Abstract
The goal of this study was to understand the mechanisms of greater weight loss by gastric bypass (GBP) compared to gastric banding (GB) surgery. Obese weight- and age-matched subjects were studied before (T0), after a 12 kg weight loss (T1) by GBP (n=11) or GB (n=9), and at 1 year after surgery (T2). PYY3-36, ghrelin, GLP-1, leptin, and amylin were measured after an oral glucose challenge. At T1, glucose-stimulated GLP-1 and PYY levels increased significantly after GBP but not GB. Ghrelin levels did not change significantly after either surgery. In spite of equivalent weight loss, leptin and amylin decreased after GBP, but not after GB. At T2, weight loss was greater after GBP than GB (p=0.003). GLP-1, PYY, and amylin levels did not significantly change from T1 to T2; leptin levels continued to decrease after GBP, but not after GB at T2. Surprisingly, ghrelin AUC increased 1 year after GBP (p=0.03). These data show that, at equivalent weight loss, favorable GLP-1 and PYY changes occur after GBP, but not GB, and could explain the difference in weight loss at 1 year. Mechanisms other than weight loss may explain changes of leptin and amylin after GBP.
Keywords: GLP-1, gastric bypass, weight loss, gastric banding
Introduction
Bariatric surgery has become the preferred option for the treatment of morbid obesity. Gastric bypass surgery (GBP) results in about 56.7-66.5% excess weight loss, while adjustable gastric banding (GB), a purely restrictive procedure, usually results in 40.7-54.2% excess weight loss (1). In addition to the malabsorptive and/or restrictive components of bariatric surgery, some studies suggest that hormonal changes that occur after GBP could contribute to the better weight loss outcome compared to GB (2-4).
Regulation of food intake is a complex interplay of central and peripheral signals. Some gastrointestinal hormones, such as glucagon-like peptide-1 (GLP-1), peptide YY3-36 (PYY) and ghrelin, show meal-to-meal variation, and the administration of GLP-1 (5) or PYY (6) decreases food intake. Post-prandial GLP-1 (7, 8) and PYY (4, 9, 10) levels increase after GBP but not after GB (11).
Reports of ghrelin levels after bariatric surgery vary widely, with reported increases (12, 13), decreases (14, 15), or no change after GBP, and no change or increase after GB (2, 16, 17).
Among other peripheral hormones associated with food intake regulation, leptin is produced in the adipocytes; leptin levels decrease with weight loss by diet (18) or by bariatric surgery, both GBP and GB (3, 19). Amylin is co-secreted with insulin from the pancreas in response to nutrients; amylin analogues inhibit food intake (20, 21). Circulating amylin levels decrease with diet-induced weight loss (22).
Whether the hormonal changes after surgery are secondary to weight loss or due to the nature of the surgery is unknown. We have recently reported that at equivalent weight loss, GBP results in greater post-prandial GLP-1 and PYY levels compared to weight loss by diet (10, 23). Most longitudinal studies that have compared the hormonal changes after GBP and GB have been conducted in patients with unmatched pre-surgery body weight, with differential weight loss between GBP and GB groups (2, 24), making the comparison between surgeries difficult to interpret. In this study, our goal was to compare the changes in levels of peptides known to regulate food intake (GLP-1, PYY, ghrelin, leptin, amylin) after GBP and GB. In order to differentiate the role of weight loss from that of surgery on peptide changes, we studied the effect of an equivalent weight loss (∼12 kg) by GBP or GB in weight-matched patients. These patients were studied again 1 year postoperatively, when weight loss was different between groups. We hypothesized that some hormonal changes (post-prandial increase in GLP-1 and PYY levels, decrease in ghrelin levels) would occur after GBP but not after GB, and be directly related to the nature of the surgery, independent of weight loss; other hormones (decrease in leptin and amylin) would change similarly with equivalent weight loss after GBP and GB.
Methods and Procedures
Subjects
Obese patients who were eligible candidates for GBP, younger than 60 years of age, were invited to participate in the study and signed an informed consent approved by our institution prior to enrollment. One group of patients was studied before GBP (T0), 1 month after (T1) and 1 year after GBP (T2). A second group of patients, fulfilling the same recruitment criteria, was studied before GB (T0), after a 12-kg weight loss (T1) and 1 year after surgery (T2). There was no time limit to achieve the matched weight loss at T1 in the GB group. GB patients were frequently contacted by the research coordinator, who assessed weight loss and referred patients to surgeons for band adjustment as needed. After the weight loss target of 10-12 kg was reached after GB, patients returned for T1 studies. Glucose tolerance was assessed by history and/or medications, and by glucose tolerance test. Patients with type 2 diabetes for less than 5 years and not on insulin, thiazolidinedione, exenatide, or dipeptidyl peptidase-IV (DPP-IV) inhibitors, with HbA1c less than 8% were included in the study.
