Abstract
BACKGROUND
Obesity is a risk factor for prostate cancer development, but the underlying mechanism is unknown. The present study tested the hypothesis that stromal cells of the adipose tissue might be recruited by cancer cells to help tumor growth.
METHODS
PC3 prostate cancer cells were transplanted into the subcutaneous space of the right flank of athymic mice. One week later, adipose tissue-derived stromal or stem cells (ADSC) or phosphate-buffered saline (PBS, as control) was transplanted similarly to the left flank. Tumor size was monitored for the next 34 days; afterwards, the mice were sacrificed and their tumors harvested for histological examination. The ability of PC3 cells to attract ADSC was tested by migration assay. The involvement of the CXCL12/CXCR4 axis was tested by migration assay in the presence of a specific inhibitor AMD3100.
RESULTS
Throughout the entire course, the average size of PC3 tumors in ADSC-treated mice was larger than in PBS-treated mice. ADSC were identified inside the tumors of ADSC-treated mice; CXCR4 expression was also detected. Migration assay indicated the involvement of the CXCL12/CXCR4 axis in the migration of ADSC toward PC3 cells. Capillary density was twice as high in the tumors of ADSC-treated mice than in the tumors of PBS-treated mice. VEGF expression was similar but FGF2 expression was significantly higher in tumors of ADSC-treated mice than in the tumors of PBS-tread mice.
CONCLUSION
Prostate cancer cells recruited ADSC by the CXCL12/CXCR4 axis. ADSC helps tumor growth by increasing tumor vascularity, and which was mediated by FGF2.
Keywords: prostate cancer, adipose tissue-derived stem cells, CXCL12, SDF-1, CXCR4, FGF2
INTRODUCTION
Recent epidemiologic studies have shown that obesity is associated with both higher incidence of aggressive prostate cancer and larger prostate tumor size [1–3]. Since obesity is especially prevalent among cancer patients, a better understanding of how it contributes to tumor progression is needed for effective cancer management. To this end, recent studies have shown that stromal cells in the adipose tissue may be a contributing factor [4–6]. Specifically, a population of cells commonly referred to as adipose tissue-derived stromal or stem cells (ADSC) may be recruited by cancer cells, and once arrived in the tumor site, these cells participate in the expansion of pre-existing vasculature and/or the formation of new blood vessels. These newly formed blood vessels then feed the growth of cancer cells.
The above-described theory regarding the connection between ADSC and cancer was based on three lines of evidence. First, earlier studies have shown that bone marrow stromal/stem cells (BMSC), which are normally present in the peripheral circulation at low frequency, migrate to tumor sites through chemotaxis and can aid tumor growth by providing various growth factors [7–9]. Second, more recent studies have shown that ADSC and BMSC share many biological properties, including the ability to respond to chemotactic factors and to migrate to sites of injuries, inflammation, and/or tumor [10–15]. Third, by definition ADSC are cells in the stromal vascular fraction (SVF) of homogenized adipose tissue. They are thus closely associated with the vasculature and may be de facto vascular progenitor/stem cells [16–18]. Thus, when recruited to tumor sites, ADSC expectedly have the ability to form new blood vessels by themselves or to secrete angiogenic factors that promote the expansion of the pre-existing tumor vasculature.
In the present study, we found that ADSC could migrate to prostate tumor and promote its growth. We also found that ADSC treatment correlated with increased tumor vascularization, and which was possibly mediated by FGF2 but not VEGF.
