Abstract
Effective stroke therapies require recanalization of occluded cerebral blood vessels. However, reperfusion can cause neurovascular injury, leading to cerebral edema, brain hemorrhage, and neuronal death by apoptosis/necrosis. These complications, which result from excess production of reactive oxygen species in mitochondria, significantly limit the benefits of stroke therapies. We have developed a focal stroke model using mice deficient in mitochondrial manganese-superoxide dismutase (SOD2−/+) to investigate neurovascular endothelial damage that occurs during reperfusion. Following focal stroke and reperfusion, SOD2−/+ mice had delayed blood-brain barrier breakdown, associated with activation of matrix metalloproteinase and high brain hemorrhage rates, whereas a decrease in apoptosis and hemorrhage was observed in SOD2 overexpressors. Thus, induction and activation of SOD2 is a novel strategy for neurovascular protection after ischemia/reperfusion. Our recent study identified the signal transducer and activator of transcription 3 (STAT3) as a transcription factor of the mouse SOD2 gene. During reperfusion, activation of STAT3 and its recruitment into the SOD2 gene were blocked, resulting in increased oxidative stress and neuronal apoptosis. In contrast, pharmacological activation of STAT3 induced SOD2 expression, which limits ischemic neuronal death. Our studies point to antioxidant-based neurovascular protective strategies as potential treatments to expand the therapeutic window of currently approved therapies.
Keywords: Cerebral ischemia, Oxidative stress, Reactive oxygen species, Mitochondria, Mn-SOD, STAT3, NADPH oxidase, CK2, Neuroprotective signaling
Introduction
Nearly 80% of strokes are caused by occlusion of a cerebral artery by a thrombus. Thus, effective stroke therapies require recanalization of occluded cerebral blood vessels. Reperfusion strategies have proven to be the most effective therapies for stroke treatment. However, early reperfusion of ischemic brain tissue can result in harmful consequences, including the breakdown of the blood-brain barrier, which can lead to cerebral edema and/or brain hemorrhage, as well as neurovascular injury and neuronal death. During cerebral ischemia, cerebral blood flow is reduced by occlusion of vessels in brain tissues that are supplied with oxygen [1]. Reperfusion after ischemia causes oxidative stress, which is a result of overproduction of reactive oxygen species (ROS) in mitochondria. This overproduction significantly limits the benefits of stroke therapies, and these ROS trigger many cellular and molecular events, including protein oxidation/nitrosylation/nitration, lipid peroxidation, and DNA damage, which can induce cell death following cerebral ischemia and reperfusion [2].
Manganese-containing superoxide dismutase (Mn-SOD or SOD2), a mitochondrial antioxidant enzyme for superoxide, is a primary cellular defense enzyme involved in protecting cells from oxidative stress [3]. Homozygous mutant mice that are deficient in SOD2 die within the first 10 days of life with dilated cardiomyopathy, accumulation of lipid in liver and skeletal muscle, and metabolic acidosis [4]. We have shown that superoxide radicals and infarction volumes increase after cerebral ischemia and reperfusion in Mn-SOD–deficient mice (SOD2−/+) [5]. SOD2 knockout (KO) mice are more susceptible to hemorrhage, but increased hemorrhage rates are absent in SOD2 transgenic (TG) mice, which have reduced vascular endothelial cell death [6]. Our recent study demonstrated that Mn-SOD is a direct target of signal transducer and activator of transcription 3 (STAT3) in ischemia reperfusion-induced neuronal cell death and that the loss of STAT3 activity reduced Mn-SOD expression after cerebral ischemia [7]. STAT3 is a transcriptional factor and an intracellular signal transducer that is activated by cytokines, growth factors, and receptor- or nonreceptor-tyrosine kinases [8, 9]. Tyrosine phosphorylation of STAT3 at Y705 is required for STAT3 activity. After phosphorylation at Y705, dimerization, and nuclear translocation, STAT3 binds to the promoters of target genes and finally induces gene expression [10]. In this review, we will discuss antioxidant-based neuroprotective strategies as potential treatments that may expand the molecular therapeutic window targeting STAT3 activity.
Cellular and Molecular Events Following Cerebral Ischemia
In mitochondria of brain tissue, approximately 2–5% of electron flow produces superoxide anion radicals (O2•−) and hydrogen peroxide (H2O2) [11]. These ROS are scavenged by SOD, glutathione peroxidase, and catalase. Other antioxidants, such as glutathione, ascorbic acid, and α-tocopherol, are also involved in the detoxification of free radicals [12].