Laparoscopic bariatric surgery
For GBP, the jejunum was divided 30 cm from the ligament of Treitz and anastomosed to a 30 mL proximal gastric pouch. The jejunum was re-anastomosed 150 cm distal to the gastrojejunostomy. For GB, a silicone band (approximately 10-12 mm diameter) was placed around the proximal portion of the stomach to create a 30 mL pouch. This band contains a volume-adjustable balloon on its inner surface that is connected to an implantable port device placed subcutaneously to allow for adjustments. Adjustments were performed by the surgeon as needed
The post-bariatric surgery nutritional recommendations included a daily intake of 600–800 kcal of purée diet, 70 g protein, and 1.8 L fluid for 1 month after surgery. This was achieved, on an individual basis, with multiple small meals, snacks, and various commercial protein supplements. After 1 month, patients were told to introduce solid food and to increase their daily caloric intake to 800-1000 kcal, with 70 g protein and 1.8 L fluid daily. The diet after surgery was monitored by food records but not directly supervised. The diet in days preceding the testing was not controlled for.
Three-hour OGTT
At T0, T1 and T2, all patients underwent a 3-hour 50 g (in 200 mL) oral glucose challenge (OGTT). Patients were fasted for 12 hours prior to testing. After intravenous catheter insertion at 0800 hours, blood samples were collected before and at 15, 30, 45, 60, 90, 120, and 180 minutes following the glucose drink, on chilled EDTA tubes with added aprotinin (500 kallikrein inhibitory U/mL blood) and DPP-IV inhibitor (Millipore Corp., St. Charles, MO) (10 μl/mL blood). Samples were then centrifuged at 4° C for plasma collection before storage at −70° C.
Assays
PYY3-36, total ghrelin, total GLP-1, leptin, and amylin were measured by radio-immunoassay (RIA) (Millipore, St. Charles, MO). All intra- and inter-assay coefficients of variance ranged from 3.4-7.4% and 4.4-7.4 %, respectively. Glucose concentration was measured at bedside by the glucose oxidase method (Beckman glucose analyzer; Beckman Coulter, Inc., Fullerton, CA). All hormonal and metabolite assays were performed at the New York Obesity Research Center.
Statistical Analyses
Total areas under the curve 0-180′ (AUC) for outcome variables were calculated using the trapezoidal method. Differences between surgical groups were assessed by unpaired t-test. General linear model (GLM) with repeated measures was used to detect changes over 1 year within each surgical group. Simple bivariate correlation analyses were performed between weight loss and outcome variables. Data are expressed as mean ± SD except in figures where mean ± SEM are reported. Statistical significance was set at p<0.05 (2-tailed). Statistical analyses were performed with SPSS 16.0 (SPSS Inc., Chicago, IL).
Results
Baseline characteristics (T0)
At T0, before surgery, patients in the GBP and GB groups had similar body weight, BMI, fasting leptin, amylin, glucose, insulin, GLP-1, PYY and ghrelin levels, and glucose-stimulated GLP-1, PYY, ghrelin, and amylin levels (Table 1). Most patients were either diabetic or glucose intolerant (11 in GBP group, 6 in GB group); the remaining 3 patients (GB group) were normoglycemic. Hemoglobin A1C (HbA1C) levels were significantly higher in the GBP group compared to the GB group at baseline (6.7±0.8% vs. 5.7±0.7%, p=0.01).
Table 1.
Subject characteristics at baselinea
| GBP (n=11) | GB (n=9) | |
|---|---|---|
| Weight (kg) | 116.1± 19.6 | 125.2± 19.9 |
| BMI (kg/m2) | 45.1±8.2 | 46.5±6.8 |
| Fasting GLP-1T (pmol/L) | 6.8±4.3 | 4.9±1.9 |
| GLP-1T Peak (pmol/L) | 15.5±5.9 | 11.9±5.4 |
| GLP-1T AUC (pmol/L/min) | 7.2±3.0 | 4.8±1.2§ |
| Fasting PYY (pmol/L) | 60.7 ±21.2 | 46.0±25.6 |
| Peak PYY (pmol/L) | 74.3 ±26.0 | 52.3 ±24.9 |
| PYY AUC (pmol/L) | 50.5±27.3 | 35.7±21.4 |
| Fasting Ghrelin (pmol/L) | 148.2±36.8 | 120.3±32.6 |
| Ghrelin Nadir (pmol/L) | 126.1±38.8 | 93.7±33.2 |
| Ghrelin AUC (pmol/L/min) | 139.3±40.4 | 111.9±30.2 |
| Fasting Leptin (ng/mL) | 34.1±9.7 | 30.4±9.1 |
| Fasting Amylin (pmol/L) | 14.5±9.5 | 20.6±25.8 |
| Amylin AUC (pmol/L/min) | 19.9±9.3 | 23.6±22.9 |
Section symbol (§) indicates significantly different from GBP (p<0.05, unpaired t-test)
Band adjustment
In the first year after GB, patients returned for a total of 6.5±1.0 follow-up visits (range 5-8) and received 2.0±2.1 band adjustments (range 0-6).
