibroblasts were seeded at 105 cells per well of a six-well dish, allowed to attach, and then infected with either single-stranded or scAAV at 104 viral genomes/cell as described above. After 24 h, the cells were trypsinized, incubated in hypotonic buffer for 5 min on ice, and lysed in non-ionic detergent. Centrifugation of the lysate allowed the nuclear fraction to be collected as the pellet, whereas the supernatant was reserved as the cytoplasmic fraction. Low molecular weight DNA from each fraction was isolated by Hirt extraction [35] and then used for quantitative PCR. Primer pairs were designed to anneal to sequences within the CMV promoter sequence. Viral genomes were detected using SYBR Green dye in an Eppendorf Mastercycler Realplex2. The results were standardized to a dilution series of vector plasmid DNA of known copy number. Three independent experiments were performed, yielding similar results. Values were then expressed as the mean of these experiments.
Animal models
All animal experiments were conducted according to protocols approved by the University of Florida Institutional Animal Care and Use Committee. All efforts were made to minimize animal suffering. Rabbits used in the study were housed separately in metal cages and maintained on commercial food and water ad libitum. The cages were kept at a constant temperature (22–25°C) and relative humidity (50–55%).
A retroviral vector, DFG-hIL-1β-neo encoding both the mature form of human IL-1β fused to the secretory polypeptide sequence from human parathyroid hormone, and the neomycin phosphotransferase genes [36] was used to transduce HIG-82 cells. After infection, transduced cells were positively selected by culture in DMEM containing 10% FBS and 0.5 mg/ml G418. After selection, this cell line was found to produce over 200 ng of IL-1β per 106 cells per 48 h. To induce arthritis in the rabbit knee, the HIG-82-IL-1β+ cells were first trypsinized from culture plates, washed twice in Gey’s balanced salts solution (GBSS) and counted using a hemocytometer. Approximately 5 × 104 of the cells were resuspended in GBSS in a 0.25-ml volume and injected via the parapatellar approach into both knees of 3–4 kg New Zealand White rabbits.
Joint lavage was utilized to monitor intra-articular transgene expression and leukocytic infiltration. For this procedure, rabbits were first anaesthetized by subcutaneous injection with a cocktail of xylazine, ketamine and acepromazine. The rabbit knee joints were then lavaged, first by direct intra-articular injection of 1 ml of GBSS. The joints were then put through several ranges of motion. The needle was re-inserted, and the fluid aspirated using the syringe. Leukocytes in recovered fluids were counted using a hemocytometer. Lavage fluids were centrifuged to pellet cells and debris, and the supernatant was aliquotted and stored at −80°C. For sacrifice of the animals, the rabbits were anaesthetized by subcutaneous injection with the xylazine, ketamine and acepromazine cocktail as above, followed by an intravenous overdose of Nembutal via the ear vein. IL-1Ra levels in recovered fluids were measured using an ELISA kit from R&D Systems in accordance with the manufacturer’s instructions.
Statistical analysis
For much of the data presented, pooled two-sample t-tests were conducted to determine significance in differences between mean values obtained. p < 0.05 was considered statistically significant. For the in vitro expression data, separate models were fit to the observations made on days 1, 3 and 7. For each model fit, the IL-1Ra levels and scAAV.Il-1Ra doses were transformed using a base-10 logarithm, and a simple linear regression was fit to the transformed data using p < 0.05 for each test.
Results
scAAV transduces rabbit synovial fibroblasts with high efficiency
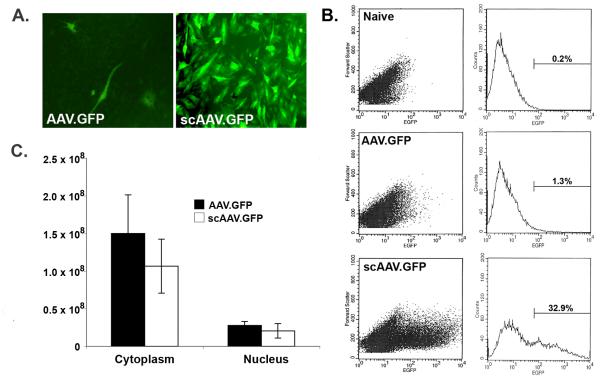
To determine the relative transduction efficiency in articular fibroblasts of the double-stranded, scAAV vector and the conventional single-stranded form, we first inserted the cDNA for GFP into the respective vector plasmids and packaged each into AAV capsid serotype 2. Articular fibroblasts isolated from synovial and capsular tissues of the joints of rabbits were cultured in multiwell plates and infected with approximately 104 viral genomes per cell of either AAV.GFP or scAAV.GFP. At periodic intervals post-infection, individual cultures were analysed by microscopy and flow cytometry for numbers of GFP+ cells and the levels of fluorescence. As shown in Figures 1A and 1B, the scAAV.GFP vector provided an aproximately 25-fold greater transduction than the conventional single-stranded vector, with onset of GFP expression within 24 h. Fluorescence was noted to diminish somewhat by day 7; however, this was attributed to the loss of the episomal AAV genomes from the cells of the rapidly dividing line in culture.
