Summary
Sec2p is the guanine nucleotide exchange factor (GEF) that activates the Rab GTPase Sec4p on secretory vesicles. Sec2p also binds a Rab acting earlier in the secretory pathway, Ypt32p-GTP, forming a RabGEF cascade. Ypt32p and the Sec4p effector Sec15p (a component of the exocyst complex) compete for binding to Sec2p. Indeed Ypt32p initially recruits Sec2p, but subsequently allows a handoff of active Sec2p/Sec4p to Sec15p. Intriguingly, Golgi-associated phosphatidylinositol 4-phosphate (PI4P) works together with Ypt32-GTP in this context. PI4P inhibits Sec2p-Sec15p interactions, promoting recruitment of Sec2p by Ypt32p as secretory vesicles form. However, PI4P levels appear to decline as vesicles reach secretory sites, allowing Sec15p to replace Ypt32p as vesicles mature. In this way, the regulation of PI4P levels may switch Sec2p/Sec4p function during vesicle maturation, from a RabGEF recruitment cascade involving Ypt32p, to an effector positive feedback loop involving Sec15p.
Introduction
Rab GTPases regulate membrane traffic by interacting with functionally diverse effector molecules that control distinct aspects of the vesicular transport reaction (Grosshans et al., 2006b). Sec4p, a rab protein associated with secretory vesicles, controls at least three different elements of the exocytic machinery in yeast. Sec4p may recruit the type V myosin, Myo2p, to secretory vesicles to promote their active transport along polarized actin cables (Govindan et al., 1995; Walch-Solimena et al., 1997). In addition, Sec4p directly binds to Sec15p, a component of the octameric exocyst complex implicated in vesicle tethering (Guo et al., 1999b). Sec4p also directly binds to Sro7p, a homolog of the lgl tumor suppressor that regulates fusion by binding to the tSNARE Sec9p (Grosshans et al., 2006a). Activation of Sec4p by its specific guanine nucleotide exchange factor (GEF), Sec2p, is necessary for these interactions (Walch-Solimena et al., 1997) (Elkind et al., 2000).
Sec2p, like its substrate Sec4p, is highly concentrated on the surface of secretory vesicles and this association is essential for the efficient activation of Sec4p (Elkind et al., 2000). We have proposed that Sec2p is recruited to membranes by binding to the rab protein, Ypt32p, in its GTP-bound conformation (Ortiz et al., 2002). Ypt32p is predominantly associated with the Golgi and regulates export from this compartment (Benli et al., 1996) (Jedd et al., 1997). The interaction of Sec2p with Ypt32p and Sec4p constitutes a rab GEF cascade in which one rab, in its GTP-bound conformation, recruits the GEF that activates the next rab along the secretory pathway. This mechanism effectively couples one stage of transport with the next and may, by orchestrating a time-dependant rab conversion, confer directionality to the pathway (Ortiz et al., 2002) (Grosshans et al., 2006b).
Sec2p also interacts with the Sec4p effector, Sec15p. By physically linking a Sec4p GEF to a Sec4p effector, a micro-domain of highly activated Sec4p and highly concentrated Sec15p could be maintained through a positive feedback loop. In fact, the interaction of Sec2p and Sec15p is normally restricted to the vesicular fraction, even though the major pools of both of these proteins are found in the cytosolic fraction (Medkova et al., 2006). The role of an effector-GEF complex in the formation of a Rab micro-domain was first established for Rabex 5 and Rabaptin on endosomes (Horiuchi et al., 1997) and may be a common feature of Rab function.
The region of Sec2p that interacts with Ypt32p lies between residues 160 and 258, just downstream of the exchange domain. The Ypt32p binding site overlaps with the Sec15p binding site and Ypt32p and Sec15p compete against each other for binding to Sec2p (Medkova et al., 2006). Interestingly, truncation or mutation of the region of Sec2p between residues 450 and 508 leads to dramatically enhanced binding to Sec15p and to an alternate conformation as revealed by partial proteolysis studies. In these mutant strains, the bulk of Sec2p is bound to the exocyst and the Sec2p-Sec15p interaction is no longer limited to the vesicular fraction, but is observed in the cytosolic pool as well. Because their interaction with Ypt32p is blocked, the mutant Sec2 proteins fail to associate with vesicles and the strains exhibit temperature sensitive growth and secretion. Overexpression of Ypt32p restores the growth of these sec2 mutants and restores the localization of the mutant Sec2 proteins by competing against the enhanced Sec15p binding (Ortiz et al., 2002) (Medkova et al., 2006).
We have proposed a working model (Medkova et al., 2006) in which Sec2p is initially recruited to membranes in one conformation by binding to Ypt32-GTP. Sec2p then adopts a different conformation that allows Sec15p to replace Ypt32p. This GEF-effector complex persists on the vesicle surface to promote transport and tethering. After tethering, Sec2p returns to its original conformation that favors displacement from Sec15p, thereby allowing Sec2p to recycle through the cytoplasm for another round of vesicle transport. The nature of the signal that triggers the changes in Sec2p conformation is not known, nor has it been established that Ypt32p binding is necessary and sufficient for recruitment of Sec2p.
Here we demonstrate that Sec2p binds phosphatidylinositol 4-phosphate (PI4P) and that the production of PI4P within the Golgi by the PI4 kinase Pik1p is necessary, in combination with Ypt32p, for Sec2p localization. Moreover, we find that the interaction of PI4P with Sec2p selectively inhibits Sec15p binding and by doing so could facilitate the recruitment of Sec2p by Ypt32p.
Results
Sec2p directly binds to PI4P
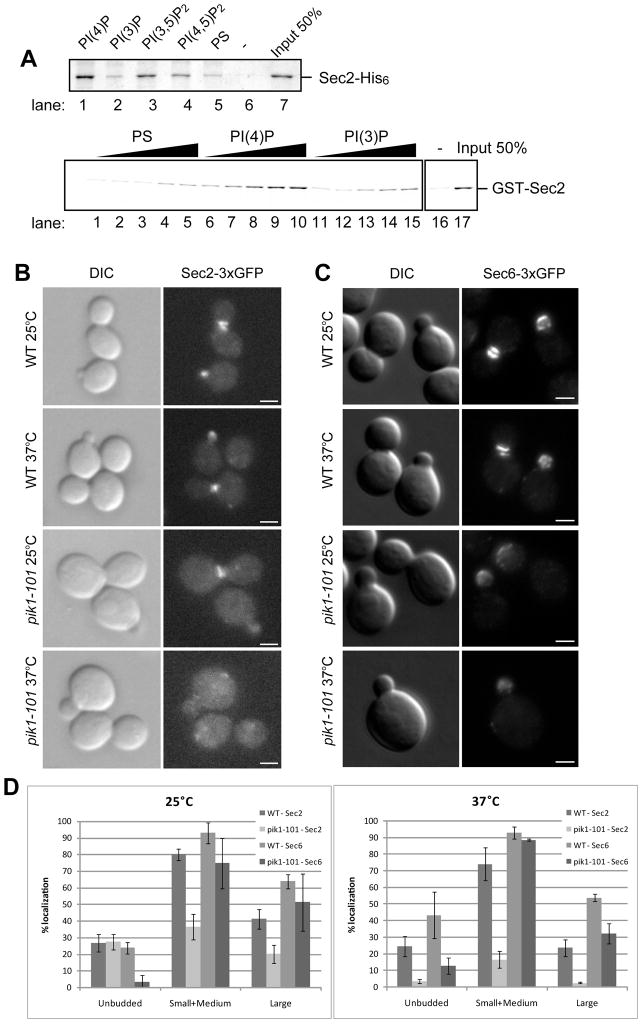
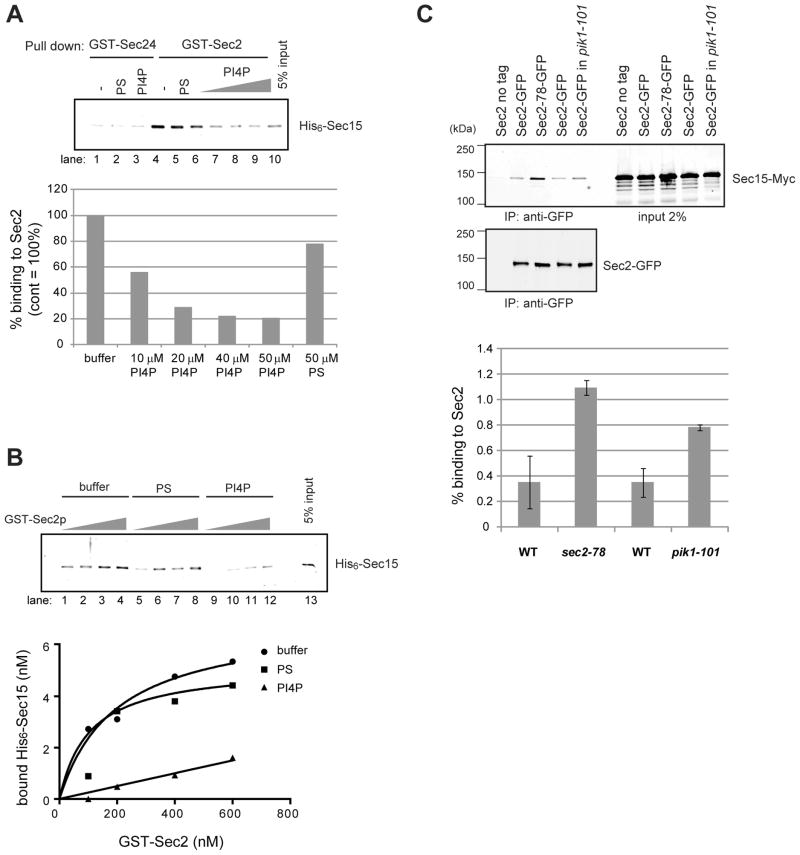
To explore a possible role of phosphoinositides in Sec2p function, we incubated Sec2p with liposomes containing various phosphoinositides. Liposome-bound Sec2p was precipitated by centrifugation and detected by Coomassie blue staining. Yeast contains four major phosphoinositides marking different compartments: endosomal PI3P, Golgi-associated PI4P, plasma membrane PI(4,5)P2 and vacuolar PI(3,5)P2 (Strahl and Thorner, 2007). Sec2p showed the highest binding to liposomes containing PI4P (Figure 1A, top panel, lane 1), yet also showed some affinity to PI(3,5)P2 and PI(4,5)P2 (lane 3 and 4). The binding to PI3P was at the same level as that to control liposomes containing phosphoserine (PS) (compare lane 2 and 5). Sec2 did not precipitate in the absence of liposomes (lane 6). The approximate Kd of Sec2p for PI4P was determined to be 185 μM by incubating equal amounts of GST-Sec2p with various concentrations of liposomes containing PS, PI3P or PI4P (Figure 1A, bottom panel).