MATERIALS AND METHODS
Isolation, Culture and Labeling of ADSC
Adipose tissue samples were obtained from patients during routine abdominoplasty following informed patient consent and according to the guidelines set by our Institution’s Committee on Human Research. The procedure of ADSC isolation and culture has been described previously [16]. Briefly, the adipose tissue was rinsed with phosphate-buffered saline (PBS) containing 1% penicillin and streptomycin, minced into small pieces, and then incubated in a solution containing 0.075% collagenase type IA (Sigma–Aldrich, St. Louis, MO) for 1 hr at 37°C with vigorous shake. The top lipid layer was removed and the remaining liquid portion was centrifuged at 220g for 10 min at room temperature. The pellet was treated with 160 mM NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in DMEM supplemented with 10% fetal bovine serum (FBS), filtered through a 40-μm cell strainer (BD Biosciences, Bedford, MA), and plated at a density of 1 × 106 cells in a 10-cm dish. After reaching 80% confluence, the cells were harvested and stored in liquid nitrogen at a density of 5 × 105 cells/ml of freezing media (DMEM, 20% FBS, and 10% DMSO). They were recovered and expanded as needed. For tracking purpose, they were labeled with thymidine analog 5-ethynyl-2-deoxyuridine (EdU; Invitrogen, Carlsbad, CA) at 10 μM for 12 hr, as described previously [19].
Transplantation of Tumor Cells and ADSC
Twenty 8-week-old male nu/nu athymic mice (Simonsen Laboratories, Gilroy, CA) were used in this experiment. All animal care, treatments, and procedures were approved by the Committee on Animal Research at our institution. They were housed in microisolator cages under sterile conditions and observed for at least 1 week to ensure proper health prior to the initiation of the study. Lighting, temperature, and humidity were centrally controlled and recorded daily. Each mouse received in its right flank a subcutaneous injection of 2 × 106 human prostate cancer PC3 cells (American Type Culture Collection, Manassas, VA) suspended in 0.2 ml of 50% growth factor reduced Matrigel (Becton Dickinson, Mountain View, CA) in PBS. They were then randomly divided into a control group (n = 10) and an ADSC transplantation group (n = 10). One week after the injection of PC3, each mouse in the control group received an injection of 200 μl PBS in the subcutaneous space of the left flank, whereas each mouse in the ADSC treatment group received an injection of 1 × 106 of EdU-labeled ADSC (200 μl PBS) in the subcutaneous of the left flank. Starting 7 days and continuing until 41 days after injection, tumor size was measured every 3 days with an electronic caliper and calculated as length (mm) × width2 (mm2) × 0.523. After the final measurement, the mice were euthanized by CO2 inhalation and their tumors harvested for histological analysis.
Immunofluorescence Staining
Tissue samples were fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 hr followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetic USA, Torrance, CA) and stored at −70°C until use. Fixed frozen tissue specimens were cut at 10 μm, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min. The slides were then placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min, and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated overnight at 4°C with primary antibodies followed by secondary antibody conjugated with FITC or Texas Red (Vector Labs, Burlingame, CA). Control tissue sections were similarly prepared except no primary antibody was added. After rinses with PBS, the slides were incubated with freshly made Click-iT reaction cocktail for 30 min at room temperature without light followed by staining with 4′, 6-diamidino-2-phenylindole (DAPI, for nuclear staining, Sigma–Aldrich). Stained tissues were examined by fluorescence microscopy. For image analysis, five randomly selected fields per tissue were photographed and recorded using Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY). Primary antibodies used in this study were anti-CXCR4, anti-CD31, anti-VEGF (Abcam, Cambridge, MA), and anti-FGF2 (BD Biosciences, San Diego, CA).
Cell Migration Assay
The migration capability of ADSC to PC3 cells was assessed in triplicate using 24-well BioCoat with an 8-mm pore size (BD Biosciences, Bedford, MA). Briefly, 1 ml of the conditioned medium of PC3 cells or human corporal smooth muscle cells (CSMC, as control) at 60 ~ 80% confluence was added to the lower chamber, and 1 × 105 ADSC in 500 μl of DMEM were added to the upper chamber. After incubation for 24 hr at 37°C in a humidified atmosphere with 5% CO2, migrated cells, which adhered to the lower surface of a glass cover slide, were stained with Calcin (Invitrogen) for 10 min and counted in 8 high-power microscopic fields. For blocking of CXCR4, AMD3100 (Sigma–Aldrich) at different concentration was added to the culture medium of ADSC for 1 hr prior to the initiation of the migration assay.
