Abstract
Calcium acts as a second messenger in many cell types, including lymphocytes. Resting lymphocytes maintain a low concentration of Ca2+. However, engagement of antigen receptors induces calcium influx from the extracellular space by several routes. A chief mechanism of Ca2+ entry in lymphocytes is through store-operated calcium (SOC) channels. The identification of two important molecular components of SOC channels, CRACM1 (the pore-forming subunit) and STIM1 (the sensor of stored calcium), has allowed genetic and molecular manipulation of the SOC entry pathway. In this review, we highlight advances in the understanding of Ca2+ signaling in lymphocytes with special emphasis on SOC entry. We also discuss outstanding questions and probable future directions of the field.
In lymphocytes, crosslinking of antigen receptors typically activates phosphoinositide-specific phospholipase C. Phospholipase C breaks down phosphatidylinositol-4,5-bisphosphate to generate inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) and diacylglycerol. Ins(1,4,5)P3 binds its receptor located on the surface of internal Ca2+ stores, mainly the endoplasmic reticulum, and activates the release of Ca2+ into the cytoplasm. This event, known as ‘store depletion’, in turn activates store-operated calcium (SOC) channels in the plasma membrane to recruit Ca2+. Lymphocytes are believed to use SOC entry (SOCE) as the main mode of Ca2+ influx. The best characterized SOC channels in lymphocytes are known as ‘calcium release–activated calcium’ (CRAC) channels1. CRAC channels are highly Ca2+-selective, low-conductance channels with a characteristic inwardly rectifying current-voltage relationship. The past few years have brought substantial progress in understanding the molecular composition of the CRAC signaling complex. High-throughput screens based on RNA-mediated interference have identified STIM1 (stromal interaction molecule 1) as the endoplasmic reticulum–resident Ca2+ sensor and CRACM1 (calcium release–activated calcium modulator 1; also called Orai1) as the pore-forming subunit of CRAC channels2–6. STIM1 has one homolog, STIM2, whereas CRACM1 has two homologous proteins, CRACM2 and CRACM3, in mice and humans.
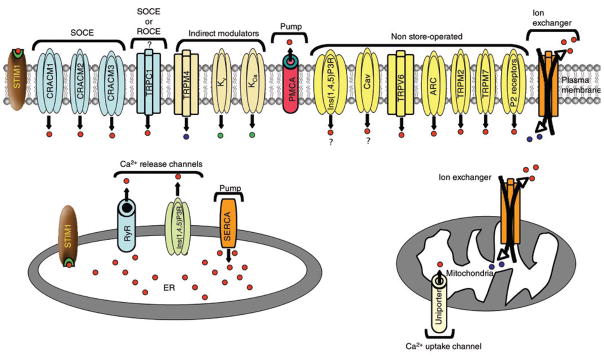
The canonical transient receptor potential (TRPC) channels have also been reported to increase intracellular Ca2+ concentrations either directly through coupled plasma membrane receptor stimulation or, arguably, through ‘store depletion’, in different cell types7–9. Of the seven mammalian TRPC channels (TRPC1–TRPC7), TRPC1 has most often been reported to form diverse channels, ranging from relatively Ca2+ selective to nonselective, in a variety of cell types by selective homomeric or heteromeric interactions with TRPC3, TRPC4 and TRPC7 (refs. 10,11). In their non-store-operated mode, TRPC3, TRPC6 and TRPC7 can also be activated by diacylglycerol8,12,13. Direct involvement of TRPCs in SOCE remains controversial with no conclusive reports of store-operated TRPC Ca2+ currents in lymphocytes. The evidence and controversies surrounding the function of TRPC channels in SOCE have been discussed1,11. There are many other non-store-operated routes of lymphocyte Ca2+ entry and modulators of lymphocyte cytosolic Ca2+ concentration (summary, Table 1 and Fig. 1).
Table 1.
Routes of cytosolic Ca2+ modulation in lymphocytes
| Route | Activation | Physiological outcome | Reference |
|---|---|---|---|
| Pumps | |||
| SERCA | ATP | Pumps Ca2+ from cytosol to ER | 74 |
| PMCA | ATP | Prevents cytosolic Ca2+ accumulation and contributes to Ca2+ signaling dynamics | 75,76 |
| Intracellular Ca2+ channels | |||
| Ins(1,4,5)P3R, ER | Ins(1,4,5)P3 | Transient increase in cytosolic Ca2+ | 77 |
| RyR | Ca2+, cADPR, L-type Ca2+ channels | Transient increase in cytosolic Ca2+ | 78,79 |
| Ca2+-selective store-operated channels | |||
| CRAC | Store-operated | Sustained increase in cytosolic Ca2+ | 4–6 |
| Nonselective (receptor- or store-operated) channels | |||
| TRPC | Diacylglycerol or store-operated? | Sustained increase in cytosolic Ca2+ | 7–9 |
| Ion exchangers | |||
| Dual-mode sodium-calcium exchanger | Forward mode: high cytosolic Ca2+ concentrations | Prevents accumulation of Ca2+ in the cytosol | 80,81 |
| Reverse mode: high cytosolic Na+ concentrations | Extrudes Na+ and contributes to increase in cytosolic Ca2+ | 76,81 | |
| Mitochondrial Ca2+ buffering | |||
| Ca2+-uptake channel: mitochondrial Ca2+ uniporter | High cytosolic Ca2+ concentrations | Buffers sub-plasmalemmal Ca2+ | 82,83 |
| Ca2+-extrusion channel: sodium-calcium exchanger | High mitochondrial Ca2+ concentrations | Buffers sub-plasmalemmal Ca2+, facilitates ER refilling | 82,83 |
| Indirect modulators of cytosolic Ca2+ | |||
| TRPM4 and TRPM5 (Na+ channels) | Membrane depolarization–activated | Inhibits the driving force for CRAC-mediated Ca2+ influx | 52,54 |
| Kv1.3 (K+ channel) | Membrane depolarization–activated | Potentiates the driving force for CRAC-mediated Ca2+ influx | 84 |
| KCa3.1 (K+ channel) | Calcium-activated | Potentiates the driving force for CRAC-mediated Ca2+ influx | 84 |
| Non-store-operated Ca2+ influx | |||
| Purinergic P2 receptors | Nucleotides | Nonselective cation influx | 85 |
| TRPV6 (CaT1) | Constitutively active; inhibited by cytosolic increase in Ca2+ | Selective Ca2+ influx | 1,86 |
| TRPM2 (LTRPC2) | Pyrimidine nucleotides, ADPR and NAD | Nonselective cation influx | 87,88 |
| TRPM7 (MIC or LTRPC7) | Intracellular Mg2+ concentrations | Nonselective cation influx | 89,90 |
| ARC channel | Phosphorylation | Selective Ca2+ influx | 91 |
| Ins(1,4,5)P3R, PM (controversial) | Ins(1,4,5)P3 | Unknown | 92 |
| Cav (controversial) | Unclear in lymphocytes | Contributes to increases in cytosolic Ca2+ and NFAT translocation | 71,72 |
CaT1, Ca2+ transport protein; ADPR, adenosine 5′ diphosphoribose; NAD, nicotinamide adenine dinucleotide; LTRPC, long transient receptor potential channel; MIC, Mg2+ inhibited cation channel; PM, plasma membrane.