SOD processes a dismutation reaction of O2•− and produces H2O2, which is then detoxified by catalase or glutathione peroxidase, and finally changed to water and oxygen (O2•− + O2•− + 2H+ → H2O2 + O2) [1]. H2O2 breaks down easily if transition metal ions are present, producing hydroxyl radical (−OH) through the superoxide-driven Fenton reaction (H2O2+Fe2+ → ·HO + Fe3+ + −OH) [13]. This hydroxyl radical is a very strong oxidizer and can attack various kinds of organic structures, such as phospholipids and nucleic acids [14]. Peroxynitrite is formed by the nonenzymatic reaction with nitric oxide and O2•− at a rate constantly close to diffusion [15]. It is also a potent oxidant/nitrating agent and can inhibit the function of SOD [15], as well as the mitochondrial respiratory chain [16], thus leading to increased O2•− and peroxynitrite formation [15]. Superoxide can lead to the inactivation of a variety of enzymes [13] and is involved in vascular dysfunction, cerebral ischemic injury [17-19], vasospasm after subarachnoid hemorrhage [20, 21], atherosclerosis [22], and meningitis [23].
During neurological disorders like cerebral ischemia and reperfusion, cerebral blood flow is reduced by occlusion of vessels, causing a reduction in the oxygen supply in the affected region of the brain. Reperfusion after ischemia causes oxidative stress in neurovascular units and various brain regions. It also causes overproduction of ROS in mitochondria, and these overproduced ROS consume antioxidants, inactivate the detoxification system, disturb the endogenous antioxidative defense system, and finally, could lead to a dramatic rise in intracellular ROS.
Numerous studies of cellular macromolecules have demonstrated that ROS are directly involved in oxidative damage such as lipid peroxidation, protein oxidation, protein nitrosylation/nitration, and nucleic acid damage in ischemic tissues, leading to cell death [3, 24]. Within the first several minutes of cerebral ischemia, reduced cerebral blood flow induces a series of biochemical events that cause metabolic dysfunction such as reduction in ATP formation, failure of the Na-K-ATPase pump, a decrease in tissue pH, membrane depolarization, Ca2+ influx through activation of voltage-operated calcium channels, and excessive release of excitatory amino acids (glutamate) [25]. Activation of glutamate receptors leads to a further increase in intracellular calcium, activation of intracellular enzymes such as phospholipase, nitric oxide synthase, protease, endonuclease, and oxidase during cerebral ischemia within several hours [25]. Recent studies have demonstrated the production of superoxide radicals by N-methyl-d-aspartate (NMDA) receptor-mediated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation [26]. These events amplify ROS production, mitochondrial dysfunction, proapoptotic protein activation, cytochrome c release, and caspase activation, and these cascades finally result in apoptotic cell death within several days/weeks after cerebral ischemia. Production of ROS after ischemia also affects DNA damage and this damage induces energy depletion of cells. Membrane damage caused by protease activation, protein malfolding by Ca2+ influx, and DNA damage results in necrotic cell death after cerebral ischemia. Finally, within several days/weeks after cerebral ischemia, brain damage such as blood-brain barrier disruption and intracerebral hemorrhage occurs.
Neuroprotective Strategies and Molecular Targets
Targeting Mn-SOD Activity and STAT3 Signaling
Superoxide dismutases are specific antioxidant enzymes that detoxify O2•− and produce H2O2. Three SODs have been identified, copper/zinc SOD (SOD1), SOD2, and extracellular SOD (SOD3) [1]. SOD1 is a copper- and zinc-containing homodimer that is distributed exclusively in intracellular cytoplasmic spaces [27]. SOD2 is a manganese-containing enzyme that localizes to the mitochondria after encoding from nuclei [28]. SOD3, the most recently identified, is an extracellular protein that exists as a copper- and zinc-containing tetramer with the capacity to remove superoxide [29].
Brain injuries such as ischemia reperfusion and stroke increase O2•− and ROS in mitochondria [3]. The important protective role of SOD2 as a first-line defense against mitochondrial ROS production after cerebral ischemia, is supported by studies using SOD2 mutant mice. SOD2 KO mice showed exacerbated infarct volume [30], increased cytochrome c release, and DNA fragmentation after permanent focal cerebral ischemia [31]. Several transgenic studies have also shown that SOD2 protects against oxidative stress-induced cellular apoptosis and injuries. SOD2 TG mice or cells that overexpress SOD2 showed reduced infarct volume after cerebral ischemia or reduced neural apoptosis against oxidative stress [32]. In cell culture models, SOD2 overexpression significantly reduced cell death mediated by the toxic effects [32-34]. Expression of human SOD2 in mitochondria protects TG mice from oxygen-induced lung injury [35]. Therefore, targeting SOD2 activity provides a potent neuroprotective advantage during brain damage such as ischemia reperfusion and stroke.
