Abstract
Impaired ability to conduct daily activities is a diagnostic criterion for dementia and a determinant of healthcare services utilization and caregiver burden. What predicts decline in instrumental activities of daily living (IADLs) is not well understood. This study examined measures of episodic memory, executive function, and MRI brain volumes in relation to baseline IADLs and as predictors of rate of IADL change. Participants were 124 elderly persons with cognitive function between normal and moderate dementia both with and without significant small vessel cerebrovascular disease. Random effects modeling showed that baseline memory and executive function (EXEC) were associated with baseline IADL scores, but only EXEC was independently associated with rate of change in IADLs. Whereas hippocampal and cortical gray matter volumes were significantly associated with baseline IADL scores, only hippocampal volume was associated with IADL change. In a model including cognitive and neuroimaging predictors, only EXEC independently predicted rate of decline in IADL scores. These findings indicate that greater executive dysfunction at initial assessment is associated with more rapid decline in IADLs. Perhaps executive function is particularly important with respect to maintaining IADLs. Alternatively, executive dysfunction may be a sentinel event indicating widespread cortical involvement and poor prognosis.
Keywords: Alzheimer’s disease, Vascular dementia, Memory, Everyday function, Neuroimaging, Frontal lobe
INTRODUCTION
Dementia, by definition, is associated with both compromised cognition and independent functioning. Deficits in daily living skills are related to increased distress and reduced quality of life for the patient and caregiver as well as increased use of healthcare services (Hope et al., 1998; Severson et al., 1994; Vetter et al., 1999). Given the significant burden of functional impairments on the patients, caregivers, and care providers, identification of factors that best predict future decline in daily functioning has important clinical implications.
The assessment of everyday functioning in older adults typically focuses on an individual’s ability to carry out activities of daily living (ADLs). Basic ADLs (BADLs) include tasks such as grooming, feeding, and toileting, whereas instrumental ADLs (IADLs) involve complex behaviors including managing finances, handling medications, and housekeeping. BADLs are highly correlated with motor functioning and coordination (Bennett et al., 2002; Boyle et al., 2002; Cahn et al., 1998). In contrast, declines in IADLs have been shown to be influenced by cognitive functioning, are affected relatively early in the course of dementia(Stern et al., 1990), and may be present in preclinical dementia states such as mild cognitive impairment (MCI; Griffith et al., 2003; Ritchie et al., 2001). Assessment of ADLs in large-scale studies is usually conducted through informant report. This method of ADL assessment has the advantage of asking family members or caregivers who are familiar with the individual’s performance in real-world environments to rate their level of functioning, and is also highly time and cost-efficient. Performance-based assessment of ADLs, in contrast, allows a trained rater to directly observe behavior in well-defined functional tasks. This method may not accurately reflect true abilities however, insofar as the assessment is conducted in a structured environment with prompts to carry out desired tasks. Furthermore, because most performance-based scales are time-consuming, they are less practical for use in large-scale research studies.
Early work in this area demonstrated a relationship between global cognitive impairment and global measures of functional status (Reed et al., 1989; Skurla et al., 1988). Recent studies have reported an association between impairments in specific cognitive abilities and global measures of daily functioning. For example, a number of studies show memory (Farias et al., 2004; Goldstein et al., 1992) and executive functions (Bell-McGinty et al., 2002; Boyle et al., 2004; Cahn-Weiner et al., 2002; Richardson et al., 1995) to be the cognitive domains most consistently associated with various measures of everyday function. Whereas these studies have shown that tests of memory and executive functioning are associated with baseline functional status, the relative importance of each of these domains has been unclear for a number of reasons. First, most studies have used neuropsychological instruments that are not psychometrically matched to have similar measurement properties across different cognitive domains. It is thus possible that different patterns of statistical significance reflect differences in scale range or reliability rather than true differences in associations between cognitive domains and daily function. Second, most studies examining the relationship between cognition and functional impairment have been cross-sectional, and have not examined how well memory and executive function at baseline predict rate of future decline in IADLs.
Longitudinal studies evaluating cognitive functioning and its influence on future disability have typically relied on global measures of cognition. Population-based longitudinal studies have shown that global measures of baseline cognitive function are associated with a faster rate of functional decline and predict future disabilities in IADLs (Barberger-Gateau & Fabrigoule, 1997; Schmeidler et al., 1998). Two more recent studies evaluated how specific cognitive domains predict future decline in functional status in patients with cerebrovascular disease (CVD). Boyle and colleagues (Boyle et al., 2004) reported that, after accounting for global cognition at baseline, baseline performance on tests of executive function accounted for a significant proportion of the variance in IADLs after one year. The predictive utility of other specific cognitive domains such as memory were not examined. Bennett and colleagues (Bennett et al., 2002) reported a six-year longitudinal study of subcortical vascular disease and functional decline and found that a measure heavily dependent on attention/visuospatial and executive functions was the only independent cognitive predictor of IADL decline. These studies suggest that executive measures predict future daily functioning in patients with CVD, but are limited to some extent in their generalizability to other aging related neurodegenerative conditions. Furthermore, similar to the cross sectional studies, neuropsychological measures of different cognitive domains have not been psychometrically equivalent, making the relative importance of the different cognitive domains difficult to evaluate.
