Abstract
The Mayo Cognitive Factor Scores were derived from a “core battery” consisting of the WAIS-R, WMS-R, and Auditory Verbal Learning Test. The present study sought to clarify the factor structure of an expanded neuropsychological battery in normal elderly controls. Confirmatory factor analysis was performed on the WAIS-III, WRAT-3 Reading, Boston Naming Test, Controlled Oral Word Association Test, Category Fluency, Rey-Osterrieth Complex Figure, Visual Form Discrimination, and Trail Making Test A & B. A base four-factor model consistent with the WAIS-III factor structure was utilized. Only one novel five factor model differentiating processing and motor speed tests improved upon this base model. Other models did not, including a factor for executive function, division of construction/visuospatial ability, or “hold”/“no hold” language abilities.
Introduction
The Mayo Older Americans Normative Studies (MOANS; Ivnik, Malec, Smith, Tangalos, & Petersen, 1996; Ivnik, Malec, Tangalos, Petersen, Kokmen & Kurland, 1990, 1992) and Mayo Older African American Normative Studies (MOAANS; Lucas et al., 2005) were undertaken to better understand test battery properties in the elderly (Smith, Ivnik, and Lucas, in press). One series of projects examined the factor structure of a “core battery” consisting of the revised Wechsler Intelligence and Memory Scales (WAIS-R, Wechsler, 1981;WMS-R, Wechsler, 1987) and Auditory Verbal Learning Test (AVLT; Rey, 1970) in normal and mildly demented Caucasian and African American individuals aged 55–97 (Pedraza et al., 2005; Smith, Ivnik, Malec, Petersen, Kokmen, & Tangalos, 1994; Smith et al., 1992; Smith, Ivnik, Malec, Petersen, & Tangalos, 1993). These were the first three “co-normed” tests presented in the MOANS project. This research identified and replicated a five factor model [the Mayo Cognitive Factor Scores (MCFS)] composed of Verbal Comprehension, Perceptual Organization, Attention, Learning, and Retention factors to best fit these measures across these populations. Knowledge gained about the construct validity of these five cognitive domains continues to be utilized at Mayo’s Alzheimer’s Disease Research Center (cf. Smith et al., 2007).
Today, MOA(A)NS normative data have expanded beyond the WAIS-R, WMS-R, and AVLT, and are widely available and utilized (e.g., Strauss, Sherman, & Spreen, 2006). These normative data have gained popularity because they provide psychometrically sound norms for those over age 55, and because tests used in the MOANS studies were either co-normed or normed using samples pooled from the same population. The tests that make up the MOANS normative data and subsequent “MOANS battery” were selected because of their individual psychometric integrity and their wide use in dementia evaluations. When administered together, these tests provide clinicians with an assessment battery that is familiar to most neuropsychologists inside and outside of the Mayo system. These additional tests have been assumed to assess cognitive domains separate from the 5 traditionally identified within the “core battery.” However, it is not known 1) if these tests actually do cluster separately from the traditional MCFS/traditional WAIS factors and 2) whether the traditional cognitive domain ascribed to tests through lesion studies or normative studies done with primarily younger adults will hold true when the tests are given to older patients. Although age differences on cognitive tasks are now a well accepted neuropsychological principle, to our knowledge, no studies have examined the factor structure of a full neuropsychological battery in an older sample.
Factor analysis has been a common method of examining the construct validity of neuropsychological tests. To be meaningful in examining cognitive domains, however, factor analysis has to be done correctly. Recently, Delis and colleagues (2003) challenged factor analysis’ use in testing construct validity as a “myth.” They conducted factor analysis of memory testing in normal, Alzheimer’s disease (AD), and Huntington’s disease patients to demonstrate a “loss” of key memory constructs when one applies factor results from one population to another. Larrabee (2003) and Bowden (2004) responded to this article, noting other limitations of improperly conceptualized factor analyses, but these authors also defended the use of factor analysis when done properly. Delis’ group (Jacobson, Delis, Hamilton, Bondi, and Salmon, 2004) published a rejoinder that incorporated these opinions, resulting in a call for researchers and clinicians to recognize the utility and limitations of factor analysis.
We take three main points from that this series of articles: 1) The sample/population in which the factor analysis is conducted is important, as generalization to different samples/populations (particularly different disease populations) may be limited or simply wrong (Bowden, 2004; Delis et al., 2003; Jacobson et al., 2004). 2) Method variance (strong correlations among variables from the same test due to similar test stimuli and instructions) may result in a collapsing of factors into an overly simple, faulty result (Larrabee, 2003). 3) Factor analysis statistics are complicated, and numerous chances for error in interpretation must be heeded (Bowden, 2004).
Most often, factor analysis is used as an examination of the properties of a single measure to see if the items fall together into a single idea or few ideas thought to represent the topic(s) that the test author wanted to measure. Perhaps the most well known example is the four factor structure of the Wechsler Adult Intelligence Scale – 3rd Edition (WAIS-III; Wechsler, 1997). Many now regard this model as a superior replacement to the traditional intelligence quotient scores (Tulsky, Saklofske, & Zhu, 2003). Moving beyond factor analysis of a single test, projects on recent co-normed versions of the WAIS-III and Wechsler Memory Scale-III (WMS-III; Wechsler, 1997; e.g., Tulsky, Ivnik, Price, & Wilkins, 2003) have added to the clinical utility of these measures when administered jointly.