Statistical Analysis
Data were analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA). For comparison between two groups, t-test was used. For comparison among multiple groups, one-way analysis of variance followed by the Tukey–Kramer post hoc test was used. Statistical significance was set at P <0.05.
RESULTS
ADSC Promote Growth of Grafted Prostate Cancer Cells
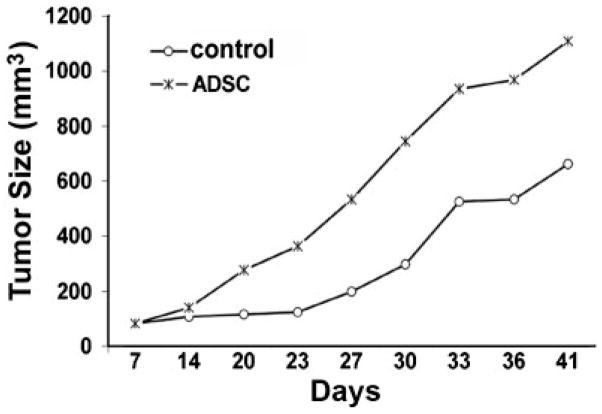
To investigate the effect of ADSC on prostate tumor growth, athymic mice were subcutaneously injected with PC3 prostate cancer cells into the right flank, followed 1 week later by the injection of ADSC or PBS (control) into the left flank. At the time of ADSC or PBS injection, a small tumor was observed at the site of PC3 injection in every mouse. These tumors then grew at variable rates during the course of the next 34 days, with those in the ADSC-treated group growing faster on average than those in the PBS-treated group (Fig. 1).
Fig. 1.
Athymic mice (n = 20) were subcutaneously injected with PC3 prostate cancer cells into the right flank, followed 1 week later by the injection of ADSC (n = 10) or PBS (control, n = 10) into the left flank. Tumor growth was then monitored for 34 days. The average calculated tumor sizes are shown for the indicated time points.
ADSC Migrate to Grafted Prostate Tumor
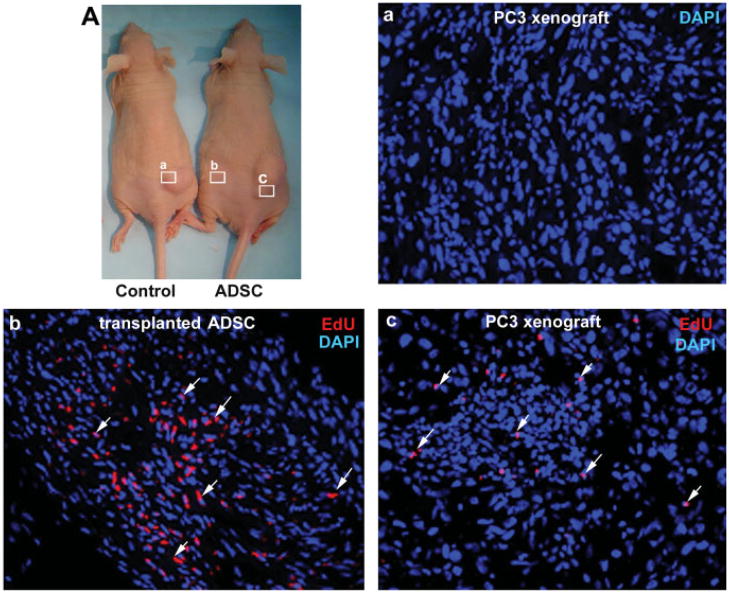
We have recently shown that a novel thymidine analog, EdU, could be used to track transplanted ADSC [19]. By using this method, we found that many of the transplanted ADSC still remained in the injection site 34 days afterward (Fig. 2b). We also found that some transplanted ADSC have migrated to the PC3 tumor, as EdU-labeled cells were identifiable in the periphery and the middle of the tumor (Fig. 2c). In addition, we found that the migration appeared to be tumor specific, as tissues such as the liver, spleen, lung, and prostate were devoid of any EdU-labeled cells (data not shown).