The relationship between IADLs and neuroimaging is not well understood. Two imaging measures that have been of particular interest are hippocampal atrophy, which is associated with memory dysfunction (Golomb et al., 1994; Grundman et al., 2003; Reed et al., 2000) and white matter abnormality and lacunes, which appear to be most strongly (but not exclusively) associated with executive dysfunction (Cummings, 1993; Reed et al., 2004). Cahn and colleagues (Cahn et al., 1996) demonstrated that elderly depressed patients with greater subcortical disease performed worse than those without significant disease on IADLs. In contrast, a study of patients with vascular dementia conducted by Boyle and colleagues (Boyle et al., 2004) reported that after accounting for the contribution of executive function to IADL performance, subcortical neuropathology on MRI did not account for additional variance. Farias and colleagues examined the relationships between MRI-based hippocampal volume (HV), white matter hyperintensity (WMH) and everyday functioning. In this large, community-based study of multicultural and multilingual individuals, HV and WMH correlated significantly with everyday functioning, but WMH continued to show an effect after accounting for age, whereas HV did not. No studies to date have yet evaluated the predictive power of brain imaging for ADLs in AD, either alone or in concert with neuropsychological measures.
The goal of the present study was to examine cognitive and MRI predictors of longitudinal change in IADL ratings. We focused on memory and executive function for the reasons outlined earlier. In addition we examined the contributions of HV and WMH in order to determine if they offer additional predictive ability, or whether they mediate the effects of the cognitive measures. The neuropsychological composite indices employed in this study have matched psychometric properties that were derived from item response theory methods, enabling us to better characterize the relative importance of memory and executive function in predicting ratings of IADL change.
METHOD
Participants
Participants were recruited from three academic dementia centers as part of a multicenter collaborative longitudinal study of aging and cerebrovascular disease, described previously (Mungas et al., 2005; Reed et al., 2004). The majority of participants with cognitive impairments (excluding controls) were initially referred for a clinical evaluation.All participants received a thorough examination, including medical history, neurological examination, appropriate laboratory tests (e.g., serum chemistry, blood count, thyroid function tests, lipid panel, syphilis serology, serum folate and vitamin B12), neuropsychological testing with a standardized test battery and interview with a collateral source to assess everyday function. In addition, participants received a standardized MRI scan of the brain at baseline. Exclusion criteria included (1) neurological illness other than AD or CVD; (2) cortical infarction on MRI; (3) significant closed head injury with loss of consciousness lasting longer than 30 minutes and resulting in significant cognitive or functional changes subsequent to the injury; (4) alcohol abuse within 5 years of the onset of cognitive loss; and (5) use of neuroleptics or antidepressants other than selective serotonin reuptake inhibitors and regular use of anxiolytics, hypnotics, or antihistamines. The institutional review boards at all participating institutions approved this study, and subjects or their legal representatives gave written informed consent.
Participants were selected from the parent sample simply on the basis of having at least two functional measure assessments with imaging and/or neuropsychological testing performed within six months of the baseline functional assessment [mean lag between neuropsychological testing and IADL = 1.2 months (SD = 1.6); mean lag between imaging and IADL = 2.2 months (SD = 1.6)]. We elected to restrict the sample to those with imaging and cognitive information within 6 months of the initial functional assessment to ensure that these measures were close to the values at baseline. The parent sample consisted of a total of 621 individuals, and of these individuals 124 persons, 52 cognitively normal, 35 MCI, and 37 demented (diagnoses at baseline evaluation) were included in the current study because they had neuropsychological testing and imaging within 6 months of the functional assessment and because they had at least two functional measure assessments. MCI was defined by any cognitive impairment sufficient to cause a Clinical Dementia Rating Scale (Hughes et al., 1982; Morris, 1993) score of .5. The CDR was determined according to previously published protocols that included a semi-structured interview of both the patient and the informant by certified CDR administrators. The CDR score was determined independently of neuro-psychological information according to published protocol. Of those with dementia, 24 had either possible or probable AD [NINCDS0ADRDA criteria; (McKhann et al., 1984)], 7 had either possible or probable ischemic vascular dementia [ADDTC criteria; (Chui et al., 1992)], and 6 had a dementia of mixed (AD0CVD) etiology.
Neuropsychological Measures
All participants underwent a standardized neuropsychological assessment. Several tests were used to derive psychometrically matched measures of memory (MEM) and executive function (EXEC) that served as the independent measures in this study. Details of scale derivation and validation have been reported previously (Mungas et al., 2003). To summarize, item response theory (IRT) analyses were performed to evaluate basic psychometric properties of items or units of measurement of potential donor scales from the neuropsychological assessment. Items were then selected for the scales so that the final scales have matched and uniformly high reliability across a broad range of ability. That is, the two scales have similar levels of reliability at all points of the ability continuum. The MEM composite (38 possible total points) was based on the MAS Word List Learning Test (Williams, 1991). It was composed of the sum of short delay free recall (12 points), short delay cued recall (12 points), Trial 3 total recall (11 points), and Trial 1 (3 points). The EXEC composite (79 possible total points) was constructed using WMS-R (Wechsler, 1987) Digit Span backward and Visual Memory Span backward total scores (12 points each), the Initiation/Perseveration subscale of the Mattis Dementia Rating Scale (13 points), (Mattis, 1973) and letter fluency [(Benton & Hamsher, 1976); FAS, 14 points for each letter]. These measures were converted to standard scores based on the mean and standard deviation of a group of normal controls from a larger sample of 400 from this project (Mungas et al., 2003). The scales have a mean of 100 and SD of 15 in the sample of controls, and have high reliability (r > .90) from about −2.0 SD below the mean of the overall development sample to 2.0 SD above the mean. These measures do not have appreciable floor or ceiling effects for participants in this sample and have linear measurement properties across a broad ability range. They are near-normally distributed, which presents advantages for statistical analyses.