The current study expanded upon the Mayo “core battery” to include commonly used additional neuropsychometric tests [Benton Visual Form Discrimination (BVFD; Benton, Sivan, & Hamsher, 1994), Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 1983), Category Fluency (Animals, Fruits, and Vegetables; Lucas et al., 1998), Controlled Oral Word Association Test (COWAT; Benton, Hamsher, & Sivan, 1994), Rey-Osterrieth Complex Figure Copy (Rey-O; Rey, 1970), Trail Making Test (TMT, Reitan, 1992), and Wide Range Achievement Test – 3rd Edition (WRAT-3, Wilkinson, 1993) Reading]. This expands upon the clinical utility of the MOANS normative data, as clinicians who utilize MOANS data and these additional tests that make up the MOANS battery will benefit from further understanding what these tests represent cognitively when used together. However, the current study was not meant solely to be an examination of the MOANS battery. It also was meant to examine the cognitive constructs of a common neuropsychometric battery in an older sample, using sound, contemporary factor analytic practices (i.e., Delis, Bowden, Larrabee).
Method
Participants
A sample of 535 community dwelling, elderly subjects collected as part of the MOANS projects (e.g., Ivnik et al., 1990) were considered for inclusion in this study. Subjects were recruited from routine visits to their primary care physician, and all were cognitively normal by physician and self-report. Of these subjects, 314 produced valid profiles for the complete neuropsychometric battery. This final sample had a mean age of 77.1 years (SD = 5.4; range = 65–95), 13.6 years of education (SD = 2.7; range 5–20), and was 58.6% female.
Measures
The neuropsychological battery included the Wechsler Adult Intelligence Scale – 3rd Edition (WAIS-III; Wechsler, 1997) subtests required to produce the four Factors of verbal comprehension, perceptual organization, working memory and processing speed. The WAIS-III was chosen rather than the WAIS-R used in previous MOANS research in anticipation that use of this most recent version of the WAIS would be more applicable to clinicians’ practices. Also included were the AVLT, WMS-R, WRAT-3 Reading subtest, 60-item BNT, COWAT, Category Fluency, Rey-O Copy, BVFD, and TMT.
Procedures
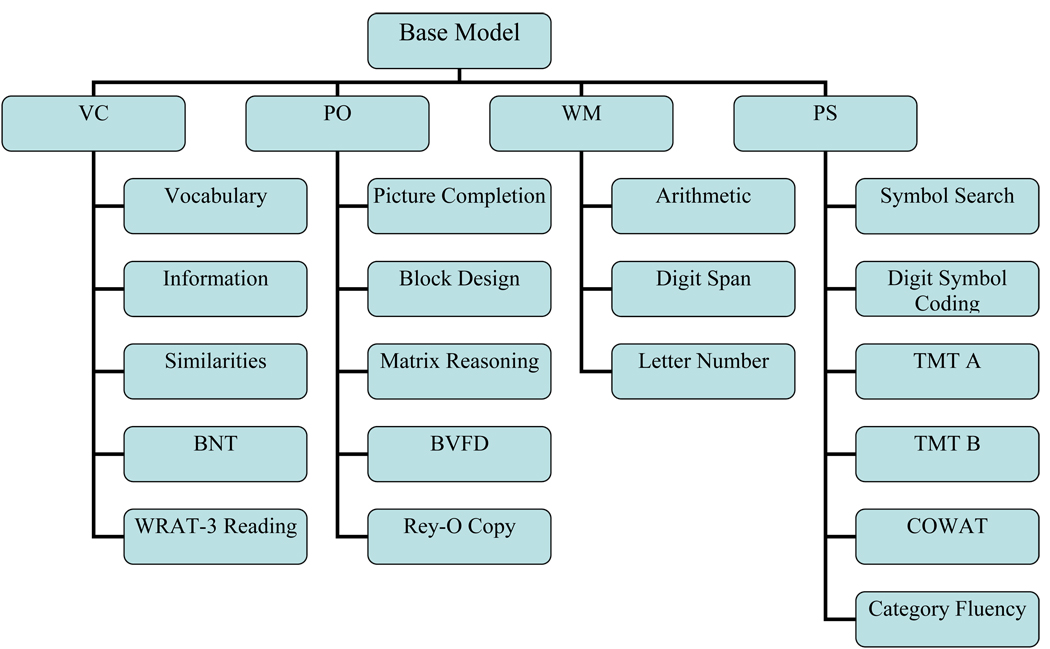
The factor structure of the WAIS-III [Verbal Comprehension (VC; Vocabulary, Similarities, Information), Perceptual Organization (PO; Picture Completion, Block Design, Matrix Reasoning), Working Memory (WM; Arithmetic, Digit Span, Letter Number Sequencing), and Processing Speed (PS; Digit Symbol Coding and Symbol Search)] was defined as the base model upon which to compare all additional factor models (Figure 1). Four theoretical 5-factor models were constructed a-priori.
Figure 1.