Fig. 2.
A: After the last size measurement, tumors (a,c) from control and ADSC-treated mice, as well as the subcutaneous tissue of the ADSC injection site (b), were excised for histological examination. The tumors and subcutaneous tissues were stained with DAPI (blue) and EdU (red) for nuclear visualization and tracking of ADSC, respectively. a: Tumors from control mice had no ADSC. b: Subcutaneous tissue from ADSC-treated mice had many ADSC (arrows). c:Tumors from ADSC-treated mice also had ADSC (arrows). Original magnification is200×. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
CXCL12/CXCR4 Mediates Migration of ADSC Toward Prostate Cancer Cells
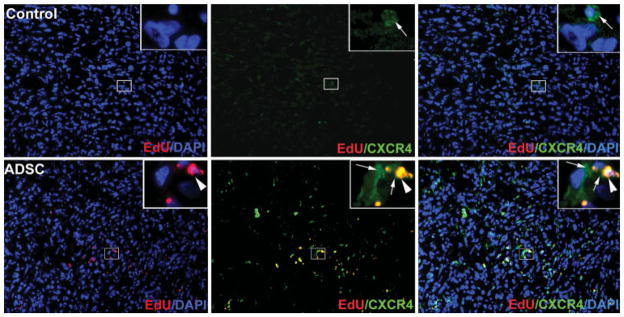
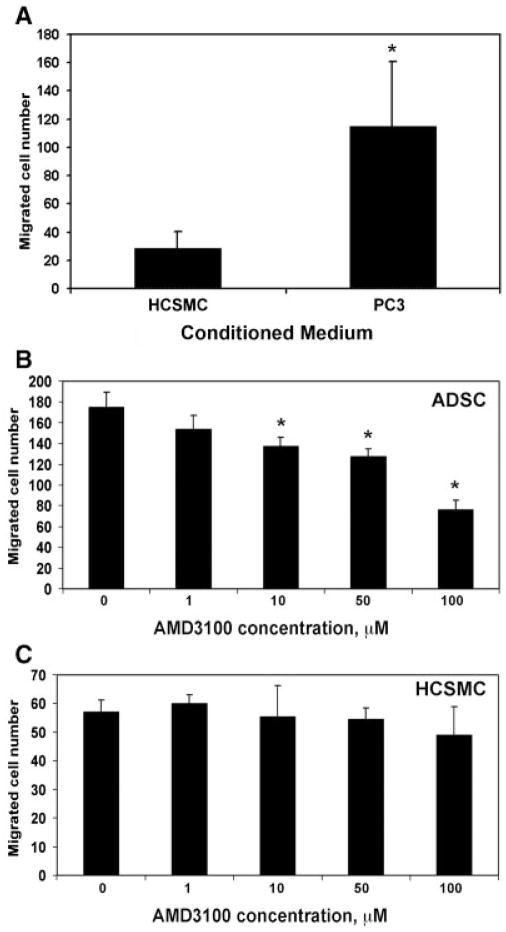
We considered the possibility that ADSC migration to PC3 tumor was mediated by homing factor SDF-1 (CXCL12) and its receptor CXCR4. To investigate this possibility, we first examined CXCR4 expression in the tumors. The results show that, while tumors from PBS-treated mice had a few cells that expressed low levels of CXCR4, tumors from ADSC-treated mice had numerous cells that expressed CXCR4 at high levels (Fig. 3). We then tested whether PC3 cells could attract ADSC through the secretion of chemoattractant factors. The results (Fig. 4A) show that the number of ADSC migrated to PC3-conditioned medium was more than fourfold higher than that migrated to a control medium (conditioned by human cavernous smooth muscle cells; HCSMC, P <0.01). Finally, we tested whether the migration of ADSC to PC3-conditioned medium was mediated by the CXCL12/CXCR4 system. By using AMD3100, a CXCR4-specific inhibitor, we found that the migration of ADSC was dose-dependently inhibited but the migration of HCSMC was not (Fig. 4B,C, P <0.05). Thus, both the in vivo and in vitro results support the notion that migration of ADSC to the PC3 tumor was mediated by the CXCL12/CXCR4 axis.