Activities of Daily Living Measure
The Blessed Roth Dementia Rating Scale (BRDRS) requires an informant to rate two groups of activities, physical ADLs (e.g., eating, dressing, and toileting) and instrumental ADLs (e.g. housekeeping and money management). For the purpose of this study, only the eight IADL items were analyzed. The eight BRDRS IADL items are shown in Table 1. They are rated by a clinician based on caregiver report of the patient’s ability to complete the task using a scale of 1 = “Unable,” 0.5 = “Some trouble,” and 0 = “Normal.” The BDRS has often been used as a measure of functional status because of its demonstrated correlation with postmortem biochemical and neuropathological changes (Blessed et al., 1968, 1988; Tomlinson, 1977).
Table 1.
Blessed-Roth Dementia Rating Scale instrumental activities of daily living items
| a. | Ability to find way around familiar streets |
| b. | Perform household tasks |
| c. | Cope with small sums of money |
| d. | Remember short lists of items |
| e. | Find way about indoors |
| f. | Interpret surroundings (e.g., to recognize whether in hospital or at home) |
| g. | Recall recent events (e.g., recent outings, visits of relatives) |
| h. | Tendency to dwell in the past |
MRI Methods
The MRI protocol included a T1-weighted coronal magnetization prepared rapid gradient echo study with 1.5-mm slices and double spin-echo axial study (Reed et al., 2001). Segmentation to obtain quantitative volumes of lacunes (LAC), WMH, cortical gray matter (CGM), and hippocampal volume (HV) was accomplished using the T-1 and T-2 weighted axial images. Image segmentation methods have been previously described elsewhere (Fein et al., 2000). A computer algorithm was used to classify brain MRI pixels first into principle tissue types of gray matter, white matter, and cerebrospinal fluid. Subsequently, an operator-guided computer algorithm was applied to further subdivide gray matter into cortical and subcortical gray matter, and white matter into white matter lesions and normal appearing white matter. In addition, total intracranial volume was computed by summing overall pixels within the intracranial vault. Normalization of regions of interest volumes was accomplished by multiplying each volume by the ratio of the average control group total intracranial volume to that particular subject’s total intracranial volume.
Semi-automated hippocampal volumetry was carried out using a commercially available high dimensional brain mapping tool (Medtronic Surgical Navigation Technologies, Louisville, CO), that has been recently validated and compared to manual tracing of the hippocampus (Hsu et al., 2002). Measurement of HV is achieved first by manually placing 22 control points as local landmarks for the hippocampal head, one at the tail, and four per image (i.e. at the superior, inferior, medial and lateral boundaries) on five equally-spaced images perpendicular to the long axis of the hippocampus. Second, fluid image transformation was used to match the individual brains to a template brain, and pixels corresponding to hippocampus were labeled and counted to obtain the volumes (Christensen et al., 1997). This method of hippocampal voluming has documented reliability as measured by intraclass coefficients of .94 (Hsu et al., 2002).
Lacunes were operationally defined as small areas of the brain (<3 mm) with increased signal compared to CSF on proton density MRI in subcortical gray and white matter. Lacunes were differentiated from perivascular spaces because only lacunes are hyperdense relative to CSF on proton density images. For all scans one board certified neuroradiologist identified the lacunes. These lacunes were subsequently outlined by hand by a trained operator according to previously described criteria (Reed et al., 2000).
Data Analysis
Two sample t-test or chi-square tests were used to compare continuous and categorical variables between the parent and current study sample. Multiple imputation methods, using a Markov Chain Monte Carlo approach, were used to impute missing IADL ratings. IADL ratings were only imputed for dates at which the functional measure was attempted but not completed either because of an insufficient caregiver available to evaluate the functional ability of the subject or an incomplete questionnaire. Only 10% of the IADL ratings were imputed and 68% of those imputed were for normal subjects. We imputed 10 data sets assuming an underlying distribution of the IADL ratings centered at the baseline mean of each diagnostic group and combined the results from each of the data sets to yield final estimates of the associations. Alternative assumptions including assuming an underlying distribution centered at no functional impairments and at the baseline mean of all subjects were also considered, and results from these analyses were similar to those presented. Repeated measures, random effects models (Laird & Ware, 1982) were used to assess associations between imaging, neuropsychological variables and change in the IADL ratings. These models incorporated random-effects to allow for between person variability in IADL scores summarized by a person’s tendency to be above or below the predicted average level at a given time and to decline faster or slower than average. They also allow for different time-lag spacing and number of assessments across subjects. Models were built separately for baseline cognitive and imaging variables. Model building began with simple models followed by joint models that included all significant variables from the earlier steps. A final joint model included the significant variables from the imaging and cognitive models. Models were adjusted for the possible confounding effects of age, education, and gender. Model assumptions of normality, linearity, constant variance, and bivariate normality of the random effects were examined using graphical diagnostics, including residual plots and Q-Q plots.
The IADL variable was not normally distributed so the IADL rating was shifted by one and then transformed using the natural logarithm. This transformed variable satisfied the assumptions of the models. The imaging variables were centered at the mean and the memory and executive scores were transformed to z-scores for ease of interpretation.
RESULTS
There were 634 IADL assessments for the 124 cases included in the analyses. Final models presented in this paper include the 106 subjects with complete imaging and neuropsychological data. The modal number of annual assessments was five, and ranged from one to eight. The average time from the initial to last assessment was 5.5 years (SD = 2.3, range = 1.0–9.6). Eighty-nine percent of participants had more than one annual re-assessment, and 82% had more than two follow-up assessments. Table 2 presents baseline demographic information for each of the three clinical groups. All participants in this study underwent a baseline clinical evaluation and at least one subsequent annual evaluation. There were no significant differences between the sample used in the current study and the original parent sample in terms of age (p = .30) and education level (p = .5). The groups also did not differ on the MMSE (p = .3). The groups did differ in gender representation with a larger percentage of men in the study sample as compared to the parent sample (p < .001). Similarly there was a slightly higher percentage of Whites in the study sample (p = .05). The drop out rate was also higher in the parent sample as compared to the study sample (30% drop out rate vs. 10%, respectively). Finally, a higher percentage (p < .01) of those included in the analyses died (38%) compared to the parent study (22%)
Table 2.