Equivalent weight loss (T1)
GBP patients were studied before and about 1 month after surgery (T1 for GBP = 39.6±20.6 days, range 22-92 days) with a mean weight loss of 13.3±6.4 kg (range 5.3 -24.9 kg, Table 2). GB patients were studied after a matched weight loss of 11.3±2.5 kg (range 8.9-17.2 kg, p=0.35 compared to GBP, Table 2), which was achieved after about 3 months (T1 for GB = 97.4±84.3 days, range 21-235 days, p=0.08 compared to GBP).
Table 2.
Changes in weight, GLP-1, PYY, ghrelin, leptin, and amylin after equivalent weight loss (T1) by GBP or GBa
| GBP (n=11) | GB (n=9) | |||||
|---|---|---|---|---|---|---|
| Baseline | Post-weight loss (T1) | Δ | Baseline | Post-weight loss (T1) | Δ | |
| Weight (kg) | 118.3±18.9 | 105.1± 18.4* | -13.3±6.4 | 122.9±20.6 | 111.7±20.7* | -11.3±2.5 |
| BMI (kg/m2) | 45.1±7.8 | 40.1± 6.8* | -5.0±2.2 | 45.7±7.5 | 41.4± 7.3* | -4.2±1.1 |
| Fasting GLP-1T (pmol/L) | 6.8±4.3 | 7.0±3.3 | 0.2±4.2 | 4.9±1.9 | 4.2±1.1§ | -0.6±2.3 |
| GLP-1T Peak (pmol/L) | 15.5±5.9 | 102.3 ±56.1* | 86.8±57.1 | 11.9±5.4 | 17.5 ±18.7§ | 5.6±19.7§ |
| GLP-1T AUC (pmol/L/min) | 7.2±3.0 | 29.0±9.9* | 21.8±9.7 | 4.8±1.2§ | 6.3±4.7§ | 1.5±5.1§ |
| Fasting PYY (pmol/L) | 60.7 ±21.2 | 48.2 ±31.2 | -12.6 ±23.8 | 46.0±25.6 | 46.8 ±11.4 | 0.9±17.0 |
| Peak PYY (pmol/L) | 74.3 ±26.0 | 124.9 ±50.4* | 50.6 ±40.5 | 52.3 ±24.9 | 59.7 ±19.4§ | 7.4 ±21.5§ |
| PYY AUC (pmol/L/min) | 50.5±27.3 | 84.4±32.6* | 33.9±28.7 | 35.7±21.4 | 41.2±13.1§ | 5.5±17.2§ |
| Fasting Ghrelin (pmol/L) | 148.2±36.8 | 135.3±64.3 | -12.9±46.5 | 120.3±32.6 | 136.7±56.3 | 16.3±36.9 |
| Ghrelin Nadir (pmol/L) | 126.1±38.8 | 105.5±45.2 | -20.7±29.5 | 93.7±33.2 | 99.8±49.1 | 6.1±27.8 |
| Ghrelin AUC (pmol/L/min) | 139.3±40.4 | 123.6±49.9 | -15.8 ±32.2 | 111.9±30.2 | 120.9±43.0 | 9.1±28.6 |
| Fasting Leptin (ng/mL) | 34.1±9.7 | 18.3 ±7.9* | -15.7 ±6.3 | 30.4±9.1 | 22.3± 6.6 | -8.1 ±11.7 |
| Fasting Amylin (pmol/L) | 14.5±9.5 | 6.4± 6.3* | -8.1±6.4 | 20.6±25.8 | 26.7± 35.2 | 6.1±14.3§ |
| Amylin AUC (pmol/L/min) | 19.9±9.3 | 9.5±5.2* | -10.4±9.7 | 23.6±22.9 | 27.0± 27.8 | 2.5±12.0§ |
Asterisk (*) indicates significantly different from baseline (p<0.05, GLM with repeated measures). Section symbol (§) indicates significantly different from GBP (p<0.05, unpaired t-test)
Fasting GLP-1 and PYY levels did not change significantly after either surgery (Table 2, Figure 1a-b) and were not significantly different between surgical groups. GLP-1 and PYY levels (AUC) significantly increased from baseline after oral glucose after GBP (Table 2, Figure 1a-b) but not after GB. The change in GLP-1 and PYY AUC from baseline was significantly different between the GBP and GB groups (GLP-1: p<0.001; PYY: p=0.018, Table 2).
Figure 1.
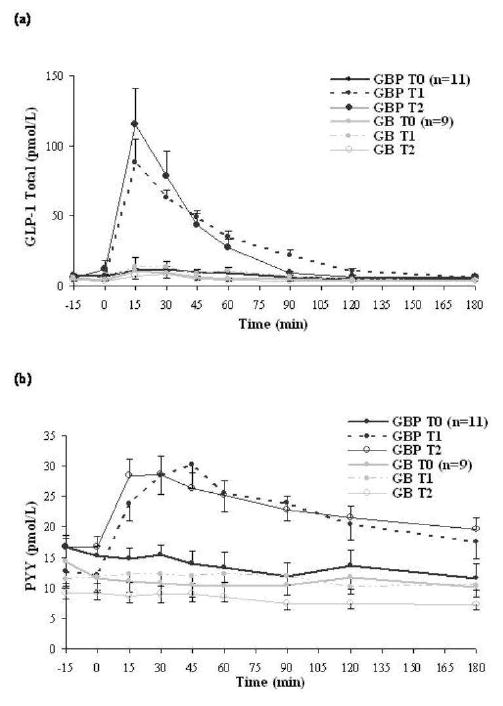
Mean post-prandial GLP-1 (a) and PYY (b) levels at T0, T1, and T2 after GBP or GB. Data are present as mean ± S.E.M. GLP-1, Glucagon-like peptide-1; PYY, peptide YY; T0, baseline; T1, at equivalent weight loss (12 kg) between surgical groups; T2, at one year post-surgery; GBP, gastric bypass; GB, gastric banding.