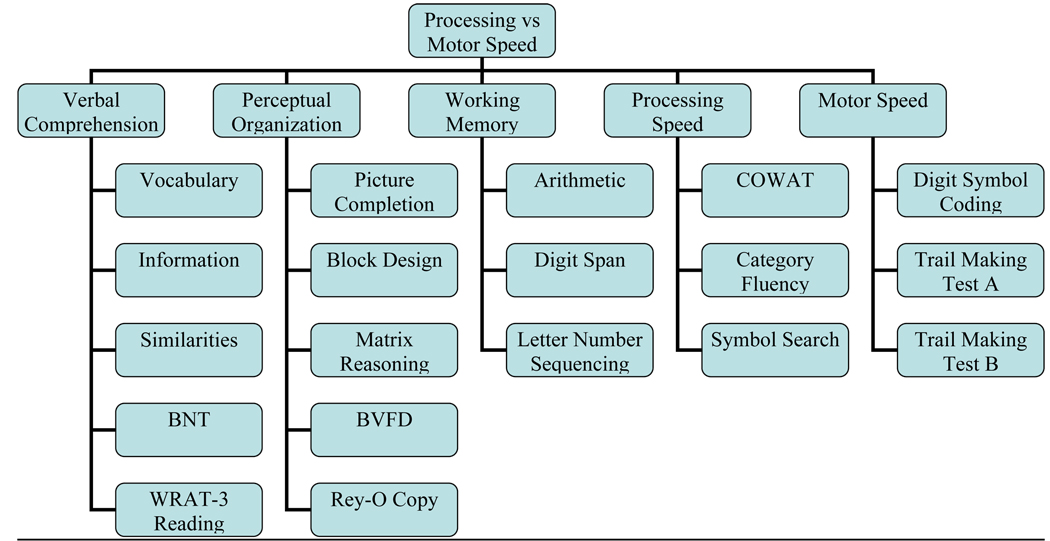
The first five-factor model explored the utility of differentiating “processing” speed from “motor” speed (see Figure 2). Tests with little or no motor demands, but requiring quick performance (COWAT, Category Fluency, and WAIS-III Symbol Search) were contrasted to those with greater motor demands (WAIS-III Digit Symbol and Trail Making A and B). Various causes of restricted hand-use (e.g., arthritis, neuropathy) may impede motor speed in cognitively normal elderly that may be confused with processing speed if not examined separately.
Figure 2.
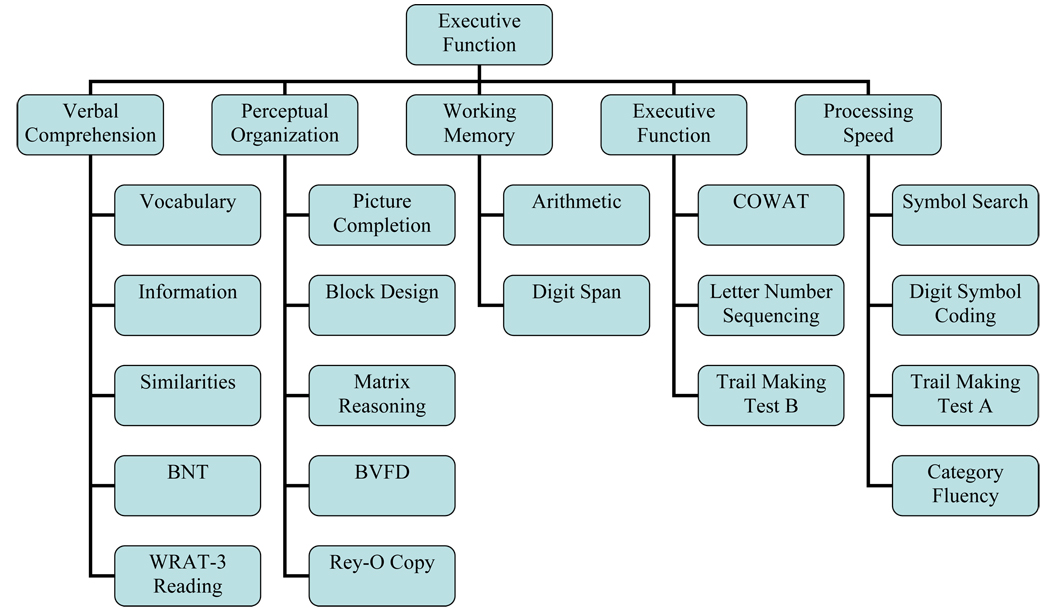
The construct of executive function was evaluated in the second five-factor model (Figure 3). Many neuropsychologists utilize TMT B, COWAT, and Letter Number Sequencing to evaluate mental flexibility and “frontal lobe/executive function.” This was contrasted with the more basic attention/concentration demands in Digit Span and Arithmetic.
Figure 3.