Fig. 3.
Tumors from control (upper panels) and ADSC-treated (lower panels) mice were stained with DAPI (blue), EdU (red), and CXCR4 (green). The insert (original magnification is 1,000×) shown at the upper right corner of each graph (original magnification is 200×) is the enlargement of the boxed area near the center of the graph. Arrowhead points to a representative nucleus, which was co-stained with EdU and CXCR4. Arrows point to cytoplasmic or membrane expression CXCR4. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 4.
A: The conditioned medium of PC3 cells or human cavernous smooth muscle cells (HCSMC) was assessed for ability to attract ADSC. The number of attracted ADSC is shown in the Y-axis. * indicates P <0.05 when compared to HCSMC control. B: The conditioned medium of PC3 cells was assessed for ability to attract ADSC, which was treated with AMD3100 at the indicated concentrations for1hr prior. * indicates P <0.05 when compared to 0 μM control. C:The conditioned medium ofPC3 cells was assessed for ability to attract HCSMC, which was treated with AMD3100 at the indicated concentrations for1hrprior.
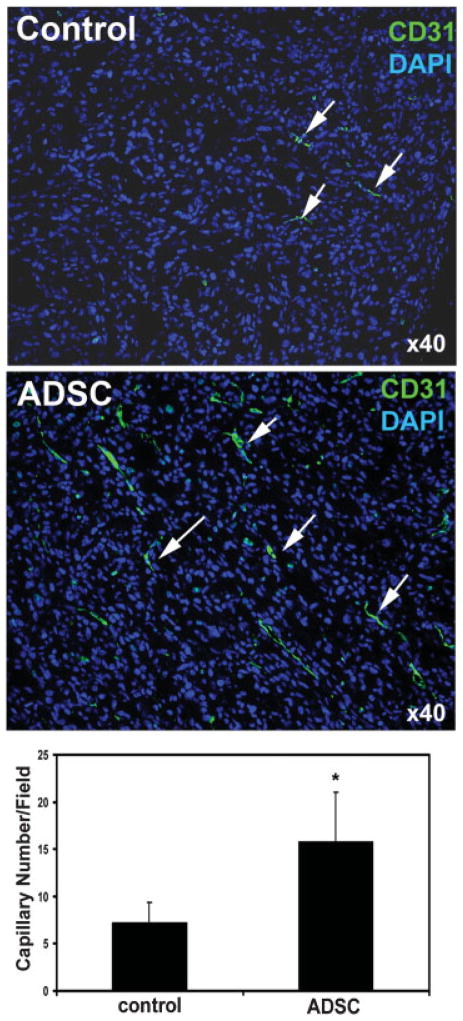
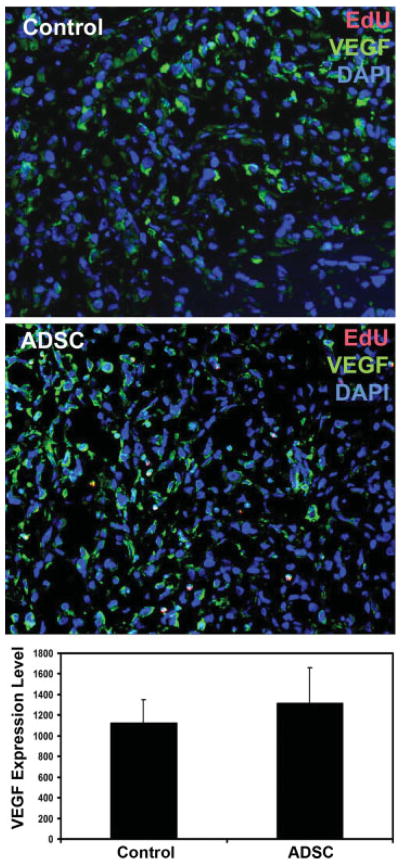
ADSC Increase Tumor Vascularity
The migrated ADSC might assist the growth of PC3 tumor by increasing tumor vascularity. To test this hypothesis, we compared the degree of vascularization—by immunofluorescent staining with endothelial-specific CD31 antibody—in the PC3 tumors of ADSC and PBS-treated mice. The results show that tumors from ADSC-treated mice had approximately twice as much CD31 staining as tumors from PBS-treated mice (Fig. 5, P <0.01). We then investigated whether vasculotrophic factors could possibly be involved in promoting vascularization in the tumor of ADSC-treated mice. The results show that VEGF was widely expressed in the PC3 tumors in both ADSC and PBS-treated mice with no significant differences (Fig. 6, P >0.05). On the other hand, FGF2 expression was vastly different between ADSC and PBS-treated mice (Fig. 7, P <0.01). Also of note is that, while VEGF expression was identifiable in a few EdU-labeled cells (migrated ADSC), colocalization of FGF expression and EdU could not be found (Fig. 7). This latter result thus suggests that ADSC promoted tumor vascularization (and therefore tumor growth) by upregulating FGF2 expression in PC3 cells.