Figure 1.
scAAV-mediated gene transfer to rabbit articular fibroblasts in vitro. Cultures of primary articular fibroblasts isolated from the joints of rabbits were infected with 104 viral genomes per cell of either conventional AAV.GFP or double-stranded, self-complementary AAV (scAAV.GFP). Parallel cultures of uninfected cells (Naïve) were used as negative controls. Both vectors were packaged in AAV serotype 2 capsid. (A) Three days later, the cultures were examined visually by fluorescence microscopy. (B) Fluorescence was then quantified using flow cytometry. In these assays, scAAV provided an approximately 25-fold greater transduction than the conventional AAV vector. For each scatter plot shown on the left, levels of fluorescence are represented on the horizontal axes, and cell size is indicated on the vertical axes. For graphs on the right, fluorescence is indicated on the horizontal axes and cell number on the vertical axes. Plots shown were representative of four replicates. (C) To track the intracellular migration of the respective viral genomes, cultures of rabbit fibroblasts were infected with either AAV.GFP or scAAV.GFP. Twenty-four hours later, the cells were harvested, and the nuclear and cytoplasmic fractions were isolated. Viral genomes in the respective fractions were determined using quantitative PCR. Values plotted for each vector and compartment represent the means of four replicates. Error bars represent one standard deviation. For both types of vectors, less than 20% of the viral genomes entered the nucleus at 24 h post-infection. For the cytoplasm and nuclear fractions, separate two-sample t-tests were conducted; using p < 0.05, there was no significant difference between the respective samples in each group.
To determine whether the enhanced transduction of the scAAV vector might be partially attributable to variation in intracellular trafficking and nuclear entry, we infected parallel cultures of cells and with both vector types and determined the viral genomes present in the cytoplasmic and nuclear fractions using quantitative PCR. As shown in Figure 1C, no significant differences were observed between the different AAV vector types; both entered the cells with similar efficiency, and similar proportions of viral DNA entered the nucleus (approximately 18% and 14% for conventional and scAAV, respectively). These data indicate, that at least within this cell type, there are no appreciable differences between the two vectors with regard to viral entry into the cell, as well as entrance into the nucleus. Consistent with previous studies, these results show that transduction of articular fibroblasts with conventional AAV vectors is severely limited by the inability of these cells to effectively achieve second-strand DNA synthesis.
To determine the levels of therapeutic protein synthesis provided by the scAAV vector, the cDNA for human IL-1Ra [37] was inserted into the pHpa-tr-SK, scAAV vector (scAAV.hIL-1Ra) and packaged into AAV serotype 2. IL-1Ra is a secreted protein that serves as competitive inhibitor of IL-1 by binding to available type I IL-1 receptors and preventing subsequent interaction with IL-1 ligand and IL-1 receptor accessory protein [38]. IL-1Ra is useful as a reporter gene because it has no known agonist activity and can be measured in biological fluids by ELISA, which distinguishes between the human form and the endogenous IL-1Ra of the experimental animal.
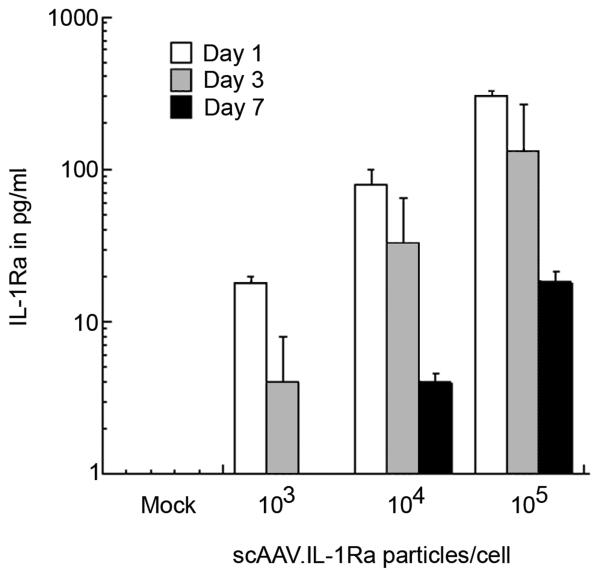
Cultures of primary rabbit articular fibroblasts were infected with increasing amounts of scAAV.IL-1Ra, ranging from 103 to 105 particles per cell. To follow the pattern of gene expression over time, the media were collected every 24 h post-infection, the cells washed and fresh culture media added. IL-1Ra levels in the media collected at days 1, 3 and 7 post-infection, were measured by ELISA. As shown in Figure 2, the lapine articular fibroblasts were amenable to transduction with scAAV.hIL-1Ra, and expressed the transgene in a dose-dependent manner. Interestingly, for all doses the greatest level of expression was measured at 24 h post-infection. As with the expression of GFP, we noted that IL-1Ra production gradually diminished over the week-long experiment, which is consistent with the loss of the episomal viral genomes from cell division.