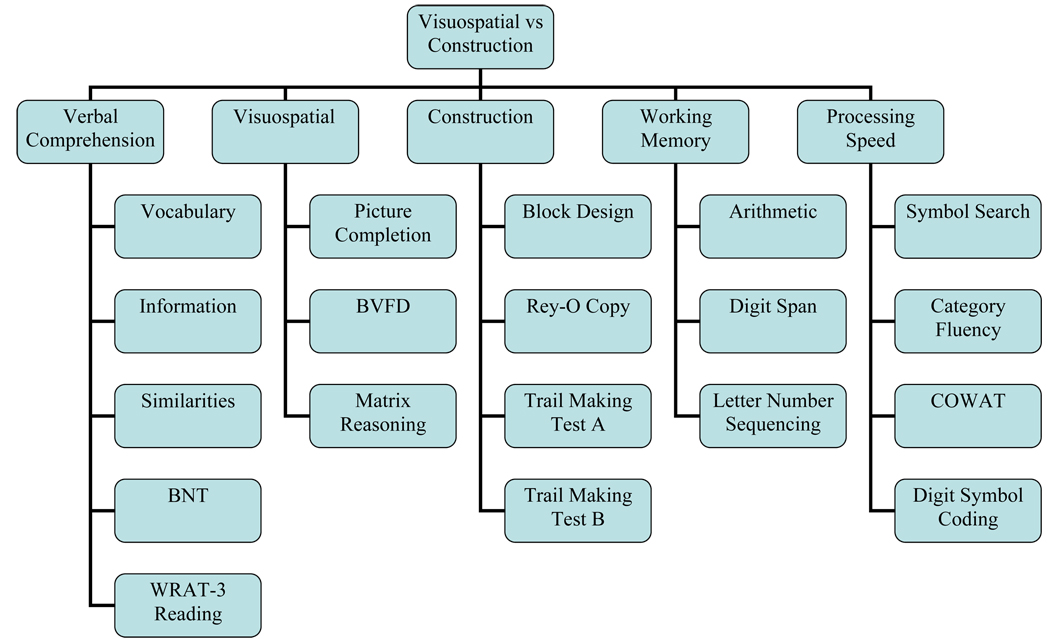
Factor model three (Figure 4) divided visuospatial abilities from constructional abilities. As with the first five-factor model, restricted hand-use may affect construction performances without impacting visuospatial ability. Visuospatial functions were assessed with WAIS-III Picture Completion and Matrix Reasoning as well as BVFD. Construction was assessed using WAIS-III Block Design, Rey-O Copy, and TMT A and B.
Figure 4.
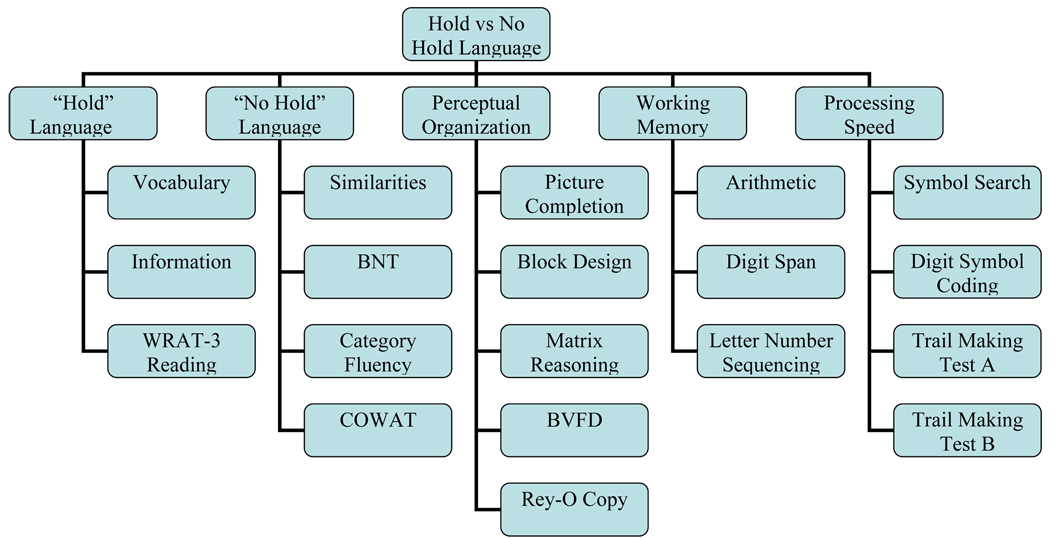
The fourth 5-factor model addressed the ability of this battery to differentiate hold and no hold language tests (Figure 5). Hold versus no hold language abilities are a well accepted construct in aging (Wechsler, 1958), and are frequently used in estimating premorbid functioning after brain damage (Smith-Seemiller, Franzen, Burgess, & Prieto, 1997). These “disease resistant” tasks may also be distinguished in dementia (Larrabee, 1985) until the severe stages of the disease or aphasia onset (Patterson, Graham, & Hodges, 1994). WAIS-III Vocabulary and Information and WRAT-3 Reading were a-priori labeled as hold tests, and WAIS-III Similarities, BNT, Category Fluency, and COWAT were considered no hold tests.
Figure 5.
Learning and memory variables were not examined in these analyses for two reasons. For one, extensive analysis of learning and memory variables in normal elderly has been examined in previous MOANS research (Smith et al., 1992). Secondly, because no novel learning and memory factors or variables were being postulated, we sought to maximize the number of variables that we could reliably examine with our sample size by eliminating extraneous data points.
Data Analysis
A covariance matrix was obtained for the 314 complete neuropsychological batteries (Table 1). Because WAIS-III and MOANS standard scores were utilized, this covariance matrix was standardized (equivalent to the correlation matrix).
Table 1.