Fig. 5.
Tumors from control and ADSC-treated mice were stained with DAPI (blue) and CD31 (green). The number of capillaries per field was determined by counting each contiguous green stain as one capillary (arrows). The average capillary numbers per field in all 10 tumors of control mice and all 10 tumors of ADSC-treated mice are shown in the bar chart. * indicates P <0.05 when compared to control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 6.
Tumors from ADSC-treated mice were stained with DAPI (blue), EdU (red), and VEGF (green). VEGF expression level was determined by counting the pixel number of all green stains per field at 200× magnification. The average pixel numbers per field in all 10 tumors of control mice and all10 tumors of ADSC-treated mice are shown in the bar chart. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 7.
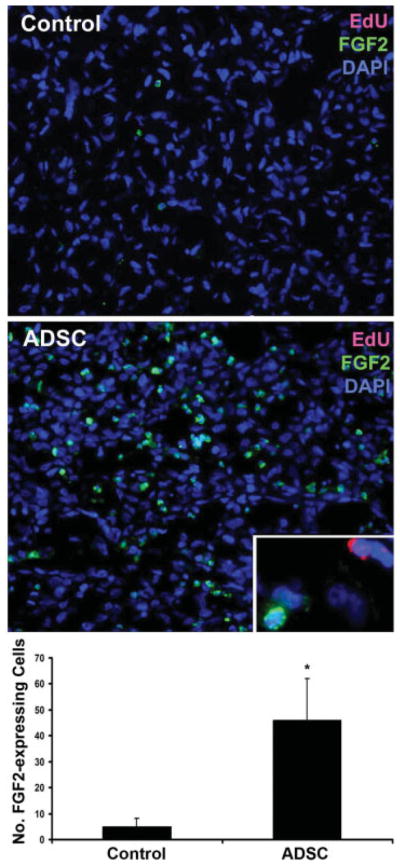
Tumors from ADSC-treated mice were stained with DAPI (blue), EdU (red), and FGF2 (green). A representative EdU+ cell, which was infrequently seen in strongly FGF2+ areas, is shown in the insert (original magnification is 1,000×). The average numbers of FGF2-expressing cells per field at 200× in all10 tumors of control mice and all 10 tumors of ADSC-treated mice are shown in the bar chart. * indicates P <0.05 when compared to control. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
Obesity has been linked to a variety of diseases, including many kinds of cancer. In regard to prostate cancer, two recent studies that analyzed large sets of data involving 38,380 and 2,302 patients respectively have also affirmed an association with obesity. However, how obesity can contribute to prostate cancer progression remains unclear. In the present study, we tested the hypothesis that mesenchymal stem cells (MSC) that normally reside in the adipose tissue might play a role. Specifically, we first investigated whether transplantation of ADSC into a prostate tumor-bearing murine host had a positive effect on tumor growth. The results show that throughout the entire course of 34 days of monitoring, the average tumor size was always larger in the ADSC-treated mice than in the PBS-treated mice.