Figure 2.
Infection of primary synovial fibroblasts with scAAV.IL-1Ra results in high level expression of the transgene. To test the function of the scAAV.IL-1Ra, cultures of primary, lapine, articular fibroblasts were plated and allowed to grow to approximately 70% confluence. The cells were then incubated with increasing amounts of the scAAV.IL-1Ra vector as indicated. At 24-h intervals post-infection, the culture media were removed and stored. The cells were washed with saline and the media replaced. IL-1Ra levels in culture supernatants from days 1, 3 and 7 were determined using ELISA. Each viral dose was tested in quadruplicate, and the bars represent the mean ± SD. For observations made on days 1, 3 and 7, IL-1Ra levels and scAAV.Il-1Ra doses were transformed using a base-10 logarithm, and a simple linear regression was fit to the transformed data. Using p < 0.05 for each test, a significant trend was detected for days 1, 3 and 7.
Taken together, the results obtained demonstrate that the scAAV.IL-1Ra vector was infectious for rabbit articular fibroblasts and thus suitable for evaluation in the rabbit knee in vivo. The levels of hIL-1Ra synthesis were comparable to those achieved previously with recombinant adenoviral and lentiviral vectors.
Intra-articular expression of AAV.IL-1Ra in normal and arthritic rabbit knee joints
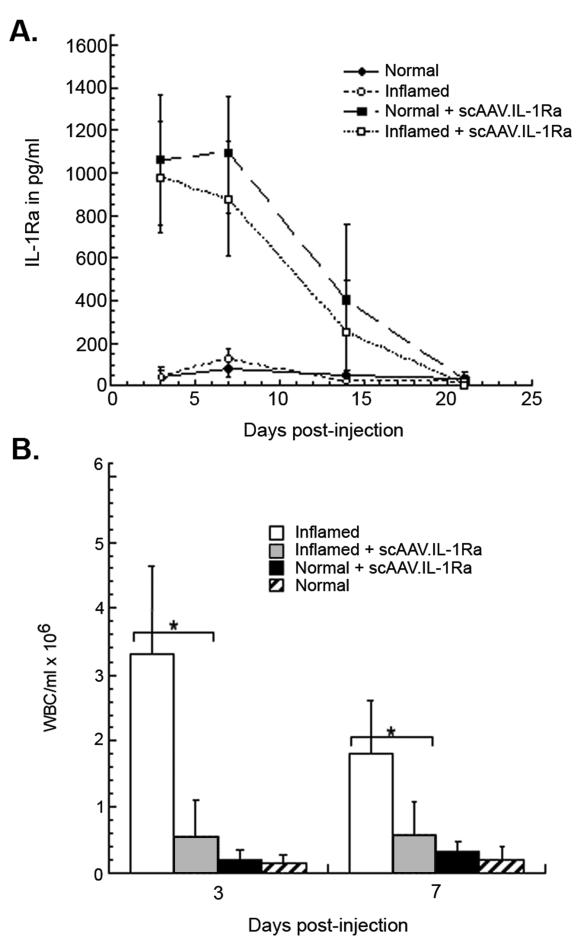
Having established that the scAAV.IL-1Ra vector was able to efficiently transduce articular cells in culture, we aimed to determine and compare the patterns of intra-articular gene expression after injection of the vector into normal and inflamed joints. To establish an inflammatory environment in the rabbit knee, approximately 5 × 104 cells of a rabbit synovial fibroblast line retrovirally transduced to constitutively express human IL-1β (HIG-82-IL-1β-neo) were injected into both knees of ten rabbits. This procedure has been shown to induce an acute inflammatory response in the joint that mimics many of the pathologies associated with rheumatoid arthritis in humans and persists for approximately 10–14 days [36]. Three days after delivery of the IL-1β+ cells, approximately 5 × 1011 particles of scAAV.hIL-1Ra were injected into both knees of five rabbits receiving the IL-1+ cells and into both knees of five normal rabbits. For negative controls, an equivalent volume of saline solution was injected into both knees of the remaining IL-1+ rabbits, as well as five additional normal rabbits. The knees of all four groups of rabbits were initially lavaged at 3 and 7 days post-injection of the vector and then weekly thereafter for 28 days. Recovered lavage fluids from each knee were analysed individually for levels of human IL-1Ra by ELISA, as well as for numbers of infiltrating leukocytes.