Figure 1.
Routes of Ca2+ influx and efflux. Routes with similar mechanisms of activation are grouped together here. The probability of activation of a particular mechanism and its eventual contribution toward an increase in cytosolic Ca2+ may vary, and all routes may not be active at a given time. Red dots, Ca2+; blue dots, Na+; green dots, K+; ?, controversial route. ROCE, receptor-operated Ca2+ entry; Kv, voltage-gated K+ channel; KCa, Ca2+-activated K+ channel; PMCA, plasma membrane Ca2+ ATPase; Ins(1,4,5)P3R, Ins(1,4,5)P3 receptor; TRPV6, transient receptor potential, vanilloid, member 6; ARC, arachidonate-regulated, Ca2+-selective; P2 receptors, purinergic receptors; RyR, ryanodine receptor; SERCA, sarco-endoplasmic reticulum Ca2+ ATPase; ER, endoplasmic reticulum.
SOC influx during antigen receptor signaling
Antigen recognition in lymphocytes typically results in the tyrosine phosphorylation of immunoreceptor tyrosine-based activation motifs and the recruitment and activation of protein tyrosine kinases. These initial signaling events, which are more or less similar in T and B lymphocytes and mast cells, trigger the phosphorylation and activation of phospholipase C, which generates Ins(1,4,5)P3 and diacylglycerol. Ins(1,4,5)P3-induced release of Ca2+ from the endoplasmic reticulum results in a transient increase in the cytosolic free Ca2+ concentration, and diacylglycerol activates protein kinase C. Protein kinase C and Ca2+ together initiate certain rapid cellular responses, such as rearrangement of the actin cytoskeleton to promote T cell motility, adhesion and formation of the immunological synapse, although maintenance of the immunological synapse probably requires a prolonged increase in the cytosolic Ca2+ concentration as a result of SOCE14. Diacylglycerol can accomplish several other functions, including directly activating non-store-operated Ca2+ influx through certain TRPC channels and stimulating the GTPase Ras–mitogen-activated protein kinase signaling cascade, which subsequently activates the AP-1 transcriptional complex8,12. In lymphocytes, AP-1 acts together with the transcription factors NFAT and NF-κB to modulate gene expression15. Ca2+ influx mediated by CRAC channels may also directly activate the Ras–mitogen-activated protein kinase cascade and thus also promote AP-1 activation16. Other transcriptional regulators that depend on Ca2+ signaling for lymphocyte activation include NFAT, NF-κB, the kinase Jnk and calmodulin-dependent kinase, although the duration and amplitude of cytosolic Ca2+ flux required for activation of each transcription factor varies17,18. For example, a prolonged increase in cytosolic Ca2+ concentration via the CRAC channels is crucial for the activation of calcineurin, a serine-threonine phosphatase needed for the dephosphorylation and nuclear localization of NFAT. Even after its translocation into the nucleus, NFAT requires a sustained increase in Ca2+ concentration to prevent its ejection19. Several other cytosolic and nuclear factors modulate the intracellular localization of NFAT. These include the calcineurin inhibitors AKAP, CABIN, CHP and DSCR1, the newly identified Homer family proteins and the NFAT kinases GSK3, CK1 and DYRK19,20. Thus, the SOCE-calcineurin-NFAT pathway acts together with several other signaling cascades to promote lymphocyte antigen receptor–induced gene transcription.
STIM proteins
STIM1 is a 77-kilodalton single-spanning transmembrane protein that resides mainly in the endoplasmic reticulum and to some extent also in the plasma membrane. STIM1 has been proposed to sense the depletion of Ca2+ stores through its amino-terminal Ca2+-binding EF hand domain. Store depletion triggers the formation of oligomers of STIM1 in the endoplasmic reticulum through the EF-SAM region and subsequent translocation to discrete ‘puncta’ at endoplasmic reticulum–plasma membrane junctions21,22. Structural insights into the mechanism of the formation of oligomers have shown that in resting, Ca2+-replete conditions, the EF-SAM domain exists as a well-folded monomer. Ca2+ depletion induces partial unfolding and exposure of hydrophobic residues in the EF-SAM region, which results in its formation of oligomers23. Consistent with those findings, forced formation of heterodimers of STIM1 results in Ca2+-independent punctae formation and activation of CRAC2,24,25. The coiled-coil domains of STIM1, which mediate constitutive homotypic interactions, may further stabilize the EF-SAM-triggered oligomers and promote the translocation of STIM1 to endoplasmic reticulum–plasma membrane junctions26. The exact mechanism by which STIM1 oligomers activate CRAC channels once located in the endoplasmic reticulum–plasma membrane junctional region still remains to be determined.