Baseline demographic, neuropsychological, and ADL information for the three subject groups [Mean (SD)]
| Cognitively normal (n = 52) |
MCI (n = 35) |
Dementia (n = 37) |
|
|---|---|---|---|
| Age | 72.5 (7.4) | 72.8 (8.4) | 73.1 (8.4) |
| Gender (% female) | 46 | 20 | 32 |
| Education (years) | 14.9 (3.0) | 14.1 (3.2) | 14.0 (3.2) |
| Ethnicity (% White) | 85 | 77 | 84 |
| MMSE Score | 29.0 (1.3) | 28.2 (1.9) | 22.3 (4.7) |
| Memory Composite+ | .74 (.63) | .14 (.5) | −.90 (64) |
| Executive Composite+ | .65 (.61) | −.02 (.9) | −.90 (.74) |
| IADL | .45 (.84) | .80 (.85) | 2.9 (1.7) |
Presented as a standardized score
Cognitive Predictors of Baseline and Change in IADL Ratings
The random effects models allowed us to investigate associations between cognitive variables and baseline level and change in IADL ratings. In a model that only considered MEM, MEM was significantly associated with baseline functional status (p < .001) and with change in IADL ratings over time (p < .01). A lower (more impaired) MEM score at baseline was associated with higher IADL scores (poorer baseline functional status) and with a faster rate of decline. In a separate model-using baseline EXEC, a similar relationship was seen with executive function significantly associated with baseline functional status (p < .001) and with change in IADL ratings (p < .01). In the final, joint model, which controlled for age and education both MEM and EXEC were associated with baseline IADL ratings, but only EXEC predicted IADL change. MEM did not reach significance in the model. Education but not age was associated with baseline IADL rating (lower education associated with worse function). These results are displayed in Table 3.
Table 3.
Results of random effects modeling of MEM and EXEC baseline and change in association with IADLs
| Effect | Estimate | Standard Error | P value |
|---|---|---|---|
| Intercept | .636 | .041 | <.001 |
| Age | .004 | .005 | .48 |
| Education | .031 | .013 | .035 |
| MEM | −.440 | .060 | <.001 |
| EXEC | −.11 | .050 | .04 |
| Time | .06 | .010 | <.001 |
| MEM × time | −.025 | .013 | .051 |
| EXEC × time | −.033 | .012 | .015 |
| Age × time | .000 | .001 | .82 |
| Edu. × time | −.001 | .003 | .68 |
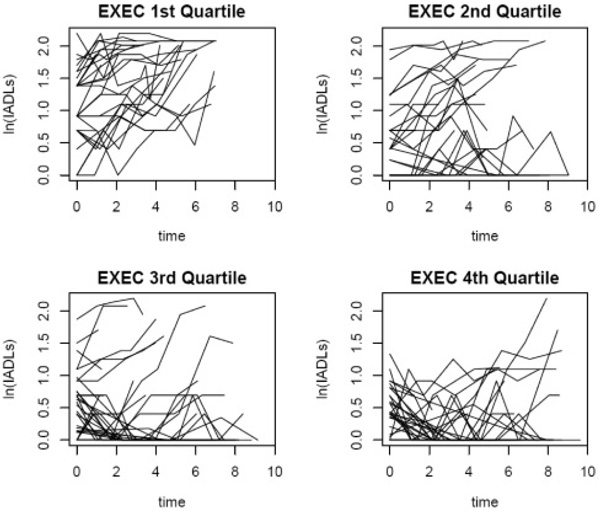
Figure 1 displays the individual trajectories of the IADL score by quartiles of EXEC. A joint model not controlling for age or education produced very similar results. There were no associations between change in IADL rating and gender, so this variable was not included in the final joint model, although results were similar to those presented in Table 3.
Fig. 1.
Individual trajectories of the IADL score by quartiles of EXEC. Those in the first quartile of EXEC have the lowest EXEC scores and their IADL trajectories suggest increasing levels of functional impairment over time. Those in the fourth quartile have the highest EXEC scores, and although there is variability, the trajectories suggest a lesser degree of functional impairment over time.
Neuroimaging Predictors of Baseline and Change in IADL Ratings
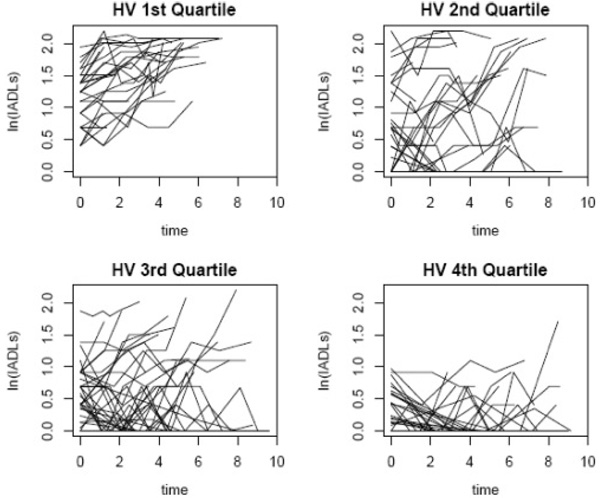
We first examined each of the imaging variables individually followed by a joint model with all significant variables. In these individual models, baseline volume measurements of CGM (p < .001) and HV (p < .001) were significantly associated with baseline IADL ratings, whereas HV was associated with change in IADL ratings (p < .01) and CGM was modestly associated with change in IADL ratings (p = .05). Individuals with more CGM and larger HV had less functional impairment at baseline. Those with smaller HV or smaller CGM volume had more rapid declines in IADL ratings over time. WMH (p = .1) and LAC (p = .2) were not associated with baseline IADL ratings or with change in IADL ratings (WMH p = .4, LAC p = .4). There were no associations with gender so this variable was not included in the final joint model. In the final joint model including CGM and HV and controlling for age and education, HV was an independent predictor of baseline IADL ratings and HV was significantly associated with change in IADL ratings. These results are displayed in Table 4. Individual trajectories of the IADL score by quartiles of HV are shown in Fig. 2.