As shown in Figure 3A, scAAV.IL-1Ra-mediated intra-articular gene delivery resulted in approximately the same level and duration of IL-1Ra expression for both inflamed and naïve joints. At days 3 and 7, mean levels of approximately 1 ng of IL-1Ra per ml of recovered lavage fluid were detected in both inflamed and normal joints. By day 14, mean IL-1Ra levels had decreased by 60–80%, and by day 21 human IL-1Ra was undetectable in lavage fluids of any animals. IL-1Ra expression was not detected in the normal and inflamed control rabbits beyond normal background levels for this procedure.
Figure 3.
Intra-articular expression of scAAV.IL-1Ra after direct injection into normal and inflamed rabbit knee joints. Ten rabbits were initially injected in both knees with 5 × 104 HIG-82-IL-1β-neo cells, which stimulates an immediate, persistent inflammatory state. Three days later, 5 × 1011 particles of scAAV.IL-1Ra were injected into both knees of five of the rabbits with inflamed knees and five normal rabbits. An equivalent volume of saline was injected into the remaining five inflamed rabbits and an additional five normal rabbits. (A) Periodically, the knees of all rabbits were lavaged with saline and the IL-1Ra content in recovered fluids measured by ELISA. Data are shown as the mean ± SD. For rabbits receiving scAAV-IL-1Ra, using a two-sample t-test and p < 0.05, no significant differences were observed between inflamed and normal knees for all days post-injection. The same test conducted for rabbits injected with saline showed no significant differences between normal and inflamed as well. (B) Infiltrating leukocytes in lavage fluids recovered at days 3 and 7 for each group were quantified using a hemocytometer. Data are shown as the mean ± SD. A pooled two-sample t-test was used to determine the significance of the differences in leukocytic infiltration in inflamed knees that were injected with scAAV.IL-1Ra and untreated controls. *p < 0.05.
A significant decrease in leukocytosis of the synovial fluid was observed at days 3 and 7 in the arthritic (inflamed) joints receiving the scAAV.IL-1Ra, relative to the saline-injected arthritic controls (p < 0.05) (Figure 3B). This is consistent with previously observed anti-inflammatory effects associated with intra-articular delivery and overexpression of the IL-1Ra cDNA in this model system [9]. Different from the intra-articular injection of adenoviral vectors, no detectable increase in leukocyte levels in synovial fluids was observed in the normal rabbits receiving the scAAV vector at any time point during the 4-week experiment (Figure 3B).
To determine whether the onset of a second inflammatory response would re-stimulate intra-articular IL-1Ra transgene expression, HIG-82-IL-1β-neo cells were injected into the knees of the previously inflamed and normal rabbits that had received the AAV.IL-1Ra 5 weeks earlier. The knees of the rabbits were then lavaged 3 days later and again, after sacrifice, at day 7. The recovered fluids were analysed individually for IL-1Ra levels by ELISA. By contrast to previous reports indicating that, once extinguished, AAV-mediated transgene expression could be re-established by a subsequent inflammatory stimulus [39,40] no IL-1Ra was detected in lavage fluids from animals of either group. PCR analyses of recovered synovial tissues did not detect the presence of scAAV.IL-1Ra genomes (data not shown).