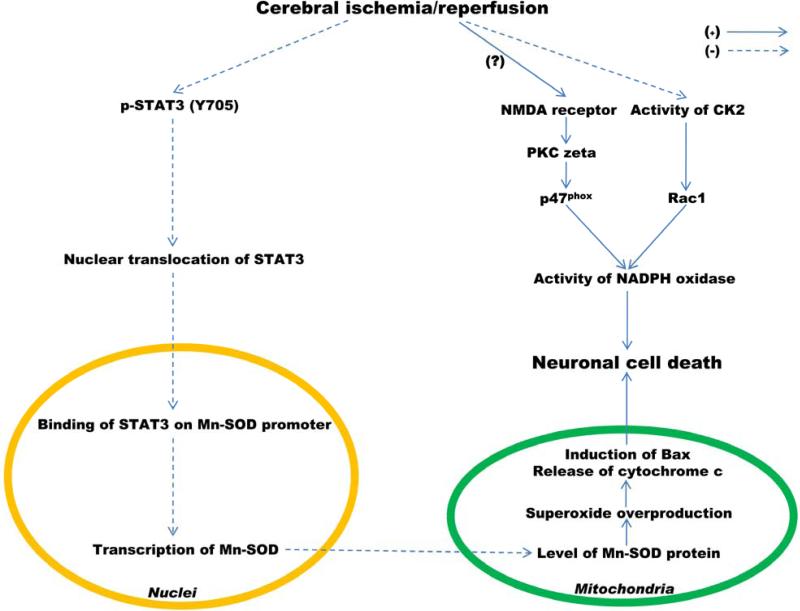
We have recently reported that targeting SOD2 expression by STAT3 activity is neuroprotective in mouse cerebral ischemia [7] (Figure 1). STAT3 upregulates transcription of the SOD2 gene in the mouse brain, and expression of SOD2 is significantly reduced by ischemic reperfusion in a mouse model of middle cerebral artery occlusion (MCAO). Expression of the SOD2 gene is highly regulated and inducible by numerous stimuli in various cells and tissue [36-38]. The levels of mRNA and protein in the SOD2 gene were significantly decreased after reperfusion, and phosphorylation of STAT3 at Y705 was also rapidly decreased at early post-reperfusion periods after transient focal ischemia [7]. Phosphorylated STAT3 is usually recruited into the mouse SOD2 promoter and upregulates transcription of the mouse SOD2 gene in the normal brain. Following an extensive promoter analysis, we found that there are multiple putative binding motifs of STAT3 in the mouse SOD2 promoter and that STAT3 binds to some of the SOD2 promoter. However, its recruitment into the SOD2 promoter was completely blocked and transcription of the mouse SOD2 gene was significantly reduced [7]. This finding was confirmed by a pharmacological approach for STAT3 inhibition using AG490 and a molecular approach for STAT3 knockdown using siRNA transfection in mouse brain tissue or mouse primary cortical neurons. SOD2 was revealed to be a gene that is a specific target of STAT3. The loss of STAT3 activity by ischemic reperfusion increases the generation of ROS and protein nitration, as well as neuronal cell death by reducing SOD2 expression. Also, more inhibition of STAT3 using AG490 under ischemia reperfusion enhances infarct volume [7] and this effect was reversed by treatment with interleukin-6 (IL-6) via recovery of SOD2 expression (Jung et al., unpublished data). IL-6 is a well-known cytokine that activates STAT3 in various cells and tissues and recovers the levels of SOD2 mRNA and protein, which were reduced by cerebral ischemia (Jung et al., unpublished data). This indicates that targeting SOD2 expression by STAT3 activation using treatment with cytokines could be a therapeutic candidate for neuroprotection in cerebral ischemic brain injury.
Fig. 1.
Induction of Mn-SOD (SOD2) expression by STAT3 is neuroprotective after cerebral ischemia and reperfusion. PKC, protein kinaseC.