Fasting and glucose-suppressed ghrelin levels tended to decrease after GBP and increase after GB, but neither ghrelin values at T1 nor the changes from baseline were significantly different between groups (Table 2, Figure 2a-b).
Figure 2.
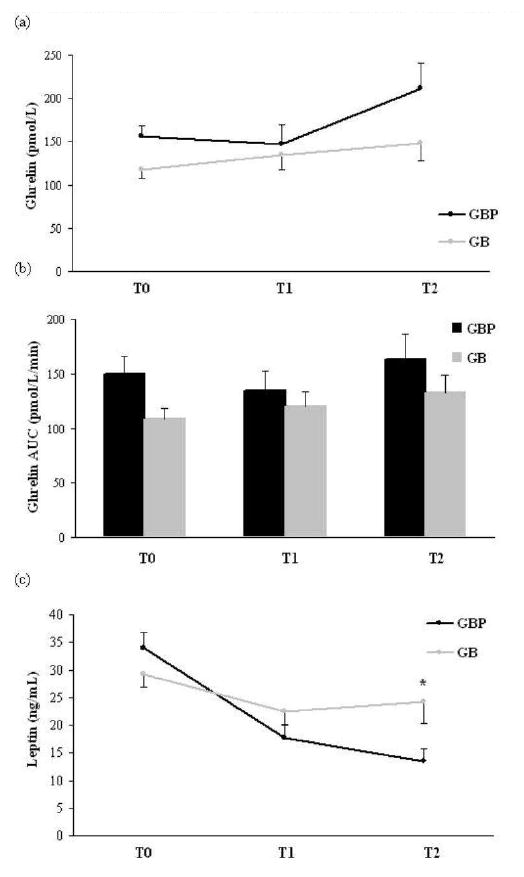
Mean fasting ghrelin (a), ghrelin AUC (b), and leptin (c) T0, T1, and T2 after GBP or GB. Data are present as mean ± S.E.M. Statistical signficance is indicated with an asterisk (*) between groups only AUC, area under the curve; T0, baseline; T1, at equivalent weight loss (12 kg) between surgical groups; T2, at one year post-surgery; GBP, gastric bypass; GB, gastric banding.
Fasting leptin levels decreased after GBP (p=0.0001) but not after GB (Table 2, Figure 2c). However, neither the leptin values at T1 nor the changes from baseline were significantly different between surgical groups. The decreases in fasting amylin and amylin AUC during the OGTT at T1 were significantly greater after GBP than GB (p=0.028 and p=0.026 for fasting amylin and amylin AUC, respectively, Table 2).
One year post-surgery (T2)
One year after surgery, both groups continued to lose weight. Patients after GBP lost almost twice as much weight as patients after GB (36.4±11.0 kg vs. 17.8±12.7 kg, p=0.01), although the range in each group varied (23.6-63.8 kg for GBP and 0.10-40.6 kg for GB) (Table 3). HbA1C decreased significantly after GBP but was not different between GBP and GB groups 1 year after surgery (5.2±0.4% vs. 5.5±0.6%, respectively, p=0.13).
Table 3.