Repeat dose of AAV.IL-1Ra does not restore transgene expression
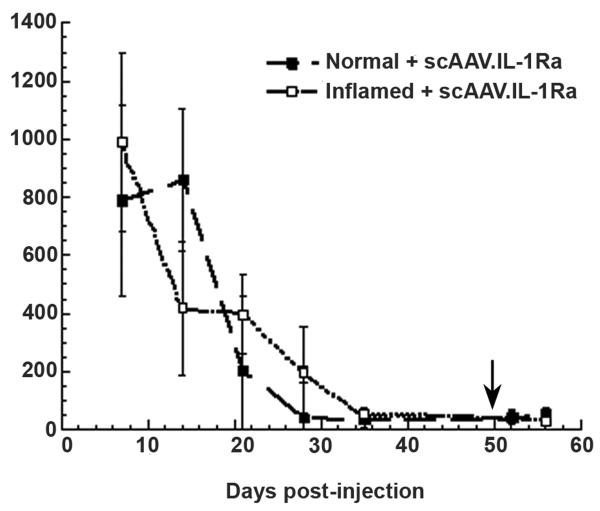
Having found no difference in levels of IL-1Ra expression after AAV-mediated gene delivery between normal and inflamed joints, patterns of transgene expression were determined after repeat dosing. As above, 5 × 104 HIG-82-IL-1β+ cells were injected into both knees of ten rabbits. Three days later, approximately 5 × 1011 particles of scAAV.IL-1Ra were injected into both knees of five of the rabbits receiving the IL-1 cells and into both knees of five normal rabbits. The knees of all rabbits were lavaged weekly until IL-1Ra expression had completely diminished. As shown in Figure 4, similar to the results of the previous experiment, both normal and inflamed joints injected with the scAAV.IL-1Ra expressed approximately 1 ng of IL-1Ra per ml of recovered lavage fluid at day 7, and IL-1Ra production gradually diminished thereafter. Somewhat different from the results obtained in Figure 3, at day 21, one rabbit each from the inflamed and the normal groups still expressed IL-1Ra above background levels in both joints. By day 28, IL-1Ra expression persisted only in the knees of one animal in the inflamed group. At day 35, no IL-1Ra was present in the joints of any of the rabbits. Two weeks after IL-1Ra expression had diminished in all the animals, a second dose of AAV.IL-1Ra was injected intra-articularly. Lavage fluids recovered at day 3 and 7 after the second injection showed no significant IL-1Ra expression at either time point.
Figure 4.
Repeat injection of scAAV.IL-1Ra does not result in rescue of transgene expression. Similar to the procedure described in Figure 3, five rabbits were injected in both knees with HIG-82-IL-1β-neo cells to establish an inflammatory state. Three days later, the inflamed rabbits and five normal rabbits were then injected with scAAV.IL-1Ra in both knees. At weekly intervals, the knees of the rabbits were lavaged with saline, and IL-1Ra levels measured using ELISA. At 49 days after the initial scAAV.IL-1Ra injection and 14 days after IL-1Ra expression had diminished in all joints, a second intra-articular injection of scAAV.IL-1Ra was administered to all rabbit knees (indicated by an arrow). The knees of all rabbits were lavaged at 3 and 7 days after the second vector injection. Values shown are the mean ± SD IL-1Ra levels at specific time points. A two-sample t-test was conducted for IL-1Ra levels between groups for each day post-injection; using p < 0.05, the only significant difference detected was at day 14 post-injection.
Discussion
In the present study, we evaluated patterns of transgene expression in articular cells after infection with scAAV vectors, first in vitro and then in vivo in normal and inflamed joints. We found that, in culture, the scAAV vector represented a significant technical advance over conventional single-stranded vectors with regard to cellular transduction, providing an approximately 25-fold enhancement in transgenic expression. The comparatively poor performance of the conventional vector indicates that second-strand DNA synthesis can be a major impediment to effective transduction of joint tissues. Concerning normal and inflamed articular environments, we found no significant difference in the levels or duration of expression of the IL-1Ra transgene after delivery of the self-complementary vector. Generally, after injection of approximately 5 × 1011 particles, sufficient levels of IL-1Ra transgene product were generated to cause a reduction in the leukocytic infiltration in joints inflamed by constitutive IL-1 production. By contrast to previous studies [39,40], we found that, after the loss of IL-1Ra transgene expression, neither re-injection of the scAAV.IL-1Ra vector, nor the induction of a second inflammatory response could generate detectable levels of IL-1Ra expression intra-articularly.
In animal studies of intra-articular transgene expression, we have found several advantages to the rabbit knee as a model system. Being approximately the same size as the metacarpophalangeal joints of the human hand, a frequent site of rheumatoid arthritis, it offers a reasonable simulation of the process of gene delivery in the treatment of human joint disease. Given that the studies published to date have been performed in rats and mice [17,22–29,41,42] with joints being 1–2 log orders smaller than those in humans, we believe the data obtained in the present study are among the first to report intra-articular transgene expression from an AAV vector in articular tissues on a clinically applicable scale. As shown by the ability of the IL-1Ra expression to alleviate leukocytosis in inflamed joints of the rabbits, the efficiency of scAAV-mediated gene delivery and ensuing expression is sufficient to induce a beneficial biological response in this context.