Figure 1.
(A) Sec2p directly binds to Phosphoinsitides. (top panel) Sec2p-His6 (120 nM) purified from E. Coli was incubated by itself (lane 6) or with 0.5 mM liposomes containing 50/30/15 mol% of PC/PE/PS and 5 mol% of the indicated phosphoinositides (lanes 1–4), or 45/30/25 mol% of PC/PE/PS (lane 5). Liposomes were precipitated by centrifugation at 100,000 × g and bound proteins were detected by Coomassie blue staining. (bottom panel) GST-Sec2p purified from E. Coli was incubated with 0.05, 0.1, 0.25, 0.375, and 0.5 mM of liposomes containing 10 mol% of PS, PI4P, or PI3P. Liposomes were precipitated by centrifugation at 100,000 x g and bound proteins were detected with anti-Sec2p antibody. (B) Sec2p is mislocalized in pik1-101 cells. Localization of Sec2-3xGFP (B) and Sec6-3xGFP (C) was examined in wildtype (WT) and pik1-101 cells. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose and then shifted to 37°C for 60 minutes. Cells were immediately fixed and examined as described in Experimental procedures. Bars are 2 μm. (D) Cells were classified into three categories (unbudded, small/medium buds, or large buds). A total of approximately 200 cells were counted for each experiment. Values indicate the percentage of cells showing bud or mother-daughter neck localization of Sec2-3xGFP or Sec6-3xGFP. Mean and S.D. of three different experiments are shown.
Localization of Sec2p requires Pik1 activity
Pik1p is an essential phosphatidylinositol 4-kinase that acts in both the nucleus and the Golgi, and is required for post-Golgi transport (Hama et al., 1999) (Audhya et al., 2000; Walch-Solimena and Novick, 1999). The pik1-101 mutation leads to inactivation of the kinase activity at 37°C, however, slow growth, a reduction of cellular PI4P levels and a partial secretion defect are observed in pik1-101 cells even at 25°C (Walch-Solimena and Novick, 1999). We analyzed the distribution of Sec2-3xGFP in wild type and pik1-101 yeast cells. The 3xGFP coding sequence was fused to the C-terminus of the SEC2 coding sequence and expressed behind the SEC2 promoter as the sole copy of SEC2 in yeast. Cells were grown at 25°C, shifted to 37°C for 60 min, then fixed with methanol and observed by epi-fluorescence microscopy. In wild type cells, Sec2-3xGFP localized to the tips of small and medium size buds, and to the mother-daughter neck of larger cells at all temperatures (Figure 1B, top four panels). This pattern reflects the association of Sec2p with secretory vesicles concentrated at sites of secretion (Elkind et al., 2000). Localization of Sec2-3xGFP to sites of secretion was observed in some pik1-101 cells, at 25°C (Figure 1B, third panels from the top), however, the percentage of the cells exhibiting this localization was only half that of wild type cells (Figure 1D, left panel). Following a shift to 37°C, Sec2-3xGFP was largely mislocalized to the cytoplasm (Figure 1B, bottom panels). A small population of Sec2 was mislocalized to puncta that were not positive for a late-Golgi marker Sec7p (not shown). The percentage of cells exhibiting normal localization was very low relative to wild type (Figure 1D, right panel).
To determine if the effect of pik1-101 on Sec2p localization is indicative of a failure in the vesicle association of Sec2p or a failure of vesicles to concentrate at exocytic sites, we examined the localization of Sec6-3xGFP. Sec6p is a component of the Exocyst complex that tethers secretory vesicles to the plasma membrane (TerBush and Novick, 1995). Most exocyst components, including Sec6p, are associated with secretory vesicles as they move towards the plasma membrane along polarized actin cables (Boyd et al., 2004). Wild type and pik1-101 cells expressing Sec6-3xGFP as the sole copy were grown at 25°C, shifted to 37°C for 60 min, then fixed with methanol and analyzed by fluorescence microscopy. Sec6-3xGFP localized to the bud tip or mother-daughter neck in wild type cells (Figure 1C, top four panels). Although the expression level of Sec6-3xGFP in pik1-101 was about 64% of wild type, leading to a somewhat weaker fluorescence signal, Sec6-3xGFP localized normally both at 25°C and 37°C (Figure 1C, bottom four panels). The percentage of cells exhibiting normal Sec6-3xGFP localization was reduced to some extent, perhaps reflecting reduced vesicle production, but less severely then Sec2-3xGFP (Figure 1D). Thus, the localization of Sec2p is more sensitive to the loss of Pik1p function than that of Sec6p, suggesting that the association of Sec2p with secretory vesicles is dependent upon the pool of PI4P generated by Pik1p.
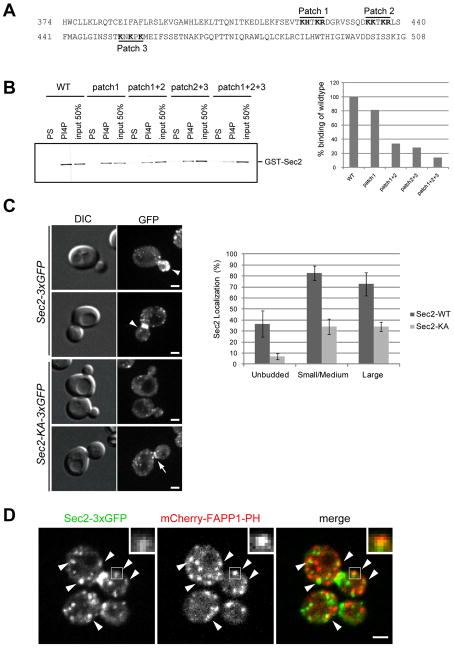
Sec2p localization depends on PI4P-binding affinity via its positively charged patches
To determine if PI4P-binding is required for Sec2p localization, we first mapped the PI4P-binding region in Sec2p. Since Sec2p has no conserved lipid-binding motif, various truncation constructs were tested in vitro for binding to PI4P liposomes. Sec2p-truncation mutants consisting of a.a. 1–160, 1–258, 1–374, and 509–759 (C-terminus) showed no specific binding to PI4P, however, a.a. 1–450, 1–508, 1–623, and 450–759 bound to PI4P (not shown), suggesting that the phosphoinositide binding region lies between a.a 374 and 508. Within this region there are three positively charged patches (Figure 2A). Mutation of positively charged amino acids to alanine in patch 1, 2, or 3 individually had only a slight effect on PI4P-binding (Figure 2B). Combinations of mutations in patch 1 and 2, or patch 2 and 3, reduced PI4P-binding affinity to about 30% of the wildtype (Figure 2B). When all three patches were mutated, PI4P-binding was reduced to about 10% of wildtype (Figure 2B), indicating that all three patches are involved in PI4P binding. Hereafter we refer to the mutant that has mutations in all three patches as the Sec2-KA mutant.
Figure 2. Sec2p-PI4P interaction is required for Sec2p localization.
