Abstract
Asthma is a very common disorder that still causes significant morbidity and mortality. A high percentage of individuals with asthma also experience exercise-induced bronchoconstriction (EIB). This article reviews the current literature and updates the reader on the safety, efficacy, and clinical applications of leukotriene modifiers in the treatment of EIB.
Asthma affects 14 to 15 million people in the United States and is responsible for more than 100 million days of restricted activity, more than 5,000 deaths, and 470,000 hospitalizations each year [1]. Previously characterized as a disease of airway smooth muscle, asthma is currently defined by the National Heart, Lung, and Blood Institute as "a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role, in particular, mast cells, eosinophils, T lymphocytes, macrophages, neutrophils, and epithelial cells." [2] Exercise-induced bronchoconstriction (EIB) occurs in approximately 80 to 90% of individuals with asthma and in approximately 11% of the general population without otherwise symptomatic asthma [3,4]. This article reviews the current literature and updates the reader on the safety, efficacy, and clinical applications of leukotriene modifiers in the treatment of EIB.
Role of Leukotrienes in Asthma Pathogenesis
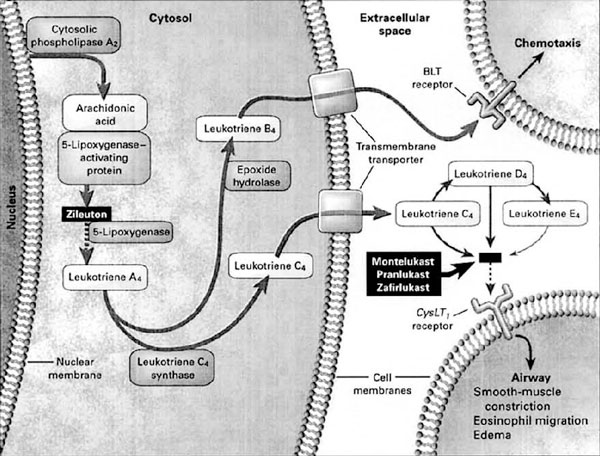
Various biologic signals (including receptor activation, antigen-antibody interaction, and physical stimuli such as cold) activate cytosolic phospholipase A2 to liberate arachidonic acid from membrane phospholipids [5]. The liberated arachidonic acid is then metabolized to various active compounds, including the leukotrienes LTB4, LTC4, LTD4, and LTE4 (Figure 1).
Figure 1.
Biosynthesis and physiologic effects of leukotrienes and pharmacologic actions of antileukotrienes. Reproduced with permission from Drazen et al. [6] BLT = B leukotriene receptor.
LTC4, LTD4, and LTE4, formerly known collectively as slow-reacting substance of anaphylaxis, are collectively called the cysteinyl leukotrienes. The dose of LTD4 required to produce clinical bronchoconstriction has been estimated to be 1,000- to 10,000-fold lower than that of histamine or methacholine, which indicates that these mediators are extremely potent [5]. The cysteinyl leukotrienes exert their biologic effects by binding to cysteinyl leukotriene receptors (specifically subtype 1, CysLT1) on airway smooth muscle and bronchial vasculature, and they contribute to the bronchospasm, increased bronchial hyperresponsiveness, mucus production and mucosal edema, enhanced smooth-muscle cell proliferation, and eosinophilia that are characteristic of the asthmatic airway [6]. Both bronchial and bronchoalveolar lavage studies have provided evidence of increased levels of cysteinyl leukotrienes in the airways of asthmatic individuals [7]. Mast cells synthesize and release leukotrienes in those who are susceptible to exercise- induced bronchoconstriction (EIB) but are probably not the only source, especially in individuals with underlying airway inflammation. Additionally, because mast cells are known to release more than one bronchoconstricting agent, EIB probably does not result from the action of a single mediator. (An in-depth discussion of the mediators involved in EIB and their cellular sources are beyond the scope of this review.)
Exercise-Induced Bronchoconstriction
EIB occurs in individuals of all ages but particularly in children and young adults for whom physical activity is common. EIB is bronchoconstriction that develops occasionally during physical activity (if the activity is of sufficient duration) but usually develops 10 to 30 minutes after physical activity in individuals with underlying airway hyperresponsiveness [4]. The occurrence of EIB in asthmatic persons is common and often signifies suboptimal control of asthma [8].
The diagnosis of EIB is confirmed in the laboratory by a drop of 15% or more in forced expiratory volume in 1 second (FEV1) after vigorous exercise for 6 minutes, according to American Thoracic Society guidelines [9]. Apostexercise drop of 10 to 15% in FEV1 would be considered "probable EIB." Minute ventilation (exercise intensity), temperature and humidity of the inspired air (climatic conditions), and underlying baseline airway responsiveness are the primary determinants of the degree of EIB a patient will experience [4]. The exact mechanism leading to EIB is not yet fully understood but probably relates to drying and/or cooling of the airway mucosa and to mediator release [3]. Many studies, however, have demonstrated the protective effect of CysLT1 receptor antagonists against EIB, providing strong evidence of an important role of cysteinyl leukotrienes in regard to EIB [10].