Table 4.
Results of random effects modeling of MRI volumes at baseline and change in association with IADLs
| Effect | Estimate | Standard error | p value |
|---|---|---|---|
| Intercept | .720 | .050 | <.001 |
| Age | −.007 | .006 | .25 |
| Education | −.0005 | .015 | .98 |
| CGM | −1.11 | 1.31 | .41 |
| HV | −585.7 | 89.4 | <.001 |
| Time | .071 | .011 | <.001 |
| HV × time | −56.08 | 18.85 | .006 |
| CGM × time | −.05 | .28 | .87 |
| Age × time | −.01 | .001 | .64 |
| Edu × time | −.007 | .003 | .04 |
CGM = cortical gray matter volume; HV = hippocampal volume.
Fig. 2.
Individual trajectories of the IADL score by quartiles of HV. Those in the first quartile of HV have the lowest HV and their IADL trajectories suggest increasing levels of functional impairment over time. Those in the fourth quartile have the highest HV and the trajectories suggest a more stable level of functional impairment over time.
Cognitive and Neuroimaging Combined Model Predicting IADL Change
Finally, using a joint model that included both the cognitive and imaging variables that were statistically significant in the previous models, we determined which variables made independent contributions to predicting baseline IADL ratings and change in IADL ratings over time. Table 5 presents the results from the model that does not include an interaction between CGM and time, because this term was not significant and did not change the results. Both MEM (p < .001) and HV (p = .03) were independently associated with baseline IADL ratings. Baseline EXEC was the only variable that independently predicted rate of decline over time in IADL ratings (p = .036).
Table 5.
Results of random effects modeling of MRI volumes and MEM and EXEC at baseline and change in association with IADLs
| Effect | Estimate | Standard error | P value |
|---|---|---|---|
| Intercept | .667 | .043 | <.001 |
| CGM | .219 | .944 | .818 |
| HV | −217.9 | 94.9 | .03 |
| MEM | −.357 | .064 | <.001 |
| EXEC | −.09 | .054 | .11 |
| Time | .064 | .011 | <.001 |
| MEM × time | −.018 | .015 | .25 |
| EXEC × time | −.029 | .013 | .036 |
| HV × time | −17.25 | 22.11 | .44 |
DISCUSSION
In this study we examined the relationship between cognition and everyday function both from a cross-sectional and longitudinal standpoint. In the cross-sectional analysis, we examined the relative associations between memory and executive dysfunction and the severity of functional impairment. The results suggest that memory and executive functioning are related to current functional impairment (with memory possibly having somewhat of a stronger relationship than executive functioning based on examination of p-values). In the longitudinal analysis we evaluated the association between baseline memory and executive function and future change, or decline in functional status. In this joint model only executive functioning was associated with a more rapid rate of decline. Thus, those individuals with greater executive dysfunction at baseline experienced a faster rate of IADL decline, whereas greater memory impairment at baseline was not independently associated with the rate of functional decline.
Why might memory function relate to contemporaneous everyday function but not to rate of future change in everyday function whereas executive function relates to both? There are at least two possible explanations. First, executive dysfunction may play a particularly important role in the progression of disability. For example, Royall (Royall, 2006; Royall et al., 2004, Royall et al.,2005) argues for the central role of executive dysfunction in causing functional disability and in the conversion of MCI to dementia. Perhaps executive functions, because of their role in regulating and coordinating other mental functions, organizing behavior and generating responses, play a central role in a variety of everyday activities. Hence, when these functions begin to fail the person cannot function as well regardless of how well other cognitive functions are maintained. Further, because of the adaptive aspect of executive functions, intact executive function may permit successful compensation for failure in other cognitive domains, for example, memory. If so, failing executive abilities would then reduce compensatory ability and compound the loss of everyday function. Consistent with the present data, Royall and colleagues (Royall et al., 2005) found that change in executive function, but not change in memory, was associated with change in functional impairment over time, also suggesting that executive dysfunction may be particularly important to longitudinal declines in everyday function.
A second possible explanation for the results of the current study is that executive dysfunction is simply a marker of greater cortical involvement. Thus, when executive functions fail other cortically-mediated functions begin to fail as well and when multiple cognitive functions fail, everyday function declines more rapidly. It is well known that AD affects the hippocampal formation early in the development of the disease (Braak & Braak 1991; Hyman et al., 1984), which explains the fact that memory is affected severely and this occurs early in the course of the disease. As the disease progresses other cognitive functions (e.g. executive functions among other domains) become increasingly affected (Welsh et al., 1992). In this context, it is possible that once executive dysfunction is evident, AD pathology is affecting multiple neocortical areas (including, but not limited to the frontal lobes). Thus, executive functioning at baseline identifies how advanced the disease process is, and perhaps those with more advanced disease processes progress faster. In contrast, if memory alone is affected, functional decline may remain relatively unchanged or may not progress. Consistent with this idea is the fact that the lag between onset of amnestic MCI and conversion to dementia (if it occurs) is highly variable. During this time, which may extend over years, functional limitations are slight and distinct, and progressive loss of function is not seen. Evidence suggests that multi-domain MCI progresses to dementia more rapidly than amnestic or single-domain MCI (Alexopoulos et al., 2006; Meyer et al., 2002), which is also consistent with the hypothesis that cortical involvement carries a poor prognosis for everyday function. Arguing against the “more cortical involvement at baseline” hypothesis is the fact that we did not find that degree of cortical volume loss at baseline was predictive of faster functional decline. Ultimately to answer the question about whether there is truly something unique to executive dysfunction that is associated with a more rapid course of functional decline we will have to include measures not only of memory and executive functioning, but also of other cognitive domains (e.g., visuospatial functions). A strength of this study over most of the previous work was the use of psychometrically matched cognitive scales derived through item response theory methods, allowing for greater confidence that the pattern of associations found was not influenced by nonspecific measurement artifacts of the tests.