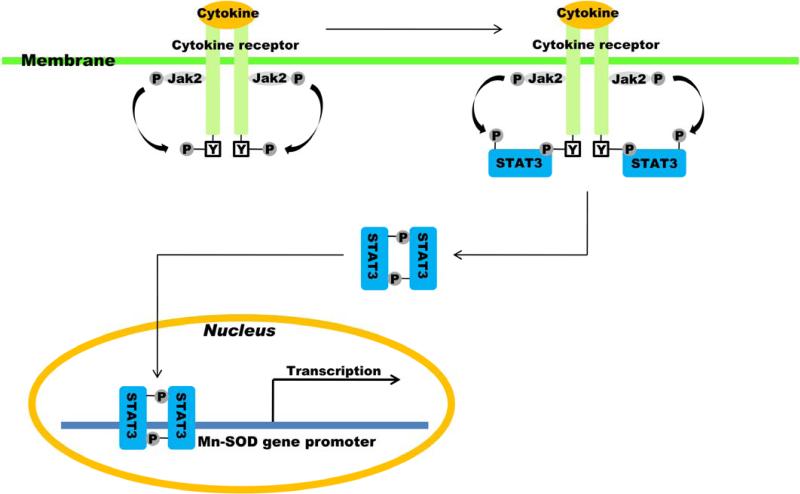
SOD2 synthesis in eukaryotic cells is dramatically upregulated by inflammatory mediators, including lipopolysaccharide, tumor necrosis factor α, IL-1 and IL-6, and interferon-γ [36, 37, 39-42]. This upregulation in SOD2 mRNA in response to some cytokines may result from an increase in the rate of transcription of SOD2 [37-39, 41]. Many studies have reported that cytokines [43-54], growth factors [55-61], and hormones [62, 63] are neuroprotectant and activate STAT3. They accomplish neuroprotection through STAT3 activation in response to multiple signaling pathways in the central nervous system (CNS) following injury (Table). Six STATs have been identified [64], but among them, STAT3 is mainly characterized as neuroprotective against various brain injuries, including cerebral ischemia. Secretoneurine reduced infarct size in rats after MCAO via STAT3 activation and protected primary cortical cells against oxygen-glucose deprivation (OGD) via STAT3-induced antiapoptotic protein expression [65]. Estradiol reduced infarct size in rats after MCAO via reduction of STAT3 phosphorylation [66]. Administration of an IL-6 receptor antibody increased apoptosis and enlarged infarct size in mice after MCAO via STAT3 dephosphorylation [67]. Therefore, cytokines (including growth factors and hormones) for neuroprotection against cerebral ischemia could be candidates for molecular therapeutic reagents via STAT3-upregulated mRNA synthesis of SOD2 (Figure 2).
Table.
Neuroprotective factors that activate STAT3
| STAT3 activators | Effects of neuroprotection | References |
|---|---|---|
| Growth factors | ||
| EGF | Reduced infarct size after cerebral ischemia | 55 |
| Induced neurogenesis after cerebral ischemia | 57 | |
| IGF | Increased neuronal survival | 59 |
| Increased neuritic length | 60 | |
| NGF | Induced neuronal differentiation | 57 |
| Increased STAT3-inducible GAP-43 gene | 58 | |
| BDNF | Increased neurite outgrowth | 56 |
| Reduced infarct size after permanent MCAO | 61 | |
| Cytokines | ||
| IL-6 | Decreased infarct size after cerebral ischemia | 46, 51 |
| Increased neuronal survival | 45 | |
| Promoted axon outgrowth, neuronal differentiation | 50 | |
| LIF | Reduced infarct size after cerebral ischemia | 54 |
| CNTF | Protected ganglion cells | 52 |
| G-CSF | Increased STAT3-inducible Bcl-2 and Pim-1 in neurons | 43, 47 |
| Increased STAT3-inducible cIAP2 in glia | 43 | |
| EPO | Decreased ischemia-induced apoptosis | 44, 53 |
| Promoted regeneration of CNS neurons | 48 | |
| Increased STAT3-inducible Bcl-2 | 49 | |
| Hormone | ||
| Estradiol | Reduced neuronal death after cerebral ischemia | 62 |
| Sustained smaller infarcts after MCAO | 63 |
EGF, epidermal growth factor; IGF, insulin-like growth factor; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; LIF, leukemia inhibitory factor; CNTF, ciliary neurotrophic factor; G-CSF, granulocyte-colony stimulating factor; EPO, erythropoietin.
Fig. 2.
Neuroprotective strategy: Targeting Mn-SOD activity via STAT3 activation. P, phosphorylation; Jak2, Janus activating kinase 2;Y, tyrosine residue.