(A) The Sec2 sequence between a.a. 374 and 508 is necessary for PI4P binding. Positively charged patches are shown in bold. (B) GST-tagged Sec2 wild-type (WT), or Sec2 with mutations in positively charged patches 1, 2, or 3, were purified from E. Coli. Proteins were incubated with liposomes containing 10 mol% PS or PI4P. Liposomes were precipitated by centrifugation at 100,000 x g and bound proteins were detected with anti-Sec2 antibody. The intensity of the bands was quantified using Image J. (C) Localization of Sec2-3xGFP or Sec2-KA-3xGFP was examined. Cells were grown overnight at 25°C in a synthetic medium containing 2% glucose. Bars are 2 μm. Arrowheads indicate normal localization of wildtype Sec2. Arrow in bottom panel shows normal localization of Sec2-KA, but with reduced intensity. A total of approximately 80 cells were counted for each experiment. Values indicate the percentage of cells showing bud or mother-daughter neck localization of Sec2-3xGFP. Mean and S.D. of three different experiments are shown. (D) The localization of Sec2-3xGFP (left), mCherry-FAPP1-PH (middle) and the merge (right) was examined in wildtype cells. Cells were grown at 25°C in a synthetic medium containing 2% glucose and then live cells were analyzed with a spinning disk confocal microscope. Insets and arrowheads show colocalization between Sec2-3xGFP and mCherry-FAPP1-PH. Bar is 2 μm.
To test the localization of Sec2-KA, the mutations were introduced into the SEC2 locus and the 3xGFP sequence was fused to the C-terminus. The localization of Sec2-KA-3xGFP was significantly affected (Figure 2C, left panels). Localization to the bud tip or bud neck was not seen in most cells, however, ~30% of the cells exhibited normal localization, but with reduced intensity (Figure 2C, left bottom, arrow). This result indicates that the PI4P-interaction is important for Sec2p localization.
Sec2p transiently associates with PI4P-enriched membranes
To determine if Sec2p localizes to sites of PI4P concentration, we generated a strain expressing both Sec2-3xGFP and mCherry-tagged to the Pleckstrin homology (PH) domain of the FAPP1 protein. This domain shows a high specificity for PI4P in vitro (Dowler et al., 2000) and several groups have shown that the FAPP1-PH domain recognizes PI4P in yeast, even though FAPP1 is a mammalian protein (Stefan et al., 2002) (Roy and Levine, 2004) (Faulhammer et al., 2005). The mCherry sequence was attached to the N-terminus of the FAPP1-PH domain and this fusion protein was expressed behind the ADH1 promoter. The mCherry-FAPP1-PH signal was lost when cells were fixed in methanol, so we observed live cells with a spinning disk confocal microscope. FAPP1-PH was distributed throughout the cells, yet showed accumulation on punctate structures (Figure 2D, middle) that have been shown to be elements of the Golgi apparatus (Levine and Munro, 2002, Stefan et al., 2002, Faulhammer et al., 2005, (Tahirovic et al., 2005). The most prominent concentration of Sec2-3xGFP localized to the bud tip and mother-daughter neck and did not colocalize with FAPP1-PH (Figure 2D, left panel). Nevertheless, a portion of Sec2-3xGFP localized to small puncta that might represent secretory vesicles or elements of the Golgi. About 43% of the Sec2-3xGFP puncta were also positive for FAPP1-PH and similarly about 38% of the FAPP1-PH puncta were also positive for Sec2-3xGFP (Figure 2D, arrowheads, inset). This result suggests that Sec2p transiently associates with sites of high PI4P concentration on the Golgi, yet PI4P levels are reduced once secretory vesicles arrive at sites of secretion.
The growth defect of pik1-101 is partially suppressed by Sec2 overexpression
The growth defect of pik1-101 might be due in part to Sec2p mislocalization, which would in turn lead to a failure in Sec4p activation. In this case, restoring Sec4p activity by either overexpressing its activator, Sec2p, or by directly overexpressing Sec4p, might be expected to at least partially rescue the growth defect of pik1-101 cells. In addition, our prior studies demonstrated that overexpression of Ypt32p could restore the localization of two Sec2p mutants (Ortiz et al., 2002). Therefore, Sec2p localization might be restored in pik1-101 by overexpressing Ypt32p, which would rescue the growth defect of pik1-101 cells. To test these possibilities, Sec2p, Sec4p, and Ypt32p, were overexpressed from high copy number plasmids in a pik1-101 strain. As shown in Figure 3A, wild type cells grew at all temperatures, whereas pik1-101 could not grow well above 30°C. When Sec2p, Sec4p, or Ypt32p were overexpressed, pik1-101 cells were able to grow well at 30°C (Figure 3A, middle panel), and grow slowly at 35°C (Figure 3A, right panel). At 37°C, no growth was evident, indicating that the rescue of pik1-101was only partial (not shown). In contrast, overexpression of Ypt1p, the rab GTPase that acts early in the secretory pathway, did not rescue the pik1-101 growth defect at any temperature tested. The partial nature of the rescue of pik1-101 by overexpression of Ypt32p, Sec2p or Sec4p indicates that PI4P must have another essential function(s) in addition to the recruitment of Sec2p. In fact, Pik1p has recently been implicated in the recruitment of a lipid flippase and a PI4P-binding protein required for budding of vesicles from the Golgi (Dippold et al., 2009; Natarajan et al., 2009).
Figure 3. Overexpression of Sec2p, Ypt32p and Sec4p partially suppresses the growth defect of pik1-101.
(A) Wild-type (WT), pik1-101, and pik1-101 cells overexpressing Sec2p, Ypt1p, Ypt32p, or Sec4p from a high-copy number, 2μ-based plasmid were spotted onto YPD plates in 5-fold serial dilutions. Cells were grown at the indicated temperatures for two days. Two independent transformants were analyzed for each plasmid. (B) Localization of Sec2-3xGFP was examined in wildtype (WT, top panels), WT harboring the Ypt32 overexpressing vector (second panels from top), pik1-101 (third panels from top), or pik1-101 harboring the Ypt32 overexpressing vector (bottom panels). Cells were grown at 25°C overnight in a synthetic medium containing 2% glucose and then shifted to 37°C for 60 minutes. Cells were immediately fixed and examined. Images shown are cells after the 37°C shift. Bars are 2μm. (C) Cells were classified into three categories (unbudded, small/medium buds, or large buds). A total of approximately 100 cells were counted for each experiment. Values indicate the percentage of cells showing bud or mother-daughter neck localization of Sec2-3xGFP. (D) Tetrads from a diploid carrying the sec2-KA, ypt31Δ and ypt32-A141D alleles. ypt31Δ ypt32-A141D spores are shown within circles. ypt31Δ ypt32-A141D sec2-KA triple mutants are shown within squares.
To determine if Ypt32p overexpression restores Sec2p localization in pik1-101 cells, we first confirmed that Ypt32 overexpressed from a high copy number plasmid rescued the growth defect of pik1-101 cells expressing Sec2-3xGFP at 30°C (Supplemental Figure S2). Next, we observed the localization of Sec2-3xGFP in cells overexpressing Ypt32p. In wildtype cells, overexpression of Ypt32p did not affect the localization of Sec2-3xGFP at either permissive (not shown) or restrictive temperature (Figure 3B, top panels). In pik1-101 cells Sec2-3xGFP was mislocalized at the restrictive temperature (Figure 3B, third panel from the top), however, when Ypt32p was overexpressed, Sec2p-3xGFP localization was largely restored (Figure 3B, bottom panel; Figure 3C), particularly in budded cells.
Both PI4P-binding and Ypt31/32p are required for growth
The restoration of Sec2p localization in pik1-101 cells by Ypt32p overexpression, suggests that Ypt32p and PI4P might act in parallel to control Sec2p localization. PI4P-binding deficient sec2 mutant cells (sec2-KA) did not show a growth defect at any temperature tested (not shown), thus we determined if there were synthetic effects when Ypt32 and PI4P-binding deficient mutants were combined. Since Ypt31 and Ypt32 are essential for growth, yet functionally redundant (Benli et al., 1996), we used a combination of mutations, ypt31Δ ypt32A141D, to disrupt their function. The ypt31Δypt32A141D strain exhibits temperature sensitive blocks in growth and secretion (Jedd et al., 1997). Diploids carrying the sec2-KA and ypt31Δypt32A141D alleles were sporulated and the resulting tetrads were dissected at 25°C. Spores carrying the ypt31Δ ypt32A141D alleles grew normally at 25°C (Figure 3D, circle), 30°C, and 32°C (not shown). In contrast, spores expressing sec2-KA and ypt31Δ ypt32A141D alleles grew extremely slowly at 25°C (Figure 3D, square), 30°C, and 32°C (not shown). Neither double (ypt31Δypt32A141D) nor triple mutants grew at 37°C (not shown). Localization of the Sec2-KA protein in the ypt31Δypt32A141D background was almost completely abolished (not shown). These results are consistent with a model in which PI4P and Ypt31/32p act in parallel to regulate Sec2p function.