Correlation Matrix
| Wrat | Voc | Sim | Info | Name | Cowat | Cat | PC | BD | MR | Rey | Vis D | Arith | DS | LNS | TmtA | TmtB | SS | DigS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wrat | 1 | ||||||||||||||||||
| Voc | .652 | 1 | |||||||||||||||||
| Sim | .486 | .688 | 1 | ||||||||||||||||
| Info | .548 | .711 | .607 | 1 | |||||||||||||||
| Name | .378 | .485 | .466 | .580 | 1 | ||||||||||||||
| Cowat | .399 | .445 | .413 | .356 | .272 | 1 | |||||||||||||
| Cat | .269 | .260 | .272 | .230 | .271 | .417 | 1 | ||||||||||||
| PC | .237 | .236 | .272 | .284 | .344 | .266 | .184 | 1 | |||||||||||
| BD | .240 | .259 | .407 | .291 | .314 | .216 | .225 | .393 | 1 | ||||||||||
| MR | .381 | .443 | .502 | .466 | .400 | .301 | .290 | .379 | .519 | 1 | |||||||||
| Rey | .252 | .193 | .203 | .162 | .196 | .171 | .040 | .197 | .338 | .351 | 1 | ||||||||
| VisD | .292 | .265 | .338 | .240 | .193 | .178 | .157 | .228 | .333 | .443 | .216 | 1 | |||||||
| Arith | .319 | .369 | .376 | .414 | .311 | .260 | .225 | .231 | .389 | .470 | .283 | .310 | 1 | ||||||
| DS | .467 | .388 | .318 | .359 | .270 | .402 | .153 | .135 | .239 | .317 | .238 | .223 | .345 | 1 | |||||
| LNS | .427 | .393 | .343 | .300 | .263 | .396 | .265 | .223 | .284 | .357 | .172 | .309 | .378 | .460 | 1 | ||||
| TMTA | .087 | .105 | .117 | .109 | .136 | .224 | .238 | .177 | .280 | .254 | .077 | .105 | .209 | .067 | .206 | 1 | |||
| TMTB | .312 | .289 | .288 | .252 | .238 | .429 | .397 | .227 | .366 | .375 | .222 | .256 | .401 | .320 | .367 | .459 | 1 | ||
| SS | .208 | .298 | .323 | .250 | .184 | .307 | .222 | .333 | .373 | .389 | .235 | .237 | .328 | .228 | .355 | .310 | .440 | 1 | |
| DigS | .288 | .343 | .345 | .232 | .203 | .413 | .324 | .173 | .291 | .335 | .161 | .327 | .281 | .218 | .358 | .346 | .508 | .504 | 1 |
Each theoretical model was then subjected to confirmatory factor analysis using LISREL (Jöreskog & Sörbom, 1993). Across all models consistent procedures were used. 1) The factor matrix (PHI) was standardized with diagonal elements=1 and off-diagonal elements free to vary. This permitted non-orthogonal factors. Error terms were assumed uncorrelated except for Trail Making A & B. These two variables could produce confounding method variance, so error (theta-delta) term for these two variables was left free to vary in all LISREL modeling. Maximum likelihood analysis provided parameter estimation and goodness of fit statistics [Goodness of Fit Index (GFI) and Adjusted Goodness of Fit Index (AGFI)] for all models.
Results
The covariance Goodness of fit statistics for the base 4 factor model and the 5 factor models are presented in Table 2. Across χ2/df, GFI, AGFI indices, neither the Hold vs. No Hold or Visuospatial vs. Construction models improved upon the base 4 factor model and were thus eliminated from further consideration. The processing speed and executive function models had slightly better χ2/df ratios. Moreover, using the standard χ2 difference relative to df difference to test of goodness of fit significance (Bentler and Bonett, 1980) suggested both models were better. Specifically, for the processing and motor speed 5-factor relative to the base 4-factor model, the χ2 difference= 13.89 df difference = 4, p<.05. For the Executive function 5-factor model relative to the base 4-factor model the χ2 difference= 15.82, df difference = 4, p<.05.
Table 2.
Goodness of Fit Values
| χ2 | Df | χ2/df | GFI | AGFI | |
|---|---|---|---|---|---|
| WAIS-III Factors | 322.01 | 145 | 2.22 | .90 | .87 |
| Modified WAIS-III | 272.12 | 142 | 1.92 | .92 | .89 |
| Factors | |||||
| Processing vs | 308.12 | 141 | 2.19 | .90 | .87 |
| Motor Speed | |||||
| Modified Processing vs | 249.48 | 138 | 1.81 | .92 | .89 |
| Motor Speed | |||||
| Executive Function | 306.19 | 141 | 2.17 | .91 | .87 |
| Modified Executive | 290.6 | 138 | 2.11 | .91 | .88 |
| Function | |||||
| Visuospatial vs | 358.79 | 141 | 2.54 | .89 | .85 |
| Construction | |||||
| “Hold” vs “No Hold” | 333.10 | 141 | 2.36 | .89 | .86 |
| Language |
Note. GFI = Goodness of Fit Index; AGFI = Adjusted Goodness of Fit Index; WAIS-III = Wechsler Adult Intelligence Scale – 3rd Edition.