STIM1 has a closely related homolog, STIM2. The function of STIM2 has remained somewhat controversial, with some early reports assigning it a positive function in SOCE and others suggesting a negative regulatory function2,27. A subsequent report has confirmed that STIM1 and STIM2 are activators of SOCE in HeLa cervical cancer cells, human embryonic kidney 293 cells and human umbilical vein endothelial cells28. More notably, knockdown of STIM1 or STIM2 expression mediated by small interfering RNA has shown that STIM2, but not STIM1, regulates mainly basal cytosolic and endoplasmic reticulum Ca2+ concentrations in these cells28. The authors suggest that to maintain basal Ca2+ concentrations, STIM2 has a Ca2+-binding EF hand domain with a lower affinity for Ca2+ than that of STIM1. As a result, even a small decrease in the endoplasmic reticulum Ca2+ concentration results in the translocation of STIM2 to endoplasmic reticulum–plasma membrane junctions to activate CRAC channels. In contrast to that report28, no obvious changes in the basal cytosolic Ca2+ or endoplasmic reticulum Ca2+ pools have been detected in ex vivo naive or in vitro–differentiated T cells isolated from Stim2−/− mice, whereas naive Stim1−/− T cells seem to have less release of endoplasmic reticulum Ca2+ induced by antibody to CD3, which indicates that Stim1−/− cells have depleted resting endoplasmic reticulum Ca2+ pools29.
Similarly, mast cells derived from Stim1−/− mice have much less antigen- or thapsigargin-stimulated release of endoplasmic reticulum Ca2+ than do Stim1+/+ mast cells16. Although the size of resting endoplasmic reticulum Ca2+ pools in Stim1−/− and Stim1+/+ mast cells was not specifically assessed in that study16, as mentioned above, it seems likely that STIM1 is crucial for the replenishment of endoplasmic reticulum Ca2+ during the resting state in mast cells and T cells. Thus, although it is clear that both STIM1 and STIM2 contribute to the maintenance of intracellular Ca2+ concentrations, their exact physiological functions seem to be complex and may differ depending on the cell type and mechanism of activation.
CRACM proteins
CRACM1 is a small protein of 32.7 kilodaltons with four transmembrane domains and amino and carboxyl ends that face the cytosol4–6. One of the first experiments to test whether CRACM1 could form the CRAC channel involved expressing CRACM1 together with STIM1 in human embryonic kidney and Jurkat cell lines. This resulted in an enormous amplification of 50- to 100-fold in CRAC currents6,30–32. Further biochemical analysis, including coimmunoprecipitation and site-directed mutagenesis of negatively charged transmembrane glutamate residues, has shown that CRACM1 creates the pore of the CRAC channel by forming homo-oligomers33–35. In experiments with heterologous expression systems, CRACM1 can also form heteropolymers with CRACM2 (Orai2) and CRACM3 (Orai3) and possibly some TRPC channel subunits36,37. A functional CRAC channel pore requires the tetrameric assembly of CRACM subunits. This has been shown by coexpression of preassembled tandem CRACM1 multimers composed of varying numbers of subunits along with a dominant negative CRACM1 mutant38,39.
Communication between STIM1 and CRACM1
A strong functional interaction between STIM1 and CRACM1 was demonstrated by many laboratories30–32. Such studies suggest that STIM1 and CRACM1 are necessary and sufficient to generate CRAC-like currents in vitro. Additional interacting molecules are either not required or are not limiting for the in vitro reconstitution of CRAC currents. After store depletion, STIM1 forms oligomers, moves to the endoplasmic reticulum–plasma membrane junctions and localizes within 10–25 nm of the plasma membrane40. Furthermore, the STIM1 clusters in the endoplasmic reticulum–plasma membrane junctions are present near the regions of Ca2+ influx from the plasma membrane. Although much insight has been gained from heterologous expression studies of CRACM1 and STIM1, many basic questions remain unanswered. For example, does STIM1 associate directly with CRACM1 to activate Ca2+ influx or is the interaction mediated by accessory proteins? Is plasma membrane STIM1 involved in SOCE? Using coimmunoprecipitation and fluorescence resonance energy transfer, various groups have shown that drosophila as well as mammalian homologs of STIM1 and CRACM1 can localize together and associate with each other33,34,41–43, with only a few exceptions that have failed to detect any interaction44. In addition, store depletion can enhance the association between drosophila STIM and CRACM proteins34. Using chemically inducible bridges of varying lengths between endoplasmic reticulum and plasma membrane, one study has shown that the estimated protrusion of CRACM1 into the cytosol is between 11 nm and 14 nm, whereas the STIM1 cytosolic tail measures only 6 nm. Although those data do not rule out the possibility of direct interaction between CRACM1 and STIM1, it is possible that unlike STIM1, CRACM1 is part of a larger molecular complex and that accessory proteins serve the function of connecting the two molecules45. One candidate for this could be Ca2+-independent phospholipase A2, a component of the Ca2+-influx factor pathway46. In fact, a drosophila ortholog of Ca2+-independent phospholipase A2, CG6718, has shown up as a fairly strong ‘hit’ in a genome-wide RNA-mediated interference screen for genes involved in regulating SOCE4. However, the hypothesis noted above can be reconciled with earlier reports6,30–32 showing amplification of about 100-fold in CRAC currents induced by coexpression of only CRACM1 and STIM1 when other intracellular components contributing to SOCE are present in nonlimiting quantities.