Sec2p localization is unaffected in a PI4P phosphatase mutant
Sac1p is a phosphatidylinositol phosphate phosphatase that acts on phosphoinositides including PI4P (Guo et al., 1999a) (Hughes et al., 2000). Inactivation of Sac1p leads to a large increase in the PI4P concentration of the ER and the Golgi membrane (Foti et al., 2001). To test whether the localization of Sec2p is affected by a shift in the distribution of PI4P, we first examined PI4P localization using the GFP-tagged FAPP1-PH domain construct in live sac1-6 cells at 30°C. While GFP-FAPP1-PH was highly concentrated on puncta in wild type cells, in sac1-6 cells GFP-FAPP1-PH localized to ring shaped structures typical of the nuclear envelope and cortical ER (Supplemental Figure S1A). Puncta were observed in sac1-6 cells, however they were not enhanced, suggesting that PI4P levels were increased most prominently in the ER rather than the Golgi. We next examined the localization of Sec2-3xGFP and found that it localized normally in sac1-6 cells (Supplemental Figure S1B). Thus, although Sec2p binds PI4P in vitro and Sec2-3xGFP requires Pik1p function for localization in vivo, Sec2-3xGFP does not shift localization solely in response to a shift in the distribution of PI4P.
PI4P and PI(4,5)P2 at the plasma membrane are not required for Sec2p localization
In yeast, another essential PI4-kinase, Stt4p, generates an independent pool of PI4P at the plasma membrane (Audhya and Emr, 2002). Mss4p, a PI4P5-kinase, then utilizes the PI4P produced by Stt4p, to generate PI(4,5)P2 at the plasma membrane (Homma et al., 1998) (Desrivieres et al., 1998). Sec2-3xGFP localized normally in stt4-4 cells at both the permissive temperature 25°C (not shown), and following a shift to 37°C for 60 min (Supplemental Figure S1C, top panels). Sec2-3xGFP also localized normally in the mss4-102 mutant at both 25°C (not shown) and following a shift to 37°C for 60 min (Supplemental Figure S1C, bottom panels). These results indicate that Sec2p localization is independent of the PI4P and PI(4,5)P2 pools at the plasma membrane.
PI(3,5)P2 on the vacuolar membrane is not required for Sec2p localization
In vitro liposome-binding experiment showed that Sec2p has significant affinity to PI(3,5)P2 (Figure 1A). To test if Sec2p localization is regulated by PI(3,5)P2, we examined the localization of Sec2p-3xGFP in a fab1Δ, PI3-5 kinase deletion mutant and found that it was unaffected (Supplemental Figure S1D, bottom panels).
Two-hybrid screen for Sec2p mutants that no longer bind Ypt32p
Overexpression of Ypt32p restores the localization of Sec2p in pik1-101 cells (Figure 3B and C), and in two Sec2p mutants, sec2-59 and sec2-78 (Ortiz et al., 2002), suggesting that Ypt32p acts together with PI4P in Sec2p membrane recruitment. To critically evaluate this proposal, we sought sec2 mutations that lost their interaction with Ypt32p, but not with Sec15p or PI4P. We used the yeast two-hybrid system to screen for Sec2p mutants that had lost the ability to interact with Ypt32p. SEC2 and YPT32 were cloned into the GAL4 activation domain vector pACTII and GAL4 DNA-binding domain vector pGBKT7, respectively. PCR random mutagenesis was performed on the 5′ end of SEC2 (codons 1–311). The mutagenized sec2 sequences were cloned back into pACTII and co-transformed with YPT32 into yeast. Co-transformants were screened for loss of interaction as determined by their inability to grow on either SC-his plates, or SC-his with 5 mM 3-amino-1,2,4-triazole (3-AT), a competitive inhibitor of His3p function. A total of six sec2 mutants were identified that showed at least some reduction in His3p function relative to wild-type. Three were shown to be -His and three were +His, yet unable to grow in the presence of 3-AT (Supplemental Figure S3A). All clones identified as having lost interaction with Ypt32p were further screened to confirm that Sec2p was still full length by immunoblot analysis using an anti-HA antibody that recognizes the N-terminal HA epitope on Sec2p (not shown).
The Ypt32p-binding domain of each sec2 mutant was sequenced and aligned with the wild-type sequence (Supplemental Figure S3B). The level of mutagenesis ranged from one amino acid change (M13 and M14) to five (M7). The sec2 mutant M19 has two changes and both M5 and M37 have three. Although the level of mutagenesis and screening did not saturate the Ypt32p-binding domain, the amino acid changes are clustered in two general areas.
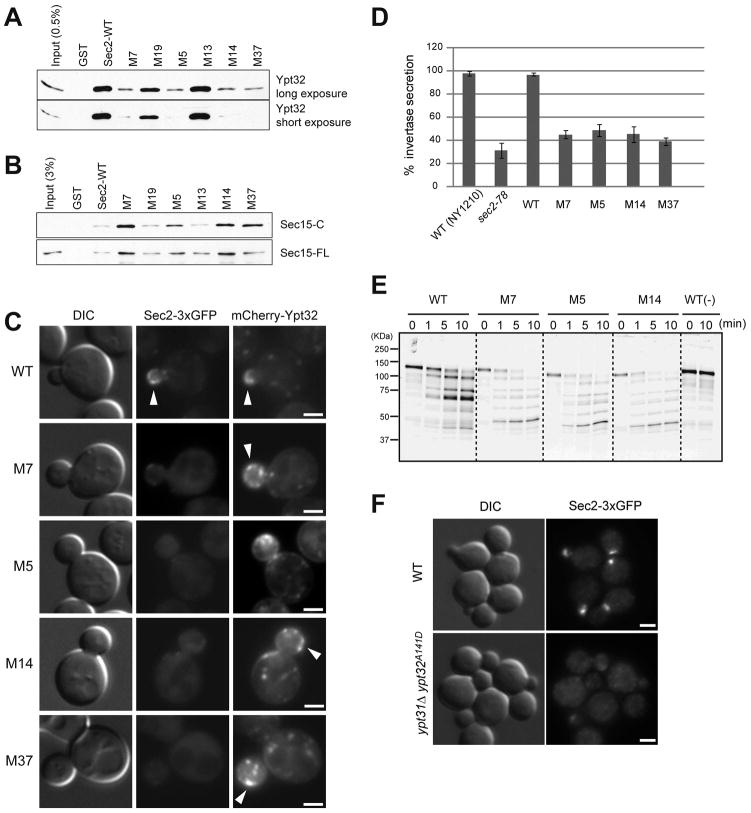
Sec2p mutants have decreased affinity for Ypt32p-GTP in vitro
The region encoding the Ypt32p-binding domain of each sec2 mutant (codons 157–339) was sub-cloned into a GST-Sec2p expression construct. The GST-Sec2 mutant proteins were purified from E. coli on glutathione beads and used in binding assays with purified, recombinant His6-Ypt32p pre-loaded with GTPγS. The amount of Ypt32p bound was evaluated by immunoblot using an anti-Ypt32p antibody. Wild-type Sec2p bound approximately 10% of the available Ypt32-GTP under these conditions (Figure 4A). Greatly reduced Ypt32-GTP binding was seen with four of the six mutants identified in the two-hybrid screen (M7, M5, M14, and M37). A longer exposure of the Western blot shows that these mutants still have some affinity for Ypt32p. The M13 sec2 mutant, although identified in the two-hybrid screen as having a weakened interaction with Ypt32p, bound levels similar to wild type in vitro. M19, which was identified as having no interaction in the two-hybrid system, bound approximately 50% of wild type levels in vitro. Since only a portion (codons 157–339) of the mutagenized region of SEC2 (codons 1–311) was transferred into the expression construct, it is possible that a mutation upstream of the sub-cloned region contributed to the loss of interaction observed in the two-hybrid screen with M13 and M19.
Figure 4. Sec2p-Ypt32p interaction is required for Sec2p localization, growth, and secretion.
(A) An in vitro-binding assay confirms the loss of the Ypt32p-Sec2p interaction in Sec2 mutants. GST-Sec2p and His6-Ypt32p were purified from E. coli. Ypt32p was eluted, preloaded with GTPγS, and used in binding reactions with Sec2p and Sec2p mutants immobilized on beads, as described in Experimental procedures. Bound proteins were detected with anti-Ypt32 antibody. (B) In vitro binding of Sec15p is increased for Sec2 mutants that show decreased binding to Ypt32p. GST-Sec2p and His6-Sec15p (Sec15-FL) or His6-Sec15p(559–901) (Sec15-C) were purified from E. coli. Sec15p was eluted and incubated with GST-Sec2p immobilized on glutathione beads. Bound proteins were detected with anti-Sec15 antibody. (C) Sec2 mutants are mislocalized. The localization of Sec2p-3xGFP and mCherry-Ypt32p were examined in wildtype (WT) and various sec2 mutants. Cells were grown overnight at 25°C in synthetic medium containing 2% glucose and live cells were observed. Arrowheads in WT indicate the bud tip localization of Sec2p and Ypt32p. Bars are 2 μm. (D) Secretion of invertase is blocked in sec2 mutants. The percentage of the secreted invertase was examined in untransformed wild type cells (NY1210), sec2-78, wild type expressing Sec2-3xGFP, and the four M-mutants fused to 3xGFP. Cells were grown overnight at 25°C in YP medium with 2% glucose. 1 OD unit of cells was resuspended in YP medium containing 0.1% glucose and cultured at 37°C for 60 min. The amount of invertase secreted during this time period was measured as described in Experimental procedures. The mean and S.D of three different experiment are shown. (E) Ypt32p-binding deficient sec2 mutant shows different conformation. GST-tagged Sec2 and various Sec2 mutant proteins were purified from yeast and the purified proteins were then incubated with or without (−) trypsin. Aliquots were withdrawn from reaction tubes at 0 min, 1 min, 5 min, and 10 min. Proteolytic fragments were detected with an anti-Sec2p antibody that recognizes the N-terminal part of Sec2p. (F) Sec2 was mislocalized in ypt31Δ ypt32A141D cells. Localization of Sec2-3xGFP was examined in wildtype (top panels) and ypt31Δ ypt32A141D (bottom panels) cells. Cells were grown overnight at 25°C in synthetic medium containing 2% glucose. Cells were fixed and examined. Bars are 2 μm.