Targeting NADPH Oxidase and Molecular Signaling
Despite the key role of SOD2 in the first-line defense against mitochondrial oxidative stress during cerebral ischemia and reperfusion, oxidative stress is also generated in cytoplasmic compartments and cytoplasm plays an important role in neuronal death. One such cytoplasmic enzyme is NADPH oxidase, a prooxidant enzyme that generates superoxide through an electron transfer from NADPH to molecular oxygen. NADPH oxidase was originally identified in neutrophils, but its expression and distribution have subsequently been found in many other cell types such as neurons, astrocytes, and microglia in the cortex, hippocampus, and cerebellum [68-71].
NADPH oxidase is a multicomponent enzyme composed of membrane-bound subunits (Nox2 and p22phox) and cytosolic subunits (p47phox, p67phox, and p40phox) [68, 72]. It is activated through migration of cytosolic subunits (p47phox, p67phox, guanosine 5'-triphosphate-Rac1) from cytoplasm to the membrane forming the active NADPH oxidase complex [68]. Rac1 participates in the multicomponent assembly in active NADPH oxidase as a membrane-bound subunit [73] and is a key activator of Nox2 [74, 75]. Expression and activity of NADPH oxidase are regulated by various stresses such as ischemic injury [76, 77] and redox stress in amyotrophic lateral sclerosis [78, 79] and are upregulated in Alzheimer disease [80-82]. Several studies have shown that NADPH oxidase plays a role in oxidative stress, contributing to ischemic brain injury. NADPH oxidase is the major source of NMDA-induced superoxide formation in neurons [26]. NMDA induces a rapid increase in neuronal superoxide production, followed by neuronal death. This increase was completely blocked by apocynin, a NADPH oxidase inhibitor. However, p47phox−/− neurons showed a much attenuated NMDA-induced superoxide production, as well as cell death relative to wild-type (WT) neurons [26]. Superoxide production and membrane translocation of p47phox were also blocked by inhibiting protein kinase C ζ, which activates the NADPH oxidase complex [26] (Figure 1). In apocynin-treated mice and gp91 KO mice, infarction volume after MCAO was significantly less than in the WT mice [76]. This indicates that inhibition of NADPH oxidase is neuroprotective after ischemia reperfusion. Crosstalk between NADPH oxidase and SOD after cerebral ischemia has also been reported. The gp91phox protein significantly increased at 1 h of reperfusion, however, the amount of gp91phox in SOD1 TG mice was much less than in WT mice after focal ischemia. In SOD1 KO mice, gp91phox was significantly more upregulated than in WT mice after focal ischemia at 1 and 4 h of reperfusion [76]. Enhanced expression of gp91phox in SOD2−/+ mice after focal cerebral ischemia and reperfusion has also been examined [83]. Death of endothelial cells in SOD1 TG mice subjected to 6 h of OGD/24 h of reoxygenation was less than in SOD1 WT mice [84], and treatment with apocynin reduced expression of p47 and death in endothelial cells subjected to OGD/reoxygenation [83]. These reports suggest that downregulation of NADPH oxidase activity can be a molecular target for neuroprotection against cerebral ischemic injury.
We recently examined casein kinase 2 (CK2), a novel negative regulator of NADPH oxidase, after cerebral ischemia in mice [85] (Figure 1). CK2 activity was rapidly reduced in mouse brains after cerebral ischemia, and this CK2 inhibition significantly increased NADPH oxidase activity via Rac1 activation. We also examined the molecular targeting mechanism of NADPH oxidase activation by CK2 activity under ischemia reperfusion. CK2 interacted directly with Rac1 in mouse brains and this interaction was significantly diminished after reperfusion in a mouse MCAO model [85]. CK2 inhibition after MCAO enhanced ischemia infarction and neuronal cell death via NADPH oxidase activation, but these effects were reversed by inhibition of NADPH oxidase activity using apocynin [85]. These results support the idea that targeting NADPH downregulation by enhancing CK2 activity after cerebral ischemia at an early post-reperfusion period can be an alternative to SOD2 as a molecular therapeutic target for neuroprotection against oxidative stress in brain injury.