Changes in weight, GLP-1, PYY, ghrelin, leptin, and amylin by GBP or GB after 1 year (T2)a
| GBP (n=11) | GB (n=9) | |||||
|---|---|---|---|---|---|---|
| Baseline | After 1 year (T2) | Δ | Baseline | After 1 year (T2) | Δ | |
| Weight (kg) | 118.3±18.9 | 81.9± 13.0* | -36.4± 10.9 | 122.9±20.6 | 105.1±18.4*§ | -17.8± 12.7 § |
| BMI (kg/m2) | 45.1±7.8 | 31.4±4.6* | -13.7±4.7 | 45.7±7.5 | 39.8± 6.9* § | -6.9±2.4 |
| Fasting GLP-1T (pmol/L) | 6.8±4.3 | 8.5±11.1 | 1.6±9.3 | 4.9±1.9 | 4.0±2.0 | -0.9±1.4 |
| GLP-1T Peak (pmol/L) | 15.5±5.9 | 127.5 ±83.5* | 112.0± 86.7 | 11.9±5.4 | 9.4±8.2§ | -2.6±8.5a |
| GLP-1T AUC (pmol/L/min) | 7.2±3.0 | 26.4 ±15.8* | 19.2±16.7 | 4.8±1.2§ | 3.8±0.9§ | -1.0±1.5§ |
| Fasting PYY (pmol/L) | 60.7 ±21.2 | 66.9 ±23.0 | 6.2 ±23.3 | 46.0±25.6 | 36.3 ±13.1§ | -9.6±20.5 |
| Peak PYY (pmol/L) | 74.3 ±26.0 | 120.6 ±38.6* | 46.3 ±35.3 | 52.3 ±24.9 | 44.2 ±14.8§ | -8.1 ±25.0 |
| PYY AUC (pmol/L/min) | 50.5±27.3 | 27.8±8.8* | -7.9±21.7 | 35.7±21.4 | 27.8±8.8§ | -7.9±21.7§ |
| Fasting Ghrelin (pmol/L) | 148.2±36.8 | 211.7±92.1 | 63.4±71.1 | 120.3±32.6 | 147.8±55.8 | 27.5±48.3 |
| Ghrelin Nadir (pmol/L) | 126.1±38.8 | 150.5±48.0 | 24.3±28.1 | 93.7±33.2 | 120.2±44.5 | 26.5±35.7 |
| Ghrelin AUC (pmol/L/min) | 139.3±40.4 | 179.8± 57.9* | 40.5±34.9 | 111.9±30.2 | 132.3 ±46.3 | 20.5±40.4 |
| Fasting Leptin (ng/mL) | 34.1±9.7 | 13.4± 7.1* | -20.7±7.3 | 30.4±9.1 | 22.9 ±11.3§ | -7.5 ±15.7§ |
| Fasting Amylin (pmol/L) | 14.5±9.5 | 3.7±3.4* | -10.7±7.4 | 20.6±25.8 | 17.8± 23.2 | -2.8±16.7 |
| Amylin AUC (pmol/L/min) | 19.9±9.3 | 7.0±3.1* | -12.9±8.3 | 23.6±22.9 | 17.4±23.7 | -3.4±15.9 |
Asterisk (*) indicates significantly different from baseline (p<0.05, GLM with repeated measures). Section symbol (§) indicates significantly different from GBP (p<0.05, unpaired t-test)
Fasting and glucose-stimulated levels of GLP-1 and PYY remained unchanged from T1 to T2 in GBP patients, with sustained elevation of glucose-stimulated GLP-1 (3-fold increase) and PYY (60% increase) levels (Table 3, Figure 1a-b). In spite of additional weight loss after GB, neither fasting nor glucose-stimulated GLP-1 and PYY levels changed at T2. As a result, the changes in glucose stimulated GLP-1 and PYY from baseline to T2 was significantly different between the GBP and GB group (GLP-1: p=0.002; PYY: p=0.002, Table 3).
Fasting and glucose-suppressed ghrelin AUC significantly increased by 46% from T1 to T2 after GBP (p<0.05, Table 3, Figure 2a and 2b). After GB, fasting and glucose-suppressed ghrelin levels continued to increase non-significantly at T2 (Table 3, Figure 2a and 2b). At T2, despite the greater amount of weight loss after GBP compared to GB, neither ghrelin levels nor the changes from baseline were significantly different between the 2 groups (Table 4).
Table 4.
Summary of changes in hormones after GBP and GBa
| Equivalent weight loss (T1) | 1 year post surgery (T2) | |||
|---|---|---|---|---|
| GBP | GB | GBP | GB | |
| GLP-1 | ||||
| Fasting | ↔ | ↔ | ↔ | ↔ |
| 3 h AUC | ↑ | ↔ | ↑ | ↔ |
| PYY | ||||
| Fasting | ↔ | ↔ | ↔ | ↔ |
| 3 h AUC | ↑ | ↔ | ↑ | ↔ |
| Ghrelin | ||||
| Fasting | ↔ | ↔ | ↔ | ↔ |
| 3 h AUC | ↔ | ↔ | ↑ | ↔ |
| Amylin | ||||
| Fasting | ↓ | ↔ | ↓ | ↔ |
| 3 h AUC | ↓ | ↔ | ↓ | ↔ |
| Fasting Leptin | ↓ | ↓↔ | ↓ | ↓↔ |
Summary of effects from baseline. Bold arrows indicate statistically significant changes from baseline.
Although patients in both groups continued to lose weight at T2 compared to T0, leptin levels continued to decrease significantly only after GBP, and did not change after GB (Figure 2c). Both the leptin values at T2 and the change in leptin from baseline to T2 was significantly different between the 2 surgical groups (p=0.04 for both comparisons, Table 3).
At 1 year, fasting and glucose-stimulated amylin levels continued to decrease nonsignificantly and were not different between surgical groups (Table 3).
Discussion
We studied hormonal signals relating to food intake after GBP and GB, as potential mechanisms for the greater weight loss after GBP. In order to distinguish between the surgery-related hormonal effects from the weight loss-related effects, we prospectively compared the changes of these hormonal signals following GBP and GB, first after a matched 12 kg weight loss, and then at 1 year, at differential weight loss.
GBP has been shown to consistently produce greater weight loss than GB (25, 26); this effect is supported in our data at 1 year. Nevertheless, some studies suggest that by 3 to 7 years post -surgery, weight loss tends to be similar between surgical groups (27). The mechanisms of sustained weight loss after these two surgeries are not yet known.