Sec2p mutants have increased affinity for Sec15p, and normal affinity for PI4P
The Sec2p mutants were next tested to see if their affinity for Sec15p had also been altered. We used both His6-tagged full-length Sec15 protein (Sec15-FL) and a C-terminal fragment (Sec15-C, a.a. 559–910) since the full-length Sec15p is more difficult to express and purify in large amounts (Medkova et al., 2006), and it interacts less efficiently than the C-terminal portion of the protein. We observed a very striking inverse correlation between Ypt32p-binding and Sec15p-binding. Those mutants that exhibited decreased binding to Ypt32p (M7, M5, M14 and M37), exhibited increased binding with both full-length Sec15p and the C-terminus of Sec15p (Figure 4B).
We next tested the effects of the mutations on the interaction of Sec2p with liposomes containing PI4P. All mutant proteins showed near normal association with liposomes containing PI4P and this level of binding was significantly greater than that seen with control liposomes containing PS instead of PI4P (Supplemental Figure S3C). Thus, these mutations do not interfere with PI4P binding.
The Sec2p-Ypt32p interaction is needed for localization of Sec2p, but not Ypt32p
The four sec2 mutants that showed greatly reduced binding to Ypt32p were tagged with 3xGFP and introduced into yeast as the sole copy of SEC2. An immunoblot using anti-Sec2p antibody shows that the levels of Sec2-3xGFP and the four, tagged mutant proteins are similar to that of endogenous Sec2p (Supplemental Figure S3D). Yeast strains were grown overnight at 25°C, harvested, washed, and visualized by fluorescence microscopy. Sec2-3xGFP was localized in small buds and mother-daughter necks of wild-type cells. All four Ypt32-binding-deficient Sec2p mutants showed diffuse fluorescence throughout the cell with little or no apparent concentration at bud tips and necks (Figure 4C; quantification shown in Supplemental Figure S3E).
While these results confirm that the localization of Sec2p requires its interaction with Ypt32p, we next asked if Ypt32p localization requires its interaction with Sec2p. An mCherry-Ypt32p construct was introduced at the URA3 locus of each of the Sec2-3xGFP strains. Ypt32p was localized normally in all strains, even though it has greatly reduced affinity for the mutant Sec2 proteins (Figure 4C, arrowheads). Thus, the localization of Ypt32p is not dependent on its interaction with Sec2p.
The Sec2p-Ypt32p interaction is needed for growth and secretion
To determine if the loss of the Sec2p-Ypt32p interaction has an effect on growth, serial dilutions of yeast strains containing Sec2-3xGFP, Sec2-78, or each of the M-mutants tagged with 3xGFP were spotted onto YPD plates and incubated at either 34 or 37°C (Supplemental Figure S3F). M7, M5, M14 and M37, although not completely dead at 37°C, as was the negative control sec2-78, were severely impaired. Overexpression of Ypt32p completely rescued the growth defects of these sec2 mutants and restored the localization of the mutant proteins (Supplemental Table SI).
We then assayed the mutant strains for their ability to secrete the cell wall enzyme invertase. As shown in Figure 4D, wild type secretes nearly all of the invertase made during a one hour period of derepressed synthesis. The mutants deficient in binding Ypt32p showed a block in secretion comparable to that of sec2-78.
Ypt32p-binding deficient sec2 mutants show a different conformation
The Sec2-78p mutant binds Sec15p more tightly than wild type and exhibits a different limited proteolysis pattern when incubated with trypsin, suggesting that it exists in an alternate conformation (Medkova et al., 2006). Since the Sec2 mutants that have lower affinity to Ypt32p show phenotypes similar to Sec2-78p (Figures 4A-D), we examined whether those mutants proteins have a conformation similar to Sec2-78p. GST-tagged wildtype Sec2 and each of the mutants were purified from yeast, and incubated with trypsin. The limited proteolysis pattern was detected with anti-Sec2 antibody that recognizes the N-terminal region. Wildtype Sec2p accumulated a major degradation product of ~70 kDa (Figure 4E). In contrast, the major degradation product of the Sec2 mutants was ~50 kDa and the overall proteolysis pattern was distinct from that of wildtype (Figure 4E). Since the pattern was similar to that of Sec2-78p, the new Sec2 mutants might have conformations similar to that of Sec2-78p despite the different sites of these mutations.
Ypt31p and Ypt32p are required for Sec2p localization
We next tested Sec2p localization in cells deficient for Ypt31p and Ypt32p. Cells were grown overnight at 25°C, fixed, and visualized by fluorescence microscopy. In ypt31Δypt32A141D cells, Sec2-3xGFP showed diffuse fluorescence throughout the cell with no concentration at the sites of secretion, even at the permissive temperature (Figure 4F). This result confirms that Ypt31 and Ypt32 are required for Sec2p localization.
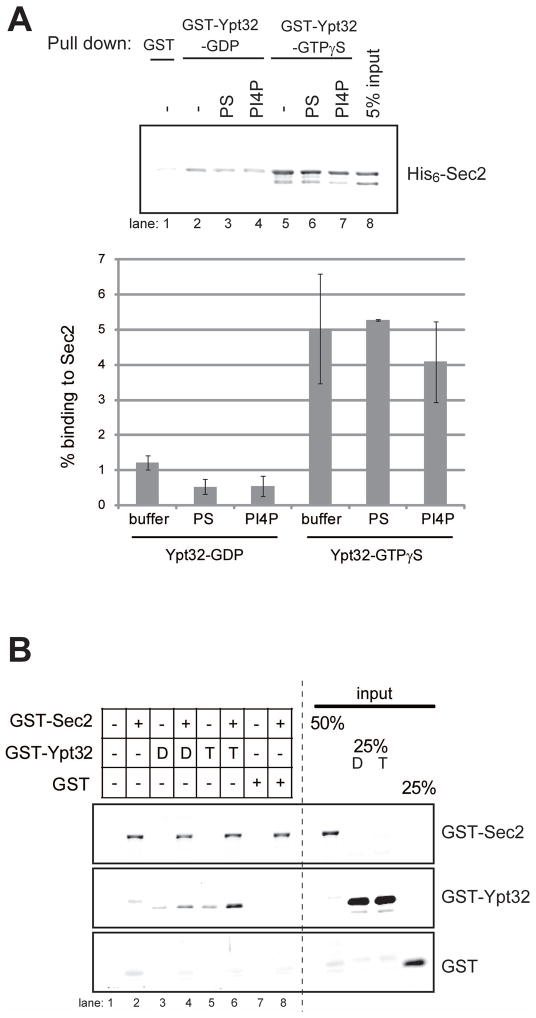
PI4P does not inhibit the binding of Sec2p to Ypt32p
To explore the possibility that PI4P binding influences the ability of Sec2p to interact with Ypt32p, we have performed in vitro binding studies. His6-Sec2p was pre-incubated in the presence or absence of liposomes containing either PS or PI4P. GST-Ypt32p was immobilized on beads and preloaded with GDP or GTPγS, and the Sec2p-liposome complex was added to the beads. After the reaction, the binding buffer and unbound proteins were removed from beads, which were then washed with buffer containing Triton X-100. Detergent was included in the wash buffer to prevent liposome-mediated precipitation. Sec2p bound more efficiently to GTPγS-loaded Ypt32p, as previously shown (Ortiz et al., 2002). Neither control liposomes nor PI4P liposomes had a significant effect on the binding of Ypt32p to Sec2p (Figure 5A).
Figure 5. Three-way interaction between Sec2p, PI4P and Ypt32-GTP.