Acknowledgements
This work was supported by grants P50 NS014543, RO1 NS025372, RO1 NS036147, and RO1 NS038653 from the National Institutes of Health, and by the James R. Doty Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, Liu J, Lee Y-S, Nito C, Kamada H, Dodd RL, Hsieh LB, Hassid B, Kim EE, González M, Chan PH. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara T, Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 3.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Huang T-T, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 5.Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice. Implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- 6.Maier CM, Hsieh L, Crandall T, Narasimhan P, Chan PH. Evaluating therapeutic targets for reperfusion-related brain hemorrhage. Ann Neurol. 2006;59:929–938. doi: 10.1002/ana.20850. [DOI] [PubMed] [Google Scholar]
- 7.Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 9.Levy DE, Lee C-k. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 11.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimura M, Tominaga T, Chan PH. Neuroprotective effect of an antioxidant in ischemic brain injury: involvement of neuronal apoptosis. Neurocrit Care. 2005;2:59–66. doi: 10.1385/NCC:2:1:059. [DOI] [PubMed] [Google Scholar]
- 13.Maier CM, Chan PH. Role of superoxide dismutases in oxidative damage and neurodegenerative disorders. Neuroscientist. 2002;8:323–334. doi: 10.1177/107385840200800408. [DOI] [PubMed] [Google Scholar]
- 14.Phillis JW. A “radical” view of cerebral ischemic injury. Prog Neurobiol. 1994;42:441–448. doi: 10.1016/0301-0082(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 15.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brorson JR, Schumacker PT, Zhang H. Nitric oxide acutely inhibits neuronal energy production. J Neurosci. 1999;19:147–158. doi: 10.1523/JNEUROSCI.19-01-00147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaizumi S, Woolworth V, Fishman RA, Chan PH. Liposome-entrapped superoxide dismutase reduces cerebral infarction in cerebral ischemia in rats. Stroke. 1990;21:1312–1317. doi: 10.1161/01.str.21.9.1312. [DOI] [PubMed] [Google Scholar]
- 18.Kinouchi H, Epstein CJ, Mizui T, Carlson E, Chen SF, Chan PH. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci USA. 1991;88:11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uyama O, Matsuyama T, Michishita H, Nakamura H, Sugita M. Protective effects of human recombinant superoxide dismutase on transient ischemic injury of CA1 neurons in gerbils. Stroke. 1992;23:75–81. doi: 10.1161/01.str.23.1.75. [DOI] [PubMed] [Google Scholar]
- 20.Kajita Y, Suzuki Y, Oyama H, Tanazawa T, Takayasu M, Shibuya M, Sugita K. Combined effect of L-arginine and superoxide dismutase on the spastic basilar artery after subarachnoid hemorrhage in dogs. J Neurosurg. 1994;80:476–483. doi: 10.3171/jns.1994.80.3.0476. [DOI] [PubMed] [Google Scholar]
- 21.Kamii H, Kato I, Kinouchi H, Chan PH, Epstein CJ, Akabane A, Okamoto H, Yoshimoto T. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn–superoxide dismutase. Stroke. 1999;30:867–871. doi: 10.1161/01.str.30.4.867. [DOI] [PubMed] [Google Scholar]
- 22.Miller FJ, Jr, Gutterman DD, Rios CD, Heistad DD, Davidson BL. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 23.Pfister H-W, Koedel U, Lorenzl S, Tomasz A. Antioxidants attenuate microvascular changes in the early phase of experimental pneumococcal meningitis in rats. Stroke. 1992;23:1798–1804. doi: 10.1161/01.str.23.12.1798. [DOI] [PubMed] [Google Scholar]
- 24.Chopp M, Chan PH, Hsu CY, Cheung ME, Jacobs TP. DNA damage and repair in central nervous system injury. National Institute of Neurological Disorders and Stroke workshop summary. Stroke. 1996;27:363–369. doi: 10.1161/01.str.27.3.363. [DOI] [PubMed] [Google Scholar]
- 25.Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies – the need for new approaches. Cerebrovasc Dis. 2004;17(suppl 1):153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- 26.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 28.St. Clair D. Manganese superoxide dismutase: genetic variation and regulation. J Nutr. 2004;134:3190S–3191S. doi: 10.1093/jn/134.11.3190S. [DOI] [PubMed] [Google Scholar]