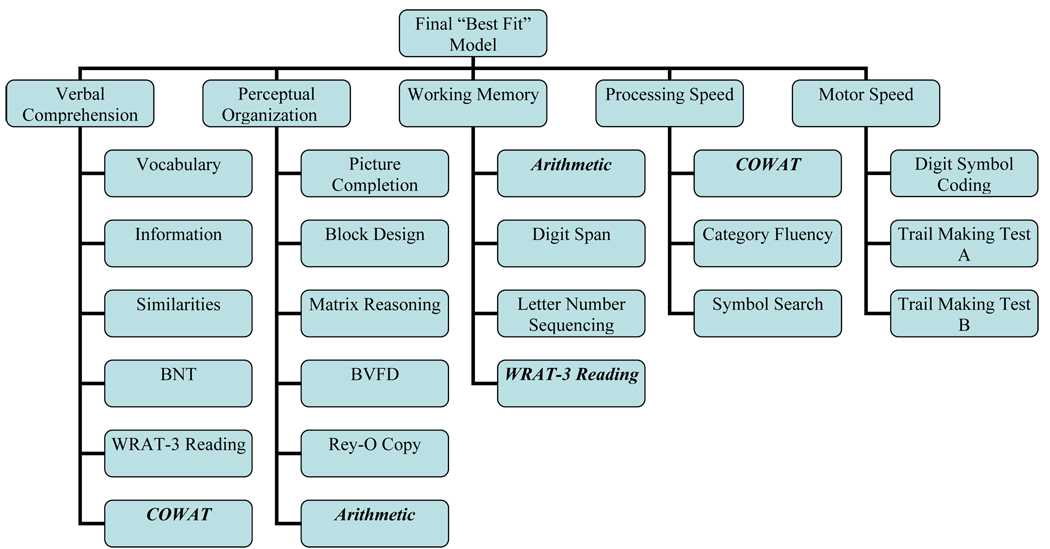
However, none of the models reached the standard of χ2/df <2. Thus, we used the modification indices provided by LISREL to guide model improvement beginning with our base model. Allowing for the following modification indices improved the base model: 1) WAIS-III Arithmetic loaded on both WM and PO factors, 2) COWAT loaded on VC and PS, and 3) WRAT-3 Reading loaded on VC and WM. Modification of these variables resulted in an adjusted goodness of fit of .89, and a chi-square to df ratio of less than 2.0. We subsequently permitted the same modifications to the processing and motor speed and executive function models to permit model improvement while maintaining nesting. For the executive function model, the modifications did not result in improvement over the base 4-factor model. However, for the processing versus motor speed 5-factor model, the modifications did push the χ2/df to less than 2.0 and less than the base 4-factor model. The χ2 difference between the processing and motor speed model was 22.6 with df difference of 4, suggesting the 5-factor model remained significantly better than the 4-factor model at p<.001. This final ‘best’ model is depicted in Figure 6.
Figure 6.
Discussion
Even though neuropsychologists routinely utilize tests to define aspects of cognition in the elderly, the construct validity of these “divisions” of cognition in a full neuropsychological battery required exploration. Additionally, we sought to understand the cognitive constructs assessed by a testing battery commonly given utilizing the MOANS normative data. These tests represent measures that are frequently used together by neuropsychologists nationwide.
The concept of cognitive domains such as executive function, visuospatial versus visuoconstruction, and processing versus motor speed largely originate from lesion studies, younger populations, or disease populations. The concept of “hold” and “no hold” tasks comes from theoretical suppositions of aging changes by Wechsler (1958), which have been supported in the literature when normal elderly and those with Alzheimer’s disease are compared (Larrabee, 1985). Given the 19 subtests used in this factor analysis, a 5-factor model involving Verbal Comprehension, Perceptual Organization, Working Memory, Processing Speed, and Motor Speed had the best fit to the covariance structure in this normal older adult sample. Other models defining an executive function domain, separate domains of construction and visuospatial ability, or separate hold and no hold tests were not better and in some cases these models were worse then the basic 4-factor model defined by the WAIS-III subtests. These results suggest that attempts to delineate additional cognitive domains of executive function, construction/visuospatial, or “hold no hold” tasks using this battery with normal elderly may be unfounded. In retrospect, it is perhaps not surprising that the concept of differentiating disease resistant and non-resistant (hold, no hold) verbal tasks is not applicable when the sample is cognitively normal and when age corrected scores are examined.
While factor analyses of specific tests or a single cognitive construct are numerous, factor analyses of full neuropsychological batteries are sparse outside of those for the Halstead-Reitan Battery (Newby, Hallenbeck, & Embretson, 1983) or batteries derived from the Luria battery (Ostrosky, Canseco, Quintanar, Navarro, & Ardila, 1985). The few existing full battery factor analysis studies have utilized younger adults. In general for younger adults, various combinations of verbal, visuospatial, attention, learning/memory, reasoning, and motor ability are the most common reported factors, depending upon the tests administered (e.g., Carroll, 1993).
In a more recent example, Frazier, Youngstrom, Chelune, Naugle, and Lineweaver (2004) used various factor analytic methods on a full neuropsychological battery that included the WAIS-III, WMS-III, WRAT-3 Reading, Finger Tapping (Halstead, 1947; Reitan, 1955), Grooved Pegboard (Matthews & Klove, 1964), TMT, BNT, COWAT, and Wisconsin Card Sorting Test (Heaton, Chelune, Talley, Kay, & Curtiss, 1993) in an overall younger sample (age range 16–93). These authors found four factors: Memory (WMS-III), Visual Motor (high loadings from TMT, Tapping, Grooved Pegboard), Language (BNT, Verbal Comprehension Index, WRAT-3 Reading) and Executive Functioning (WCST). Comparison of the overlapping tests with the current MOANS study (TMT, BNT, WAIS-III, WRAT-3 Reading) reveals loadings on similar factors between both studies. One exception is the Perceptual Organization Index which loaded moderately on both Language and Visual Motor factors for the younger adults, but was clearly its own factor for our older sample. Comparison to this study also highlights how factor loadings are dependent on the tests used. For example, it is unknown if inclusion of the WCST in our battery would have produced an Executive Functioning factor.