Treatment of Exercise-Induced Bronchoconstriction
Nonpharmacologic Measures
A warm-up period of light exercise lasting at least 10 minutes may lessen the degree of EIB experienced for 40 minutes to 3 hours [11]. Exercising in a warm humidified environment (if possible) and gradually lowering the intensity of exercise have also been proposed to lessen the degree of EIB experienced by patients [11].
Pharmacologic Measures
Short-Acting β2 Agonists
A short-acting β2 agonist given 15 minutes to 1 hour before exercise can prevent EIB symptoms for up to 4 hours [12], but this bronchoprotective effect has been observed to significantly decrease after 1 week of regular use [13].
Long-Acting β2 Agonists
The long-acting β2 agonists formoterol and salmeterol both will inhibit EIB for up to 12 hours, but formoterol is more rapidly effective [12]. However, regular use of long-acting inhaled β2 agonists has resulted in tachyphylaxis [12], as evidenced by diminished bronchoprotection by 6 to 9 hours [14].
Cromones
Cromolyn and nedocromil inhibit EIB when used prior to exercise. However, they are not as effective as inhaled β2 agonists are in the management of EIB [12].
Other Agents
Anticholinergics, antihistamines, α agonists, and oral β2 agonists have also been investigated for the treatment of EIB [12]. Results are varied; routine use of these types of pharmacologic intervention is not recommended as primary treatment of EIB [12]. Other therapies are still being investigated [12].
Inhaled Corticosteroids
Regular use of inhaled corticosteroids is effective maintenance therapy and reduces EIB [15]. An acute protective effect has been observed 4 hours after inhalation in one small study [16].
Thromboxane Inhibitors
Thromboxane A2 synthesis inhibitors, especially if combined with leukotriene receptor antagonists, have been shown to protect against EIB [17].
Leukotriene Modifiers
Leukotriene Synthesis Inhibitors
The physiologic effects of leukotrienes are inhibited by drugs known as leukotriene modifiers. The blocking of leukotriene-mediated effects can be achieved by administering receptor antagonists (zafirlukast, montelukast) or by targeting enzymes involved in leukotriene biosynthesis. Zileuton is a 5-lipoxygenase inhibitor that inhibits the formation of LTA4 from arachidonic acid, thereby preventing cysteinyl leukotriene synthesis (see Figure 1). Blocking arachidonic enzymatic conversion by the use of 5-lipoxygenase inhibitors does protect against EIB [18] but to a lesser degree and for a shorter duration when compared with the use of receptor antagonists [19].
Leukotriene Receptor Antagonists
Leukotriene receptor antagonists (LTRAs) have been shown to decrease airway responsiveness to methacholine, allergens, and cold air [7]. In aspirin-sensitive individuals, LTRAs inhibit the response to acetylsalicylic acid challenge and improve asthma control [7]. LTRAs may also have a role as corticosteroid-sparing agents [1]. For asthmatic individuals, zafirlukast provides protection against EIB when administered immediately prior to exercise [4], and a single oral dose has been shown to attenuate EIB in children [20] and in adults [19]. Montelukast has been the most extensively studied LTRA. Its protective effects against EIB have been seen to occur as early as 1 hour [19] and up to 24 hours after a single oral dose [14,21]. When montelukast is administered on a regular basis, protection against EIB is maintained over 12 weeks, without the development of tolerance [22].