(A) PI4P does not significantly affect Ypt32-binding to Sec2. GST or GST-Ypt32p (0.60 μM) were immobilized on glutathione-Sepharose beads and preloaded with GDP or GTPγS. His6-Sec2p (0.11 μM) was incubated with buffer (−) or liposome containing 10 mol% (50 μM) of PS or PI4P for 30 min. Then the Sec2-liposome complex was added to the Ypt32-conjugated beads and incubated for an additional 60 min. After the reaction, beads were washed with buffer containing 0.05% Triton X-100. Bound proteins were detected with anti-Ypt32p antibody. The intensity of the bands was quantified using Image J. The percentage of bound Sec2p was calculated and indicated. The mean and S.D of three different experiments are shown. (B) PI4P and Ypt32p can bind Sec2p at the same time. GST-Sec2p, GST-Ypt32p or GST were purified from E. Coli and eluted from glutathione-Sepharose beads. GST-Ypt32p was preloaded with GDP (D) or GTPγS (T). GST-Sec2p (0.15 μM), nucleotide-loaded GST-Ypt32p (0.60 μM), or GST (0.60 μM) were incubated with liposome containing 10 mol% PI4P for 30 min. Liposomes were precipitated by centrifugation at 100,000 × g and bound proteins were analyzed with anti-GST antibody. Lane 6 shows increased association of Ypt32-GTP with PI4P liposomes in the presence of Sec2p, suggesting the formation of a ternary complex.
Sec2p, Ypt32p and PI4P can form a ternary complex
The localization studies suggest that Ypt32p and PI4P act in parallel in Sec2p recruitment (Figures 1, 2 and 3). These observations prompted us to ask whether Sec2p can bind Ypt32p and PI4P at the same time. To address this question, we performed in vitro liposome binding experiments. GST-Sec2p, GST-Ypt32p, or GST were purified from E. Coli and eluted from Glutathione beads. Ypt32p was preloaded with either GDP or GTPγS. GST-Sec2p, nucleotide-loaded GST-Ypt32p, or GST were incubated with liposomes containing PI4P. Liposome-bound proteins were precipitated by centrifugation and analyzed with anti-GST antibody. Only trace amounts of Ypt32p were precipitated with PI4P-liposomes in the absence of Sec2p (Figure 5B, lane 3,5), indicating that Ypt32p does not have an ability to directly bind PI4P. However, in the presence of Sec2p, Ypt32p was precipitated with PI4P-liposomes, more efficiently in its GTP-bound form (Figure 5B, compare lane 4 and 6). This result implies that Sec2p, Ypt32p and PI4P can form a ternary complex.
PI4P inhibits the binding of Sec15p binding to Sec2p
We next tested if PI4P-binding influences the ability of Sec2p to interact with Sec15p in vitro. GST-tagged Sec2p was immobilized on beads, pre-incubated in the presence or absence of liposomes containing either 50 μM of PS, or 10 to 50 μM of PI4P. Sec24p, which is similar in size to Sec2p, was used as a negative control. Bacterially purified His6-Sec15p was added to the Sec2p or Sec24p-liposome mixture, and bound complex was pulled down with Glutathione beads. After the reaction, the beads were washed with buffer containing Triton X-100. Detergent was included in the wash buffer to prevent liposome-mediated precipitation of Sec15p. Since Sec15p binds both control liposomes and PI4P liposomes (not shown), if the experiment were done in the absence of detergent, GST-Sec2p would pull down PI4P liposomes which would then pull down Sec15p regardless of its ability to directly bind Sec2p. Sec2p bound Sec15p and control liposomes containing PS had little effect on the binding (Figure 6A, lane 4 and 5). PI4P liposomes strongly inhibited the Sec15p-Sec2p interaction in a dose dependent manner (Figure 6A, lane 6–9). GST-Sec24p did not bind to Sec15p (Figure 6A, lane 1–3).
Figure 6. PI4P inhibits binding of Sec15p to Sec2p.
(A) PI4P inhibits the binding of Sec15p to Sec2p. GST-Sec24p or GST-Sec2p (0.10 μM) were immobilized on glutathione-Sepharose beads and preincubated with buffer (−) or 0.5 mM liposome containing 50 μM of PS, or 10 to 50 μM of PI4P for 30 min. Then 50 nM of purified His6-Sec15p was added to the beads and incubated for an additional 60 min. After the reaction, beads were washed with buffer containing 0.05% Triton X-100. Bound Sec15p was detected with anti-Sec15p antibody. The intensity of the bands was quantified using Image J. (B) 100 to 600 nM of GST-Sec2p was immobilized on glutathione-Sepharose beads and preincubated with buffer (−) or 0.5 mM liposome containing 50 μM of PS, or 50 μM of PI4P for 30 min. Then 50 nM of purified His6-Sec15p was added to the beads and incubated for an additional 60 min. After the reaction, beads were washed with buffer containing 0.05% Triton X-100. Bound proteins were detected with anti-Sec15p antibody. The intensity of the bands was quantified using Image J. Results shown here are representative of three independent experiments. (C) The Sec2p-Sec15p interaction is enhanced in pik1-101 cells. Sec2p-GFP was immunoprecipitated with anti-GFP antibody from wild-type or pik1-101 cell lysates. Sec2-78p-GFP was immunoprecipitated from the sec2-78 mutant. As a negative control, cells expressing untagged Sec2p were used. Co-precipitated Sec15p-13xMyc was detected with anti-Myc antibody (top panel). Sec2-GFP in the immunoprecipitates was detected by immunoblotting with anti-GFP antibody (bottom panel). The intensity of the bands was quantified using Image J. The percentage of Sec15p in the immunoprecipitate was calculated and indicated. The mean and S.D of three different experiment are shown.
To determine if PI4P affects the binding affinity between Sec2p and Sec15p, various concentration of GST-Sec2p immobilized on beads were pre-incubated with liposomes containing PS or PI4P, and then purified His6 -Sec15p was added to the beads. As shown in Figure 6B, control liposome containing PS did not affect the Sec2-Sec15 interaction, however, PI4P significantly reduced the affinity between Sec2p and Sec15p over a range of Sec2p concentrations (Figure 6B).
The Sec2p-Sec15p interaction is enhanced in a pik1-101 mutant
To test the effect of PI4P on the interaction of Sec2p with Sec15p in a more physiological setting we compared the efficiency of co-precipitation of Sec15-13xMyc with Sec2-GFP from pik1-101 mutant and wild type lysates. Cells were grown overnight at the permissive temperature and Sec2-GFP was precipitated from lysates with anti-GFP antibody. As shown in Figure 6C, significantly more Sec15-13xMyc was co-precipitated with Sec2p from a pik1-101 lysate than from a wild type lysate, although this increase was not as dramatic as the increase seen in a sec2-78 lysate. Cytosolic and membrane-bound pools of Sec2p were not resolved in this experiment. This result supports the hypothesis that the PI4P made by Pik1p acts to inhibit the binding of Sec15p to Sec2p.
Discussion
Rab GTPases are key regulators of membrane traffic, controlling various aspects of each stage of transport along the exocytic and endocytic pathways. Understanding how rab exchange factors are themselves regulated is therefore of central importance. Our prior studies had suggested a working model in which Sec2p is initially recruited to membranes by binding to Ypt32-GTP in one conformation, yet after recruitment, Sec2p shifts to an alternate conformation that allows Sec15p to displace Ypt32p (Ortiz et al., 2002) (Medkova et al., 2006). The Sec2p-Sec15p interaction on secretory vesicles would constitute a positive feedback loop that ensures a high concentration of activated Sec4p together with its downstream effector, Sec15p and other components of the exocyst tethering complex (Grosshans et al., 2006b). However, the mechanism by which these alternative binding modes of Sec2p are specified has been unclear. Here we show that the phosphoinositide PI4P plays two interrelated roles in Sec2p function: it acts in parallel with Ypt32p in the initial recruitment of Sec2p to membranes, yet it also controls the choice of Sec2p’s binding partners by selectively inhibiting the binding of Sec15p to Sec2p.
While our earlier study had suggested that Ypt32p acts to recruit Sec2p (Ortiz et al., 2002), we now provide more definitive proof. We have generated sec2 mutants that specifically disrupt the Ypt32p binding site and show that this correlates with loss of Sec2p localization, inhibition of the secretory pathway and impaired growth at elevated temperatures. We also observed enhanced binding to Sec15p that inversely correlated with the reduction in Ypt32p-binding (Supplemental Table SI). We propose that Sec2p has two conformations, one that favors binding to Ypt32p and one that favors binding to Sec15p. These new mutations appear to shift Sec2p to a conformation that favors binding to Sec15p rather than to Ypt32p. This phenotype is distinct from that of mutations in the region downstream of amino acid 450, such as sec2-78. Those mutations also led to increased Sec15p binding, however they did so without affecting the ability of Sec2p to bind Ypt32p.
Four independent lines of evidence point to a role for PI4P in Sec2p function: Sec2p binds PI4P containing liposomes, but not control liposomes of the same net charge; Sec2p becomes mislocalized in a pik1-101 mutant, even though another vesicle associated protein is largely unaffected; mutations within Sec2 that block PI4P-binding reduce its localization and overexpression of Ypt32p, Sec2p or Sec4p partially suppresses the temperature-sensitive growth defects of pik1-101 cells. The simplest interpretation of these results is that PI4P formed at the Golgi by Pik1p acts in parallel with Ypt32p to recruit Sec2p; a model that is analogous to the recruitment of various proteins to the endosome by the combined signals of rab5-GTP and PI3P (Christoforidis et al., 1999a; Christoforidis et al., 1999b; Nielsen et al., 2000; Simonsen et al., 1998) (Schnatwinkel et al., 2004). The use of activated Rabs and phosphoinositides as combinatorial signals may be a common theme in the regulation of membrane traffic in all eukaryotic cells.