Not all tasks loaded as traditionally expected in this older sample when allowing for modification indices: Category Fluency loaded as a processing speed task (not verbal) and phonemic fluency loaded as a verbal and processing speed task [not executive functioning; consistent with Frazier et al.’s (2004) findings]. These results highlight how completion of a task often requires more than one skill. When looking at a normal elderly population, certain skills are perhaps more salient than others. Hence, while speed is perhaps always a prerequisite to good performance on fluency tasks, it may be the most important aspect in the elderly. Thus, tasks such as category fluency become more of a speed task than a verbal task, and phonemic fluency is a mix of the two. In addition, WRAT-3 Reading loaded as both a verbal and an attention task. Good attention to the WRAT-3 task (e.g., reading “beatify” versus “beautify”) as well as verbal skills are required for good performance.
Implications
As Delis et al. (2003) highlighted, the results of factor analysis are dependent upon the characteristics of the sample used. However, it appears that neuropsychologists may have a tendency to violate this basic tenant in research when applying cognitive domains across populations: generalizability is limited to the population(s) under examination. This is undoubtedly due to the simple fact that very little literature exists to look at construct validity of our traditional cognitive domains across various demographic and patient populations.
The consequence of not knowing what a specific test in a battery may be measuring for a particular population is the spread misinformation. When researchers report findings, they tend to simplify the neuropsychological results by cognitive domains for their abstract and conclusions, e.g., memory impairment, executive dysfunction, etc. The assignment of a cognitive domain label is based on that researcher’s assumptions of what construct their test(s) assessed. If this attribution is faulty, incorrect conclusions about the strengths and weaknesses of various normal and disease populations results. The clinician, in turn, depends upon the summaries provided by the researcher, and such misinformation can lead to misunderstandings about patient diagnosis and prognosis.
For example, phonemic fluency has been used in some studies as the sole measure of “executive function.” However, both the current study in normal elderly and the results of Frazier et al. (2004) in younger adults do not support phonemic fluency as loading on an executive function factor. Clinicians reading conclusions to studies reporting impaired or unimpaired executive function in a certain population based on phonemic fluency results may be misled, and make faulty diagnostic conclusions or predictions about their patients using that misinformation.
Limitations and Future Research
The current study did not re-examine the factor structure of the AVLT and WMS-R as done by Smith et al. (1992). We had no theoretical basis to assume that AVLT and WMS-R would load any differently beyond the Learning and Memory factors they originally created (plus a loading on the Perceptual Organization factor for Visual Reproduction I). Also, as more variables are added to a factor analytic study, the sample size required can become prohibitive. We instead chose to conserve our sample for previously unexamined tasks. While we did not utilize the original MCFS sample in this study, the sample was selected based on the same recruitment procedures, inclusion and exclusion criteria, and were drawn from the same largely southeastern Minnesota population. We suspect that when these two tests are added back to the battery used in this study, we would have a 7 factor model: Verbal Comprehension, Perceptual Organization, Attention, Processing Speed, Motor Speed, Learning, and Memory. It is also important to note that we do not know how the factor structure would change with use of the WMS-III.
The results of the current study can only be generalized to normal elderly given the “Mayo battery.” We also caution that it may be incorrect to assume that cognitively impaired elderly will exhibit the five cognitive domains of verbal comprehension, perceptual organization, processing speed, motor speed, and attention that are identified here. Future research will be needed to determine how the factor structure of the Mayo battery changes in various disease populations.
Conclusions
Use of the Mayo battery of neuropsychological tests in normal elderly produces five cognitive domains consistent with the WAIS-III factor structure plus and additional domain of motor speed. Extrapolation of additional domains such as executive functioning, visuospatial and visuoconstruction, and “hold” “no hold” verbal domains may be unfounded, producing misleading conclusions.
Acknowledgments
Supported in part by NIA grant, Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation, Mayo Alzheimer’s Disease Research Center P50 AG16574, and the Mayo Alzheimer’s Disease Patient Registry UO1 AG06786
References
- Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin. 1980;88:588–606. [Google Scholar]
- Benton AL, Hamsher KdeS, Sivan AB. Multilingual Aphasia Examination. 3rd ed. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- Benton AL, Sivan AB, Hamsher KdeS. Contributions to neuropsychological assessment. A clinical manual. 2nd ed. New York: Oxford University Press; 1994. [Google Scholar]
- Bowden SC. The role of factor analysis in construct validity: Is it a myth? Journal of the International Neuropsychological Society. 2004;10:1018–1019. doi: 10.1017/s1355617704107091. [DOI] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Delis DC, Jacobson M, Bondi MW, Hamilton JM, Salmon DP. The myth of testing construct validity using factor analysis or correlations with normal or mixed clinical populations: Lessons from memory assessment. Journal of the International Neuropsychological Society. 2003;9:936–946. doi: 10.1017/S1355617703960139. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Youngstrom EA, Chelune GJ, Naugle RI, Lineweaver TT. Increasing the reliability of ipsative interpretations in neuropsychology: A comparison of reliable components analysis and other factor analytic methods. Journal of the International Neuropsychological Society. 2004;10:578–589. doi: 10.1017/S1355617704104049. [DOI] [PubMed] [Google Scholar]