Montelukast Comparison Studies
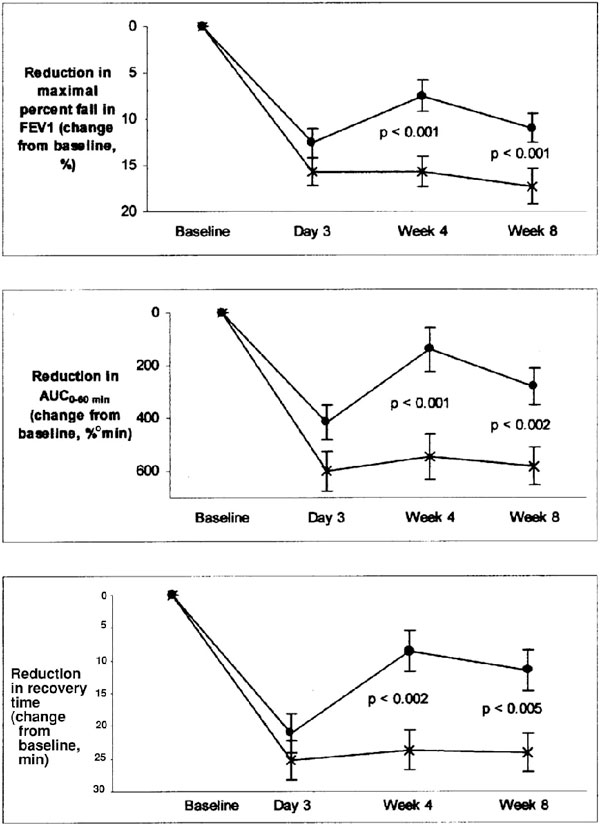
Literature that directly compares the use of montelukast with the use of other bronchoprotective anti-inflammatory or bronchodilator agents is accumulating. To date, studies comparing salmeterol with montelukast and studies comparing budesonide with montelukast have been published. Villaran and colleagues [23] compared 10 mg of oral montelukast administered daily to 50 μg of inhaled salmeterol administered twice daily and found no significant difference in protection against EIB after 3 days of treatment. However, after 4 and 8 weeks of regular dosing, montelukast was significantly more effective than salmeterol in attenuating EIB, as evidenced by a greater reduction in FEV1 drop, area under the curve (0-60 minutes), and time to recovery (Figure 2). The difference is attributed to the development of tolerance following regular administration of a long-acting β2 agonist and the absence of tolerance with regular LTRA administration. Another group reproduced these findings by showing similar protection against EIB during the first 3 days of treatment with either montelukast or salmeterol, but again, the protection was lost in the salmeterol group after 4 weeks of treatment. Protection was maintained in the montelukast group through the study's duration of 8 weeks [14].
Figure 2.
Comparison of montelukast (×) with salmeterol (•) in change from baseline in maximum percentage fall in FEV1 after exercise (top), AUC0-60min (middle) and time to recovery (bottom). Reproduced with permission from Villaran C et al. [23] AUC = area under the curve; FEV1 = forced expiratory volume in 1 second.
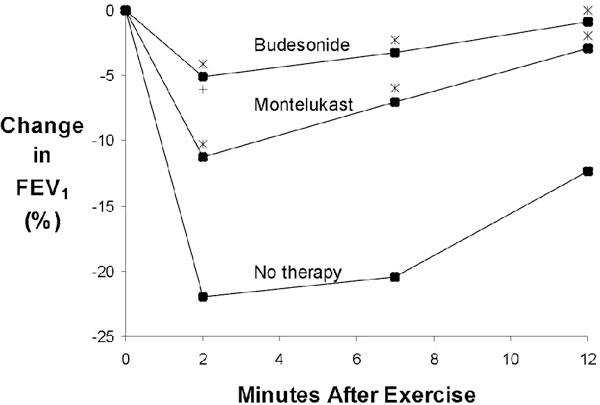
A recent investigation comparing the protective effect of montelukast (10 mg per day for 3 days) and budesonide (400 μg twice daily for 15 days) in 20 patients with EIB showed both treatments to be effective in reducing the percentage of decrease in FEV1 after exercise when compared to placebo. Additionally, budesonide treatment demonstrated a trend toward better protection than did montelukast treatment at three postexercise time points (2, 7, and 12 minutes), but the difference was significant only at the 2-minute endpoint (Figure 3). Although both treatments were proven to be effective, significant individual variation was evident.
Figure 3.
Change in forced expiratory volume in 1 second (FEV1) after exercise at baseline, after budesonide administration, and after montelukast administration in patients with exercise-induced bronchoconstriction. Reproduced with permission from Vidal C et al. [8]
Summary
As a class, the cysteinyl leukotriene receptor antagonists (LTRAs) are effective in the treatment of exercise-induced bronchoconstriction (EIB). LTRAs can be used as an alternative to lowdose inhaled corticosteroids or can replace inhaled corticosteroids when side effects, poor inhaler administration technique, or noncompliance is suspected. The beneficial effects of LTRAs include increased pulmonary function, decreased symptoms, and decreased use of rescue medication. Montelukast has several advantages over other LTRAs, including formulation, onset of action, duration of action, and a low incidence of adverse effects. Perhaps most important, chronic daily use does not result in the development of tolerance. Montelukast is therefore clinically useful for protection against EIB in children and adults, resulting in increased physical activity and quality of life.