In attempts to elucidate the molecular mechanism of activation of CRAC channels, extensive delineation of the domains of STIM1 has identified regions involved in targeting STIM1 to the endoplasmic reticulum–plasma membrane junction versus those involved in activation or CRACM1 binding22,26,41,47. Some data indicate that the STIM1 carboxy-terminal cytosolic tail containing a conserved ezrin-radixin-moesin domain and a lysine-rich region is sufficient to constitutively activate CRAC currents in human embryonic kidney cells41. However, another study found the polycationic region to be dispensable for CRAC activation47. That study also reported that the coiled-coil domain is essential for STIM1 aggregation and the serine-proline domain is essential for targeting to the endoplasmic reticulum–plasma membrane junction47. Furthermore, others have proposed that the carboxy-terminal domain of STIM1 dynamically binds a putative coiled-coil domain in the carboxy-terminal tail of CRACM1 in a Ca2+ store–dependent way43. One group used chemical crosslinking and single-molecule imaging of green fluorescent protein–tagged CRACM1 to show that CRACM1 exists as a dimer in resting conditions. Furthermore, expression of green fluorescent protein–tagged CRACM1 together with the carboxyl terminus of STIM1 induces the ‘dimerization’ of CRACM1 dimers, resulting in active tetrameric CRAC channels in a store-independent way48. Further studies in this direction will delineate the precise molecular domains and steps involved in CRAC channel gating.
Functions of CRAC in mast cells
Mast cells are the key effectors that work at the interface of innate and adaptive immune responses49. Although mast cells can be activated in many different ways and thus serve many different functions, crosslinking of the high-affinity immunoglobulin E receptor FcεRI is the main mechanism of mast cell activation. Crosslinking of FcεRI initiates two parallel pathways activated by the Src-family protein tyrosine kinases Lyn and Fyn. Until recently it was generally accepted that in mast cells the Lyn–Syk–membrane adaptor Lat–phospholipase C axis is the main contributor to Ca2+ mobilization, whereas the Fyn–adaptor Gab2–phosphatidylinositol-3-OH kinase–protein kinase C axis controls degranulation. Notably, Syk-deficient mast cells show defects in Ca2+ mobilization as well as degranulation, which indicates that the two pathways are not entirely independent of each other50. One distinct point of convergence between the two pathways seems to be at the level of protein kinase C activation and the increase in the cytosolic Ca2+ concentration, both of which probably act in concert to activate degranulation. The importance of the increase in cytosolic Ca2+ concentrations to mast cell degranulation is well documented. However, a specific requirement for CRAC channel–mediated Ca2+ influx has been evaluated only recently with mast cells derived from CRACM1- and STIM1-knockout mice16,51.
Mast cells lacking either STIM1 or CRACM1 show a considerable defect in degranulation. FcεRI-induced in vivo anaphylaxis is also strongly inhibited in CRACM1-knockout mice and is much lower in Stim1+/− mice16,51. As the release of Ca2+ from the endoplasmic reticulum is similar in wild-type and CRACM1-knockout mast cells, CRAC-mediated Ca2+ influx is crucial for mast cell degranulation. Further support for the idea that CRAC channels are involved in mast cell degranulation and anaphylaxis comes from Trpm4−/− mice. TRPM4 (transient receptor potential, melastatin, member 4) is a Ca2+-activated nonselective cation channel that decreases the driving force for Ca2+ entry through CRAC channels by modulating the membrane potential52,53. Trpm4−/− bone marrow–derived mast cells show more SOCE than their Trpm4+/+ counterparts do in response to FcεRI stimulation. Furthermore, bone marrow–derived mast cells from Trpm4−/− mice show augmented degranulation and a more severe immunoglobulin E–mediated acute passive cutaneous anaphylactic response54. Degranulation involves the translocation, docking and fusion of granules with the plasma membrane. Therefore, it is possible that CRAC channel–mediated sub-plasmalemmal increases in the cytosolic Ca2+ concentration activate or amplify certain ‘downstream’ signaling pathways involved in granule secretion55 or simply promote granule exocytosis by binding to Ca2+-sensing proteins, such as synaptotagmins, which are part of the SNARE complex involved in membrane fusion56. Unexpectedly, leukotriene secretion is also strongly inhibited in CRACM1-knockout mice, which suggests the involvement of CRAC channels in the secretion of proinflammatory lipid mediators51. Increases in cytosolic Ca2+ concentrations activate NFAT and NF-κB, which regulate the transcription of genes encoding cytokines. It is therefore not unexpected that overall cytokine secretion is lower in CRACM1-knockout and Stim1−/− mast cells and augmented in Trpm4−/− mast cells. However, careful analysis shows that individual cytokines are affected differently. For example, secretion of tumor necrosis factor is much lower in Stim1−/− and CRACM1-knockout mast cells and higher in Trpm4−/− mast cells. However, secretion of interleukin 6 is much lower, less affected or unaffected in Stim1−/−, CRACM1-knockout or Trpm4−/− mast cells, respectively16,51,54. Secretion of interleukin 6 in mast cells depends on nuclear translocation of NF-κB57, which in turn depends on a large transient increase in the cytosolic Ca2+ concentration. NFAT translocation, however, requires a more sustained increase in Ca2+. It is likely that the smaller basal endoplasmic reticulum Ca2+ pools in Stim1−/− mast cells fail to provide the required large transient increase in cytosolic Ca2+ concentrations and thus result in less secretion of interleukin 6 (ref. 16). In agreement with that hypothesis, CRACM1-deficient and Trpm4−/− mast cells show no substantial change in resting endoplasmic reticulum Ca2+ pools16,51,54. Therefore, it is possible that other CRACM homologs or store-operated channels are involved in maintaining the basal cytosolic and resting endoplasmic reticulum Ca2+ concentrations in mast cells. Such a scenario would also be consistent with the general idea that STIM1 acts as a global store sensor and activator of all store-operated channels.