The patterns of transgene expression observed with the self-complementary vector in rabbits differ somewhat from those observed in the joints of mice injected with conventional AAV. In the murine system, AAV mediated transgene expression was found to onset significantly earlier in arthritic joints, and levels of expression were greater than in normal joints [17]. This was primarily attributed to differences in the synthesis of the second DNA strand of the AAV vector within the infected cell, and increased production of DNA synthesis/repair enzymes in cells receiving inflammatory stimuli. With the self-complementary vector, AAV-mediated transgene expression from normal rabbit synovial fibroblasts in culture as well as in vivo in normal joints had a rapid onset, with no evidence of delay relative to arthritic joints. Thus, the self-complementary vector bypasses the variability associated with conventional AAV vectors in the arthritic environment, and thereby provides a more predictable gene delivery reagent. The observation that cells within both normal and inflamed joints of the rabbit are equally capable of being transduced by an scAAV-based vector indicates that this system may have application in a spectrum of articular ailments. These include inflammatory conditions, such as rheumatoid arthritis, as well as those not directly associated with chronic inflammation, such as osteoarthritis, and repair of joint tissues, such as meniscus and ligament.
Although intra-articular gene transfer accompanied by limited inflammation has been previously reported with AAV, the capacity of this vector to enable persistent transgenic expression in joint tissues has yet to be fully assessed. In the present study, we found that, within the rabbit knee, expression of the human IL-1Ra transgene was gradually lost over a period of a few weeks and that re-administration of the vector could not restore expression. Unfortunately, the human IL-1Ra transgene product used in these experiments, although extremely useful as a secretable marker, is at the same time immunogenic when administered across species boundaries to the joints of normal, immunocompetent animals [15]. We have found similar patterns of abbreviated intra-articular expression after the use of other xenogenic transgene products, regardless of whether they are secreted or intracellular [15]. Recent studies have shown that, in the absence of specific T-cell mediated immunity directed against nonself proteins of transgenic or viral vector origin, cells within fibrous tissues of the joint can support long-term (> 6 months) transgenic expression [15,16]. The capacity with which AAV vectors can infect and transduce these particular cell types is currently unknown, but is an area of ongoing study within our group, as well as others.
AAV-mediated transduction of the target cell involves several key steps that broadly include viral attachment and entry [43,44], intracellular trafficking to the nucleus [45–47], nuclear entry and uncoating [48,49], and conversion of the single-stranded genome into a double-stranded form [50,51]. In attempting to improve the efficiency of AAV transduction, the development of methods to cross-package vector genomes in alternate capsid serotypes has dramatically expanded the host cell range of the widely used, AAV serotype 2-based vectors. Furthermore, the development of scAAV vectors bypasses the limitations associated with second-strand DNA synthesis. As shown in the present study, as well as those conducted previously, important barriers to transduction of articular fibroblasts appear to still remain at the level of intracellular trafficking [31,52,53]. Relative to other viral vectors, high numbers of viral particles are required to achieve transduction of human, rat and rabbit synovial fibroblasts in culture, typically in the range of 104–105 viral particles per cell and, as shown in the present study, only between 10% and 20% of AAV genomes enter the nucleus. Several lines of evidence implicate the ubiquitin-proteasome pathway as a key hurdle to efficient intracellular trafficking by AAV2-based vectors and other serotypes. Jennings et al. [53] showed that the addition of proteasome inhibitors, such as carbobenzoxy-l-leucyl-l-leucyl-l-leucinal (zLLL), dramatically enhanced nuclear uptake of AAV genomes in human synovial fibroblasts and was accompanied by a proportional increase in transgenic expression. More recently, Zhong et al. [54] demonstrated that cellular phosphorylation of specific tyrosine residues on the AAV capsid surface led to increased ubiquitination of the viral particle and enhanced proteasome degradation. Site-directed mutagenesis of these tyrosines to phenylalanine blocked ubiquitination and led to an approximately ten-fold enhancement of transduction efficiency [55]. The relative utility of these modified capsids in articular cells has not been investigated but, similar to the self-complementary vectors, they have the potential to significantly enhance the efficiency of AAV-mediated gene transfer.
Acknowledgements
This work was supported by grants AR048566 and AR05249 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. S.C.G. and P.D.R. are founders of Molecular Orthopaedics, Inc., a company that develops gene-based treatments for musculoskeletal diseases.