Our data confirm, as previously shown, a rapid and substantial rise of GLP-1 (7, 23) and PYY (10) 1 month after GBP, but not after equivalent weight loss by GB. In contrast to previous studies, either cross-sectional or performed under different weight loss conditions between groups (11, 24), our patients were matched at baseline for both body weight and endocrine parameters. These results suggest that the dramatic increase in GLP-1 and PYY after GBP, but not after GB or diet (10, 23), is likely related to the nature of the bypass surgery itself, and not to the weight loss. The post-prandial increase in GLP-1 and PYY 1 month after GBP remains unchanged 1 year after surgery, despite continued weight loss. This further supports the idea that the gut peptide increases are independent of weight loss and related to the GBP procedure.
A recent study by de Carvalho et al. (28) reported that glucose-stimulated GLP-1 levels 9 months after GBP were nonsignificantly higher in patients with pre-operative diabetes or glucose intolerance compared to normoglycemic patients. This finding suggests that the differences in glucose-stimulated GLP-1 levels between the 2 surgical groups may be attributable to differences in glucose tolerance status between the GBP and GB groups. However, the magnitude of the change in GLP-1 levels between the 2 surgical groups in our study is considerably greater (10-fold) compared to the change between the glucose intolerant and normoglycemic group observed in the study by de Carvalho et al. (28). This supports the idea that the changes that we observed in GLP-1 and PYY levels between the GBP and GB groups are primarily due to the surgical differences and are most likely minimally affected by the slight differences in glucose tolerance status between the 2 groups.
Both GLP-1 and PYY administration reduce food intake in animal models and in humans (5, 29), an effect mediated by gut vagal afferents (30), or ligand-receptor interactions in the hypothalamus (31). Nonspecific blockade of GLP-1 and PYY with octreotide after GBP or GB decreased satiety and increased meal size (32). A recent study reported that brain activation changes after a peripheral administration of PYY3-36 decreases food intake in humans (33), further characterizing the role of gut-brain interactions and their effects on energy intake.
PYY administration decreases adiposity in rats (34), and weekly subcutaneous administration of a long-acting GLP-1 analogue for 15 weeks significantly reduced body weight in obese diabetic patients compared to placebo (35). It is possible that the increase in GLP-1 and PYY after GBP may be, independently and/or additively, partially responsible for its weight loss effects, via meal-to-meal regulation.
Ghrelin is the only peripheral peptide known to stimulate food intake (36, 37). The decrease of ghrelin levels (14, 15), or the absence of elevation of ghrelin levels (3), often reported after GBP, are believed to contribute to the weight loss effects after this surgery. Our data confirm this rapid effect of GBP on the decrease of ghrelin levels, in spite of a 12 kg weight loss, 1 month after the surgery. However, surprisingly, ghrelin levels increased with further weight loss 1 year after GBP. This is in agreement with another study (38). It is possible that temporary vagal dysfunction results in the initial decrease of ghrelin levels after GBP, with a subsequent increase as a function of weight loss. The late increase one year after GBP is similar to that seen after weight loss by diet and GB (2, 14). The discrepancy between our study and others (2, 14, 24) on ghrelin levels immediately after GBP may be related to the status of energy balance of the patients, the alteration of the vagal nerve, possible Helicobacter pylori-related infections, or the diet composition and frequency of meals of the subjects prior to measurement (12, 16, 38).
Leptin is secreted from the adipocytes in proportion to fat mass; its primary function is to act centrally on the hypothalamus to reduce food intake (39). Leptin levels consistently decrease with various modes of weight loss, including diet (18), GB (17), and GBP (19). Interestingly, our data show that an equivalent 12 kg weight loss decreases leptin levels after GBP but not after GB. In addition, the change in leptin levels after either surgery at both T1 and T2 did not correlate to weight loss (data not shown), as shown in previous studies (17, 24). This finding suggests that changes in leptin after GBP may be partially related to the nature of the surgery, although the role of caloric restriction, changes in body fat composition and the large variability in this small sample size cannot be ruled out. One can speculate that lower leptin levels after GBP may indicate reduced leptin resistance (40) and a lower threshold necessary to induce a reduction in food intake. One year after surgery, leptin levels decreased further after GBP with continued weight loss, but not after GB, despite significant and continued weight loss. One limitation of this study is that body composition and fat mass were not measured. As fat mass is a stronger predictor of leptin levels compared to body weight, it is possible that a minimal reduction in body fat mass after GB is responsible for the lack of change in leptin levels. However, many studies have reported a significant reduction in fat mass one year after GB (41-43), suggesting that leptin levels would also decrease after this surgery. It will be interesting to see if these differential effects persist in a larger group when all subjects are at weight maintenance.