Notably, CRACM1-knockout and Stim1−/− mast cells show no defects in proliferation or differentiation in vitro, and tissue mast cell numbers in CRACM1-knockout and Stim1−/− mice are similar to those in wild-type mice. These data suggest that a non-store-operated mode of Ca2+ entry may be crucial for the differentiation and proliferation of mast cells, but SOCE is not.
Calcium signaling in thymocytes
Calcium signaling has long been proposed to be crucial for thymocyte maturation. Early studies using cyclosporine A (a calcineurin inhibitor) showed impaired development of CD4+CD8+ double-positive thymocytes into CD4+CD8− or CD4−CD8+ single-positive thymocytes, as well as defects in thymocyte negative selection58,59. In addition, targeted disruption of the catalytic subunit (calcineurin Aβ)60 as well as the regulatory subunit (calcineurin B1)61 of calcineurin results in a distinct defect in positive selection, with fewer single-positive cells, although negative selection seems normal61. Similarly, disruption of NFATc3, which is ‘preferentially’ expressed in double-positive thymocytes, results in fewer single-positive thymocytes and is associated with defects in expression of the antiapoptosis protein Bcl-2 by double-positive cells62. Notably, CRACM1-deficient and Stim1−/− Stim2−/− mice show no defect in the thymic development of conventional T cells29,51,63. These observations are unexpected, given the reports described above linking calcium-calcineurin-NFAT signaling to T cell development.
As CRACM1 has two closely related homologs (CRACM2 and CRACM3), one obvious issue that arises from the studies discussed above is whether CRACM2 and/or CRACM3 form functional channels to recruit Ca2+ in thymocytes. Both CRACM2 and CRACM3 have been shown to be able to conduct CRAC currents in in vitro overexpression systems36,64. Indeed, CRACM2 mRNA is much more abundant than CRACM1 mRNA in wild-type mouse thymocytes51. Moreover, the lymphoid regions of thymus and spleen in a ‘gene-trap’ CRACM1-deficient mouse are devoid of CRACM1 protein expression, as indicated by β-galactosidase reporter activity51, whereas another report has detected faint expression of CRACM1 protein in wild-type thymus and lymph nodes63. Thus, CRACM2 may be the dominant homolog in mouse thymocytes, different CRACM homologs may be expressed and function during different stages of T cell development and maturation, and/or thymocytes may use a non-store-operated method of sustaining Ca2+ signaling during positive selection. Further studies with CRACM2- and CRACM3-deficient mouse models should be able to test these various possibilities.
Notably, deletion of TRPM7 results in a block in thymocyte development at the CD4−CD8− double-negative stage65. TRPM7 is a non-store-operated cation channel that conducts Ca2+ and Mg2+ ions and has an intrinsic kinase activity. Although the exact mechanism of this defect remains to be demonstrated, the Mg2+ homeostasis of TRPM7-deficient thymocytes seems to be normal65.
CRAC channels in naive and differentiated T cells
Since the discovery of the CRAC current, crucial functions for SOCE in all aspects of lymphocyte activation, including proliferation and cytokine secretion, have been proposed66,67. Moreover, analysis of peripheral blood lymphocytes68 and T cell lines69 derived from patients with severe combined immunodeficiency (SCID) who have a loss-of-function mutation in the gene encoding CRACM1 has identified defective T cell proliferation and cytokine secretion. Two independent analyses of CRACM1-deficient mice have provided several new insights into the function of CRAC channels in T cell activation. One group has used gene-trap technology to ‘knock down’ CRACM1 expression51, whereas the other group has generated CRACM1-deficient mice by targeted gene ablation63. Unexpectedly, naive T cell populations analyzed ex vivo show either no defect51 or an insubstantial decrease63 in SOC influx in the presence of physiological concentrations of extracellular Ca2+. Furthermore, proliferation is unaffected, although cytokine secretion by naive T lymphocytes is impaired in CRACM1-deficient mice51,63. In addition, thymocyte development is normal in CRACM1-deficient mice29,51,63. Therefore, the phenotype of thymocytes and naive T cells from CRACM1-deficient mice contrasts considerably with the phenotype of T cells from patients with SCID who have mutations in the gene encoding CRACM1 (refs. 68,69). These differences may be due to species-specific dependency on CRACM1 and/or CRACM2 and CRACM3. The other important difference is the activation status of the cells when naive T cells from CRACM1-deficient mice are compared with immortal T cell lines developed in vitro from human patients with SCID. Indeed, analysis of in vitro–differentiated helper T cell populations has reconciled some (but not all) of these phenotypic differences5,63,68,69. Differentiated helper T cells from CRACM1-deficient mice have much less SOC influx as well as cytokine release, although T cell proliferation remains unaffected, in contrast to the earlier reported proliferation defects in T cells from patients with SCID 63,68,69.