References
- 1.Evans CH, Ghivizzani SC, Robbins PD. Gene therapy for arthritis: what next? Arthritis Rheum. 2006;54:1714–1729. doi: 10.1002/art.21886. [DOI] [PubMed] [Google Scholar]
- 2.Bandara G, Mueller GM, Galea-Lauri J, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci USA. 1993;90:10764–1078. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans CH, Robbins PD, Ghivizzani SC, et al. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261–1280. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- 4.Adriaansen J, Kuhlman RR, van Holten J, Kaynor C, Vervoordeldonk MJ, Tak PP. Intraarticular interferon-beta gene therapy ameliorates adjuvant arthritis in rats. Hum Gene Ther. 2006;17:985–996. doi: 10.1089/hum.2006.17.985. [DOI] [PubMed] [Google Scholar]
- 5.Lechman ER, Keravala A, Nash J, Kim SH, Mi Z, Robbins PD. The contralateral effect conferred by intra-articular adenovirus-mediated gene transfer of viral IL-10 is specific to the immunizing antigen. Gene Ther. 2003;10:2029–2035. doi: 10.1038/sj.gt.3302109. [DOI] [PubMed] [Google Scholar]
- 6.Evans CH, Ghivizzani SC, Oligino TA, Robbins PD. Future of adenoviruses in the gene therapy of arthritis. Arthritis Res. 2001;3:142–146. doi: 10.1186/ar291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lubberts E, Joosten LA, van Den Bersselaar L, et al. Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. J Immunol. 1999;163:4546–4556. [PubMed] [Google Scholar]
- 8.Nita I, Ghivizzani SC, Galea-Lauri J, et al. Direct gene delivery to synovium. An evaluation of potential vectors in vitro and in vivo. Arthritis Rheum. 1996;39:820–828. doi: 10.1002/art.1780390515. [DOI] [PubMed] [Google Scholar]
- 9.Oligino T, Ghivizzani S, Wolfe D, et al. Intra-articular delivery of a herpes simplex virus IL-1Ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. 1999;6:1713–1720. doi: 10.1038/sj.gt.3301014. [DOI] [PubMed] [Google Scholar]
- 10.Ghivizzani SC, Lechman ER, Kang R, et al. Direct adenovirus-mediated gene transfer of interleukin 1 and tumor necrosis factor alpha soluble receptors to rabbit knees with experimental arthritis has local and distal anti-arthritic effects. Proc Natl Acad Sci USA. 1998;95:4613–4618. doi: 10.1073/pnas.95.8.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Gao G, Clayburne G, Schumacher HR. Elimination of rheumatoid synovium in situ using a Fas ligand ‘gene scalpel’. Arthritis Res Ther. 2005;7:R1235–R1243. doi: 10.1186/ar1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechman ER, Jaffurs D, Ghivizzani SC, et al. Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees. J Immunol. 1999;163:2202–2208. [PubMed] [Google Scholar]
- 13.Ijima K, Murakami M, Okamoto H, et al. Successful gene therapy via intraarticular injection of adenovirus vector containing CTLA4IgG in a murine model of type II collagen-induced arthritis. Hum Gene Ther. 2001;12:1063–1077. doi: 10.1089/104303401750214285. [DOI] [PubMed] [Google Scholar]
- 14.Ghivizzani SC, Oligino TJ, Glorioso JC, Robbins PD, Evans CH. Direct gene delivery strategies for the treatment of rheumatoid arthritis. Drug Discov Today. 2001;6:259–267. doi: 10.1016/s1359-6446(01)01685-3. [DOI] [PubMed] [Google Scholar]
- 15.Gouze E, Gouze JN, Palmer GD, Pilapil C, Evans CH, Ghivizzani SC. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol Ther. 2007;15:1114–1120. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- 16.Gouze E, Pawliuk R, Gouze JN, et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7:460–466. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 17.Goater J, Muller R, Kollias G, et al. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J Rheumatol. 2000;27:983–989. [PubMed] [Google Scholar]
- 18.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Bowles DE, van Dyke T, Samulski RJ. Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther. 2005;12:913–925. doi: 10.1038/sj.cgt.7700876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 22.Adriaansen J, Fallaux FJ, de Cortie CJ, Vervoordeldonk MJ, Tak PP. Local delivery of beta interferon using an adeno-associated virus type 5 effectively inhibits adjuvant arthritis in rats. J Gen Virol. 2007;88:1717–1721. doi: 10.1099/vir.0.82603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cottard V, Mulleman D, Bouille P, Mezzina M, Boissier MC, Bessis N. Adeno-associated virus-mediated delivery of IL-4 prevents collagen-induced arthritis. Gene Ther. 2000;7:1930–1939. doi: 10.1038/sj.gt.3301324. [DOI] [PubMed] [Google Scholar]