Unfortunately, additional analysis of subgroups of patients (e.g., those with MCI versus those with AD) was underpowered in the current study because of small sample sizes, and therefore such results were not presented. Preliminary, post-hoc analysis of our results indicated that in the MCI sample, there was a trend for baseline MEM to be associated with baseline IADL ratings but EXEC to be associated with longitudinal change in everyday function (very similar to the results that are presented using the entire sample). Interestingly MEM was primarily associated with baseline IADL ratings and change in IADL ratings in the AD group, suggesting that prominent memory dysfunction in this group may largely drive the progression of functional impairment. However, a better understanding of how the current results relate to specific subgroups of older adults awaits larger studies.
In addition to examining cognitive predictors of baseline and change in IADL ratings, we were also interested in examining neuroimaging predictors. In the final multivariate model we found that although hippocampal volume was associated with functional status in the cross-sectional analysis, baseline hippocampal volumes were not independently associated with rate of decline. That is, when the cognitive and neuroimaging measures were entered jointly as predictors, only executive functioning accounted for a significant proportion of variance in future daily function. Our results are consistent with the findings of Boyle and colleagues (Boyle et al., 2004) who found that MRI measures of white matter disease did not improve the prediction of IADL performance in patients with vascular dementia over and above the contributions of cognitive variables. Both studies found that cognitive measures predicted daily function better than did brain imaging markers. Whether this is a generalizable finding, however, requires further study. For example, the research on the comparative value of imaging and cognitive predictors of conversion from MCI to dementia, a change defined in part by decreasing everyday function, is quite mixed (DeCarli et al., 2004; Korf et al., 2004; Visser et al., 1999).
Although some previous studies (Boyle et al., 2004; Farias et al., 2004) have suggested that neuroimaging markers of CVD (e.g., WMH) relate to functional status, we did not find this relationship in the present study. In this study, WMH and lacunes were related to neither baseline functional status nor rate of change. The differences between studies may be partially related to differences in sample characteristics. The study by Boyle and colleagues (Boyle et al., 2004) focused on individuals specifically selected because of CVD, and the study by Farias and colleagues (Farias et al., 2004) examined a community-based group with low rates of dementia. Thus, the imaging predictors of rate of change may vary depending on the sample and in particular the distribution and severity of different brain pathologies within that sample. In the present case, both the distribution of clinical diagnoses and the results of neuropathology examinations in deceased patients from the parent sample (Reed et al., 2004) suggest that the underlying pathology in this sample, while including a significant degree of CVD, is predominantly AD. For this reason it may be that neither lacunes nor WMH are particularly important markers of disease in this sample. Analyses of neuroimaging data from the parent study have shown that HV (and cortical gray matter) tend to be more strongly associated with cognition than WMH and volume of lacunes (Mungas et al., 2005; Mungas et al., 2001). It is therefore possible that the contribution of white matter disease in this sample with substantial AD is lesser.
A number of limitations deserve mention. How well our results may generalize to the aged population at large is unknown, but in this context the heterogeneity of this sample is strength. Community-based studies using autopsy confirmation of diagnosis have generally shown a substantial frequency of cases with “mixed dementia,” with reported rates ranging from 40% to 77% (Barker et al., 2002; White et al., 2005). Some degree of caution about the generaliz-ability of the results does seem warranted given differences between the study and parent samples. Although there were no differences in age, education, and global level of cognitive impairment (as measured by the MMSE) between the parent sample and current study sample, there were differences in some demographic variables (gender and race). Additionally, there was a higher drop out rate in the parent sample, although this did not appear to be a result of greater cognitive impairment in this larger group. There was no difference in diagnosis between those who dropped out and those who continued in the parent study, and the distribution of diagnoses was similar between the two groups at the baseline evaluation. The study sample likely contained a higher percentage of more frail or ill individuals, as evidenced by a higher mortality rate than the parent sample (38% vs. 22%). Reasons for attrition in the study sample are not known; therefore it is possible that subjects may not have returned for additional assessments because they were either too impaired, or alternatively, because they were functioning sufficiently well that they were less interested in participating in the study. As such, the relationships reported here may be specific to the sample studied, and cross-validation would be necessary for determining the generalizability of the findings.