- 29.Petersen SV, Enghild JJ. Extracellular superoxide dismutase: structural and functional considerations of a protein shaped by two different disulfide bridge patterns. Biomed Pharmacother. 2005;59:175–182. doi: 10.1016/j.biopha.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujimura M, Morita-Fujimura Y, Kawase M, Copin J-C, Calagui B, Epstein CJ, Chan PH. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome c and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19:3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller JN, Kindy MS, Holtsberg FW, St. Clair DK, Yen H-C, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong GHW, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Zulueta M, Ensz LM, Mukhina G, Lebovitz RM, Zwacka RM, Engelhardt JF, Oberley LW, Dawson VL, Dawson TM. Manganese superoxide dismutase protects nNOS neurons from NMDA and nitric oxide-mediated neurotoxicity. J Neurosci. 1998;18:2040–2055. doi: 10.1523/JNEUROSCI.18-06-02040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wispé JR, Warner BB, Clark JC, Dey CR, Neuman J, Glasser SW, Crapo JD, Chang L-Y, Whitsett JA. Human Mn-superoxide dismutase in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992;267:23937–23941. [PubMed] [Google Scholar]
- 36.Dougall WC, Nick HS. Manganese superoxide dismutase: a hepatic acute phase protein regulated by interleukin-6 and glucocorticoids. Endocrinology. 1991;129:2376–2384. doi: 10.1210/endo-129-5-2376. [DOI] [PubMed] [Google Scholar]
- 37.Wong GHW, Goeddel DV. Induction of manganous superoxide dismutase by tumor necrosis factor: possible protective mechanism. Science. 1988;242:941–944. doi: 10.1126/science.3263703. [DOI] [PubMed] [Google Scholar]
- 38.Visner GA, Dougall WC, Wilson JM, Burr IA, Nick HS. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem. 1990;265:2856–2864. [PubMed] [Google Scholar]
- 39.Valentine JF, Nick HS. Acute-phase induction of manganese superoxide dismutase in intestinal epithelial cell lines. Gastroenterology. 1992;103:905–912. doi: 10.1016/0016-5085(92)90024-s. [DOI] [PubMed] [Google Scholar]
- 40.Akashi M, Hachiya M, Paquette RL, Osawa Y, Shimizu S, Suzuki G. Irradiation increases manganese superoxide dismutase mRNA levels in human fibroblasts. Possible mechanisms for its accumulation. J Biol Chem. 1995;270:15864–15869. doi: 10.1074/jbc.270.26.15864. [DOI] [PubMed] [Google Scholar]
- 41.Borg LAH, Cagliero E, Sandler S, Welsh N, Eizirik DL. Interleukin-1β increases the activity of superoxide dismutase in rat pancreatic islets. Endocrinology. 1992;130:2851–2857. doi: 10.1210/endo.130.5.1533363. [DOI] [PubMed] [Google Scholar]
- 42.Whitsett JA, Clark JC, Wispé JR, Pryhuber GS. Effects of TNF-α and phorbol ester on human surfactant protein and MnSOD gene transcription in vitro. Am J Physiol Lung Cell Mol Physiol. 1992;6:L688–L693. doi: 10.1152/ajplung.1992.262.6.L688. [DOI] [PubMed] [Google Scholar]
- 43.Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143:965–974. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck H-H, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Rüther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Sirén A-L. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 45.Hama T, Kushima Y, Miyamoto M, Kubota M, Takei N, Hatanaka H. Interleukin-6 improves the survival of mesencephalic catecholaminergic and septal cholinergic neurons from postnatal, two-week-old rats in cultures. Neuroscience. 1991;40:445–452. doi: 10.1016/0306-4522(91)90132-8. [DOI] [PubMed] [Google Scholar]
- 46.Herrmann O, Tarabin V, Suzuki S, Attigah N, Coserea I, Schneider A, Vogel J, Prinz S, Schwab S, Monyer H, Brombacher F, Schwaninger M. Regulation of body temperature and neuroprotection by endogenous interleukin-6 in cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- 47.Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, Mochizuki H, Mizuno Y, Urabe T. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26:402–413. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 48.Kretz A, Happold CJ, Marticke JK, Isenmann S. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci. 2005;29:569–579. doi: 10.1016/j.mcn.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Kumral A, Genc S, Ozer E, Yilmaz O, Gokmen N, Koroglu TF, Duman N, Genc K, Ozkan H. Erythropoietin downregulates Bax and DP5 proapoptotic gene expression in neonatal hypoxic-ischemic brain injury. Biol Neonate. 2006;89:205–210. doi: 10.1159/000089951. [DOI] [PubMed] [Google Scholar]
- 50.Kushima Y, Hatanaka H. Interleukin-6 and leukemia inhibitory factor promote the survival of acetylcholinesterase-positive neurons in culture from embryonic rat spinal cord. Neurosci Lett. 1992;143:110–114. doi: 10.1016/0304-3940(92)90244-2. [DOI] [PubMed] [Google Scholar]
- 51.Loddick SA, Turnbull AV, Rothwell NJ. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 52.MacLaren RE, Buch PK, Smith AJ, Balaggan KS, MacNeil A, Taylor JS, Osborne NN, Ali RR. CNTF gene transfer protects ganglion cells in rat retinae undergoing focal injury and branch vessel occlusion. Exp Eye Res. 2006;83:1118–1127. doi: 10.1016/j.exer.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 53.Solaroglu I, Solaroglu A, Kaptanoglu E, Dede S, Haberal A, Beskonakli E, Kilinc K. Erythropoietin prevents ischemia-reperfusion from inducing oxidative damage in fetal rat brain. Childs Nerv Syst. 2003;19:19–22. doi: 10.1007/s00381-002-0680-2. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki S, Yamashita T, Tanaka K, Hattori H, Sawamoto K, Okano H, Suzuki N. Activation of cytokine signaling through leukemia inhibitory factor receptor (LIFR)/gp130 attenuates ischemic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:685–693. doi: 10.1038/sj.jcbfm.9600061. [DOI] [PubMed] [Google Scholar]