- 24.Adriaansen J, Khoury M, de Cortie CJ, et al. Reduction of arthritis following intra-articular administration of an adeno-associated virus serotype 5 expressing a disease-inducible TNF-blocking agent. Ann Rheum Dis. 2007;66:1143–1150. doi: 10.1136/ard.2006.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adriaansen J, Tas SW, Klarenbeek PL, et al. Enhanced gene transfer to arthritic joints using adeno-associated virus type 5: implications for intra-articular gene therapy. Ann Rheum Dis. 2005;64:1677–1684. doi: 10.1136/ard.2004.035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan JM, Villarreal G, Jin WW, Stepan T, Burstein H, Wahl SM. Intraarticular gene transfer of TNFR: Fc suppresses experimental arthritis with reduced systemic distribution of the gene product. Mol Ther. 2002;6:727–736. doi: 10.1006/mthe.2002.0808. [DOI] [PubMed] [Google Scholar]
- 27.Khoury M, Adriaansen J, Vervoordeldonk MJ, et al. Inflammation-inducible anti-TNF gene expression mediated by intra-articular injection of serotype 5 adeno-associated virus reduces arthritis. J Gene Med. 2007;9:596–604. doi: 10.1002/jgm.1053. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi H, Kato K, Miyake K, Hirai Y, Yoshino S, Shimada T. Adeno-associated virus vector-mediated anti-angiogenic gene therapy for collagen-induced arthritis in mice. Clin Exp Rheumatol. 2005;23:455–461. [PubMed] [Google Scholar]
- 29.Tas SW, Adriaansen J, Hajji N, et al. Amelioration of arthritis by intraarticular dominant negative Ikk beta gene therapy using adeno-associated virus type 5. Hum Gene Ther. 2006;17:821–832. doi: 10.1089/hum.2006.17.821. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich-Vinther M, Duch MR, Soballe K, O’Keefe RJ, Schwarz EM, Pedersen FS. In vivo gene delivery to articular chondrocytes mediated by an adeno-associated virus vector. J Orthop Res. 2004;22:726–734. doi: 10.1016/j.orthres.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Katakura S, Jennings K, Watanabe S, et al. Recombinant adeno-associated virus preferentially transduces human, compared to mouse, synovium: implications for arthritis therapy. Mod Rheumatol. 2004;14:18–24. doi: 10.1007/s10165-003-0260-7. [DOI] [PubMed] [Google Scholar]
- 32.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 33.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W, Zhong L, Wu J, et al. Role of cellular FKBP52 protein in intracellular trafficking of recombinant adeno-associated virus 2 vectors. Virology. 2006;353:283–293. doi: 10.1016/j.virol.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 36.Ghivizzani SC, Kang R, Georgescu HI, et al. Constitutive intra-articular expression of human IL-1 beta following gene transfer to rabbit synovium produces all major pathologies of human rheumatoid arthritis. J Immunol. 1997;159:3604–3612. [PubMed] [Google Scholar]
- 37.Arend WP, Gabay C. Physiologic role of interleukin-1 receptor antagonist. Arthritis Res. 2000;2:245–248. doi: 10.1186/ar94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Pan RY, Chen SL, Xiao X, Liu DW, Peng HJ, Tsao YP. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000;43:289–297. doi: 10.1002/1529-0131(200002)43:2<289::AID-ANR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Pan RY, Xiao X, Chen SL, et al. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol. 1999;73:3410–3417. doi: 10.1128/jvi.73.4.3410-3417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HG, Xie J, Yang P, et al. Adeno-associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum Gene Ther. 2000;11:2431–2442. doi: 10.1089/104303400750038525. [DOI] [PubMed] [Google Scholar]
- 42.Apparailly F, Khoury M, Vervoordeldonk MJ, et al. Adeno-associated virus pseudotype 5 vector improves gene transfer in arthritic joints. Hum Gene Ther. 2005;16:426–434. doi: 10.1089/hum.2005.16.426. [DOI] [PubMed] [Google Scholar]
- 43.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 44.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J Virol. 2000;74:992–996. doi: 10.1128/jvi.74.2.992-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen J, Qing K, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J Virol. 2001;75:4080–4090. doi: 10.1128/JVI.75.9.4080-4090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhong L, Li W, Yang Z, et al. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum Gene Ther. 2004;15:1207–1218. doi: 10.1089/hum.2004.15.1207. [DOI] [PubMed] [Google Scholar]
- 50.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traister RS, Fabre S, Wang Z, Xiao X, Hirsch R. Inflammatory cytokine regulation of transgene expression in human fibroblast-like synoviocytes infected with adeno-associated virus. Arthritis Rheum. 2006;54:2119–2126. doi: 10.1002/art.21940. [DOI] [PubMed] [Google Scholar]
- 53.Jennings K, Miyamae T, Traister R, et al. Proteasome inhibition enhances AAV-mediated transgene expression in human synoviocytes in vitro and in vivo. Mol Ther. 2005;11:600–607. doi: 10.1016/j.ymthe.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Zhong L, Zhao W, Wu J, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 55.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]