- Halstead WC. Brain and intelligence. Chicago: University of Chicago Press; 1947. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological testing norms above age 55: COWAT, BNT, MAE TOKEN, WRAT-R Reading, AMNART, Stroop, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. The Auditory Verbal Learning Test (AVLT):Norms for ages 55 and older. Psychological Assessment. 1990;2:304–312. [Google Scholar]
- Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. The Auditory Verbal Learning Test (AVLT):Updated norms for ages 56 to 97. The Clinical Neuropsychologist. 1992;6:83–104. [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8: Structural equation modeling with the SIMPLIS command language. Chicago: Scientific Software International; 1993. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea &Febiger; 1983. [Google Scholar]
- Larrabee GJ. Lessons on measuring construct validity: A commentary on Delis, Jacobson, Bondi, Hamilton, and Salmon. Journal of the International Neuropsychological Society. 2003;9:947–953. doi: 10.1017/S1355617703960140. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Largen JW, Levin HS. Sensitivity of age-decline resistant (“hold”) WAIS subtests to Alzheimer’s disease. Journal of Clinical and Experimental Neuropsychology. 1985;7:497–504. doi: 10.1080/01688638508401281. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, et al. Mayo’s Older Americans Normative Study:Category fluency norms. Journal of Clinical and Experimental Neuropsychology. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Willis FB, Ferman TJ, Smith GE, Parfitt FC, et al. Mayo’s Older African Americans Normative Studies:Normative data for commonly used clinical neuropsychological measures. The Clinical Neuropsychologist. 2005;19:162–183. doi: 10.1080/13854040590945265. [DOI] [PubMed] [Google Scholar]
- Matthews CG, Klove K. Instruction manual for the Adult Neuropsychology Test Battery. Madison, WI: U of Wisconsin Medical School; 1964. [Google Scholar]
- Newby RF, Hallenbeck CE, Embretson S. Confirmatory factor analysis of four general neuropsychological models with a modified Halstead-Reitan Battery. Journal of Clinical Neuropsychology. 1983;5:115–133. doi: 10.1080/01688638308401159. [DOI] [PubMed] [Google Scholar]
- Ostrosky F, Canseco E, Quintanar L, Navarro E, Ardilla A. Sociocultural effects in neuropsychological assessment. International Journal of Neuroscience. 1985;27:53–66. doi: 10.3109/00207458509149134. [DOI] [PubMed] [Google Scholar]
- Patterson K, Graham N, Hodges JR. Reading in dementia of the Alzheimer type: A preserved ability? Neuropsychology. 1994;8:395–407. [Google Scholar]
- Pedraza O, Lucas JA, Smith GE, Willis FB, Graff-Radford NR, Ferman TJ, et al. Mayo’s older African American normative studies:confirmatory factor analysis of a core battery. Journal of the International Neuropsychological Society. 2005;11:184–191. doi: 10.1017/s1355617705050204. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Investigation of the validity of Halstead’s measures of biological intelligence. Archives of Neurology and Psychiatry. 1955;73:28–35. doi: 10.1001/archneurpsyc.1955.02330070030005. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail Making Test:Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1970. [Google Scholar]
- Smith GE, Ivnik RJ, Malec JF, Kokmen E, Tangalos EG, Kurland LT. Mayo’s Older Americans Normative Studies (MOANS): Factor structure of a core battery. Psychological Assessment. 1992;4:382–390. [Google Scholar]
- Smith GE, Ivnik RJ, Malec JF, Petersen RC, Tangalos EG. Factor structure of the Mayo Older Americans Normative Sample (MOANS) core battery: Replication in a clinical sample. Psychological Assessment. 1993;5:121–124. [Google Scholar]
- Smith GE, Ivnik RJ, Malec JF, Petersen RC, Kokmen E, Tangalos EG. Mayo cognitive factors scales:Derivation of a short battery and norms for factor scores. Neuropsychology. 1994;8:194–202. [Google Scholar]
- Smith GE, Pankratz VS, Negash S, Machulda MM, Petersen RC, Boeve BF, et al. A plateau in pre-Alzheimer memory decline: Evidence for compensatory mechanisms? Neurology. 2007;69:133–139. doi: 10.1212/01.wnl.0000265594.23511.16. [DOI] [PubMed] [Google Scholar]
- Smith GE, Ivnik RJ, Lucas JA. Assessment techniques: Tests, test batteries, norms and methodological approaches. In: Morgan J, Ricker J, editors. Textbook of Clinical Neuropsychology. New York: Oxford Press; (in press) [Google Scholar]
- Smith-Seemiller L, Franzen MD, Burgess EJ, Prieto LR. Neuropsychologists’ practice patterns in assessing premorbid intelligence. Archives of Clinical Neuropsychology. 1997;12:739–744. [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Tulsky DS, Ivnik RJ, Price LR, Wilkins C, et al. Assessment of cognitive functioning with the WAIS-III and WMS-III: Development of a six-factor model. In: Tulsky DS, editor. Clinical interpretation of the WAIS-III and WMS-III. New York: Academic Press; 2003. pp. 147–179. [Google Scholar]
- Tulsky DS, Saklofske DH, Zhu J, et al. Revising a standard: An evaluation of the origin and development of the WAIS-III. In: Tulsky DS, editor. Clinical interpretation of the WAIS-III and WMS-III. New York: Academic Press; 2003. pp. 147–179. [Google Scholar]
- Wechsler D. The Measurement and Appraisal of Adult Intelligence. 4th ed. Baltimore, MD: Williams & Wilkins Co; 1958. Mental deterioration and its appraisal; pp. 199–213. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised: Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third Edition: Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Third Edition: Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test-3. Wilmington, DE: Wide Range, Inc.; 1993. [Google Scholar]