References
- Blake KV. Montelukast: data from clinical trials in the managementof asthma. Ann Pharmacother. 1999;33:1299–314. doi: 10.1345/aph.18430. [DOI] [PubMed] [Google Scholar]
- Expert Panel report II. Bethesda (MD):US Department of Health and Human Services; 1997. National Heart, Lung, and Blood Institute, National Asthma Education Program. Guidelines for the diagnosis and management of asthma. Pub. No.: 97-4051. [Google Scholar]
- Gotshall RW. Exercise-induced bronchoconstriction. Drugs. 2002;62:1725–39. doi: 10.2165/00003495-200262120-00003. [DOI] [PubMed] [Google Scholar]
- Marciniuk DD, Cockcroft DW. Exercise-induced bronchoconstriction:the role of leukotriene modifiers in therapy. Can J Allergy Clin Immun. 1998;3:298–303. [Google Scholar]
- Salvi SS, Krishna MT, Sampson AP, Holgate ST. The anti-inflammatory effects of leukotriene-modifying drugs and their use in asthma. Chest. 2001;119:1533–46. doi: 10.1378/chest.119.5.1533. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Israel E, O'Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- Renzi PM. Antileukotriene agents in asthma: the dart that kills the elephant? CMAJ. 1999;160:217–223. [PMC free article] [PubMed] [Google Scholar]
- Vidal C, Fernandez-Ovide E, Pineiro J. Comparison of montelukast versus budesonide in the treatment of exercise-induced bronchoconstriction. Ann Allergy Asthma Immunol. 2001;86:655–8. doi: 10.1016/S1081-1206(10)62294-6. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM. Leukotriene bronchoconstriction induced by allergen and exercise. Am J Respir Crit Care Med. 2000;161:S68–72. doi: 10.1164/ajrccm.161.supplement_1.ltta-14. [DOI] [PubMed] [Google Scholar]
- Tan RA, Spector SL. In: Clinical exercise testing. Weisman IM, Zeballos RJ, editor. Basel: Karger; 2002. pp. 205–16. [Google Scholar]
- Tan TA, Spector SL. Exercise-induced asthma: diagnosis and management. Ann Allergy Asthma Immunol. 2002;89:226–36. doi: 10.1016/S1081-1206(10)61948-5. [DOI] [PubMed] [Google Scholar]
- Inman MD, O'Byrne PM. The effect of regular inhaled albuterol on exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 1996;153:65–9. doi: 10.1164/ajrccm.153.1.8542164. [DOI] [PubMed] [Google Scholar]
- Edelman JM, Turpin JA, Bronsky EA. Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. Ann Intern Med. 2000;132:97–104. doi: 10.7326/0003-4819-132-2-200001180-00002. [DOI] [PubMed] [Google Scholar]
- Jonasson G, Carlsen KH, Hultquis C. Low-dose budesonide improves exercise-induced bronchospasm in schoolchildren. Pediatr AllergyImmunol. 2000;11:120–5. doi: 10.1034/j.1399-3038.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- Thio BJ, Slingerland GL, Nagelkerke AF. Effects of single-dose fluticasone on exercise induced asthma in asthmatic children: a pilot study. Pediatr Pulmonol. 2001;32:115–21. doi: 10.1002/ppul.1097. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Ishibashi Y, Murakami Y. Beneficial effect of combination therapy with ozagrel and pranlukast in exercise-induced asthma demonstrated by krypton-81 m ventilation scintigraphy-- a case report. Ann Acad Med Singapore. 2000;29:766–9. [PubMed] [Google Scholar]
- Lehnigk B, Rabe KF, Dent G. Effects of a 5-lipoxygenase inhibitor, ABT-761, on exercise induced bronchoconstriction and urinary LTE4 in asthmatic patients. Eur Respir J. 1998;11:617–23. [PubMed] [Google Scholar]
- Coreno A, Skowronski M, Kotaru C, McFadden ER. Comparative effects of long-acting {158}2- agonists, leukotriene receptor antagonists, and a 5-lipoxygenase inhibitor on exercise-induced asthma. J Allergy Clin Immunol. 2000;106:500–6. doi: 10.1067/mai.2000.109425. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Ostrom NK, Bronsky EA. The leukotriene D4-receptor antagonist zafirlukast attenuates exercise-induced bronchoconstriction in children. J Pediatr. 1999;134:273–9. doi: 10.1016/S0022-3476(99)70449-X. [DOI] [PubMed] [Google Scholar]
- Reiss TF, Hill JB, Harman E. Increased urinary excretion of LTE4 after exercise and attenuation of exercise-induced bronchospasm by montelukast, a cysteinyl leukotriene receptor antagonist. Thorax. 1997;52:1030–5. doi: 10.1136/thx.52.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff JA, Busse WW, Pearlman D. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise induced bronchoconstriction. N Engl J Med. 1998;16(339):147–52. doi: 10.1056/NEJM199807163390302. [DOI] [PubMed] [Google Scholar]
- Villaran C, O'Neill SJ, Helbling A. Montelukast versus salmeterol in patients with asthma and exercise-induced bronchoconstriction. Montelukast/Salmeterol Exercise Study Group. J Allergy Clin Immunol. 1999;104:547–53. doi: 10.1016/S0091-6749(99)70322-2. [DOI] [PubMed] [Google Scholar]