The current study focused on two cognitive domains, memory and executive function because prior studies had identified them as especially important to daily function. The measurement of other cognitive domains such as language and visuospatial abilities were not included. Thus the question as to whether executive functions play a special role in predicting functional decline or whether it is a marker of a more general effect of greater cortical involvement cannot be directly addressed by this study. If it is a more general effect we would expect that other measures of neocortical functions such as language and visuospatial skills may also be associated with rate of change. Using psychometrically matched instruments assessing a wider range of cognitive domains is an important direction for future research. A second issue is that the particular executive function scale used here was derived from working memory and verbal fluency tests and thus cannot be said to measure executive function in a comprehensive sense. Different measures of executive abilities might well yield different results. This is to an extent true of the memory composite as well, although the weighting of the memory measure—delayed verbal episodic memory—is at the core of a range of clinical memory tests and clearly represents a central aspect of memory function. The IADL rating scale, while widely used, is brief and non-comprehensive. Use of both informant- and performance-based instruments might permit more detailed and complex relationships between cognitive functions and everyday function to emerge. Additionally, although this study employed a broader range of MRI markers than previous studies, we nevertheless did not have a specific measure of frontal lobe volume or specific frontal white matter changes, which may be important for determining the contribution of this brain structure to the prediction of functional disability. Dysfunction of the prefrontal cortex may arise from direct neuronal loss, loss of input from neocortical association areas, and damage to frontal-striatal circuitry. Unfortunately, we are unable to identify the pathway or the etiology of this dysfunction in this study, and future studies with more specific MRI volumetric measures of frontal lobe volume are needed. Additionally, the reliability of identifying lacunes is known to be problematic. In this study one rater (a board certified neurologist) identified lacunes on MRI scans for all participants. Further evaluation of this approach is needed.
In summary, previous studies have shown that individuals with greater global cognitive dysfunction at baseline experience a more rapid rate of decline in IADLs than individuals with milder impairment overall (Feldman et al., 2001; Schmeidler et al., 1998). The current results extend these findings to show that in particular, those patients with a greater degree of executive dysfunction at initial assessment tend to experience a more rapid decline in ratings of their functional status over time. Although more atrophic hippocampi at baseline were associated with a faster rate of decline in everyday function, this was not independent of the effects of executive dysfunction. Thus, whereas these data suggest that executive abilities may play a particularly important role in predicting decline of everyday function, various issues leave that an open question. However, these observations provide strong support for the value of exploring the effects of specific domains of cognitive impairment on daily function and demonstrate that the factors predicting current status may differ from those that predict change. Understanding the difference may refine our understandings of how brain pathology is eventually expressed in disability.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute on Aging AG10129, AG021511, AG12435, P50AG16570, by the California Department of Health Services Alzheimer’s Disease Program and the Veterans Affairs Northern California Health Care System.
REFERENCES
- Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dementia and Geriatric Cognitive Disorders. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- Barberger-Gateau P, Fabrigoule C. Disability and cognitive impairment in the elderly. Disability and Rehabilitation. 1997;19:175–193. doi: 10.3109/09638289709166525. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer’s Disease and Associated Disorders. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bell-McGinty S, Podell K, Franzen M, Baird AD, Williams MJ. Standard measures of executive function in predicting instrumental activities of daily living in older adults. International Journal of Geriatric Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- Bennett HP, Corbett AJ, Gaden S, Grayson DA, Kril JJ, Broe GA. Subcortical vascular disease and functional decline: A 6-year predictor study. Journal of the American Geriatric Society. 2002;50:1969–1977. doi: 10.1046/j.1532-5415.2002.50608.x. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. Blessed-Roth Dementia Scale (DS) Psychopharmacology Bulletin. 1988;24:705–708. [PubMed] [Google Scholar]
- Boyle PA, Cohen RA, Paul R, Moser D, Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. International Journal of Geriatric Psychiatry. 2002;17:164–169. doi: 10.1002/gps.539. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Paul RH, Moser DJ, Cohen RA. Executive impairments predict functional declines in vascular dementia. The Clinical Neuropsychologist. 2004;18:75–82. doi: 10.1080/13854040490507172. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cahn DA, Malloy PF, Salloway S, Rogg J, Gillard E, Kohn R, Tung G, Richardson ED, Westlake R. Subcortical hyperintensities on MRI and activities of daily living in geriatric depression. Journal of Neuropsychiatry and Clinical Neurosciences. 1996;8:404–411. doi: 10.1176/jnp.8.4.404. [DOI] [PubMed] [Google Scholar]
- Cahn DA, Sullivan EV, Shear PK, Pfefferbaum A, Heit G, Silverberg G. Differential contributions of cognitive and motor component processes to physical and instrumental activities of daily living in Parkinson’s disease. Archives of Clinical Neuropsychology. 1998;13:575–583. [PubMed] [Google Scholar]
- Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Applied Neuropsychology. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- Christensen GE, Joshi SC, Miller MI. Volumetric transformation of brain anatomy. IEEE Trans Med Imaging. 1997;16:864–877. doi: 10.1109/42.650882. [DOI] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed B, Haan MN, Jagust WJ. Everyday functioning in relation to cognitive functioning and neuroimaging in community-dwelling Hispanic and non-Hispanic older adults. Journal of the International Neuropsychological Society. 2004;10:342–354. doi: 10.1017/S1355617704103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H, Sauter A, Donald A, Gelinas I, Gauthier S, Torfs K, Parys W, Mehnert A. The Disability Assessment for Dementia Scale: A 12-month study of functional ability in mild to moderate severity Alzheimer disease. Alzheimer Disease and Associated Disorders. 2001;15:89–95. doi: 10.1097/00002093-200104000-00008. [DOI] [PubMed] [Google Scholar]