- 55.Jin K, Sun Y, Xie L, Childs J, Mao XO, Greenberg DA. Post-ischemic administration of heparin-binding epidermal growth factor-like growth factor (HB-EGF) reduces infarct size and modifies neurogenesis after focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 2004;24:399–408. doi: 10.1097/00004647-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Labelle C, Leclerc N. Exogenous BDNF, NT-3 and NT-4 differentially regulate neurite outgrowth in cultured hippocampal neurons. Dev Brain Res. 2000;123:1–11. doi: 10.1016/s0165-3806(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 57.Nakatomi H, Kuriu T, Okabe S, Yamamoto S-i, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 58.Schicho R, Schuligoi R, Sirinathsinghji DJS, Donnerer J. Increased expression of GAP-43 in small sensory neurons after stimulation by NGF indicative of neuroregeneration in capsaicin-treated rats. Regul Pept. 1999;83:87–95. doi: 10.1016/s0167-0115(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 59.Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- 61.Yamashita K, Wiessner C, Lindholm D, Thoenen H, Hossmann K-A. Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab Brain Dis. 1997;12:271–280. doi: 10.1007/BF02674671. [DOI] [PubMed] [Google Scholar]
- 62.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17β-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 64.Levy DE, Darnell JE., Jr STATs: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 65.Shyu W-C, Lin S-Z, Chiang M-F, Chen D-C, Su C-Y, Wang H-J, Liu R-S, Tsai C-H, Li H. Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest. 2008;118:133–148. doi: 10.1172/JCI32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dziennis S, Alkayed NJ. Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev Neurosci. 2008;19:341–361. doi: 10.1515/revneuro.2008.19.4-5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamashita T, Sawamoto K, Suzuki S, Suzuki N, Adachi K, Kawase T, Mihara M, Ohsugi Y, Abe K, Okano H. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: possible involvement of Stat3 activation in the protection of neurons. J Neurochem. 2005;94:459–468. doi: 10.1111/j.1471-4159.2005.03227.x. [DOI] [PubMed] [Google Scholar]
- 68.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 69.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu G-Y, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim MJ, Shin K-S, Chung Y-B, Jung KW, Cha CI, Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- 71.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- 72.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 73.Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin Sci. 2005;109:217–226. doi: 10.1042/CS20050067. [DOI] [PubMed] [Google Scholar]
- 74.Hordijk PL. Regulation of NADPH oxidases. The role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 75.Miyano K, Sumimoto H. Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie. 2007;89:1133–1144. doi: 10.1016/j.biochi.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia–reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li B, Guo Y-S, Sun M-M, Dong H, Wu S-Y, Wu D-X, Li C-Y. The NADPH oxidase is involved in lipopolysaccharide-mediated motor neuron injury. Brain Res. 2008;1226:199–208. doi: 10.1016/j.brainres.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 79.Wu D-C, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis in mice. Proc Natl Acad Sci USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Virgilio F. New pathways for reactive oxygen species generation in inflammation and potential novel pharmacological targets. Curr Pharm Des. 2004;10:1647–1652. doi: 10.2174/1381612043384727. [DOI] [PubMed] [Google Scholar]
- 81.Block ML. NADPH oxidase as a therapeutic target in Alzheimer's disease. BMC Neurosci. 2008;9(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. doi:10.1186/1471-2202-1189-S1182-S1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zekry D, Epperson TK, Krause K-H. A role for NOX NADPH oxidases in Alzheimer's disease and other types of dementia? IUBMB Life. 2003;55:307–313. doi: 10.1080/1521654031000153049. [DOI] [PubMed] [Google Scholar]
- 83.Maier CM, Narasimhan P, Song YS, CHAN PH. Neuroscience. San Diego: Nov 3-7, 2007. 2007. NADPH oxidase expression following cerebral ischemia-reperfusion in SOD2-KO mice may be associated with endothelial cell damage. Presentation No. 447.9. [Google Scholar]
- 84.Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]