To explore how ADSC could promote tumor growth, we conducted histological examinations and found that all PC3 tumors from ADSC-treated mice invariably harbored ADSC. We then found that while tumors from PBS-treated mice were essentially devoid of CXCR4 expression, tumors from ADSC-treated mice had numerous cells that expressed CXCR4 at high levels. Noteworthy is that, while all ADSC (EdU+) were CXCR4+, many CXCR+ cells were EdU−. At present, the identity of these CXCR4+ EdU− cells is unknown, but possibilities include (i) ADSC that have lost the EdU label; (ii) endogenous stem cells that were mobilized by ADSC; and (iii) PC3 cells that were activated by ADSC. Also of note is that while CXCR4 is normally a cell surface receptor, its nuclear localization, as seen in Figure 3, has been reported previously [20,21]. In addition, CXCR4 expression in ADSC [22–24] and CXCL12 expression in PC3 cells [25] have been reported previously. Finally, based on our migration assay and the specific inhibitory effect of AMD3100 on CXCL12 binding to CXCR4, we conclude that ADSC migration to PC3 tumors was mediated by the CXCL12/CXCR4 axis.
After migrating to the tumor, ADSC might help tumor growth by increasing its vascularity. Indeed, our results show that tumors from ADSC-treated mice had twice as much CD31 staining as tumors from PBS-treated mice. Furthermore, we found that, while VEGF was expressed at similar levels in the tumors of PBS and ADSC-treated mice, FGF2 was expressed at a significantly higher level in the tumors of ADSC-treated mice. However, both the VEGF and FGF2-expressing cells were largely EdU−, suggesting that they were PC3 cells, or ADSC that have lost the EdU label, or certain host cells that were recruited by the ADSC-activated tumor. In any event, ADSC-induced upregulation of FGF2 was probably responsible for the increased vascularity and tumor growth. Thus, the present study provides cellular and molecular evidence for the association between obesity and prostate tumor development. Specifically, we show that adipose stromal cells could migrate to prostate tumor, increased tumor vascularity, and thereby increased tumor growth. However, this conclusion does not exclude the possibility that other types of stromal cells (e.g., BMSC) can also aid tumor growth. Rather, we consider the possibility that the increased number of stromal cells in obese patients due to enlarged adipose tissue may contribute to tumor growth.
Acknowledgments
Arthur Rock Foundation; Grant sponsor: National Institutes of Health; Grant number: DK045370.
This work was supported by grants from the Arthur Rock Foundation and the National Institutes of Health (DK045370).
References
- 1.Rodriguez C, Freedland SJ, Deka A, Jacobs EJ, McCullough ML, Patel AV, Thun MJ, Calle EE. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(1):63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 2.Park JH, Cho BL, Kwon HT, Lee CM, Han HJ. Effect of body mass index and waist circumference on prostate specific antigen and prostate volume in a generally healthy Korean population. J Urol. 2009;182(1):106–110. doi: 10.1016/j.juro.2009.02.130. discussion 110–101. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Banez LL, Sun LL, Fitzsimons NJ, Moul JW. Obese men have higher-grade and larger tumors: An analysis of the duke prostate center database. Prostate Cancer Prostatic Dis. 2009;12(3):259–263. doi: 10.1038/pcan.2009.11. [DOI] [PubMed] [Google Scholar]
- 4.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, Liu W, Arlinghaus RB, Alt EU. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30(4):589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 5.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69(12):5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazennec G, Jorgensen C. Concise review: Adult multipotent stromal cells and cancer: Risk or benefit? Stem Cells. 2008;26(6):1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra PJ, Merlino G. A traitor in our midst: Mesenchymal stem cells contribute to tumor progression and metastasis. Future Oncol. 2008;4(6):745–749. doi: 10.2217/14796694.4.6.745. [DOI] [PubMed] [Google Scholar]