It is unlikely that both of the strains of CRACM1-deficient mice described above are hypomorphic; thus, the other logical possibilities are discussed here. The higher expression of CRACM2 than of CRACM1 and CRACM3 mRNA in mouse thymocytes and naive T cells suggests that these populations may use CRACM2 as the dominant CRACM homolog to form the Ca2+-recruiting SOC channel51. In agreement with that hypothesis, relatively less CRACM2 mRNA is detected in the in vitro–differentiated helper T cell subsets63. Another possibility is that, as reported for TRPC channels, CRACM homologs may form distinct heteromultimeric CRAC channels in vivo with variable cell- or tissue-specific composition. Thus, the expression of CRACM homologs and/or the composition of CRAC channels in T cells may vary with the differentiation status of the cell. Although CRACM2 has been reported to result in store-operated currents when overexpressed with STIM1 (refs. 36,64), it is important to note that the biophysical and pharmacological properties of the resulting currents are distinct from those of native CRAC channels. CRACM homologs also presumably have different activation thresholds and Ca2+-dependent inactivation properties36,64. As these differences may not be apparent in assays using fluorescence-based Ca2+ indicators (such as Fura-2), direct recording from developing and naive T cells, although challenging, is crucial and may provide insights into the exact route of Ca2+ influx in these T cell populations.
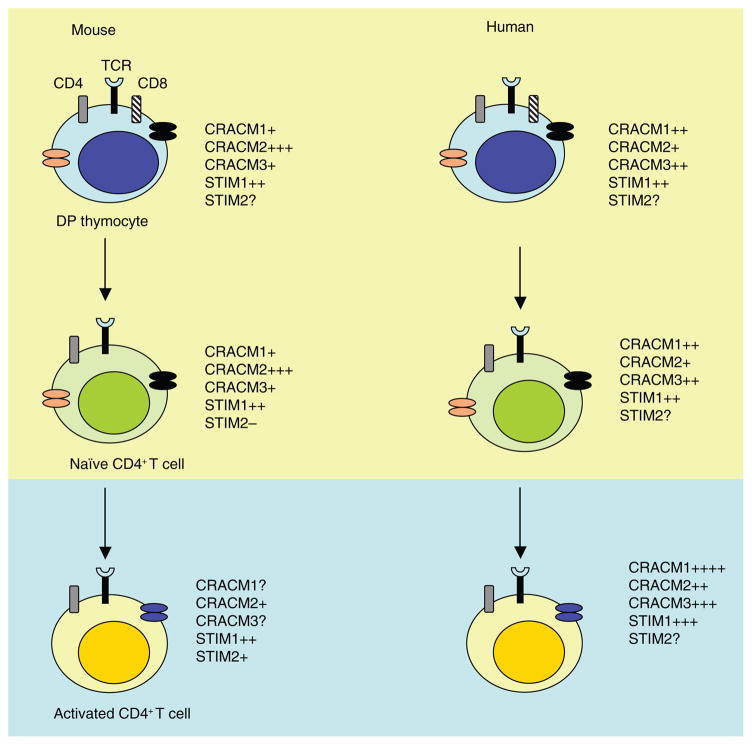
Another independent observation that supports the hypothesis outlined above is that STIM2 expression, although negligible in naive T cells, is higher in helper T cells differentiated in vitro29. Moreover, the hypothesis that STIM2 translocates to endoplasmic reticulum–plasma membrane junctions to activate CRAC channels after sensing even a slight depletion in endoplasmic reticulum Ca2+ stores28 fits well with the finding that antigen-experienced, differentiated or memory T cell populations require a relatively suboptimal stimulus to trigger cytokine release. We have provided a summary of the proposed expression pattern for STIM and CRACM homologs on the basis of published and unpublished data (Fig. 2). Notably, Stim1−/− Stim2−/− mice show no obvious defect in the thymic differentiation of conventional T cells and only a partial defect in the proliferation of naive T cells29. Given that STIM proteins are considered global activators of SOCE, these data also suggest that a non-store-operated method of Ca2+ influx may contribute to the regulation of Ca2+ signaling in naive T cells. However, Stim1−/− Stim2−/− mice do have a defect in regulatory T cell numbers and develop lymphoproliferative disorders29. Such observations are consistent with the lymphocyte hyperproliferation reported in mice lacking both NFATc1 and NFATc2 (ref. 70).
Figure 2.
Hypothetical model of the various modes of Ca2+ influx in developing, mature and activated T lymphocytes. Top (yellow shaded region), unknown chief source of Ca2+ influx; bottom (blue shaded region), SOCE is the main mode of Ca2+ influx; +, ++ and +++, reported changes (increases) in the expression of CRACM and STIM mRNA; ?, untested and unknown; −, undetectable; orange ovals, store-independent channels; black ovals, store-dependent channels. TCR, T cell antigen receptor; DP, double-positive.
Non-store-operated Ca2+ signaling in T cells
T lymphocytes also express voltage-gated Ca2+ channels (Cav channels), but whether these channels are functional in lymphocytes remains debatable71,72. Cav channels are expressed and functional mainly in excitable cells such as neurons and muscle cells, where they are activated in response to membrane depolarization. CD4+ T cells lacking the Cav regulatory β4 or β3 subunits show impaired Ca2+ responses in response to stimulation of the T cell antigen receptor72. These Ca2+ responses seemed to be independent of the release of Ca2+ from endoplasmic reticulum stores, as no defect is found in response to thapsigargin, which mediates passive release of Ca2+ from the endoplasmic reticulum and activates CRAC channels. Nuclear translocation of both NFAT subtypes, NFATc2 and NFATc1, is inhibited in both β-subunit-deficient T cell strains, which shows that the entire calcium-calcineurin-NFAT pathway is affected. Although the proliferation of β4-mutant CD4 T lymphocytes is intact, cytokine secretion from these cells is lower. These data collectively suggest that Cav channels are necessary for a normal T cell antigen receptor–mediated Ca2+ response in CD4+ T cells.