We observed about 40% overlap between Sec2-GFP fluorescence and mCherry-FAPP1-PH, a marker of high PI4P concentration on late Golgi compartments. However, this co-localization did not extend to the regions of high Sec2p concentration at sites of secretion, such as the tip of the bud or the mother-bud neck. The pattern of overlap between mCherry-FAPP1-PH and Sec2-GFP suggests that Sec2p only transiently resides on a PI4P-positive compartment. Sec2p may associate with PI4P as it is recruited to a late compartment of the Golgi, yet be rapidly packaged into vesicles that have a much lower concentration of PI4P. While mCherry-FAPP1-PH might not detect all pools of PI4P, genetic evidence strongly supports the hypothesis that large amounts of PI4P are not transported from the Golgi to the plasma membrane by secretory vesicles. The requirement for Stt4p, the PI4-kinase at the plasma membrane, cannot be satisfied by overexpression of Pik1p, the PI4-kinase on the Golgi (Cutler et al., 1997). It is not known what becomes of the Golgi pool of PI4P as secretory vesicles are formed. While it could be dephosphorylated by a phosphoinositide phosphatase or converted into PI(4,5)P2 by a PI4P5-kinase, the most likely possibility is that it is retained within the Golgi by a sorting mechanism as secretory vesicles bud off this compartment. Selective enrichment of ergosterol and sphingolipids within yeast secretory vesicles has recently been demonstrated (Klemm et al., 2009), supporting the possibility of lipid sorting at this step.
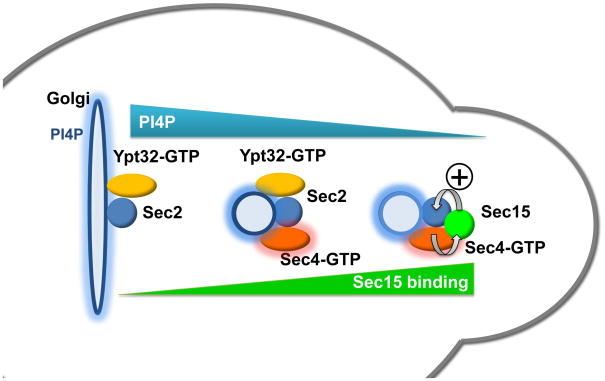
Our in vitro binding studies showing selective inhibition of Sec15p binding by PI4P suggest an interesting scenario (Figure 7); elevated levels of PI4P on the late Golgi or nascent vesicles could act locally to block the interaction of Sec15p with Sec2p. Since Sec15p and Ypt32p compete for binding to Sec2p, and the affinity of Sec2p for Sec15p is several fold higher than its affinity for Ypt32p (Medkova et al., 2006), PI4P may be needed to inhibit the Sec2p-Sec15p interaction at the Golgi so that Ypt32-GTP can recruit Sec2p. In a pik1-101 mutant, the binding of Sec15p by Sec2p would be uninhibited, blocking the recruitment of Sec2p by Ypt32p. The increased co-precipitation of Sec15p with Sec2p in a pik1-101 mutant supports this possibility. The localization pattern of tagged FAPP1-PH suggests that the level of PI4P is reduced once secretory vesicles reach exocytic sites at bud tips and bud necks. This reduction in PI4P levels would relieve the inhibition of Sec15p binding, allowing Sec15p to displace Ypt32p from its binding site on Sec2p. In this fashion, PI4P levels could control the relative affinity of Sec2p’s binding partners, leading first to membrane recruitment by Ypt32-GTP in a Rab GEF cascade and then, as PI4P levels fall, to the formation of a Rab GEF-effector positive feedback loop that facilitates vesicle tethering.
Figure 7. A model for recruitment and regulation of Sec2p by PI4P.
From left to right: Sec2p is initially recruited to the Golgi membrane through the combined signals from PI4P and Ypt32-GTP. The interaction of PI4P with Sec2p also serves to block the binding of Sec15p with Sec2p, thereby facilitating the interaction of Sec2p with Ypt32-GTP. Vesicles bud off of the Golgi carrying Sec2p and Ypt32-GTP, but containing reduced levels of PI4P. Sec2p activates Sec4p, which then recruits its effector, Sec15p. The reduced level of PI4P on the vesicle membrane allows Sec15p to competitively replace Ypt32-GTP on Sec2p. This generates a positive feedback loop in which Sec2p activates Sec4p, Sec4-GTP recruits Sec15p and Sec15p helps retains Sec2p on the vesicle. The elevated levels of Sec4-GTP and Sec15p prepare the vesicle to tether, dock and fuse with the plasma membrane.
Experimental Procedures
Plasmid Construction and Strains
Liposome preparation
In vitro liposome binding assay
GST-Sec2p and Sec2-His6 were purified from E. coli as described in Medkova et al. (2006) with the exception using 0.1 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) overnight at 16°C. Bound protein was eluted with a buffer containing 1x PBS, 160 mM NaCl, and 300 mM Imidazole (pH 8.0) for His-tagged protein or 20 mM Glutathione, 100 mM Tris-HCl (pH 8.0), and 300 mM NaCl for GST-tagged protein.
100–120 nM of Sec2p was incubated with liposomes at different concentrations in a buffer containing 1x PBS and 0.4 mg/ml BSA for 30 minutes at 25°C. The volume of the assay mix was 100 μl. After the reaction, lipid vesicles were pelleted at 100,000 x g (55,000 rpm) in a TLA120.2 rotor for 30 minutes at 25°C. Lipid pellets were suspended in 50 μl of 1x SDS-PAGE buffer. Sec2p was visualized by western blotting with anti-Sec2 antibody (1:1000). To define a binding curve, the intensity of the bands was analyzed with Image J and GraphPad Prism 5.
Fluorescence Microscopy
Yeast cells were grown overnight to mid-log phase in a synthetic medium containing 2% glucose. For most experiments, cells were fixed with methanol as previously described (Elkind et al., 2000). For detail, see Supplemental Experimental Procedures.
Two-hybrid screen
In vitro binding assay
The Ypt32p-binding domain of Sec2p was cloned from the two-hybrid vector into a GST-Sec2p expression construct (NRB1152; (Ortiz et al., 2002) using restriction enzymes NsiI and XbaI. GST-Sec2p and His6-Ypt32p (NRB845; (Du and Novick, 2001) were purified from E. coli as described in Medkova et al. (2006) with the exception that Rosetta2 cells (Novagen) were grown in Terrific Broth and induced using 0.1 mM IPTG overnight at 16°C.
The 3′ end of SEC15 (bp 1675–2733) was PCR amplified and cloned into pQLinkG (Scheich et al., 2007) using BamHI and NotI (NRB1424). Expression and purification for this construct was done as above, except TEV protease (Invitrogen) was used to cleave the protein from the beads.
In vitro binding and washing conditions for GST-Sec2p and either Ypt32-GTP or Sec15p are described in (Medkova et al., 2006), with the exception that the binding reaction volume was decreased to 200 μl and 0.05% Triton-X was added to the wash buffer.
Invertase assay
Invertase activity was measured as described previously (Nair et al., 1990).
Limited Proteolysis assay
GST-Sec2p and GST-Sec2-mutant proteins purified from protease-deficient yeast strains, and limited tryptic digestion was performed as described previously (Medkova et al., 2006).
In vitro binding assay with liposomes
GST-Ypt32p, His6-Sec15p, and His6- or GST-Sec2p were expressed and purified from E. Coli strain BL21 as described previously (Medkova et al., 2006), with the exception that protein expression was induced overnight at 16°C. Ypt32p was preloaded with guanosine diphoshphate (GDP) or guanosine 5′-O-(3-thio)triphosphate (GTPγS, Roche) in a buffer containing 1x PBS, 1 mM nucleotide, 1 mg/ml BSA, 1 mM DTT, and 1 mM EDTA for 1 h at RT. Afterwards, 4 mM MgCl2 was added to the reaction and it was incubated another 15 min.