- 9.Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Mesenchymal stem cells: Flip side of the coin. Cancer Res. 2009;69(4):1255–1258. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]
- 10.Kubis N, Tomita Y, Tran-Dinh A, Planat-Benard V, Andre M, Karaszewski B, Waeckel L, Penicaud L, Silvestre JS, Casteilla L, Seylaz J, Pinard E. Vascular fate of adipose tissue-derived adult stromal cells in the ischemic murine brain: A combined imaging-histological study. Neuroimage. 2007;34(1):1–11. doi: 10.1016/j.neuroimage.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Kim JM, Lee ST, Chu K, Jung KH, Song EC, Kim SJ, Sinn DI, Kim JH, Park DK, Kang KM, Hyung Hong N, Park HK, Won CH, Kim KH, Kim M, Kun Lee S, Roh JK. Systemic transplantation of human adipose stem cells attenuated cerebral inflammation and degeneration in a hemorrhagic stroke model. Brain Res. 2007;1183:43–50. doi: 10.1016/j.brainres.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Kim U, Shin DG, Park JS, Kim YJ, Park SI, Moon YM, Jeong KS. Homing of adipose-derived stem cells to radiofrequency catheter ablated canine atrium and differentiation into cardio-myocyte-like cells. Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 13.Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galie M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27(10):2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 14.Lamfers M, Idema S, van Milligen F, Schouten T, van der Valk P, Vandertop P, Dirven C, Noske D. Homing properties of adipose-derived stem cells to intracerebral glioma and the effects of adenovirus infection. Cancer Lett. 2009;274(1):78–87. doi: 10.1016/j.canlet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Lee DH, Ahn Y, Kim SU, Wang KC, Cho BK, Phi JH, Park IH, Black PM, Carroll RS, Lee J, Kim SK. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15(15):4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 16.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17(6):1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Silva Meirelles L, Sand TT, Harman RJ, Lennon DP, Caplan AI. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15(2):221–229. doi: 10.1089/ten.tea.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CS, Xin ZC, Deng CH, Lin G, Ning H, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. doi: 10.14670/HH-25.807. in press. [DOI] [PubMed] [Google Scholar]
- 19.Lin G, Huang YC, Shindel AW, Banie L, Wang G, Lue TF, Lin CS. Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;7:864–873. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Speetjens FM, Liefers GJ, Korbee CJ, Mesker WE, van de Velde CJ, van Vlierberghe RL, Morreau H, Tollenaar RA, Kuppen PJ. Nuclear localization of CXCR4 determines prognosis for colorectal cancer patients. Cancer Microenviron. 2009;2(1):1–7. doi: 10.1007/s12307-008-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang LH, Liu Q, Xu B, Chen W, Yang Q, Wang ZX, Sun YH. Identification of nuclear localization sequence of CXCR4 in renal cell carcinoma by constructing expression plasmids of different deletants. Plasmid. 2010;63(1):68–72. doi: 10.1016/j.plasmid.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Cho HH, Kyoung KM, Seo MJ, Kim YJ, Bae YC, Jung JS. Overexpression of CXCR4 increases migration and proliferation of human adipose tissue stromal cells. Stem Cells Dev. 2006;15(6):853–864. doi: 10.1089/scd.2006.15.853. [DOI] [PubMed] [Google Scholar]
- 23.Sengenes C, Miranville A, Maumus M, de Barros S, Busse R, Bouloumie A. Chemotaxis and differentiation of human adipose tissue CD34+/CD31− progenitor cells: Role of stromal derived factor-1 released by adipose tissue capillary endothelial cells. Stem Cells. 2007;25(9):2269–2276. doi: 10.1634/stemcells.2007-0180. [DOI] [PubMed] [Google Scholar]
- 24.Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Heydarkhan S, Xu Y, Zuk PA, MacLellan WR, Beygui RE. Human adipose stem cells: A potential cell source for cardiovascular tissue engineering. Cells Tissues Organs. 2008;187(4):263–274. doi: 10.1159/000113407. [DOI] [PubMed] [Google Scholar]
- 25.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–473. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]