- Goldstein G, McCue M, Rogers J, Nussbaum PD. Diagnostic differences in memory test based predictions of functional capacity in the elderly. Neuropsychological Rehabilitation. 1992;2:307–317. [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Convit A, Mittelman MS, Cohen J, Rusinek H, De Santi S, George AE. Hippocampal formation size in normal human aging: A correlate of delayed secondary memory performance. Learning and Memory. 1994;1:45–54. [PubMed] [Google Scholar]
- Griffith HR, Belue K, Sicola A, Krzywanski S, Zamrini E, Harrell L, Marson DC. Impaired financial abilities in mild cognitive impairment: A direct assessment approach. Neurology. 2003;60:449–457. doi: 10.1212/wnl.60.3.449. [DOI] [PubMed] [Google Scholar]
- Grundman M, Jack CR, Jr, Petersen RC, Kim HT, Taylor C, Datvian M, Weiner MF, DeCarli C, DeKosky ST, van Dyck C, Darvesh S, Yaffe K, Kaye J, Ferris SH, Thomas RG, Thal LJ. Hippocampal volume is associated with memory but not nonmemory cognitive performance in patients with mild cognitive impairment. Journal of Molecular Neuroscience. 2003;20:241–248. doi: 10.1385/jmn:20:3:241. [DOI] [PubMed] [Google Scholar]
- Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. International Journal of Geriatric Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hsu YY, Schuff N, Amend DL, Du AT, Norman D, Chui HC, Jagust WJ, Weiner MW. Quantitative magnetic resonance imaging differences between Alzheimer disease with and without subcortical lacunes. Alzheimer’s Disease and Associated Disorders. 2002;16:58–64. doi: 10.1097/01.WAD.0000013690.85676.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. The British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Korf ES, Wahlund LO, Visser PJ, Scheltens P. Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology. 2004;63:94–100. doi: 10.1212/01.wnl.0000133114.92694.93. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale, Professional Manual. Odessa, FL: Psychological Assessment Resources; 1973. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Meyer J, Xu G, Thornby J, Chowdhury M, Quach M. Longitudinal analysis of abnormal domains comprising mild cognitive impairment (MCI) during aging. Journal of the Neurological Sciences. 2002;201:19–25. doi: 10.1016/s0022-510x(02)00159-4. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- Reed BR, Eberling JL, Mungas D, Weiner M, Jagust WJ. Frontal lobe hypometabolism predicts cognitive decline in patients with lacunar infarcts. Archives of Neurology. 2001;58:493–497. doi: 10.1001/archneur.58.3.493. [DOI] [PubMed] [Google Scholar]
- Reed BR, Eberling JL, Mungas D, Weiner M, Kramer JH, Jagust WJ. Effects of white matter lesions and lacunes on cortical function. Archives of Neurology. 2004;61:1545–1550. doi: 10.1001/archneur.61.10.1545. [DOI] [PubMed] [Google Scholar]
- Reed BR, Eberling JL, Mungas D, Weiner MW, Jagust WJ. Memory failure has different mechanisms in subcortical stroke and Alzheimer’s disease. Annals of Neurology. 2000;48:275–284. [PMC free article] [PubMed] [Google Scholar]
- Reed BR, Jagust WJ, Seab JP. Mental status as a predictor of daily function in progressive dementia. The Gerontologist. 1989;29:804–807. doi: 10.1093/geront/29.6.804. [DOI] [PubMed] [Google Scholar]
- Richardson ED, Nadler JD, Malloy PF. Neuropsychologic prediction of performance measures of daily living skills in geriatric patients. Neuropsychology. 1995;9:565–572. [Google Scholar]
- Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: A population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- Royall DR. Mild cognitive impairment and functional status. Journal of the American Geriatric Society. 2006;54:163–165. doi: 10.1111/j.1532-5415.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. Journal of the American Geriatric Society. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Executive control mediates memory’s association with change in instrumental activities of daily living: The Freedom House Study. Journal of the American Geriatric Society. 2005;53:11–17. doi: 10.1111/j.1532-5415.2005.53004.x. [DOI] [PubMed] [Google Scholar]
- Schmeidler J, Mohs RC, Aryan M. Relationship of disease severity to decline on specific cognitive and functional measures in Alzheimer disease. Alzheimers Disease and Associated Disorders. 1998;12:146–151. doi: 10.1097/00002093-199809000-00005. [DOI] [PubMed] [Google Scholar]
- Severson MA, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Ivnik RJ, Atkinson EJ, Kurland LT. Patterns and predictors of institutionalization in community-based dementia patients. Journal of the American Geriatric Society. 1994;42:181–185. doi: 10.1111/j.1532-5415.1994.tb04949.x. [DOI] [PubMed] [Google Scholar]
- Skurla E, Rogers JC, Sunderland T. Direct assessment of activities of daily living in Alzheimer’s disease. A controlled study. Journal of the American Geriatric Society. 1988;36:97–103. doi: 10.1111/j.1532-5415.1988.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Stern Y, Hesdorffer D, Sano M, Mayeux R. Measurement and prediction of functional capacity in Alzheimer’s disease. Neurology. 1990;40:8–14. doi: 10.1212/wnl.40.1.8. [DOI] [PubMed] [Google Scholar]
- Tomlinson BE. Morphological changes and dementia in old age. In: Smith WL, Kinsbourne M, editors. Aging and Dementia. Holliswood, NY: Spectrum; 1977. pp. 25–57. [Google Scholar]
- Vetter PH, Krauss S, Steiner O, Kropp P, Moller WD, Moises HW, Koller O. Vascular dementia versus dementia of Alzheimer’s type: Do they have differential effects on caregivers’ burden? Journals of Gerontology (B) 1999;54:S93–S98. doi: 10.1093/geronb/54b.2.s93. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. Journal of Neurology. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale–Revised (WMS-R) San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Archives of Neurology. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, Hardman J, Davis D, Nelson J, Markesbery W. Recent clinical-pathologic research on the causes of dementia in late life: Update from the Honolulu-Asia Aging Study. Journal of Geriatric Psychiatry and Neurology. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- Williams JM. Memory Assessment Scales. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]