Amylin is co-secreted with insulin from the pancreas. In addition to its effects on glycemic control, administration of amylin analogues reduce food intake (20, 21), and one study showed that circulating amylin levels were reduced after a diet-induced weight loss in a population of obese nondiabetic children (22). We report that fasting and postprandial amylin levels decreased after GBP and did not change after GB, following a 12 kg weight loss. To our knowledge this is the first report on the changes in amylin levels after bariatric surgery. GB patients exhibited a non-significant decrease in fasting and glucose-stimulated amylin levels, implying that weight loss may also contribute to the reduction in amylin levels. It cannot, however, be ruled that the differences in amylin changes between the 2 surgical groups is due to the slight differences in glucose tolerance status. As amylin is co-secreted with insulin, reduced insulin secretion after GBP may account for some of the changes observed in amylin levels. Insulin AUC decreased non-significantly after GBP (data not shown). Interestingly, the pattern of amylin levels in response to an OGTT mirrors previously reported changes observed with insulin, C-peptide and pro-insulin levels after GBP (7).
Although the absence of an effect of GB on stimulated GLP-1 and PYY levels was expected, our data surprisingly showed no change in ghrelin, leptin, and amylin levels after GB, despite significant weight loss. The results in this study suggest that hormonal signals related to food intake do not play a major role in the weight loss effects of GB. However, it is possible that our small study group may not have given us enough power to detect a significant change in these hormone levels after GB. Larger clinical trials will confirm whether or not there are hormonal changes that occur after GB and if these changes contribute to its weight loss effects.
A limitation of our study is that we did not measure food intake. Food intake measurements by food records are often inconsistent (44) and in the laboratory can be particularly challenging after bariatric surgery, due to the size of the pouch and or the level of band adjustment. Hunger and satiety, assessed by visual analogue scale, has been studied after bariatric surgery (8, 45), showing a decrease in hunger and increase in satiety after GBP, but no correlation with gut peptide levels. Unfortunately these measures are not sensitive enough in a small group of patients (45) and do not always correlate with food intake (46). More long term studies (>1 year post surgery) may be able to clearly determine whether the changes in hormone levels do indeed relate to changes in food intake after GBP and GB.
If one postulates that the increase in GLP-1 and PYY and the decrease in leptin and amylin may participate in the decrease food intake after GBP, these changes should predict future weight loss. However, we found no correlations between fasting or stimulated hormone levels, with body weight or weight loss at one year (data not shown); the small number of patients may not provide enough power to observe such correlations. Another beneficial effect of gut peptide changes, specifically GLP-1, may be related to glucose homoeostasis after GBP. A recent study in rats reported blunted glucose tolerance in diabetic Goto-Kakizaki rats treated with exendin-9, a GLP-1 antagonist after duodenal jejunal bypass surgery (47). Whether the changes in gut hormones, which may modify appetite and food intake (32), also affect energy balance and body weight after GBP remains to be demonstrated.
Concluding remarks
Our study confirms that the changes of the gut hormones GLP-1 and PYY are consistent and of large magnitude after GBP. Although it is tempting to assume a role of these gut peptide changes in the sustained weight loss after GBP, studies to date remain purely associative. The use of specific gut hormone receptor agonists and antagonists in studies such as these may provide categorical evidence as to the potential hormonal mechanisms of appetite control, meal regulation, and weight loss after GBP.
Our study demonstrates the importance of well-controlled longitudinal studies to interpret the differential changes of ghrelin after GBP. Our data suggest that the early and late changes in ghrelin levels may occur by different mechanisms.
GB is a powerful tool to achieve weight loss in morbidly obese patients, yet its mechanism of action remains unknown and is likely not mediated by changes in gut peptides. Our data show unexpectedly that leptin and ghrelin did not respond to weight change after GB. Future studies assessing daily meal composition, size, and frequency, and body composition after surgery will help determine mechanisms of weight loss after various types of bariatric surgery.
Acknowledgments
This work was funded by grants from the American Diabetes Association CR-7-05 CR-18, NIH R01-DK67561, GCRC 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05, and NIH T32-DK07559. We would like to thank our volunteer participants, the CRC team, Ping Zhou and Yim Dam for their technical help, and Sara Arias and Nicholas Swerdlow for assistance in manuscript preparation.