However, membrane depolarization does not result in the activation of Cav channels in T lymphocytes, which suggests the existence of an alternative gating mechanism for these channels72. An independent study has shown that a carboxy-terminal fragment of Cav1.2 (also called the ‘L-type voltage-gated calcium channel’) can translocate to the nucleus and regulate transcription73. This calcium channel–associated transcription regulator can bind to a nuclear protein and an endogenous promoter and to regulate the expression of a wide variety of genes essential for signaling and excitability in neurons. The nuclear localization of the calcium channel–associated transcription regulator is regulated by changes in intracellular Ca2+ concentrations, among other parameters. In summary, the operation and functional importance of store-independent modes of Ca2+ entry in lymphocytes remains to be determined.
Conclusions
Molecular and genetic approaches have demonstrated that components of the SOCE signaling pathway are crucial for the function of lymphocytes. However, several alternative routes of Ca2+ influx exist and may be implemented at various stages of the development and maturation of lymphocytes. Future studies of how individual lymphocyte types use different sources of Ca2+ influx during their lifespan should provide useful insights.
Acknowledgments
We thank P. Rao and D. Okuhara for discussions and J.W. Putney for critical reading of this manuscript, and we apologize to those colleagues whose work we could not cite because of space limitations. Supported by the Cancer Research Institute–Irvington Institute Fellowship Program (M.V.) and the US National Institutes of Health (GM 053950 to J.-P.K.).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/natureimmunology/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Liou J, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang SL, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vig M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 6.Zhang SL, et al. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philipp S, et al. TRPC3 mediates T-cell receptor-dependent calcium entry in human T-lymphocytes. J Biol Chem. 2003;278:26629–26638. doi: 10.1074/jbc.M304044200. [DOI] [PubMed] [Google Scholar]
- 8.Venkatachalam K, Ma HT, Ford DL, Gill DL. Expression of functional receptor-coupled TRPC3 channels in DT40 triple receptor InsP3 knockout cells. J Biol Chem. 2001;276:33980–33985. doi: 10.1074/jbc.C100321200. [DOI] [PubMed] [Google Scholar]
- 9.Putney JW., Jr Capacitative calcium entry: sensing the calcium stores. J Cell Biol. 2005;169:381–382. doi: 10.1083/jcb.200503161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villereal ML. Mechanism and functional significance of TRPC channel multimerization. Semin Cell Dev Biol. 2006;17:618–629. doi: 10.1016/j.semcdb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie RC. TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol. 2007;578:9–24. doi: 10.1113/jphysiol.2006.118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao GK, Kaminski NE. Induction of intracellular calcium elevation by Delta9-tetrahydrocannabinol in T cells involves TRPC1 channels. J Leukoc Biol. 2006;79:202–213. doi: 10.1189/jlb.0505274. [DOI] [PubMed] [Google Scholar]
- 14.Lioudyno MI, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci USA. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 16.Baba Y, et al. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2008;9:81–88. doi: 10.1038/ni1546. [DOI] [PubMed] [Google Scholar]
- 17.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 18.Ho N, Gullberg M, Chatila T. Activation protein 1-dependent transcriptional activation of interleukin 2 gene by Ca2+/calmodulin kinase type IV/Gr. J Exp Med. 1996;184:101–112. doi: 10.1084/jem.184.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109:S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 20.Huang GN, et al. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science. 2008;319:476–481. doi: 10.1126/science.1151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 22.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135:110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baba Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soboloff J, et al. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ entry. Curr Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 28.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh-Hora M, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peinelt C, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercer JC, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soboloff J, et al. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 33.Vig M, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeromin AV, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 36.Lis A, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol. 2007;17:794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong HL, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mignen O, Thompson JL, Shuttleworth TJ. Orai1 subunit stoichiometry of the mammalian CRAC channel pore. J Physiol. 2008;586:419–425. doi: 10.1113/jphysiol.2007.147249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji W, et al. Functional stoichiometry of the unitary calcium-release-activated calcium channel. Proc Natl Acad Sci USA. 2008;105:13668–13673. doi: 10.1073/pnas.0806499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang GN, et al. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 42.Barr VA, et al. Dynamic movement of the calcium sensor STIM1 and the calcium channel Orai1 in activated T-cells: puncta and distal caps. Mol Biol Cell. 2008;19:2802–2817. doi: 10.1091/mbc.E08-02-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muik M, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–8022. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 44.Gwack Y, et al. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 45.Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 46.Smani T, et al. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, et al. Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J Biol Chem. 2007;282:29448–29456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 48.Penna A, et al. The CRAC channel consists of a tetramer formed by STIM-induced dimerization of Orai dimers. Nature. 2008;456:116–120. doi: 10.1038/nature07338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leslie M. Mast cells show their might. Science. 2007;317:614–616. doi: 10.1126/science.317.5838.614. [DOI] [PubMed] [Google Scholar]
- 50.Nadler MJ, Kinet JP. Uncovering new complexities in mast cell signaling. Nat Immunol. 2002;3:707–708. doi: 10.1038/ni0802-707. [DOI] [PubMed] [Google Scholar]