For the in vitro liposome competition assay, 150 nM GST or GST-Sec2p were immobilized on beads and incubated with liposomes suspended in PBS in a buffer containing 1x PBS, 1 mg/ml BSA, 1 mM MgCl2, and 1 mM DTT for 30 min at RT. Then 50 nM His6-Sec15p or 600 nM GST-Ypt32p preloaded with GDP or GTPγS were added to the reactions and incubated another 60 min. After the reaction, buffer and unbound proteins were removed from the beads which were then washed with a buffer containing 1x PBS, 5 mM MgCl2, 1 mM DTT, 10 μM GDP or GTPγS, and 0.05% Triton X-100. Bound proteins were analyzed with anti-Sec15 (1:1000 dilution) or anti-Ypt32 (1:3000 dilution) antibodies for primary antibody. DyLight 800-conjugated goat anti-rabbit IgG (1:10,000 dilution, Thermo Fisher Scientific, Rockford, IL) was used for secondary antibody and detected with Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Immunoprecipitation assay
Cells were grown overnight in a YP medium containing 2% glucose at 25°C. Approximately 50 OD units of cells were lysed by using a Bead Beater in 1.5 ml of lysis buffer containing 20 mM Hepes (pH 7.5), 150 mM KCl, 0.5 mM DTT, 2 mM EDTA, 1 mM PMSF and protease inhibitors. 0.5% of Triton X-100 was added to the lysates, and the lysates were incubated at 4°C for 15 min then centrifuged at 15,000 × g for 15 min. The total protein concentration in the supernatant was measured by Bradford protein assay (Bio-Rad Laboratories, Hercules, CA). Lysates were precleared by incubation with 10 μl of protein G-Sepharose for 60 min at 4°C. Sec2-GFP was immunoprecipitated overnight with anti-GFP polyclonal antibody at 4°C. 10 μl of Protein G-Sepharose was added and incubated for 120 min at 4°C. Beads were then washed for three times with 1 ml of wash buffer containing 20 mM Hepes (pH 7.5), 150 mM KCl and 0.05% TritonX-100. Immunoprecipitates were analyzed by western blotting with anti-Myc monoclonal antibody (1:1000 dilution, Cell Signaling Technology, Danvers, MA) and anti-GFP polyclonal antibody (1:2000 dilution). For secondary antibody, DyLight 800 conjugated goat anti-mouse or DyLight 680 conjugated goat anti-rabbit IgG were used (1:15000 dilution) and detected by Odyssey Infrared Imaging system.
Supplementary Material
Acknowledgments
We thank Susan Ferro-Novick for providing anti-Ypt32 and anti-GFP antibodies, and SFNB490, SFNB823 and SFNB1351 vectors. We thank Louise Lucast and Pietro De Camilli at Yale for help in liposome preparation and for providing pEGFPC2-FAPP1-PH. We thank Ohkyung Kwon and Hiroyuki Hakozaki at National Center for Microscopy & Imaging Research, and Jennifer Meerloo at UCSD Microscopy Shared Facility for technical support. We thank Daniel Kümmel for supplying the GST-Sec15p(559–910) construct in pQLinkG, Felix Rivera-Molina for NRB1324 and NRB1325, Dan Williams for NRB1448, and members of the Novick laboratory for helpful comments and suggestions throughout this study. This study was supported by grants GM35370 and GM082861 from the N.I.H. to P.N. E.M.Y. was supported by a fellowship from HFSP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audhya A, Emr SD. Stt4 PI 4-kinase localizes to the plasma membrane and functions in the Pkc1-mediated MAP kinase cascade. Developmental cell. 2002;2:593–605. doi: 10.1016/s1534-5807(02)00168-5. [DOI] [PubMed] [Google Scholar]
- Audhya A, Foti M, Emr SD. Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Molecular biology of the cell. 2000;11:2673–2689. doi: 10.1091/mbc.11.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benli M, Doring F, Robinson DG, Yang X, Gallwitz D. Two GTPase isoforms, Ypt31p and Ypt32p, are essential for Golgi function in yeast. The EMBO journal. 1996;15:6460–6475. [PMC free article] [PubMed] [Google Scholar]
- Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. The Journal of cell biology. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, McBride HM, Burgoyne RD, Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999a;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature cell biology. 1999b;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- Cutler NS, Heitman J, Cardenas ME. STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. The Journal of biological chemistry. 1997;272:27671–27677. doi: 10.1074/jbc.272.44.27671. [DOI] [PubMed] [Google Scholar]
- Desrivieres S, Cooke FT, Parker PJ, Hall MN. MSS4, a phosphatidylinositol-4-phosphate 5-kinase required for organization of the actin cytoskeleton in Saccharomyces cerevisiae. The Journal of biological chemistry. 1998;273:15787–15793. doi: 10.1074/jbc.273.25.15787. [DOI] [PubMed] [Google Scholar]
- Dippold HC, Ng MM, Farber-Katz SE, Lee SK, Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu S, et al. GOLPH3 bridges phosphatidylinositol-4- phosphate and actomyosin to stretch and shape the Golgi to promote budding. Cell. 2009;139:337–351. doi: 10.1016/j.cell.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. The Biochemical journal. 2000;351:19–31. doi: 10.1042/0264-6021:3510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du LL, Novick P. Purification and properties of a GTPase-activating protein for yeast Rab GTPases. Methods in enzymology. 2001;329:91–99. doi: 10.1016/s0076-6879(01)29070-3. [DOI] [PubMed] [Google Scholar]
- Elkind NB, Walch-Solimena C, Novick PJ. The role of the COOH terminus of Sec2p in the transport of post-Golgi vesicles. The Journal of cell biology. 2000;149:95–110. doi: 10.1083/jcb.149.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulhammer F, Konrad G, Brankatschk B, Tahirovic S, Knodler A, Mayinger P. Cell growth-dependent coordination of lipid signaling and glycosylation is mediated by interactions between Sac1p and Dpm1p. The Journal of cell biology. 2005;168:185–191. doi: 10.1083/jcb.200407118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti M, Audhya A, Emr SD. Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Molecular biology of the cell. 2001;12:2396–2411. doi: 10.1091/mbc.12.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. The Journal of cell biology. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Andreeva A, Gangar A, Niessen S, Yates JR, 3rd, Brennwald P, Novick P. The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. The Journal of cell biology. 2006a;172:55–66. doi: 10.1083/jcb.200510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proceedings of the National Academy of Sciences of the United States of America. 2006b;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Stolz LE, Lemrow SM, York JD. SAC1-like domains of yeast SAC1, INP52, and INP53 and of human synaptojanin encode polyphosphoinositide phosphatases. The Journal of biological chemistry. 1999a;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- Guo W, Roth D, Walch-Solimena C, Novick P. The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. The EMBO journal. 1999b;18:1071–1080. doi: 10.1093/emboj/18.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H, Schnieders EA, Thorner J, Takemoto JY, DeWald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. The Journal of biological chemistry. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- Homma K, Terui S, Minemura M, Qadota H, Anraku Y, Kanaho Y, Ohya Y. Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. The Journal of biological chemistry. 1998;273:15779–15786. doi: 10.1074/jbc.273.25.15779. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Hughes WE, Woscholski R, Cooke FT, Patrick RS, Dove SK, McDonald NQ, Parker PJ. SAC1 encodes a regulated lipid phosphoinositide phosphatase, defects in which can be suppressed by the homologous Inp52p and Inp53p phosphatases. The Journal of biological chemistry. 2000;275:801–808. doi: 10.1074/jbc.275.2.801. [DOI] [PubMed] [Google Scholar]
- Jedd G, Mulholland J, Segev N. Two new Ypt GTPases are required for exit from the yeast trans-Golgi compartment. The Journal of cell biology. 1997;137:563–580. doi: 10.1083/jcb.137.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. The Journal of cell biology. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medkova M, France YE, Coleman J, Novick P. The rab exchange factor Sec2p reversibly associates with the exocyst. Molecular biology of the cell. 2006;17:2757–2769. doi: 10.1091/mbc.E05-10-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J, Muller H, Peterson M, Novick P. Sec2 protein contains a coiled-coil domain essential for vesicular transport and a dispensable carboxy terminal domain. The Journal of cell biology. 1990;110:1897–1909. doi: 10.1083/jcb.110.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nature cell biology. 2009;11:1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. The Journal of cell biology. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. The Journal of cell biology. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Levine TP. Multiple pools of phosphatidylinositol 4-phosphate detected using the pleckstrin homology domain of Osh2p. The Journal of biological chemistry. 2004;279:44683–44689. doi: 10.1074/jbc.M401583200. [DOI] [PubMed] [Google Scholar]
- Scheich C, Kummel D, Soumailakakis D, Heinemann U, Bussow K. Vectors for co-expression of an unrestricted number of proteins. Nucleic acids research. 2007;35:e43. doi: 10.1093/nar/gkm067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS biology. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Stefan CJ, Audhya A, Emr SD. The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4,5)-bisphosphate. Molecular biology of the cell. 2002;13:542–557. doi: 10.1091/mbc.01-10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl T, Thorner J. Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochimica et biophysica acta. 2007;1771:353–404. doi: 10.1016/j.bbalip.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahirovic S, Schorr M, Mayinger P. Regulation of intracellular phosphatidylinositol-4-phosphate by the Sac1 lipid phosphatase. Traffic (Copenhagen, Denmark) 2005;6:116–130. doi: 10.1111/j.1600-0854.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. The Journal of cell biology. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Collins RN, Novick PJ. Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. The Journal of cell biology. 1997;137:1495–1509. doi: 10.1083/jcb.137.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nature cell biology. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.