Footnotes
Disclosure Statement: This work was funded by grants from the American Diabetes Association CR-7-05 CR-18, NIH R01-DK67561, 1 UL1 RR024156-03, ORC DK-26687, DERC DK-63068-05, Merck Investigator Initiated Studies Program, Amylin Investigator Initiated Studies Program. B.L. received grant support through Merck and Amylin Investigator Initiated Studies Program in 2007. J.T is the recipient of an educational grant from Ethicon Endosurgery/ Johnson & Johnson. M.B., S.M., B.O., J.T., J.J.M., B.B., N.K., A.C., and B.L. have nothing to declare.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–50. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 3.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–61. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 4.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naslund E, Gutniak M, Skogar S, Rossner S, Hellstrom PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–30. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- 6.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 7.Laferrère B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–16. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–40. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 9.Morinigo R, Vidal J, Lacy AM, Delgado S, Casamitjana R, Gomis R. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–5. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 10.Olivan B, Teixeira J, Bose M, et al. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg. 2009;249:948–53. doi: 10.1097/SLA.0b013e3181a6cdb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 12.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 13.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–83. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 15.Morinigo R, Casamitjana R, Moize V, et al. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–16. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 16.Fruhbeck G, Rotellar F, Hernandez-Lizoain JL, et al. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–15. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 17.Shak JR, Roper J, Perez-Perez GI, et al. The Effect of Laparoscopic Gastric Banding Surgery on Plasma Levels of Appetite-Control, Insulinotropic, and Digestive Hormones. Obes Surg. 2008 doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geldszus R, Mayr B, Horn R, Geisthovel F, von zur Muhlen A, Brabant G. Serum leptin and weight reduction in female obesity. Eur J Endocrinol. 1996;135:659–62. doi: 10.1530/eje.0.1350659. [DOI] [PubMed] [Google Scholar]
- 19.Geloneze B, Tambascia MA, Pareja JC, Repetto EM, Magna LA, Pereira SG. Serum leptin levels after bariatric surgery across a range of glucose tolerance from normal to diabetes. Obes Surg. 2001;11:693–8. doi: 10.1381/09608920160558623. [DOI] [PubMed] [Google Scholar]
- 20.Smith SR, Blundell JE, Burns C, et al. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: a 6-wk translational research study. Am J Physiol Endocrinol Metab. 2007;293:E620–7. doi: 10.1152/ajpendo.00217.2007. [DOI] [PubMed] [Google Scholar]
- 21.Chapman I, Parker B, Doran S, et al. Low-dose pramlintide reduced food intake and meal duration in healthy, normal-weight subjects. Obesity (Silver Spring) 2007;15:1179–86. doi: 10.1038/oby.2007.626. [DOI] [PubMed] [Google Scholar]
- 22.Reinehr T, de Sousa G, Niklowitz P, Roth CL. Amylin and its relation to insulin and lipids in obese children before and after weight loss. Obesity (Silver Spring) 2007;15:2006–11. doi: 10.1038/oby.2007.239. [DOI] [PubMed] [Google Scholar]
- 23.Laferrère B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angrisani L, Lorenzo M, Borrelli V. Laparoscopic adjustable gastric banding versus Roux-en-Y gastric bypass: 5-year results of a prospective randomized trial. Surg Obes Relat Dis. 2007;3:127–32. doi: 10.1016/j.soard.2006.12.005. discussion 32-3. [DOI] [PubMed] [Google Scholar]
- 26.Tice JA, Karliner L, Walsh J, Petersen AJ, Feldman MD. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am J Med. 2008;121:885–93. doi: 10.1016/j.amjmed.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–40. doi: 10.1381/096089206778026316. [DOI] [PubMed] [Google Scholar]
- 28.de Carvalho CP, Marin DM, de Souza AL, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19:313–20. doi: 10.1007/s11695-008-9678-5. [DOI] [PubMed] [Google Scholar]
- 29.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 30.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–5. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 33.Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–9. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 34.Reidelberger RD, Haver AC, Chelikani PK, Buescher JL. Effects of different intermittent peptide YY (3-36) dosing strategies on food intake, body weight, and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R449–58. doi: 10.1152/ajpregu.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–93. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 36.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 37.Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005;29:1130–6. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 38.Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–10. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 39.Pelleymounter MA, Cullen MJ, Baker MB, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 40.Jequier E, Tappy L. Regulation of body weight in humans. Physiol Rev. 1999;79:451–80. doi: 10.1152/physrev.1999.79.2.451. [DOI] [PubMed] [Google Scholar]
- 41.Pontiroli AE, Pizzocri P, Librenti MC, et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. J Clin Endocrinol Metab. 2002;87:3555–61. doi: 10.1210/jcem.87.8.8708. [DOI] [PubMed] [Google Scholar]
- 42.Savastano S, Angrisani L, Di Somma C, et al. Relationship Between Growth Hormone/Insulin-Like Growth Factor-1 Axis Integrity and Voluntary Weight Loss After Gastric Banding Surgery for Severe Obesity. Obes Surg. 2009 doi: 10.1007/s11695-009-9926-3. [DOI] [PubMed] [Google Scholar]
- 43.Heath ML, Kow L, Slavotinek JP, Valentine R, Toouli J, Thompson CH. Abdominal adiposity and liver fat content 3 and 12 months after gastric banding surgery. Metabolism. 2009;58:753–8. doi: 10.1016/j.metabol.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Todd KS, Hudes M, Calloway DH. Food intake measurement: problems and approaches. Am J Clin Nutr. 1983;37:139–46. doi: 10.1093/ajcn/37.1.139. [DOI] [PubMed] [Google Scholar]
- 45.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 46.Pi-Sunyer X, Kissileff HR, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in obese men. Physiol Behav. 1982;29:627–30. doi: 10.1016/0031-9384(82)90230-x. [DOI] [PubMed] [Google Scholar]
- 47.Kindel TL, Yoder SM, Seeley RJ, D'Alessio DA, Tso P. Duodenal-Jejunal Exclusion Improves Glucose Tolerance in the Diabetic, Goto-Kakizaki Rat by a GLP-1 Receptor-Mediated Mechanism. J Gastrointest Surg. 2009 doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]