- 51.Vig M, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release–activated calcium channels. Nat Immunol. 2008;9:89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Launay P, et al. TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- 53.Launay P, et al. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- 54.Vennekens R, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- 55.Chang WC, et al. Local Ca2+ influx through Ca2+ release-activated Ca2+ (CRAC) channels stimulates production of an intracellular messenger and an intercellular pro-inflammatory signal. J Biol Chem. 2008;283:4622–4631. doi: 10.1074/jbc.M705002200. [DOI] [PubMed] [Google Scholar]
- 56.Rettig J, Neher E. Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- 57.Kalesnikoff J, et al. SHIP negatively regulates IgE + antigen-induced IL-6 production in mast cells by inhibiting NF-κB activity. J Immunol. 2002;168:4737–4746. doi: 10.4049/jimmunol.168.9.4737. [DOI] [PubMed] [Google Scholar]
- 58.Jenkins MK, Schwartz RH, Pardoll DM. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988;241:1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- 59.Gao EK, Lo D, Cheney R, Kanagawa O, Sprent J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature. 1988;336:176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- 60.Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin A β-deficient mice. Proc Natl Acad Sci USA. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 62.Oukka M, et al. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9:295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 63.Gwack Y, et al. Hair loss and defective T and B cell function in mice lacking ORAI1. Mol Cell Biol. 2008 doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeHaven WI, Smyth JT, Boyles RR, Putney JW., Jr Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem. 2007;282:17548–17556. doi: 10.1074/jbc.M611374200. [DOI] [PubMed] [Google Scholar]
- 65.Jin J, et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Partiseti M, et al. The calcium current activated by T cell receptor and store depletion in human lymphocytes is absent in a primary immunodeficiency. J Biol Chem. 1994;269:32327–32335. [PubMed] [Google Scholar]
- 69.Feske S, et al. Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur J Immunol. 1996;26:2119–2126. doi: 10.1002/eji.1830260924. [DOI] [PubMed] [Google Scholar]
- 70.Peng SL, Gerth AJ, Ranger AM, Glimcher LH. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity. 2001;14:13–20. doi: 10.1016/s1074-7613(01)00085-1. [DOI] [PubMed] [Google Scholar]
- 71.Kotturi MF, Hunt SV, Jefferies WA. Roles of CRAC and Cav-like channels in T cells: more than one gatekeeper? Trends Pharmacol Sci. 2006;27:360–367. doi: 10.1016/j.tips.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Badou A, et al. Critical role for the β regulatory subunits of Cav channels in T lymphocyte function. Proc Natl Acad Sci USA. 2006;103:15529–15534. doi: 10.1073/pnas.0607262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Cav1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gouy H, Cefai D, Christensen SB, Debre P, Bismuth G. Ca2+ influx in human T lymphocytes is induced independently of inositol phosphate production by mobilization of intracellular Ca2+ stores. A study with the Ca2+ endoplasmic reticulum-ATPase inhibitor thapsigargin. Eur J Immunol. 1990;20:2269–2275. doi: 10.1002/eji.1830201016. [DOI] [PubMed] [Google Scholar]
- 75.Telford WG, Miller RA. Detection of plasma membrane Ca2+-ATPase activity in mouse T lymphocytes by flow cytometry using fluo-3-loaded vesicles. Cytometry. 1996;24:243–250. doi: 10.1002/(SICI)1097-0320(19960701)24:3<243::AID-CYTO7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 76.Bautista DM, Hoth M, Lewis RS. Enhancement of calcium signalling dynamics and stability by delayed modulation of the plasma-membrane calcium-ATPase in human T cells. J Physiol (Lond) 2002;541:877–894. doi: 10.1113/jphysiol.2001.016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jayaraman T, Ondriasova E, Ondrias K, Harnick DJ, Marks AR. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proc Natl Acad Sci USA. 1995;92:6007–6011. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guse AH, et al. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- 79.Sei Y, Gallagher KL, Basile AS. Skeletal muscle type ryanodine receptor is involved in calcium signaling in human B lymphocytes. J Biol Chem. 1999;274:5995–6002. doi: 10.1074/jbc.274.9.5995. [DOI] [PubMed] [Google Scholar]
- 80.Guerini D, Coletto L, Carafoli E. Exporting calcium from cells. Cell Calcium. 2005;38:281–289. doi: 10.1016/j.ceca.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 81.Balasubramanyam M, Rohowsky-Kochan C, Reeves JP, Gardner JP. Na+/Ca2+ exchange-mediated calcium entry in human lymphocytes. J Clin Invest. 1994;94:2002–2008. doi: 10.1172/JCI117553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saris NE, Carafoli E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry (Mosc) 2005;70:187–194. doi: 10.1007/s10541-005-0100-9. [DOI] [PubMed] [Google Scholar]
- 83.Hoth M, Fanger CM, Lewis RS. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J Cell Biol. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beeton C, Chandy KG. Potassium channels, memory T cells, and multiple sclerosis. Neuroscientist. 2005;11:550–562. doi: 10.1177/1073858405278016. [DOI] [PubMed] [Google Scholar]
- 85.Cowen DS, et al. Extracellular adenosine triphosphate activates calcium mobilization in human phagocytic leukocytes and neutrophil/monocyte progenitor cells. J Clin Invest. 1989;83:1651–1660. doi: 10.1172/JCI114064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui J, Bian JS, Kagan A, McDonald TV. CaT1 contributes to the stores-operated calcium current in Jurkat T-lymphocytes. J Biol Chem. 2002;277:47175–47183. doi: 10.1074/jbc.M205870200. [DOI] [PubMed] [Google Scholar]
- 87.Perraud AL, et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. doi: 10.1038/35079100. [DOI] [PubMed] [Google Scholar]
- 88.Sano Y, et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. doi: 10.1126/science.1062473. [DOI] [PubMed] [Google Scholar]
- 89.Nadler MJ, et al. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 90.Prakriya M, Lewis RS. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J Gen Physiol. 2002;119:487–507. doi: 10.1085/jgp.20028551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shuttleworth TJ. What drives calcium entry during [Ca2+]i oscillations? –challenging the capacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- 92.Dellis O, et al. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]